ABSTRACT
Batrachospermum turfosum Bory is one of the generalists among the few red algae that have adapted to freshwater habitats, occurring in a variety of primarily shaded, nutrient-poor micro-habitats with lotic (running) or lentic (standing) waters. Seasonal variations in water level and canopy cover can expose this sessile alga to widely fluctuating temperatures, solar irradiation and nutrient availability. Here we report on the ecophysiology of B. turfosum collected from an ultra-oligotrophic bog pool in the Austrian Alps. Photosynthesis as a function of photon fluence density (PFD) and temperature was studied by measuring oxygen evolution in combination with chlorophyll fluorescence. In addition, the effects of ultraviolet radiation (UVR) on photosynthetic pigments were analysed using HPLC and spectrophotometric methods, and cellular ultrastructure was studied using transmission electron microscopy. We found that B. turfosum is adapted to low light, with a light compensation point (Ic) and a light saturation point (Ik) of 8.4 and 29.7 µmol photons m–2 s–1, respectively, but also tolerates higher PFDs of ~1000 µmol photons m–2 s–1, and is capable of net photosynthesis at temperatures between 5°C and 35°C. Exposure to either UV-A or UV-AB for 102 h led to a strong transient drop in effective quantum yield (ΔF/FM’), followed by an acclimation to about 70% of initial ΔF/FM’ values. Ultrastructural changes included the accumulation of plastoglobules and dilated membranes after UVR treatment. Although all photosynthetic pigments strongly decreased upon UVR exposure and no UV-photoprotectants (e.g. mycosporine-like amino acids) could be detected, the alga was capable of recovering ΔF/FM’ and phycobiliproteins after UVR treatment. In summary, B. turfosum tolerates a wide range of irradiation and temperature regimes, and these traits may be the basis for its successful adaptation to challenging environments.
Introduction
The majority of red algae (Rhodophyta) are marine organisms, and only about 3% (~180 species) occur in freshwater habitats (Guiry, Citation2012). Most of these freshwater red algae are found in light- and nutrient-poor running waters and accordingly, their ecophysiological adaptations differ from those of most marine red algae. However, freshwater Rhodophyta are important constituents in river ecosystems, where they can be abundant and widely distributed (Necchi, Citation2016). Three freshwater red algae, Compsopogon caeruleus, Kumanoa mahlacensis and Batrachospermum turfosum (Sheath & Vis, Citation2015), are typical inhabitants of lentic freshwater bodies, such as lakes and ponds. There are also other species with a widespread distribution, e.g. Bostrychia moritziana and Chroodactylon ornatum (Sheath & Vis, Citation2015), but these are marine invaders inhabiting estuaries and brackish waters, i.e. are not strictly limited to fresh water.
The broad latitudinal and altitudinal distribution of B. turfosum is unusual for freshwater red algae, which are usually restricted to elevations below 900m above sea level (Israelson, Citation1942; Sheath & Vis, Citation2015). In North America B. turfosum occurs from Alaska to central Mexico (Sheath et al., Citation1994; Müller et al., Citation1997) and in Europe is abundant in Finland and Sweden, but rare in central Europe (Eloranta & Kwandrans, Citation2007). Batrachospermum turfosum occurs in mountainous to subalpine microhabitats characterized by dark-coloured, oligotrophic (i.e. nutrient-poor) humic waters (Parker et al., Citation1973; Kumano, Citation2002), often attached to solid substrata like stones or wooden branches. In such conditions algae can form colourless hair cells hosting phosphatase enzymes that mediate the uptake of phosphate from organic P-compounds (Gibson & Whitton, Citation1987), inhibited in the presence of Fe(III). These microhabitats can exhibit significant seasonal fluctuations in temperature and water levels, and light penetration through canopy gaps (Canham et al., Citation1990). In shallow-water ponds inhabited by B. turfosum temperatures can fluctuate from 34°C in the summer (Gregor et al., Citation2003) to around freezing point in the winter (Müller et al., Citation1997), and elevated photosynthetically active radiation (PAR) and ultraviolet radiation (UVR) are additional stress factors at high altitudes (Blumthaler et al., Citation1997).
Numerous studies on photosynthesis of freshwater red algae showed that they are typically adapted to shaded environments, with a low light compensation point (Ic) and light saturation point (Ik) in combination with high photosynthetic efficiency (α) in the light-limiting range (Karsten et al., Citation1993; Leukart & Hanelt, Citation1995), and a capability of down-regulating photosynthesis by dynamic photoinhibition (Necchi, Citation2005; Drerup et al., Citation2015). Parker et al. (Citation1973) described bleaching and disintegration of the thalli after transplantation, and suggested that B. turfosum (formerly B. vagum) is sensitive to bright light, although related species (e.g. B. gelatinosum) can tolerate a wide range of irradiance (Rider & Wagner, Citation1972). More recent studies on photoacclimation in freshwater red algae showed that they can adjust components of their photosynthetic apparatus and can decrease chlorophyll a contents after high light exposure (Bautista & Necchi, Citation2007). Ultraviolet radiation can cause damage to nucleic acids, proteins and pigments (Karsten, Citation2008 and references therein) and can directly damage photosystem II (PSII) by photoinactivation of the oxygen-evolving complex or the D1 protein (Tyystjarvi, Citation2008; Vass, Citation2012). In contrast, photosystem I seems to be less affected by UVR absorption, but can suffer oxidative damage through reactive oxygen species (ROS) when photoprotective mechanisms fail (Vass, Citation2012).
The Rhodophyta are highly diverse in their photoprotective mechanisms, and their capabilities for effective high energy quenching (qE) of overexcited photosystems are somewhat enigmatic (Bruce & Vasil’ev, Citation2004), because typical key qE components are missing (PsbS proteins) or uncommon (xanthophyll cycle). Carotenoid profiles of Rhodophyta have been classified into three groups, with predominantly zeaxanthin, lutein or xanthophyll cycle-related pigments (Schubert et al., Citation2006). Batrachospermum sp. belongs to the second group (Schagerl & Donabaum, Citation2009). Photoprotective responses to enhanced UVR have been extensively investigated in marine red macroalgae (Bischof et al., Citation2006), identifying an array of UV-absorbing compounds such as mycosporine-like amino acids (MAAs; Karsten & West, Citation2000; Hoyer et al., Citation2001), phenolic compounds (Blunt et al., Citation2015) and carotenoids (Schubert et al., Citation2006), but there are few data for freshwater red algae. The phycobilisomes (PBS) in the antenna of red algae contain phycobiliproteins (PBPs), which efficiently absorb PAR and transfer excitons to chlorophyll a in PSII. Kaczmarczyk & Sheath (Citation1991) showed that antenna pigments in Sheathia boryana (formerly B. boryanum) were altered in response to light quality, although without changes in photosynthetic rates. Ultraviolet radiation triggers the dissociation of PBS from the thylakoid membrane (Lao & Glazer, Citation1996) and further disassembly and breakage of the anchor linker (ApcE; Six et al., Citation2007), leading to a partially uncoupled energy transfer (Sah et al., Citation1998).
In summary, hardly any data exist on the photoprotective mechanisms against UVR damage in freshwater red algae, and even less is known about the ecophysiology of the generalist B. turfosum. Here, we provide baseline data for light and temperature requirements of photosynthesis and respiration, assessed by oxygen evolution together with measurements of the optimum quantum yield (FV/FM), and for photoprotection against UVR by studying the kinetics of quantum efficiency, pigment composition and changes in ultrastructure in response to either UV-A or UV-AB exposure.
Materials and methods
Algal material and environmental parameters
Thalli of Batrachospermum turfosum Bory attached to wooden branches were collected from a humic bog pool (Supplementary Fig. S1) in Austria and identified after Knappe & Huth (Citation2014). The algal material was transported in light-protected boxes to the lab, cleaned of peat particles with MilliQ water (Barnstead, Thermo Fisher Scientific, Waltham, USA), and acclimated at 10–15 µmol photons m–2 s–1 at 6–10°C for 4–8 days before experimental treatments. Parameters of solar radiation were recorded in the shade and in the light on a sunny, cloudless day on 18 July 2014 (13:00, CET + 1) with a PMA 2100 radiometer (Solar Light, Glenside, USA) equipped with PMA 2132, PMA 2110 and PMA 2106 sensors for PAR, UV-A and UV-B, respectively. Bog water was sampled for nutrient analysis (Spectroquant test kits, Merck, Darmstadt, Germany), electrical conductivity and pH (WTW, Cond 330i, Weilheim, Germany). Phenol contents were calculated as gallic acid equivalents (GAE) and measured according to Everette et al. (Citation2010).
Light and temperature requirements for photosynthesis and respiration
Respiratory oxygen consumption in the dark and photosynthetic oxygen production rates under increasing photon fluence density (PFD; PI curve) were measured with a Fibox 3 oxygen optode (Presens, Regensburg, Germany) at 20°C using a 3 ml thermostatic acrylic chamber (DW1, Hansatech, Norfolk, UK) after Remias et al. (Citation2010). Algal filaments were suspended in 2.5% Bold’s Basal Medium (BBM), enriched with 0.2 ml NaHCO3 solution (75 mM; Merck, Darmstadt, Germany) to produce a 3 ml suspension with a final inorganic carbon concentration of about 2 mM. PFDs from 0 to 1050 µmol photons m–2 s–1 PAR emitted from a halogen light source were calibrated inside the chamber using a radiometer (QRT1 sensor, Hansatech, Norfolk, UK) and were controlled with combinations of neutral density filters (10%, 25%, 50%, 80%; Nr. 980214, Hansatech, Norfolk, UK).
Photosynthetic oxygen evolution rates at temperatures from 5 to 50°C (in increments of 5°C) were measured with the above-mentioned equipment, by irradiating the algal material with 100 µmol photons m–2 s–1 PAR using a temperature-controlled water bath (K20/DC 10, Thermo Haake, Karlsruhe, Germany). Oxygen evolution rates were measured for 10 min each in the light, and in the dark after a 20 min pre-acclimation phase for each temperature. FV/FM was recorded using a pulse-amplitude modulated (PAM) fluorometer (PAM 2500, Heinz Walz, Effeltrich, Germany) and a KS-2500 cuvette (Heinz Walz). FV/FM measurements were conducted after Schubert et al. (Citation2011) with minor modifications, in the same temperature range as oxygen evolution rates using red LED as actinic light (Emmax = 630 nm) and far red LED (Emmax = 750 nm) to ensure full oxidation of the electron-transport chain (state 1). Thalli were dark-acclimated for 30 min, followed by a 5-s far-red pulse and another dark incubation for 5 min, and then FV/FM was measured with saturating pulses (SP) of ~1000 µmol photons m–2 s–1. Oxygen evolution for each PFD increment and time interval was normalized to the concentration of total chlorophyll a and dry weight (DW). For this, algal material was filtered after each measurement onto GF/C glass fibre filters (Whatman, Dassel, Germany), immediately frozen in liquid nitrogen, freeze-dried (Lyovac GT2, Leybold, Hanau, Germany), weighed and analysed by high performance liquid chromatography (HPLC; see chromatographic analysis).
Light, confocal laser scanning and transmission electron microscopy
Light and confocal laser scanning microscopy (CLSM) were conducted with a Zeiss Axiovert 200 M microscope (Carl Zeiss AG, Jena, Germany), either in bright field mode or by differential interference contrast (DIC). Images were captured by a Zeiss Axiocam MRc5 camera operated by Zeiss Axiovision (release V 4.7.1.0) software. Batrachospermum turfosum cells were excited by an argon laser beam (488 nm) and emission was collected with a long pass filter (LP 560 nm; false coloured red). Z-stack projections were generated, with optimized optical slice distances, covering approximately half of the width of an individual whorl cell. For transmission electron microscopy (TEM) thalli were chemically fixed as previously described by Holzinger et al. (Citation2011) with minor modifications. Briefly, samples were fixed in 1.25% glutaraldehyde in 25 mM sodium cacodylate buffer (pH 6.8) for 1 h, post-fixed with 1% OsO4 at 4°C for 24 h, rinsed and dehydrated in increasing ethanol concentrations and propylene oxide, and embedded using Spurr’s low viscosity embedding kit (Science Services, Munich, Germany). Ultrathin sections were prepared with a Reichert Ultra-microtome (Leica, Wetzlar, Germany), counterstained with 2% uranyl acetate and Reynold’s lead citrate, and investigated at a Zeiss LIBRA 120 transmission electron microscope (Carl Zeiss AG, Oberkochen, Germany) at 80 kV. Images were taken with a Proscan 2 k SSCCD camera (Proscan Electronic Systems, Lagerlechfeld, Germany) and processed with Adobe Photoshop 7.0 software (Adobe Systems Inc., San Jose, CA, USA).
Irradiation and quantum efficiency of PSII
For monitoring effective quantum yield ΔF/FM’ before and after UV-exposure, thalli were wetted in 2.5% BBM (pH 5.5) and placed on GF/C glass fibre filters (13 mm; Pall Corporation, Dreieich, Germany), followed by mild vacuum filtration (> 99 mPa) for 2 s and rehydration with one drop of BBM. ΔF/FM’ was measured using a red LED as actinic light source (Emmax = 630 nm; SP ~1000 µmol photons m–2 s–1) at 20–25 µmol photons m–2 s–1 for 5–10 min at ambient temperature (~22°C). Subsequently, the thalli were transferred to sterile multi-well plates (Greiner Bio-One, Frickenhausen, Germany) filled with 4 ml of 2.5% BBM (pH 5.5) and irradiated at 16°C in a temperature-controlled growth cabinet (CLF-Percival, Wertingen, Germany). Algae were exposed for 3, 6, 30, 54, 78 and 102 h at a 18/6 light/dark cycle using a combination of white fluorescent tubes (Alto 17 W, Philips, Amsterdam, the Netherlands), UV-A fluorescent tubes (Actinic BL TL-D 18 W/10, Philips, Amsterdam, the Netherlands) and UV-B incandescent bulbs (ReptiGlo 25 W/10, Hagen GmbH, Holm, Germany) to provide PAR (400–700 nm), UV-A (320–400 nm) and UV-B (280–320 nm) irradiation, respectively. To obtain the desired irradiation regimes, multi-well plates were covered with transparent cut-off filter foils for 320 nm (type 6088329, Corporate Express, Stuttgart, Germany) and 380 nm (type 2041, Bruxsafol, Hammelburg, Germany); the latter foil is transparent for a low amount of UV-A (< 5%) between 380 and 400 nm and the coverlid of the multi-well plates filtered irradiation lower than 290 nm.
Thalli were exposed either to PAR (P), or PAR with UV-A (PA) or PAR with UV-A and UV-B (PAB). PAR was 20 µmol photons m–2 s–1 for all irradiations, the UV-A was 4.10 W m–2 for PA and 5.03 W m–2 for PAB, and the UV-B was 0.026 W m–2 for PA and 0.132 W m–2 for PAB. To ensure uniform irradiation of all samples, multi-well plates were placed on rotating discs (four rotations per min). After UVR exposure for 102 h thalli were allowed to recover for 24 h at 16°C and 20 µmol photons m–2 s–1 PAR (L36W/77, Osram Fluora, Munich, Germany) without UVR in a diurnal cycle (18L/6D). Thalli were transferred to GF/C glass fibre filters (13 mm; Pall Corp., Dreieich, Germany), ΔF/FM’ was determined as described above, and thalli were frozen in liquid nitrogen and freeze-dried.
Pigment analysis
Freeze-dried thalli were ground with glass beads using a laboratory mill (Tissuelyser II, Qiagen, Venlo, the Netherlands) at 30 Hz for 10 min, and extracted as described by Aigner et al. (Citation2013) with minor modifications. The powder was suspended in 4 ml methyl-tert-butylether (MTBE, SigmaAldrich, St. Louis, USA) containing 0.1% butylated hydroxytoluene (BHT, SigmaAldrich, St. Louis, USA) to prevent oxidation of pigments. The extract was sonicated for 15 min at 0°C, and then 2 ml of 20% methanol (v/v; Roth, Karlsruhe, Germany) was added, the mixture vortexed and frozen overnight at −20°C for quantitative extraction. This extract was then centrifuged (1000 g, 5 min) at 4°C to support phase separation of the lipophilic supernatant (MTBE-phase) and the hydrophilic lower (methanol) phase. The upper and the lower phases were separated, evaporated to dryness in a SpeedVac (SPD111V, Thermo Fisher Scientific, Waltham, USA) with a refrigerated vapour-trap (RVT5105, Thermo Fisher Scientific, Waltham, USA), and then re-suspended in, respectively, 500 µl N,N-dimethylformamide (DMF, Scharlau, Sentmenat, Spain) and 500 µl methanol (HPLC grade, Roth, Karlsruhe, Germany). The extracts were centrifuged (15 000 g, 45 min, 4°C) prior to injection into the HPLC. Primary pigments in the lipophilic phase and secondary pigments in the hydrophilic phase were separated using an Agilent 1100 Series system (Waldbronn, Germany) equipped with a vacuum degasser, a binary pump, a cooled autosampler (4°C), a diode array detector (DAD) and a fluorescence detector (FLD).
Photosynthetic primary pigments (chlorophylls, carotenoids and phycobiliproteins)
Primary pigments were analysed according to Remias et al. (Citation2005) with minor modifications. Primary pigments were separated on a LiChroCART (C18, 100 × 4.6 mm, 5 µm, 120 A) column (Agilent, Waldbronn, Germany) at 30°C at a flow rate of 1 ml min–1 using solvent A (acetonitril:methanol = 74:6) and solvent B (methanol:hexane = 5:1). The system was started at 0% solvent B for 4 min, followed by a gradient to 100% solvent B from 4 to 9 min, which was maintained for 9 min, followed by a 5 min post-run with 100% solvent A. Whole absorbance spectra were recorded each second and primary pigments were identified by retention time and absorption spectra at 440 nm with a DAD, and quantified using a calibration curve of external standards. Chlorophyll a was obtained from SigmaAldrich, St. Louis, USA; antheraxanthin and violaxanthin from DHI C14, Centralen, Denmark; zeaxanthin and lutein from Carl Roth, Karlsruhe, Germany; ß-carotene from Calbiochem, Darmstadt, Germany. Neoxanthin and chlorophyll b from spinach extracts were gathered with a fraction collector (1200 Series, Agilent, Waldbronn, Germany) and concentrations calculated using the specific absorption coefficients.
The phycobiliproteins R-phycoerythrin (PE), R-phycocyanin (PC) and R-allophycocyanin (APC) were extracted according to Cornish et al. (Citation2013) with minor modifications. Briefly, ground material was suspended at 4°C in 500 µl extraction solution consisting of 74.5 ml Sørensen’s phosphate buffer (50 mM, pH 7), 0.5 ml Triton X-100, 10.5 µl β-mercaptoethanol and 10 protease inhibitor tablets (cOmplete, Roche Diagnostics GmbH, Mannheim, Germany). The extracts were vortexed, sonicated for 15 min at 0°C and subjected to two freeze-thaw cycles from −25°C to 4°C. The samples were centrifuged (17 000 g, 20 min) at 4°C and supernatants collected for spectrophotometric analysis. Concentrations were calculated using the equations of Beer & Eshel (Citation1985) for PC and PE and the equation of Kursar et al. (Citation1983) for APC.
Secondary photoprotective compounds (phenolic compounds and MAAs)
To test for the presence of putative photoprotective compounds, secondary hydrophilic phenolic compounds were analysed on a Synergi hydro column (RP18, 150 × 2.0 mm, 4 µm, 100 A; Phenomenex, Aschaffenburg, Germany) with a RP-18 guard cartridge (20 × 4 mm) at 25°C at a flow rate of 0.3 ml min–1 and an injection volume of 25 µl using solvent A (water with 0.5% formic acid) and solvent B (methanol with 0.5% formic acid) starting at 0% solvent B, with a gradient to 100% solvent B within 40 min, followed by a 8 min post run with 100% solvent A. Whole absorbance spectra were recorded each second and DAD detection wavelengths were set to 280 nm and 350 nm, respectively, after Aigner et al. (Citation2013).
The presence of MAAs was tested using a Supelcosil LC-NH2 column (RP18, 150 × 4.6 mm, 3 µm; Supelco, St. Louis, USA), protected with a RP18 guard cartridge (20 × 4.6 mm) at 30°C at a flow rate of 1 ml min–1 using solvent A (0.1% ammonium formiate, pH 3.14) and solvent B (methanol), starting with 75% solvent B, followed by a gradient to 30% solvent B within 5 min, then to 0% solvent B from 7 to 8 min, to 30% solvent B at 10 min, and then 75% solvent B at 15 min, followed by a 5 min post run with 100% solvent A. Whole absorbance spectra were recorded each second at wavelengths between 310 nm and 330 nm. MAA standards isolated from marine red algae (asterina-330, mycosporine-glycine, palythine, porphyra-334, and shinorine) were made available from former studies (Karsten & West, Citation2000).
Statistical analysis
Data for oxygen evolution (n = 4), FV/FM (n = 4), nutrient contents in the bog water (n = 5), primary and accessory pigments (n = 5), and ΔF/FM’-measurements (n = 10) were tested for significance using a one-way analysis of variance (ANOVA), with a p-value ≤ 0.05, p ≤ 0.01, and p ≤ 0.001, or two-way ANOVA (p ≤ 0.01), as appropriate. Data for oxygen evolution and FV/FM were further tested by Fisher’s LSD post-hoc test, and all other data with the Bonferroni post-hoc test. All analyses were carried out using SPSS 18.0 (IBM Corp., Somer, NY, USA), Origin Pro 9.1 (Originlab, Northampton, USA) or the software package R (version 3.3.1).
Results
Algal material and environmental parameters
The collection site of B. turfosum is characterized by diurnally fluctuating PFDs, which differed about 14-fold, 5-fold and 3.5-fold for PAR, UV-A and UV-B, respectively (Supplementary Table S1) when comparing shaded conditions under the tree canopy with direct insolation. Irradiation, measured on a sunny, cloudless summer day, reached values of 1700 ± 35 µmol photons m–2 s–1 PAR, 39 ± 1.3 W m–2 UV-A and 2.1 ± 0.04 W m–2 UV-B, typical for this altitude and season, and the habitat received between 700–900 KWh m–2 a–1 radiant energy over the course of the year (Supplementary Fig. S2). Solar irradiation at one hour of full exposure at noon in the sun varied by less than 5%, for PAR, UV-A and UV-B whereas in the shade PAR varied by almost 50%, UV-A by up to 20% and UV-B by about 12%. However, only a small proportion of radiation was able to penetrate through the brown-coloured water (Supplementary Fig. S3; Kd UV-B ~0.01). On the various sampling dates the water had very low conductivity (22.3–24.5 µS cm–1) and was acidic-soft (pH values were 5.7 to 4.6 and water hardness was between 1 and 1.5 dH). The nutrient concentrations were very low for ammonia (0.02–0.04 mg l–1), nitrate (0.05–0.08 mg l–1) and below detection limit for phosphate (< 0.01 mg l–1), whereas the iron content was very high on all three sampling dates (1.2–2.7 mg l–1), due to the iron-rich sandstone in the first geological formation at the collection site. In the bog pool water phenolic compounds were characterized as polyphenols and the concentrations varied between spring and summer (1.1–1.9 mg l–1). Both iron and phenolic compounds are responsible for the brown colouration of the bog water, which was most pronounced in autumn. Calculations of the proportion of humic acids showed that these compounds are responsible for 20–55% of the brown colouration (Supplementary Table S2), whereas iron most probably accounted for the remaining absorbance.
Light and confocal laser scanning microscopy (CLSM)
Chloroplasts in whorl cells were ribbon-shaped in a parietal arrangement and formed a spiral, which was corroborated by CLSM images (Z-stacks). The chlorophyll autofluorescence allowed us to illustrate the parietal arrangement in median optical CLSM sections (Supplementary Fig. S1).
Photosynthetic performance under varying light and temperature regimes
Light requirements for photosynthesis
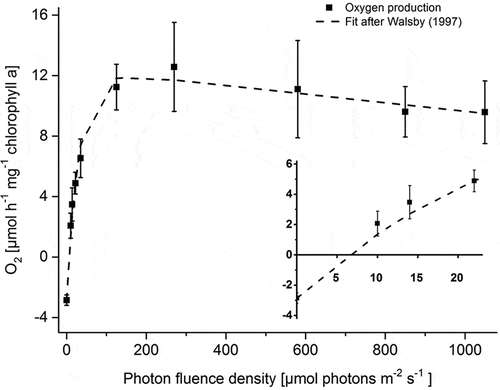
Increasing PFDs stimulated photosynthetic oxygen production at 20°C in B. turfosum up to 275 µmol photons m–2 s–1, followed by a gentle decline at higher PFDs (). Based on a fit according to Walsby (Citation1997), values for Ic and Ik were 8.4 µmol photons m–2 s–1 and 29.7 µmol photons m–2 s–1, respectively, and in the light-limited range α was 0.4978, clearly indicating very low light requirements for photosynthesis (inset of ). In the light-saturated range, the maximal net photosynthesis (Pmax) was 11.9 ± 0.29 µmol O2 h–1 mg–1 chlorophyll a and the degree of photoinhibition (β) was determined to be −0.002 µmol O2 h–1 mg–1 chlorophyll a, although the alga showed relatively high photosynthetic activity up to 1050 µmol photons m–2 s–1 (for ease of comparison with other studies DW-based calculations are shown in Supplementary Fig. S4A).
Fig. 1. Effects of photon fluence rates on oxygen evolution of Batrachospermum turfosum. The inset shows the PI curve in low light. Data were fitted according to the model of Walsby (Citation1997). All measurements were conducted at 20°C (n = 4, means ± SD).
Photosynthetic activity under increasing temperatures
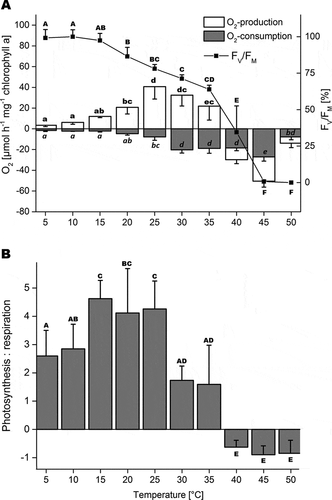
The effect of increasing temperature (from 5°C to 50°C) on photosynthetic oxygen production (at 100 µmol photons m–2 s–1) and respiration (in the dark) together with measurements of FV/FM in B. turfosum revealed strong differences in sensitivity of the two physiological processes (). Net oxygen production was observed between 5°C and 35°C, whereas at 25°C the highest oxygen production of 40.7 ± 11.94 µmol O2 h–1 mg–1 chlorophyll a was found, which was significantly different from all other temperatures except 30°C (p < 0.05). Oxygen production was not detectable above 35°C, and negative net production of oxygen was observed at temperatures above 40°C. Dark respiration (oxygen consumption) was low up to 15°C (–2.1 ± 0.9 µmol O2 h–1 mg–1 chlorophyll a), increased about 2-fold at 20°C, about 3-fold at 25°C, and about 10-fold above 30°C (), where it was greatest (−20.2 ± 3.1 µmol O2 h–1 mg–1 chlorophyll a). Chlorophyll a concentration did not change significantly during the temperature treatment (Supplementary Table S3) and DW-based calculations for oxygen evolution are shown in Supplementary Fig. S4B. The highest FV/FM values were observed at 10°C (0.584 ± 0.028), which significantly declined between 10°C and 20°C (p < 0.05). FV/FM further decreased, dropped to ~30% at 40°C (0.202 ± 0.105) and reached 0 at 45°C. The highest ratio between photosynthesis and respiration was 4 to 4.5 between 15°C and 25°C. Ratios of 2.5 to 3, and 1.5 to 2, respectively, were found between 5°C and 10°C, and between 30°C and 35°C ().
Fig. 2. Temperature dependence of photosynthesis in Batrachospermum turfosum. A. Oxygen production at 100 µmol photons m–2 s–1, dark-respiration and optimum quantum yield (FV/FM). Negative values are shown as stacked bars and significant differences between means are marked with different letters. B. Ratio between photosynthesis and respiration as a function of temperature. Significant differences between mean values were calculated by one-way ANOVA followed by the Fisher’s LSD post hoc test (p ≤ 0.05; n = 4, means ± SD).
Response to ultraviolet radiation
Kinetics of effective quantum yield
The decrease in ΔF/FM’, expressed as a percentage of initial ΔF/FM’ of untreated thalli (P: 0.48 ± 0.002; PA: 0.48 ± 0.005; PAB: 0.48 ± 0.006) was compared with ΔF/FM’ values of thalli irradiated with P, PA und PAB. All three irradiation treatments differed significantly from each other (p < 0.01; ). Within each treatment, algae exhibited a decrease in ΔF/FM’ between 0 h and 6 h, and those treated with PAB showed a significantly sharper transient drop in ΔF/FM’ compared with PA at 3 h (p < 0.01). Under PA and PAB ΔF/FM’ values significantly increased between 6 h and 54 h of treatment (p < 0.01), then plateaued at around 70% of their initial values (PA: 73.2 ± 10.3%, PAB: 75.3 ± 5.5%) until the end of UVR exposure at 102 h when P-irradiated algae reached 95.8 ± 3.8% of initial values ().
Fig. 3. Changes in effective quantum yield (ΔF/FM’) in Batrachospermum turfosum in response to irradiation. P = PAR; PA = UV-A; PAB = UV-AB. Data points (n = 10, means ± SD) labelled with the same letter do not differ significantly from each other (two-way ANOVA and Bonferroni’s post-hoc test; p ≤ 0.01). Light (18 h) and dark (6 h) phases are indicated by grey and black shading, respectively, for the irradiation treatments, and by white and black shading for the recovery phase.
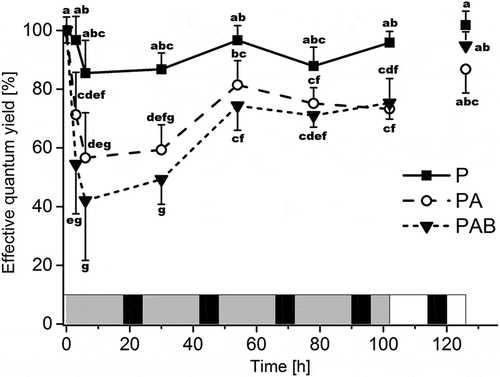
None of the treatments damaged or chronically affected the alga. After a recovery period of 24 h (without UVR) ΔF/FM’ of thalli from all treatments increased almost to the initial values (P: 101.8 ± 4.7%; PAB: 94.8 ± 4.7%; PA: 86.8 ± 8.0%).
Photosynthetic primary pigments
Untreated thalli contained chlorophyll a (8.33 ± 1.86 nmol mg–1 DW), lutein (3.04 ± 0.91 nmol mg–1 DW), zeaxanthin (0.27 ± 0.07 nmol mg–1 DW; ) and trace amounts of α- and β-carotene (Supplementary Table S3). After 102 h chlorophyll a levels dropped by about two-thirds, which was significant for both PA and PAB (p < 0.001), compared with P (p < 0.01). Upon irradiation, lutein contents were halved compared with 0 h (p < 0.05), whereas zeaxanthin contents remained unchanged. During the 24 h recovery period (without UVR) chlorophyll a levels partially recovered after the P and PAB treatments, but not after PA (P: p < 0.05, PA: p < 0.01). Lutein levels fully recovered after all treatments, and under PAB the zeaxanthin content slightly increased compared with 0 h (p < 0.05; ).
Fig. 4. Primary and accessory pigments in Batrachospermum turfosum upon irradiation and recovery. A. Primary pigments B. Phycobiliproteins. P = PAR; PA = UV-A; PAB = UV-AB. Thalli were exposed to irradiation treatments (P, PA or PAB) for 102 h, followed by 24 h of recovery in the absence of UVR (n = 5, means ± SD). Significances between control (0 h) and irradiated samples were tested by one-way ANOVA followed by Bonferroni’s post-hoc test. Statistical differences between means are indicated by 1, 2 or 3 asterisks for p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively. PC = phycocyanin; APC = allophycocyanin.
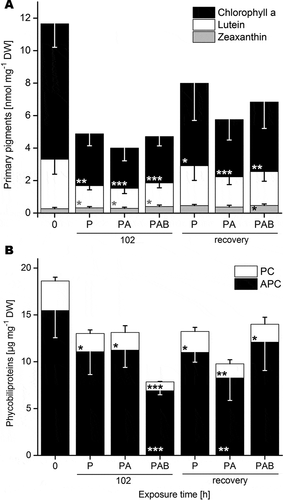
Phycobiliproteins were represented by APC and PC, whereas PE was not detectable with the spectrophotometric method used (). All irradiation treatments led to a decrease in PC (p < 0.05), was most pronounced for PAB which declined by two-thirds (p < 0.001). In PAB-exposed algae APC was halved (p < 0.001). During the 24 h recovery phase, PC and APC remained unchanged in P-treated thalli and decreased further in PA-treated ones, but significantly increased after PAB treatment (). The presence of putative photoprotective secondary metabolites such as phenolic compounds and/or MAAs was tested for, but none were detected.
Transmission electron microscopy
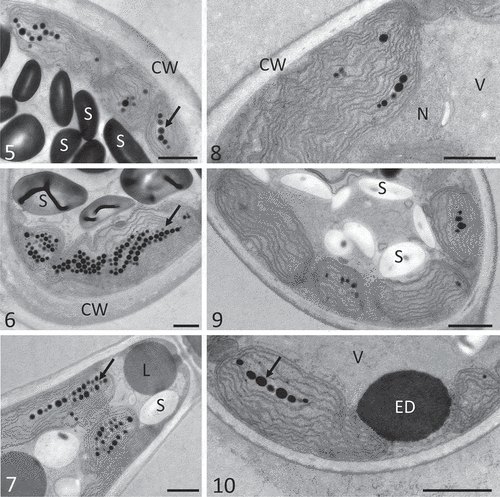
Whorl cells of B. turfosum exposed for 102 h under P conditions had intact chloroplasts with few plastoglobules and abundant cytosolic floridean starch (). In contrast, cells exposed for 102 h to PA () exhibited dense accumulations of plastoglobules, and the thylakoid membranes occasionally showed dilations. However, numerous starch grains were apparent in the cell centre. Exposure to PAB (102 h) also resulted in accumulation of plastoglobules and cytoplasmic lipid bodies (). After recovery (24 h), cells did not change in ultrastructural appearance () when compared with P. After recovery from PA () thylakoid membranes were arranged in a more parallel manner, but plastoglobules and starch granules could still be observed (). A similar effect of the recovery was observed for PAB-treated cells (), with a reduced number of plastoglobules and parallel thylakoid membranes. Additionally, these cells contained electron-dense bodies with a diameter of up to 1 µm.
Figs 5–10. Transmission electron micrographs of Batrachospermum turfosum whorl cells upon irradiation and recovery. Thalli were exposed to PAR, UV-A and UV-AB ( & , respectively) for 102 h followed by recovery in the absence of UVR ( & ). . Parietal chloroplasts with plastoglobules (arrow) and cytoplasmic starch grains. . Accumulation of plastoglobules (arrow). . Plastoglobules (arrow), cytoplasmic lipid bodies. . Chloroplast with parallel thylakoid membranes. . Intact chloroplasts. . Reduced number of plastoglobules (arrow), electron-dense body in the cell cortex. Abbreviations: CW, cell wall; ED, electron-dense body; L, lipid body; N, nucleus; S, starch; V, vacuole. Scale bars: 1 µm.
Discussion
This study shows that Batrachospermum turfosum is adapted to a broad range of temperature and irradiance conditions, including UVR, which are typically found in habitats at elevated altitudes and northern latitudes characterized by ultra-oligotrophic waters that may contain unfavourable organic and inorganic compounds.
Light and temperature requirements for photosynthesis
The photosynthetic performance of B. turfosum is characterized by very low Ik and Ic values together with a high photosynthetic efficiency even at low PFDs (), in agreement with reports on other freshwater red algae (Karsten et al., Citation1993; Leukart & Hanelt, Citation1995; Necchi, Citation2005). However, B. turfosum also tolerates PFDs of ~1000 µmol photons m–2 s–1. PFDs between 1000 and 2400 µmol photons m–2 s–1 were also reported to occur in the habitats of Compsopogon caeruleus and B. delicatulum (Necchi & Zucchi, Citation2001). Tolerance to this wide range of light conditions is supported by the plasticity of the photosynthetic apparatus (Necchi, Citation2005), which in freshwater red algae can vary between populations (Necchi & Vis, Citation2005), between field-collected and cultured specimens (Necchi, Citation2005, Drerup et al., Citation2015) and between algae acclimated to low light and high light conditions (Bautista & Necchi, Citation2007).
The temperature dependence of photosynthesis was assessed by maximum chlorophyll a fluorescence and oxygen evolution from PSII (Gilbert et al., Citation2000), showing oxygen production in a broad temperature range between 5°C and 35°C (), in accordance with the temperatures reported for B. turfosum habitats (Müller et al., Citation1997; Gregor et al., Citation2003). Highest photosynthetic efficiency between photosynthesis and respiration (P:R ratio) was found between 15 and 25°C (), supporting previous reports on other Batrachospermum species and for C. caeruleus (Necchi & Zucchi, Citation2001; Necchi, Citation2004). At temperatures above 20°C oxygen consumption started to increase, and was maximal at 30°C, indicative of unfavourable conditions for net photosynthesis. However, B. turfosum can also withstand high temperatures of up to 35°C for short periods, as would be expected for an alga occurring in standing waterbodies in which temperatures were reported to fluctuate up to 34°C (Gregor et al., Citation2003). The two methodological approaches for the assessment of photosynthesis, i.e. through chlorophyll a fluorescence and oxygen measurements, provided different results (), as reported earlier (Hanelt et al., Citation1994; Gilbert et al., Citation2000). FV/FM steadily declined with increasing temperatures above 15°C, to ~60% of its initial signal at 35°C, where the P:R ratio was still positive, to ~30% at 40°C, where no oxygen production was detectable. Therefore, the increase in oxygen consumption at 40°C is probably induced by higher mitochondrial activity masking net photosynthesis. Above 40°C, photosynthetic activity collapsed, as reported for higher plants (Pospisil et al., Citation1998), presumably due to increasing thermal denaturation of proteins such as the D1 of PSII. In summary, in B. turfosum optimal conditions for photosynthesis are low light, around 100 µmol photons m–2 s–1, and temperatures between 15 and 25°C, although photosynthetic acitivty was also recorded at much higher PFDs, and at lower and higher temperatures, supporting euryoecious adaptation.
Dynamic photoinhibition in response to ultraviolet radiation
The effective quantum yield of thalli exposed to PA and PAB differed significantly from P, and from each other throughout the entire exposure period (). In UVR-treated thalli, but not in the P-treated thalli, ΔF/FM’ showed a significant, rapid, transient decrease, suggesting that PSII was impaired, followed by an increase, indicative of photoacclimation, and then ΔF/FM’ plateaued. Similar kinetics were also described for higher plants (Tyystjarvi, Citation2008) and cyanobacteria (Allakhverdiev & Murata, Citation2004), in which the initial decline in ΔF/FM’ was suggested to be due to damage to PSII components, such as the oxygen-evolving complex, and to insufficient repair mechanisms (Vass, Citation2012). Furthermore, damage to PSII by UV-A alone is generally lower compared with UV-AB, although both have similar effects on PSII (Vass, Citation2012). In B. turfosum the initial drop in ΔF/FM’ was greater in PAB-irradiated thalli than in PA-irradiated ones, but in both treatments thalli were able to acclimate, as shown by their ability to reach about 70% of their initial ΔF/FM’ values. In marine red algae UVR contributed to dynamic photoinhibition, resulting in a 30% drop in photosynthetic performance in Lithophyllum incrustans and Sphaerococcus coronopifolium (Figueroa & Gomez, Citation2001), and in young gametophytes of Gelidium floridanum (Schmidt et al., Citation2012). When B. turfosum thalli were allowed to recover in the absence of UVR, they almost re-established their initial ΔF/FM’ values (). Interestingly, PAB-irradiated thalli recovered better than PA-irradiated ones, as also observed in the red alga Gracilaria lemaneiformis (Xu & Gao, Citation2010). UV-B-induced repair mechanisms for D1 and D2 proteins were also found in cyanobacteria (Vass et al., Citation2013). Taken together with the mostly intact ultrastructure after the recovery phase (–), it appears that UVR did not incur chronic photoinhibition in the conditions tested, providing further evidence for the presence of a flexible, plastic photosynthetic apparatus.
The pigment composition of B. turfosum is dominated by chlorophyll a and lutein and it also contains small amounts of zeaxanthin (), typical of Batrachospermales (Schagerl & Donabaum, Citation2009). Exposure to P, PA and PAB induced a strong decline in chlorophyll a and lutein concentrations, also in the P-treated controls in which no significant changes in ΔF/FM’ were found. This indicates that the alga suffered some stress, or acclimated by down-regulating photosynthesis, in response to the treatment, even at the dim light of 20 µmol photons m–2 s–1 used. The phycobiliproteins, APC and PC, were also strongly affected by UVR, whereby PAB-irradiation had the greatest effect, especially on APC, of which the concentration was halved. In contrast, P- and PA-irradiation had only a small effect on APC, whereas PC was also significantly lowered. Figueroa & Gomez (Citation2001) suggested that state transitions in combination with cyclic electron transport by photosystem I greatly contribute to non-photochemical quenching in red algae. Direct involvement of PBS in energy dissipation was shown in Porphyridium cruentum (Kana et al., Citation2014), but the contribution of PBS to qE in B. turfosum remains to be elucidated. In the recovery phase, PBS composition remained unchanged in P-irradiated thalli, whereas APC significantly increased in the PAB-irradiated thalli. Upon recovery of B. turfosum PBPs re-synthesis was apparently enhanced in thalli treated with PAB compared with PA. High photosynthetic plasticity and fast re-synthesis of PBPs were also described for polar marine red algae after long periods of darkness (Lüder et al., Citation2002). In the present study, re-synthesis of PBPs was observed although the experiments were conducted under nutrient-poor conditions. In contrast, another generalist red alga, C. caeruleus, an indicator of nutrient-enriched environments, suffered from nitrogen starvation, which caused PBS degradation (Bautista & Necchi, Citation2014). Therefore, the capacity to re-synthesize PBPs could reflect the adaptation of B. turfosum to ultra-oligotrophic environments.
Algae from the eulittoral are known to synthesize numerous UV-photoprotective compounds, mainly MAAs (Karsten, Citation2008), in response to elevated UVR, whereas algae from deeper waters lack this capability (Hoyer et al., Citation2001). The presence of such UV-sunscreens can alleviate ultrastructural damage, including to chloroplasts, as shown for Palmaria decipiens and Bangia atropurpurea (Poppe et al., Citation2003; Holzinger et al., Citation2004). In B. turfosum no putative UV-sunscreens, like MAAs or phenolic compounds, were found. However, the alga may benefit from UV-absorbing organic and inorganic compounds, such as polyphenolic compounds and iron, which are abundantly present in bog water. On the other hand, these compounds can lead to ROS formation (Roach et al., Citation2015). Batrachospermum spp. are characterized by a thick extracellular mucilage, which is why they are sometimes named ‘frog spawn’. This mucilage could act as a physical barrier for ROS and polyphenols. Moreover, the dense whorls of B. turfosum will provide some self-shading, which may also contribute to photoprotection. Nonetheless, B. turfosum has a notable tolerance of UVR compared with shade-adapted marine red algae from the lower sublittoral (Ic values < 30 μmol m–2 s–1), which were extremely sensitive to mild UVR treatment (e.g. Phycodrys austrogeorgica; Poppe et al., Citation2003), and showed no or only weak recovery (Bischof et al., Citation2006). By contrast, the ultrastructure of B. turfosum was still intact after the irradiation treatments applied although accumulation of plastoglobules was observed (–). Plastoglobules are lipoprotein particles inside chloroplasts that accumulate upon stress, including high light, senescence and desiccation (Austin II et al., Citation2006; Bréhélin et al., Citation2007; Holzinger et al., Citation2011), and that have also been suggested to function as reserves in red algae (Schmidt et al., Citation2012).
In conclusion, B. turfosum is adapted to low light, but also tolerates high PFDs, at least in the short-term, and is capable of net photosynthesis at a wide range of temperatures. Moreover, B. turfosum can acclimate to UVR by dynamic photoinhibition, and although it does not use MAAs for photoprotection, is capable of recovering ΔF/FM’ and PBPs after UVR treatment. These traits of B. turfosum may facilitate its broad occurrence in ultra-oligotrophic water bodies, such as bog pools, from northern latitudes to subalpine regions, characterized by widely fluctuating light and temperature conditions.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://10.1080/09670262.2016.1274430
Supplementary Table S1. Physical parameters and nutrient contents of the bog pool water. Measurements were taken on 30 May, 18 July and 5 October 2014. Phenols are expressed as gallic acid equivalents (mg l–1 GAE). Different capital letters represent significant differences between sampling dates, assessed by one-way ANOVA followed by Bonferroni’s post hoc test (P ≤ 0.05; n = 5, means ± SD). Non-detected compounds are shown as n.d.; irradiance values for the collection site in the sun and shade (taken on 18 July 2014 at 13:00, CET + 1) are shown for PAR, UV-A and UV-B radiation.
Supplementary Table S2. Contribution of humic acid to bog water colour, measured in gallic acid equivalents (GAE).
Supplementary Table S3. Chlorophyll and carotenoid concentrations in Batrachospermum turfosum on a dry weight basis.
Supplementary Fig. S1. Collection site and morphology of Batrachospermum turfosum. Sampling site (A–B), macroscopic view (C), light- (D–F) and confocal scanning microscopy (CLSM; H–I) are shown. The bog pool is located in a subalpine peat bog (1026 m a.s.l.; 12°30.763′N and 47°53.315′E). A. Partly shaded bog pool at noon. B. Submersed wooden branch covered by the algae. C. Macroscopic view of a thallus. D. Apical whorls. E. Spermatangia (arrows). F. Long colourless hair cells (≥ 200 µm) emerging from terminal whorl cells. G. Ribbon-shaped, spiral chloroplasts. H. Z-stack, the chloroplast autofluorescence illustrates the spiral shape. I. The median focal plane demonstrates the parietal arrangement of the chloroplast. Scale bars: C = 2 cm; D = 50 µm; E = 20 µm; F = 40 µm; G–I = 10 µm.
Supplementary Fig. S2. Habitat and collection site of Batrachospermum turfosum. The habitat in the ‘Moorkomplex Biedringer Platte’ is located in a subalpine peat bog at ~1000 m a.s.l., consisting of a kermi-type bog (A) and a blanket bog (B). In contrast to the surrounding forest (C), the bog pool (black ellipse in the inset) receives strong radiant energy over the course of the year (700–900 KWh m–2 a–1). Report of annual solar potential in Tyrol: http://www.tirol.gv.at/statistik-budget/tiris (accessed 04.07.2016).
Supplementary Fig. S3. Spectral absorbance characteristics of the bog pool water.
Supplementary Fig. S4. Temperature dependence of photosynthesis in Batrachospermum turfosum on a dry weight basis. A. Oxygen production at 100 µmol photons m–2 s–1, dark-respiration and optimum quantum yield (FV/FM). Negative values are shown as stacked bars and significant differences between means are marked with different letters. B. Ratio between photosynthesis and respiration as a function of temperature. Significant differences between mean values were calculated by one-way ANOVA followed by the Fisher’s LSD post hoc test (p ≤ 0.05; n = 4, means ± SD).
Author contributions
S. Aigner collected B. turfosum, designed and conducted the experiments, and drafted the manuscript; A. Holzinger contributed TEM images: A. Holzinger, U. Karsten and I. Kranner gave advise on experimental design and statistical procedures, discussed the data and edited the manuscript; I. Kranner supervised the work, provided lab space, equipment and materials and together with S. Aigner produced the final version of the paper.
Supplementary_material.zip
Download Zip (3.3 MB)Acknowledgements
The authors would like to thank E. Rott and J. Knappe for help with identifying the alga, K. Herburger for help with PAM and oxygen measurements, W. Stöggl, T. Roach and E. Arc for support regarding the biochemical analyses, B. Jungwirth and S. Obwegeser for excellent technical assistance with TEM, and three anonymous reviewers for very helpful comments on earlier drafts of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aigner, S., Remias, D., Karsten, U. & Holzinger, A. (2013). Unusual phenolic compounds contribute to ecophysiological performance in the purple-colored green alga Zygogonium ericetorum (Zygnematophyceae, Streptophyta) from a high-alpine habitat. Journal of Phycology, 49: 648–660.
- Allakhverdiev, S.I. & Murata, N. (2004). Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage–repair cycle of photosystem II in Synechocystis sp PCC 6803. Biochimica et Biophysica Acta-Bioenergetics, 1657: 23–32.
- Austin II, J.R., Frost, E., Vidi, P.A., Kessler, F. & Staehelin, L.A. 2006. Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell, 18: 1693–1703.
- Bautista, A.I.N. & Necchi, O. Jr. (2007). Photoacclimation in three species of freshwater red algae. Brazilian Journal of Plant Physiology, 19: 23–34.
- Bautista, A.I.N. & Necchi, O. Jr. (2014). Physiological performances of two populations of Compsopogon caeruleus (Rhodophyta) to inorganic and phosphorus impoverishment. Brazilian Journal of Botany, 37: 391–398.
- Beer, S. & Eshel, A. (1985). Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Australian Journal of Marine and Freshwater Research, 36: 785–792.
- Bischof, K., Gómez, I., Molis, M., Hanelt, D., Karsten, U., Lüder, U., Roleda, M.Y., Zacher, K. & Wiencke, C. (2006). Ultraviolet radiation shapes seaweed communities. Reviews in Environmental Sciences and Biotechnology, 5: 141–166.
- Blumthaler, M., Ambach, W. & Ellinger, R. (1997). Increase in solar UV radiation with altitude. Journal of Photochemistry and Photobiology B-Biology, 39: 130–134.
- Blunt, J.W., Copp, B.R., Keyzers, R.A., Munro, M.H.G. & Prinsep, M.R. (2015). Marine natural products. Natural Product Reports, 32: 116–211.
- Bréhélin, C., Kessler, F. & van Wijk, K.J. (2007). Plastoglobules: versatile lipoprotein particles in plastids. Trends in Plant Science, 12: 260–266.
- Bruce, D. & Vasil’ev, S. (2004). Excess light stress: multiple dissipative processes of excess excitation. In Chlorophyll a Fluorescence: A Signature of Photosynthesis (Papageorgiou, G.C. & Govindjee, editors), 497–523. Springer, Dordrecht.
- Canham, C.D., Denslow, J.S., Platt, W.J., Runkle, J.R., Spies, T.A. & White, P.S. (1990). Light regimes beneath closed canopies and tree-fall gaps in temperate and tropical forests. Canadian Journal of Forest Research – Revue Canadienne De Recherche Forestière, 20: 620–631.
- Cornish, M.L., O’Leary, S.J.B. & Garbary, D.J. (2013). Phycobilisome composition in Chondrus crispus (Gigartinales, Rhodophyta) from a wild type strain and its vegetatively derived green mutant. Algae, 28: 121–129.
- Drerup, S.A., Gonzalez, D.A. & Vis, M.L. (2015). Photosynthetic characteristics of some common temperate freshwater red algal taxa (Rhodophyta). Phycologia, 54: 609–616.
- Eloranta, P. & Kwandrans, J. (2007). Freshwater red algae (Rhodophyta). Identification guide to European taxa, particularly to those in Finland. Norrlinia, 15: 1–103.
- Everette, J.D., Bryant, Q.M., Green, A.M., Abbey, Y.A., Wangila, G.W. & Walker, R.B. (2010). Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. Journal of Agricultural and Food Chemistry, 58: 8139–8144.
- Figueroa, F.L. & Gomez, I. (2001). Photosynthetic acclimation to solar UV radiation of marine red algae from the warm-temperate coast of southern Spain: a review. Journal of Applied Phycology, 13: 235–248.
- Gibson, M.T. & Whitton, B.A. (1987). Hairs, phospatase activity and environmental chemistry in Stigeoclonium, Chaetophora and Draparnaldia (Chaetophorales). British Phycological Journal, 22: 11–22.
- Gilbert, M., Wilhelm, C., & Richter, M. (2000). Bio-optical modelling of oxygen evolution using in vivo fluorescence: comparison of measured and calculated photosynthesis/irradiance (P-I) curves in four representative phytoplankton species. Journal of Plant Physiology, 157: 307–314.
- Gregor, T., Kiele, E. & Timmermann, E. (2003). Wiederfunde von Batrachospermum turfosum Bory in Niedersachsen. Lauterbornia, 46: 185–189.
- Guiry, M.D. (2012). How many species of algae are there? Journal of Phycology, 48: 1057–1063.
- Hanelt, D., Jaramillo, M.J., Nultsch, W., Senger, S. & Westermeier, R. (1994). Photoinhibition as a regulative mechanism of photosynthesis in marine algae of Antarctica. Serie Científica Instituto Antártico Chileno, 44: 67–77.
- Holzinger, A., Lütz, C. & Karsten, U. (2011). Desiccation stress causes structural and ultrastructural alterations in the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust. Journal of Phycology, 47: 591–602.
- Holzinger, A., Lütz, C., Karsten, U. & Wiencke, C. (2004). The effect of ultraviolet radiation on ultrastructure and photosynthesis in the red macroalgae Palmaria palmata and Odonthalia dentata from arctic waters. Plant Biology, 6: 568–577.
- Hoyer, K., Karsten, U., Sawall, T. & Wiencke, C. (2001). Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Marine Ecology Progress Series, 211: 117–129.
- Israelson, G. (1942). The freshwater Florideae of Sweden. Symbolae botanicae Upsalienses, 6: 1–134.
- Kaczmarczyk, D. & Sheath, R.G. (1991). The effect of light regime on the photosynthetic apparatus of the freshwater red alga Batrachospermum boryanum. Cryptogamie Algologie, 12: 249–263.
- Kana, R., Kotabova, E., Lukes, M., Papacek, S., Matonoha, C., Liu, L.N., Prasil, O. & Mullineaux, C.W. (2014). Phycobilisome mobility and its role in the regulation of light harvesting in red algae. Plant Physiology, 165: 1618–1631.
- Karsten, U. (2008). Defense strategies of algae and cyanobacteria against solar ultraviolet radiation. In Algal Chemical Ecology (Amsler C.D., editor), 273–296. Springer, Berlin.
- Karsten, U. & West, J.A. (2000). Living in the intertidal zone – seasonal effects on heterosides and sun-screen compounds in the red alga Bangia atropurpurea (Bangiales). Journal of Experimental Marine Biology and Ecology, 254: 221–234.
- Karsten, U., West, J.A. & Ganesan, E.K. (1993). Comparative physiological ecology of Bostrychia moritziana (Ceramiales, Rhodophyta) from freshwater and marine habitats. Phycologia, 32: 401–409.
- Knappe, J. & Huth, K. (2014). Rotalgen des Süßwassers in Deutschland und angrenzenden Gebieten. Bibliotheca Phycologia, 118: 73–83.
- Kumano, S. (2002). Freshwater Red Algae of the World. Biopress Ltd, Dorset.
- Kursar, T.A., Vandermeer, J. & Alberte, R.S. (1983). Light-harvesting system of the red alga Gracilaria tikvahiae. 1. Biochemical analyses of pigment mutations. Plant Physiology, 73: 353–360.
- Lao, K.Q. & Glazer, A.N. (1996). Ultraviolet-B photodestruction of a light-harvesting complex. Proceedings of the National Academy of Sciences USA, 93: 5258–5263.
- Leukart, P. & Hanelt, D. (1995). Light requirements for photosynthesis and growth in several macroalgae from a small soft-water stream in the Spessart Mountains, Germany. Phycologia, 34: 528–532.
- Lüder, U.H., Wiencke, C. & Knoetzel, J. (2002). Acclimation of photosynthesis and pigments during and after six months of darkness in Palmaria decipiens (Rhodophyta): a study to simulate Antarctic winter sea ice cover. Journal of Phycology, 38: 904–913.
- Müller, K.M., Vis, M.L., Chiasson, W.B., Whittick, A. & Sheath, R.G. (1997). Phenology of a Batrachospermum population in a boreal pond and its implications for the systematics of section Turfosa (Batrachospermales, Rhodophyta). Phycologia, 36: 68–75.
- Necchi, O. Jr. (2004). Photosynthetic responses to temperature in tropical lotic macroalgae. Phycological Research, 52: 140–148.
- Necchi, O. Jr. (2005). Light-related photosynthetic characteristics of freshwater rhodophytes. Aquatic Botany, 82: 193–209.
- Necchi, O. Jr. (2016). Red algae (Rhodophyta) in rivers. In River Algae (Necchi, O. Jr., editor), 65–92. Springer, Berlin.
- Necchi, O. Jr. & Vis, M.L. (2005). Reproductive ecology of the freshwater red alga Batrachospermum delicatulum (Batrachospermales, Rhodophyta) in three tropical streams. Phycological Research, 53: 194–200.
- Necchi, O. Jr. & Zucchi, M.R. (2001). Photosynthetic performance of freshwater Rhodophyta in response to temperature, irradiance, pH and diurnal rhythm. Phycological Research, 49: 305–318.
- Parker, B.C., Samsel, S.G.L. & Prescott, G.W. (1973). Comparison of microhabitats of macroscopic subalpine stream algae. American Midland Naturalist, 90: 143–153.
- Poppe, F., Schmidt, R.A.M., Hanelt, D. & Wiencke, C. (2003). Effects of UV radiation on the ultrastructure of several red algae. Phycological Research, 51: 11–19.
- Pospisil, P., Skotnica, J. & Naus, J. (1998). Low and high temperature dependence of minimum F0 and maximum FM chlorophyll fluorescence in vivo. Biochimica et Biophysica Acta-Bioenergetics, 1363: 95–99.
- Remias, D., Lütz-Meindl, U. & Lütz, C. (2005). Photosynthesis, pigments and ultrastructure of the alpine snow alga Chlamydomonas nivalis. European Journal of Phycology, 40: 259–268.
- Remias, D., Karsten, U., Lütz, C. & Leya, T. (2010). Physiological and morphological processes in the alpine snow alga Chloromonas nivalis (Chlorophyceae) during cyst formation. Protoplasma, 243: 73–86.
- Rider, D.E. & Wagner, R.H. (1972). Relationship of light, temperature, and current to seasonal distribution of Batrachospermum (Rhodophyta). Journal of Phycology, 8: 323–331.
- Roach, T., Miller, R., Aigner, S. & Kranner, I. (2015). Diurnal changes in the xanthophyll cycle pigments of freshwater algae correlate with the environmental hydrogen peroxide concentration rather than non-photochemical quenching. Annals of Botany, 116: 519–527.
- Sah, J.F., Krishna, K.B., Srivastava, M. & Mohanty, P. (1998). Effects of ultraviolet-B radiation on phycobilisomes of Synechococcus PCC 7942: alterations in conformation and energy transfer characteristics. Biochemistry and Molecular Biology International, 44: 245–257.
- Schagerl, M. & Donabaum, K. (2009). Patterns of major photosynthetic pigments in freshwater algae. 1. Cyanoprokaryota, Rhodophyta and Cryptophyta. Annales de Limnologie – International Journal of Limnology, 39: 35–47.
- Schmidt, E.C., dos Santos, R.W., de Faveri, C., Horta, P.A., Martins, R.D., Latini, A., Ramlov, F., Maraschin, M. & Bouzon, Z.L. (2012). Response of the agarophyte Gelidium floridanum after in vitro exposure to ultraviolet radiation B: changes in ultrastructure, pigments, and antioxidant systems. Journal of Applied Phycology, 24: 1341–1352.
- Schubert, N., García-Mendoza, E. & Pacheco-Ruiz, I. (2006). Carotenoid composition of marine red algae. Journal of Phycology, 42: 1208–1216.
- Schubert, N., García-Mendoza, E. & Enríquez, S. (2011). Is the photo-acclimatory response of Rhodophyta conditioned by the species carotenoid profile? Limnology and Oceanography, 56: 2347–2361.
- Sheath, R.G. & Vis, M.L. (2015). Red algae. In Freshwater Algae of North America. 2nd ed. (Wehr, J.D., Sheath, R.G. & Kociolek, J.P., editors), 237–260. Elsevier Academic Press, London.
- Sheath, R.G., Vis, M.L. & Cole, K.M. (1994). Distribution and systematics of Batrachospermum (Batrachospermales, Rhodophyta) in North America. 6. Section Turfosa. Journal of Phycology, 30: 872–884.
- Six, C., Joubin, L., Partensky, F., Holtzendorff, J. & Garczarek, L. (2007). UV-induced phycobilisome dismantling in the marine picocyanobacterium Synechococcus sp. WH8102. Photosynthesis Research, 92: 75–86.
- Tyystjarvi, E. (2008). Photoinhibition of photosystem II and photodamage of the oxygen evolving manganese cluster. Coordination Chemistry Reviews, 252: 361–376.
- Vass, I. (2012). Molecular mechanisms of photodamage in the photosystem II complex. Biochimica et Biophysica Acta, 1817: 209–217.
- Vass, I.Z., Kos, P.B., Sass, L., Nagy, C.I. & Vass, I. (2013). The ability of cyanobacterial cells to restore UV-B radiation induced damage to photosystem II is influenced by photolyase dependent DNA repair. Photochemistry and Photobiology, 89: 384–390.
- Walsby, A.E. (1997). Numerical integration of phytoplankton photosynthesis through time and depth in a water column. New Phytologist, 136: 189–209.
- Xu, J.T. & Gao, K.S. (2010). UV-A enhanced growth and UV-B induced positive effects in the recovery of photochemical yield in Gracilaria lemaneiformis (Rhodophyta). Journal of Photochemistry and Photobiology B-Biology, 100: 117–122.
