Abstract
A previously unknown member of the Bacillariaceae was discovered almost simultaneously in four different brackish coastal wetlands on the Atlantic and Mediterranean coasts of the Iberian Peninsula. It appears to tolerate a wide range of salinities but was never common in samples where it occurred. The frustules were consistently hantzschioid (i.e. with the raphe systems always on the same side of the frustule) and the valve outline was asymmetrical about the apical plane, two features that have until recently been considered characteristic of Hantzschia. Molecular phylogenies based on rbcL and LSU rDNA indicated, however, that the new species does not belong in Hantzschia but among the several disparate lineages that comprise the paraphyletic genus Nitzschia. This finding, coupled with the recent discovery of other diatoms with constant hantzschioid symmetry but with a morphology very similar to the type species of Nitzschia, is discussed in relation to the status and characterization of Hantzschia as an independent genus. It is concluded that, while a core of hantzschioid species may exist that can be classified together, corresponding to the traditional understanding of the genus Hantzschia, there is no single morphological feature common to all of them that can be used to diagnose the group and differentiate it from the various hantzschioid lineages that are separate from true Hantzschia and currently placed in e.g. Nitzschia or Cymbellonitzschia. Testing whether a hantzschioid species does or does not belong to Hantzschia will in many cases require molecular evidence. Although the new coastal species does not belong to the same lineage as the type species of Nitzschia, N. sigmoidea, it is described for the moment as N. varelae Carballeira, D.G. Mann & Trobajo, sp. nov., until there is a better understanding of generic limits in the Bacillariaceae following a wider molecular and morphological survey of that family.
Introduction
Frustule symmetry has long been recognized as a relevant taxonomic character in the family Bacillariaceae (Hustedt, Citation1930; Round et al., Citation1990), and has been considered especially important for separating the genera Nitzschia and Hantzschia. Grunow (Citation1877) established Hantzschia, separating it from Nitzschia on the basis that the raphes of the two valves lie on the same side of the frustule in Hantzschia but on opposite sides in Nitzschia. These two distinctive symmetries are usually referred to as ‘hantzschioid’ and ‘nitzschioid’ respectively (Mann, Citation1980a). The production of hantzschioid and nitzschioid symmetries has been shown in some cases to reflect differences in the positioning of the nucleus, microtubule centre and silicon deposition vesicle during cell division (Pickett-Heaps & Kowalski, Citation1981; Pickett-Heaps, Citation1983; Pickett-Heaps et al., Citation1990). For about a century this distinction between the two genera using frustule symmetry was generally accepted (Hustedt, Citation1930; Cleve-Euler, Citation1952; Hendey, Citation1964) but after the discovery that some Nitzschia species can produce both symmetries (e.g. Lauritis et al., Citation1967; Geitler, Citation1968), the basis for separating Hantzschia and Nitzschia had to be reconsidered. The first suggestion was to use not frustule symmetry but instead the types of cell division present (Mann, Citation1980a; Pickett-Heaps, Citation1983), because in Nitzschia, hantzschioid cells were thought to produce one nitzschioid and one hantzschioid daughter cell at each cell division, whereas the cells of Hantzschia species (and most species of Cymbellonitzschia) always and exclusively produce hantzschioid cells (Mann, Citation1980a; Mann & Trobajo, Citation2014). However, recent work has shown that three exclusively hantzschioid species are very similar to the type species of Nitzschia (N. sigmoidea) in valve ultrastructure, chloroplast morphology and auxosporulation (Mann & Trobajo, Citation2014), but differ from Hantzschia in these characteristics, suggesting that cell division patterns are not in themselves a satisfactory basis for separating Hantzschia and Nitzschia.
Recently, we have simultaneously discovered an undescribed hantzschioid diatom in four coastal lagoons along the Atlantic and Mediterranean coasts of Spain. Our aims here are: (i) to describe this species; (ii) to use sequences from two genes (rbcL and LSU rDNA) to test the phylogenetic relationship between it and the other groups with consistently hantzschioid symmetry known so far in the Bacillariaceae; and (iii) to review the features that have been suggested previously to characterize Hantzschia species.
Materials and methods
Study sites and sampling
The new species was found at four sites in the Iberian Peninsula: the Valdoviño (or Frouxeira) and Muro lagoons, both on the Atlantic coast of Galicia (NW Spain); the Clot lagoon in the Ebro Delta, on the Mediterranean coast of Catalonia (NE Spain); and a salt marsh near Alfacada lagoon, about 12 km from Clot lagoon ().
Fig. 1. Locations of the four coastal lagoons (Valdoviño, Muro, Alfacada and Clot) where Nitzschia varelae was found. (A) Positions of the four coastal lagoons in the Iberian Peninsula: Valdoviño and Muro lagoons on the Atlantic coast, and the Ebro Delta with Alfacada and Clot lagoons on the Mediterranean coast. (B) Map detail of the Ebro Delta and locations of the Alfacada and El Clot lagoons. (C–F) Orthophotographs of the lagoons showing locations of sampling points (open circles). (C) Valdoviño lagoon. (D) Muro lagoon. (E) Clot lagoon. (F) Alfacada lagoon.
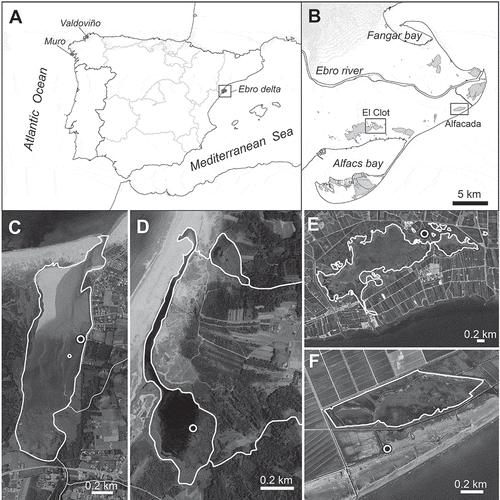
Valdoviño lagoon is located in the town of Valdoviño (A Coruña, Galicia). It is a mesohaline–polyhaline shallow coastal lagoon with 115.5 ha of area and a maximum depth of 2 m, although there are major water level fluctuations depending on the opening of the sand barrier, seasonal rainfall and freshwater input through two tributary streams. The shallow depth allows the development of brackish macrophytic vegetation across the lake’s surface in summer. The vegetation is composed mainly of the hydrophytes Ruppia maritima and Potamogeton pectinatus, with a minor presence of charophytes (Chara, Tolypella), and dominance along the shore line by the helophyte Phragmites australis in the inner part. The basin of the lagoon is dominated by schistous metamorphic rocks, with land use comprising a mosaic of suburban areas and sets of small agricultural parcels. A general description of the lagoon is given by Dalda-González (Citation1968). The site sampled (UTM coordinates 29T X 568153, Y 4828484; Datum WGS84) was in a muddy sediment zone near the middle of one margin of the lagoon shore.
Muro lagoon, near Porto do Son (A Coruña, Galicia), is an oligohaline–mesohaline shallow coastal lagoon of 27 ha and a maximum depth of 1.75 m. The lagoon is fed by a small freshwater stream and experiences a severe drop in water levels in summer, or even dries out completely. The lagoon has seasonal contact with the sea through a long channel parallel to the coast and the sea water intrusion determines salinity fluctuations throughout the year. The main macrophytes are R. maritima and P. pectinatus. The site sampled (29T X 496685, Y 4719284; Datum WGS84) was in a zone of muddy sediment near the centre of the lagoon. The basin of the lagoon is dominated by schistous metamorphic rocks, with major wooded and shrubby areas and little human activity (limited to small parcels of agricultural land).
The samples in Valdoviño and Muro lagoons were collected seasonally from different habitats (phytoplankton, epiphyton, periphyton and sediment) during 2007, and in autumn 2012 and spring 2013.
El Clot lagoon is situated in the Ebro Delta (Catalonia). It is a small (56 ha) shallow lagoon, with a mean depth of about 53 cm (maximum depth around 90 cm). The hydrology of the lagoon is dominated by the main agricultural activity of the Delta (rice cultivation). The lagoon receives seawater via a narrow channel from the neighbouring Encanyissada lagoon (directly connected to Alfacs Bay, ) but its hydrology and its salinity in particular are greatly affected by freshwater inputs from the Ebro River through irrigation channels, especially during the rice cultivation season (May–September); the lagoon salinity varies between about 3 in summer and 13 psu in winter (Prado et al., Citation2013). The pH is alkaline (c. 7.82–7.93, Rodríguez-Climent et al., Citation2013; Benito et al., Citation2015). The shallowness of the lagoon and its oligo-mesohaline waters favour the development of P. pectinatus, which is abundant in the lagoon throughout the year (Prado et al., Citation2013). The basin of the lagoon is formed in deltaic sediments (mainly mud and sand in combination with organic matter). The site sampled (UTM coordinates 31T X 304120, Y 4503127; Datum WGS84), was very close to the principal freshwater inflow and the salinity at the time of sampling (16 March 2011) was 4.88 psu. The sample comprised periphyton on submerged plants.
The site near Alfacada (or Aufacada) lagoon where the new species was found is a Salicornia marsh between the lagoon and Alfacs Bay (Mediterranean Sea) (). The marsh lies more or less at sea level – it is almost permanently flooded with saline water (to an average depth of c. 10 cm), has high soil salinity, and is subject to periodic sea storm events. The site UTM coordinates are 31T X 316668, Y 4505010 (Datum WGS84). The surface sediment was sampled three times for diatom analysis, in November 2012, and April and August 2013 (see Benito et al., Citation2015 for further sampling details), though the new species was found only in the November sample and in low abundance.
Chemical analyses of water samples
Valdoviño and Muro lagoons were sampled on eight occasions between 2007 and 2013 (February, March, July and November 2007, September–October 2012 and May–June 2013). Temperature, conductivity, salinity, pH and dissolved oxygen saturation were measured in situ with a multiparameter handheld meter Multi 350i (WTW, Germany). Water samples (1 litre) were collected for nutrient analyses. Dissolved organic carbon (DOC) was measured with a Shimadzu TOC 5000A Total Organic Carbon Analyzer. Inorganic dissolved nutrients in water (NO3–, NO2–, NH4+, PO43–, SiO44–) were analysed from filtered waters using an Integral Futura autoanalyser system (Alliance Instruments), which uses separate analytical lines to determine NO3–, NO2–, PO43–, NH4+, and SiO44–, according to standard colorimetric methods (Hansen & Koroleff, Citation1999) (see Ferreira, Citation2013, for further analytical details). All analyses were performed at the Research Support Services of the University of A Coruña (Servizos de Apoio á Investigación, SAI).
The physical and chemical variables measured at the Salicornia marsh site in the Ebro Delta were: temperature, pH, dissolved oxygen saturation, conductivity, salinity, sediment sand and organic matter content, and inorganic dissolved nutrient content in the water; see Benito et al. (Citation2015) for details of the analyses.
Unfortunately no chemical analyses of the water from Clot lagoon are available for 16 March 2011, when we collected the sample from which Nit952CAT was isolated (see below). However, the site was the same as site 9 of Benito et al. (Citation2015, Supplementary table S2), for which physical and chemical data (i.e. pH, dissolved oxygen saturation, conductivity, sediment sand and organic matter content, and inorganic dissolved nutrient content in water) are reproduced here in . However, Benito did not find the new species in his site 9 sample (the only Clot lagoon sample he studied, collected in November 2012).
Table 1. Environmental data for the three sites where Nitzschia varelae was found: Valdoviño lagoon (Valdoviño, Galicia), Muro lagoon (Porto do Son, Galicia), Salicornia marsh of Alfacada lagoon (Ebro Delta, Catalonia) and Clot lagoon (Ebro Delta, Catalonia).
Clone isolation and cell culture
An aliquot of the Clot lagoon sample was spread over the surface of a 2% agar plate (made with 35 psu seawater) and a monoclonal culture of the new species (Nit952CAT) was established by isolating a single cell from the agar surface by micropipette. The culture was maintained in a 1:1 mixture of WC (Guillard & Lorenzen, Citation1972) and R (Chepurnov & Mann, Citation1997) medium.
Diatom preparation, microscopy and morphological characterization
The diatom samples from Valdoviño, Muro and from the Alfacada in Ebro Delta were treated with a mixture of H2O2 (30%) and HCl (35%) in a water bath at 60°C to remove organic matter, followed by successive centrifugations at 900 × g for 4 min and washes with distilled water to dilute out the remaining acid. Cultured material (from Clot lagoon) was cleaned by boiling in 70% HNO3 and rinsed several times with distilled water by repeated sedimentation (for at least 6 h) and decanting.
For light microscopy (LM), aliquots were placed on clean coverslips and allowed to air dry. For the preparation of permanent slides, coverslips were mounted on glass slides using Naphrax resin (refractive index= 1.74; Brunel Microscopes: http://www.brunelmicroscopes.co.uk/). A voucher slide of culture Nit952CAT was incorporated into the diatom herbarium at the Royal Botanic Garden Edinburgh (RBGE), as accession E5469 and stored DNA in the RBGE DNA bank as EDNA11-0022523. Voucher slides and DNA vouchers for the other new sequences used in this paper are listed in Supplementary table S1.
Diatoms were examined with either a Nikon Eclipse E600 equipped with a Plan-Apochromat 100× objective (N.A. 1.32), or a Zeiss Axio Imager M2 with a Plan-Apochromat 100× objective (N.A. 1.4), in both cases with differential interference contrast (Nomarski) optics. LM photographs were taken either with an AxioCam ICc5 Zeiss digital camera (Nikon E600) or an AxioCam HRc (Zeiss M2).
Samples for scanning electron microscopy (SEM) were either prepared as described by Mann & Trobajo (Citation2014) and examined with a Leo Supra Field Emission SEM, or mounted on aluminium stubs, sputtered with gold (40 nm) for 3 min with a Bio Rad Microscience Division SEM coating system and studied with a Zeiss Ultra Plus Field Emission SEM, operated at 5 kV.
Morphological data for the clones and taxa included in the molecular phylogenetic study were sourced either from our own observations or from the literature, as described in Supplementary tables S4–S6.
DNA sequencing and phylogenetic analysis
New rbcL and LSU sequences were obtained from clone Nit952CAT and from several Nitzschia species selected to provide phylogenetic context (Supplementary table S1). DNA was extracted during exponential growth phase using either a DNeasy Plant Kit (Qiagen, Crawley, UK) or a high-throughput genomic DNA extractor (QIAxtractor, Qiagen). RbcL and partial LSU were used for phylogenetic analyses because of their proven value in Bacillariaceae (Trobajo et al., Citation2006, Citation2009, Citation2010; Amato et al., Citation2007) and other diatoms (e.g. Sarno et al., Citation2005; Mann & Evans, Citation2007; Theriot et al., Citation2010). Regions within the LSU and rbcL genes are also currently recommended for use as diatom barcodes (Mann et al., Citation2010; Hamsher et al., Citation2011) and hence the sequences provided here may be useful for identification of N. varelae in metabarcoding analyses of environmental samples.
RbcL (~1400 bp) was amplified using the DPrbcL1 and DPrbcL7 primers (Daugbjerg & Andersen, Citation1997) as modified by Jones et al. (Citation2005), viz. 5′–AAGGAGAAATHAATGTCT–3′ and 5′–AARCAACCTTGTGTAAGTCTC–3′. Amplification was carried out in 25 μl volumes containing 10 ng DNA, 1 mM dNTPs, 1× Roche diagnostics PCR reaction buffer (Roche Diagnostics GmbH, Mannheim, Germany), 1 unit Taq DNA polymerase (Roche), and 0.5 μM 94°C of each primer. PCR cycling comprised an initial denaturing phase for 3 min (94°C), followed by 30–40 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 1.5 min, with a final extension of 72°C for 5 min.
Partial LSU rDNA (including the D1–D3 hypervariable domains, ~800 bp) was amplified with primers D1R (Scholin et al., Citation1994) and D3R (Nunn et al., Citation1996). Reaction volumes were 25 μl and contained 1 μl of DNA template, 2.5 μl of 10× NH4 buffer, 2.5 μl of 50 mM MgCl2, 2.5 μl of 2 mM dNTPs, 0.75 μl of 10 μM forward and reverse primers, and 0.125 μl of BIOTAQ Taq polymerase (Bioline, London, UK). PCR involved an initial denaturing at 94°C for 4 min, followed by 35 cycles of 1 min at 94°C, 40 s at 56°C and 1 min at 72°C, and a final extension of 7 min at 72°C. PCR products were purified with ExoSAP-IT (GE Healthcare Life Sciences, Little Chalfont, UK) and inspected by agarose gel electrophoresis against known standards.
The sequencing primers used for rbcL were the amplification primers, together with NDrbcL5 and NDrbcL11 (Daugbjerg & Andersen, Citation1997); for LSU, we used the amplification primers and, in some cases, also the D2C primer (Scholin et al., Citation1994). The sequencing reactions were conducted with Big Dye terminator v. 3.1 sequencing chemistry (Applied Biosystems, Foster City, USA); reaction volumes were 10 μl and contained 1 μl of DNA template, 2 μl of 5× sequencing buffer, 0.32 μl of 10 μM sequencing primers, and 0.5 μl of BigDye terminator. Excess dye-labelled nucleotides were removed using the Performa DTR V3 clean-up system (EdgeBio, Gaithersburg, USA). Samples were sent to Edinburgh Genomics, University of Edinburgh for capillary analysis using an ABI 3730XL sequencer (Applied Biosystems). Forward and reverse reads were assembled using Sequencher 4.8 (Gene Codes Corporation, Ann Arbor, USA) or SeqMan (DNASTAR, Madison, Wisconsin, USA) and aligned together with all publicly available rbcL and LSU sequences judged to be of suitable length and coverage (> 1000 bp for rbcL, although finally no sequence shorter than 1190 bp was included; > 500 bp for LSU, with rare exceptions to increase taxon sampling) (Supplementary tables S2, S3). In the case of rbcL, two close relatives of the Bacillariaceae have been identified in a study of amphoroid diatoms by Stepanek & Kociolek (Citation2014). Amphora laevissima and A. obtusa var. crassa are not closely related to the type species of Amphora, A. ovalis. They were added to the Bacillariaceae matrix as an outgroup. However, no LSU sequences are available for these two species. The Eunotiophycidae (sensu Round et al., Citation1990) were also added as an outgroup for both LSU and rbcL: analyses indicate that they are sister to all other raphid diatoms (the Bacillariophycidae sensu Round et al., Citation1990) and that the Bacillariaceae branch off close to the base of the Bacillariophycidae (e.g. Sims et al., Citation2006; Theriot et al., Citation2010).
Alignment of rbcL using MUSCLE implemented in MEGA6 (Tamura et al., Citation2013) was straightforward; nine nucleotides in the rbcL sequence of Nitzschia palea clone Japan B1 (GenBank FN557019), corresponding to an insertion of three amino acids, were excluded from analysis. In the case of LSU, alignment was first performed using MUSCLE but then refined by eye, taking into account the secondary structure of the D1–D3 region of LSU rRNA as determined for the bryophyte Funaria hygrometrica (GenBank accession X74114: Capesius & Van de Peer, Citation1997) and the diatom Navicula cryptocephala (GenBank FN397590: Poulíčková et al., Citation2010), and also the results of LOCARNA analyses (Smith et al., Citation2010) of LSU rDNA for selected species from across the Bacillariaceae. The resulting matrix was then analysed non-intensively by maximum likelihood (ML), as implemented in MEGA6 (with a GTR model, ‘partial deletion’ and ‘weak’ branch swapping), in order to identify identical sequences, which were then eliminated (in some cases, however, the saved sequences were non-identical but became identical when ambiguously aligned regions were excluded), and closely related sequences, which were re-inspected for errors in alignment. Finally, ambiguously aligned positions from the most variable parts of LSU and rare insertions were excluded and the ends of both the LSU and rbcL alignments were trimmed to reduce gaps. The final rbcL and LSU alignments, containing 175 and 195 sequences respectively, are available as Supplementary alignments 1 and 2. No attempt was made to concatenate sequences to make a two-gene dataset because of major differences in taxon sampling. Furthermore, even where the same taxon was represented in both datasets, the clone used sometimes differed (for example, in Bacillaria paxillifera, Hantzschia amphioxys var. major, H. elongata, Nitzschia draveillensis, N. sigmoidea, Tryblionella apiculata and various Pseudo-nitzschia, Fragilariopsis and Cylindrotheca species). Hence, given that many species are probably not homogeneous (e.g. Mann, Citation1999; Sarno et al., Citation2005; Rovira et al., Citation2015), concatenation of sequences according to taxon could result in chimaera formation, even if clones have been identified correctly according to current taxonomy.
ML analyses of the final rbcL and LSU alignments were performed with General Time Reversible + Gamma models, using RAxML (Stamatakis, Citation2014) via the raxmlGUI interface (Silvestro & Michalak, Citation2012), version 1.5 beta. The rbcL alignment was partitioned by codon position. Robustness of the trees obtained was assessed by bootstrapping (1000 replicates). The Approximately Unbiased (AU) test, developed by Shimodaira (Citation2002), and the Shimodaira–Hasegawa (SH) test, both as implemented in the CONSEL package (Shimodaira & Hasegawa, Citation2001), were used to examine whether particular constrained trees were significantly worse than the most likely trees.
Results
For practical purposes we describe the new species first (to avoid having to refer to it as ‘Nit952CAT’), before justifying why we place it in Nitzschia.
Species description
Nitzschia varelae Carballeira, D.G. Mann & Trobajo, sp. nov. –,
Table 2. Valve morphometric data of N. varelae from Valdoviño, Muro and Clot lagoons.
Figs 2–13. Nitzschia varelae, sp. nov. from Valdoviño Lagoon (Figs 2–7, 10 and 11) and Clot Lagoon (clone NIT952cat: Figs 8, 9, 12 and 13), LM, differential interference contrast optics. Figs 2, 4, 6 & 11. Frustules in valve view: note that the raphe systems of the two valves lie on the same side (left). Figs 2–13. Valves at different stages of size reduction, with the raphe always on the more convex side. Note the tendency of the valves to become more strongly differentiated into a linear central section and narrowly rostrate ends during size reduction, the wider separation of the central fibulae in all specimens, and the central nodule (e.g. arrowheads), which marks the position of the central raphe endings. Scale bar = 10 µm.

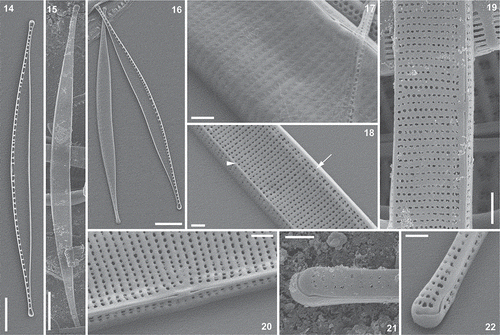
Figs 14–22. Nitzschia varelae, sp. nov., clone NIT952cat from Clot Lagoon, SEM (in Figs 14, 16–18, 20 and 22, the specimens were tilted through 25°, except Fig. 14, for which no tilt was applied) and specimens from Valdoviño Lagoon, SEM (Figs 15, 19, 21 in all specimens no tilt was applied). Fig. 14. Whole valve, interior. Fig. 15. Whole valve, exterior. Fig. 16. Valves of a disassembled frustule, exterior (left) and interior. Fig. 17. Central part of valve, exterior, showing poroids closed externally by hymenes and a rounded transition between the valve face and the distal mantle. Fig. 18. Valve exterior with a sharp transition between the valve face and distal mantle, marked by a small marginal ridge (arrowhead); note also that each stria is represented within the raphe canal by two poroids (e.g. arrow). Figs 19, 20. Valve centre outside, showing the raphe endings and the double row of poroids within the raphe canal. Figs 21, 22. Valve pole, exterior: note that the terminal raphe fissure bends towards the distal (more concave) side of the valve. Scale bars = 5 µm (Figs 14–16), 1 µm (Fig. 19) or 500 nm (Figs 17, 18, 20–22).
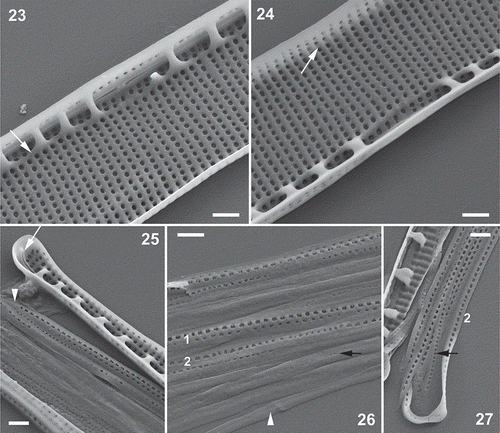
Figs 23–27. Nitzschia varelae, sp. nov., clone NIT952cat from Clot Lagoon, SEM (all specimens tilted through 25°). Fig. 23. Valve centre inside, showing raphe endings and widely separated rib-like fibulae linked at their bases by a shallow longitudinal ridge (arrow). The raphe slits end symmetrically in a small nodule resembling a double helictoglossa. Note that each uniseriate stria (apart from two at the centre) is represented within the raphe canal by two poroids. Fig. 24. Valve centre inside, looking towards the distal mantle: note the slightly wider separation of the poroids at the junction of valve face and mantle (arrow). Fig. 25. Valve pole inside with helictoglossa (arrow). Note also the open end of band 1 (valvocopula; arrowhead), which bears two longitudinal rows of poroids. Figs 26, 27. Girdle bands: Fig. 26 shows the central part of a disassembled girdle, while Fig. 27 shows a polar view of the most advalvar bands. Band 1 (‘1’) bears two rows of relatively widely spaced poroids, while another advalvar band (probably band 2: ‘2’) bears only one row of slightly more densely spaced poroids; bands further from the valve (e.g. black arrows) have much smaller and more densely spaced poroids or are plain (arrowhead, Fig. 26). Scale bars = 500 nm.
Description: Frustules always hantzschioid (i.e. with the raphes of both valves on the same side of the cell: , , , ). Valves arcuate (less strongly in short valves), tapering to long, narrow, rostrate apices (, , –, , ), the convex margin being evenly curved in all specimens except near the apices (where it becomes straighter), the opposite margin being concave throughout in longer valves (–) but becoming almost straight in the central part of the valve in shorter valves (–). Valves 40.4–84.8 μm long and 2.1–3.2 μm wide. Raphe eccentric, lying along the convex margin, with central raphe endings (detectable in LM as a very small thickening of the convex margin: arrowheads in –). Fibulae 12.7–16.0 in 10 μm, the central pair being noticeably more widely spaced than any of the others (–). Striae 53.8–60.2 in 10 μm, undetectable in LM (–) even with NA 1.4 objectives and the condenser oiled.
SEM revealed the striae to be uniseriate, each comprising a line of round poroids, which (even in the same clonal material) can be continuous from the raphe to the distal margin () or be interrupted at the junction of valve face and mantle (; this can also be visible internally, ), where there may even be a slight longitudinal ridge. Within the raphe canal the striae are represented on the valve face by two sets of poroids, which form two longitudinal rows parallel to the raphe (, , , , ). In lightly cleaned valves, the poroids can be seen to be occluded by sieve plates (hymenes), which lie at the outer apertures of the poroids ().
The raphe is raised on a slight keel and the central raphe endings are coaxial and terminate in a slight ridge both internally () and externally (, , ). At the poles, the raphe is hooked towards the concave side of the valve externally (, ), while internally there is a simple helictoglossa (). Internally, the raphe canal is separated from the rest of the valve by a longitudinal ridge running parallel to the raphe (, , ). The fibulae extend from this ridge across to the proximal mantle; they are slender arched ribs (–). At the centre, the longitudinal ridge linking the fibula bases is more feebly developed and the fibulae are much more widely separated.
The girdle comprises very narrow open bands (), of which at least the most advalvar (the valvocopula) bears two longitudinal rows of small poroids, while the more abvalvar bands bear either one row or are plain (–).
Holotype: Slide BM 101 850, deposited in the Natural History Museum in London, bears a number of specimens of which the holotype specimen, lying in valve view, can be located with England finder (Graticules Ltd, Tonbridge, UK) coordinates G-56-3. It measures 66.7 μm in length and 2.7 μm in width and has 15 fibulae in 10 μm.
Isotype: Slide BM 101 851 has been deposited at the Natural History Museum in London and bears many specimens; an isotype, lying in valve view, can be located with England finder coordinates W-15-2. It measures 73.3 μm in length and 2.7 μm in width and has 14 fibulae in 10 μm.
Type locality: Shallow coastal lagoon of Valdoviño or Frouxeira (UTM 29T X 568153, Y 4828484; Datum WGS84). Valdoviño, A Coruña (Galicia), Spain. Date: 16/11/2007. Sample of the epiphyton.
Etymology: This new taxon is dedicated to Dr Manuel Varela Rodríguez of the Spanish Institute of Oceanography, in recognition of the contributions he has made throughout his career to Galician diatom studies in freshwater, brackish and marine environments.
Ecology: In Valdoviño lagoon, in samples collected on 16 November 2007, Nitzschia varelae was observed in all types of habitats studied (i.e. sediment, epiphyton, periphyton and plankton) but only in low abundance (< 1%). It was observed again in Valdoviño on 7 October 2012 in a sample of epiphyton. In Muro lagoon it was found in sediment samples collected on 9 September 2012, again in low abundance (< 1%). In Clot lagoon it was observed only in a periphyton sample taken from submerged vegetation on 16 March 2011. It was not observed in a sediment sample taken in November 2012 by Benito et al. (Citation2015). Among the surface sediment samples from Salicornia marsh near the Alfacada lagoon in the Ebro Delta, N. valerae was observed in only one sample (November 2012) and in low abundance (0.6%). Physical and chemical data for water at the four sites are provided in and show that this species tolerates salinities ranging at least from 2.5 to 28 mS cm–1, and pH from 6.8 to 9.
Comparison with similar species: Nitzschia varelae can be distinguished from other species of Bacillariaceae by the combination of constant hantzschioid symmetry, arcuate shape with the raphe on the more convex side, and delicate structure, with a stria density of > 40 in 10 μm and a valve width < 4 μm. All the Bacillariaceae with constant hantzschioid symmetry are like N. varelae in having an asymmetrical valve outline. However, in most of them [including the three recently described hantzschioid species of Nitzschia subgen. Nitzschia (Mann & Trobajo, Citation2014), Hantzschia species (e.g. Joh, Citation2014), and three species of Cymbellonitzschia (the freshwater C. diluviana and two marine species, C. banzuensis and C. szulczewskii, but not the type species C. minima: see Jewson & Lowry, Citation1993; Cocquyt & Jewson, Citation1994; Stepanek et al., Citation2016)] the raphe lies on the concave or straight side of the frustule, not on the convex side, in contrast to N. varelae. It should be noted here that C. minima, the type species of Cymbellonitzschia, does not have constant hantzschioid symmetry; in this species, therefore, because of the formation of nitzschioid cells, the raphe can lie on either the convex or the straight margin.
Among Hantzschia species, the most similar to N. varelae is H. weyprechtii, which also has rather widely spaced fibulae (relative to the striae) and a clearly wider central interspace (Cleve & Grunow, Citation1880; Mann, Citation1978, figs 133, 134). However, although H. weyprechtii is much more delicate than most Hantzschia, it is much more robust than N. varelae, with 33–35 striae in 10 μm and valve widths of 6–7 μm (Cleve & Grunow Citation1880); moreover, only the poles of H. weyprechtii show any tendency to reflex, the remainder of the ventral (raphe-bearing) margin being straight (Mann, Citation1978).
Among species traditionally assigned to Nitzschia those morphologically most similar to N. varelae belong to sections Nitzschiella and Lanceolatae. In Nitzschia hummii, which is arcuate like N. varelae, the raphe can lie on either the concave or the convex side (Hustedt, Citation1955; Simonsen, Citation1987), and this species must therefore produce at least some nitzschioid cells, like C. minima but unlike N. varelae; N. hummii cells also differ from N. varelae in lacking central raphe endings, having a lower striation density (c. 30 in 10 µm) and in being twisted about the apical axis. Nitzschia varelae resembles N. acicularis and N. draveillensis (Krammer & Lange-Bertalot, Citation1988) and some other similar species (e.g. the needle-shaped species from Lake Victoria studied by Sitoki et al., Citation2013) in having delicate, narrow valves with protracted apices and striae that are essentially unresolvable in LM (with densities > 40 in 10 μm). However, all of these species are straight, not arcuate, and several, including N. acicularis, lack central raphe endings and a wider central interspace. Cylindrotheca closterium is often arcuate, like N. varelae, and it is also extremely delicately structured (in both species, the striae are invisible in LM), but the raphes appear on opposite sides of the frustule in C. closterium, not on one side (Hustedt, Citation1955 pl. 16, fig. 18; Krammer & Lange-Bertalot, Citation1988, pl. 87, figs 1, 2). Furthermore, C. closterium cells have a clearly differentiated central ‘body’ and needle-like ends, in contrast to N. varelae, in which the ends are more gradually attenuated.
Molecular phylogeny
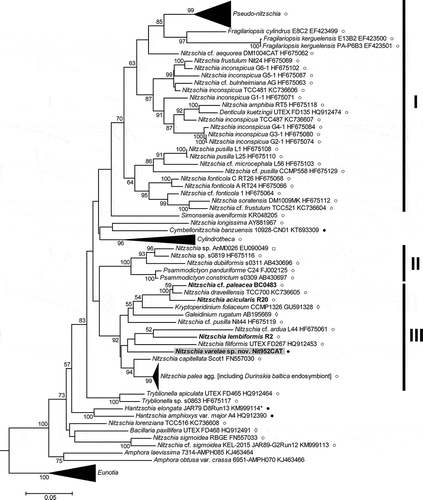
In the rbcL tree (), the family Bacillariaceae appeared as a moderately well supported clade (BS 73%), with the two Amphora species (A. laevissima and A. obtusa var. crassa) as its sister group. The genus Nitzschia was not monophyletic, forming a number of clades interspersed among representatives of the genera Pseudo-nitzschia and Fragilariopsis (which together formed a fairly well supported clade), Denticula, Simonsenia, Cymbellonitzschia, Cylindrotheca, Psammodictyon, Tryblionella, Hantzschia and Bacillaria. Many of the deepest nodes within the Bacillariaceae were without significant support but some nodes demonstrating the non-monophyly of Nitzschia were better supported, including a moderately well supported clade (, clade I) containing Pseudo-nitzschia, Fragilariopsis, Denticula, Simonsenia and some Nitzschia species belonging to the sect. Lanceolatae, and a well-supported clade (clade II) containing Psammodictyon and species of Nitzschia sect. Dubiae. The type species of Nitzschia, N. sigmoidea, fell outside both of these clades, and also outside a very well supported clade (clade III) composed of Nitzschia species belonging mostly to sections Lanceolatae and Nitzschiella. Tryblionella and Bacillaria branched off near the base of the Bacillariaceae, as did Nitzschia lorenziana, with no clear evidence of their relationship to each other or to clades I–III. The type species of Hantzschia, H. amphioxys, grouped with a sequence (KM999114) previously labelled in GenBank as Nitzschia linearis (see below for further comment on this accession).
Fig. 28. Maximum likelihood phylogenetic tree of Bacillariaceae based on rbcL sequences, rooted by Eunotiaceae. Bootstrap values > 50% are plotted at the nodes. New sequences are in bold type. New sequences are in bold type. The taxonomic identities (as given in GenBank), clone identifiers and GenBank accession numbers are given for each sequence included, except in the clades of Pseudo-nitzschia spp., Cylindrotheca spp., the Nitzschia palea complex, and Eunotia spp., which have been collapsed to aid presentation. The GenBank accessions included in these clades are listed in Supplementary tables S2 & S4. I–III are major well-supported (> 70% BS) clades. KM999114 (*) was originally classified in GenBank as ‘Nitzschia linearis’ but its identity has been corrected to Hantzschia elongata (see text). ○ = some or all frustules are nitzschioid (i.e. H→H+H is not the only type of cell division present); ● = all frustules are hantzschioid (i.e. H→H+H is the only type of cell division present); □ = no data; ◊ = raphe central or frustules absent (in endosymbiotic species) (see Supplementary table S4 for data sources).
The LSU tree () had a similar topology to the rbcL tree and clades I, II and III were again present, with moderate to high support. However, with more of the diversity of the Bacillariaceae sampled, LSU revealed several extra clades, including a group of apochlorotic species (clade IV: not all the apochlorotic Bacillariaceae species were contained in this) and a clade of freshwater Hantzschia species. Again, Tryblionella, Bacillaria and the type group of Nitzschia (here represented by N. sigmoidea and N. dissipata) branched off from low nodes in the tree, but there was no support for the relationships implied between them and the rest of the Bacillariaceae.
Fig. 29. Maximum likelihood phylogenetic tree of Bacillariaceae based on LSU rDNA sequences, rooted by Eunotiaceae. Bootstrap values > 50% are plotted at the nodes. The taxonomic identities (as given in GenBank), clone identifiers and GenBank accession numbers are given for each sequence included, except in the clades of Pseudo-nitzschia and related genera (Fragilariopsis and Neodenticula), Cylindrotheca spp., Nitzschia navis-varingica, the Nitzschia palea complex and N. capitellata, and Eunotia spp., which have been collapsed to aid presentation (the accessions included are listed in Supplementary tables S3 & S5). Labelling conventions as in ; data sources in Supplementary table S5.
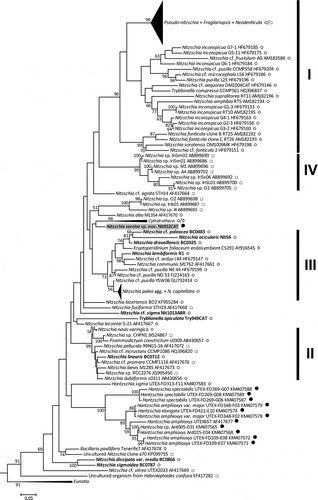
In both rbcL and LSU, Cylindrotheca emerged as a monophyletic group and was clearly separate from the Nitzschia sect. Nitzschiella species with which C. closterium was long placed (Hustedt, Citation1930; Krammer & Lange-Bertalot, Citation1988), which include N. acicularis, N. draveillensis and N. longissima (for the last only rbcL was available).
Nitzschia varelae was not associated with Hantzschia in either tree, nor with any other taxa known to produce hantzschioid cells, e.g. Cymbellonitzschia banzuensis (rbcL, ), which only produces hantzschioid cells, or N. alba (LSU only, ) and N. sigmoidea (, ), which both produce mixtures of hantzschioid and nitzschioid cells. In the rbcL tree N. varelae was placed in clade III, which also contained the Nitzschia sect. Nitzschiella species N. acicularis and N. draveillensis. In the LSU tree, it was outside clade III but formed part (with Cylindrotheca) of its sister group.
Given the low support for many nodes in both trees, and hence uncertainty about where N. varelae actually belongs, two explicit hypotheses were examined using AU tests. Hypothesis 1 was that N. varelae + Hantzschia comprised a monophyletic group, characterized morphologically by constant hantzschioid symmetry. In the case of rbcL, two variants of hypothesis 1 were examined, according to the assumed identity of GenBank KM999114, which is the sister of H. amphioxys var. major in the rbcL tree (). KM999114 was obtained by sequencing a single cell, which was documented before DNA extraction but for which no voucher remained afterwards (Hamilton et al., Citation2015). The sequence was originally uploaded to GenBank as ‘Nitzschia linearis’ but we were doubtful about this given the different position of N. linearis (which is consistently nitzschioid, not hantzschioid: Mann & Trobajo, Citation2014) in our LSU tree (), where it appears in clade II. Dr Paul Hamilton very kindly checked records of the original material from which KM999114 was obtained and informed us that KM999114 should be reclassified as belonging to Hantzschia elongata; this correction has since been made in GenBank. However, to avoid the possibility of error, we examined two variants of hypothesis 1, according to whether the population represented by KM999114 had constant hantzschioid symmetry (hypothesis 1A – in other words, the test was of a tree in which N. varelae was constrained to group with both KM999114 and H. amphioxys, both being considered representatives of ‘true’ Hantzschia) or not (hypothesis 1B).
Hypothesis 2 was that N. varelae was part of a monophyletic group with N. acicularis, N. paleacea and N. draveillensis, characterized by a needle-like (spicular) morphology, protracted valve apices, and high stria density (> 40 in 10 μm). In the case of rbcL, AU tests strongly rejected hypotheses 1A (P = 0.009) and 1B (P = 6 × 10–6) but not hypothesis 2 (P = 0.18) (SH results were 0.02, 4 × 10–4, and 0.37, respectively). With LSU, neither hypothesis could be rejected at P < 0.05, though hypothesis 1 (P = 0.07) appeared less credible than hypothesis 2 (P = 0.13) (SH results were 0.15 and 0.20, respectively).
Discussion
The systematic position of Nitzschia varelae
The results of our phylogenetic analyses are broadly similar to some previously published trees (e.g. Trobajo et al., Citation2006, Citation2009; Rimet et al., Citation2011; Rovira et al., Citation2015; Witkowski et al., Citation2015b; Stepanek et al., Citation2016) in showing (1) non-monophyly of Nitzschia; (2) support for groupings corresponding to our clades I, II and III; and (3) separation of each of these three clades from the clade containing the type species of Nitzschia, N. sigmoidea. In addition, (4) Cylindrotheca is now consistently monophyletic (see also Vanormelingen et al., Citation2013), in contrast to an earlier analysis when fewer sequences were available (Trobajo et al., Citation2009); (5) the consistently hantzschioid Cymbellonitzschia banzuensis does not group with Hantzschia in analyses of either rbcL () or SSU (Stepanek et al., Citation2016); and (6) the Hantzschia species that have been sampled (various isolates of H. spectabilis, H. elongata, H. sigma and the type species, H. amphioxys) form a clade (in LSU analyses, ; no rbcL data are available for these isolates; for SSU, see Stepanek et al., Citation2016), although the position of the sigmoid species H. sigma is equivocal. Interestingly, this species, an outlier to the clade containing other Hantzschia species (; see also the SSU tree of Stepanek et al., Citation2016), is not consistently hantzschioid: Simonsen (Citation1987, p. 241) records that nitzschioid frustules are present in the type material of H. sigma.
The molecular data indicate (strongly in the case of rbcL, weakly with LSU) that Nitzschia varelae does not belong in Hantzschia, nor is it close to it, despite its consistently hantzschioid symmetry. There is also no indication that it is close to Cymbellonitzschia banzuensis, the other consistently hantzschioid species for which there are molecular data. Rather, N. varelae belongs in or close to clade III Bacillariaceae, which contains a variety of freshwater, brackish and marine Nitzschia species and the three ‘dinotoms’ (i.e. the diatom endosymbionts of the dinoflagellates Kryptoperidinium foliaceum, Galeidinium rugatum and Durinskia baltica).
Among the Nitzschia species of clade III, N. varelae morphologically most resembles N. acicularis and N. draveillensis, because of its marginal raphe system, narrow spicular cells and protracted apices. This morphology would previously have led to the classification of all three species together in Nitzschia sect. Nitzschiella (Grunow in Cleve & Grunow, Citation1880; Krammer & Lange-Bertalot, Citation1988). These species also have in common an extremely delicate striation (> 40 uniseriate striae in 10 μm). In the most-likely rbcL and LSU trees, N. varelae does not group with N. acicularis and N. draveillensis, but AU tests indicate that it is possible that N. varelae, N. acicularis and N. draveillensis could in fact comprise a monophyletic group that also includes N. paleacea. Even if they do, however, the original concept of sect. Nitzschiella as a group of Nitzschia species characterized by an eccentric raphe and protracted apices (“Schaalen mit excentrischem Kiel und lang vorgezogenen Spitzen”: Cleve & Grunow, Citation1880, p. 100) would still be severely undermined because of the transfer of N. closterium to Cylindrotheca and the distant relationships between Cylindrotheca, N. acicularis and N. draveillensis, N. lorenziana, and N. longissima (all classified in the ‘Nitzschiellae’ by Krammer & Lange-Bertalot, Citation1988) in the rbcL tree. The spicular morphology of these species has apparently evolved independently.
The present work, together with previous studies (e.g. Rimet et al., Citation2011), suggests, therefore, that two of the ‘key characters’ traditionally used to separate genera and sections within the Bacillariaceae – frustule symmetry and valve outline – do not always distinguish natural groups and that, in the case of N. varelae, they may give a misleading impression of its systematic position.
Since molecular phylogenies (ours and those cited above) show that Nitzschia is not monophyletic, many species currently classified there will need to be reassigned or transferred in order to create a natural system. Similar problems were faced by Ruck et al. (Citation2016) with respect to the Surirellales and Rhopalodiales, and they have been able to suggest a realignment of genera that reflects relationships while minimizing nomenclatural changes. However, the Bacillariaceae have not yet been sampled sufficiently extensively to provide a basis for an equivalent reorganization. Our molecular phylogenies suggest that it would not be easy to develop a phylogenetically coherent classification of Bacillariaceae in which N. varelae would remain classified in Nitzschia, given that the type species of Nitzschia remains N. sigmoidea. Nevertheless, for the moment we think it is best to assign N. varelae to Nitzschia, rather than, for example, to a new genus comprising our clade III (which would be premature), or to Hantzschia (since this would clearly be wrong).
What now characterizes Hantzschia?
As explained in the introduction, the discovery that some Nitzschia species can produce hantzschioid cells prompted a reexamination of Hantzschia and a redefinition of the genus (Mann, Citation1977, Citation1980a; Round et al., Citation1990). According to the suggested redefinition, it is not cell symmetry that separates Hantzschia and Nitzschia but the pattern of cell division: in Hantzschia, hantzschioid cells always divide to give hantzschioid daughter cells, whereas in Nitzschia they never do. The discovery by Mann & Trobajo (Citation2014) of three marine species with consistently hantzschioid symmetry but the same frustule and cell structure as the type species of Nitzschia, N. sigmoidea, indicated that the suggested redefinition of Hantzschia is inadequate, and our study of N. varelae corroborates this, showing that consistent hantzschioid symmetry is present in several unrelated lineages (Hantzschia species, Cymbellonitzschia banzuensis and N. varelae) and cannot be used to characterize Hantzschia.
Mann (Citation1980a) suggested six other characters apart from the cell division pattern that may help to characterize and identify true Hantzschia (i.e. species closely related to the type species, H. amphioxys). Most of these characters have already proved unsatisfactory (Supplementary text 1). The two remaining ones that deserve some discussion are the form of the central internal raphe endings and the morphology of the chloroplasts. In N. varelae, as in most other Bacillariaceae species with central raphe endings (in some Bacillariaceae the raphe is continuous from pole to pole so they do not have central raphe endings: see Mann, Citation1982 for examples), the internal raphe fissures approach each other symmetrically at the centre and end in a small nodule (e.g. Mann, Citation1978; Trobajo et al., Citation2013). In Hantzschia, on the other hand, when internal central raphe endings are present, they are usually found to be deflected in opposite directions, both in H. amphioxys and its allies and in marine Hantzschia species such as H. marina, H. wittii and H. distinctepunctata (Mann, Citation1978, Citation1980b, Citation1981; Round et al., Citation1990; see Supplementary table S6). However, the utility of this character is thrown into doubt by the recent discovery of N. sancti-francisci (Witkowski et al., Citation2015a), in which the frustules are said to be nitzschioid but which has oppositely deflected internal raphe endings.
Most Bacillariaceae have two simple plate-like chloroplasts (illustrated by e.g. Heinzerling, Citation1908; Mann, Citation1978, Citation1981; Cox, Citation1996; Round et al., Citation1990; Trobajo et al., Citation2006; Mann & Trobajo, Citation2014; see also Supplementary tables S4 & S5) arranged one above the other along the length of the cell. A few have more elaborate, lobed chloroplasts (e.g. Mereschkowsky, Citation1903a, b; Mann & Trobajo, Citation2014) and very rarely there are more than two chloroplasts per cell (e.g. in N. longissima: Karsten, Citation1897), but as far as we know, no other Bacillariaceae have been reported to have the complex type of chloroplast present in the H. amphioxys and H. virgata groups of species or in H. weyprechtii. In all of these, each chloroplast comprises two girdle-appressed plates linked by one or two bridges containing a large ± isodiametric pyrenoid (Heinzerling, Citation1908; Round, Citation1970; Mann, Citation1978). However, the diagnostic value of this character is compromised by the fact that it is not universal within species currently assigned to Hantzschia (see Supplementary table S6). Thus, although H. marina and H. distinctepunctata have offset internal raphe endings (see Supplementary table S6), they have simpler chloroplasts than in H. amphioxys, H. virgata and H. weyprechtii, more like those in other Bacillariaceae. Hence the complex chloroplast morphology exemplified in H. amphioxys characterizes a group or groups within Hantzschia, rather than the genus as a whole.
In summary, although it is quite possible that the whole set of species traditionally assigned to the genus Hantzschia (including those listed in Supplementary table S6) does comprise a monophyletic group, there is no single morphological, cytological or frustular character that can be used on its own to characterize it. The most efficient way to test whether a consistently hantzschioid species belongs to Hantzschia is probably via molecular phylogenetics, given that extensive sequence information is already available for H. amphioxys and its allies (Supplementary table S3; Souffreau et al., Citation2013).
Why has N. varelae not been described previously?
Even though there have been many fewer studies of diatoms in brackish habitats and coastal wetlands than in fresh water, there is nevertheless a significant body of work on these environments in the Iberian Peninsula (e.g. Azpeitia, Citation1911; Tomàs Citation1988; Cambra et al., Citation1991; Trobajo et al., Citation2004; Clavero i Oms, Citation2009; Rovira et al., Citation2009, Citation2012; Ribeiro, Citation2010; Ferreira, Citation2013; Álvarez-Blanco & Blanco, Citation2014; Gomes, Citation2014; Benito et al., Citation2015). Despite this, there appear to be no published records or illustrations of Nitzschia varelae from the Iberian Peninsula, either as a Hantzschia or a Nitzschia species, nor from similar environments from elsewhere (e.g. Peragallo & Peragallo, Citation1897–1908; Cholnoky, Citation1963; Archibald, Citation1983; Kuylenstierna, Citation1990; Poulin et al., Citation1990; Snoeijs et al., 1993–98; Witkowski et al., Citation2000; Flower, Citation2001; Zalat & Vildary, Citation2005; Della Bella et al., 2007; Álvarez-Blanco & Blanco, Citation2014). At least with respect to conductivity/salinity, our data suggest that N. varelae does not have narrow requirements and indeed it is possible that the species is favoured by the environmental fluctuations that are characteristic of coastal wetlands and lagoons (e.g. salinity). In any case, because of its wide tolerance one might have expected that N. varelae would have been observed before. However, we found it only in very low abundance (< 1%), and if this is also true elsewhere it is perhaps not surprising that it has been overlooked or not documented, even though its characteristic arcuate shape, high stria density and constant hantzschioid symmetry should make it easy to recognize. On the other hand, since the arcuate shape is much more subtle in small cells of N. varelae (i.e. cells at the end of size reduction), it is possible that such cells, especially when found in low abundance, may have been confused with delicate and finely striated species of Nitzschia sections Lanceolatae or Nitzschiella.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2017.1309575
Supplementary table S1: new Nitzschia clones sequenced for this study.
Supplementary table S2: rbcL sequences downloaded from GenBank.
Supplementary table S3: LSU sequences downloaded from GenBank.
Supplementary table S4: Morphological information for rbcL accessions.
Supplementary table S5: Morphological information for LSU accessions.
Supplementary table S6: Morphological data on Hantzschia species.
Supplementary text: Brief explanation of characters not useful for distinguishing Hantzschia.
Supplementary alignment 1: phylip formatted rbcL alignment used to construct (i.e. after exclusions).
Supplementary alignment 2: phylip formatted LSU alignment used to construct (i.e. after exclusions).
Supp_material.zip
Download Zip (136.5 KB)Acknowledgements
We thank Ruth Hollands (RBGE, UK) for the new LSU sequences of N. acicularis, N. dissipata var. media, N. draveillensis, N. linearis, N. paleacea and N. sigmoidea; and David Mateu (IRTA Aquatic Ecosystems, Catalonia, Spain) for collecting the Clot sample from which the N. varelae clone was isolated. The UK Environment Agency, contract SC140024, provided the rbcL sequence of the N. paleacea clone. We thank Xosé Lois Otero Pérez (USC) for helping us to obtain environmental data from Valdoviño and Muro lagoons (2012–2013). We thank Dr Roberto Bao (UDC) for the use of the laboratory and microscopy equipment. We are also most grateful to Dr Nina Lundholm, Dr Alberto Amato and Dr Chui Pin Leaw for responding to our queries and generously sharing information about cell symmetry and chloroplast arrangement in those Bacillariaceae they have cultured and sequenced, especially in Pseudo-nitzschia, as detailed in Supplementary tables S4 & S5; and to Dr Paul Hamilton for checking the identity of the cell used to obtain KM999114. We also thank the reviewers who made constructive suggestions for improving the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Rafael Carballeira
R. Carballeira: original concept, drafting and editing manuscript, light and electron microscopy, chemical water analysis; R. Trobajo: original concept, drafting and editing manuscript, culture diatom isolates, light and electron microscopy, chemical water analysis; M. Leira: drafting manuscript; X. Benito: drafting manuscript, light microscopy; S. Sato: culture diatom isolates, light microscopy; D.G. Mann: original concept, drafting and editing manuscript, analysis of molecular data.
Rosa Trobajo
R. Carballeira: original concept, drafting and editing manuscript, light and electron microscopy, chemical water analysis; R. Trobajo: original concept, drafting and editing manuscript, culture diatom isolates, light and electron microscopy, chemical water analysis; M. Leira: drafting manuscript; X. Benito: drafting manuscript, light microscopy; S. Sato: culture diatom isolates, light microscopy; D.G. Mann: original concept, drafting and editing manuscript, analysis of molecular data.
Manel Leira
R. Carballeira: original concept, drafting and editing manuscript, light and electron microscopy, chemical water analysis; R. Trobajo: original concept, drafting and editing manuscript, culture diatom isolates, light and electron microscopy, chemical water analysis; M. Leira: drafting manuscript; X. Benito: drafting manuscript, light microscopy; S. Sato: culture diatom isolates, light microscopy; D.G. Mann: original concept, drafting and editing manuscript, analysis of molecular data.
Xavier Benito
R. Carballeira: original concept, drafting and editing manuscript, light and electron microscopy, chemical water analysis; R. Trobajo: original concept, drafting and editing manuscript, culture diatom isolates, light and electron microscopy, chemical water analysis; M. Leira: drafting manuscript; X. Benito: drafting manuscript, light microscopy; S. Sato: culture diatom isolates, light microscopy; D.G. Mann: original concept, drafting and editing manuscript, analysis of molecular data.
Shinya Sato
R. Carballeira: original concept, drafting and editing manuscript, light and electron microscopy, chemical water analysis; R. Trobajo: original concept, drafting and editing manuscript, culture diatom isolates, light and electron microscopy, chemical water analysis; M. Leira: drafting manuscript; X. Benito: drafting manuscript, light microscopy; S. Sato: culture diatom isolates, light microscopy; D.G. Mann: original concept, drafting and editing manuscript, analysis of molecular data.
David G. Mann
R. Carballeira: original concept, drafting and editing manuscript, light and electron microscopy, chemical water analysis; R. Trobajo: original concept, drafting and editing manuscript, culture diatom isolates, light and electron microscopy, chemical water analysis; M. Leira: drafting manuscript; X. Benito: drafting manuscript, light microscopy; S. Sato: culture diatom isolates, light microscopy; D.G. Mann: original concept, drafting and editing manuscript, analysis of molecular data.
References
- Archibald, R.E.M. (1983). The diatoms of the Sundays and Great Fish rivers in the Eastern Cape Province of South Africa. Bibliotheca Diatomologica, 1: 1–392.
- Álvarez-Blanco, I. & Blanco, S. (2014). Benthic diatoms from Mediterranean coasts. Bibliotheca Diatomologica, 60: 1–409.
- Amato, A., Kooistra, W.H.C.F., Levialdi Ghiron, J.H., Mann, D.G., Pröschold, T. & Montresor, M. (2007). Reproductive isolation among sympatric cryptic species in marine diatoms. Protist, 158: 193–207.
- Azpeitia, F. (1911). La diatomología española en los comienzos del siglo XX. Asociación Española para el progreso de las Ciencias. Sección 3a Ciencias Naturales. E. Arias, Madrid.
- Benito, X., Trobajo, R. & Ibañez, C. (2015). Benthic diatoms in a Mediterranean delta: ecological indicators and a conductivity transfer function for paleoenvironmental studies. Journal of Paleolimnology, 54: 171–188.
- Cambra, J., Sabater, S. & Tomàs, X. (1991). Diatom check-list from Catalonian countries (eastern Spain). Butlletí de la Institució Catalana d’Història Natural, 59: 41–55.
- Capesius, I. & Van de Peer, Y. (1997). Secondary structure of the large ribosomal subunit RNA of the moss Funaria hygrometrica. Journal of Plant Physiology, 151: 239–241.
- Chepurnov, V.A. & Mann, D.G. (1997). Variation in the sexual behaviour of natural clones of Achnanthes longipes (Bacillariophyta). European Journal of Phycology, 32: 147–154.
- Cholnoky, B.J. (1963). Beiträge zur Kenntnis des marinen Litorals von Südafrica. Botanica Marina, 5: 38–83.
- Clavero i Oms, E. (2009). Diatomees d’ambients hipersalins costaners: taxonomia, distribució i empremtes en el registre sedimentari. Institut d’Estudis Catalans, Barcelona.
- Cleve-Euler, A. (1952). Die Diatomeen von Schweden und Finnland, Teil V. (Schluss). Kungliga Svenska VetenskapsAkademiens Handlingar, ser. 4, 3: 1–153.
- Cleve, P.T. & Grunow, A. (1880). Beiträge zur Kenntniss der arctischen Diatomeen. Kongliga Svenska Vetenskaps-Akademiens Handlingar, 17: 1–121.
- Cocquyt, C. & Jewson, D.H. (1994). Cymbellonitzschia minima Hustedt (Bacillariophyceae), a light and electron microscopic study. Diatom Research, 9: 239–247.
- Cox, E.J. (1996). Identification of freshwater diatoms from live material. Chapman & Hall, London.
- Dalda González, J. (1968). Estudio fitoecológico de la laguna de Valdoviño, en La Coruña. Botanica Complutensis, 1: 15–50.
- Daugbjerg, N. & Andersen, R.A. (1997). A molecular phylogeny of the heterokont algae based on analyses of chloroplast-encoded rbcL sequence data. Journal of Phycology, 33: 1031–1041.
- Della Bella, V., Puccinelli, C., Marcheggiani, S. & Mancini, L. (2007). Benthic diatom communities and their relationship to water chemistry in wetlands of central Italy. Annales de Limnologie – International Journal of Limnology, 43: 89–99.
- Ferreira, T. (2013). Diatom-based characterization of Iberian coastal environments at different time scales. PhD dissertation. University of Lisbon, Lisbon.
- Flower, R.J. (2001). Change, stress, sustainability and aquatic ecosystem resilience in North African wetland lakes during the 20th century: an introduction to integrated biodiversity studies within the CASSARINA Project. Aquatic Ecology, 35: 261–280.
- Geitler, L. (1968). Die Lage der Raphen in den Zellen von Nitzschia-Arten. Berichte der Deutschen Botanischen Gesellschaft, 81: 411–413.
- Gomes, A. (2014). Alterações ambientais na costa Algarvia durante o Holocénico: um estudo com base em diatomáceas. PhD dissertation. University of Algarve, Algarve.
- Grunow, A. (1877). New diatoms from Honduras. Monthly Microscopical Journal, 18: 165–187.
- Guillard, R.R.L. & Lorenzen, C.L. (1972). Yellow-green algae with chlorophyllide c. Journal of Phycology, 8: 10–14.
- Hamilton, P.B., Lefebvre, K.E. & Bull, R.D. (2015). Single cell PCR amplification of diatoms using fresh and preserved samples. Frontiers in Microbiology, 6: 1084.
- Hamsher, S.E., Evans, K.M., Mann, D.G., Poulíčková, A. & Saunders, G.W. (2011). Barcoding diatoms: exploring alternatives to COI-5P. Protist, 162: 405–422.
- Hansen, H.P. & Koroleff, F. (1999). Determination of nutrients. In Methods of Seawater Analysis, 3rd ed. (Grasshoff, K., Kremling, K., Ehrhardt, M., editors), 159–228. Wiley-VCH, Weinheim, Weinheim.
- Heinzerling, O. (1908). Der Bau der Diatomeenzelle. Bibliotheca Botanica, 69: 1–88.
- Hendey, N.I. (1964). An Introductory Account of the Smaller Algae of British Coastal Waters. Part V: Bacillariophyceae (Diatoms). Ministry of Agriculture, Fisheries and Food, Fishery Investigations series IV. Her Majesty’s Stationery Office, London.
- Hustedt, F. (1930). Bacillariophyta. In Die Süsswasser-Flora Mitteleuropas, vol. 10 (Pascher, A., editor), 1–466. G. Fischer, Jena.
- Hustedt, F. (1955). Marine littoral diatoms of Beaufort, North Carolina. Duke University Marine Station Bulletin, 6: 1–67.
- Jewson, D.H. & Lowry, S. (1993). Cymbellonitzschia diluviana Hustedt (Bacillariophyceae): habitat and auxosporulation. Hydrobiologia, 269: 87–96.
- Joh, G. (2014). The diverse species of the genus Hantzschia (Bacillariophyta) in sand flats of the Nakdong River estuary in Korea. Journal of Ecology and Environment, 37: 245–255.
- Jones, H.M., Simpson, G.E., Stickle, A.J. & Mann, D.G. (2005). Life history and systematics of Petroneis (Bacillariophyta), with special reference to British waters. European Journal of Phycology, 40: 43–71.
- Karsten, G. (1897). Untersuchungen über Diatomeen III. Flora, 89: 404–433.
- Krammer, K. & Lange-Bertalot, H. (1988). Bacillariophyceae 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süßwasserflora von Mitteleuropa, vol. 2 (2) (Ettl, H., Gerloff, J., Heynig, H. & Mollenhauer, D., editors), 1–596. G. Fischer, Stuttgart and New York.
- Kuylenstierna, M. (1990). Benthic algal vegetation in the Nordre Älv Estuary (Swedish West Coast). PhD. Dissertation, University of Göteborg, Göteborg.
- Lauritis, J.A., Hemmingsen, B.B. & Volcani, B.E. (1967). Propagation of Hantzschia sp. Grunow daughter cells by Nitzschia alba Lewin and Lewin. Journal of Phycology, 3: 236–237.
- Mann, D.G. (1977). The diatom genus Hantzschia Grunow – an appraisal. Nova Hedwigia, Beiheft, 54: 323–354.
- Mann, D.G. (1978). Studies in the family Nitzschiaceae (Bacillariophyta). PhD Dissertation. University of Bristol.
- Mann, D.G. (1980a). Hantzschia fenestrata Hust. (Bacillariophyta) – Hantzschia or Nitzschia? British Phycological Journal, 15: 249–260.
- Mann, D.G. (1980b). Studies in the diatom genus Hantzschia II. H. distinctepunctata. Nova Hedwigia, 33: 341–352.
- Mann, D.G. (1981). Studies in the diatom genus Hantzschia 3. Infraspecific variation in H. virgata. Annals of Botany, 47: 377–395.
- Mann, D.G. (1982). The use of the central raphe endings as a taxonomic character (Notes for a monograph of the Bacillariaceae 1). Plant Systematics and Evolution, 141: 143–152.
- Mann, D.G. (1999). The species concept in diatoms. Phycologia, 38: 437–495.
- Mann, D.G. & Evans, K.M. (2007). Molecular genetics and the neglected art of diatomics. In Unravelling the Algae – the Past, Present and Future of Algal Molecular Systematics (Brodie, J. & Lewis, J.M., editors), 231–265. CRC Press, Boca Raton, FL.
- Mann, D.G. & Trobajo, R. (2014). Symmetry and sex in Bacillariaceae (Bacillariophyta), with descriptions of three new Nitzschia species. European Journal of Phycology, 49: 276–297.
- Mann, D.G., Sato, S., Trobajo, R., Vanormelingen, P. & Souffreau, C. (2010). DNA barcoding for species identification and discovery in diatoms. Cryptogamie: Algologie, 31: 557–577.
- Mereschkowsky, C. (1903a). Les types de l´endochrome chez les Diatomées. Scripta Botanica, Horti Universitatis Imperialis Petropolitanae, 21: 107–193.
- Mereschkowsky, C. (1903b). Nouvelles recherches sur la structure et la division des diatomées. Rapport préliminaire. Bulletin de la Société Impériale des Naturalistes de Moscou, 1903: 149–172.
- Nunn, G., Theisen, B., Christensen, B. & Arctander, P. (1996). Simplicity-correlated size growth of the nuclear 28S ribosomal RNA D3 expansion segment in the crustacean order Isopoda. Journal of Molecular Evolution, 42: 211–223.
- Peragallo, H. & Peragallo, M. (1897–1908). Diatomées marines de France et des districts maritimes voisines. Micrographe-Editeur, Grez-sur-Loing.
- Pickett-Heaps, J. D. (1983). Valve morphogenesis and the microtubule center in three species of the diatom Nitzschia. Journal of Phycology, 19: 259–281.
- Pickett-Heaps, J.D. & Kowalski, S.E. (1981). Valve morphogenesis and the microtubule center of the diatom Hantzschia amphioxys. European Journal of Cell Biology, 25: 150–170.
- Pickett-Heaps, J.D., Schmid, A.-M.M. & Edgar, L.A. (1990). The cell biology of diatom valve formation. Progress in Phycological Research, 7: 1–168.
- Poulíčková, A., Vesela, J., Neustupa, J. & Skaloud, P. (2010). Pseudocryptic diversity versus cosmopolitanism in diatoms: a case study on Navicula cryptocephala Kütz. (Bacillariophyceae) and morphologically similar taxa. Protist, 161: 353–369.
- Poulin, M., Bérard-Therriault, L., Cardinal, A. & Hamilton, P.B. (1990). Les diatomées (Bacillariophyta) benthiques de substrats durs des eaux marines et saumâtres du Québec. 9. Bacillariaceae. Le Naturaliste Canadien, 117: 73–101.
- Prado, P., Caiola, N. & Ibáñez, C. (2013). Spatio-temporal patterns of submerged macrophytes in three hydrologically altered Mediterranean coastal lagoons. Estuaries and Coasts, 36: 414–429.
- Ribeiro, L. (2010). Intertidal benthic diatoms of the Tagus estuary: Taxonomic composition and spatial-temporal variation. PhD dissertation, University of Lisbon, Lisbon.
- Rimet, F., Kermarrec, L., Bouchez, A., Hoffmann, L., Ector, L. & Medlin, L.K. (2011). Molecular phylogeny of the family Bacillariaceae based on 18S rDNA sequences: focus on freshwater Nitzschia of the section Lanceolatae. Diatom Research, 26: 273–291.
- Rodríguez-Climent, S., Caiola, N. & Ibáñez, C. (2013). Salinity as the main factor structuring small-bodied fish assemblages in hydrologically altered Mediterranean coastal lagoons. Scientia Marina, 77: 37–47.
- Rovira, L., Trobajo, R. & Ibáñez, C. (2009). Periphytic diatom communities in a Mediterranean salt wedge estuary: the Ebre estuary (NE Iberian Peninsula): preliminary results. Acta Botanica Croatia, 68: 285–300.
- Rovira, L., Trobajo, R., Leira, M. & Ibáñez, C. (2012). The effect of hydrological dynamics on benthic diatom community in a stratified estuary: the case of the Ebro Estuary (Catalonia, Spain). Estuarine, Coastal & Shelf Science, 101: 1–14.
- Rovira, L., Trobajo, R., Sato, S., Ibáñez, C. & Mann, D.G. (2015). Genetic and physiological diversity in the diatom Nitzschia inconspicua. Journal of Eukaryotic Microbiology, 62: 815–832.
- Round, F.E. (1970). The genus Hantzschia with particular reference to H. virgata v. intermedia (Grun.) comb. nov. Annals of Botany, 34: 75–91.
- Round, F.E., Crawford, R.M. & Mann, D.G. (1990). The Diatoms: Biology and Morphology of the Genera. Cambridge University Press, Cambridge.
- Ruck, E.C., Nakov, T., Alverson, A.J. & Theriot, E.C. (2016). Phylogeny, ecology, morphological evolution, and reclassification of the diatom orders Surirellales and Rhopalodiales. Molecular Phylogenetics and Evolution, 103: 155–171.
- Sarno, D., Kooistra, W.H.C.F., Medlin, L.K., Percopo, I. & Zingone, A. (2005). Diversity in the genus Skeletonema (Bacillariophyceae). II. An assessment of the taxonomy of S. costatum-like species with the description of four new species. Journal of Phycology, 41: 151–176.
- Scholin, C. A., Herzog, M., Sogin, M. & Anderson, D. M. (1994). Identification for group-and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene1. Journal of Phycology, 30: 999–1011.
- Shimodaira, H. (2002). An approximately unbiased test of phylogenetic tree selection. Systematic Biology, 51: 492–508.
- Shimodaira, H. & Hasegawa, M. (2001). CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics, 17: 1246–1247.
- Silvestro, D. & Michalak, I. (2012). raxmlGUI: a graphical front-end for RAxML. Organisms Diversity and Evolution, 12: 335–337.
- Simonsen, R. (1987). Atlas and Catalogue of the Diatom Types of Friedrich Hustedt. Vol. 1: Catalogue, 1–525. Vol. 2: Atlas, pl. 1–395. Vol. 3: Atlas, pl. 396–772. Berlin, Stuttgart, J. Cramer.
- Sims, P.A., Mann, D.G. & Medlin, L.K. (2006). Evolution of the diatoms: insights from fossil, biological and molecular data. Phycologia, 45: 361–402.
- Sitoki, L., Kofler, W. & Rott, E. (2013). Planktonic needle-shaped Nitzschia species from Lake Victoria, Africa, revisited. Diatom Research, 28: 165–174.
- Smith, C., Heyne, S., Richter, A.S., Will, S. & Backofen, R. (2010). Freiburg RNA Tools: a web server integrating IntaRNA, ExpaRNA and LocARNA. Nucleic Acids Research, 38, Supplement: W373–377.
- Snoeijs, P., Vilbaste, S., Potapova, M., Kasperoviciene, J. & Balashova, J. (editors) (1993–1998). Intercalibration and distribution of diatom species in the Baltic sea. 5 vols. Opulus Press, Uppsala.
- Souffreau, C., Vanormelingen, P., Van de Vijver, B., Isheva, T., Verleyen, E., Sabbe, K & Vyverman, W. (2013). Molecular evidence for distinct Antarctic lineages in the cosmopolitan terrestrial diatoms Pinnularia borealis and Hantzschia amphioxys. Protist, 164:101–115.
- Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312–1313.
- Stepanek, J.G. & Kociolek, J.P. (2014). Molecular phylogeny of Amphora sensu lato (Bacillariophyta): an investigation into the monophyly and classification of the amphoroid diatoms. Protist, 165: 177–195.
- Stepanek, J.G., Hamsher, S.E., Mayama, S., Jewson, D.H. & Kociolek, J.P. (2016). Observations of two marine members of the genus Cymbellonitzschia (Bacillariophyta) from Tokyo Bay, Japan, with the description of the new species Cymbellonitzschia banzuensis. Phycological Research, 64: 26–34.
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution, 30: 2725–2729.
- Theriot, E.C., Ashworth, M., Ruck, E., Nakov, T. & Jansen, R.K. (2010). A preliminary multigene phylogeny of the diatoms (Bacillariophyta): challenges for future research. Plant Ecology and Evolution, 143: 278–296.
- Tomàs, X. (1988). Diatomeas de las aguas epicontinentales saladas del litoral mediterráneo de la Península Ibérica. PhD dissertation, University of Barcelona, Barcelona.
- Trobajo, R., Quintana, X.D. & Sabater, S. (2004). Factors affecting the periphytic diatom community in Mediterranean coastal wetlands (Emporda wetlands, NE Spain). Archiv für Hydrobiologie, 160: 375–399.
- Trobajo, R., Mann, D.G., Chepurnov, V.A., Clavero, E. & Cox, E.J. (2006). Taxonomy, life cycle, and auxosporulation of Nitzschia fonticola (Bacillariophyta). Journal of Phycology, 42: 1353–1372.
- Trobajo, R., Clavero, E., Chepurnov, V.A., Sabbe, K., Mann, D.G., Ishihara, S. & Cox, E.J. (2009). Morphological, genetic, and mating diversity within the widespread bioindicator Nitzschia palea (Bacillariophyceae). Phycologia, 48: 443–459.
- Trobajo, R., Mann, D.G., Clavero, E., Evans, K.M., Vanormelingen, P. & McGregor, R.C. (2010). The use of partial cox1, rbcL and LSU rDNA sequences for phylogenetics and species identification within the Nitzschia palea complex (Bacillariophyceae). European Journal of Phycology, 45: 413–425.
- Trobajo, R., Rovira, L., Ector, L., Wetzel, C.E., Kelly, M. & Mann, D.G. (2013). Morphology and identity of some ecologically important small Nitzschia species. Diatom Research, 28: 37–59.
- Vanormelingen, P., Vanelslander, B., Sato, S., Gillard, J., Trobajo, R., Sabbe, K. & Vyverman, W. (2013). Heterothallic sexual reproduction in the model diatom Cylindrotheca. European Journal of Phycology, 48: 93–105.
- Witkowski, A., Lange-Bertalot, H. & Metzeltin, D. (2000). Diatom flora of marine coasts I. Iconographia Diatomologica, 7: 1–925.
- Witkowski, A., Lange-Bertalot, H., Kociolek, J.P., Bąk, M., Kulikovskiy, M.S. & Kuznetsova, I. (2015a). Diatom flora of San Francisco Bay and vicinity III. New species in the genus Nitzschia Hassall. Nova Hedwigia, Beiheft, 144: 211–228.
- Witkowski, A., Gomes, A., Mann, D.G., Trobajo, R., Li, C., Barka, F., Gusev, E., Dąbek, P., Grzonka, J., Kurzydłowski, K.J., Zgłobicka, I., Harrison, M. & Boski, T. (2015b). Simonsenia aveniformis sp. nov. (Bacillariophyceae), molecular phylogeny and systematics of the genus, and a new type of canal raphe system. Scientific Reports, 5: 17115.
- Zalat, A. & Vildary, S. (2005). Distribution of diatom assemblages and their relationship to environmental variables in the surface sediments of three northern Egyptian lakes. Journal of Paleolimnology, 34: 159–174.
