ABSTRACT
How the variation in phenotypic traits like cell size and motility impacts predator-induced cellular aggregation is not known. Furthermore the genetic composition of cell groups in mixed populations of Chlamydomonas has not been investigated. An examination of these two questions will not only enhance our understanding of Chlamydomonas ecology, but also shed light on the primordial steps before integrated multicellular groups were established. Group living comes with viability and reproductive costs and it is not known how these are shared if groups are genetically heterogeneous. We observed that the natural predator Peranema trichophorum (Euglenoidea) induced clumping in Chlamydomonas. When co-cultured with P. trichophorum cells protected themselves by forming facultative groups (reverting back to a unicellular lifestyle once predators were removed). The dynamics of group formation in different Chlamydomonas species and strains correlated with cell size and swimming speed. Small or less motile strains aggregated more readily than large, fast-swimming ones. Interestingly, Chlamydomonas groups were both intra-species and inter-species chimaeric. This suggests that the predator-induced group formation in Chlamydomonas involved cells coming together rather than staying together and during aggregation cells showed little or no discrimination between self and non-self. These data demonstrate that the dynamics of cell aggregation, in unicellular volvocines at least, depends on phenotypic traits like cell size and motility and high genetic relatedness is not mandatory at this initial stage. These findings further our understanding of aggregation in mixed Chlamydomonas populations and have implications for understanding the very first steps on the road to simple multicellularity.
INTRODUCTION
Cellular aggregation is a phenomenon observed in many unicellular and facultatively multicellular organisms and there are several environmental factors that lead to this phenotype (Raper, Citation1984; Boraas et al., Citation1998; Kaiser, Citation2003; Grosberg & Strathmann, Citation2007; Bonner, Citation2009; Niklasa & Newman, Citation2013; Tarnita et al., Citation2013). In the model unicellular organism Chlamydomonas (Chlorophyceae) some of the selective pressures inducing cell aggregation, for example predation or changes in the microenvironment, are well established (Lürling & Beekman, Citation2006; Schlesinger et al., Citation2012) but the effects of phenotypic variation of traits such as cell size and swimming speed on the dynamics of aggregation are unknown. Addressing this gap in knowledge will further our understanding of the ecology of Chlamydomonas species. For example, in natural populations it is not known whether cells of different sizes or swimming speeds aggregate with comparable efficiencies in stressful environments. This would be important since small, slower cells would have a greater probability of being engulfed by predators. It is also proposed that the first multicellular organisms were groups of undifferentiated cells and predation was the dominant ecological selective pressure driving this phenotype in several organisms including volvocines and other chlorophytes (Bell, Citation1985; Boraas et al., Citation1998; Lürling & Van Donk, Citation2000; Lürling & Beekman, Citation2006; Grosberg & Strathmann, Citation2007). Chlamydomonas reinhardtii is used as a model organism for investigating the selective forces and questions concerning cells coming together or staying together at the very beginning of multicellular evolution (Lürling & Beekman, Citation2006; Ratcliff et al., Citation2013; Moulton & Bell, Citation2013). In addition, the genetic makeup of predator-induced cell aggregates is largely unexplored and in the laboratory setting, group formation is typically induced using clonal populations.
In this study, the roles of cell size and motility in aggregation were examined and the idea that the earliest groups on the road to multicellularity were clonal is challenged. Unicellular Chlamydomonas cells with different cell sizes and motility were co-cultured with a protozoan predator Peranema trichophorum (Euglenoidea) and their responses investigated. Peranema, a flagellated free-living protist which occurs in freshwater ponds and lakes, can engulf small unicellular living or dead prey but not large cell aggregates (Chen, Citation1950; our observation). This unique predator–prey system provides an opportunity to study the correlation between organismal phenotypes and the dynamics of aggregation as well as the genetic structure of groups. In the presence of predators or their culture filtrate, all the species and strains of Chlamydomonas examined here formed groups within 18–48 h and comprised 10–100,000 cells. The smaller, slower-swimming strains formed groups more readily than larger, faster-swimming forms. In addition, groups were inter- and intra-species chimaeric, indicating that aggregates formed by cells coming together rather than staying together. These data show that the dynamics of cell aggregation, in unicellular volvocines at least, depends on traits like cell size and swimming speed. In addition, high genetic relatedness is not a pre-condition for aggregation, which is an important finding for our understanding of how heterogeneous populations of Chlamydomonas respond to predators in natural settings as well as the very first steps on the road to simple multicellularity.
MATERIALS AND METHODS
Chlamydomonas strains and species
Six strains belonging to three species of Chlamydomonas were used as prey: C. reinhardtii UTEX-89, C. reinhardtii CC-125, C. reinhardtii CC-2342, C. reinhardtii CC-2931, C. debaryana UTEX-1344 and C. moewusii UTEX-9. Organisms were obtained from the Chlamydomonas resource centre, University of Minnesota, USA and the University of Texas, Austin, USA. Peranema trichophorum (Carolina Biological Supply Company, Burlington, NC) was used as a predator. The unresolved issues regarding Chlamydomonas taxonomy and phylogeny (Buchheim et al., Citation1996; Pröschold et al., Citation2001) are appreciated; however, this would not have any effect on the experimental aims and objectives.
Growth of Chlamydomonas and Peranema
All Chlamydomonas strains were streaked on Tris-Acetate-Phosphate (TAP) agar plates to obtain pure isolates. Cultures of single colonies were grown axenically in standard TAP medium (Gorman & Levine, Citation1965) at 22°C in a 12 h:12 h light-dark cycle. Peranema trichophorum was grown axenically at 22°C under fluorescent room light during the day and in the dark at night; prior to inoculation in the growth medium the Peranema cells were rendered bacteria-free using the method of Saito et al. (Citation2003). Peranema growth medium was prepared as follows: 2–3 wheat grains and egg yolk (100–200 mg of boiled egg yolk suspended in 1 ml sterile water and vortex mixed) in 100 ml spring water (Valpre; pH 7.3); boiled for 5 min and allowed to cool. Within 5–6 days a large number of (1–5 × 105 ml–1) elongated, healthy, motile Peranema were produced and remained alive for 3–4 weeks after which they typically become thin, curled, immotile and died. After every 2–3 weeks a fresh culture was started by inoculating 1 ml old Peranema culture into a fresh 100 ml medium. Both Chlamydomonas and Peranema cultures were plated periodically on nutrient agar plates to screen for bacterial contamination.
Chlamydomonas cell size, motility and genetic differences
All Chlamydomonas strains were characterized with respect to size and motility and measurements performed on exponentially growing cells. Cell size was also measured in aggregates. For size measurements, cells growing in TAP broth (liquid) and TAP agar (solid) were harvested at the end of light and dark cycles. Cells were suspended in TAP medium and photographed using 100 × oil immersion lens (Olympus BX4-1) and a DP72 (Olympus) colour camera. Similarly, the cell aggregates were photographed after 24 h of formation and used for measuring cell size. Cell size was measured using Cellsens Dimension 1.7 digital imaging software in two different ways (–): (i) the length from papilla to the posterior end; and (ii) the mean of the two longest diameters. For cell motility, an aliquot was loaded on a haemocytometer and recorded for 2–3 min using the microscope-camera setup mentioned above. Cell motility was not quantified but cells were easily categorized as either slow or fast swimmers based on obvious qualitative differences. Genetic similarity indices of C. reinhardtii UTEX-89, C. reinhardtii CC-125, C. reinhardtii CC-2342, C. reinhardtii CC-2931 (four strains of the same species) were calculated from published data for four polymorphic microsatellite DNA markers (Kang & Fawley, Citation1997). The genetic similarity between two strains is given by the number of similar microsatellite DNA bands resulting from a given primer pair over the total number of different DNA bands produced by that primer pair over all the four strains. The genetic similarity values were averaged over all the four primer pairs. The three Chlamydomonas species used in the study are phylogenetically distinct based on nuclear-encoded SSU rRNA sequence data (Pröschold et al., Citation2001).
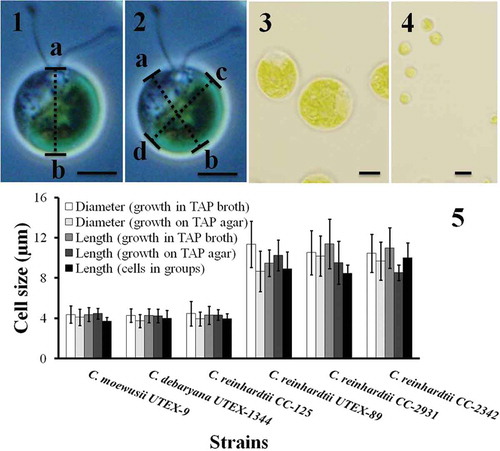
Figs 1–5. Cell size of Chlamydomonas strains grown under different conditions. Cell size was measured after cells were harvested from TAP agar, TAP broth and after they had formed groups. The cell size was measured in two different ways. In the first case the length from papilla to the posterior end (length ‘ab‘ in ) was measured. In the second case the cell diameter was measured at two different angles (the mean of diameter ‘ab‘ and ‘cd‘ in ). The results are mean ± SD; n = 40–50 cells of each strain. Chlamydomonas strains/species had different cell size (–): C. moewusii UTEX-9, C. debaryana UTEX-1344 and C. reinhardtii CC-125 were significantly smaller than C. reinhardtii UTEX-89, C. reinhardtii CC-2931 and C. reinhardtii CC-2342. A typical big cell and a small cell is shown in and respectively. Scale bars: –, 10 µm.
Aggregation by Chlamydomonas species
Filtrate of predator cultures was used to see whether the physical presence of the predators was necessary for inducing aggregation. Chlamydomonas cells growing in TAP medium were harvested from the logarithmic phase of growth using an Eppendorf (5702R) centrifuge at 3000 rpm for 3 min washed in Standard Volvox Medium (SVM: Kirk & Kirk, Citation1983) and re-suspended in 20 ml SVM at a density of 2 × 106 cells ml–1 in 100 ml flasks to which P. trichophorum cells (washed with SVM) were added at a density of 1 × 104 ml–1. SVM was used for the predation experiments because P. trichophorum cells (after growing in the medium described above) survived longer in SVM than in TAP. In controls the same amount of Peranema-naïve growth medium (that is, sterile medium without exposure to predators or their culture filtrate) was added. In another set of predation experiments predators were previously grown on unicellular Chlamydomonas cells as the sole food source instead of Peranema growth medium. Briefly, 102–103 Peranema cells were co-inoculated with a suspension of C. reinhardtii UTEX-89 (1–5 × 104 ml–1) in SVM and the flasks were kept shaking at 22°C in a cycle of 12h dark:12h light). Chlamydomonas reinhardtii UTEX 89 was used as food source because it is a relatively large, highly motile strain which remained unicellular for a longer period in the presence of predators (see results). After every 24 h a small amount of fresh unicellular C. reinhardtii UTEX 89 cells was added to the cultures. In 2–3 days a large number of predators were available. In predation experiments the number of predators and prey would have changed during the course of the experiment as Chlamydomonas can grow and divide in SVM but is engulfed by Peranema which will grow, divide and eventually die. In experiments where the role of Peranema culture filtrate in group formation was tested the Chlamydomonas cells were prepared as mentioned above and inoculated in a six-well plate (Nunclon surface; 35 mm diameter) at a density of 1 × 107 cells well–1. 2–3-week-old P. trichophorum culture was collected and filtered (0.2 µm pore size; Pall Life Sciences, South Africa) to remove any cells. Filtrate was added to wells (four different volumes well–1: 0.5 ml, 1 ml, 2 ml and 4 ml). In controls either Peranema cells (positive control) or sterile Peranema growth medium (negative control) were used. The Chlamydomonas cell density (2 × 106 cells ml–1) and the total volume (5 ml well–1) was adjusted with SVM. For quantifying aggregates, a wet mount of a 10 µl sample (from the flasks containing Chlamydomonas cells co-inoculated with the predators) was made and observed microscopically as above. In related experiments we monitored aggregation in the presence of predators on solid SVM agar plates. The aim was to see whether Chlamydomonas cells (which are less motile on solid agar plates) can avoid predation even if they are constrained from joining together and forming groups. Chlamydomonas and Peranema cells were co-inoculated on 10 cm SVM agar plates at a density of 1×105 cm−2 and 1×103 cm−2 respectively; control plates contained only Chlamydomonas cells at the same density. Since the Peranema were added directly from the culture flasks the inoculum contained the chemicals which would be present in their culture filtrate too. Plates were incubated at 22°C in a daily cycle of 12h dark followed by 12h light. Plates were observed for the presence of algal growth and their intensity after 4–5 days using 10× lens of Olympus BX4-1 microscope.
Genetic structure of aggregates formed in the presence of predators
To investigate whether groups formed in the presence of predators were clonal or chimaeric, Chlamydomonas cells were harvested from the growth medium (TAP) and the cells of two strains/species mixed in a 1:1 ratio (1 × 106 cells ml–1 of each), predators were added (1 × 104 ml–1) and the flasks were incubated at 22°C. Chlamydomonas reinhardtii CC-125 (a small strain) was mixed with three big strains (C. reinhardtii UTEX-89, C. reinhardtii CC-2342 and C. reinhardtii CC-2931) to make three intra-species mixes. Similarly, C. debaryana and C. moewusii (both small strains) were mixed with three big C. reinhardtii strains (six inter-species combinations). Chlamydomonas strains/species in mix cultures were distinguishable by (i) size differences, and (ii) staining one of the strains or species with the fluorescent dye SYBR Green I (S7563; Molecular Probes, USA), which intercalates irreversibly in dsDNA. Chlamydomonas cells were suspended in SVM medium and stained with 2X SYBR Green I for 2 h at 22°C with constant shaking in dark at 25 RPM (Intelli-mixer; ELMI). After staining cells were washed three times in cold SVM to remove any remaining dye from the suspension and re-suspended in SVM. Stained cells of one strain were mixed with unstained cells of another stain; in another set the stained strain was reversed. It was confirmed that all the cells stained with SYBR green were fluorescent green (that is, the efficiency of staining was 100%). Controls involved mixing stained and unstained cells of the same strain.
Costs of group formation
Chlamydomonas aggregates were assessed for viability, growth and division after every 24 h. Cell viability was assessed using a vital dye Trypan blue (Sigma; 0.4%). 100 µl each of Trypan blue and an aliquot containing groups of cells formed in the presence of Peranema were mixed and incubated at room temperature for 5 min. Cells were loaded on a haemocytometer and observed using a 40× objective lens (Olympus BX4-1). Dead cells cannot exclude the dye and appear blue. Cell viability was confirmed by transferring the groups (which were collected under the microscope using micropipettes) into Chlamydomonas growth medium (TAP) without predators to see if the groups reverted back to the unicellular state. Cell division in groups was monitored microscopically and photographed every 24 h. The physical constraints in aggregates meant that daughter cells could be observed because of their proximity to the mother cell.
RESULTS
Cell size and motility of Chlamydomonas species and strains
The six strains used in this study formed two distinct morphotypes based on their size and motility: three strains were small and slow swimmers; three were larger and faster swimmers (–; Suppl. movies S1–S3). Chlamydomonas moewusii UTEX-9, C. debaryana UTEX-1344 and C. reinhardtii CC-125 were smaller (mean diameter of the three strains 4.4 ± 0.1 µm when grown in liquid TAP and 3.9 ± 0.2 µm when grown on TAP agar) than C. reinhardtii UTEX-89, C. reinhardtii CC-2342 and C. reinhardtii CC-2931 (mean diameter of the three strains 10.5 ± 0.6 µm when grown in liquid TAP and 9.8 ± 0.8 µm when grown on TAP agar). The difference in size was statistically significant (t-test for means assuming unequal variances; P < 0.001; t = 17.53, df = 5; n = 370 cells measured from TAP broth and 191 cells from TAP agar). Cell sizes grown on TAP agar vs. TAP broth were similar (t-test; P > 0.05). Differences in cell size within three small strains (C. moewusii UTEX-9, C. debaryana UTEX-1344 and C. reinhardtii CC-125) and three large strains (C. reinhardtii UTEX-89, C. reinhardtii CC-2342 and C. reinhardtii CC-2931) were not significant (t-test; P > 0.05). The second set of size measurements using cell length from papilla to the posterior end () gave the same result. Here too, C. moewusii UTEX-9, C. debaryana UTEX-1344 and C. reinhardtii CC-125 were significantly smaller than C. reinhardtii UTEX-89, C. reinhardtii CC-2342 and C. reinhardtii CC-2931 (–). The cell size differences mentioned above were sustained even after ~ 24 h of group formation (). Cell motility was compared between all six strains of Chlamydomonas grown in TAP liquid medium under similar growth conditions. At any given time point, ~50–60% of individual cells in the population were motile. Differences in cell motility were qualitatively obvious (Suppl. movies S1–S3) and not quantified further. Chlamydomonas moewusii UTEX-9, C. debaryana UTEX-1344 and C. reinhardtii CC-125 (small strains) were slow swimmers compared with the three large strains (C. reinhardtii UTEX-89, C. reinhardtii CC-2342 and C. reinhardtii CC-2931). In each experiment it was confirmed that the Chlamydomonas cultures were pure and the observed differences in cell size and motility were reproducible.
Peranema is a voracious and natural predator of Chlamydomonas
The transparent body of Peranema and the dark green colour of Chlamydomonas allowed visualization and enumeration of engulfed algal cells by light microscopy (–). Peranema was a voracious predator. In less than 2 h of co-culture with Chlamydomonas, 63.8% (23/36) of Peranema cells had already engulfed algal cells and after 24 h almost all Peranema had engulfed Chlamydomonas cells (). Peranema were able to feed on all the six strains of Chlamydomonas. However, strains were engulfed at different rates; on average small size and slow-swimming strains were eaten more often than larger, fast-swimming strains (small: 27.5 ± 10.2 cells/Peranema; big: 3.8 ± 6.2 cells/Peranema; mean ± SD; n = 20–25 Peranema in each strain; t-test; P < 0.001; t = 9.45, df = 131). Among the small strains C. moewusii UTEX-9 (32.4 ± 9.7 cells/Peranema) was eaten more often than C. debaryana UTEX-1344 (20.0 ± 7.4) and C. reinhardtii CC-125 (21.5 ± 6.1). The cells of large strains were engulfed at similar rates: C. reinhardtii UTEX-89 (17.2 ± 7.4/Peranema); C. reinhardtii CC-2342 (18.0 ± 7.1); C. reinhardtii CC-2931 (13.9 ± 6.3). The predation rates mentioned here were monitored in the initial few hours of co-culture when Peranema were actively feeding and had not accumulated enough algal biomass to affect predation rates on small vs. large cells.
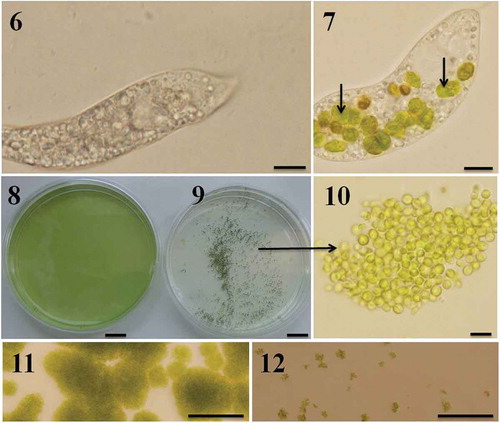
Figs 6–12. Predator-induced group formation in Chlamydomonas. Peranema raised in the laboratory using a mixture of spring water, boiled egg yellow and wheat grains is transparent and accumulates a large amount of starch granules (). Immediately after the Peranema cells were transferred into the suspension containing Chlamydomonas cells (in SVM medium) they started preying on green algal cells; intact Chlamydomonas cells are indicated with arrows (). Chlamydomonas cells formed groups soon after they were exposed to the predator. Shown here are C. moewusii UTEX-9 cells growing in the absence () and presence () of the predator, P. trichophorum. Note the groups formed by C. moewusii UTEX-9 cells in the presence of the predator (–). Predation was also studied on solid medium; Chlamydomonas cells were inoculated on SVM agar plates in the absence () and presence () of P. trichophorum and the plates were observed after 5 days of incubation at 22ºC. Since the motility of Chlamydomonas cells (not of Peranema) was restricted on solid plates they did not form groups and were heavily predated by Peranema (). In a control where Peranema was not added, algal growth was unaffected (). Scale bars: –, µm; –, cm; , 10 µm; –, 200 µm.
Facultative chimaeric aggregation
In the presence of predators unicellular Chlamydomonas cells formed aggregates of 10–100,000 cells (–). Groups were tightly compacted (shaking them continuously at 150 rpm during the course of the experiment did not separate the cells from groups), visible with the naked eye, and had a tendency to sink to the bottom of the flasks or plates (). All strains formed groups but there were appreciable differences with respect to the number of cells per group and the time taken to do so. Small slow-moving strains (C. moewusii UTEX-9, C. debaryana UTEX-1344 and C. reinhardtii CC-125) formed groups in 18–48 h. Chlamydomonas moewusii, which was eaten rapidly by Peranema, was the fastest; they formed groups within 18–24 h. In the case of small strains, 86.1 ± 12.4% of cells (mean ± SD) formed groups (~ 14% cells stayed unicellular). The remaining three strains (large and fast swimmers: C. reinhardtii CC-2342, C. reinhardtii CC-2931 and C. reinhardtii UTEX-89) were slow responders (they took 3–4 days to form groups) and only 41.7 ± 13.5% cells entered into groups. These differences between small and big strains with respect to the time required for group formation and the number of cells entering groups had a significant impact on the viability of both Chlamydomonas and Peranema. If the Chlamydomonas strains took longer to form groups, predators survived longer, presumably because the unicellular food source was available. Peranema starved and died much sooner when grown in cultures of small strains than in cultures of large strains. This is presumably because small strains aggregated quickly with fewer cells available whereas the large strains formed groups much more slowly and a relatively higher proportion of cells remained solitary and so accessible to the predators. Chlamydomonas cells were predated heavily by Peranema if the predation experiments were performed on solid plates (–), presumably because this limited their motility and aggregation.
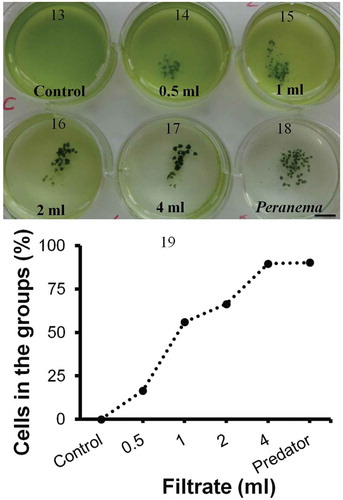
Interestingly, Chlamydomonas also formed groups in the presence of Peranema culture filtrate (–). Aggregation was even more rapid than the physical presence of Peranema possibly because of the signalling molecules already present in the filtrate (aggregation in the presence of culture filtrate: 10–12 h vs. Peranema: 18–48 h). The effect was quantitative: more cells entered groups as the proportion of Peranema culture filtrate increased (). The culture filtrate too induced a stronger, more rapid response in smaller, slower-moving strains compared with the larger, faster-moving ones. These results indicate that the physical presence of the predator is not necessary to induce group formation. The presence of predators can be detected through unknown chemicals (kairomones) secreted by Peranema into the medium.
Figs 13–19. Group formation by Chlamydomonas in the presence of Peranema culture filtrate. Chlamydomonas cells were suspended in SVM and increasing amounts of Peranema culture filtrate added (0.5–4 ml; –). In a ‘control’ well () culture filtrate was not added; instead, the same amount of sterile, Peranema- naïve growth medium was added. In a ‘Peranema’ well () living Peranema cells were added. Chlamydomonas formed groups in the presence of Peranema culture filtrate and as the amount of filtrate was increased the number of cells in the groups (compared with free swimming) increased (). Shown here is a typical outcome from one of three such experiments in C. moewusii UTEX-9. In the mean values from three technical replicates from one experiment are plotted; coefficient of variation within an experiment is 5–15%. Scale bar: 1 cm.
Group dynamics
Chlamydomonas cells stayed in aggregates as long as the living predators or active kairomones were present in cultures. Peranema cells became thin, immotile, curled and eventually died due to lack of an available food source (no algal cells were seen in their corpses and there was no alternative energy source in the medium). Predators died earlier in the cultures containing small Chlamydomonas strains than the cultures containing big Chlamydomonas strains. The difference in predator viability when grown on small cells opposed to large cells was probably because small strains formed tight groups within 18–48 h with almost no free-swimming cells to be predated and big cells formed groups after only 3–4 days with ~ 60% free swimming unicellular cells. Soon after Peranema died (and probably the factors secreted by them were degraded/evaporated), groups disaggregated and free-swimming single cells were seen. The group dissolution was not 100% efficient – a small number of cells still remained clumped even after predators died. Subsequent addition of fresh Peranema once again induced aggregation.
Genetic heterogeneity in the groups
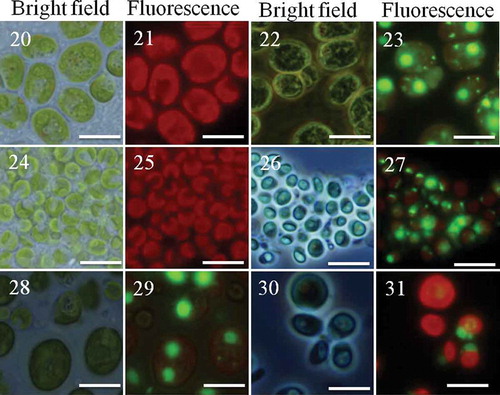
The four strains of C. reinhardtii used in the study were genetically distinct. Genetic similarity between these strains varied from 30.7 % to 69.2 % (). The three species of Chlamydomonas (C. reinhardtii, C. debaryana and C. moewusii) occupy different clades in a nuclear-encoded SSU rRNA sequence phylogeny (Pröschold et al., Citation2001). Two strains/species of Chlamydomonas were mixed and co-cultured in the presence of Peranema. In mixed cultures predator-induced aggregation was observed and all three intra-species and six inter-species mixes formed chimaeric aggregates (– and Suppl. –). This indicates that Chlamydomonas cells exhibit little or no self/non-self recognition during aggregation. The initial 1:1 mixing ratio was probably not maintained prior to group formation. The number of large strain cells was probably less due to the significantly slower doubling time than for the small strain cells in the absence of predators (data not shown). Large strains also took longer to aggregate and their number were further decreased by predation. Both intra-species and inter-species chimaeric groups had significantly more small cells than large ones although the distribution was patchy. Overall, however, in intra-species mixes 85 out of 87 (97.7%) groups were chimaeric and 83.1 ± 5.6 % of the cells were of the small strains (mean ± SD; n = 85 groups, on average 93 cells per group). Where two species (as opposed to strains) were present in mixes, 104 out of 120 (86.6%) groups were chimaeric and 91.8 ± 9.3% cells were of the small phenotype (mean ± SD; n = 104 groups, on average 126 cells per group). In cases where groups comprised one cell type only, they were of the small cell phenotype.
Table 1. Genetic similarity in Chlamydomonas reinhardtii strains. Genetic similarity was calculated from the published data (Kang & Fawley, Citation1997). The values in the table indicate the genetic similarity (%); in parentheses are the number of similar characters (DNA bands)/total characters (DNA bands).
Figs 20–31. Clonal and chimaeric groups formed by Chlamydomonas in the presence of Peranema. Chlamydomonas cell cultures either as clones or binary mixes of two strains/species were made and inoculated with the predator Peranema. Chlamydomonas cells belonging to two different strains were identified based on their size and SYBR green I stain. Groups were formed after 18–48 h; the groups were observed under bright field or fluorescence (Green fluorescence protein filter). Shown here are groups formed by C. reinhardtii CC-2931(large cells) and C. moewusii UTEX-9 (small cells): – (C. reinhardtii CC-2931, unstained); – (C. reinhardtii CC-2931, stained); – (C. moewusii UTEX-9, unstained); – (C. moewusii UTEX-9, stained); – (chimaeric groups formed by a mix of C. reinhardtii CC-2931(large and stained cells) and small, unstained cells of C. moewusii UTEX-9); – (the chimaeric groups formed by the same pair except the stained strain was reversed; that is, small cells of C. moewusii UTEX-9 were stained and mixed with large, unstained cells of C. reinhardtii CC-2931). Scale bars: –, 10 µm.
Cell viability, division and death in Chlamydomonas groups
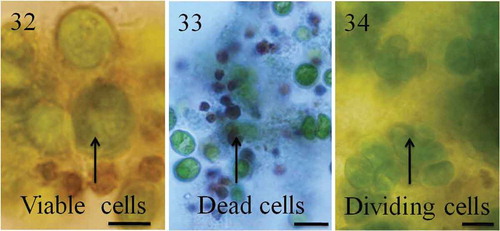
The chimaeric nature of aggregates means that Chlamydomonas cells must have come together as opposed to staying together in the presence of Peranema or their culture filtrate and remained in groups as long as the predators were present. The groups (maintained in flasks at 22°C, shaking at 140 rpm with dark: light cycle) were picked manually and observed for morphological changes or stained using Trypan blue to assess cell viability. Cells in the group started dying after 4–5 days of aggregation. The dead cells in groups shrank, became dark brown in colour and were unable to exclude the vital dye Trypan blue (–). When observed after 2–3 weeks, cell death was more prominent in groups formed by the large, fast swimmers compared with the smaller, slow-swimming strains (big cells = 70.7 ± 25.0% dead cells/group; small cells = 15.8 ± 10.9%; mean ± SD; n = 80 groups; t-test, P < 0.001). Chlamydomonas cultures growing in the absence of predators (controls) had no signs of cell death in this period. Simultaneously, a few cells in the groups were growing and actively dividing (). The recently divided daughter cells remained with mother cells, probably because daughter cell movement was physically constrained by the presence of other cells in the group. Once predator-induced aggregates (after 2–3 weeks) were transferred to fresh TAP medium, cells reverted to their unicellular free-living state indicating that aggregation was indeed the response to the presence of predators or their filtrate.
Figs 32–34. Cell turnover in groups. Chlamydomonas cells formed tight groups in the presence of predators or their culture filtrates. Within groups, some cells grew or divided, others died. Cells in the groups are protected from predators but for unknown reasons a large number of cells in the groups die. indicates green viable cells and dark brown dead cells. Cell death was confirmed using Trypan blue stain; dead cells appear blue as they fail to exclude Trypan blue (). Recently divided cells are shown in . Scale bars: –, 10 µm.
DISCUSSION
Cellular aggregation forms part of the life cycle or ecology of many unicellular organisms and is particularly interesting for addressing questions concerning the evolution of group formation and multicellularity. Here, we examined the dynamics of aggregation in different Chlamydomonas species and strains with respect to cell size and motility and genetic relatedness. In agreement with previous studies (Boraas et al., Citation1998; Lürling & Beekman, Citation2006), all strains and species of Chlamydomonas rapidly aggregated in the presence of predators (in this case Peranema) or its culture filtrate. Groups also formed in the presence of other predators (rotifers and ciliates; not shown) suggesting aggregation is a non-specific defence strategy used by unicellular algae in response to a variety of predation pressures. Interestingly, the stimulus for aggregation was not the physical presence of the predator itself, but dissolved chemical compounds (kairomones; see Brown et al., Citation1970 for a discussion of possible mechanisms) present in the culture filtrate. It is likely that Peranema, whether growing naturally or in the growth medium described above, secretes kairomones, which are detected by Chlamydomonas. A large number of phytoplankton and zooplankton species protect themselves from predators using various morphological, behavioural and life history changes in response to kairomones produced by predators (Brown et al., Citation1970; Boeing et al., Citation2006). For example, Chlamydomonas cells were shown to alter their phototaxis in response to kairomones produced by zooplankton (Daphnia) and even in response to the kairomones produced by fish, which feed on Daphnia (Latta IV et al., Citation2009). It would be interesting to know whether kairomone detection in Chlamydomonas uses a common pathway or whether the mechanisms are predator-kairomone specific.
In Chlamydomonas, the role of phenotypic traits such as cell size and motility in the aggregation of cells when exposed to predators has been neglected. In this study it was observed that small, slow-swimming Chlamydomonas strains aggregated much faster than large, fast swimmers. The reason is presumably the greater intensity of the selective pressure. Peranema trichophorum engulfed the small, slow-swimming Chlamydomonas strains more readily.
In Chlamydomonas and other unicellular chlorophytes the genetic structure of aggregates formed in the presence of predators has also not been explicitly addressed (Boraas et al., Citation1998). Typically, genetic heterogeneity is not examined directly or aggregates are assumed to be clonal. In this study Chlamydomonas cells mixed freely with individuals of other strains or species and formed both intra-species and inter-species chimaeric groups. These data suggest that, in the volvocines at least, if cells came together as opposed to stayed together at the very beginning of multicellularity, discrimination between self and non-self was not a prerequisite or even a major factor in aggregation. Phenotypic differences may have been more important than anticipated. It is, of course, likely that following initial group formation, physical separation of genotypes within groups based on kin recognition mechanisms ameliorated the costs associated with group living. In the cellular slime mould or Dictyostelid amoebae (another model system for the evolution of cooperation and multicellular development) cells also come together and form multicellular aggregates and fruiting bodies upon starvation. Similar to the findings reported here for Chlamydomonas, aggregates and fruiting bodies of Dictyostelium amoebae can be chimaeric (Kaushik et al., Citation2006; Sathe et al., Citation2010, 2014).
Colonial living is typically associated with fitness costs (Michod, Citation1999; Lürling & Van Donk, Citation2000) and in this study, cell aggregates sank to the bottom of cultures flasks away from light sources. Nutrient uptake by individual cells was probably compromised due to limited access and it was observed that aggregation induced cell death in a proportion of individuals. The death mechanism was not examined explicitly, although it is likely to be a form of programmed cell death (PCD) since cells showed the typical shrinkage phenotype under light microscopy. Furthermore, PCD affects the fitness of close relatives positively (Durand et al., Citation2011) and that of non-relatives negatively (Durand et al., Citation2014); which may explain how the chimaeric aggregates described here evolved into clonal groups that gave rise to the Tetrabaenaceae and other multicellular volvocines. Chlamydomonas cells can also form groups by staying together if subjected to artificial settling selection (Ratcliff et al., Citation2013). Cells in these groups also displayed a PCD-like phenotype, although in this case groups were clonal and PCD facilitated the separation of cells and formation of new daughter groups.
This study demonstrates that predator-induced aggregation in a Chlamydomonas-like unicellular ancestor may have depended more on organismal phenotypes than anticipated. Before multicellularity evolved in the volvocines, the earliest groups may have formed by cells coming together rather than staying together as a defence against predation. Subsequently, clonal groups emerged via PCD such that the costs of group living were shared among relatives, giving rise to the Tetrabaenaceae and other multicellular volvocines.
SUPPLEMENTARY INFORMATION
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2015.1107759
Supplementary figs S1–4. Genetic heterogeneity in clumps formed by Chlamydomonas in the presence of Peranema.
Supplementary movie 1. Cell motility of a small strain C. moewusii UTEX 9.
Supplementary movie 2. Cell motility of a large strain C. reinhardtii CC2342.
Supplementary movie 3. Cell motility of a large strain C. reinhardtii CC2931.
Supplementary Movie 3
Download QuickTime Video (39.1 KB)Supplementary Movie 2
Download QuickTime Video (144 KB)Supplementary Movie 1
Download QuickTime Video (60.2 KB)Supplementary Figs S1-4
Download PDF (170.3 KB)ACKNOWLEDGEMENTS
SS acknowledges a URC postdoctoral fellowship from the University of Witwatersrand, Johannesburg, South Africa. This work was supported by grants to PMD from Medical Research Council and the University of Witwatersrand, Johannesburg, South Africa and National Aeronautics and Space Administration (#NNX13AH41G) grant to Richard E. Michod, Patrick J. Ferris and PMD at the University of Arizona. SS acknowledges financial support from the Durand Foundation for Evolutionary Biology and Phycology. The authors thank Cristian Solari and Stuart Sym for helpful comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Santosh Sathe
S. Sathe: original concept, study design, experiments, data analysis and manuscript preparation; P.M. Durand: original concept, study design and manuscript preparation.
Pierre M. Durand
S. Sathe: original concept, study design, experiments, data analysis and manuscript preparation; P.M. Durand: original concept, study design and manuscript preparation.
REFERENCES
- Bell, G. (1985). The origin and early evolution of germ cells as illustrated in the Volvocales. In The Origin and Evolution of Sex (Halverson, H.O. & Monroy, A., editors), 221–256. Alan R. Liss, New York.
- Boeing, W.J., Ramcharan, C.W. & Riessen, H.P. (2006). Multiple predator defence strategies in Daphnia pulex and their relation to native habitat. Journal of Plankton Research, 26: 571–584.
- Bonner, J.T. (2009). The Social Amoebae. Princeton University Press, Princeton, NJ.
- Boraas, M.E., Seale, D.B. & Boxhorn, J.E. (1998). Phagotrophy by a flagellate selects for colonial prey: a possible origin of multicellularity. Evolutionary Ecology, 12: 153–164.
- Brown, W.L., Eisner, T. & Whittaker, R.H. (1970). Allomones and kairomones: transspecific chemical messengers. BioScience, 20: 21–22.
- Buchheim, M.A., Lemieux, C., Otis, C., Gutell, R.R., Chapman, R.L. & Turmel, M. (1996). Phylogeny of the Chlamydomonadales (Chlorophyceae): a comparison of ribosomal RNA gene sequences from the nucleus and the chloroplast. Molecular Phylogenetics and Evolution, 5: 391–402.
- Chen, Y.T. (1950). Investigations of the biology of Peranema trichophorum (Euglenineae). Quarterly Journal of Microscopic Science, 91: 279–308.
- Durand, P.M., Rashidi, A. & Michod, R.E. (2011). How an organism dies affects the fitness of its neighbors. American Naturalist, 177: 224–232.
- Durand, P.M., Choudhury, R., Rashidi, A. & Michod, R.E. (2014). Programmed death in a unicellular organism has species-specific fitness effects. Biology Letters, 10: 20131088.
- Gorman, D.S. & Levine, R.P. (1965). Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proceedings of the National Academy of Sciences USA, 54: 1665–1669.
- Grosberg, R.K. & Strathmann, R.R. (2007). The evolution of multicellularity: a minor major transition? Annual Review of Ecology, Evolution, and Systematics, 38: 621–654.
- Kaiser, D. (2003). Coupling cell movement to multicellular development in myxobacteria. Nature Reviews Microbiology, 1: 45–54.
- Kang, T.-J. & Fawley, M.W. (1997). Variable (CA/GT)n simple sequence repeat DNA in the alga Chlamydomonas. Plant Molecular Biology, 35: 943–948.
- Kaushik, S., Katoch, B. & Nanjundiah, V. (2006). Social behaviour in genetically heterogeneous groups of Dictyostelium giganteum. Behavioral Ecology and Sociobiology, 59: 521–530.
- Kirk, D.L. & Kirk, M.M. (1983). Protein synthetic patterns during the asexual life cycle of Volvox carteri. Developmental Biology, 96: 493–506.
- Latta IV, L.C., O’Donnell, R.P. & Pfrender, M.E. (2009). Vertical distribution of Chlamydomonas changes in response to grazer and predator kairomones. Oikos, 118: 853–858.
- Lürling, M. & Beekman, W. (2006). Palmelloids formation in Chlamydomonas reinhardtii: defence against rotifer predators? Annales de Limnologie – International Journal of Limnology, 42: 65–72.
- Lürling, M. & Van Donk, E. (2000). Grazer-induced colony formation in Scenedesmus: are there costs to being colonial? Oikos, 88: 111–118.
- Michod, R.E. (1999). Darwinian Dynamics: Evolutionary Transitions in Fitness and Individuality. Princeton University Press, Princeton, NJ.
- Moulton, T. & Bell, G. (2013). Selecting for multicellularity in the unicellular alga Chlamydomonas reinhardtii. McGill Science Undergraduate Research Journal, 8: 30–38.
- Niklasa, K.J. & Newman, S.A. (2013). The origins of multicellular organisms. Evolution & Development, 15: 41–52.
- Pröschold, T., Marin, B., Schlösser, U.G. & Melkonian, M. (2001). Molecular phylogeny and taxonomic revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas Ehrenberg and Chloromonas Gobi, and description of Oogamochlamys gen. nov. and Lobochlamys gen. nov. Protist, 152: 265–300.
- Raper, K.B. (1984). The Dictyostelids. Princeton University Press, Princeton, NJ.
- Ratcliff, W.C., Herron, M.D., Howell, K., Pentz, J.T., Rosenzweig, F. & Travisano, M. (2013). Experimental evolution of an alternating uni- and multicellular life cycle in Chlamydomonas reinhardtii. Nature Communications, 4: 2742.
- Saito, A., Suetomo, Y., Arikawa, M., Omura, G., Mostafa Kamal Khan, S.M., Kakuta, S., Suzaki, E., Kataoka, K., & Suzaki, T. (2003). Gliding movement in Peranema trichophorum is powered by flagellar surface motility. Cell Motility and the Cytoskeleton, 55: 244–253.
- Sathe, S., Kaushik, S., Lalremruata, A., Aggarwal, R.K., Cavender, J.C. & Nanjundiah, V. (2010). Genetic heterogeneity in wild isolates of cellular slime mold social groups. Microbial Ecology, 60: 137–148.
- Sathe, S., Khetan, N., & Nanjundiah, V. (2014). Interspecies and intraspecies interactions in social amoebae. Journal of Evolutionary Biology, 27: 349–362.
- Schlesinger, A., Eisenstadt, D., Bar-Gil, A., Carmely, H., Einbinder, S., & Gressel, J. (2012). Inexpensive non-toxic flocculation of microalgae contradicts theories; overcoming a major hurdle to bulk algal production. Biotechnology Advances, 30: 1023–1030.
- Tarnita, C.E., Taubes, C.H. & Nowak, M.A. (2013). Evolutionary construction by staying together and coming together. Journal of Theoretical Biology, 320: 10–22.
