Abstract
Hannaea baicalensis is a benthic pennate diatom that predominantly grows at depths of less than a metre attached to rocks and filamentous algae in Lake Baikal, Russia. This narrow zone at the edge of the lake is subject to frequent wave action and lake level fluctuations, which combine with other factors to affect seasonal abundance. During ice cover from January to May in 2008, when lake levels decreased from 42 to 14 cm above datum, H. baicalensis cell abundance remained low (0.39 × 106 cells cm–2). The main period of net cell increase occurred in autumn, when there was a period of stable lake level (±10 cm changes in water depth) that coincided with the return of nutrients during autumn overturn. Cell abundance reached 1.52 × 106 cells cm–2 on 31 October. Alongside the changes in abundance, cyclic size changes in cell apical lengths were found (40 to 144 µm), which were associated with timing of the length of the life cycle. Size decline occurred in both spring and autumn, with an average decrease in apical length of 36 µm per year. It took two years for the mean apical length of a single cohort to decrease from 128 µm to 56 µm, which was then below the threshold (< 65 µm) for initiation of size regeneration.
Introduction
Hannaea baicalensis Genkal, Popovskaya & Kulikovsky (Genkal et al., Citation2008) is a benthic, araphid diatom endemic to Lake Baikal. This species mainly occurs along rocky shores in water less than 1 metre deep, attached to plants and stones (10–40 cm diameter) in the Ulothrix zonata Kütz. zone (Flower et al., Citation2004; Pomazkina & Rodionova, Citation2004; Pomazkina & Sherbakova, Citation2011; Pomazkina et al., Citation2008, Citation2013). Cell abundance decreases rapidly from the surface down to 2 m depth, but low cell abundances have been found as deep as 5–10 m in autumn (Flower et al., Citation2004; Pomazkina & Rodionova, Citation2004; Pomazkina & Sherbakova, Citation2011), which may be the result of redistribution of cells in a season when strong wave action frequently affects shallower depths. Hannaea has been recorded around the whole coast of Lake Baikal, but was rare on the eastern shore of the south basin (Flower et al., Citation2004). Hannaea baicalensis, and a sister species, Hannaea superiorensis R.J. Bixby and M.B. Edlund, belong to a genus that has evolved from riverine into lacustrine habitats and, in the case of these species, into the turbulent wave zones of large lakes (e.g. Bixby et al., Citation2005). This is an extreme habitat, where even a relatively small rise or fall in water level can either cause drying out or the water becoming too deep for optimal growth. Therefore, one aim of this study was to investigate whether lake level might contribute to changes in seasonal cell abundance. Also, we studied the rate of size decline to see whether this was affected by seasonal factors. Cycles of size change in diatoms have been studied since the pioneering work to develop the MacDonald–Pfitzer hypothesis (MacDonald, Citation1869; Pfitzer, Citation1869, Citation1871) and they have been linked to the timing of the length of life cycles (see Drebes, Citation1977; Lewis Citation1983, Citation1984; Mann, Citation1988, Citation2011; Jewson Citation1992a, b; Edlund & Stoermer, Citation1997; Potapova & Snoeijs, Citation1997; D’Alelio et al., Citation2010). However, although we now know that there is considerable variation in the basic adaptation, including some species that do not undergo size decline or that regenerate vegetatively (Chepurnov et al., Citation2004), we need more information on how, or if, population dynamics affects the timing and length of the life cycle in natural diatom populations, especially in benthic pennate species (e.g. Drebes, Citation1977; Kociolek & Stoermer, Citation1988; Potapova & Snoeijs, Citation1997).
MATERIALS and Method
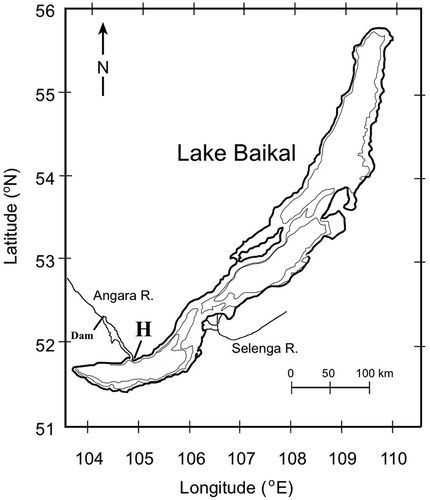
Samples of H. baicalensis were collected at 2- or 3-week intervals from the surface of stones in 0.25 m water depth at a fixed location beside the Baikal Museum harbour (51.868 °N, 104.830 °E) between 15 October 2007 and 30 October 2009, with a final sampling in October 2010. The site is near where the River Angara exits from Lake Baikal, Siberia (), which made sampling possible throughout the year, because the collection site remained ice-free due to water discharge from the lake into the river. An area of 2 × 2 cm was cleared off the surface of boulders and the sample transferred to a 30 ml sample bottle with added Lugol’s iodine. Temperature was recorded at the site using a glass mercury thermometer. Measurements of lake level were made approximately 4 km east from the sampling site, at the harbour of the Limnology Institute of the Russian Academy of Sciences, using a continuously recording Vaidal float recorder and stilling well (Sinyukovich, Citation2005) permanently fixed to a pier. Zero was 454.38 m above sea level using the Baltic Sea system. Measurements of abundance and apical length were made in settling chambers on an inverted Olympus IM microscope at ×400 using a digital video measurement and analysis system, model RMC-D4 (Brian Reece Scientific, Newbury), with measurements made to the nearest 1 μm. Measurements of 100 valves per sample were used to follow the relatively large size declines in the single age class of Hannaea, but more measurements may be appropriate in other circumstances, e.g. if there are a mixture of age classes in the population (Spaulding et al., Citation2012). Measurement of apical valve length was the chord length between the valve apices (Bixby & Zeek, Citation2010; Spaulding et al., Citation2012).
Fig. 1. Map of Lake Baikal, showing the sampling site of Hannaea baicalensis adjacent to the harbour of the Baikal Museum, near the outlet of the Angara River (‘H’). The site of the dam on the Angara River, which raised the lake level after 1959, is also marked. The depth contours are included for 500 and 1000 m.
Results
Seasonality and lake level change
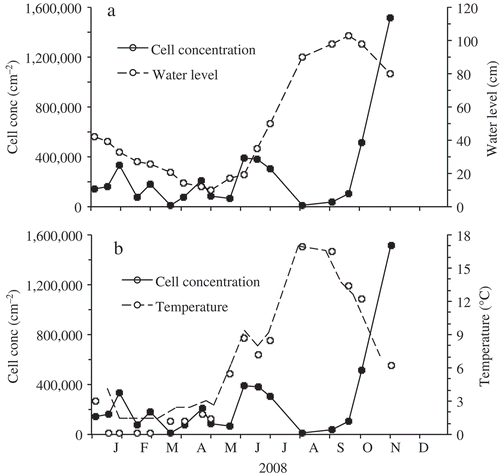
Cells of Hannaea were usually found singly or attached valve face to valve face, which meant that due to their elongated and curved form their cells formed into small colonies in a shape resembling a baseball glove. Hannaea was most often associated with the dense green mats of Ulothrix zonata (Weber et Mohr) Kütz., which formed a distinct narrow zone (approximately 5 to 30 cm depth) on rocks and boulders below the water surface at the edge of the lake. The diatom Didymosphenia was also frequently present in this complex. During 3 years of study, the population density of H. baicalensis reached a low of 0.012 × 106 cells cm–2 on 2 August 2008 and a maximum of 1.52 × 106 cm–2 on 31 October 2008, i.e. with over two orders of magnitude difference. The mean cell abundance from 22 sample dates in 2007 and 2008 was 0.25 × 106 cm–2. The largest changes in cell abundance during 2008 occurred when lake levels were stable and temperatures were above 12°C (, ). Lake levels in Lake Baikal usually decrease during winter ice cover, and increase in April, as the run-off from rivers starts to increase again. In this study, lake levels reached a peak in September (). In 2008, the lake was frozen from 21 January to 29 April and the water level decreased by 0.32 m, which coincided with a decline in cell abundance (). The period following ice break-up during May and early June is usually the time of maximum phytoplankton cell abundance, ahead of summer stratification and at a time when nutrients, such as nitrate and phosphate, become limiting (see Jewson et al., Citation2008, Citation2010; Jewson & Granin Citation2014). There was an initial net increase in Hannaea cell abundance between 20 May and 3 June during a period of relatively stable lake levels of 17 to 19 cm. There was then a rapid rise in lake levels from 19 to 90 cm between 3 June and 2 August which probably prevented a build-up of cells at their preferred depth, because cells will have had to keep recolonizing previously bare and dry rocks. Cell abundance then dropped to 0.012 × 106 cells cm–2 by 2 August, the lowest recorded in the study. However, there was then a period of relatively stable lake level (± 10 cm) at the same time as the water temperature began to decrease from a high of 16.9°C (), indicating the start of the autumn overturn and the availability of nutrient-rich water again. During this period, from 2 August to 31 October 2008, the fastest net increase in abundance was recorded, with the population doubling approximately every 11 days (from 0.012 to 1.52 × 106 cells cm–2).
Fig. 2. Seasonal changes inshore at the site beside the Museum Harbour in Lake Baikal during 2008, (a) in cell abundance of H. baicalensis (cells cm–2) in the Ulothrix zone and lake level (cm) and (b) in cell abundance of H. baicalensis (cells cm–2) and water temperature (°C). Zero for lake level was 454.38 m asl using the Baltic sea system.
Changes in apical cell length
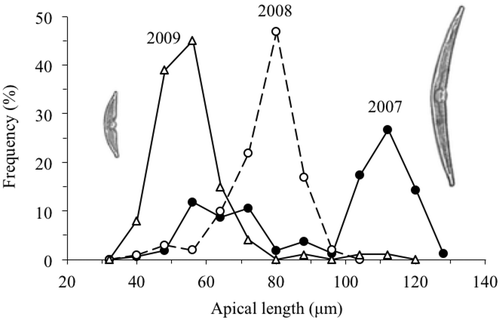
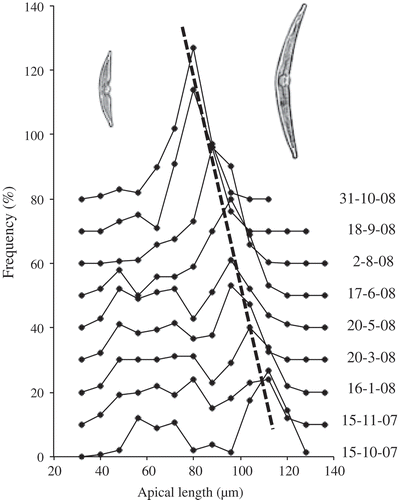
To determine whether changes in abundance affected the length of the life cycle of H. baicalensis, changes in apical length were followed over 3 years from 2007 to 2010. At the time of the first sampling on 15 October 2007, size regeneration was in progress. Some large cells with apical lengths up to 128 µm were found, but the modal length was 112 µm (). These cells above 70 µm made up 59% of the total population. The shorter cells (below 70 µm), which were those undergoing size regeneration, then decreased gradually in abundance over the next 8 months until only a small residual number of cells of this size were left by 30 June 2008 (). The newly size-regenerated cells then dominated the population (–p), but continued to decrease in size (), as cells divided and the population increased in abundance (see ). Over the next two years, the apical length of this age class continued to decline (), along with the presence of a low number of cells (less than 5%) in the population from both younger and older age classes (i.e. greater and lesser apical lengths). By 31 October 2008, the apical length of the dominant size class had decreased to 80 µm and by 30 October 2009, it had decreased to 56 µm (), which was similar in size to the smaller cohort of cells that were size regenerating in October 2007 () and 50% of the mean length of the size-regenerated cohort (112 µm). The rate of decline in apical length was not constant, but occurred in three periods during the regular sampling of 2007 and 2008. Between 15 November and 14 December 2007 there was a decrease from 112 to 104 µm in the apical length of the larger age cohort. This was equivalent to a decrease of 0.13 µm d–1 over 60 days. The apical length then did not change until 28 February 2008, when it began to decrease again, falling to 96 µm on 20 March, a rate of 0.38 µm d–1 over 21 days. There was then no further decline in apical length until August, when the highest rate of decline coincided with the main increase in abundance. The apical length declined from 96 to 84 µm over 30 days from 2 August to 1 September 2008, a rate of 0.40 µm d–1. At this time, one cell was found with an apical length of 144 µm, the longest measured during the whole study. Overall, the mean rate of decline in apical length of the dominant size class between 15 October 2007 and 30 October 2009 was from 112 to 56 µm, which was equivalent to an average decrease of 28 µm in apical length per year. The last samples collected on 15 November 2010 showed that the majority of the population below the size threshold in 2009 (< 65 µm) had size regenerated and were already declining in apical length again, with a single dominant size class at 88 µm.
Fig. 3. Relative frequency of the size distributions of apical lengths of H. baicalensis in Lake Baikal from 15 October 2007 to 2 August 2008. See for individual sample dates combined into a single figure.
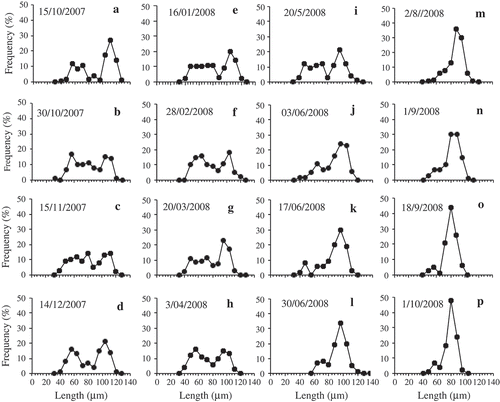
Fig. 4. Relative frequency of the size distributions of apical lengths of H. baicalensis in Lake Baikal during 2007 and 2008, but with the curves offset by adding 10% to each subsequent sampling date. The dashed line indicates the decline in apical length in the age class that size regenerated in October 2007 (size classes are ± 4 μm). For clarity, only every other sample date is presented. The individual curves for each sample date are shown in .
Discussion
To maintain a population in the Ulothrix zone, Hannaea has to adjust to lake level changes in addition to other factors affecting diatom populations, such as nutrient limitation and temperature fluctuations (Jewson et al., Citation2008, Citation2010; Jewson & Granin, Citation2014). In Lake Baikal, changes in lake level are the result of a combination of both natural and anthropogenic factors. The natural factors include seiches up to 20 cm (Shimaraev et al., Citation1994; Smirnov et al., Citation2014) and even earthquakes (Granin et al., Citation2012). In the latter case, the most extreme example was a 24 cm displacement that followed the Sumatra earthquake in 2012, but generally the impacts are much less (Granin et al., Citation2012). In terms of statutory controls, the construction of a dam at Irkutsk on the Angara River in 1959 (see ) resulted in a rise in lake level of 80 cm, followed by an average annual variation in lake level height of 89 cm (Sinyukovich, Citation2005). During our study in 2008, the variation in level was slightly above this at 93 cm (). Given that the majority of the Hannaea population prefer to live in water that is considerably less than 1 m deep (Flower et al., Citation2004; Pomazkina & Rodionova, Citation2004; Pomazkina & Sherbakova, Citation2011), sustaining a population at the preferred depth involves recolonizing surfaces as the water level rises and falls. Helping this recolonization may be a ‘reservoir’ of cells at low abundances down to 2 m water depth and also redistributed cells at depths of 5–10 m in autumn (Flower et al., Citation2004; Pomazkina & Rodionova, Citation2004; Pomazkina & Sherbakova, Citation2011). The mechanisms for recolonization are not known, but could include cells attached to the dense filaments of Ulothrix that become dislodged during wave action. As a result, there are likely to be high losses from the diatom population, as some cells become entrained in tychoplankton during storms. Although these losses are costly in population terms, they do have the benefit of aiding dispersal, especially when associated with changes in lake level. This is important with Hannaea mainly occurring in such a narrow depth zone. During rising lake levels cells must recolonize bare surfaces, but during falling water levels the cells are in danger of dehydration. Therefore, the standing crop of cells at the preferred depth of 25 cm is frequently less than the potential crop. For cell abundances to increase significantly in these shallow depths, water levels must be stable for periods of several weeks (). Consequently, the main growth period in this study was in autumn, as nutrients returned, temperature declined, and lake levels stabilized (). In other years, conditions may be different, such as when the main peak in cell abundance was reported in July (Flower et al., Citation2004; Pomazkina & Rodionova, Citation2004; Pomazkina & Sherbakova, Citation2011). In the future, experiments to investigate recolonization could include use of artificial surfaces, transfer of stones into deeper water and even survival after desiccation. However, there are now additional threats to this habitat. Since this study was carried out, mass growths of the green algae dominated by Spirogyra and Stigeoclonium have been found along the lake-edge of the north basin (Timoshkin et al., Citation2015). These have now spread to other areas of the lake, including the site used in this investigation.
Another factor potentially affecting the population dynamics of Hannaea, which is often overlooked, is the cycle of size decline and size regeneration linked to the timing of the life cycle. For a long time, it was thought that size decline was a consequence of having a rigid silica cell wall, but we know now that size diminution can be avoided, as some species do not decline in size (see Chepurnov et al., Citation2004) and most pennate diatoms decline only in one dimension, i.e., apical length, including Hannaea baicalensis (e.g. –). Hannaea belongs within the paraphyletic lineages of ‘araphid‘ diatoms, which have evolved improved methods for increasing the success of sexual reproduction compared with the older paraphyletic groups of ‘centric‘ diatoms (see Chepurnov et al., Citation2004), which release motile gametes into the water. In araphid species such as Tabularia (Davidovich et al., Citation2012), gametes have been found that use filopodium-like projections to help locate female gametes and in Pseudostaurosira trainorii, Sato et al. (Citation2011) found structures resembling flagella. In Hannaea, there appeared to be a much higher success rate of size-regeneration, with the newly size regenerated cells forming up to 59% of the population. This contrasts with most centric species, such as Aulacoseira, where the size-regenerated cohort can be less than 3% of the total population (Jewson Citation1992a, b; Jewson et al. Citation2008). Future modelling may show how improvements in recruitment success can impact the length of the life cycle, however, at present, there are still very few field studies quantifying this in natural populations. One is for a planktonic raphid diatom Pseudonitzschia, which has a 2-year life cycle (D’Alelio et al., Citation2010) and also the benthic araphid species Diatoma moniliformis (Potapova & Snoeijs, Citation1997), which may have a 2- or 3-year life cycle. Other evidence comes from a study of sexual reproduction in the raphid pennate Gomphoneis herculeana by Kociolek & Stoermer (Citation1988), where size analysis indicated a reduction in apical length that was likely to result in a life cycle of just one year. This compares to 3 or 4 years for centric species (Jewson Citation1992a, b; Jewson & Granin Citation2014). An important determinant of the length of the life cycle in any habitat is the length of the growing season and the number of divisions possible in any one year. For Hannaea in Lake Baikal, changes in standing crop were not a reliable guide to when the population was most actively growing and dividing.
In summary, Hannaea populations in Lake Baikal need to recolonize their preferred habitat if lake levels change. As a result, cell abundances remain low during periods of rapid lake level change, with increases in abundance mainly restricted to times when lake levels stabilize, especially if this coincides with favourable water temperatures and nutrient availability (). Also, our results showed that declines in cell apical length were not just restricted to periods of increasing abundance (–), but could occur during periods when abundances showed little change or even decreased. Such situations arose when cells were dividing, but there was a similar or higher rate of cell loss, such as during times of lake level change. Therefore, in this study, size decline in apical length, which is a major factor affecting the length of diatom life cycles, occurred under a range of conditions. For example, in October–December and February–March, size declines were during stable or declining standing crops during falling lake levels, but the size declines in August 2008 were during increasing cell abundance when there were stable lake levels. The net result is that the rate of size decline found in Hannaea () suggests that the life cycle could be completed in 2 years.
Acknowledgements
We wish to thank L. Olbolkina, who collected the Hannaea samples in all weathers. We also thank members of the Limnology Institute, especially N. G. Granin and A. A. Zhdanov for the lake level data, as well as G. Pomazkina, T. Sherbakova, M. A. Grachev, Ye. Likoshway and N. Bondarenko for their help. We are also very grateful for the help of S. Spaulding, A. Mackay, D. M. Williams and R. Flower.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
Notes on contributors
David H. Jewson
D. H. Jewson: original concept, drafting and editing manuscript; R. J. Bixby: concept, drafting and editing.
Rebecca J. Bixby
D. H. Jewson: original concept, drafting and editing manuscript; R. J. Bixby: concept, drafting and editing.
References
- Bixby, R.J. & Zeek, E.C. (2010). A simple method for calculating valve curvature. Proceedings of the Academy of Natural Sciences of Philadelphia, 160: 73–81.
- Bixby, R.J., Edlund, M.B. & Stoermer, E.F. (2005). Hannaea superiorensis sp. nov., an endemic diatom from the Laurentian Great Lakes. Diatom Research, 20: 227–240.
- Chepurnov, V.A., Mann, D.G., Sabbe, K. & Vyverman, W. (2004). Experimental studies on sexual reproduction in diatoms. International Review of Cytology, 237: 91–154.
- D’Alelio, D., d’ Alcala, M.R., Dubroca, L., Sarno, D., Zingone, A. & Montresor, M. (2010). The time for sex: a biennial life cycle in a marine planktonic diatom. Limnology and Oceanography, 55: 106–114.
- Davidovich, N. A., Kaczmarska, I., Karpov, S.A., Davidovich, O. I., MacGillivary, M.L. & Mather, L. (2012). Mechanism of male gamete motility in araphid pennate diatoms from the genus Tabularia (Bacillariophyta). Protist, 163: 480–494.
- Drebes, G. (1977). Sexuality. In The Biology of Diatoms (Werner, D., editor), 250–283. University of California Press, Oakland, CA.
- Edlund, M.B. & Stoermer, E.F. (1997). Ecological, evolutionary, and systematic significance of diatom life histories. Journal of Phycology, 33: 897–918.
- Flower, R.J., Pomazkina, G., Rodionova, E. & Williams, D.M. (2004). Local and mesoscale diversity patterns of benthic diatoms in Lake Baikal. In Proceedings of the 17th International. Diatom Symposium, Ottawa, Canada, 25–31 August 2002 (Poulin, M., editor), 69–92. Biopress Ltd., Bristol.
- Genkal, S.I., Popovskaya, G.I. & Kulikovsky, M.S. (2008). New for science species from the genus Hannaea (Bacillariophyta). International Journal of Algae, 10: 321–329.
- Granin, N.G., Radziminovich, N.A., Kucher, K.M. & Checdel’nitskii, V.V. (2012). Generation of Lake Baikal level oscillations by remote strong earthquakes. Doklady Earth Sciences, 455: 331–335.
- Jewson, D.H. (1992a). Size reduction, reproductive strategy and the life cycle of a centric diatom. Philosophical Transactions of the Royal Society, London B, 336: 191–213.
- Jewson, D.H. (1992b). Life cycle of a Stephanodiscus sp. (Bacillariophyta). Journal of Phycology, 28: 856–866.
- Jewson, D.H. & Granin, N.G. (2014). Cyclical size change and population dynamics of a planktonic diatom, Aulacoseira baicalensis, in Lake Baikal. European Journal of Phycology, 50: 1–19.
- Jewson, D.H., Granin, N.G., Zhdarnov, A.A., Gorbunova, L.A., Bondarenko, N.A. & Yu Gnatovsky, R. (2008). Resting stages and ecology of the planktonic diatom Aulacoseira skvortzowii in Lake Baikal. Limnology and Oceanography, 53: 1125–1136.
- Jewson, D.H., Granin, N.G., Zhdarnov, A.A., Gorbunova, L.A. & Yu Gnatovsky, R. (2010). Vertical mixing, size change and resting stage formation of the planktonic diatom Aulacoseira baicalensis. European Journal of Phycology, 45: 354–364.
- Kociolek, J.P. & Stoermer, E.F. (1988). Taxonomy, ultrastructure and distribution of Gomphoneis herculeana, G. eriense and closely related species (Naviculales: Gomphonemataceae). Proceedings of the Academy of Natural Sciences of Philadelphia, 140: 24–97.
- Lewis, W.M. (1983). Interruption of synthesis as a cost of sex in small organisms. American Naturalist, 121: 825–833.
- Lewis, W.M. (1984). The diatom sex clock and its evolutionary importance. American Naturalist, 123: 73–80.
- MacDonald, J.D. (1869). On the structure of the diatomaceous frustule, and its genetic cycle. Annals and Magazine of Natural History Series 4, 3: 1–8.
- Mann, D.G. (1988). Why didn’t Lund see sex in Asterionella? A discussion of the diatom life cycle in nature. In Algae and the Aquatic Environment (Round, F.E., editor), 383–412. Biopress Ltd., Bristol.
- Mann, D.G. (2011). Size and sex. In The Diatom World (Cellular Origin, Life in Extreme Habitats and Astrobiology) (Seckbach J. & Kociolek, J.P., editors), 145–165. Springer, Dordrecht.
- Pfitzer, E. (1869). Über den Bau und die Zellteilung der Diatomeen. Botanische Zeitung, 27: 774–776.
- Pfitzer, E. (1871). Untersuchungen über Bau und Entwicklung der Bacillariaceen (Diatomaceen). Botanische Abhandlungen, 2: 1–189.
- Pomazkina, G.V. & Rodionova, E.V. (2004). Benthic Bacillariophyta in Southern Baikal. Algologia, 14: 62–72 (in Russian).
- Pomazkina, G.V. & Sherbakova, T.A. (2011). Prevailing species of Bacillariophyta of the littoral zone of Lake Baikal (Russia). International Journal of Algae, 13: 101–106.
- Pomazkina, G.V., Rodionova, E.V. & Yu. Mushnikova, O. (2008). Microphitobenthos of Southern Baikal. Algologia, 18: 160–172.
- Pomazkina, G.V., Rodionova, E.V. & Yu. Mushnikova, O. (2013). Uniqueness of diatom flora from the Strait of Olkhonskie Vorota and the Gulf of Maloe More, Lake Baikal (Russia). International Journal of Algae, 15: 201–217.
- Potapova, M. & Snoeijs, P. (1997). The natural life cycle in wild populations of Diatoma moniliformis (Bacillariophyceae) and its disruption in an aberrant environment. Journal of Phycology, 33: 924–937.
- Sato, S., Beakes, G., Masahiko, I., Nagumo, T. & Mann, D.G. (2011). Novel sex cells and evidence for sex pheromones in diatoms. PloS ONE, 6: 1–15.
- Shimaraev, M.N., Verbolov, V.I., Granin, N.G. & Sherstyankin, P.P. (1994). In Physical Limnology of Lake Baikal: A Review (Shimaraev, M.N. & Okuda, S., editors). Irkutsk, Okayama.
- Sinyukovich, V.N. (2005). Reconstruction of the natural level regime of Lake Baikal after the construction of the Irkutsk hydroelectric power plant. Meteorology and Hydrology, 7: 70–76.
- Smirnov, S.V., Kucher, K.M., Granin, N.G. & Sturova, I.V. (2014). Seiche oscillations in Lake Baikal. Atmospheric and Oceanic Physics, 50: 92–102.
- Spaulding, S.A., Jewson, D.H., Bixby, R.J., Nelson, H. & McKnight, D.M. (2012). Automated measurement of diatom size. Limnology and Oceanography: Methods, 10: 882–890.
- Timoshkin, O.A., Bondarenko, N.A., Volkova, Ye. A., Tomberg, I.V., Vishnyakov, V.S. & Malnik, V.V. (2015). Mass development of green filamentous algae of the genera Spirogyra and Stigeoclonium (Chlorophyta) in the littoral zone of the southern part of Lake Baikal. Hydrobiological Journal, 51: 13–23 (in Russian).