Abstract
Species of Prasiolales (Trebouxiophyceae, Chlorophyta) are among the most common terrestrial and freshwater algae in polar regions. Comprehensive molecular studies of this group are available for Antarctica, but not yet for Arctic regions. We examined the diversity of the Prasiolales in the Svalbard Archipelago combining morphological observations of field-collected material, culture studies, molecular data (plastid rbcL and tufA sequences) and literature records. We confirmed the widespread occurrence of Prasiola crispa and P. fluviatilis, species recorded from Spitsbergen since the 19th century. Molecular phylogenetic analyses led to the discovery of two new genera of Prasiolales. Prasionema payeri is morphologically identical to filamentous stages of P. crispa, but represents an early-diverging lineage in the order. Prasionella wendyae is a colonial alga reproducing by aplanospores; its phylogenetic position is among the basal lineages of the order, but it could not be reliably reconstructed due to weak statistical support. The inclusion of P. wendyae in the prasiolalean phylogeny determined the paraphyly of Rosenvingiella, requiring the establishment of the new genus Rosenvingiellopsis for R. constricta. A poorly known species described from Spitsbergen, Ulothrix discifera, is transferred here to Rosenvingiella. Whereas some species of Prasiolales have bipolar distribution (P. crispa), others appear to be restricted to one or other of the poles. Our results suggest that polar regions are still a major repository of unknown algal diversity and highlight the importance of continued field surveys and the use of molecular data.
Introduction
Green microalgae and cyanobacteria represent the main photosynthesizers in many terrestrial and freshwater habitats of polar regions (Broady, Citation1996; Skulberg, Citation1996). Communities formed by these organisms occur on a variety of substrata and play a key role in supplying organic matter and increasing nitrogen content, contributing to soil formation in deglaciated areas (Kaštovská et al., Citation2005). The existence of terrestrial and freshwater microalgae in polar habitats has been known for well over a century, but in recent years these organisms have received special attention due to their extremophilic nature. Molecular studies conducted in the past two decades have revealed surprising phylogenetic diversity hidden under often simple and uniform morphology (e.g., Moniz et al., Citation2012a; Souffreau et al., Citation2013). Molecular data have also provided valuable insights into the distribution and phylogeography of polar microalgae, clarifying the spatial structure of some communities (Bielewicz et al., Citation2011) and revealing that, whereas some lineages are amphipolar and occur both in the Arctic and Antarctica (Jungblut et al., Citation2012), others are endemic to Antarctica and may have survived there over glacial periods (De Wever et al., Citation2009; Strunecký et al., Citation2010; Souffreau et al., Citation2013).
The green algal order Prasiolales is associated with cold climates and includes some of the most common terrestrial and freshwater algae of polar regions (Kobayasi, Citation1967; Moniz et al., Citation2012a; Garbary & Tarakhovskaya, Citation2013). This group is characterized by a unique combination of morphological and ultrastructural features (O’Kelly et al., Citation1989; Van den Hoek et al., Citation1995; Sherwood et al., Citation2000) and is well known for its great ecological versatility, as it comprises marine, freshwater and terrestrial species (Knebel, Citation1936; Printz, Citation1964; Broady, Citation1989). In its traditional circumscription, the Prasiolales consist of the single family Prasiolaceae and about 45 species belonging to the genera Prasiococcus Vischer, Prasiola Meneghini, Prasiolopsis Vischer and Rosenvingiella P.C. Silva. Polar species of Prasiolales belong mainly to Prasiola and commonly occur in habitats subjected to enrichment in organic nitrogen, such as nesting sites of birds (Jakubas et al., Citation2008; Moniz et al., Citation2012a; Zmudczynska et al., Citation2012). Prasiola crispa (Lightfoot) Kützing is the species most frequently recorded in the Arctic and Antarctica (Kobayasi, Citation1967; Wiencke & Clayton, Citation2002). Polar populations of this alga are considered model extremophiles (CAREX, Citation2009), and several studies have examined in detail their tolerance to extreme desiccation (Jacob et al., Citation1992; Bock et al., Citation1996) and UV radiation (Jackson & Seppelt, Citation1995, Citation1997; Hoyer et al., Citation2001; Lud et al., Citation2001; Holzinger et al., Citation2006).
The phylogenetic position and genus-level classification of the Prasiolales were surrounded by great uncertainty for a long time, but molecular data produced in the last 15 years (Sherwood et al., Citation2000; Naw & Hara, Citation2002, Rindi et al., Citation2004, Citation2007; Heesch et al., Citation2012; Moniz et al., Citation2012a, Citation2012b, Citation2014) have led to considerable progress in this regard. The position of this order in the class Trebouxiophyceae is robustly established (Lemieux et al., Citation2014) and the data available are now unraveling a complex scenario in its evolution. The class Trebouxiophyceae is predominantly terrestrial and the closest relatives of the Prasiolales are unicellullar or colonial subaerial genera, such as Stichococcus and Diplosphaera (Darienko et al., Citation2010; Thüs et al., Citation2011; Lemieux et al., Citation2014). This suggests that marine and freshwater species of Prasiolales are the result of secondary recolonization of aquatic habitats, an event that in algae is very difficult to demonstrate (in constrast to the well-documented transitions from aquatic habitats to land, which have occurred multiple times; Lewis & Lewis, Citation2005). Recent studies extended the molecular sampling for this order to regions of the austral hemisphere, including Antarctica, with results that led to some major taxonomic and biogeographic surprises (Heesch et al., Citation2012; Moniz et al., Citation2012a, Citation2012b). Moniz et al. (Citation2012a) published the first molecular study on the Prasiolales of Antarctica and showed that Antarctic Prasiola crispa represented a complex of three different cryptic species, identified as P. antarctica Kützing and P. glacialis Moniz, Rindi, Novis, Broady & Guiry, and the genuine P. crispa. These discoveries call for a reassessment of many previous studies on the Prasiolales of Antarctica and Antarctic islands, and have implications for phylodiversity, biogeography and dispersal of polar algae that go beyond the limits of this group.
The Svalbard Archipelago is located between 74° and 81° North latitude and represents the best-investigated area of the Arctic in terms of natural history. The presence of Prasiola on Spitsbergen was documented more than a century ago (Jessen, Citation1848; Lagerstedt Citation1869). Not less than five species of Prasiolales have been recorded in this archipelago (Kjellman, Citation1877; Borge Citation1911; Hansen & Haugen, Citation1989; Vinogradova, Citation1995; Skulberg, Citation1996; Gulliksen et al., Citation1999), plus some taxa of dubious taxonomic identity presumably referable to this order (Kjellman, Citation1877; Wittrock, Citation1883). In consideration of the recent discoveries made on Prasiolales of Antarctica, a similar reassessment for the Prasiolales of the Svalbard Archipelago becomes of special interest from a biogeographic and taxonomic point of view. In recent years we were able to collect samples of Prasiolales on Spitsbergen and obtain from them DNA sequence data, which allowed us to assess the identity of the most common species and discover two new genera. We present here a reassessment of the diversity of the Prasiolales in the Svalbard Archipelago based on these data.
Materials and Methods
Natural history, field collections and culture studies
We critically reviewed records of Prasiolales from the Svalbard Archipelago available in the literature and the information available about their distribution (Supplementary Table 1). Collections of Prasiolales were made by MP in the area of Billefjorden (western coast of Spitsbergen) in August 2012 and by Jakub Žárský and Josef Elster in the summers of 2013 and 2014, respectively (). The samples were stored in sealed ziploc bags prior to examination in the laboratory. Microphotographs were taken with a Nikon DXM1200 digital camera.
Table 1. Details of field-collected and cultured specimens of Prasiolales examined in the study.
Selected strains from field-collected samples were isolated into unialgal cultures by SH by serial subcultivation in the laboratory at NUI, Galway. Cultures were grown in sterile polystyrene Petri dishes (Sterilin, Stone, UK) containing approximately 30 ml of liquid Von Stosch medium with 20 ml enrichment per 1 l water (Guiry & Cunningham, Citation1984), prepared using either fresh water (‘Highland Spring’ or ‘Volvic’ still mineral water, UK or France, respectively) or full strength autoclaved seawater (collected in Carna, Co. Galway, Ireland), or on the same media solidified with 1% agar (UltraPure™, Invitrogen, Carlsbad). At NUIG, the strains were maintained in a controlled culture chamber (Sanyo MLR-351, Sanyo Electric Co., Osaka, Japan) at 10°C, 20–50 µmol photons m–2 s–1, 18:6 h light:dark. Voucher specimens were deposited in the Phycological Herbarium, National University of Ireland, Galway (GALW), the Natural History Museum, London (BM), and the herbarium of the Smithsonian Institution, Washington DC (US). Duplicates of the cultures of the type strains of Prasionema payeri and Prasionella wendyae were deposited in the Culture Collection for Autotroph Organisms (CCALA, http://ccala.butbn.cas.cz/), Czech Republic, and in the Culture Collection for Algae and Protozoa (CCAP, http://www.ccap.ac.uk/), UK.
DNA extractions, PCR amplification and sequencing
Some of the strains of Prasiolales collected from Spitsbergen were used for DNA extraction. DNA was extracted from fresh cultured material (~10 mg) using the NucleoSpin® Plant II kit (Macherey-Nagel, Düren, Germany) or from silica-dried material using the Qiagen DNeasy Plant Mini Kit (Qiagen, Crawley, UK), following the manufacturer’s instructions. The plastid-encoded genes for the large subunit of RuBisCO (rbcL) and for the elongation factor Tu (tufA) were amplified via polymerase chain reactions (PCR) using published primers: SH F5 with SH R8 (Heesch et al., Citation2012) and RH1 (Manhart, Citation1994) with RT1134 (Rindi et al., Citation2008) for rbcL; and tufGF4 with tufAR for tufA (Saunders & Kucera, Citation2010). A Techne TC-3000 thermal cycler (Techne, Stone, UK) was used for the PCR amplifications. PCR reactions contained 2.5 µl of DNA extract, 12.5 µl of a premix including Taq polymerase (GoTaq® Green Master Mix, Promega, Madison, Wisconsin, USA), 0.5 µl 10 μM of each primer (Eurofins, Germany) and 9 µl of sterile deionized water. The PCR protocol comprised an initial denaturation step of 2 min at 94°C, followed by 35 cycles of denaturation for 30 s at 94°C, annealing for 1 min at 50°C (rbcL) or 55°C (tufA) and elongation for 2 min at 72°C, and ending with a final elongation for 10 min at 72°C. The amount of DNA in PCR products was quantified visually on agarose gels using HyperLadder II (Bioline, London, UK) as reference. The PCR products were cleaned using the PureLink® PCR Purification Kit (Invitrogen, Germany) and custom-sequenced by GATC (Cologne, Germany) using the same primers, on an Applied Biosystems 3730xl DNA Analyzer.
Sequence alignment and phylogenetic analyses
The quality of the sequence data was verified by visual inspection of the electropherograms in 4Peaks v.1.7.2 (Griekspoor & Groothuis, Citation2005). The new sequences produced in this study and included in the datasets are based on high-quality bidirectional readings. The new sequences were added to alignments of Prasiolales prepared on the basis of recent molecular studies (Rindi et al., Citation2007; Heesch et al., Citation2012; Moniz et al., Citation2012a, Citation2012b, Citation2014); care was taken to include in the analyses all lineages currently known in the Prasiolales. Two alignments were used for the analyses: an alignment for the rbcL gene and a concatenated alignment rbcL-tufA. The alignments were prepared and adjusted manually in Se-Al version 2.0a11 (http://tree.bio.ed.ac.uk/software/seal/). Some sequences of additional trebouxiophycean algae closely related to the Prasiolales were included in the datasets for outgroup-rooting purposes. For the rbcL alignment, these were Chlorella mirabilis (rbcL sequence obtained from plastid genome KM462865), Diplosphaera mucosa AM260444, Diplosphaera sp. AM260445, Pabia signiensis (from plastid genome KM462866) and Stichococcus bacillaris AM260442; for the concatenated rbcL-tufA alignment, Chlorella mirabilis (both genes from plastid genome KM462865) and Pabia signiensis (both genes from plastid genome KM462866). The rbcL alignment consisted of 43 taxa and was 1067 base pairs long; the concatenated rbcL-tufA alignment consisted of 28 taxa and was 1814 base pairs long. Sequences of the tufA gene were not available for some taxa (Prasiola mexicana, Prasionella wendyae, Rosenvingiella polyrhiza). For these taxa, the portions of the alignment corresponding to tufA were entered as missing data.
Phylogenetic reconstructions were performed for both datasets using the Maximum Likelihood criterion (ML) in RAxML version 1.3 (Silvestro & Michalak, Citation2011), while Bayesian Inference (BI) was carried out in MrBayes v3.2.5 x64 (Huelsenbeck & Ronquist, Citation2001; Ronquist et al., Citation2012). Prior to analyses, alignments were transferred to respective file formats in ALTER (Glez-Peña et al., Citation2010), and the datasets were partitioned for codon positions (both datasets) and genes (concatenated rbcL-tufA dataset). ML analyses were run using the default GTR model in RAxML, with node support assessed from the results of bootstrapping (BP) analyses based on 1000 pseudoreplicates. Bayesian Posterior Probabilities (PP) were approximated in four Monte Carlo Markov Chains run for 1 million generations. Priors and probability proposals set as default in MrBayes were used. The stationary distribution of the runs was verified before stopping the analysis, by ensuring that the average standard deviations of split frequencies between the runs reached levels well below 0.01. Trees were sampled every 100 generations, with the first 2000 discarded as burn-in. 50% majority rule consensus trees were calculated from the remaining trees.
Results
Field surveys and species recorded
In the course of the study we collected and identified four species of Prasiolales: Prasiola crispa, P. fluviatilis, Prasionema payeri gen. et sp. nov. and Prasionella wendyae gen. et sp. nov. We were not able to rediscover four other prasiolalean species previously reported from the Svalbard Archipelago: Prasiola furfuracea (Mertens ex Hornemann) Trevisan, Prasiola stipitata Suhr ex Jessen, Rosenvingiella polyrhiza (Rosenvinge) P.C. Silva and Rosenvingiella discifera (Kjellman) comb. nov. (formerly Ulothrix discifera Kjellman; Supplementary material 1).
Molecular phylogeny
We obtained rbcL sequences for four species of Prasiolales from Svalbard: Prasiola crispa, Prasiola fluviatilis, Prasionella wendyae and Prasionema payeri (). For Prasionema payeri, the tufA sequence was also obtained. These taxa were recovered with high support as members of the Prasiolales both in the rbcL () and in the concatenated rbcL-tufA () phylogenies.
Fig. 1. Phylogram inferred from Maximum Likelihood analysis of the rbcL dataset of Prasiolales with ML bootstrap support (BP) and Bayesian posterior probabilities (PP) indicated at the nodes. BP values lower than 60% and PP lower than 0.9 are not reported. Samples from Svalbard are marked in bold; asterisks indicate new sequences generated in this study.
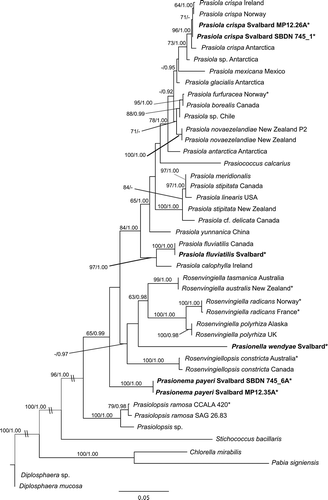
Fig. 2. Phylogram inferred from Maximum Likelihood analysis of the concatenated rbcL-tufA dataset of Prasiolales with ML bootstrap support (BP) and Bayesian posterior probabilities (PP) indicated at the nodes. BP values lower than 60% and PP lower than 0.9 are not reported. Samples from Svalbard are marked in bold; asterisks indicate new sequences generated in this study.
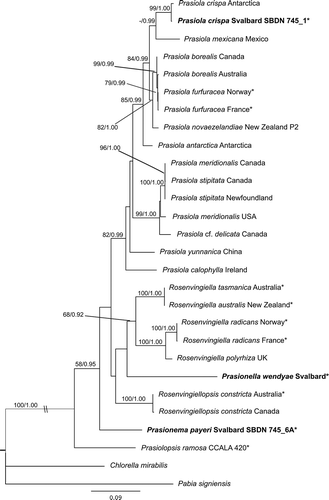
For Prasiola crispa and P. fluviatilis, the rbcL sequences confirmed the morphological identifications. Prasiola crispa from Spitsbergen belonged with high support to the P. crispa clade (). This is the most derived clade in the order, unambiguously identified by the link with the type specimen of P. crispa (sequenced by Moniz et al., Citation2012a). Prasiola fluviatilis from Spitsbergen had an identical rbcL sequence to the only strain of this species for which molecular data are currently available (a strain from Arctic Canada sequenced by Sherwood et al., Citation2000); the position of this species as sister taxon to Prasiola calophylla (Carmichael ex Greville) Kützing recovered in previous studies is confirmed here. Prasionema payeri and Prasionella wendyae formed two early-diverging lineages among the Prasiolales, but their relationships could not be clarified. In both phylogenies, Prasionema payeri was sister taxon to a clade including all Prasiolales except Prasiolopsis, whereas Prasionella wendyae was sister to a clade formed by species of Rosenvingiella (R. australis S. Heesch & W.A. Nelson, R. radicans (Kützing) Rindi, McIvor & Guiry, R. tasmanica Moniz, Rindi & Guiry); however, these relationships did not receive significant support in bootstrap analyses (–). The inclusion of Prasionella wendyae in the analyses had another important implication for the taxonomy of the order: the genus Rosenvingiella became paraphyletic, because Rosenvingiella constricta was separated in an independent lineage from the other species (–). We therefore propose the transfer of this species to the new genus Rosenvingiellopsis.
Morphology and taxonomic treatment
Prasiola crispa ()
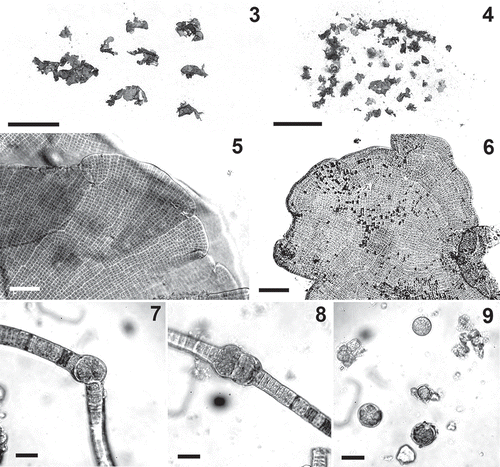
Specimens of P. crispa were collected from wet soil and formed dense green spinach-like growths or thin carpets at sites affected by inputs of organic nitrogen (). This alga occurred in different morphological forms. Specimens with the typical leafy habit consisted of crisped blades dark green in colour, irregularly shaped, up to 2 cm long and wide, monostromatic, 12–21 µm thick (–). In the blades the cells were arranged in horizontal and vertical rows; areolae were not evident, but some thickened lines were often observed in surface view (–). The cells were square or rectangular, 5–9 µm long and wide. Uniseriate filamentous specimens (‘Hormidium stage’, , –) were also observed, often mixed with pluriseriate ribbon-like thalli (‘Schizogonium stage’); a complete range of morphological forms from uniseriate filaments to well-developed blades with all intermediate forms was observed in some samples (). The uniseriate filaments were 10–20 μm wide. In some samples, presumed to be this species, some uniseriate filaments possessed intercalary portions formed by enlarged globular cells, 20–25 µm wide, produced either singly or in series of 2–5 (–). These cells appeared to behave as aplanosporangia (); upon detachment from the filaments, they had their contents divided into many small cells, which were released and produced new filaments.
Figs 3–9. Prasiola crispa. . Habit of leafy form. . Mixture of leafy and filamentous forms. . Detail of margin of blades. . Surface view of blade. . A filament producing a presumptive aplanosporangium. . A filament with two enlarged intercalary cells forming putative aplanosporangia. . Putative aplanosporangia and spores. Scale bars: , 4=1 cm; , 6=50 μm; –9=20 μm.
Prasiola fluviatilis (–)
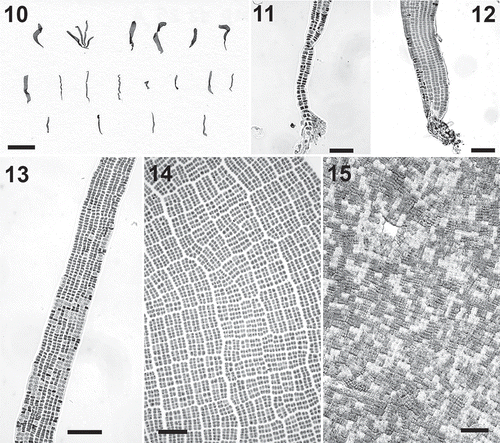
Specimens of P. fluviatilis were found in streams, where they produced thin carpets tightly adherent to rocks. Individual specimens consisted of ribbon-like thalli up to 1.5 cm long and 2 mm wide, attached to the rock by a thin stipe that gradually expanded into a linear blade (–). The stipe was pluriseriate throughout its length (–); its basal cells produced unicellular rhizoids similar to those of Rosenvingiella (). In the vegetative blades the cells were arranged in vertical and horizontal rows and had mostly thin walls (). Evident areolae were usually lacking; in the apical portions of large blades, however, clear areolae and thickened longitudinal lines were present (). The blades were 15–18 μm thick, and in surface view the cells were 5–10 μm long and wide. Some large specimens appeared to be close to reproductive maturation. In the apical portions the tissue was divided in patches with cells of different sizes and colours, as in gametangial portions of Prasiola stipitata (). The light portions (presumptive male gametangia) had small cells 2.5–4 μm in length and width; the dark portions (presumptive female gametangia) had cells 6–10 μm in length and width. Release of gametes was not observed.
Figs 10–15. Prasiola fluviatilis. . Habit. . Basal part of a specimen showing uniseriate rhizoids. . Detail of the basal portion of a specimen. . Detail of blade with ribbon-like habit. . Surface view of blade showing evident areolae. . Detail of apical part of a blade with presumptive gametangial portions. Scale bar: Scale bars: Fig. 10=1 cm; Figs 11–15=40 μm.
Prasionema Heesch, M. Pažoutová & Rindi, gen. nov
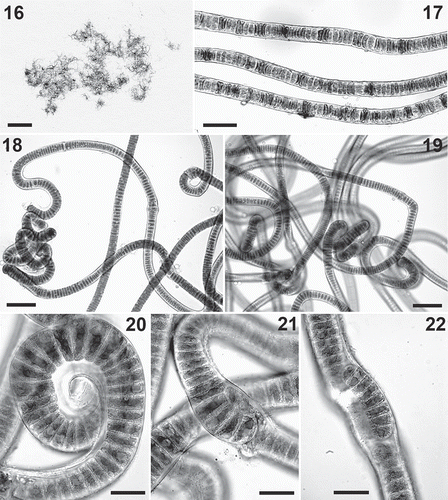
Diagnosis: thalli formed by uniseriate filaments, 18–22 µm wide, with a few short biseriate portions, reproducing by vegetative fragmentation. Cells disc-shaped, 0.2–0.5 times as long as wide, containing a single axial chloroplast with a central pyrenoid. It differs from similar prasiolalean algae in rbcL and tufA sequences.
Holotype species: Prasionema payeri Heesch, M. Pažoutová & Rindi
Prasionema payeri Heesch, M. Pažoutová & Rindi, sp. nov
(–)
Diagnosis: thallus consisting of uniseriate filaments, 18–22 µm wide, with a few short biseriate portions. Cells disc-shaped, 0.2–0.5 times as long as wide, containing a single axial chloroplast with a central pyrenoid. Cell walls slightly corrugated in field-collected specimens, smooth in cultured specimens. Specialized forms of reproduction not observed. Reproduction by vegetative fragmentation, which takes place by separation of short filaments in biseriate portions.
Holotype specimen: GALW15829. Billefjorden, Petuniabutka, Spitsbergen, Czech field station. Soil on side of wooden latrine, with rich supply of moisture and nutrients. M. Pažoutová, 13 August 2012.
Isotype specimens: BM and US.
Paratype specimens: GALW15830, GALW15831.
rbcL sequence of holotype specimen: LN877814.
Type locality: Billefjorden, Petuniabutka, Spitsbergen, Czech field station.
Etymology: The name of the genus indicates the prasiolalean nature of this alga, whereas the specific epithet honours the Austro-Hungarian military officer Julius von Payer (1841–1915), for his contributions to Arctic explorations.
Prasionema payeri formed a dense patch on moist soil with nutrient enrichment at the type locality. Its morphology (–) is identical to that of thick filamentous forms of Prasiola crispa, with which this alga was initially confused; rbcL and tufA sequences, however, clearly demonstrated the separation of the two species. The morphology of P. payeri showed very limited variation between field conditions and culture conditions (–), and the only form of reproduction observed was by vegetative fragmentation, with release of short filaments from the biseriate portions of the thallus (–). At present, the known geographic distribution of P. payeri is restricted to Spitsbergen.
Figs 16–22. Prasionema payeri. . Habit of some field-collected filaments from holotype material (GALW15829). . Details of some field-collected filaments from holotype material (GALW15829). . Filaments from material isolated in culture . . Filaments from material isolated in culture. . Detail of a specimen isolated in culture. . Detail of a filament forming biseriate portions. . Detail of a short filament produced by fragmentation from a biseriate part of the thallus. Scale bars: =1 cm; =40 μm; =60 μm; –22=20 μm.
Prasionella Heesch, M. Pažoutová & Rindi, gen. nov
Diagnosis: Thalli formed by colonies up to 120 µm in diameter, sarcinoid, cushion-shaped, or subglobular, containing a few to some hundreds of cells enclosed in a common envelope. Cells 5–6 µm long and wide (6–14 µm in small colonies), each containing a single axial chloroplast with a central pyrenoid. Asexual reproduction taking place by cell division or by production and release of aplanospores. It differs from other trebouxiophycean algae in its rbcL sequence.
Holotype species: Prasionella wendyae S. Heesch, M. Pažoutová & F. Rindi.
Prasionella wendyae Heesch, M. Pažoutová & Rindi, sp. nov
(–)
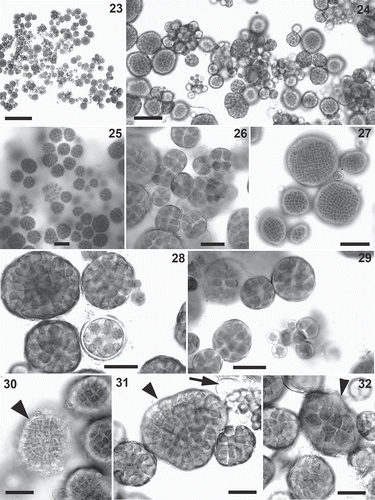
Diagnosis: Thalli consisting of small colonies up to 120 µm in diameter, sarcinoid, cushion-shaped or subglobular. Most colonies are formed by a few cells to some tens of cells, with the largest colonies consisting of several hundreds of cells. Cells are mainly 5–6 µm long and wide (6–14 µm in small colonies), each containing a single axial chloroplast with a central pyrenoid. In some colonies (that are presumed to be male colonies reproducing sexually) cells are light green, smaller, 2–3 µm long and wide; in other colonies, presumed to be female, cells are darker and larger, 10–18 µm long and wide. All cells of a colony are enclosed in a common envelope. Reproduction takes place either by cell division or by production and release of aplanospores. Individual cells of a colony become aplanosporangia, that produce either a single or multiple aplanospores (up to about 30 in an aplanosporangium). Upon release, each aplanospore is spherical, 5–10 µm in diameter; it subsequently enlarges and undergoes multiple cell divisions, producing many cells that remain enclosed in a common envelope, thus forming a new colony.
Holotype specimen: GALW15826. Gåsöyane Islands, Spitsbergen. On basalt rock just above tidal zone; the locality is a protected bird-nesting site, nitrogen-enriched by bird guano. J. Elster, 25 August 2014.
Isotype specimens: BM and US.
rbcL sequence of holotype specimen: LN877817.
Holotype locality: Gåsöyane Islands, Spitsbergen.
Etymology: The name of the genus indicates the prasiolalean nature of this alga. The specific epithet honours Wendy Guiry, in recognition of the invaluable contribution that she has offered for many years to the phycological community by curating information in AlgaeBase.
This alga was not common in field-collected samples, where it was occasionally observed mixed with filamentous Prasiola crispa. At the type locality, it occurred on bare muddy rock, nutrient-enriched by bird guano, mixed with uniseriate filaments of presumptive Prasiola crispa. Prasionella wendyae was fully characterized and sequenced after isolation in culture, where it assumed a distinctive habit. It consisted of colonies varying in shape and dimensions, from a few cells to some hundreds of cells, usually sarcinoid and cushion-shaped when small, nearly spherical when large (–). In culture, Prasionella wendyae grew both in liquid and agar media, in marine as well as fresh water. Growth appeared to be generally very slow, compared with other Prasiolales under the same culture conditions; the colonies appeared to be slightly sticky and tended to aggregate in groups. The only form of reproduction that could be clearly observed was by production of aplanosporangia (), from which either one or multiple aplanospores (, ) were released. The life history of this alga, however, could not be clarified. We observed some light-coloured colonies with small cells, 2–3 µm long and wide (–), reminiscent of the male gametangial parts observed in other prasiolalean algae reproducing sexually (Prasiola stipitata, Rosenvingiella constricta (Setchell & N.L. Gardner) P.C. Silva). Conversely, other colonies were formed by darker and larger cells, 10–18 µm long and wide (), reminiscent of the female gametangial parts of the same species. We therefore presume that these might be dioecious gametangial colonies reproducing sexually; however, we were not able to observe directly release of gametes and subsequent fertilization.
Figs 23–32. Prasionella wendyae. Culture of the holotype strain SBDN 1141_D. . Habit. . A mixture of colonies of various sizes. . Some nearly spherical colonies. . Detail of young colonies. . Detail of large colonies. . Detail of some colonies and an aplanosporangium. . Detail of some colonies and some aplanospores. . Detail of a presumptive male gametangial colony (arrowhead). . Detail of a presumptive male gametangial colony (arrowhead) and an aplanosporangium releasing aplanospores (arrow). . Detail of a presumptive female gametangial colony (arrowhead). Scale bars: Fig. 23=200 μm; Figs 24, 25, 27=50 μm; Figs 26, 28–32=20 μm.
Rosenvingiellopsis Heesch, M. Pažoutová & Rindi, gen. nov
Diagnosis: Thallus formed by basal uniseriate filaments enlarging into pluriseriate, pseudoparenchymatous axes. The uniseriate filaments are 18–20 μm wide and bear long unicellular rhizoids produced as extensions of the cells; the rhizoids may be produced either singly or in short series. The pseudoparenchymatous axes are up to 175 μm thick, rounded in transversal section, with constrictions at frequent intervals, club-shaped in the apical portions. Sexual reproduction takes place by oogamy; the life history is presumed to be haplontic, and adult specimens are presumed to be dioecious haplonts, with meiosis taking place in the zygote. It differs from Rosenvingiella in rbcL sequences.
Etymology: The name of the genus indicates the morphological similarity with Rosenvingiella.
Holotype species: Rosenvingiellopsis constricta (Setchell & N.L. Gardner) Heesch, M. Pažoutová & Rindi
Rosenvingiellopsis constricta (Setchell & N.L. Gardner) Heesch, M. Pažoutová & Rindi, comb. nov.
Basionym: Gayella constricta Setchell & N.L. Gardner in N.L. Gardner Citation1917: 384, pl. 32: fig. 5; pl. 33, figs 5–9.
Gardner, N.L. (Citation1917). New Pacific coast marine algae I. University of California Publications in Botany, 6: 377–416, plates 31–35.
Homotypic synonyms:
Rosenvingiella constricta (Setchell & N.L. Gardner) P.C. Silva
Schizomeris constricta (Setchell & N.L. Gardner) Berger & Knebel
Type specimen: UC205701.
Type locality: Near the mouth of Tomales Bay, Marin County, California.
Rosenvingiella discifera (Kjellman) Heesch, M. Pažoutová & Rindi, comb. nov.
Basionym: Ulothrix discifera Kjellman Citation1877: 52, plate V, figs 10–14.
Kjellman, F.R. Citation1877. Om Spetsbergens marina klorofyllforande Thallophyter. II. Bihang till Kongelige Svenska Vetenskaps-Akademiens Handlingar, 4: 1–60.
Homotypic synonym: Gayella discifera (Kjellman) Rosenvinge
Lectotype (designated here): fig. 13 of plate V in Kjellman (Citation1877) (Supplementary Figure 1).
Syntype localities: Fairhaven and Duympoint, Spitsbergen.
Discussion
Prasionema and Prasionella as examples of hidden cryptic diversity in polar microalgae
The discovery of two new genera, Prasionema and Prasionella, confirms that our knowledge of polar algae is still far from complete. Due to the logistical difficulties of sampling in polar regions, detailed studies on polar algae have focused on relatively few sites. It is therefore not surprising that microalgae from these regions are still a major source of discoveries. Prasionema payeri and Prasionella wendyae represent yet more examples of genetic cryptic diversity hidden behind a simple morphology in green microalgae. Morphological convergence is a widespread phenomenon in all algal groups (De Clerck et al., Citation2013) and it tends to reach extreme levels in groups with simple morphology, as is the case in microchlorophytes (Krienitz & Bock, Citation2012; Fučiková et al., Citation2014; Krienitz et al., Citation2015). Prasionema payeri has a uniseriate filamentous habit, a type of morphology that is typical of some of the most widespread and cosmopolitan green algae (Rindi & Guiry, Citation2004; Pichrtová et al., Citation2014; Ryšánek et al., Citation2015). This habit has arisen at least three times in the Prasiolales, as it is found in three unrelated taxa: Prasionema payeri, Rosenvingiella radicans and Prasiola crispa. The relationship between Rosenvingiella radicans and the filamentous form of Prasiola crispa was a major source of confusion that plagued the taxonomy of the Prasiolales for over two centuries and was resolved by Rindi et al. (Citation2004) combining culture studies and molecular data. It is likely that for Arctic regions similar confusion has occurred for Prasionema payeri and filamentous Prasiola crispa. These two algae are morphologically indistinguishable and can be recognized with certainty only using DNA sequence data obtained from unialgal cultures. We expect that the geographic distribution of Prasionema payeri will turn out to be much wider than is currently known, but further studies will be necessary to clarify this. Prasionella wendyae has a morphology reminiscent of Prasiococcus calcarius, a subaerial alga originally described from central Europe (Vischer, Citation1953) but also reported from polar regions (Broady, Citation1983). Small-sized colonies of Prasionella wendyae are impossible to discriminate from Prasiococcus calcarius, and the two species share the same mode of reproduction by aplanospores (Bourrelly, Citation1966; Broady, Citation1983; Ettl & Gärtner, Citation1995). Fully developed colonies of Prasionella, however, are generally larger (up to some hundreds of cells) than those of Prasiococcus (2–8 cells: Broady, Citation1983; Ettl & Gärtner, Citation1995), and the rbcL phylogeny provides undisputable evidence of their separation.
Taxonomy of Rosenvingiella and establishment of Rosenvingiellopsis
The discovery of Prasionella has also major implications on the taxonomy of Rosenvingiella. Rosenvingiella was a new name designated by Silva (Citation1957) for the former genus Gayella Rosenvinge, illegitimate homonym of Gayella Pierre (Silva, Citation1957). Silva included in the genus two species, the generitype Rosenvingiella polyrhiza (Rosenvinge) P.C. Silva and Rosenvingiella constricta (Setchell & N.L. Gardner) P.C. Silva; subsequent studies (Vinogradova, Citation1984; Rindi et al., Citation2004; Heesch et al., Citation2012; Moniz et al., Citation2012b) have increased the number of species to six (Guiry & Guiry, Citation2015). Morphologically, species of Rosenvingiella consist of unbranched pseudoparenchymatous axes attached to the substratum by horizontal uniseriate filaments issuing unicellular rhizoids either singly or in short series (Rindi, Citation2007; Guiry & Guiry, Citation2015). This genus includes both terrestrial and marine (upper intertidal) species. Whereas the only species widespread in terrestrial habitats (R. radicans) consists mostly of uniseriate filaments, in the marine species the pluriseriate pseudoparenchymatous portions usually form the majority of the thallus.
Rosenvingiella constricta was described (as Gayella constricta Setchell & N.L Gardner) by Setchell & Gardner in Gardner (Citation1917) based on specimens from Tomales Point, California. The alga exhibits the morphology typical of Rosenvingiella; Gardner (Citation1917) separated it from R. polyrhiza based on the deeper constrictions in the pluriseriate axes and the fewer and longer rhizoids. In previous rbcL phylogenies Rosenvingiella was monophyletic and R. constricta was recovered within the clade corresponding to this genus, but only with moderate (Rindi et al., Citation2007; Heesch et al., Citation2012; Moniz et al., Citation2012b) or weak (Moniz et al., Citation2012a) support. In a recent tufA phylogeny (Moniz et al., Citation2014) the only two species of Rosenvingiella included, R. constricta and R. radicans, formed separate lineages and the monophyly of the genus was not supported. Taken together, these studies indicated that R. constricta represented a separate lineage from all other species of Rosenvingiella. Our results have confirmed this situation and provided strong support for the separation of R. constricta in the new genus Rosenvingiellopsis. Further studies will be necessary to clarify if this separation is also supported by additional morphological or ultrastructural characters, or life history traits. Gardner (Citation1917) did not provide details about the reproduction and life history of Rosenvingiella constricta in the population originally used for the description. Our current knowledge of reproduction and life history of this species is inferred from the studies of Kornmann & Sahling (Citation1974) for populations from Helgoland and O’Kelly et al. (Citation1989) for populations from British Columbia. These studies concluded that R. constricta is a dioecious haplont that reproduces by oogamy and in which meiosis and sex determination take place in the zygote.
We propose here Rosenvingiella discifera as a new combination for the species described by Kjellman (Citation1877: 52) as Ulothrix discifera, based on material from Fairhaven and Duympoint. At the time when Kjellman described the species, the circumscription of many genera of green algae was different from the current one and Ulothrix accommodated many filamentous green algae that are now separated in different genera. In order to clarify the identity of this alga, we looked for authentic specimens of Ulothrix discifera in UPS (the main repository of Kjellman’s collections) and other herbaria that are strong candidates to host collections of this author (BM, FI, LD, O, PC, S, TRH, TROM, TU, US). Unfortunately, the curators of these herbaria were not able to locate any specimens of Ulothrix discifera collected or examined personally by Kjellman. In any case, Kjellman’s (Citation1877) description and illustrations (Supplementary Figure 1) strongly suggest that this alga is a member of the genus Rosenvingiella as currently circumscribed; this was also the opinion of Rosenvinge (Citation1893: 938), who transferred this species to Gayella. Unfortunately in the course of our field surveys we were not able to collect any specimens referable to this species, so the specific identity of Rosenvingiella discifera remains in need of clarification based on new collections from the type localities. Morphologically, Rosenvingiella discifera is very similar to R. polyrhiza, and for many characters there is complete agreement between the two species. Rosenvinge (Citation1893: 938) considered them distinct, remarking that R. discifera is devoid of rhizoids and has cells arranged in less regular rows than R. polyrhiza. We believe, however, that without accurate culture studies the taxonomic significance of these characters is impossible to verify and that the examination of field collections alone is not sufficient to assess their stability. Ultimately, the only way to clarify the status of R. discifera will be to sequence material collected from the type localities in good correspondence with the original description. Since this alga is prone to confusion with R. polyrhiza and R. constricta, which have both been reported from subpolar and cold temperate regions of the North Atlantic (Rosenvinge, Citation1893; Waern, Citation1952; Kornmann & Sahling, Citation1974; Rindi, Citation2007; Heesch, unpublished information), this should be considered a priority for future studies on the taxonomy of the Prasiolales.
Wittrock (Citation1883: plate 3, figs 25–26) illustrated a filamentous alga from Fairhaven that he designated as Ulothrix discifera β nivalis; however, he did not provide a diagnosis for this taxon, which was therefore not validly published. This is a filamentous uniseriate alga, with the habit typical of a prasiolalean species; in consideration of the impossibility to distinguish easily Prasionema and filamentous Prasiola crispa, the identity of this alga cannot be assessed.
Biogeography
Biogeography and diversity of eukaryotic microorganisms have been the focus of much discussion in the last 10 years (Caron, Citation2009; Ryšánek et al., Citation2015). On one side, it is believed that protists have ubiquitous distribution and unlimited dispersal, implying much lower total diversity compared with that of macroorganisms (Finlay et al., Citation2001; Finlay, Citation2002); this view is synthesized by the well-known Baas Becking’s (Citation1934) statement ‘everything is everywhere, but the environment selects’. The opposite viewpoint argues that global protist species diversity is extremely high because most species have limited geographic distribution (Foissner, Citation2006, Citation2008), a perspective that in recent years has received support from numerous molecular studies. Based on the information presently available, the Prasiolales do not appear to conform perfectly to either of these two models, as the situation differs among species. Prasiola crispa can be considered genuinely cosmopolitan; there is now solid molecular confirmation that this species occurs in polar and cold temperate regions of both hemispheres (Rindi et al., Citation2004, Citation2007; Heesch et al., Citation2012; Moniz et al., Citation2012a; Heesch et al., this study). This alga probably exists in any habitat where conditions suitable for its persistence and reproduction occur. It would be interesting to search for Prasiola crispa in areas located in tropical regions but with glacial conditions similar to those of polar regions (e.g. the Himalayas; Mount Kilimanjaro; Peruvian, Ecuadorian and Colombian Andes). It can be expected that in habitats with suitable conditions (especially enrichment in organic nitrogen) Prasiola crispa will be present, which would strongly support the hypothesis of global dispersal of this species. This type of biogeographic scenario has been documented recently for other microchlorophytes (Schmidt et al., Citation2011). This situation applies also to the marine species Prasiola stipitata and P. meridionalis, which are widely distributed on rocky shores of cold temperate regions of both hemispheres (Rindi et al., Citation2004, Citation2007; Heesch et al., Citation2012; for a detailed discussion of the taxonomic relationships between these species based on molecular data, see Moniz et al., Citation2014). Conversely, other species appear to have a more limited distribution, e.g. Prasiola calophylla (so far only confirmed from northeastern Atlantic and central European regions), P. fluviatilis (polar and subpolar regions of the northern hemisphere), Prasiola novaezelandiae S. Heesch & W.A. Nelson (New Zealand), Rosenvingiella australis and R. tasmanica (New Zealand and Tasmania, respectively). For certain species the distribution is restricted to a single region and type of habitat (e.g. Prasiola linearis Jao, only known from coastal lagoons in Washington State and British Columbia; Rindi, Citation2010). We suspect that to some extent such differences in distribution may be related to the type of habitat occupied. Terrestrial species, that are dispersed by the action of wind, rain and animals (primarily birds), can be expected to have a wider distribution than aquatic species, especially freshwater species, that release their spores and gametes in the water bodies in which they occur. Indeed for green microalgae the examples of widespread distribution that are currently best supported by molecular evidence concern subaerial forms (Hodač et al., Citation2012; Ryšánek et al., Citation2015); conversely, some of the strongest cases of restricted distribution and dispersal limitations concern freshwater microalgae, particularly diatoms (Vyverman et al., Citation2007; De Wever et al., Citation2009; Evans et al., Citation2009). We acknowledge, however, that further investigations of the diversity and distribution of Prasiolales in little-explored regions are highly desirable in order to draw stronger generalizations.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2015.1115557
Supplementary table 1. Details of records of species of Prasiolales for the Svalbard Archipelago available in the literature.
Supplementary table 2. Origin and GenBank accession numbers of taxa included in the sequence alignments, with collection details of additional new samples from outside Svalbard for which new sequences were generated in this study
Supplementary fig. 1. Plate V of Kjellman (Citation1877). is the designated lectotype of Rosenvingiella discifera.
Supplementary Figure 1
Download JPEG Image (1.2 MB)Supplementary Tables 1 and 2
Download MS Word (41 KB)Acknowledgements
MP is grateful to Josef Elster for overall support and to Jana Kvíderová and Jakub Žárský for assistance with fieldwork. Alex Sašenka Bernardová and Jan Jeník Kavan should be mentioned for their care for Centre for Polar Ecology. Dr Thomas Leya and the CCCryo Culture Collection are gratefully acknowledged for useful information and for arranging the loan of the strain 066-99.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Svenja Heesch
S. Heesch: sample collection, culture work, DNA extraction, PCR and sequencing, molecular phylogenetic analyses, microphotography, manuscript editing. M. Pazoutova: sample collection, microphotography. M.B.J. Moniz: DNA extraction, PCR and sequencing, molecular phylogenetic analyses. F. Rindi: original concept, drafting and editing manuscript, microphotography.
Marie Pažoutová
S. Heesch: sample collection, culture work, DNA extraction, PCR and sequencing, molecular phylogenetic analyses, microphotography, manuscript editing. M. Pazoutova: sample collection, microphotography. M.B.J. Moniz: DNA extraction, PCR and sequencing, molecular phylogenetic analyses. F. Rindi: original concept, drafting and editing manuscript, microphotography.
Mónica B.J. Moniz
S. Heesch: sample collection, culture work, DNA extraction, PCR and sequencing, molecular phylogenetic analyses, microphotography, manuscript editing. M. Pazoutova: sample collection, microphotography. M.B.J. Moniz: DNA extraction, PCR and sequencing, molecular phylogenetic analyses. F. Rindi: original concept, drafting and editing manuscript, microphotography.
Fabio Rindi
S. Heesch: sample collection, culture work, DNA extraction, PCR and sequencing, molecular phylogenetic analyses, microphotography, manuscript editing. M. Pazoutova: sample collection, microphotography. M.B.J. Moniz: DNA extraction, PCR and sequencing, molecular phylogenetic analyses. F. Rindi: original concept, drafting and editing manuscript, microphotography.
References
- Baas Becking, L.G.M. (1934). Geobiologie of inleiding tot de milieukunde. W.P. Van Stockum & Zoon, The Hague.
- Bielewicz, S., Bell, E., Kong, W., Friedberg, I., Priscu, J.C. & Morgan-Kiss, R.M. (2011). Protist diversity in a permanently ice-covered Antarctic lake during the polar night transition. ISME Journal, 5: 1559–1564.
- Bock, C., Jacob, A. Kirst, G.O., Leibfritz, D. & Mayer, A. (1996). Metabolic changes of the Antarctic green alga Prasiola crispa subjected to water stress investigated by in vivo P-31 NMR. Journal of Experimental Botany, 47: 241–249.
- Borge, O. (1911). Die süsswasseralgenflora Spitzbergens. Videnskapsselskapet Skrifter I – Matematisk-Naturvidenskabelig Klasse, 11: 1–39.
- Bourrelly, P. (1966). Les algues d’eau douce. Initiation à la systématique. Tome I: Les Algues vertes. pp. 1–511. Boubée & Cie, Paris.
- Broady, P.A. (1983). The antarctic distribution and ecology of the terrestrial chlorophytan alga Prasiococcus calcarius (Boye Petersen) Vischer. Polar Biology, 1: 211–216.
- Broady, P.A. (1989). The distribution of Prasiola calophylla (Carmich.) Menegh. (Chlorophyta) in Antarctic freshwater and terrestrial habitats. Antarctic Science, 1: 109–118.
- Broady, P.A. (1996). Diversity, distribution and dispersal of Antarctic terrestrial algae. Biodiversity and Conservation, 5: 1307–1335.
- CAREX (Coordination Action for Research Activities on life in Extreme Environments). (2009). CAREX model organisms workshop. Compilation of inputs received. CAREX, Strasbourg.
- Caron, D.A. (2009) Past president’s address: protistan biogeography: why all the fuss? Journal of Eukaryotic Microbiology, 56: 105–112.
- Darienko, T., Gustavs, L., Mudimu, O., Rad Menendez, C., Schumann, R., Karsten, U., Friedl, T. & Pröschold, T. (2010). Chloroidium, a common terrestrial green alga previously assigned to Chlorella (Trebouxiophyceae, Chlorophyta). European Journal of Phycology, 45: 79–95.
- De Clerck, O., Guiry, M.D., Leliaert, F., Samyn, Y. & Verbruggen, H. (2013). Algal taxonomy: a road to nowhere? Journal of Phycology, 49: 215–225.
- De Wever, A., Leliaert, F., Verleyen, E., Vanormelingen, P., Van der Gucht, K., Hodgson, D.A., Sabbe, K. & Vyverman, W. (2009). Hidden levels of phylodiversity in Antarctic green algae: further evidence for the existence of glacial refugia. Proceedings of the Royal Society B, 276: 3591–3599.
- Ettl, H. & Gärtner, G. (1995). Syllabus der Boden-, Luft- und Flechtenalgen. Gustav Fischer Verlag, Stuttgart.
- Evans, K.M., Chepurnov, V.A., Sluiman, H.J., Thomas, S.J., Spears, B.M. & Mann, D.G. (2009). Highly differentiated populations of the freshwater diatom Sellaphora capitata suggest limited dispersal and opportunities for allopatric speciation. Protist, 160: 386–396.
- Finlay, B.J. (2002). Global dispersal of free-living microbial eukaryote species. Science, 296: 1061–1063.
- Finlay, B.J., Esteban, G.F., Clarke, K.J. & Olmo, J.L. (2001). Biodiversity of terrestrial protozoa appears homogenous across local and global spatial scales. Protist, 152: 355–366.
- Foissner, W. (2006). Biogeography and dispersal of microorganisms: a review emphasizing protists. Acta Protozoologica, 45: 111–136.
- Foissner, W. (2008). Protist diversity and distribution: some basic considerations. Biodiversity and Conservation, 17: 235–242.
- Fučiková, K., Lewis, P.O. & Lewis, L.A. (2014). Putting incertae sedis taxa in their place: a proposal for ten new families, and three new genera in Sphaeropleales (Chlorophyceae, Chlorophyta). Journal of Phycology, 50: 14–25.
- Garbary, D.J. & Tarakhovskaya, E.R. (2013). Marine macroalgae and associated flowering plants from the Keret Archipelago, White Sea, Russia. Algae, 28: 267–280.
- Gardner, N.L. (1917). New Pacific coast marine algae I. University of California Publications in Botany, 6: 377–416.
- Glez-Peña, D., Gómez-Blanco, D., Reboiro-Jato, M., Fdez-Riverola, F. & Posada, D. (2010). ALTER: program-oriented format conversion of DNA and protein alignments. Nucleic Acids Research, 38: 14–18.
- Griekspoor, A. & Groothuis, T. (2005). 4Peaks. www.mekentosj.com.
- Guiry, M.D. & Cunningham, E.M. (1984). Photoperiodic and temperature responses in the reproduction of north-eastern Atlantic Gigartina acicularis (Rhodophyta: Gigartinales). Phycologia, 23: 357–367.
- Guiry, M.D. & Guiry, G.M. (2015). AlgaeBase. World-wide electronic publication. National University of Ireland, Galway. Available at http://www.algaebase.org. [Accessed 14 May 2015].
- Gulliksen, B., Palerud, R., Brattegard, T. & Sneli, J. (1999). Distribution of Marine Benthic Macro-organisms at Svalbard (including Bear Island) and Jan Mayen. Research Report for DN 1999–4. Directorate for Nature Management, Trondheim.
- Hansen, J.R. & Haugen, I. (1989). Some observations of intertidal communities on Spitsbergen (79”N), Norwegian Arctic. Polar Research, 7: 23–27.
- Heesch, S., Sutherland, J.E. & Nelson, W. (2012). Marine Prasiolales (Trebouxiophyceae, Chlorophyta) from New Zealand and the Balleny Islands, with descriptions of Prasiola novaezelandiae sp. nov. and Rosenvingiella australis sp. nov. Phycologia, 51: 217–227.
- Hodač, L., Hallmann, C., Rosenkranz, H., Faßhauer, F. & Friedl, T. (2012). Molecular evidence for the wide distribution of two lineages of terrestrial green algae (Chlorophyta) over tropics to temperate zone. ISRN Ecology, 2012: 1–9.
- Holzinger, A., Karsten, U., Lütz, C. & Wiencke, C. (2006). Ultrastructure and photosynthesis in the supralittoral green macroalga Prasiola crispa from Spitsbergen (Norway) under UV exposure. Phycologia, 45: 168–177.
- Hoyer, K., Karsten, U., Sawall, T. & Wiencke, C. (2001). Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Marine Ecology Progress Series, 211: 117–129.
- Huelsenbeck, J.P. & Ronquist, F. (2001). MrBayes: Bayesian inference of phylogeny. Bioinformatics, 17: 754–755.
- Jackson, A.E. & Seppelt, R.D. (1995). The accumulation of proline in Prasiola crispa during winter in Antarctica. Physiologia Plantarum, 94: 25–30.
- Jackson, A.E. & Seppelt, R.D. (1997). Physiological adaptations to freezing and UV radiation exposure in Prasiola crispa, an Antarctic terrestrial alga. In Antarctic Communities: Species, Structure, and Survival (Battaglia, B., Valencia, J. & Walton, D.W.H., editors), 226–233. Cambridge University Press, Cambridge.
- Jacob, A., Wiencke, C., Lehmann, H. & Kirst, G.O. (1992). Physiology and ultrastructure of desiccation in the green alga Prasiola crispa from Antarctica. Botanica Marina 35: 297–303.
- Jakubas, D., Zmudczynska, K., Wojczulanis-Jakubas, K. & Stempniewicz, L. (2008). Faeces deposition and numbers of vertebrate herbivores in the vicinity of planktivorous and piscivorous seabird colonies in Hornsund, Spitsbergen. Polish Polar Research, 29: 45–58.
- Jessen, C.F.G. (1848). Prasiolae generis algarum monographia. Dissertatio inauguralis botanica. Libraria Academica, Kiliae (Kiel).
- Jungblut, A.D., Vincent, W.F. & Lovejoy, C. (2012). Eukaryotes in Arctic and Antarctic cyanobacterial mats. FEMS Microbiology Ecology, 82: 416–428.
- Kaštovska, K., Elster, J., Stibal, M. & Šantručková, H. (2005). Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (High Arctic). Microbial Ecology, 50: 396–407.
- Kjellman, F.R. (1877). Om Spetsbergens marina klorofyllforande Thallophyter. II. Bihang till Kongelige Svenska Vetenskaps-Akademiens Handlingar, 4: 1–60.
- Knebel, G. (1936). Monographie der Algenreihe von Prasiolales, insbesondere von Prasiola crispa. Hedwigia, 75: 1–20.
- Kobayasi, Y. (1967). Prasiola crispa and its allies in the Alaskan Arctic and Antarctica. Bulletin of the Natural Sciences Museum of Tokyo, 10: 211–220.
- Kornmann, P. & Sahling, P.H. (1974). Prasiolales (Chlorophyta) from Helgoland. Helgoländer Meeresuntersuchungen, 26: 99–133.
- Krienitz, L. & Bock, C. (2012). Present state of the systematic of planktonic coccoid green algae of inland waters. Hydrobiologia, 698: 295–326.
- Krienitz, L., Huss, V.A.R. & Bock, C. (2015). Chlorella: 125 years of the green survivalist. Trends in Plant Science, 20: 67–69.
- Lagerstedt, N.G.W. (1869). Om algslägtet Prasiola. Försök till en Monographi. W. Schultzò Boktryckeri, Uppsala.
- Lemieux, C., Otis, C. & Turmel, M. (2014). Chloroplast phylogenomic analysis resolves deep-level relationships within the green algal class Trebouxiophyceae. BMC Evolutionary Biology, 14: 211.
- Lewis, L.A. & Lewis, P.O. (2005). Unearthing the molecular phylodiversity of desert soil green algae (Chlorophyta). Systematic Biology, 54: 936–947.
- Lud, D., Buma, A.G.J., Van de Poll, W., Moerdijk, T.C.W. & Huiskes, A.H.L. (2001). DNA damage and photosynthetic performance in the Antarctic terrestrial alga Prasiola crispa ssp. antarctica (Chlorophyta) under manipulated UV-B radiation. Journal of Phycology, 37: 459–467.
- Manhart, J. R. (1994). Phylogenetic analysis of green plant rbcL sequences. Molecular Phylogenetics and Evolution, 3: 114–127.
- Moniz, M.B.J., Rindi, F., Novis, P.M., Broady, P.A. & Guiry, M.D. (2012a). Molecular phylogeny of Antarctic Prasiola reveals extreme cryptic diversity. Journal of Phycology, 48: 940–955.
- Moniz, M.B.J., Rindi, F. & Guiry, M.D. (2012b). Phylogeny and taxonomy of Prasiolales (Trebouxiophyceae, Chlorophyta) from Tasmania, including Rosenvingiella tasmanica sp. nov. Phycologia, 51: 86–97.
- Moniz, M.B.J., Guiry, M.D. & Rindi, F. (2014). tufA phylogeny and species boundaries in the green algal order Prasiolales (Trebouxiophyceae, Chlorophyta). Phycologia, 53: 396–406.
- Naw, M.W.D. & Hara, Y. (2002). Morphology and molecular phylogeny of Prasiola sp. (Prasiolales, Chlorophyta) from Myanmar. Phycological Research, 50: 175–182.
- O’Kelly, C.J., Garbary, D.J. & Floyd, G.L. (1989). Flagellar apparatus of male gametes and other aspects of gamete and zygote ultrastructure in Prasiola and Rosenvingiella (Chlorophyta, Prasiolales) from British Columbia. Canadian Journal of Botany, 67: 505–514.
- Pichrtová, M., Hájek., T. & Elster, J. (2014). Osmotic stress and recovery in field populations of Zygnema sp. (Zygnematophyceae, Streptophyta) on Svalbard (High Arctic) subjected to natural desiccation. FEMS Microbiology Ecology, 89: 270–280.
- Printz, H. (1964). Die Chaetophoralen der binnengewässer. Eine systematische übersicht. Hydrobiologia, 24: 1–376.
- Rindi, F. (2007). Trebouxiophyceae – Prasiolales. In Green Seaweeds of Britain and Ireland (Brodie, J., Maggs, C.A. & John, D.M., editors), 13–31. British Phycological Society, London.
- Rindi, F. (2010). Reproduction and life history of the green alga Prasiola linearis Jao (Trebouxiophyceae, Chlorophyta). Botanica Marina, 53: 1–7.
- Rindi, F. & Guiry, M.D. (2004). Composition and spatial variability of terrestrial algal assemblages occurring at the bases of urban walls in Europe. Phycologia, 43: 225–235.
- Rindi, F., McIvor, L. & Guiry, M.D. (2004). The Prasiolales (Chlorophyta) of Atlantic Europe: an assessment based on morphological, molecular and ecological data, including the characterization of Rosenvingiella radicans (Kützing) comb. nov. Journal of Phycology, 40: 977–997.
- Rindi, F., McIvor, L., Sherwood, A.R., Friedl, T., Guiry, M.D. & Sheath, R.G. (2007). Molecular phylogeny of the green algal order Prasiolales (Trebouxiophyceae, Chlorophyta). Journal of Phycology, 43: 811–822.
- Rindi, F., Guiry, M.D. & López-Bautista, J. M. (2008). Distribution, morphology and phylogeny of Klebsormidium (Klebsormidiales, Charophyceae) in urban environments in Europe. Journal of Phycology, 44: 1529–1540.
- Ronquist, F., Teslenko, M., Van der Mark, P., Ayres, D., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Rosenvinge, L.K. (1893). Grønlands Havalger. Meddelelser om Grønland, 3: 763–981.
- Ryšánek, D., Hrčková, K. & Škaloud, P. (2015). Global ubiquity and local endemism of free-living terrestrial protists: phylogeographic assessment of the streptophyte alga Klebsormidium. Environmental Microbiology, 17: 689–698.
- Saunders, G.W. & Kucera, H. (2010). An evaluation of rbcL, tufA, UPA, LSU and ITS as DNA barcode markers for the marine green macroalgae. Cryptogamie, Algologie, 31: 487–528.
- Schmidt, S.K., Lynch, R.C., King, A.J., Karki, D., Robeson, M.S., Nagy, L., Williams, M.W., Mitter, M.S. & Freeman, K.R. (2011). Phylogeography of microbial phototrophs in the dry valleys of the high Himalayas and Antarctica. Proceedings of the Royal Society B, 278: 702–708.
- Sherwood, A.R., Garbary, D.J. & Sheath, R.G. (2000). Assessing the phylogenetic position of the Prasiolales (Chlorophyta) using rbcL and 18S rRNA sequence data. Phycologia, 39: 139–146.
- Silva, P.C. (1957). Notes on Pacific marine algae. Madroño, 14: 41–51.
- Silvestro, D. & Michalak, I. (2011). RAxMLGUI: a graphical front-end for RAxML. Available at https://sites.google.com/site/raxmlgui/.
- Skulberg, O.M. (1996). Terrestrial and limnic algae and cyanobacteria (Svalbard). In A catalogue of Svalbard plants, fungi, algae and cyanobacteria (Elvebakk, A. & Prestrud, P., editors), 383–395. Skrifter 198. Norsk Polarinstitutt, Oslo.
- Souffreau, C., Vanormelingen, P., Van de Vijver, B., Isheva, T., Verleyen, E., Sabbe, K. & Vyverman, W. (2013). Molecular evidence for distinct Antarctic lineages in the cosmopolitan terrestrial diatoms Pinnularia borealis and Hantzschia amphioxys. Protist, 164: 101–115.
- Strunecký, O., Elster, J. & Komárek, J. (2010). Phylogenetic relationships between geographically separate Phormidium cyanobacteria: is there a link between north and south polar regions? Polar Biology, 33: 1419–1428.
- Thüs, W., Muggia, L., Perez-Ortega, S., Favero-Longo, S.E., Joneson, S., O’Brien, H., Nelsen, M.P., Duque-Thüs, R., Grube, M., Friedl, T., Brodie, J., Andrew, C.J., Lücking, R., Lutzoni, F. & Gueidan, C. (2011). Revisiting photobiont diversity in the lichen family Verrucariaceae (Ascomycota). European Journal of Phycology, 46: 399–415.
- Van den Hoek, C., Mann, D.G. & Jahns, H.M. 1995. Algae: An Introduction to Phycology. Cambridge University Press, Cambridge.
- Vinogradova, K.L. (1984). Ad flora chlorophytorum ex Antarctide. Novosti Sistematiki Nizshikh Rastenii, 20: 10–18.
- Vinogradova, K.L. (1995). The checklist of the marine algae from Spitsbergen. Botanical Journal, 80: 50–61.
- Vischer, W. (1953). Über primitivste Landpflanzen. Berichte der Schweizerischen Botanischen Gesellschaft, 63: 169–193.
- Vyverman, W., Verleyen, E., Sabbe, K., Vanhoutte, K., Sterken, M., Hodgson, D.A., Mann, D.G., Juggins, S., Van de Vjiver, B., Jones, V. & Flower, R. (2007). Historical processes constrain patterns in global diatom diversity. Ecology, 88: 1924–1931.
- Waern, M. (1952). Rocky-shore algae in the Öregrund Archipelago. Acta Phytogeographica Suecica, 30: 1–298.
- Wiencke, C. & Clayton, M.N. (2002). Antarctic Seaweeds. Synopses of the Antarctic Benthos Vol. 9. A.R.G. Gantner Verlag KG, Ruggell, Lichtenstein.
- Wittrock, V.B. (1883). Om snöns och isens flora, särskildt i de arktiska trakterna. A. E. Nordenskiöld, Studier och forskningar föranledda af mina resor i hoga norden. F. & G. Beijers Förlag, Stockholm.
- Zmudczynska, K., Olejniczak, I., Zwolicki, A., Iliszko, L., Convey, P. & Stempniewicz, L. (2012). Influence of allochthonous nutrients delivered by colonial seabirds on soil collembolan communities on Spitsbergen. Polar Biology, 35: 1233–1245.
