ABSTRACT
A previous study of available diatom sequences tested the value of multiple outgroups in analyses of the 18S ribosomal RNA gene aimed at recovering the three diatom Classes (Coscinodiscophyceae, Mediophyceae, Bacillariophyceae) as monophyletic groups. Of the 34 datasets tested in that study, three recovered these Classes and two of these are explored here in more detail. The major differences between these two datasets were the number of outgroups, the number of nucleotides selected for the analysis and the addition to the dataset of short clone library sequences, primarily from raphid pennate taxa. The addition of short sequences resulted in a smaller dataset in terms of total nucleotides analysed because of the filter selected in the ARB program to generate the final dataset for phylogenetic analysis. The 50% parsimony filter selects a base for the final dataset if that position is parsimony-informative in at least 50% of the sequences chosen for the analysis. Thus, with short sequences, fewer bases are available for selection in the final dataset. A final dataset with fewer nucleotides is produced from the original alignment than from a similar one with no filter applied. If no filter is applied, then a final dataset is produced with all full-length sequences. From these analyses, it was determined that the addition of short clone library sequences recovered sister relationships for certain raphid diatoms that go against conventional wisdom with regard to valve morphology. Thus, the smaller database caused some of the groupings to be unnatural. It was determined that the optimal resolution of the taxa in these datasets resulted from using only full length sequences and selected multiple outgroups. Results obtained from the reduced dataset would be a cause for concern with next generation sequencing in which short amplicons are used to identify taxa.
Introduction
The diatoms (Bacillariophyta), with over 10,000 described species and potentially many more cryptic species (Mann, Citation1999), are one of the most successful microalgal groups in both aquatic and terrestrial habitats. Their complex bipartite siliceous cell walls (valves and girdle bands) are unique among the algae. The pattern of cell size reduction in one of the daughter cells following mitosis is also unique and results in a population of cells of smaller sizes that, normally, can only be restored to the cell’s maximum cell size following sexual reproduction (see reviews in Mann & Marchant, Citation1989; Kaczmarska et al., Citation2013).
Since the 19th century, diatom classification has been based on the intricate designs of their cell walls (for a review of the history of classification see Williams, Citation2007). Since the early 1990s, much work has been directed towards understanding diatom classification. Medlin et al. (Citation1993) produced the first phylogeny of the diatoms using molecular data and showed that the centric and araphid diatoms were not monophyletic. Based on nearly 20 years of mismatch between molecular and morphological classifications, Medlin & Kaczmarska (Citation2004) defined two new subphyla, Coscinodiscophytina and Bacillariophytina, and one class of centric diatoms, Mediophyceae, and amended the description of the other two diatom classes, the Coscinodiscophyceae and Bacillariophyceae. These three classes (Coscinodiscophyceae = radial centric diatoms; Mediophyceae = polar centric diatoms + radial Thalassiosirales; Bacillariophyceae = pennate diatoms) more accurately reflect the evolution and diversity of the diatoms than does the three-class system of centrics, araphid pennates and raphid pennates presented in Round et al. (Citation1990). The three classes defined by Medlin & Kaczmarska (Citation2004) were originally defined morphologically by (1) the type of sexual reproduction and resultant auxospore formation, (2) the presence/absence of a tube or process (in the case of the centric diatoms) or raphe/sternum (in the pennate diatoms) inside the annulus (the initiation point for silicification in the diatoms), (3) symmetry of the valves and (4) the arrangement of the Golgi bodies in the cells (Medlin & Kaczmarska, Citation2004). Medlin (Citation2014) added the position of the cribrum in loculate areolae as another defining character to separate the two centric classes. Kaczmarska & Ehrman (Citation2015) found the spore-like structure of the auxospore to be another character separating the three classes. Exceptions to each character have been noted and the placement of the radial Thalassiosirales in the polar centric clade is one of the biggest exceptions to the features defining each class. Retention of an ancestral polymorphism (scales) and loss of the ability to make bands to mould a radial centric into a polar one have been invoked to explain why the radial Thalassiosirales are placed in the polar diatom lineage (Medlin, Citation2016a).
Medlin & Kaczmarska (Citation2004) proposed that the recovery of the two centric clades as monophyletic groups is highly dependent on an alignment based on the secondary structure of the SSU rRNA gene and the use of multiple outgroups. The effect of the secondary structure alignment on the topology of the rRNA tree has been documented in several studies (Medlin et al., Citation1993, Citation2008b; Medlin, Citation2010; Rimet et al., Citation2011). The use of multiple outgroups has been tested with a single gene (Medlin, Citation2014) and multiple genes (Sato, Citation2008; Medlin & Desdevies, Citation2016).
Theriot et al. (Citation2009) concluded that the use of the SSU rRNA gene was insufficient to recover the monophyletic classes as proposed by Medlin & Kaczmarska (Citation2004) and directed their subsequent research into multi-gene analysis using a single outgroup, the bolidomonads (Theriot et al., Citation2010; Ashworth et al., Citation2012, Citation2013). Based on the bioinformatic study by Piganeau et al. (Citation2012) showing that the ribosomal RNA genes contained more information and better resolution for the protists than protein-coding genes, Medlin (Citation2014) provided evidence supporting the phylogenetic value of SSU gene analyses. In that study Medlin explored the use of the SSU rRNA gene with multiple outgroups for the resolution of the centric classes to determine whether or not they were monophyletic, and if not, how many clades were recovered. She used 34 datasets with various combinations of outgroups, ingroups and numbers of nucleotides to study the effect of multiple outgroups on the ability of analyses of a single gene, the SSU rRNA gene, to recover monophyletic classes. She also looked at the effects of weighting the frequency of base substitutions per site if maximum parsimony analyses were used for large datasets. In her study, three of the datasets recovered the proposed taxonomic groups as monophyletic clades. Datasets 11 and 25 from Medlin (Citation2014) are examined here in more detail, to determine whether the number of nucleotides and the inclusion of short clone library sequences affected the relationships among the diatom taxa in the analyses.
Materials and methods
Taxon sampling
The database used in Medlin (Citation2014) included all diatom sequences that were in GenBank as of 2009 plus unpublished clone library sequences from Thomas Friedl and new strains from Belgium (K. Sabbe and W. Vyverman) and is shown in Supplementary Table 1.
Data analysis
In Medlin (Citation2014), 34 datasets with varying numbers of ingroup and outgroup taxa were tested with both Bayesian Inference (BI) and RAxML (Stamatakis, Citation2014) analyses within the ARB program to determine the effects of these variables on the ability to recover monophyletic clades at the class level in the diatoms. Two of these datasets showed monophyletic Classes and details are presented in this study. Dataset 11 contained the new sequences from University of Ghent plus all the diatom sequences in GenBank as of 2004 (Supplementary Table 1) and dataset 25 contained all the diatom taxa in GenBank as of 2009 plus all clone library sequences available in the ARB database release from 1996 that could be assigned to diatoms plus some unpublished clone library sequences from Thomas Friedl.
rRNA sequences from the diatoms were uploaded and aligned to the SILVA SSU rRNA sequence alignment in the ARB program Version 5.5 using maximum primary and secondary structural similarity (Ludwig et al., Citation2004). The ARB database release used in these analyses contained over 325,362 eukaryotic and prokaryotic sequences. Bases were aligned with one another based on their pairing across a helix. The ARB program generates a maximum parsimony (MP) tree from all sequences and all positions in the database as its reference tree. Various filters can be applied to the dataset to export a dataset for further phylogenetic analyses. Nucleotides can be exported from ARB using either no filter, thus including all the aligned bases (none should be unambiguously aligned because of the secondary structure, with the exception of the loops, which are aligned using primary sequence structure) or, more commonly, using a 50% base frequency filter across all groups. The 50% parsimony filter selects only those bases in the alignment where the base is parsimony-informative in at least 50% of the taxa used in the analysis. If any filter is used, then the number of nucleotides selected for the phylogenetic analysis varies with the taxa selected for the analysis, their sequence length and the filter selected. 50% parsimony filters can also be constructed for each taxon of interest, e.g. all eukaryotes, all prokaryotes, all heterokonts, etc. and applied to any subset of the group for which a parsimony filter has been constructed. Thus if many short sequences are included in the analysis and the 50% parsimony filter is used, the resultant dataset contains fewer nucleotides (nts). In this study, dataset 11 was generated with no filter and dataset 25 with the 50% parsimony filter. Dataset 11 had 427 taxa and 2994 nts, all full-length sequences. Dataset 25 had 594 taxa with 1834 nts, only those regions included in the clone library sequences.
Some minor differences between the diatom species in each dataset were also noted. Dataset 11 included Ellerbeckia sol but dataset 25 did not and vice versa for Paralia sulcata. In dataset 11, multiple bolidomonads plus one species of Mallomonas were used as outgroups. In dataset 25, multiple outgroups (ciliates, haptophytes and chlorophytes) outside the heterokonts, plus heterotrophic heterokonts, Mallomonas and two bolidomonads as well as clone library sequences were used.
Datasets presented here were analysed using BI from within ARB (see Medlin, Citation2014 for more details). The last 100 trees were used to construct the consensus tree from both datasets. The posterior probabilities (PP) are reported as whole numbers (e.g. 0.99 is reported as 99) or by adjusting the thickness of the line for each node to reflect the strength of the PP. Because the taxon sampling between the two datasets is very similar, the consensus trees from dataset 11 are presented as a cladogram to show the branching order and those from dataset 25 as a phylogram to show the genetic distance between taxa. The consensus tree from dataset 11 was uploaded into PAUP and exported as a cladogram in PICT format, whereas for dataset 25, the last tree was uploaded into FIGTREE and exported from that program as a phylogram.
Results
In-depth analysis of datasets 11 and 25
In two datasets from Medlin (Citation2014), monophyletic Classes were obtained (). These Classes are the radial centrics (Coscinodiscophyceae), the bipolar centrics plus the radial Thalassiosirales (Mediophyceae) and the pennate diatoms (Bacillariophyceae). Each Class has moderate to high posterior probability support. Each Class is discussed below for each dataset and illustrated side by side in –; in Supplementary Fig. 1 the clades of the raphid taxa from dataset 11 are discussed in more detail. The raphid taxa from dataset 25 are not discussed in detail because there were many misplaced taxa presumably caused by the lower number of nucleotides as a result of the clone library sequences being used in this dataset. These misplaced taxa are randomly found in correct places in some of the individual trees in dataset 25 but the consensus tree places them incorrectly as a summary of their placements in the last 100 trees.
Figure 1. Consensus tree from the last 100 trees of the BI analysis of the diatoms from datasets 11 (A) and 25 (B) showing the outgroups used in each analysis and the monophyletic classes collapsed into triangles. Triangle 1 = Class Coscinodiscophyceae, Triangle 2 = Class Mediophyceae, Triangle 3 = Class Bacillariophyceae, Triangle 4 = Class Bolidophyceae/phyta. Posterior probabilities expressed as whole numbers from the BI analysis are shown on the relevant branch nodes. Scale bar in B represents 0.3 substitutions/site.
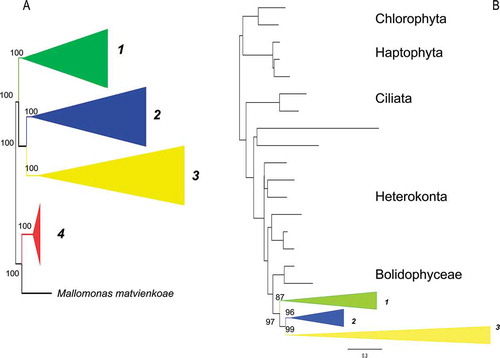
Figure 2. Consensus BI tree from the last 100 trees of the analysis of the Class Coscinodiscophyceae from datasets 11 (A) and 25 (B). Thickest lines in A represent 100% PP support for that clade, next thickest line represents >50 % support. Clades are numbered and lettered going from left to right in the tree as discussed in the text, e.g. 1, a. Scale bar in B represents 0.3 substitutions/site.
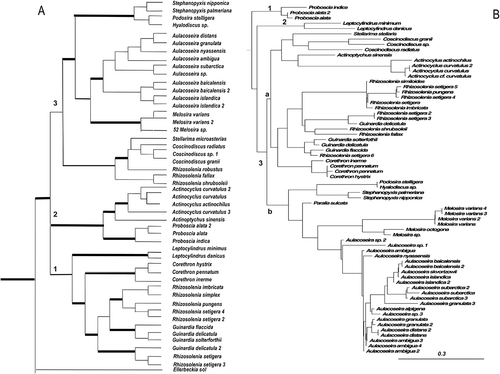
Figure 3. Consensus tree from the last 100 trees of the BI analysis of the Class Mediophyceae from datasets 11 (A) and 25 (B). Thickest lines in A represent 100% PP support for that clade, next thickest line represents >50 % support. Clades are numbered and lettered going from left to right in the tree as discussed in the text, e.g. 1> a> i. Scale bar in B represents 0.3 substitutions/site. Arrows mark two invasions of fresh waters.
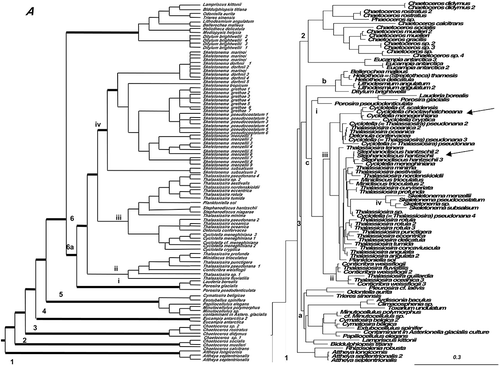
Figure 4. Consensus tree from the last 100 trees of the BI analysis of the araphid diatoms from the Class Bacillariophyceae from datasets 11 (A) and 25 (B). Thickest lines in A represent 100% PP support for that clade, next thickest line represents >50 % support. Clades are numbered and lettered going from left to right in the tree as discussed in the text, e.g. 1> a> i. Scale bar in B represents 0.3 substitutions/site.
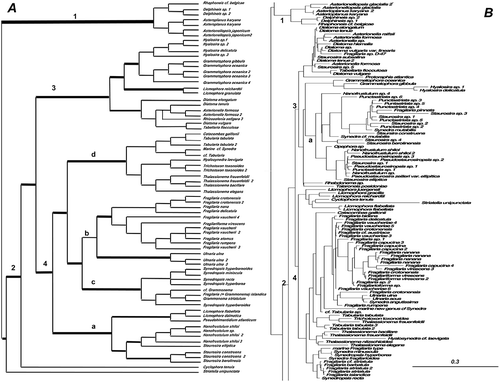
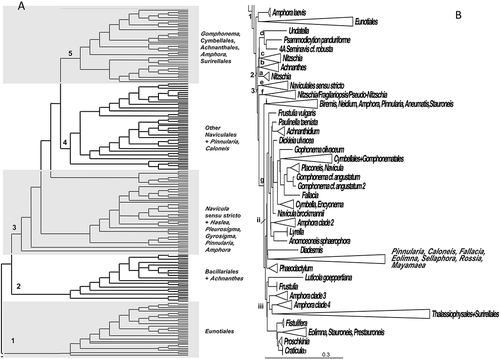
Figure 5. Consensus tree from the last 100 trees of the BI analysis of the raphid diatoms from the Class Bacillariophyceae from dataset 11 (A) and dataset 25 (B). All other centric clades removed from the tree. Every other clade is shaded in A and each clade is shown in detail in Supplementary . Thickest lines represent 100% PP support for that clade, next thickest line represents >50 % support. Clades are numbered and lettered going from left to right in the tree as discussed in the text, e.g. 1> a> i. Scale bar in B represents 0.3 substitutions/site.
Coscinodiscophyceae
For the coscinodiscophyte clade (), similar sister group relationships were recovered from the two datasets, although their branching order differed slightly. In dataset 11, Ellerbeckia sol was the first diatom to diverge, followed by a polytomy of three major clades (). Clade 1 only contained diatoms with an elongated pervalvar axis. Leptocylindrus and Corethron were recovered as monophyletic genera. Clade 2 contained two subclades: one the elongated Proboscia spp. and other radial genera of the family Coscinodiscophyceae. In clade 3, Melosira/Aulacoseira was sister to a clade with Hyalodiscus/Stephanopyxis/Podosira. Rhizosolenia and Guinardia were recovered in this clade but were not monophyletic: one subclade contained only Rhizosolenia species, a second subclade only Guinardia, and a third had a mixture of the two genera.
In dataset 25, without Ellerbeckia, there was only one coscinodiscophycean clade with a progression of divergences () that resolved the polytomy found in dataset 11 (bootstrap support ranged from 88 to 92, data not shown). The sister relationships in the subclades were identical in the two datasets with the exception that in dataset 25, Proboscia and Leptocylindrus were the first two divergences. The third clade consisted of two subclades. In the first subclade 3a, there was a divergence between the radial family Coscinodiscaceae and taxa with an elongated pervalvar axis belonging to three families. In this latter group, Guinardia and Rhizosolenia were mixed together in two clades and in a third, only Rhizosolenia species were recovered. In the second subclade 3b, the same sister relationships as in dataset 11 were obtained between Melosira/Aulacoseira and Hyalodiscus/Stephanopyxis with Podosira with Paralia sulcata interspersed between them.
In both datasets, potential cryptic species complexes were identified. Rhizosolenia setigera fell into multiple clades in , B and Aulacoseira ambigua and A. granulata were not monophyletic in . In some of the other trees with different outgroups, Proboscia fell inside the mediophycean clade (Medlin, Citation2014).
Mediophyceae
The mediophycean clade generated from both datasets (, ) was represented by a series of divergences, the first being that of Attheya, followed in dataset 11 by two clades of mixed unicellular and colonial Chaetoceros, then (4) Hemiaulales, followed by (5) Cymatosiraceae. The final divergence (6) was between the Thalassiosirales and Lithodesmiales/Eupodiscales/Triceratiales. In dataset 25, Rhizosolenia robusta was basal to the Attheya clade (1) as a misplaced taxon because it has been found in the coscinodiscophycean clade in other analyses with other outgroups (Medlin, Citation2014). In the thalassiosiralean clade, Stephanodiscus and Minidiscus both arose from within Thalassiosira; Lauderia arose from within Porosira, and Detonula arose from within Cyclotella, making, in each case, the parent genus paraphyletic.
Clade 2 in dataset 25 () contained the Chaetocerotales and Hemiaulales as sister groups, whereas in dataset 11 they were sequential divergences (clades 2–4). The subgenus Phaeoceros arose from within Chaetoceros, sister to Chaetoceros rostratus and not as an independent clade. Clade 3 contained the remaining mediophycean taxa that diverge into two large subclades. Subclade a contained the Eupodiscales and Triceratiales followed by Cymatosiraceae, sister to Toxariales. Pleurosira arose from within an Odontella/Trieres clade. A clearer picture of the polyphyletic nature of many of the genera in the Eupodiscales can be found in Ashworth et al. (Citation2013). Subclade b contained the Lithodesmiales sister to the Thalassiosirales (c).
In the thalassiosiralean clade (c) of dataset 11 (), there were four minor clades moving from left to right within subclade 6a: the first (i) contained Lauderia and Porosira with Lauderia arising from within Porosira; the second (ii) was a clade of small-celled species of Thalassiosira and Minidiscus. Some of these small-celled species of Thalassiosira have now been moved into a new genus Conticribra Stachura-Suchoples & Williams (2012). In the third minor clade (iii), there was a divergence between Cyclotella, sister to Detonula, plus a cluster of small Thalassiosira spp., one of which, Thalassiosira pseudonana, has been moved back into Cyclotella (Alverson et al., Citation2011) and a clade containing Stephanodiscus, then Planktoniella, followed by Thalassiosira. Strains identified as T. pseudonana were placed in two other clades and probably represent different species. The fourth minor clade (iv) contained Skeletonema but was embedded in Thalassiosira. As first noted in Sims et al. (Citation2006) and re-confirmed with a larger dataset in Kaczmarska et al. (Citation2006), there appear to have been two freshwater invasions in the thalassiosiralean lineages. This observation has been confirmed by Alverson et al. (Citation2007, Citation2011) and Alverson (Citation2013) using more genes.
In dataset 25 (), there were three subclades in clade b that contained Thalassiosira spp. and they were almost identical to those in dataset 11, although the taxon sampling was different. Subclade a contained small Thalassiosira spp. and could represent the genus Conticribra. The close relationship of Thalassiosira oceanica with T. weissflogii suggests that Conticribra has radiated into marine waters. Most Cyclotella spp. were in the next subclade, b, except for one sequence of Cyclotella menegheniana that was basal to the divergence of Stephanodiscus spp., sister to the final subclade. The final subclade (c) contained most of the Thalassiosira spp. Planktoniella sol and Skeletonema both arose from within Thalassiosira. One strain identified as Thalassiosira pseudonana (now in Cyclotella; Alverson et al., Citation2011) was placed in this clade. There were four sequences with the name Thalassiosira pseudonana, one being from CCMP 1335, the strain from which the genome has been sequenced that is the only one correctly termed Cyclotella pseudonana. There were two sequences of taxa identified as T. oceanica. The two freshwater clades, containing Stephanodiscus and Cyclotella, were not as distant from one another as they were in dataset 11.
Bacillariophyceae: araphids
In both datasets (, ), the araphids were recovered in two clades: the basal araphids (1) diverged first then the core araphids (2) diverged from the raphid diatoms, even though dataset 25 had more araphid taxa. The basal araphids (1) contained the Rhaphoneidales sister to Asterionellopsis and Asteroplanus spp. in both datasets. The core araphids had a basal divergence of Striatella and Cyclophora in dataset 11 followed by a divergence into two major clades (3 = taxa with septa and 4 = taxa basically without septa and taxa with labiate processes sister to those without). In dataset 25 there was no basal divergence and the tree diverged into two clades (2 = taxa with septa intermixed with taxa with no labiate processes and 3 = taxa without septa and with labiate processes). Two clades of Licmophora spp. representing the pad-forming colonial group and the stipe-forming colonial group, respectively, are in dataset 11 () more distantly separated than they are in dataset 25. Striatella unipunctata is relatively unstable in its position in various trees and has been included in the basal araphid clade (Li et al., Citation2015). In dataset 11 (), Clade 3 contained both freshwater and marine genera with septa. Clade 4 contained three subclades. The first subclade (a) contained taxa with no labiate processes (Staurosira spp.), sister to taxa with both labiate processes and septa (Licmophora pad-attached species). This was followed by two subclades, one (b) with Synedra (sequences in tree named Ulnaria ulna) and Fragilaria as sister taxa to (c) Synedropsis with Grammonema (= Fragilaria islandica and relatives) arising from within in it and another with marine genera, some of which were removed from Synedra (Williams & Round, Citation1986). The other clade (d) contained the stalk-forming Licmophora spp.
In dataset 25 (), the core araphids (clade 2) diverged into two clades (3 and 4). Clade 3 diverged into several subclades with septa, leading to a final divergence of taxa (a) without septa and without labiate processes, such as Staurosira and related genera. The paraphyletic nature of all of the new genera separated from Fragilaria by Williams & Round (Citation1986) is substantiated by this analysis. Diatoma is paraphyletic. Clade 4 contains taxa without septa and has marine taxa as its initial divergences leading to a final divergence of freshwater taxa. Synedra is sister to freshwater Fragilaria sensu stricto. In this freshwater clade, there were many taxa with multiple replicates in the tree, indicating potential cryptic species, such as Fragilaria vaucheria, F. capucina and F. nanana.
Bacillariophyceae: raphids dataset 11 (, Suppl. Fig. 1)
The raphid subclass from dataset 11 () is shown clade by clade in Supplementary Fig. 1. The raphid subclass showed major differences in the two analyses (, ). Dataset 11 had an araphid taxon as the basal divergence but in dataset 25 two raphid taxa fell at the base of the araphid clade. In dataset 11, there was a series of divergences beginning with an initial divergence of Rhabdonema, followed by Frustulia, which is also considered as a misplaced taxon because in dataset 25 it falls with other naviculoid taxa.
Dataset 11 showed a grade of clades in the raphid taxa () commencing with Eunotiales and ending with Surirellales. All of these clades have strong PP support (> 75) in the BI analysis except for the Eunotiales clade, which had only 51. This probably reflects the fact that Eunotiales are not always recovered at the base of the raphid clade but are sometimes inside it and sister to other taxa as shown in dataset 25. There were three misplaced taxa in the clade, which occur in their correct positions in other trees, thus lowering the PP values for the Eunotiales.
Each of the clades, alternately marked in grey in , is discussed below in detail and illustrated in Supplementary Fig. 1. Figure S1A shows clade 1 in detail to be comprised solely of Eunotiales with Neidium affine, Toxarium undulatum and Melosira octogona as misplaced taxa in this clade. In dataset 25, these taxa fell correctly in clades with other naviculoid, araphid and Melosira taxa, respectively. Two of the misplaced taxa were the last divergences. In the individual trees, all of the misplaced taxa were on extremely long branches and a visual inspection of the nexus file did not reveal any shifts in the alignment. Thus, their placement in the basal clade of the Eunotiales probably reflects long branch attraction. Actinella and Eunophora arose from within a basal clade of other Eunotia spp. and all of the species in this clade were from Australia/Asia. Multiple isolates of Eunotia bilunaris do not form a monophyletic group and it is clear that the strains identified as this species represent several other as yet unidentified species. A more in-depth analysis of this species from Belgium using ITS data has recovered three well-supported clades, which produced largely non-fertile F1 progeny (Vanormelingen et al., Citation2008), whereas similar isolates from Australia were considered to be a single, but separate species (Vanormelingen et al., Citation2007). The data also suggested that some forms and varieties of species deserve higher rankings, e.g. Eunotia pectinalis f. minor and E. bilunaris var. linearis.
The next divergence was of a clade (Fig. S1B) containing marine Achnanthes spp. sister to a grade of nitzschioid taxa, including Cylindrotheca, Pseudo-nitzschia and Fragilariopsis. One Amphora sequence and Diadesmis were misplaced in this clade. Fragilariopsis sublineata fell inside Pseudo-nitzschia making Fragilariopsis paraphyletic.
The next two divergences contained only two taxa each, the first Undatella and Psammodictyon and the second two strains of Amphora laevis (Fig. S1C). Navicula Section Lanceolatae and related genera diverged next. This clade began with a divergence of Gyrosigma sister to Pleurosigma, followed by Haslea, then Hippondonta. The last divergence was of Navicula lanceolata and embedded in this clade were the asymmetrical genera, Pseudogomphonema and Seminavis. The next clade, shown above the previous one, was a small mixed clade of several Pinnularia, Gomphonema, Amphora, Luticola and Brachysira spp., all probably misplaced in the consensus tree (Fig. S1C).
Figure S1D shows a clade of primarily new genera split from Navicula, sister to a clade containing Mayamaea, Pinnularia, Caloneis and one Amphora sp., which may be misplaced in this analysis but Stephanek & Kociolek (Citation2014) found some Amphora spp. of the section Oxyamphora some distance from other paraphyletic clades of Amphora. In this analysis, Caloneis spp. fell into two clades leading to a final divergence of Pinnularia. In the clade with the new genera split from Navicula, the analysis recovered a paraphyletic Sellaphora because Eolimna arose from within it, sister to a monophyletic Fallacia and Rossia. The initial divergence in this clade was of a group of Navicula species from which Stauroneis arose as a monophyletic group.
The final clade (Fig. S1E) to diverge in the raphid tree was that of the Gomphonematales, sister to the freshwater Achnanthales with Lyrella embedded in them, followed by a sequential divergence of Phaeodactylum, then two clades of Thalassiophysales and finally the Surirellales. Taxa similar to the Amphora strains used in this analysis were also found in two different clades using a three-gene analysis (Stephanek & Kociolek, Citation2014). Strains of Amphora coffeaeformis and Entomoneis alata did not group together. Entomoneis fell into two sequential clades and Stenopterobia emerged from Surirella, making that genus paraphyletic.
Bacillariophyceae: raphids dataset 25
The consensus tree from dataset 25 contained many misplaced taxa, i.e. the grouping of taxa goes against conventional wisdom in the diatoms so this dataset has had its clades reduced in to show generic groupings and is discussed by groups of genera rather than discussed in detail as in dataset 11. The PP support varied greatly and low values were associated with the misplaced taxa (data not shown). Both datasets recovered Pinnularia falling into two groups and Amphora falling into four groups.
The raphid diatoms in dataset 25 () did not resolve into straightforward groups. After an initial divergence of (1) Eunotiales plus two strains of Amphora laevis, the tree diverged into two groups, basically equivalent to (2) nitzschioid vs (3) naviculoid diatoms with one exception. Some Nitzschia and Pseudo-nitzschia with Fragilariopsis were placed in the naviculoid lineage making Bacillariales not monophyletic (Rimet et al., Citation2011), The nitzschioid group (2) contained four major clades: Nitzschia, Achnanthes, Nitzschia followed by Undatella, Psammodictyon and the naviculoid Seminavis. One Amphora sp. was misplaced in this nitzschioid clade.
In the naviculoid clades there were three major clades: (e) Navicula sensu stricto, (f) Pseudo-Nitzschia with Fragilariopsis and (g) Naviculales. In clade 3g (), the tree diverged rapidly into several smaller subclades: Group i contained some Pinnularia, some Amphora and Stauroneis sister to Neidium, Biremis and uncultured taxa. A sister relationship between Biremis and Neidium was also recovered by Witkowski et al. (Citation2014) and was supported by non-molecular data, such as cytological, raphe and auxospore features. Group ii diverges with Phaeodactylum, followed by a divergence of Pinnularia/Caloneis/Diadesmis from a large clade containing Amphora, other naviculoid genera and Gomphonematales/Cymbellales. In the gomphonematoid lineage, Frustulia vulgaris was the first divergence followed by one clade with sequential clades of freshwater monoraphid diatoms, then Dickieia, Navicula brockmanii and the final divergence of the Gomphonematales/Cymbellales and another clade containing some Amphora sister to Lyrella and Anoemoneis. In the Gomphonematales were three clades of Cymbella, three of Gomphonema, Encyonema, Placoneis and Navicula spp., Fallacia and most of the clone library sequences, which are probably distorting phylogenetic relationships in this subclade. Cymbella and Gomphonema were not monophyletic but Encyonema was. Group iii contained a basal divergence of Frustulia sister to Luticola, followed by sequential divergence of two more clades of Amphora leading to a final divergence of Thalassiophysales+Surirellales, which were sister to another clade with a basal divergence of Fistulifera, followed by a clade containing Prestauroneis, Stauroneis and Eolimna, sister to Proschkinia and Craticula. A clade with Fistulifera, Craticula and Stauroneis was also recovered by Witkowski et al. (Citation2014) in a three gene phylogenetic analysis but Fistulifera was a terminal not basal divergence in that study.
Discussion
All molecular analyses since the proposed re-classification by Medlin & Kaczmarska (Citation2004) have been controversial. Theriot et al. (Citation2009) concluded that the new system for higher level systematics of diatoms proposed by Medlin & Kaczmarska (Citation2004) was premature because their extensive re-analysis rejected their results, albeit very weakly; this has been discussed by Medlin (Citation2014). The addition of multiple genes (Theriot et al., Citation2010; Ashworth et al., Citation2012, Citation2013) has rendered the Mediophyceae monophyletic but the Coscinododiscophyceae are still a grade of clades in their analyses but not in those by Medlin. Theriot et al. (Citation2009) used a cladistic analysis of auxospore envelope features to show that ontogenetic and morphological characters associated with the post-sexual cell of diatoms (auxospore) did not support the molecular division of diatoms (their fig. 9) because the order Thalassiosirales possess auxospore characters of a class to which molecular analyses did not assign them. However, the presence of only scales in the Thalassiosirales could be a loss of the perizonial band character and reversion to an ancestral polymorphism, the possession of only scales on the auxospore (Medlin, Citation2016a). The formal cladistic analysis, presented in Medlin (Citation2014), used other valve features that pull the Thalassiosirales into the Mediophyceae in contrast to the cladistic analysis performed by Theriot et al. (Citation2009) based on auxospore features only.
The datasets analysed in Medlin (Citation2014) clearly show that increasing the number of outgroups and the identity of the outgroups have an effect on the number of clades recovered in the diatom phylogeny. Using one or multiple bolidomonads as outgroup, although they are the correct sister group for the diatoms (Guillou et al., Citation1999), resulted in numerous clades in both centric groups (see Medlin, Citation2016a table 1) for a comparison of the number of centric clades recovered by various workers/analyses). The addition of multiple genes with this single outgroup, bolidomonads, has in general recovered the mediophyte clade as monophyletic but has still left the coscinodiscophyte group with multiple clades (Theriot et al., Citation2009; Ashworth et al., Citation2012, Citation2013). The study by Li et al. (Citation2015) is the only exception to date where using a single bolidomonad and multiple genes has recovered three monophyletic classes. The high percentage of araphids in that study and that of Sato (Citation2008) and Medlin & Desdevies (Citation2016) suggests that the inclusion of a higher percentage of these taxa in the final dataset may influence the outcomes. To support the notion that multiple outgroups recover the monophyly of all three diatom clades, Sato (Citation2008) used multiple genes and multiple outgroups with BI analyses. Recent reanalysis of his datasets using a different model for amino acid substitutions has resulted in monophyletic classes with high bootstrap support in both Ml and BI analysis (Medlin & Desdevies, Citation2016).
The following combinations of bolidomonads plus Mallomonas (dataset 11) illustrated here resulted in three monophyletic classes. Adding Mallomonas and non-pigmented heterokonts, plus haptophytes, ciliates and chlorophytes outside the heterokonts (dataset 25) also recovered monophyletic classes. Besides the differences in outgroups, dataset 25 contained many clone library sequences, which when exported with the 50% base frequency filter produced a smaller dataset (1834 nts). These short clone library sequences along with the reduced number of nucleotides taken for analysis have probably distorted some of the phylogenetic relationships in this analysis. Of the clone library sequences used in dataset 25, none of them are in the centric lineages. As a result, most of the aberrant relationships recovered are in the pennate class.
In-depth analysis of datasets 11 and 25
In both datasets, the relationships among the taxa in the coscinodiscophycean and mediophycean lineages are in accord with accepted assignments at the order and family levels in these centric diatoms and were very similar between the two analyses. Attheya, which has been considered by many workers (Rampen et al., Citation2010; Theriot et al., Citation2010) to be a problematic taxon, is in a basal position in dataset 11 and at an intermediate position in dataset 25 but was never found as sister to pennates as in the analyses by Sorhannus & Fox (Citation2012). Toxarium and its relatives occupied the basal position in dataset 25.
The araphid phylogenies, in both datasets, were reasonable, continuing to separate Synedra (= Ulnaria Compère) from Fragilaria and to show that the new genera separated from Fragilaria are not monophyletic but clearly unrelated to Fragilaria. These new genera should all be placed in one genus with the derived feature of the loss of labiate processes as the defining feature. A new family has been described to accommodate this clade (Medlin & Desdevies, Citation2016). Medlin et al. (Citation2008a) and Williams (Citation2011) discussed the problem with the use of the name Ulnaria vs. Synedra for this group of freshwater taxa. Synedra is a marine genus because its type species is a marine species and is structurally different from freshwater Synedra spp.
The raphid phylogenies in the two datasets were clearly very different and this is likely caused by the inclusion of many short raphid diatom sequences from clone libraries in dataset 25, which presumably distorted the analyses. The consensus tree showed the most distortions in taxon affiliations as compared with visual inspection of various trees among the last 100 trees saved (data not shown). Bayesian inference provides a range of phylogenies based on the alignment. Depending on the situation, the spread might be narrow or wide and in this case the spread of phylogenies is quite broad, hence the distortion in the consensus tree. The analysis for dataset 11 had an araphid taxon as the basal divergence in the raphid tree and this should be investigated further to determine if this relationship is substantiated by other features.
The relationships of raphid diatoms obtained from dataset 11 are easily interpreted in terms of defined families and orders of diatoms. The most unusual feature in the raphid phylogeny is the close sister relationship of the marine Achnanthes with Bacillariales, which is a relationship recovered in most molecular analyses. The only immediately obvious shared feature that could ally the two groups is that the position of the cribrum is closer to the valve interior in the poroid areolae in both. However, perhaps the movement of the sternum to an eccentric position from a central one is a prerequisite for the raphe of the Bacillariales moving to an eccentric position. This relationship should be investigated more thoroughly. Both analyses recover two clades with canal raphes as initially shown in Medlin et al. (Citation2000) and later confirmed in Medlin & Kaczmarska (Citation2004), Sims et al. (Citation2006), Sorhannus (Citation2004, Citation2007) and Ruck (Citation2011).
Amphora occurred in multiple clades in both datasets as first shown in Medlin & Kaczmarska (Citation2004); the most recent three-gene phylogeny of this genus has also recovered multiple clades that correspond more closely to Mereschkowsky’s plastid types for the group rather than the valve morphologies upon which Cleve based his sections of the genus (Stephanek & Kociolek, Citation2014).
The Cymbellales are monophyletic but Cymbella is not. The close relationship of Anoemoneis and Dickieia to the Cymbellales as found by Bruder & Medlin (Citation2008) is also supported here with higher bootstrap support. A more in-depth analysis of this order using 5 genes can be found in Nakov et al. (Citation2014).
Taking bolidomonads as a single outgroup has never shown all three classes to be monophyletic in any type of analysis; however taking multiple outgroups from within the heterokonts has done so in several analyses (see Table 2 in Medlin Citation2016a). Ciliates, haptophytes and chlorophytes seem to be appropriate taxa for outgroups but cryptomonad, dinoflagellates, euglenoids and Giardia are not because they are too divergent from the main crown group radiation (Medlin, Citation2014). The few multiple gene analyses with a single outgroup continue to find only the Mediophyceae monophyletic (Theriot et al., Citation2010; Ashworth et al., Citation2012, Citation2013), whereas multiple outgroups recover both classes as monophyletic (Medlin & Kaczmarska, Citation2004; Sato, Citation2008; Medlin, Citation2014; Medlin & Desdevies, Citation2016). Hopefully, more such data will continue to become available in time and more outgroups inside and outside the heterokonts can be used to further investigate the monophyly of the classes with all types of analyses. With a secondary structure analysis, all of the bases in the SSU rRNA gene can be used in the analyses. Full length or nearly full-length sequences are preferable to including clone library sequences because these short sequences can distort the analyses as shown here in the analysis of dataset 25 (see effect of short sequences in the analyses in Luddington et al., Citation2012). Consequently, the field will develop as our knowledge and available instrumentation advance, additional tests are performed using new approaches, and hypotheses are updated and refined (compare trees in Medlin et al., Citation1993, Citation1996a, b, Citation2000, Citation2008a, b; Kooistra & Medlin, Citation1996; Medlin & Kaczmarska, Citation2004; Sims et al., Citation2006).
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2016.1228124
Supplementary table 1. Summary of all sequences used in this study.
Supplementary figure 1. Consensus tree from the last 100 trees of the BI analysis of the raphid diatoms from the Class Bacillariophyceae from dataset 11. A. Clade 1, B. Clade 2, C. Clade 3, D. Clade 4, E. Clade 5. Thickest lines represent 100% PP support for that clade, next thickest line represents >50 % support.
Supplementary Figure S1B
Download JPEG Image (2.2 MB)Supplementary Figure S1A
Download JPEG Image (2.5 MB)Supplementary table 1
Download MS Word (826 KB)Acknowledgements
Drs Bank Beszteri and R.M. Crawford critically read the manuscript and made useful comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Alverson, A., Jansen, R.K. & Theriot, E.C. (2007). Bridging the Rubicon: phylogenetic analysis reveals repeated colonizations of marine and fresh waters by thalassiosiroid diatoms. Molecular Phylogenetics and Evolution, 45: 193–210.
- Alverson, A.J., Beszter, B., Julius, M.L. & Theriot, E.C. (2011). The model marine diatom Thalassiosira pseudonana likely descended from a freshwater ancestor in the genus Cyclotella. BMC Evolutionary Biology, 11: 125.
- Alverson, A.J. (2013). Timing marine–freshwater transitions in the diatom order Thalassiosirales. Paleobiology, 40: 91–101.
- Ashworth, A., Ruck, E., Lobban, C., Romanovicz, R. & Theriot, E. (2012). A revision of the genus Cyclophora and description of Astrosyne gen. nov. (Bacillariophyta), two genera with the pyrenoids contained within pseudosepta. Phycologia, 51: 684–699.
- Ashworth, M.P., Nako, T. & Theriot, E.C. (2013). Revisiting Ross and Sims (1971): toward a molecular phylogeny of the Biddulphiaceae and Eupodiscaceae (Bacillariophyceae). Journal of Phycology, 49: 1207–1222.
- Bruder K. & Medlin L.K. (2008). Molecular assessment of phylogenetic relationships in selected species/genera in the naviculoid diatoms (Bacillariophyta). II. Selected genera and families. Diatom Research, 23: 331–347.
- Guillou, L., Chretiennot-Dinet, M-J., Medlin, L.K., Claustre, H., Loiseaux-de Goer, S. & Vaulot, D. (1999). Bolidomonas: a new genus with two species belonging to a new algal class, the Bolidophyceae (Heterokonta). Journal of Phycology, 35: 368–381.
- Kaczmarska, I., Beaton, M., Benoit, A.C. & Medlin, L.K. (2006). Molecular phylogeny of selected members of the Order Thalassiosirales (Bacillariophyta) and evolution of the fultoportula. Journal of Phycology, 42: 121–138.
- Kaczmarska I. & Ehrman J.M. (2015). Auxosporulation in Paralia guyana MacGillivary (Bacillariophyta) and possible new insights into the habit of the earliest diatoms. PLoS ONE 10(10): e0141150. doi: 10.1371/journal.pone.0141150.
- Kaczmarska, I., Poulíčková, A., Sato, S., Edlund, M.B., Idei, M., Watanabe, T. & Mann, D.G. (2013). Proposals for a terminology for diatom sexual reproduction, auxospores and resting stages. Diatom Research, 28: 1–32.
- Kooistra, W.H.C.F. & Medlin, L.K. (1996). Evolution of the diatoms (Bacillariophyta): IV. A reconstruction of their age from small subunit rRNA coding regions and the fossil record. Molecular Phylogenetics and Evolution, 6: 391–407.
- Li, C., Ashworth, M.P., Witkowski, A., Dąbek, P., Medlin, L.K., Kooistra, W.H.C.F., Sato, S., Zgłobicka, I., Kurzydłowski, K.J., Theriot, E.C., Sabir, J.S.M., Khiyami, M.A., Mutwakil, M.H.Z., Sabir, M.H., Alharbi, N.S., Hajara, H.N.H., Qing, S. & Jansen, R.K. (2015). New insights into Plagiogrammaceae (Bacillariophyta) based on multigene phylogenies and morphological characteristics with the description of a new genus and three new species. PLoS ONE 10(10): e0139300.
- Ludwig, W., Strunk, O., Westram, R., Richter, L., Meier, H., Yadhukumar, Buchner, A., Lai, T., Steppi, S., Jobb, G., Förster, W., Brettske, I., Gerber, S., Ginhart, A.W., Gross, O., Grumann, S., Hermann, S., Jost, R., König, A., Liss, T., Ralph Lüẞmann, May. M., Nonhoff, B., Reichel, B., Strehlow, R., Stamatakis, A., Stuckmann, N., Vilbig, A., Lenke, M., Ludwig, T., Arndt Bode, A. & Schleifer, K-H. (2004). ARB: a software environment for sequence data. Nucleic Acids Research, 32: 1363–1371.
- Luddington, I.A., Kaczmarska, I. & Lovejoy, C. (2012). Distance and character-based evaluation of the v4 region of the 18S rRNA gene for the identification of diatoms (Bacillariophyceae). PLoS ONE 7(9): e45664. doi: 10.1371/journal.pone.0045664.
- Mann, D.G. (1999). The species concept in diatoms. Phycologia, 38: 437–495.
- Mann, D.G. & Marchant, H.J. (1989). The origin of the diatom and its life cycle. In The Chromophyte Algae: Problems and Perspectives (Green, J.C., Leadbeater, B.S.C. & Diver, W.L., editors), 307–323. Clarendon Press, Oxford.
- Medlin, L.K. (2010). Pursuit of a natural classification of diatoms: an incorrect comparison of published data. European Journal of Phycology, 45: 155–166.
- Medlin, L.K. (2014). Evolution of the diatoms: VIII. Re-examination of the SSU-rRNA gene using multiple outgroups and a cladistic analysis of valve features. Journal of Biodiversity, Bioprocessing and Development, 1: 129. doi: 10.4172/2376-0214.1000129.
- Medlin, L.K. (2016a). Coalescent models explain deep diatom divergences and argue for acceptance of paraphyletic taxa and for a revised classification for araphid diatoms. Nova Hedwigia, 102: 107–123.
- Medlin, L.K. (2016b). Evolution of the diatoms: major steps in their evolution and a review of the supporting molecular and morphological evidence. Phycologia, 55: 79–103.
- Medlin, L.K. & Kaczmarska, I. (2004). Evolution of the diatoms: V. Morphological and cytological support for the major clades and a taxonomic revision. Phycologia, 43: 245–270.
- Medlin, L.K. & Desdevies, Y. (2016). Phylogeny of ‘araphid’ diatoms inferred from SSU and LSU rDNA, rbcL and psbA sequences. Vie et Milieu, 65: 129–154.
- Medlin, L.K., Williams, D.M. & Sims, P.A. (1993). The evolution of the diatoms (Bacillariophyta). I. Origin of the group and assessment of the monophyly of its major divisions. European Journal of Phycology, 28: 261–275.
- Medlin, L.K., Gersonde, R., Kooistra, W.H.C.F. & Wellbrock, U. (1996a). Evolution of the diatoms Bacillariophyta. II. Nuclear-encoded small-subunit rRNA sequence comparisons confirm a paraphyletic origin for the centric diatoms. Molecular Biology and Evolution, 13: 67–75.
- Medlin, L.K., Gersonde, R., Kooistra, W.H.C.F. & Wellbrock, U. (1996b). Evolution of the diatoms (Bacillariophyta). III. Molecular evidence for the origin of the Thalassiosirales. Desikachary Festschrift Nova Hedwigia, 112: 221–234.
- Medlin, L.K., Kooistra, W.H.C.F. & Schmid, A.M.M. (2000). A review of the evolution of the diatoms – a total approach using molecules, morphology and geology. In The Origin and Early Evolution of the Diatoms: Fossil, Molecular and Biogeographical Approaches (Witkowski, A & Sieminska, J., editors), 13–35. Szafer Institute of Botany, Polish Academy of Science, Cracow, Poland.
- Medlin, L.K., Jung, I., Bahulikar, R., Mendgen, K., Kroth, P. & Medlin, L.K. (2008a). Evolution of the diatoms. VI. Assessment of the new genera in the araphids using molecular data. Nova Hedwigia Beihefte, 133: 81–100.
- Medlin, L.K., Sato, S., Mann, D.G. & Kooistra, W.C.H.F. (2008b). Molecular evidence confirms sister relationship of Ardissonea, Climacosphenia, and Toxarium within the bipolar centric diatoms (Bacillariophyta, Mediophyceae), and cladistic analyses confirm that extremely elongated shape has arisen twice in the diatoms. Journal of Phycology, 44: 1340–1348.
- Nakov, T., Elizabeth C., Ruck, E.C., Galachyants, Y., Spaulding, S.A. & Theriot, E.C. (2014). Molecular phylogeny of the Cymbellales (Bacillariophyceae, Heterokontophyta) with a comparison of models for accommodating rate variation across sites. Phycologia, 53: 359–373.
- Piganeau, G., Eyre-Walker, A., Grimsley, N. & Moreau, H. (2012). How and why DNA barcodes underestimate the diversity of microbial eukaryotes. PLoS ONE, 7: 10. 1371/annotation/c12aac06-71d2-4749-91de-46c458e7a4eb.
- Rampen, S.W., Schouten, S., Elda-Panoto, F., Brink, M., Andersen, R.A. & Theriot, E.C. (2010). Phylogenetic position of Attheya longicornis and Attheya septentrionalis (Bacillariophyta). Journal of Phycology, 45: 444–453.
- Rimet, F., Kermarrec, L., Bouchez, A., Hoffmann, L., Ector, L. & Medlin, L.K. (2011). Molecular phylogeny of the family Bacillariaceae based on 18S rDNA sequences: focus on freshwater Nitzschia of the Lanceolatae section. Diatom Research, 26: 1–20.
- Round F.E., Crawford R.M. & Mann D.G. (1990). The Diatoms: Biology and Morphology of the Genera. Cambridge University Press, Cambridge, UK. 747 pp.
- Ruck, E. (2011). Phylogenetic systematics of the canal raphe bearing orders Surirellales and Rhopalodiales (Bacillariophyta). PhD Dissertation. University of Texas. http://repositories. lib. utexas. edu/handle/2152/ETD-UT-2010-08-1900.
- Sato, S. (2008). Phylogeny of araphid diatoms inferred from morphological and molecular data. PhD Dissertation. University of Bremen. http://elib. suub. uni-bremen. de/diss/docs/00011057).pdf
- Sims, P.A., Mann, D.G. & Medlin, L.K. (2006). Evolution of the diatoms: insights from fossil biological and molecular data. Phycologia, 45: 361–402.
- Sorhannus, U. (2004). Diatom phylogenetics inferred based on direct optimization of nuclear-encoded SSU rRNA sequences. Cladistics, 20: 487–497.
- Sorhannus, U. (2007). A nuclear-encoded small-subunit ribosomal RNA timescale for diatom evolution. Marine Micropaleontology, 65: 1–12.
- Sorhannus, U. & Fox, M. (2012). Phylogenetic analyses of a combined data set suggest that the Attheya lineage is the closest living relative of the pennate diatoms (Bacillariophyceae). Protist, 163: 252–262.
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312–1313. http://dx.doi.org/10.1093/bioinformatics/btu033.
- Stephanek, J.G. & Kociolek, J.P. (2014). Molecular phylogeny of Amphora sensu lato (Bacillariophyta): an investigation into the monophyly and classification of the amphoroid diatoms. Protist, 165: 177–195.
- Theriot, E., Alverson, A. & Gutell, R. (2009). The limits of nuclear-encoded SSU rDNA for resolving the diatom phylogeny. European Journal of Phycology, 44: 277–290.
- Theriot, E.C., Ashworth, M., Ruck, E., Nakov, T. & Jansen, R.K. (2010). A preliminary multigene phylogeny of the diatoms. Plant Ecology and Evolution, 143: 278–296.
- Vanormelingen, P., Chepurnov, V.A., Mann, D.G., Cousin, S. & Vyverman, W. (2007). Congruence of morphological reproductive and ITS rDNA sequence data in some Australasian Eunotia bilunaris (Bacillariophyta). European Journal of Phycology, 42: 61–79.
- Vanormelingen, P., Chepurnov, V.A., Mann, D.G., Sabbe, K. & Vyverman, W. (2008). Genetic divergence and reproductive barriers among morphologically heterogeneous sympatric clones of Eunotia bilunaris sensu lato (Bacillariophyta). Protist, 159: 73–90.
- Williams, D.M. (2007). Classification and diatom systematics: the past, the present and the future. In Unravelling the Algae: Past, Present and Future of Algal Systematics (Lewis, J. & Brodie, J., editors), 57–92. CRC Press, London.
- Williams, D.M. (2011). Synedra/Ulnaria: definitions and descriptions – a partial resolution. Diatom Research, 26: 149–153.
- Williams, D.M. & Round, F.E. (1986). Revision of the genus Synedra. Diatom Research, 1: 319–339.
- Witkowski, A., Barka, F., Mann, D.G., Li, C., Weisenborn, J.L.F., Ashworth, M.P., Kurzylowski, K.J. Zglobicka, I. & Dobosz, S. (2014). A description of Biremis panamae sp. nov., a new diatom species from the marine littoral, with an account of the phylogenetic position of Biremis D.G. Mann et E.J. Cox (Bacillariophyceae). PLoS ONE 9(12): e114508. doi: 10.1371/journal.pone.0114508.
