Abstract
Culture collections of microorganisms can still hold undiscovered biodiversity; with molecular techniques, considerable progress has been made in characterizing microalgae which were isolated in the past and misidentified due to a lack of morphological features. However, many strains are still awaiting taxonomic reassessment. Here we analysed the phylogenetic position, morphology and ultrastructure of the strain CCALA 307 previously identified as Coccomyxa cf. gloeobotrydiformis Reysigl isolated in 1987 from field soil in South Bohemia, Czech Republic. Molecular phylogenetic analyses based on SSU rDNA and the plastid rbcL gene revealed that the strain CCALA 307 formed a distinct sister lineage to Neocystis and Prasiola clades within the Trebouxiophyceae. We describe this strain as a new genus and species, Lunachloris lukesovae. Multiple conserved nucleotide positions identified in the secondary structures of the highly variable ITS2 rDNA barcoding marker provide further evidence of the phylogenetic position of Lunachloris. Minute vegetative cells of this newly recognized species are spherical or ellipsoid, with a single parietal chloroplast without a pyrenoid. Asexually, it reproduces by the formation of 2–6 autospores. Since the majority of recent attention has been paid to algae from the tropics or extreme habitats, the biodiversity of terrestrial microalgae in temperate regions is still notably unexplored and even a ‘common’ habitat like agricultural soil can contain new, as yet unknown species. Moreover, this study emphasizes the importance of culture collections of microorganisms even in the era of culture-independent biodiversity research, because they may harbour novel and undescribed organisms as well as preserving strains for future studies.
Introduction
The diversity of small unicellular green coccoid algae has always been underestimated owing to their simple and uniform morphology, which has made precise identification extremely challenging given the limitations of technological resources and previously available equipment. The ongoing application of molecular methods has not only helped to reveal the actual biodiversity of many ‘little green balls’ (Komárek & Fott, Citation1983) but has also enabled a more acceptable delimitation of species. In particular, the green algal class Trebouxiophyceae has recently been enriched with many new lineages (Zhang et al., Citation2008; Eliáš & Neustupa, Citation2009; Neustupa et al., Citation2009, Citation2011, Citation2013a, Citation2013b; Bock et al., Citation2010; Fučíková et al., Citation2014; Song et al., Citation2015) and has gone through a number of revisions, encompassing the genera Chloroidium (Darienko et al., Citation2010), Chlorella (Bock et al., Citation2011), Auxenochlorella (Darienko & Pröschold, Citation2015), Coccomyxa (Darienko et al., Citation2015; Malavasi et al., Citation2016), Elliptochloris and Pseudochlorella (Darienko et al., Citation2016) and Dictyochloropsis (Škaloud et al., Citation2016), with descriptions of new species and/or new taxonomic combinations. Unsurprisingly, their molecular diversity greatly exceeded the morphological diversity previously established. For example, the widely distributed genus Coccomyxa appears to be phylogenetically extremely diversified and impossible to identify just by visual examination (Darienko et al., Citation2015; Malavasi et al., Citation2016).
More and more new species continue to be described based on a combination of phylogenetic, morphological and ecological data and the majority of recently described terrestrial green algae (Chlorophyta) have been isolated from various habitats, mostly in the tropics (Zhang et al., Citation2008; Eliáš & Neustupa, Citation2009; Neustupa et al., Citation2009, Citation2011; Eliáš et al., Citation2010; Němcová et al., Citation2011), or in extremely dry deserts (Fučíková et al., Citation2014). Because these poorly explored and often extreme environments are more attractive for current biodiversity research, the terrestrial green microalgae of temperate regions have long been neglected. Only a few examples of new genera and species, accurately described using a polyphasic approach, are known from temperate Europe, e.g. Leptochlorella and Kalinella apyrenoidosa (Neustupa et al., Citation2013a), Parachloroidium (Neustupa et al., Citation2013b) and Jenufa aeroterrestrica (Procházková et al., Citation2015), while numerous other new species from Europe have been defined via the revision of the cryptic genera as mentioned above.
Many newly reported species and genera of terrestrial green algae fall within well-established clades. Prominent examples are the Watanabea clade (including the terrestrial genera Heveochlorella Zhang, Huss, Sun, Chang & Pang, Kalinella Neustupa, Němcová, Eliáš & Škaloud, Parachloroidium Neustupa & Škaloud, Desertella Fučíková, Lewis & Lewis and Polulichloris Song, Zhang, Liu & Hu) or the Prasiola clade (e.g. Pseudomarvania Eliáš & Neustupa). So far, modern molecular studies have revealed only a handful of novel terrestrial lineages within the green algal classes Chlorophyceae (e.g. Jenufa Němcová, Eliáš, Škaloud & Neustupa) and Trebouxiophyceae (e.g. Xylochloris Neustupa, Eliáš & Škaloud, Eremochloris along with Xerochlorella Fučíková, Lewis & Lewis and Leptochlorella Neustupa, Veselá, Němcová & Škaloud). However, the molecular diversity of terrestrial algae in Central Europe is still far from understood, and new lineages or clades might be expected in aero-terrestrial habitats such as tree bark (Neustupa et al., Citation2013a, Citation2013b; Procházková et al., Citation2015) and particularly in soils (Hodač, Citation2016). Although such potentially undescribed microorganisms can be obtained from current field sampling, re-examination of established algal cultures is also required. In particular the public collections of algal strains, e.g. Culture Collection of Algae at University of Göttingen, Germany (SAG), Culture Collection of Algae of Charles University in Prague (CAUP) and Culture Collection of Autotrophic Organisms in Třeboň, Czech Republic (CCALA), preserve isolates from Central Europe and hold numerous terrestrial taxa waiting for a taxonomic reassessment. Recently, multiple algal strains deposited in public culture collections have been described as new species or even genera based on molecular phylogenetics. For example, the strain CAUP H 5502, isolated in 1975, was later described as Ooplanctella Pažoutová, Škaloud & Nemjová (Pažoutová et al., Citation2010). Similarly, SAG 12.86 isolated in 1983 was recently circumscribed as Symbiochloris (Škaloud et al., Citation2016) and Chlorella vulgaris KIEG 1904 isolated in 1977 (and stored in a private culture collection) was described as Planktochlorella Škaloud & Němcová (Škaloud et al., Citation2014).
CCALA is one of the world’s oldest culture collections of algal strains, containing many terrestrial coccoid green algae deposited before the advent of molecular phylogenetics. Many strains are still waiting for inspection and here we aim to extend this effort and focus on a Coccomyxa-like strain CCALA 307 isolated in the late 1980s.
Materials and methods
Origin of the strain and culturing technique
The algal strain 307 was obtained from CCALA where it was referred to as Coccomyxa cf. gloeobotrydiformis Reisigl. It was isolated in 1987 during a 3-year study of secondary succession of abandoned arable fields near Chelčice, South Bohemia, Czech Republic. The strain was isolated from a wet meadow close to Dlouhá Ves (~ 49°06’N, 14°07’E), formed after several years of field abandonment (Lukešová, Citation1993). We cultivated the strain in Bold’s Basal Medium (BBM; Bischoff & Bold, Citation1963) in a Q-Cell 200 incubator (PolLab, Bielsko-Biała, Poland). Light (20 µmol photons m–2 s–1) was provided continuously by a cool white fluorescent tube (Standard 8W, Sylvania, USA) and the cultivation temperature was 20°C. Aliquots of liquid monocultures were used for microscopic observations and genetic analysis.
Light and electron microscopy
The morphology of the strain was investigated using both Nikon Eclipse E400 (Nikon Inc., Tokyo, Japan) and Olympus BX-51 (Olympus Corp., Tokyo, Japan) light microscopes. Chloroplast morphology was investigated using a Zeiss LSM 880 laser scanning confocal microscope (Zeiss, Jena, Germany) equipped with a Helium-Neon laser. We used a 633 nm excitation line collecting emitted light between 645 and 721 nm. A C-Apochromat 63x/1.2 W Korr water immersion objective with a M 27 adapter was employed. A series of optical sections through the chloroplast were captured and used for three-dimensional (3D) morphology reconstruction. Chlorophyll autofluorescence was used for visualization of chloroplast structure. For reconstruction of the chloroplast, 3D morphology ImageJ version 1.50g (Schneider et al., Citation2012) was used.
For transmission electron microscopy (TEM), the sample was fixed for 24 h in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) and post-fixed in 2% OsO4 in the same buffer. Fixed cells were dehydrated in a graded ethanol series (35%, 50%, 70%, 80%, 96%, 100% for 15 min), transferred to acetone (3 × 100% for 15 min) and finally embedded in Araldite – Poly/Bed® 812 mixture (Polysciences Inc., Hirschberg an der Bergstraße, Germany). Ultrathin sections were cut on a Reichert-Jung Ultracut E ultramicrotome and stained using uranyl acetate and lead citrate. Sections were examined using a JEOL JEM-1011 electron microscope (JEOL Ltd, Tokyo, Japan). Photomicrographs were obtained using a Veleta CCD camera (EMSIS GmbH, Münster, Germany) equipped with image analysis software Olympus Soft Imaging Solution GmbH (Münster, Germany) and later modified by Inkscape 0.91 (Free Software Foundation Inc., Boston, USA).
DNA isolation, PCR and sequencing
DNA extraction from fresh material was performed with the DNeasy Plant Mini Kit (Qiagen GmbH, Hilden, Germany). PCR reactions were done using PPP Master Mix (Top-Bio s.r.o., Prague, Czech Republic) in a total volume of 25 µl. 18S rDNA region was amplified using forward eukaryote specific primer 20F (Thüs et al., Citation2011) and reverse green algae specific primer CH1750R (Hallmann et al., Citation2013). Sequencing of this segment used standard sequencing primers 34F, 370R, 1122F (Pažoutová et al., Citation2010), 895R, 1422F (Remias et al., Citation2012), 891F, 1122R, 1422R (Friedl, unpublished). The ITS1-5.8S-rDNA-ITS2 region was amplified using forward primer AL1500af (Helms et al., Citation2001) and reverse primer LR3 (Vilgalys & Hester, Citation1990). The region was sequenced with primers ITS1, ITS4 (White et al., Citation1990), 5.8SbF (Mikhailyuk et al., Citation2008) and nr-SSU-1780-5´Algal, nr-LSU-0012-3´Algal (Piercey-Normore & DePriest, Citation2001). The large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (rbcL) was amplified and sequenced using a set of primers rbcL1F, rbcL2F, rbcL4R, rbcL7R, rbcL8F, rbcL9R, rbcL10F, rbcL14R, rbcL19F, rbcL20F (Hoham et al., Citation2002). Detailed primer information is given in Supplementary Table S1. All PCR reactions were performed in a thermocycler GeneTouch (BioER, Hangzhou, China) using the following program for the primer set 20F/CH1750R: initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 54°C for 1 min, extension at 72°C for 3 min and final extension at 72°C for 10 min. For ITS2 primer set AL1500af/LR3, an initial denaturation at 95°C for 5 min was followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 54°C for 30 s, extension at 72°C for 25 s and final extension at 72°C for 5 min. For rbcL primer sets we used a PCR cycle as indicated by Hoham et al. (Citation2002) with a slightly modified annealing temperature (52.8°C). Aliquots of 2 µl of PCR products were quantified on a 1% agarose gel stained with GelRed in 0.5 TBE buffer (130 V 30 min). PCR products were purified using ethanol and sent for sequencing to Macrogen (Amsterdam, the Netherlands). Sequences were deposited in GenBank under accession numbers KX620913 and KX620914.
Phylogenetic analyses
SSU rDNA and rbcL phylogenetic analyses
The closest related SSU, ITS and rbcL sequences to the strain CCALA 307 and other representatives of the class Trebouxiophyceae were acquired from GenBank employing the BLAST algorithm (Altschul et al., Citation1997). All new sequences were checked for chimeras using Bellerophon (Huber et al., Citation2004). Two separate trebouxiophycean datasets were compiled, one for SSU sequences and one for rbcL sequences; the sequence alignments were computed using MAFFT v.6 (Katoh & Toh, Citation2008). The aligned sequences were checked for possible misaligned positions in BioEdit 7.0.9.0 (Hall, Citation1999). The SSU alignment of the Trebouxiophyceae included 87 sequences/1755 positions (686 variable, 487 parsimony informative). The rbcL alignment comprised 57 sequences/1173 positions (623 variable, 489 parsimony informative). Based on the AIC criterion in jModelTest 0.1.1 (Posada, Citation2008), the GTR+Γ+I nucleotide substitution model was selected as best fitting both the datasets. A maximum-likelihood phylogeny was computed in RAxML 7.0.4 (Stamatakis et al., Citation2008) under the proposed model, and statistical support values were derived from rapid bootstrapping (1000 replicates) in the same program. For additional statistical support, Bayesian posterior probabilities were computed in MrBayes 3.2.1 x64 (Ronquist et al., Citation2012). We carried out two MCMC runs for one million generations each with one cold and three heated chains under the GTR+Γ+I evolutionary model (parameters were estimated from the data); trees were sampled every 100 generations. The final trees were visualized using FigTree (Rambaut, Citation2007). For additional sequence comparisons, Kimura-2-parameter p-distances were computed in MEGA6 (Tamura et al., Citation2013).
ITS2 rDNA secondary structure analysis
Precise annotation of the internal transcribed spacer 2 including the 5.8 and 28S flanking regions was accomplished by the ITS2 online database (Schultz et al., Citation2006; Selig et al., Citation2008; Keller et al., Citation2009; Koetschan et al., Citation2010, Citation2012). The annotation of the ITS1 spacer was assessed via a comparison with annotated Neocystis sequences available in GenBank. Minimum energy secondary structure model of ITS2 was computed with RNAstructure 5.3 (Reuter & Mathews, Citation2010) and visualized by Varna 3.8 (Darty et al., Citation2009). Subsequently, a sequence + structure alignment including CCALA 307, Neocystis brevis CAUP D 802, N. mucosa KR 1989/14, Gloeocystis polydermatica CCAP 31/5 and Stichococcus bacillaris SAG 379-1b was built employing the ClustalW algorithm implemented in 4SALE 1.7. (Seibel et al., Citation2006, Citation2008). The same software computes compensatory base changes (CBCs; Wolf et al., Citation2013) among sequences.
Results
Cell morphology and ultrastructure
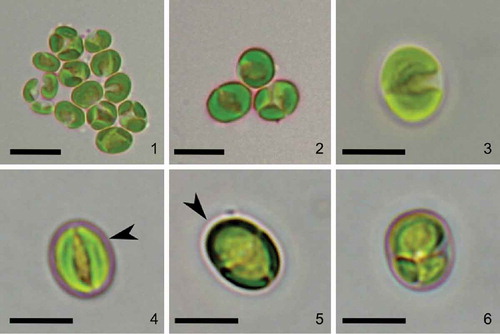
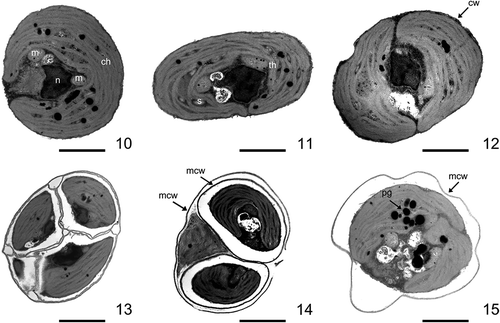
Strain CCALA 307 was coccoid unicellular with a thin, smooth and unornamented cell wall. The cell shapes varied from spherical to ellipsoid (–). Mature vegetative cells were (3.0–) 4.3–5.4 (–6.7) µm in diameter. Old, mature cells were mostly globular, had few lipid droplets in the cytoplasm and reached up to 7.7 µm in diameter. A single parietal moon-shaped chloroplast occupied the majority of the cell volume (–). Starch granules were observed in the inter-thylakoid space () but no pyrenoids. The chloroplast was usually divided into two lobes separated by a narrow incision (–, , , , ). The cells had one nucleus which was located in the middle and embraced by a chloroplast (–). The space between the nucleus and the chloroplast was occupied by mitochondria and small vacuoles (–). Plastoglobuli were visible in cross-sections (). The cells reproduced by autosporulation, forming 2–6 autospores of the same size (, , , ) which were later released through a rupture of a mother cell wall at one side of the cell (). The daughter cells stayed covered by the persistent remains of the mother cell wall (, , ). Neither zoospore formation nor sexual reproduction were observed.
Figs 1–6. Morphology of Lunachloris lukesovae gen. et sp. nov. CCALA 307. Figs 1–5. mature cells Fig. 6. autosporangium. Arrowheads point to the margins of the parental cell wall. Scale = 5 µm.
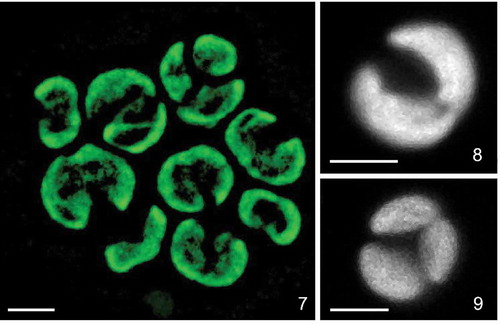
Figs 7–9. Confocal reconstructions of the chloroplast structure of Lunachloris lukesovae CCALA 307. Figs 7–8 mature vegetative cells. Fig. 9. autosporangium. Scale = 3 µm.
Figs 10–15. Ultrastructure of Lunachloris lukesovae CCALA 307. Figs 10–12. Vegetative cells (remnants of mother cell wall are not shown). Figs 13–14. Autosporangia. Fig. 15. Vegetative cell surrounded by mother cell wall after autospore release. ch, chloroplast; m, mitochondrion; n, nucleus, s, starch; th, thylakoids; cw, cell wall; mcw, mother cell wall; pg, plastoglobuli. Scale = 1 µm.
Molecular phylogeny and ITS2 secondary structure analysis
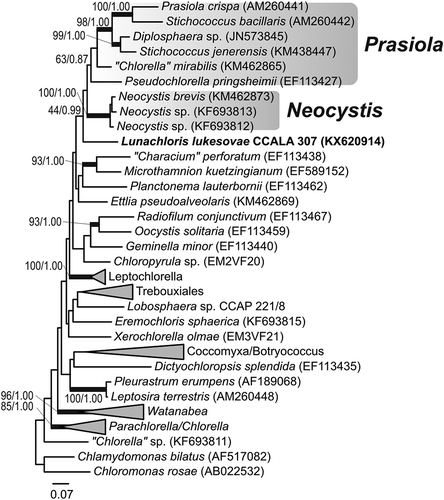
The strain CCALA 307 showed 97% SSU sequence similarity to Neocystis brevis SAG 850-1 (KM020044), Neocystis mucosa SAG 40.88 (JQ920367; Neocystis-clade) and Coenocystis inconstans (AB017435) (the closest cultured species available in GenBank (October 2016)). The rbcL comparisons revealed a relatively low similarity of 89% with the closest match, ‘Chlorella’ mirabilis SAG 38.88 (KM462865; Prasiola clade). Both the SSU () and rbcL () analyses congruently pointed out that strain CCALA 307 represents a phylogenetically isolated lineage within the Trebouxiophyceae, possibly a sister branch of the Neocystis and Prasiola clades (–), yet with low statistical support. The phylogenetic placement of the studied strain was identical in the SSU and rbcL tree topologies, for both the maximum-likelihood and the Bayesian inferences. Considering the genetic similarity of CCALA 307 with GenBank sequences of uncultured organisms, the closest SSU relative was Uncultured Dunaliellaceae clone Amb_18S_930 (EF023670; 97%) and the closest rbcL relative was Uncultured Trebouxia photobiont clone L-68 (AM158969; 89%).
Fig. 16. Maximum-likelihood tree of SSU sequences from L. lukesovae CCALA 307 and other Trebouxiophyceae. Numbers next to branches indicate statistical support values (maximum-likelihood bootstraps/Bayesian posterior probabilities). Thick lines indicate branches with high statistical support.
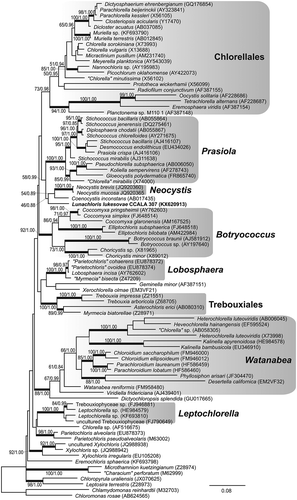
Fig. 17. Maximum-likelihood tree of rbcL sequences from L. lukesovae CCALA 307 and other Trebouxiophyceae. Numbers next to branches indicate statistical support values (maximum-likelihood bootstraps/Bayesian posterior probabilities). Thick lines indicate branches with high statistical support.
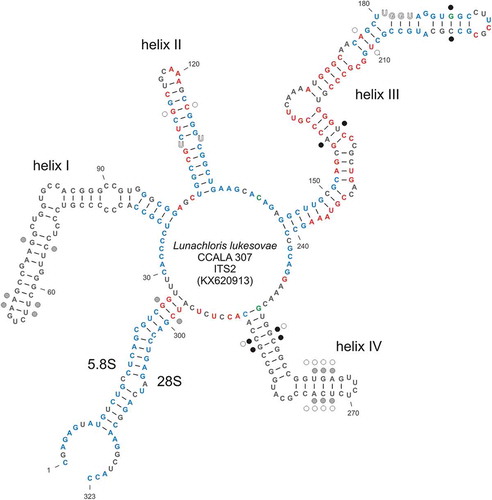
Regarding the ITS1-5.8S-ITS2 spacer region, the closest GenBank relatives of CCALA 307 comprised several Neocystis species (e.g. Neocystis mucosa SAG 40.88) with 81% similarity. The ITS2 secondary structure model of CCALA 307 showed features characteristic of other green algae, consisting of four helices and well identifiable conserved motifs (U-U in helix II and UGGU in helix III; ). Particularly helix II and helix III are highly conserved among CCALA 307 and both Neocystis species (blue and red coloured nucleotide positions in ). The strain CCALA 307 also shares some structural features with the representatives of the Prasiola clade (blue and green coloured nucleotide positions in ), but the major parts of helix I and helix IV are specific for CCALA 307 and divergent from both the Neocystis and Prasiola clade representatives (grey coloured nucleotide positions in ). The closer relationship of CCALA 307 to the Neocystis clade than to the Prasiola clade was supported by the analysis of compensatory base changes (CBCs) within the ITS2 secondary structure. CCALA 307 differed by four CBCs from Neocystis spp. (black dots in ) and by seven CBCs from the members of the Prasiola clade (grey and white dots in ).
Fig. 18. Secondary structure of the ribosomal internal transcribed spacer 2 (ITS2 rDNA) of L. lukesovae CCALA 307. Nucleotide sites which are conserved among L. lukesovae CCALA 307 and relatives from the Neocystis clade (Neocystis brevis CAUP D 802, N. mucosa KR 1989/14) and from the Prasiola clade (Gloeocystis polydermatica CCAP 31/5, Stichococcus bacillaris SAG 379-1b) are indicated by blue bold type. Nucleotides highlighted in green are common to L. lukesovae CCALA 307 and the Prasiola clade and are not present in Neocystis spp. Nucleotides highlighted in red are common only to L. lukesovae CCALA 307 and Neocystis spp. Dots near to some nucleotide sites mark compensatory base changes (CBCs). Black dots represent CBCs among L. lukesovae and Neocystis spp. Grey and white dots represent CBCs among L. lukesovae and G. polydermatica and S. bacillaris, respectively.
Lunachloris Barcytė & Hodač, gen. nov.
Diagnosis: Vegetative cells solitary, spherical or ellipsoid, uninucleate. Single parietal smooth two-lobed chloroplast lacks pyrenoid. Asexual reproduction via 2–6 autospores which are liberated through rupture of mother cell wall at one side. Size of autospores is the same within single autosporangium. Sexual reproduction or production of zoospores were not observed. Lipid droplets may be present in cytoplasm. The genus differs from other genera in SSU, ITS2 and rbcL sequences.
Type Species: Lunachloris lukesovae Barcytė & Hodač.
Etymology: Genus name comes from a Latin word luna meaning ‘moon’, emphasizing moon-shaped chloroplast and Greek word khloros/χλωρός meaning ‘pale green’.
Lunachloris lukesovae Barcytė & Hodač, sp. nov.
Diagnosis: Vegetative cells are solitary, uninucleate with spherical or ellipsoid outline, (3.0–) 4.3–5.4 (–6.7) µm in diameter and covered by remnants of mother cell wall when cultivated in liquid (BBM) media. Old cells are mostly globular. No flagella were observed. The single chloroplast is smooth, parietal and composed of two lobes. No pyrenoid. The nucleus is central, 2–4 mitochondria in the cytoplasm. Asexual reproduction is via 2–6 autospores. Sexual reproduction and production of zoospores were not observed. Lipid droplets present in older cells. The species differs from other species in SSU, ITS2 and rbcL sequences. DNA sequences available for the type strain: nuclear SSU, ITS1-5.8S-ITS2 rDNA KX620913 and plastid rbcL KX620914.
Holotype: The authentic strain CCALA 307 is permanently cryopreserved at CCALA in the metabolically inactive stage. – show the morphology of the holotype.
Type Locality: Meadow soil by Dlouhá Ves (near Chelčice), Czech Republic.
Etymology: The species name is after Dr Alena Lukešová, a prominent Czech soil biologist, who isolated the strain.
Discussion
Morphological characteristics of the strain investigated in this study fit with the typical description of many coccoid green algae and, therefore, we were not able to reveal its precise taxonomic position based merely on morphological and ultrastructural characters. Among the known algal genera it resembles, for example, Coccomyxa Schmidle (as it was originally assigned) and Choricystis (Skuja) Fott or Neocystis Hindák, all with very similar size and shape of the cells (Komárek & Fott, Citation1983; Ettl & Gärtner, Citation2014) and a parietal chloroplast without a pyrenoid. However the shape of the chloroplast is most similar to Neocystis (Eliáš et al., Citation2013) but no mucilage covering the cells was detected as is typical for this genus. Interestingly, the cells of Lunachloris were always surrounded by the persistent remnants of a mother cell wall as is also known, for example, in Coenochloris Korshikov and some species in Radiococcus Schmidle. This feature was seen not only using a light microscope but also confirmed by TEM microphotographs. However, this character can be induced only while growing the alga in liquid medium as, for example, noticed in Hylodesmus (Eliáš et al., Citation2010). The strain reproduced by autosporulation and we did not detect any zoospores.
The nuclear 18S-ITS1-5.8S-ITS2 rDNA region of Lunachloris was highly divergent compared with other trebouxiophycean taxa, indicating an early split from the nearest neighbours and thus the isolated phylogenetic position (). These findings were in agreement with phylogenetic analysis of the chloroplast rbcL gene (). The alga was placed on a solitary branch as a sister group to Neocystis and Prasiola (Karsten et al., Citation2005; Krienitz et al., Citation2011) clades and the distinct, lineage-forming Coenocystis inconstans (Hanagata & Chihara, Citation1999), belonging to core trebouxiophyceans (Lemieux et al., Citation2014). Given that the affiliation to the above-mentioned clades and species was not statistically supported in any analyses, Lunachloris might represent a novel lineage most closely related to the Neocystis clade and Coenocystis inconstans, known from tree bark (Hanagata & Chihara, Citation1999). In contrast to Lunachloris, both genera exhibit a mucilaginous layer covering the cells. In addition, C. inconstans also has a pyrenoid. Therefore, these three phylogenetically closely related genera could be discriminated by their appearance, although not by very conspicuous characteristics, especially in the case of Lunachloris and Neocystis. Moreover, they are an example of how vegetative morphology can develop relatively quickly in different directions within close relatives. On the other hand, the features seen in cultures, e.g. mucilage production, could be induced by cultivation conditions, as is known, for example, in Coccomyxa (Darienko et al., Citation2015). Therefore, the morphology of many coccoid autosporic algae should be interpreted with caution.
Trebouxiophytes are extremely diverse ecologically (Leliaert et al., Citation2012), however, the majority of them are reported from various terrestrial or aero-terrestrial habitats, including soil, tree bark, wet rocks or artificial hard substrates (Hallmann et al., Citation2013 and references therein). For example, Chloroidium belonging to the Watanabea clade, Myrmecia and Trebouxia in the Trebouxiales, Coccomyxa and Elliptochloris in the Elliptochloris clade, Stichococcus, Diplosphaera and Pseudochlorella from the Prasiola clade, and Dictyochloropsis are all well-known from both aerophytic substrata and soil (Darienko et al., Citation2010, Citation2015, Citation2016; Hallmann, Citation2015; Hodač et al., Citation2016; Malavasi et al., Citation2016; Škaloud et al., Citation2016). The majority of Neocystis strains, which are the closest relatives to Lunachloris, were isolated from soil (Eliáš et al., Citation2013) while Coenocystis inconstans is an aerial alga (Hanagata & Chihara, Citation1999). Lunachloris was isolated from a wet meadow where water is usually at, or near, the surface of the soil throughout most of the year. However, considering the ecology of its closest relatives, we speculate that Lunachloris might be a true terrestrial/soil alga rather than an aquatic species occurring in soil water.
Generally, minute soil algae tend to disperse over long distances and, therefore, are expected to be common (Hodač et al., Citation2016). However, Lunachloris has not been detected before, even by culture-independent environmental sequencing. Multiple microalgae have been re-detected by both culture-dependent and culture-independent approaches. However, Lunachloris lukesovae might represent an example of a rare or rarely accessible species. On the other hand, the total biodiversity of soil microalgae is still poorly understood and requires extended sampling for diversity evaluation (Hodač et al., Citation2016). Since the recently described genus Chloropyrula (isolated from soil in the Ural Mountains and represented by a single isolate; Gaysina et al., Citation2013) has since been detected again in a soil crust in the Californian desert (Fučíková et al., Citation2014), Lunachloris also could be found elsewhere in the future. Due to the common problem of morphological misidentification, we cannot exclude the possibility that Lunachloris has been isolated and preserved elsewhere. Consequently, the molecular reassessment of historical strains might reveal rare or as yet undiscovered species (Hoshina, Citation2014).
For many years, the discovery of new algal species has relied on the isolation and cultivation of unialgal strains. Many of these are still accessible thanks to various microbial culture collections whose fundamental aim is to collect, preserve and make strains available to the public. Moreover, culture collections serve as biodiversity stores, that are especially important in light of recent, rapid biodiversity loss (Pimm & Raven, Citation2000; Cary & Fierer, Citation2014). Traditional isolation methods discriminate against very small, rare or difficult-to-culture algal species, however, many culture-independent methods, i.e. DNA metabarcoding and metagenomics, have recently been developed to study microbial communities directly in their environment and without doubt generally show a much more quantitatively complete view of the existing biodiversity. Nevertheless, these novel, advanced methods are not ideal and also have limitations. For example, next-generation sequencing (NGS) technologies generally produce shorter sequences (with higher error rates) in comparison to traditional genomic sequencing, leading to lower taxonomic resolution. Consequently, in this paper we have demonstrated that isolation and cultivation of strains could still provide a valuable complementary approach for biodiversity studies even in the era of culture-independent biodiversity research.
Supplementary Information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org.10.1080/09670262.2017.1283541
Supplementary table S1. List of primers included in the study.
Table_S1.xlsx
Download MS Excel (12.7 KB)Acknowledgements
We thank Dr Alena Lukešová for providing us with the information about the origin of the strain.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Dovilė Barcytė
D. Barcytė: original concept, molecular lab work, microscopy, drafting and editing of manuscript; L. Hodač: phylogenetic analyses, drafting and editing of manuscript; L. Nedbalová: financial support and writting of manuscript.
Ladislav Hodač
D. Barcytė: original concept, molecular lab work, microscopy, drafting and editing of manuscript; L. Hodač: phylogenetic analyses, drafting and editing of manuscript; L. Nedbalová: financial support and writting of manuscript.
Linda Nedbalová
D. Barcytė: original concept, molecular lab work, microscopy, drafting and editing of manuscript; L. Hodač: phylogenetic analyses, drafting and editing of manuscript; L. Nedbalová: financial support and writting of manuscript.
References
- Altschul, S.F., Madden, T.L., Schäffer, A.A., Zhang, J., Zhang, Z., Miller, W. & Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research, 25: 3389–3402.
- Bischoff, H.W. & Bold, H.C. (1963). Phycological studies IV. Some soil algae from Enchanted Rock and related algal species. University of Texas Publication, 6318: 1–95.
- Bock, C., Pröschold, T. & Krienitz, L. (2010). Two new phylogenetic lineages of Dictyosphaerium-morphotype within Chlorellaceae (Trebouxiophyceae): Heynigia gen. nov. and Hindakia gen. nov. European Journal of Phycology, 45: 267–277.
- Bock, C., Krienitz, L. & Pröschold, T. (2011). Taxonomic reassessment of the genus Chlorella (Trebouxiophyceae) using molecular signatures (barcodes), including description of seven new species. Fottea, 11: 293–312.
- Cary, S.C. & Fierer, N. (2014). The importance of sample archiving in microbial ecology. Nature Reviews Microbiology, 12: 789–790.
- Darienko, T. & Pröschold, T. (2015). Genetic variability and taxonomic revision of the genus Auxenochlorella (Shihira et Krauss) Kalina et Puncocharova (Trebouxiophyceae, Chlorophyta). Journal of Phycology, 51: 394–400.
- Darienko, T., Gustavs, L., Mudimu, O., Rad Menendez, C., Schumann, R., Karsten, U., Friedl, T. & Pröschold, T. (2010). Chloroidium, a common terrestrial coccoid green alga previously assigned to Chlorella (Trebouxiophyceae, Chlorophyta). European Journal of Phycology, 45: 79–95.
- Darienko, T., Gustavs, L., Eggert, A., Wolf, W. & Pröschold, T. (2015). Evaluating the species boundaries of green microalgae (Coccomyxa, Trebouxiophyceae, Chlorophyta) using integrative taxonomy and DNA barcoding with further implications for the species identification in environmental samples. PLoS ONE, 10: e0127838.
- Darienko, T., Gustavs, L. & Pröschold, T. (2016). Species concept and nomenclatural changes within the genera Elliptochloris and Pseudochlorella (Trebouxiophyceae) based on an integrative approach. Journal of Phycology, 52: 1125–1145.
- Darty, K., Denise, A. & Ponty, Y. (2009). VARNA: Interactive drawing and editing of the RNA secondary structure. Bioinformatics, 25: 1974–1975.
- Eliáš, M. & Neustupa, J. (2009). Pseudomarvania, gen. nov. (Chlorophyta, Trebouxiophyceae), a new genus for “budding” subaerial green algae Marvania aerophytica Neustupa et Šejnohová and Stichococcus ampulliformis Handa. Fottea, 9: 169–177.
- Eliáš, M., Němcová, Y., Škaloud, P., Neustupa, J., Kaufnerová, V. & Šejnohová, L. (2010). Hylodesmus singaporensis gen. et sp. nov., a new autosporic subaerial green alga (Scenedesmaceae, Chlorophyta) from Singapore. International Journal of Systematic and Evolutionary Microbiology, 60: 1224–1235.
- Eliáš, M., Neustupa, J., Pažoutová, M. & Škaloud, P. (2013). A case of taxonomic inflation in coccoid algae: Ellipsoidion parvum and Neocystis vischeri are conspecific with Neocystis (=Nephrodiella) brevis (Chlorophyta, Trebouxiophyceae). Phytotaxa, 76: 15–27.
- Ettl, H. & Gärtner, G. (2014). Syllabus der Boden-, Luft- und Flechtenalgen. 2 Auflage. Springer Spektrum, Berlin.
- Fučíková, K., Lewis, P.O. & Lewis, L.A. (2014). Widespread desert affiliation of trebouxiophycean algae (Trebouxiophyceae, Chlorophyta) including discovery of three new desert genera. Phycological Research, 62: 294–305.
- Gaysina, L., Němcová, Y., Škaloud, P., Ševčíková, T. & Eliaš, M. (2013). Chloropyrula uraliensis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta), a new green coccoid alga with a unique ultrastructure, isolated from soil in South Urals. Journal of Systematics and Evolution, 51: 476–484.
- Hall, T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41: 95–98. Oxford University Press, Oxford.
- Hallmann, C. (2015). Biodiversity of terrestrial algal communities from soil and air-exposed substrates using a molecular approach. PhD thesis, Georg August University of Göttingen.
- Hallmann, C., Stannek, L., Fritzlar, D., Hause-Reitner, D., Friedl, T. & Hoppert, M. (2013). Molecular diversity of phototrophic biofilms on building stone. FEMS Microbiology Ecology, 84: 355–372.
- Hanagata, N. & Chihara, M. (1999). Coenocystis inconstans, a new species of bark-inhabiting green algae (Chlorophyceae, Chlorophyta). Journal of Japanese Botany, 74: 204–211.
- Helms, G., Friedl, T., Rambold, G. & Mayrhofer, H. (2001). Identification of photobionts from the lichen family Physciaceae using algal-specific ITS rDNA sequencing. The Lichenologist, 33: 73–86.
- Hodač, L. (2016). Green algae in soil: assessing their biodiversity and biogeography with molecular-phylogenetic methods based on cultures. PhD thesis, Georg August University of Göttingen. In, 188.
- Hodač, L., Hallmann, C., Spitzer, K., Elster, J., Faßhauer, F., Brinkmann, N., Lepka, D., Diwan, V. & Friedl, T. (2016). Widespread green algae Chlorella and Stichococcus exhibit polar-temperate and tropical-temperate biogeography. FEMS Microbiology Ecology, 92: fiw122.
- Hoham, R.W., Bonome, T.A., Martin, C.W. & Leebens-Mack, J.H. (2002). A combined 18S rDNA and RbCl phylogenetic analysis of Chloromonas and Chlamydomonas (Chlorophyceae, Volvocales) emphasizing snow and other cold-temperature habitats. Journal of Phycology, 38: 1051–1064.
- Hoshina, R. (2014). DNA analyses of a private collection of microbial green algae contribute to a better understanding of microbial diversity. BMC Research Notes, 7: 592.
- Huber, T., Faulkner, G. & Hugenholtz, P. (2004). Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics, 20: 2317–2319.
- Karsten, U., Friedl, T., Schumann, R., Hoyer, K. & Lembcke, S. (2005). Mycosporine-like amino acids and phylogenies in green algae: Prasiola and its relatives from the Trebouxiophyceae (Chlorophyta). Journal of Phycology, 41: 557‒566.
- Katoh, K. & Toh, H. (2008). Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics, 9: 286–298.
- Keller, A., Schleicher, T., Schultz, J., Müller, T., Dandekar, T. & Wolf, M. (2009). 5.8S-28S rRNA interaction and HMM-based ITS2 annotation. Gene, 430: 50–57.
- Koetschan, C., Förster, F., Keller, A., Schleicher, T., Ruderisch, B., Schwarz, R., Müller, T., Wolf, M. & Schultz, J. (2010). The ITS2 Database III – sequences and structures for phylogeny. Nucleic Acids Research, 38: 275–279.
- Koetschan, C., Hackl, T., Müller, T., Wolf, M., Förster, F. & Schultz, J. (2012). ITS2 database IV: interactive taxon sampling for internal transcribed spacer 2 based phylogenies. Molecular Biology and Evolution, 63: 585–588.
- Komárek, J. & Fott, B. (1983). Chlorophyceae (Grünalgen), Ordnung: Chlorococcales. In Das Phytoplankton des Susswasers; Systematik und Biologie (Huber-Pestalozzi, G., editor). E. Schweizerbart’sche Verlagsbuchhhandlung, Stuttgart.
- Krienitz, L., Bock, C., Nozaki, H. & Wolf, M. (2011). SSU rRNA gene phylogeny of morphospecies affiliated to the bioassay alga “Selenastrum capricornutum” recoverd the polyphyletic origin of crescent-shaped Chlorophyta. Journal of Phycology, 47: 880‒893.
- Leliaert, F., Smith, D.R., Moreau, H., Herron, M.D., Verbruggen, H., Delwiche, C.F. & De Clerck, O. (2012). Phylogeny and molecular evolution of the green algae. Critical Reviews in Plant Sciences, 31: 1–46.
- Lemieux, C., Otis, C. & Turmel, M. (2014). Chloroplast phylogenomic analysis resolves deep-level relationships within the green algal class Trebouxiophyceae. BMC Evolutionary Biology, 14: 211.
- Lukešová, A. (1993). Soil algae in four secondary successional stages on abandoned fields. Algological Studies, 71: 81–102.
- Malavasi, V., Škaloud, P., Rindi, F., Tempesta, S., Paoletti, M. & Pasqualetti, M. (2016). DNA-based taxonomy in ecologically versatile microalgae: a re-evaluation of the species concept within the coccoid green algal genus Coccomyxa (Trebouxiophyceae, Chlorophyta). PLoS ONE, 11: e0151137.
- Mikhailyuk, T.I., Sluiman, H.J., Massalski, A., Mudimu, O., Demchenko, E.M., Kondratyuk, S.Y. & Friedl, T. (2008). New streptophyte green algae from terrestrial habitats and an assessment of the genus Interfilum (Klebsormidiophyceae, Streptophyta). Journal of Phycology, 44: 1586–1603.
- Němcová, Y., Eliaš, M., Škaloud, P., Hodač, L. & Neustupa, J. (2011). Jenufa gen. nov.: a new genus of coccoid green algae (Chlorophyceae, incertae sedis) previously recorded by environmental sequencing. Journal of Phycology, 47: 928–938.
- Neustupa, J., Němcová, Y., Eliaš, M. & Škaloud, P. (2009). Kalinella bambusicola gen. et sp. nov. (Treboux-iophyceae, Chlorophyta), a novel coccoid Chlorella-like subaerial alga from Southeast Asia. Phycological Research, 57: 159–169.
- Neustupa, J., Eliaš, M., Škaloud, P., Němcová, Y. & Šejnohová, L. (2011). Xylochloris irregularis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta), a novel subaerial coccoid green alga. Phycologia, 50: 57–66.
- Neustupa, J., Němcová, Y., Veselá, J., Steinová, J. & Škaloud, P. (2013a). Leptochlorella corticola gen. et sp. nov. and Kalinella apyrenoidosa sp. nov.: two novel Chlorella-like green microalgae (Trebouxiophyceae, Chlorophyta) from subaerial habitats. International Journal of Systematic and Evolutionary Microbiology, 63: 377–387.
- Neustupa, J., Němcová, Y., Veselá, J., Steinová, J. & Škaloud, P. (2013b). Parachloroidium gen. nov. (Trebouxiophyceae, Chlorophyta), a novel genus of coccoid green algae from subaerial corticolous biofilms. Phycologia, 52: 411–421.
- Pažoutová, M., Škaloud, P. & Nemjová, K. (2010). Phylogenetic position of Ooplanctella planoconvexa, gen. et comb. nova and Echinocoleum elegans (Oocystaceae, Trebouxiophyceae, Chlorophyta). Fottea, 10: 75–82.
- Piercey-Normore, M.D. & Depriest, P.T. (2001). Algal switching among lichen symbioses. American Journal of Botany, 88: 1490–1498.
- Pimm, S.L. & Raven, P. (2000). Biodiversity: extinction by numbers. Nature, 403: 843–845.
- Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25: 1253–1256.
- Procházková, K., Němcová, Y. & Neustupa, J. (2015). A new species Jenufa aeroterrestrica (Chlorophyceae incertae sedis, Viridiplantae), described from Europe. Preslia, 87: 403–416.
- Rambaut, A. (2007). FigTree, a graphical viewer of phylogenetic trees. In http://tree.bio.ed.ac.uk/software/figtree.
- Remias, D., Schwaiger, S., Aigner, S., Leya, T., Stuppner, H. & Lütz, C. (2012). Characterization of an UV- and VIS-absorbing, purpurogallin-derived secondary pigment new to algae and highly abundant in Mesotaenium berggrenii (Zygnematophyceae, Chlorophyta), an extremophyte living on glaciers. FEMS Microbiology Ecology, 79: 638–648.
- Reuter, J.S. & Mathews, D.H. (2010). RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinformatics, 11: 129.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Schneider, C.A., Rasband, W.S. & Eliceiri, K.W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9: 671–675.
- Schultz, J., Müller, T., Achtziger, M., Seibel, P.N., Dandekar, T. & Wolf, M. (2006). The internal transcribed spacer 2 database – a web server for (not only) low level phylogenetic analyses. Nucleic Acids Research, 34: 704–707.
- Seibel, P.N., Müller, T., Dandekar, T., Schultz, J. & Wolf, M. (2006). 4SALE – a tool for synchronous RNA sequence and secondary structure alignment and editing. BMC Bioinformatics, 7: 498.
- Seibel, P.N., Müller, T., Dandekar, T. & Wolf, M. (2008). Synchronous visual analysis and editing of RNA sequence and secondary structure alignments using 4SALE. BMC Research Notes, 1: 91.
- Selig, C., Wolf, M., Müller, T., Dandekar, T. & Schultz, J. (2008). The ITS2 Database II: homology modelling RNA structure for molecular systematics. Nucleic Acids Research, 36: 377–380.
- Škaloud, P., Němcová, Y., Pytela, J., Bogdanov, N.I., Bock, C. & Pickinpaugh, S.H. (2014). Planktochlorella nurekis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta), a novel coccoid green alga carrying significant biotechnological potential. Fottea, 14: 53–62.
- Škaloud, P., Friedl, T., Hallmann, C., Beck, A. & Dal Grande, F. (2016). Taxonomic revision and species delimitation of coccoid green algae currently assigned to the genus Dictyochloropsis (Trebouxiophyceae, Chloro-phyta). Journal of Phycology, 52: 599–617.
- Song, H.Y., Zhang, Q., Liu, G.X. & Hu, Z.Y. (2015). Polulichloris henanensis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta), a novel subaerial coccoid green alga. Phytotaxa, 218: 137–146.
- Stamatakis, A., Hoover, P. & Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology, 57: 758–771.
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30: 2725–2729.
- Thüs, H., Muggia, L., Pérez-Ortega, S., Favero-Longo, S.E., Joneson, S., O’Brien, H., Nelsen, M.P., Duque-Thüs, R., Grube, M., Friedl, T., Brodie, J., Andrew, C.J., Lücking, R., Lutzoni, F. & Gueidan, C. (2011). Revisiting photobiont diversity in the lichen family Verrucariaceae (Ascomycota). European Journal of Phycology, 46: 399–415.
- Vilgalys, R. & Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology, 172: 4238–4246.
- White, T.J., Bruns, T., Lee, S. & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide to Methods and Applications, 18: 315–322.
- Wolf, M., Chen, S., Song, J., Ankenbrand, M. & Müller, T. (2013). Compensatory base changes in ITS2 secondary structures correlate with the biological species concept despite intragenomic variability in ITS2 sequences – a proof of concept. PLoS ONE, 8: e66726.
- Zhang, J., Huss, V.A.R., Sun, X., Chang, K. & Pang, D. (2008). Morphology and phylogenetic position of a trebouxiophycean green alga (Chlorophyta) growing on the rubber tree, Hevea brasiliensis, with the description of a new genus and species. European Journal of Phycology, 43: 185–193.
