ABSTRACT
The cultivation of red seaweeds for food (nori), agar and carrageenans is the basis of a valuable industry. However, taxonomic knowledge of these cultivated seaweeds and their wild relatives has not kept pace with advances in molecular systematics despite the fundamental importance of being able to identify commercially important species and strains, discover cryptic and endemic taxa and recognize non-native species with potentially damaging diseases and epiphytes. This review focuses on molecular taxonomic advances in the cultivated red algae with the highest commercial value globally: Eucheuma and Kappaphycus, Porphyra sensu lato and Gracilaria. All three groups are similarly taxonomically challenging: speciose, morphologically plastic, with poorly resolved species boundaries. Eucheuma and Kappaphycus are frequently misidentified and the molecular markers cox2-3 spacer, cox1 and RuBisCO spacer have helped in understanding phylogenetic relationships and identifying new species and haplotypes. In Porphyra sensu lato (Bangiales) species identification and phylogenetic relationships were highly problematic until a taxonomic revision based on a two-gene phylogeny (18S and rbcL) resulted in nine genera of bladed species. Pyropia, with at least 89 species, three in nori cultivation, has potential for new commercial evaluation. In Gracilaria sensu lato, earlier efforts to resolve species-level taxonomy and generic descriptions were superseded by application of molecular tools, including DNA sequences of the RuBisCO spacer, rbcL gene, 18S and the ITS region. Studies of these cultivated red algal genera highlight the need for a robust taxonomy, a more standardized approach to the molecular markers used and a comprehensive dataset for each representative species. Current work on DNA-based species delimitation, the emergence of high throughput sequencing, multi-gene phylogenies, publication of whole genomes (e.g. Porphyra umbilicalis) and genomes in the pipeline (e.g. Gracilaria) are increasingly improving our understanding of phylogenomic relationships and species relationships. This knowledge, in turn, can then be applied to improving red seaweed aquaculture.
Introduction
Red seaweeds have been collected from the wild for food and other products for thousands of years (Tseng, Citation1935; Brodie & Irvine, Citation2003; Collén et al., Citation2014; Ramírez et al., Citation2014 and references therein). The main uses of red algae, apart from food, have been as a source of the gelling hydrocolloids agar and carrageenan (Craigie, Citation1990). Until the Second World War (WWII, 1939–1945), seaweeds were mostly harvested from natural populations (Marshall et al., Citation1949), although Porphyra sensu lato has been cultivated in China and Japan for hundreds of years as food (Blouin et al., Citation2011; Yang et al., Citation2017). After WWII, in Asia the need for a more reliable crop after a major failure of the nori harvest in Japan led to the development of the modern nori industry (Yang et al., Citation2017). Growing demand for products over the second half of the 20th century (e.g. Marshall et al., Citation1949; Kim, Citation2012) saw a fundamental shift from wild harvesting in the North Atlantic of species including Chondrus crispus to farmed crops, such as Eucheuma in warmer tropical areas, particularly in the Pacific (Doty et al., Citation1986; Porse & Rudolph, Citation2017). A more recent drive towards the development and commercialization of functional foods, nutriceuticals, pharmaceuticals and bioactives from seaweeds is pushing up demand and leading to innovative methods of production (e.g. Hafting et al., Citation2012; Gutiérrez Cuesta et al., Citation2016). Current research indicates that macroalgal proteins contain all essential amino acids for food products and have additional bioactives (Garcia-Vaquero & Hayes, Citation2016).
Twelve red algal taxa are listed as currently in aquaculture production (FAO, Citation2015) and/or have been cultivated for consumption between 1990 and 2015 (), although the number and identity of many of these species are uncertain. The main taxa in cultivation are species of Kappaphycus, Eucheuma, Porphyra sensu lato and Gracilaria. Estimates of their wet weight harvest per continent are given in . However, the reliability of these data is questionable as the figures are based on reported ‘output from aquaculture activities designated for final harvest for consumption’ (FAO, Citation2015). For details of dry tonnage of agarophyte and carrageenophyte seaweeds for 2009 and 2015 see Porse & Rudolph (Citation2017). The main sources of carrageenan are Eucheuma denticulatum, Kappaphycus alvarezii and Kappaphycus striatum (Ask & Azanza, Citation2002), with Eucheuma “cottonii” making up 73% of world consumption (Porse & Rudolph, Citation2017). Agar from cultivated red seaweeds comes mostly from the genus Gracilaria not identified to species level (FAO, Citation2015; Porse & Rudolph, Citation2017). Gelidium yields agar of better quality than Gracilaria but it is not possible yet to grow it in cultivation and wild stocks have been severely over-exploited (Porse & Rudolph, Citation2017).
Table 1. Red seaweeds with countries where they are or have been cultivated between 1990 and 2015. Source: FAO (Citation2015).
Table 2. Global aquaculture production by continent for red seaweeds (wet weight). Source: Tonnage and value of 2015 Food and Agriculture Organization of the United Nations (FAO) statistics. F = FAO estimate; data estimated from available source of information or calculation based on specific assumptions.
Commercial marine seaweed cultivation is practiced heavily in the Asian Pacific region, with China, Indonesia and the Philippines contributing 88.7% (21 million tonnes) of the global farmed algal production in 2012 (FAO, Citation2014; Valderrama et al., Citation2015). The carrageenan-producing seaweeds Kappaphycus and Eucheuma make up approximately 33% of total algal production (FAO, Citation2014). From 1990 to 2012, the farming of these red seaweeds steadily increased in tandem with the rising demand for carrageenan (FAO, Citation2014; Hehre & Meeuwig, Citation2016). This is especially evident in Indonesia, currently the largest producer of K. alvarezii and Eucheuma spp. (FAO, Citation2014; Porse & Rudolph, Citation2017). The country produced 6.5 million metric tons of dried seaweed in 2012 (13.6% increase from 2009), of which c. 60% was from Kappaphycus and Eucheuma (KKP, Citation2013; Safari & Dardak, Citation2015). In 2013, Indonesia utilized 45% (343 643 hectares) of its suitable coastal areas for seaweed farming (KKP, Citation2013) and it has vast potential to increase its seaweed production (Hurtado et al., Citation2016).
Despite the fundamental shift in the production and supply of red seaweeds and the range of taxonomic tools now available, in most genera relatively little attention has been given to the molecular taxonomy of species under cultivation. In general, the application of molecular techniques in red algal taxonomy has revolutionized species concepts and taxonomic relationships, uncovered cryptic diversity (Robba et al., Citation2006; Leliaert et al., Citation2014; Filloramo & Saunders, Citation2016; Díaz-Tapia et al., Citation2017) and provided a greater understanding of species distributions in different geographic areas (Brodie et al., Citation2007), including evidence of much greater endemism than originally thought based on morphological identification (Brodie et al., Citation2008; Payo et al., Citation2013; Dumilag & Aguinaldo, Citation2017). Molecular analysis has also revealed that in many groups of red seaweeds considerable genetic diversity is not reflected in the morphology at the species level (e.g. Sutherland et al., Citation2011; Leliaert et al., Citation2014; Saengkaew et al., Citation2016).
Determining the correct taxonomic status of species in cultivation is crucial. Confusion in the taxonomy and systematics of cultivated red seaweed species has arisen due to the different names used in farming and commerce and the lack of material for proper identification. The names used by the FAO (Citation2015) provide a general overview and do not take into account recent taxonomic changes. Eucheuma, Kappaphycus, Gracilaria and Porphyra sensu lato have particularly challenging taxonomies: species are cosmopolitan, often lack reliable morphological characters for identification, and some have been accidentally or deliberately introduced to different parts of the world. For example, Pyropia yezoensis has been reported from the North-west Atlantic where it was most likely introduced from Japan (West et al., Citation2005; Mathieson et al., Citation2008; Neefus et al., Citation2008). Kappaphycus and Eucheuma species, which have been introduced for aquaculture in many different parts of the world (), are successful invaders (Williams & Smith, Citation2007; Sellers et al., Citation2014). The introduction of Kappaphycus spp. into Hawaii, for example, has resulted in negative impacts on coral reef ecosystems (Rodgers & Cox, Citation1999; Conklin & Smith, Citation2005) and the spread of K. alvarezii outside its cultivation sites in Panama has caused impacts on native biota (Sellers et al., Citation2014).
Introductions of non-native species for aquaculture can have consequences for the introduced species and for the indigenous flora. For example, cultivars with limited genetic stock are potentially susceptible to disease and epiphyte outbreaks (Cottier-Cook et al., Citation2016). Invasions from cultivated stocks of indigenous species have also been demonstrated in P. yezoensis in Japan, where there is evidence of plastid introgression from crops to wild populations (Niwa et al., Citation2009). This highlights the importance of, as well as the risks to, the genetic resource of wild species in natural populations for improvement of cultivated strains.
Products from different species and varieties can vary: agar polysaccharides from Gracilaria species have been shown to have different gel strengths (e.g. Marinho-Soriano, Citation2001), and different species of Eucheuma vary in their carrageenans (Phang et al., Citation2010). In nori cultivation, Pyropia tenera is considered to have a better texture than cultivated P. yezoensis (Niwa et al., Citation2005). Wild populations remain the source for new stocks for cultivation. At the same time as the seaweed industry is expanding in size and value (), environmental change due to increasing pressures on coastlines (Yang et al., Citation2017), loss of habitat due to land reclamation (Niwa et al., Citation2005) and climate change (Brodie et al., Citation2014) are all impacting on seaweed populations.
Fig. 1. Comparison of production weights and values globally (the great majority in Asia), based on data from FAO (), arranged by value. Eucheuma spp. and Kappaphycus alvarezii are high volume, low value crops, whereas Gracilaria spp. are produced in much smaller quantities but are high value. Porphyra/Pyropia spp. are intermediate in volume and value.
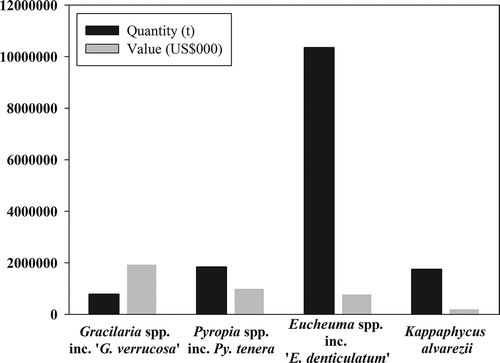
In this review we focus on molecular taxonomic advances in the red algal genera Eucheuma, Kappaphycus, Porphyra sensu lato (including Pyropia) and Gracilaria, which have the greatest harvests and/or the highest commercial value globally (–, ).
Kappaphycus and Eucheuma
The foundations of the modern taxonomy of Kappaphycus and Eucheuma (Solieraceae, Gigartinales) are Max S. Doty’s studies based on examination of tetrasporophytic, carposporophytic and gametophytic material (e.g. Doty & Alvarez, Citation1975; Doty, Citation1985, Citation1988; Doty & Norris, Citation1985). Eucheuma was originally divided into four sections, Cottoniformia, Eucheuma, Gelatiformia and Anaxiferae, of which the section Cottoniformia was later established as the segregate genus Kappaphycus (Doty, Citation1988). Betaphycus, proposed by Doty (Citation1995) for B. philippinensis based partly on its carrageenan type, was validated by Silva et al. (Citation1996), and there are currently three recognized species (Guiry & Guiry, Citation2017). The morphological characters described by Doty () are still in use today. Currently there are six taxonomically accepted Kappaphycus species and 30 Eucheuma species (Guiry & Guiry, Citation2017). Kappaphycus alvarezii, K. striatus and E. denticulatum are among the best known because of their commercial value. All six species of Kappaphycus are generally well documented in terms of morphology and to a certain extent, genetically (Tan et al., Citation2014); in contrast, in Eucheuma the lack of specimens and taxonomic research has impeded progress over the years.
Table 3. General differences between Kappaphycus, Eucheuma and Betaphycus (Doty, Citation1985, Citation1995).
In spite of their commercial importance, Kappaphycus and Eucheuma are often misidentified as a result of morphological plasticity and the widespread and often indifferent use of colloquial, commercial (cottonii and spinosum) and local names (Doty, Citation1985; Zuccarello et al., Citation2006; Tan et al., Citation2013; Hurtado, Citation2013). In the Philippines, four varieties of K. alvarezii and three varieties of K. striatus have been reported (Hurtado, Citation2013). Likewise, six varieties of K. alvarezii were reported from Malaysia, each with its own local name (Tan et al., Citation2013; Lim et al., Citation2014b). This phenomenon was also seen in China (Zhao & He, Citation2011) and Brazil (de Barros-Barreto et al., Citation2013) and is likely to be prevalent where these seaweeds are commercially cultivated. The plasticity of Kappaphycus and Eucheuma often results in the cultivation of mixed populations by local farmers (Tan et al., Citation2013), which hinders the processing of kappa- (from Kappaphycus) and iota- (from Eucheuma) carrageenans, requiring prior separation of these seaweeds (Lim et al., Citation2014b). Morphological examination is often challenging due to the lack of cystocarpic specimens which exhibit more distinctive characters and aggravated by the fact that upon drying specimens lose some of their form and structure.
Zuccarello et al. (Citation2006) sequenced the mitochondrial cox2-3 spacer and plastid RuBisCO spacer to better understand the phylogeny and genetic variation of Kappaphycus and Eucheuma worldwide. Their molecular analyses supported the genetic distinction between K. alvarezii and K. striatus, as well as revealing several distinct genotypes of K. alvarezii and E. denticulatum, some of which are unique to certain regions (e.g. Hawaii, Africa; Zuccarello et al., Citation2006). The study also demonstrated the feasibility of using molecular markers in species identification, which was corroborated by Tan and co-workers (Citation2013) who applied a combination of markers to verify Kappaphycus and Eucheuma varieties in Malaysia, leading to the description of K. malesianus (Tan et al., Citation2014). Currently genetic data are available (at least one molecular marker in published literature) for 83% and 10% of species of Kappaphycus and Eucheuma, respectively. Of the DNA markers used, the mitochondrial cox2-3 spacer was the preferred one due to its resolution of inter- and intraspecific relationships. Over the years, the cox2-3 spacer has been used for DNA barcoding (Tan et al., Citation2012), molecular identification and systematics (Zhao & He, Citation2011; Araújo et al., Citation2013; Tan et al., Citation2013; Dumilag & Lluisma, Citation2014), species description (Ganzon-Fortes et al., Citation2012; Tan et al., Citation2014) and detection of bioinvasions (Conklin et al., Citation2009). The cox2-3 spacer was also combined with the mitochondrial cox1 gene in a collaborative study by the major carrageenan producers of South-east Asia to document the genetic diversity of Kappaphycus and Eucheuma within the region (Lim et al., Citation2014a). Although not exhaustive, the study revealed several new haplotypes or potential species of Kappaphycus and Eucheuma, some of which were already being farmed commercially. The establishment of an improved genetic database of these carrageenophytes would undoubtedly help in marker-assisted selection or breeding, a technique already applied in agriculture and animal breeding.
The application of molecular markers has provided insight into the taxonomy of Kappaphycus, Eucheuma and Betaphycus (). Apart from allowing the identification of tiny, dried or deformed specimens, the use of genetic markers has provided an independent approach to phylogenetic reconstruction. Although uninformative for phylogenetic relationships among the genera Kappaphycus, Eucheuma and Betaphycus (), the cox2-3 spacer is remarkably accurate for inter- and intraspecific delineation within a genus (Zuccarello et al., Citation2006; Tan et al., Citation2012). Taxonomically, the use of this marker has revealed: (i) three genotypes in commercial strains of K. alvarezii, presumably originating from the Philippines (Ask & Azanza, Citation2002; Ask et al., Citation2003; Hurtado et al., Citation2015), as well as other strains unique to Africa and Hawaii; (ii) two potentially cryptic species of K. striatus in South-east Asia; (iii) genetic differences between K. malesianus, K. inermis and K. cottonii; (iv) three genotypes of E. denticulatum – commercially farmed strains, ‘Endong’ strains from South-east Asia and strains unique to Africa; (v) several genotypes that are to date not assessed; and (vi) potentially misidentified taxa, e.g. E. isiforme (Zuccarello et al., Citation2006; Conklin et al., Citation2009; Ganzon-Fortes et al., Citation2012; Tan et al., Citation2012, Citation2013, Citation2014; Dumilag & Lluisma, Citation2014; Lim et al., Citation2014a).
Fig. 2. Simplified phylogeny of Kappaphycus, Eucheuma and Betaphycus based on cox2-3 spacer datasets from Zuccarello et al. (Citation2006), Conklin et al. (Citation2009), Dumilag & Lluisma (Citation2014), Dumilag et al. (Citation2014), Lim et al. (Citation2014a), Tan et al. (Citation2012, Citation2014) and relevant GenBank sequences. Supplementary details are summarized in Table S1. Maximum likelihood bootstrap support/Bayesian posterior probabilities. * indicates 100%/1.00 support.
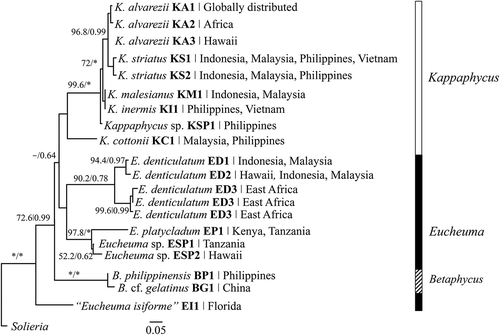
Multiple genetic markers are required to elucidate the phylogeny of Kappaphycus, Eucheuma and Betaphycus at generic and family levels. The degree of genetic variation in different DNA markers (especially from different organelles) would be normalized when analysed together, and would provide a better representation of evolutionary pathways. The use of a concatenated dataset of cox1+ cox2-3 spacer sequences for a small number of species () resolved some nodes better than the cox2-3 spacer alone. This dataset is expected to better resolve the relationship between Kappaphycus and Eucheuma when more genetic data for K. cottonii and E. arnoldii (seaweeds suspected to be ‘intermediary’ between these genera) becomes available. However, the ‘multigene’ approach will only be possible when sequences are available for each representative species, which in turn requires the standardization of the molecular markers utilized.
Fig. 3. Phylogeny of Kappaphycus spp. based on concatenated cox1 + cox2-3 spacer molecular markers. DNA sequences were based on Zuccarello et al. (Citation2006), Conklin et al. (Citation2009), Dumilag & Lluisma (Citation2014), Lim et al. (Citation2014a), Tan et al. (Citation2012, Citation2014) and relevant GenBank sequences. Supplementary details are summarized in Table S1. Maximum likelihood bootstrap support/Bayesian posterior probabilities (expressed in percentages). Diagrams not drawn to scale. * indicates 100%/1.00 support.
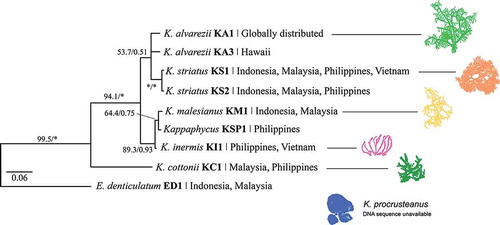
Under-sampling is a major hurdle for the advancement of Kappaphycus and Eucheuma (and Betaphycus) taxonomy. Although specimens have been collected worldwide, of the few that have been sequenced, the majority were either procured from markets or seaweed farms, leading to a general underestimation of biodiversity and genetic diversity as cultivars were typically vegetatively propagated from the same few commercial strains. For example, specimens of K. procrusteanus have not been collected after its first description and attempts to sequence DNA from the type specimen proved futile (Tan et al., Citation2014). In contrast, preliminary results on genetic diversity in South-east Asia (Lim et al., Citation2014a) have revealed numerous unidentified genotypes and potential species, suggesting that more genotypes are yet to be discovered. Therefore, future sampling efforts should focus on unsampled areas or places distant from seaweed farms. However, the extensive area involved will require coordination and concerted effort between stakeholders, industry players and academia.
Porphyra sensu lato (including Pyropia)
The Bangiales is a diverse, cosmopolitan order of red algae and a major economic resource in the production of nori (Guillemin et al., Citation2016). Species of Porphyra sensu lato (bladed Bangiales) have been a food source for thousands of years in different parts of the world, for example, in Wales (laver), Chile (luche or luchi), Japan (nori) and China (Tsu-Tsai) (Blouin et al., Citation2011; Brodie & Irvine, Citation2003; Brodie et al., Citation2008; Ramírez et al., Citation2014; Guillemin et al., Citation2016; Yang et al., Citation2017). Porphyra sensu lato may have been the first seaweed to be cultivated (Kain, Citation1991; Blouin et al., Citation2011) and its cultivation in Tokyo Bay, Japan, can be traced back to at least 1640 (Miura, Citation1975).
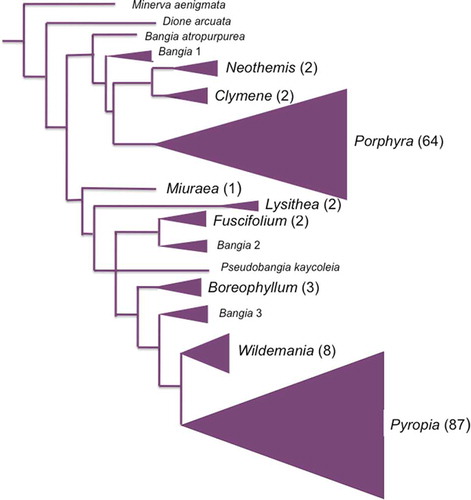
Until the application of molecular techniques, the identification and taxonomic placement of taxa within the bladed Bangiales was highly problematic due to the simple morphology and variation within and between species (Brodie et al., Citation2008; Gunnarsson et al., Citation2016). However, a concerted effort by a group of scientists from around the world focussing on the taxonomy of the Bangiales led to a major taxonomic revision of the order based on a two-gene phylogeny (Sutherland et al., Citation2011). The result was that the bladed Bangiales were split into eight genera: Boreophyllum, Clymene, Fuscifolium, Lysithea, Miuraea, Porphyra, Pyropia and Wildemania. A re-evaluation of the taxonomy of the bladed Bangiales from other parts of the world has led to a ninth bladed genus, Neothemis, being described based on a study in the western Mediterranean (; Sánchez et al., Citation2014).
Fig. 4. Phylogeny of eight genera of bladed Bangiales. Triangles represent relative number of species (numbers in parentheses) in each genus. Filamentous (Bangia) forms shown in small font. All clades shown have >75%/0.9 maximum likelihood/Bayesian support. Outgroups have been trimmed off. Sources: Phylogenetic tree is simplified from Sánchez et al. (Citation2014); numbers of species derived from Guiry & Guiry (Citation2017) and Yang & Brodie (personal observations).
There are over 160 described species of bladed Bangiales but there is thought to be considerably more diversity. For example, recently 17 new species of Porphyra, Pyropia and Wildemania were discovered in the south-eastern Pacific (Ramírez et al., Citation2014; Guillemin et al., Citation2016) and four new Pyropia species were described from the west coast of North America (Lindstrom et al., Citation2015). A re-evaluation of the bladed Bangiales along the coast of China indicates that there is a rich Pyropia flora (Yang et al., unpublished data).
With 89 species to date (some of which are yet to be described), Pyropia (Py.) is the most speciose genus of the Bangiales (Brodie & Yang, personal observations). According to Sutherland et al. (Citation2011), the Pyropia clade is strongly supported as monophyletic, although various constituent clades (at least eight) were also resolved with strong support. In a more up to date phylogeny, five intrageneric clades are resolved (Yang et al., unpublished data) with a strong biogeographic signal. Pyropia also contains most of the economically important nori species: Py. yezoensis, Py. tenera and Py. haitanensis (–) are cultivated in Japan, China, and Korea and the industry is worth about US$1.3 billion per year (Blouin et al., Citation2011). Py. yezoensisis is the main species in cultivation in all three countries (although Py. haitanensis is also cultivated in China), and its main products are known as nori (のり) in Japan and Hai-Tai (海苔) in China (Yang et al., Citation2017).
Figs 5–7. Pyropia species used in aquaculture. Fig. 5. P. tenera, collected from Penglai, Shandong Province, China on 28 March 2016 by L.E. Yang. Morphology is atypical for this species but the identity was verified with molecular data. Fig. 6. P. haitanenesis collected from Pingyu Island, Guangdong Province, China on 16 December 2012 by Weizhou Chen. Fig. 7. P. yezoensis collected from Yantai, Shandong Province on 24 March 2016 by L.E. Yang.

In China, different Pyropia strains have been developed and used in the nori cultivation industry. Two novel cultivars of Py. yezoensis have been certified by the National Certification Committee for Aquatic Varieties (NCCAV) and named Su-Tong Nos 1 and 2 (Yang et al., Citation2016). These cultivars are extensively used in the industry. Four novel cultivars of Py. haitanensis certified by NCCAV are named as Shen-Fu Nos 1 and 2 (Song, Citation2016), Min-Feng No. 1 (Wang et al., Citation2013) and Zhe-Dong No. 1 (Luo et al., Citation2015). These cultivars can be distinguished by genetic markers including AFLP (Yang et al., Citation2016) and ITS-5.8S sequences (Xie et al., Citation2013). Many other strains are being studied (Cao et al., Citation2016; Yang et al., Citation2016), but have not yet been named. Porphyra haitanensis has been transferred to Pyropia haitanensis (T.J.Chang & B.F. Zheng) N.Kikuchi & M.Miyata (Sutherland et al., Citation2011), but the generic position of the three varieties described by Zheng & Li (Citation2009) as var. culta, var. grandidentata and var. schizophylla still needs to be verified. Whether these varieties are extensively used in the industry remains unknown.
In Japan, Pyropia tenera (as Porphyra tenera Kjellman) was extensively cultivated before the artificial seeding of conchospores was developed (Ueda, Citation1932). Later, P. tenera Kjellman var. tamatsuensis Miura and P. yezoensis Ueda f. narawaensis Miura were described and extensively cultivated in Japan (Miura, Citation1984). With the impact of environmental change, P. tenera var. tamatsuensis was endangered and P. yezoensis f. narawaensis became the main cultivar in Japan (Niwa et al., Citation2005). After the transfer of P. tenera Kjellman and P. yezoensis Ueda to Pyropia, these two cultivars in Japan were transferred, respectively, to Pyropia tenera (Kjellman) N. Kikuchi, M. Miyata, M.S. Hwang & H.G. Choi var. tamatsuensis (A. Miura) N. Kikuchi, Niwa & Nakada and Pyropia yezoensis (Ueda) M.S. Hwang & H.G. Choi f. narawaensis (A. Miura) N. Kikuchi, Niwa & Nakada (Kikuchi et al., Citation2015). In Korea, Py. yezoensis is the main cultivated species although the form or variety is currently unknown. Hwang et al. (Citation2014) sequenced the mitochondrial genome of Py. yezoensis cultivated in Korea (KF561997) but the data have not yet been released yet which might enable us to resolve the question.
For those taxa of the bladed Bangiales that are used as food but are not in cultivation, there is some uncertainty as to the species involved. It is probable that several species of Porphyra are used as laver in Britain (Brodie & Irvine, Citation2003). The species used for luche or luchi in Chile has traditionally been called Porphyra columbina, although this species does not appear to occur there (Nelson & Broom, Citation2010). Specimens collected under this name have been shown to belong to three recently diverged haplotypes of Pyropia orbicularis (Ramírez et al., Citation2014; Guillemin et al., Citation2016).
Given the extent of the diversity both at the species and generic level within the bladed Bangiales, there is potential for new species and/or strains from different parts of the world to be brought into culture. Molecular taxonomic/phylogenetic analysis has been valuable in demonstrating species relationships. Clearly there is a considerable amount of taxonomy still to be undertaken with the aim of better understanding species and genera.
Gracilaria and Gracilariopsis
Gracilaria sensu lato has been the major world source of food-grade agar for several decades (McHugh, Citation1991; Hurd et al., Citation2014). Its high commercial value led to widespread efforts in the 1980s and 1990s to resolve species-level taxonomy and generic circumscriptions. Bird & McLachlan (Citation1982) noted that Gracilaria species were poorly defined, due to their notorious plasticity, with over 300 described species including multiple synonyms of the 100 recognized species. Gracilaria sensu lato was thus an important element of the Taxonomy of Economic Seaweeds workshops initiated by Isabella Abbott and Jim Norris in 1984, which addressed the difficulties in establishing correct names for commercially important seaweeds. In the proceedings of the first workshop, the economically significant species in Japan and China were considered to be the flat, digitate G. textorii (Suringar) De Toni, knobbly G. eucheumatoides Harvey (as G. eucheumoides), compressed G. bursa-pastoris (S.G.Gmelin) P.C.Silva, and the terete species Gracilariopsis (as Gracilaria) lemanieformis (Bory) Weber van Bosse, G. ‘verrucosa’, G. tenuistipitata C.F.Chang & B.M.Xia, G. vermiculophylla Ohmi, G. chorda Holmes and G. hainanensis C.F.Chang & B.M.Xia (Bangmei & Yamamoto, Citation1985). Abbott et al. (Citation1985) noted the problems in finding diagnostic morphological features in terete species. In particular, the seaweed known as G. verrucosa (Hudson) Papenfuss, reported to occur almost worldwide, was clearly heterogeneous at both species and genus levels. Gracilaria verrucosa was then considered to be the type species, originally described from the British Isles, so the nomenclature of this economically important genus was conserved by designating Gracilaria compressa (C.A.Agardh) Greville (a synonym of Gracilaria bursa-pastoris) as the lectotype of the genus (Steentoft et al., Citation1995).
The most useful morphological characters were found in the spermatangial structures (Yamamoto, Citation1978; Bird & McLachlan, Citation1982) which are distributed in superficial layers or in conceptacles of different types: shallow crypts (textorii type), single, deep crypts (verrucosa type), or deep, confluent compound crypts (henriquesiana type, used to segregate Polycavernosa C.F.Chang & B.M.Xia (a synonym of Hydropuntia Montagne) in 1963 from Gracilaria species). However, morphological overlap casts doubt on the diagnostic value of spermatangial arrangement (Abbott et al., Citation1991). Female reproductive characters were employed by Fredericq & Hommersand (Citation1989b) to show that Gracilariopsis E.Y.Dawson, which had been regarded for decades as a synonym of Gracilaria, was distinct. Fundamental differences in both female and male reproductive morphology were used to separate the Gracilariales from the Gigartinales, which aligned with the formation of agar by the Gracilariales in contrast to the carageenans of the Gigartinales (Fredericq & Hommersand, Citation1989a). The Gracilariales currently contains only the families Gracilariaceae and the parasitic Pterocladiophilaceae (Guiry & Guiry, Citation2017).
The large number of species and the paucity of morphological characters were so challenging that, as soon as molecular tools became available to phycologists, they were applied to define and circumscribe members of the Gracilariales. Rice & Bird (Citation1990) applied RFLP markers to 11 populations of ‘G. verrucosa’ from around the world and found that they were markedly heterogeneous (including what was later understood to be Gracilariopsis spp.). The first sequence data for the RuBisCO spacer (Destombe & Douglas, Citation1991), the 18S rDNA gene (Bird et al., Citation1990, Citation1992) and the ITS (Goff et al., Citation1994) all showed high divergences between Gracilaria and Gracilariopsis. Gurgel & Fredericq (Citation2004) reviewed molecular work to date, which had provided strong evidence supporting the taxonomic distinctiveness of the genera Curdiea, Melanthalia, Gracilaria and Gracilariopsis, but had not resolved the position of Hydropuntia. Using rbcL sequences for a then relatively large taxon set, Gurgel & Fredericq (Citation2004) resurrected Hydropuntia (type species: H. urvillei Montagne, a synonym of Gracilaria edulis (S.G.Gmelin) P.C.Silva; see Guiry & Guiry, Citation2017) for algae including the commercial crop species Hydropuntia (formerly Gracilaria) eucheumatoides and Hydropuntia (formerly Gracilaria) edulis. They also identified a clade with two commercial species, G. chilensis, the basis of the modern seaweed aquaculture industry in Chile (Buschmann et al., Citation2008; Bixler & Porse, Citation2011) and G. vermiculophylla (as ‘Gracilaria aff. tenuistipitata’) that they felt merited generic status, but which was never formally described. The spermatangial characters used to divide the genus by earlier workers were not diagnostic for lineages – the textorii type of spermatangial conceptacle had arisen at least twice in the evolutionary history of Gracilaria sensu lato (Gurgel & Fredericq, Citation2004).
Subsequent phylogenetic analyses using various molecular markers have reported broadly congruent trees to those of Gurgel & Fredericq (Citation2004), but taxonomic and nomenclatural interpretations have differed according to authors. While the relationships between clades have now been established fairly robustly using a three-gene dataset (; Lyra et al., Citation2015), taxonomic treatment of the genera has not yet stabilized, even for economically significant species. Lyra et al. (Citation2015) recovered Gurgel & Fredericq’s (Citation2004) Gracilaria chilensis/G. vermiculophylla clade (, clade II) within Gracilaria. Multiple generic reassignments were necessitated as Hydropuntia was again subsumed in Gracilaria (Lyra et al., Citation2015; ). Currently, of the six recognized genera in the Gracilariaceae, only Gracilaria and Gracilariopsis are of major commercial interest; they can be distinguished by spermatangial distribution ().
Fig. 8. Phylogenetic analysis of some Gracilaria, Gracilariopsis and Hydropuntia species based on three genes (rbcL, UPA and cox1), rooted with Rhodymenia and Gelidium. Values above branches are ML bootstrap values (left) and Bayesian posterior probabilities expressed as percentages (right), with full support indicated by an asterisk. The genera Melanthalia and Curdiea (not shown) are basal to Gracilaria and Gracilariopsis. Gracilaria consists of clades I–V, Hydropuntia is paraphyletic, and spermatangia and thallus type are mapped to the right of the phylogeny (reproduced from Lyra et al., Citation2015, fig. 1, with permission).
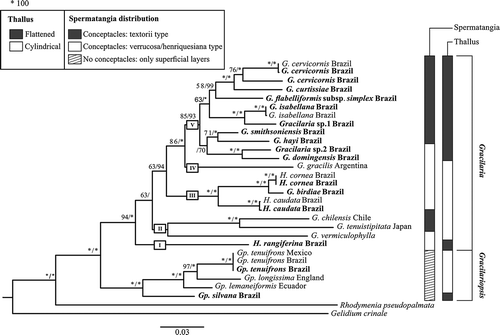
At the species level, many new taxa are still being described, recognized or transferred between genera, and there is still a lot of uncertainty concerning the biodiversity and taxonomy of this group. In particular, lack of morphological characters for the terete species has led to ongoing confusion both locally and globally, such that species are being newly discovered even in well-studied areas (e.g. Gracilaria dura was confused with G. gracilis in the British Isles; Destombe et al., Citation2010). Species circumscriptions are not always resolved by molecular data: hybridization between these two species was revealed by comparing organellar and nuclear DNA sequence markers, and cryptic species are present in the Atlantic and Mediterranean regions (Destombe et al., Citation2010). Gracilaria dura is considered to be an economically important species in India with the potential for aquaculture production of agarose (e.g. Veeragurunathan et al., Citation2015). However, the lack of reference to type materials and the high sequence divergence of purportedly conspecific samples in GenBank (Pareek et al., Citation2010) indicate that this is another example where further investigation is required for correct identification. Even when type materials are consulted, these may consist of multiple species or even genera due to the lack of diagnostic features (Muangmai et al., Citation2014).
Aquaculture of Gracilaria, with a large part of the production in Chile and Indonesia, has ensured that it remains the main genus used for agar and the price is stable (Bixler & Porse, Citation2011; FAO, Citation2015; Porse & Rudolph, Citation2017). As Steentoft et al. (Citation1995) noted, a revised definition of agar should include the correct name of the species of origin to ensure a uniform product. Molecular markers have been and will continue to be critical in developing a new taxonomy of the Gracilariaceae (Lyra et al., Citation2015). As an example, a recent cox1 barcoding study of the family in Australasia found five of the 22 discrete species to be unknown and potentially undescribed (Yang & Kim, Citation2015).
The way forward/future perspectives
All three groups considered here are highly speciose, morphologically plastic, and boundaries between species are often poorly resolved with evidence of incipient or recent speciation (e.g. Destombe et al., Citation2010; Guillemin et al., Citation2016). In the Eucheuma/Kappaphycus lineage, the main challenges are at the level of species and cultivars. Relationships between cultivated strains and wild strains are almost unknown. Phylogenetic and phylogeographic approaches could assist in the search for possible sources of additional species to cultivate, and in the search for disease-resistant strains. A clearer view of species boundaries will provide opportunities to better understand the distribution of species and their value as genetic resources, both for conservation and management. A concerted global DNA barcoding approach with common markers (e.g. cox1, cox2-3 spacer, partial rbcL sequences) would clarify which species are in cultivation and their distributions, as well as providing information on relationships among populations (e.g. Yow et al., Citation2013). For Porphyra sensu lato, multiple lineages have been resolved, and the evidence so far suggests that although just a very tiny number of species are used in cultivation, there is scope for a new phylogenomic evaluation of this group of red algae. The publication of the Porphyra umbilicalis genome (Brawley et al., Citation2017) opens up this quest. We have the potential to find new genetic markers for identification and, coupled with high-throughput sequencing, the possibility that multi-gene phylogenies will enable us to undertake this evaluation. In the Gracilariales, Lyra et al. (Citation2017) report on their ongoing phylogenomic analysis of sequences of nuclear genes and organellar genomes from 38 species. Their multicoalescent nuclear species tree analysis inferred a strongly supported phylogeny, indicating relationships among the clades and genera within this order. New developments in phylogenomics and high-throughput sequencing, as well as advances in bioinformatics, are expected to contribute greatly to clarifying the molecular systematics of these three economically important groups within the next five years.
1365174_Supp_material.docx
Download MS Word (17.3 KB)Acknowledgements
Li-En Yang acknowledges funding from the China Scholarship Council (No. 201608320018), which has enabled him to conduct his research on the bladed Bangiales of China at the Natural History Museum, London.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Phaik-Eem Lim
P.-E. Lim: drafted review on Eucheuma and Kappaphycus; J. Tan: contributed to molecular analysis for Eucheuma and Kappaphycus; L.-E. Yang: contributed to review and molecular analysis for Porphyra sensu lato; C.A. Maggs: reviewed Gracilariacae and collated the review of all groups; J. Brodie: reviewed seaweed industry and led review of Porphyra sensu lato.
References
- Abbott, I.A., Chiang, Y.M., Fredericq, S., Norris, J.N, Tsuda, R.T., Bangmei, X. & Yamamoto, H. (1985). The red alga Gracilaria Greville (Gracilariales, Gigartinales): Introduction. In Taxonomy of Economic Seaweeds with Reference to some Pacific and Caribbean Species (Abbott, I.A. & Norris, J.N., editors), 67–68. California Sea Grant College Program, La Jolla, California.
- Abbott, I.A., Zhang, J. & Xia, B. (1991). Gracilaria mixta sp. nov. and other western Pacific species of the genus (Rhodophyta: Gracilariaceae). Pacific Science, 45: 12–27.
- Araújo, P.G., Miranda, G.E.C., Barros-Barreto, M.B. & Fujii, M.T. (2013). Molecular identification of the exotic lineage of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) cultivated in the tropical region of Brazil. Phytotaxa, 109: 17.
- Ask, E.I. & Azanza, R.V. (2002). Advances in cultivation technology of commercial eucheumatoid species: a review with suggestions for future research. Aquaculture, 206: 257–277.
- Ask, E.I., Batibasaga, A., Zertuche-González, J.A. & de San, M. (2003). Three decades of Kappaphycus alvarezii (Rhodophyta) introduction to non-endemic locations. Proceedings of the International Seaweed Symposium, 17: 49–57.
- Bangmei, X. & Yamamoto, H. (1985). Gracilaria species from both China and Japan: key, list and distribution of common and economically important species. In Taxonomy of Economic Seaweeds with Reference to some Pacific and Caribbean Species (Abbott, I.A. & Norris, J.N., editors), 69–70. California Sea Grant College Program, La Jolla, California.
- Bird, C.J. & McLachlan, J. (1982). Taxonomy of Gracilaria: taxonomic criteria in Gracilaria (Rhodophyta, Gigartinales). Botanica Marina, 25: 557–562.
- Bird, C.J., Rice, E.L., Murphy, C.A., Liu, Q.Y., & Ragan, M.A. (1990). Nucleotide sequences of 18S ribosomal RNA genes from the red algae Gracilaria tikvahiae McLachlan, Gracilaria verrucosa (Hudson) Papenfuss and Gracilariopsis sp. Nucleic Acids Research, 18: 4023–4024.
- Bird, C.J., Rice, E.L., Murphy, C.A. & Ragan, M.A. (1992). Phylogenetic relationships in the Gracilariales (Rhodophyta) as determined by 18S rDNA sequences. Phycologia, 31: 510–522.
- Bixler, H.J. & Porse, H. (2011). A decade of change in the seaweed hydrocolloids industry. Journal of Applied Phycology, 23: 321–335.
- Blouin, N., Brodie, J., Grossman, A., Xu, P. & Brawley, S.H. (2011). The organism and the crop. Trends in Plant Sciences, 16: 29–37.
- Brawley, S.H. et al. (2017). Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta). Proceedings of the National Academy of Sciences of the United States of America. doi:10.1073/pnas.1703088114.
- Brodie, J.A. & Irvine, L.M. (2003). Seaweeds of the British Isles. Volume 1. Rhodophyta. Part 3B Bangiophycidae. Intercept Ltd, Hampshire.
- Brodie, J., Bartsch, I., Neefus, C., Orfanidis, S., Bray, T. & Mathieson, A. (2007). New insights into the cryptic diversity of the North Atlantic-Mediterranean ‘Porphyra leucosticta’ complex: P. olivii sp. nov. and P. rosengurttii (Bangiales, Rhodophyta). European Journal of Phycology, 42: 3–28.
- Brodie, J., Mols Mortensen, A.M., Ramírez, M.E., Russell, S. & Rinkel, B. (2008). Making the links: towards a global taxonomy for the red algal genus Porphyra (Bangiales, Rhodophyta). Journal of Applied Phycology, 20: 939–949.
- Brodie, J., Williamson, C.J., Smale, D.A. Kamenos, N.A., Mieszkowska, N., Santos, R., Cunliffe, M., Steinke, M., Yesson, Y., Anderson, K.M., Asnaghi, V., Brownlee, C., Burdett, H.L., Burrows, M., Collins, S., Donohughe, P., Harvey, B., Foggo, A., Noisette, F., Nunes, J., Raggazola, F., Raven, J.A., Schmidt, D.N., Suggett, D., Teichberg, M. & Hall-Spencer, J.M. (2014). The future of the NE Atlantic benthic flora in a high CO2 world. Ecology and Evolution, 4: 2787–2798.
- Buschmann, A.J., Hernández-Gonález, M.C. & Varela, D. (2008). Seaweed future cultivation in Chile: perspectives and challenges. International Journal of Environmental Pollution, 33: 432–455.
- Cao, Y., Wang, W.J., Liang, Z.R., Liu, F.L., Sun, X.T., Yao, H.Q., Li, X.L. & Wang, F.J. (2016). Genetic and nutrient analysis of new Pyropia yezoensis strain “Huangyou No. 1”. Guangxi Sciences, 23: 131–137. (In Chinese with English abstract)
- Collén, J., Cornish, M.L., Craigie, J., Ficko-Blean, E., Hervé, C., Krueger-Hadfield, S.A., Leblanc, C., Gurvan, M., Potin, P., Tonon, T. & Boyen, C. (2014). Chondrus crispus – a present and historical model organism for red seaweeds. Advances in Botanical Research, 71: 53–89.
- Conklin, E.J. & Smith, J.E. (2005). Abundance and spread of the invasive red algae, Kappaphycus spp., in Kane’ohe Bay, Hawai’i and an experimental assessment of management options. Biological Invasions, 7: 1029–1039. http://dx.doi.org/10.1007/s10530-004-3125-x.
- Conklin, K.Y., Kurihara, A. & Sherwood, A.R. (2009). A molecular method for identification of the morphologically plastic invasive algal genera Eucheuma and Kappaphycus (Rhodophyta, Gigartinales) in Hawaii. Journal of Applied Phycology, 21: 691–699.
- Cottier-Cook, E.J. et al. (2016). Safeguarding the Future of the Global Seaweed Aquaculture Industry. 12. United Nations University (INWEH) and Scottish Association for Marine Science, UK.
- Craigie, J.S. (1990). Cell walls. In Biology of the Red Algae (Cole, K.M. & Sheath, R.G., editors), 221–257. Cambridge University Press, Cambridge.
- De Barros-Barreto, M.B.B., Marinho, L.C., Reis, R.P., Souza da Mata, C. & Gomes Ferreira, P.C. (2013). Kappaphycus alvarezii (Gigartinales, Rhodophyta) cultivated in Brazil: is it only one species? Journal of Applied Phycology, 25: 1143. doi: 10.1007/s10811-012-9952-8.
- Destombe, C. & Douglas, S.E. (1991). Rubisco spacer sequence divergence in the rhodophyte alga Gracilaria verrucosa and closely related species. Current Genetics, 19: 395–398.
- Destombe, C., Valero, M. & Guillemin, M.L. (2010). Delineation of two sibling red algal species, Gracilaria gracilis and Gracilaria dura (Gracilariales, Rhodophyta), using multiple DNA markers: resurrection of the species G. dura previously described in the Northern Atlantic 200 years ago. Journal of Phycology, 46: 720–727.
- Díaz-Tapia, P., McIvor, L., Freshwater, D.W., Verbruggen, H., Wynne, M.J. & Maggs, C.A. (2017). The genera Melanothamnus Bornet & Falkenberg and Vertebrata S.F. Gray constitute well-defined clades of the red algal tribe Polysiphonieae (Rhodomelaceae, Ceramiales). European Journal of Phycology, 52: 1–30.
- Doty, M.S. (1985). Eucheuma alvarezii, sp. nov. (Gigartinales, Rhodophyta) from Malaysia. In Taxonomy of Economic Seaweeds: with Reference to Some Pacific and Caribbean Species (Abbott, I.A. & Norris, J.N., editors), 37–45. California Sea Grant College Program, La Jolla, California.
- Doty, M.S. (1988). Prodomus ad systematica eucheumatoideorum: A tribe of commercial seaweeds related to Eucheuma (Solieriaceae, Gigartinales). In Taxonomy of Economic Seaweeds (Abbott, I.A., editor), 47–61. California Sea Grant Program, La Jolla, California.
- Doty, M.S. (1995). Betaphycus philippinensis gen. et sp. nov. and related species (Solieriaceae, Gigartinales). In Taxonomy of Economic Seaweeds Vol. 5 (Abbott, I.A., editor), 237–245. California Sea Grant Program, La Jolla, California.
- Doty, M.S. & Alvarez, V.B. (1975). Status, problems, advances and economics of Eucheuma farms. Marine Technology Society Journal, 9: 30–35.
- Doty, M.S. & Norris J.N. (1985). Eucheuma species (Solieriaceae, Rhodophyta) that are major sources of carrageenan. In Taxonomy of Economic Seaweeds: with Reference to Some Pacific and Caribbean Species (Abbott, I.A. & Norris, J.N., editors), 47–61. California Sea Grant College Program, La Jolla, California.
- Doty, M.S., Caddy, J.F. & Santelices, B. (1986). Case studies of seven commercial seaweed resources. FAO Fisheries Technical Papers, 281: 1–311.
- Dumilag, R.V. & Aguinaldo, Z.-Z.A. (2017). Genetic differentiation and distribution of Pyropia acanthophora (Bangiales, Rhodophyta) in the Philippines. European Journal of Phycology, 52: 104–115.
- Dumilag, R.V., Liao, L.M. & Lluisma, A.O. (2014). Phylogeny of Betaphycus (Gigartinales, Rhodophyta) as inferred from COI sequences and morphological observations on B. philippinensis. Journal of Applied Phycology, 26: 587–595.
- Dumilag, R.V. & Lluisma, A.O. (2014). Resolving the phylogenetic affinities of Kappaphycus inermis within the genus Kappaphycus (Gigartinales, Solieriaceae) using mitochondrial and plastid markers. Phytotaxa, 162: 223–231.
- Filloramo, G.V. & Saunders, G.W. (2016). Molecular-assisted alpha taxonomy of the genus Rhodymenia (Rhodymeniaceae, Rhodymeniales) from Australia reveals overlooked species diversity. European Journal of Phycology, 51: 354–367.
- Food and Agriculture Organization of the United Nations (FAO). (2014). FishStatJ – Software for Fishery Statistical Time Series. Retrieved from http://www.fao.org/fishery/statistics/software/fishstatj/en.
- Food and Agriculture Organization of the United Nations (FAO). (2015). Global Aquaculture Production Dataset. Retrieved from http://www.fao.org/fishery/statistics/global-aquaculture-production/query/en
- Fredericq, S. & Hommersand, M.H. (1989a). Proposal of the Gracilariales ord. nov. (Rhodophyta) based on an analysis of the reproductive development of Gracilaria verrucosa. Journal of Phycology, 25: 213–227.
- Fredericq, S. & Hommersand, M.H. (1989b). Comparative morphology and taxonomic status of Gracilariopsis (Gracilariales, Rhodophyta). Journal of Phycology, 25: 228–241.
- Ganzon-Fortes, E.T., Trono, G.C., Villanueva, R.D., Romero, J.B. & Montaño, M.N.E. (2012). ‘Endong’, a rare variety of the farmed carrageenophyte Eucheuma denticulatum (Burman) Collins and Hervey from the Philippines. Journal of Applied Phycology, 24: 1107–1111.
- Garcia-Vaquero, M. & Hayes, M. (2016). Red and green macroalgae for fish and animal feed and human functional food development. Food Reviews International, 32: 15–45.
- Goff, L.J., Moon, D.A. & Coleman, A.W. (1994). Molecular delineation of species and species relationships in the red algal agarophytes Gracilariopsis and Gracilaria. Journal of Phycology, 30: 521–537.
- Guillemin, M.-L., Contreras-Porcia, L., Ramírez M.-E., Macaya, E., Flores-Molina, M.R., Contador, C.B., Woods, H., Wyatt, C. & Brodie, J. (2016). The bladed Bangiales (Rhodophyta) of the South Eastern Pacific: a molecular species delimitation reveals extensive diversity. Molecular Phylogenetics and Evolution, 94: 814–826.
- Guiry, M.D. & Guiry, G.M. (2017). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; last searched on 29 June 2017.
- Gunnarsson, K., Nielsen, R., Egilsdóttir, E. & Brodie, J. (2016). A collections-based approach to the species and their distribution based on the bladed Bangiales (Rhodophyta) of Iceland. Botanica Marina, 59: 223–229.
- Gurgel, C.F.D. & Fredericq, S. (2004). Systematics of the Gracilariaceae (Gracilariales, Rhodophyta): a critical assessment based on rbcL sequence analyses. Journal of Phycology, 40: 138–159.
- Gutiérrez Cuesta, R., Gonzalez Garcia, K.L., Valdes Iglesias, O.R., Hernández Rivera, Y. & Acosta Suárez, Y. (2016). Seaweeds as sources of bioactive compounds in the benefit of human health: a review. Biotecnia, 18: 20–27.
- Hafting, J.T., Critchley, A.T., Cornish, M.L., Hubley, S.A. & Archibald, A.F. (2012). On-land cultivation of functional seaweed products for human usage. Journal of Applied Phycology, 24: 385–392.
- Hehre, E.J. & Meeuwig, J.J. (2016). A global analysis of the relationship between farmed seaweed production and herbivorous fish catch. PloS ONE, 11: 1–17. https://doi.org/10.1371/journal.pone.0148250.
- Hurd, C.L., Harrison, P.J., Bischof, K. & Lobban, C.S. (2014). Seaweed Ecology and Physiology. 2nd ed. Cambridge University Press, Cambridge.
- Hurtado, A.Q. (2013). Different colour morphotypes of Kappaphycus alvarezii and Kappaphycus striatum used in commercial farming. In Taxonomy of Southeast Asian Seaweeds II (Phang, S.M. & Lim, P.E., editors), 83–92. University of Malaya Press, Kuala Lumpur.
- Hurtado, A.Q., Neish, I.C. & Critchley, A.T. (2015). Developments in production technology of Kappaphycus in the Philippines: more than four decades of farming. Journal of Applied Phycology, 27: 1945–1961.
- Hurtado, A.Q., Lim, P.E., Tan, J., Phang, S.M., Neish, I.C, & Critchley, A.T. (2016). Biodiversity and biogeography of commercial tropical carrageenophytes in the southeast Asian Region. In Carrageenans: Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects (Pereira, L., editor), 51–74. Nova Science Publishers, New York.
- Hwang, M.S., Kim, S.O., Ha, D.S., Lee, J.E. & Lee, S.R. (2014). Complete mitochondrial genome sequence of Pyropia yezoensis (Bangiales, Rhodophyta) from Korea. Plant Biotechnology Reports, 8: 221–227.
- Kain, J.M. (1991). Cultivation of attached seaweeds. In Seaweed Resources in Europe: Uses and Potential (Guiry, M.D. & Blunden, G., editors), 309–377. John Wiley & Sons, Chichester.
- Kikuchi, N., Nakada, T. & Niwa, K. (2015). Proposals of a new combination and a valid name for two Bangiales taxa (Rhodophyta) used for nori cultivation in Japan. Journal of Japanese Botany, 90: 380–385.
- Kim, S.K. (2012) Handbook of Marine Macroalgae: Biotechnology and Applied Phycology. Wiley-Blackwell, Chichester.
- KKP (2013). Master plan program budidaya laut 2012, Kementerian Kelautan dan Perikanan Indonesia.
- Leliaert, F., Verbruggen, H., Vanormelingen, P., Steen, F., López-Bautista, J.M., Zuccarello, G.C. & De Clerck, O. (2014). DNA-based species delimitation in algae. European Journal of Phycology, 49: 179–196.
- Lim, P.E., Tan, J., Phang, S.M., Nikmatullah, A., Dang, D.H., Sunarpi, H. & Hurtado, A.Q. (2014a). Genetic diversity of Kappaphycus Doty and Eucheuma J. Agardh (Solieriaceae, Rhodophyta) in Southeast Asia. Journal of Applied Phycology, 26: 1253–1272.
- Lim, P.E., Tan, J., Phang, S.M. & Rahiman, A.A. (2014b). A guide to Kappaphycus and Eucheuma seaweeds in Malaysia. University of Malaya Press, Kuala Lumpur.
- Lindstrom, S.C., Hughey, J.R. & Rosas, L.E.A. (2015). Four new species of Pyropia (Bangiales, Rhodophyta) from the west coast of North America: the Pyropia lanceolata species complex updated. PhytoKeys, 52: 1–22.
- Luo, Q.J., Yang, R., Lin, S.Z., Chen, H.M., Wang, Y.J., Wang, T.G., Yan, X.J., Sun, Q.H., & Zhang, P. (2015). A new cultivar of Pyropia haitanensis, “Zhe-Dong No. 1” (紫菜 “浙东1号”). Chinese Fisheries (中国水产), 11: 57–59. (In Chinese)
- Lyra, G.D.M., Costa, E.D.S., Jesus, P.B. de, Matos, J.C.G. de, Caires, T.A., Oliveira, M.C., Oliveira, E.C., Xi, Z., Nunes, J.M.D.C. & Davis, C.C. (2015). Phylogeny of Gracilariaceae (Rhodophyta): evidence from plastid and mitochondrial nucleotide sequences. Journal of Phycology, 51: 356–366.
- Lyra, G.M., Grassa, C.J., Iha, C., Blouin, N., Gurgel, C.D., Fredericq, S., Oliveira, M.C., Nunes, J.C., Lane, C. & Davis, C.C. (2017). Phylogenomics of the red algal clade Gracilariaceae (Rhodophyta). Abstracts, Phycological Society of America, 2017 annual meeting.
- Marinho-Soriano, E. (2001). Agar polysaccharides from Gracilaria species (Rhodophyta, Gracilariaceae). Journal of Biotechnology, 89: 81–84.
- Marshall, S.M., Newton, L. & Orr, A.P. (1949). A Study of Certain British Seaweeds and their Utilization in the Preparation of Agar. HMSO, London.
- Mathieson, A.C., Pederson, J.R., Neefus, C.D., Dawes, C.J. & Bray, T.L. (2008). Multiple assessments of introduced seaweeds in the Northwest Atlantic. ICES Journal of Marine Science, 65: 730–741.
- McHugh, D.J. (1991). Worldwide distribution of commercial resources of seaweeds including Gelidium. Hydrobiologia, 220/221: 19–29.
- Miura, A. (1975). Porphyra cultivation in Japan. In Advance of Phycology in Japan (Tokida, J. & Hirose, H., editors), 273–303. The Hague: Dr. W. Junk B. V. Publisher.
- Miura, A. (1984). A new variety and a new form of Porphyra (Bangiales, Rhodophyta) from Japan: Porphyra tenera Kjellman var. tamatsuensis Miura, var. nov. and P. yezoensis Ueda form. narawaensi Miura, form. nov. Journal of the Tokyo University of Fisheries, 71: 1–37.
- Muangmai, N., Yamagishi, Y., Zuccarello, G.C., Chirapart, A. & Lewmanomont, K. (2014). Transferring Gracilaria irregularis (Gracilariaceae, Rhodophyta) from Thailand to Gracilariopsis based on morphological and molecular analyses. Phycological Research, 62: 29–35.
- Neefus, C.D., Mathieson, A.C. & Bray, T.L. (2008). The distribution, morphology, and ecology of three introduced Asiatic species of Porphyra (Bangiales, Rhodophyta) in the northwestern Atlantic. Journal of Phycology, 44: 1399–1414.
- Nelson, W.A. & Broom, J.E.S. (2010). The identity of Porphyra columbina (Bangiales, Rhodophyta) originally described from the New Zealand subantarctic islands. Australian Systematic Botany, 23: 16–26.
- Niwa, K., Iida, S., Kato, A., Kawai, H., Kikuchi, N., Kobiyama, A. & Aruga, Y. (2009). Genetic diversity and introgression in two cultivated species (Porphyra yezoensis and Porphyra tenera) and closely related wild species of Porphyra (Bangiales, Rhodophyta). Journal of Phycology, 45: 493–502.
- Niwa, K., Kikuchi, N. & Aruga, Y. (2005). Morphological and molecular analysis of the endangered species Porphyra tenera (Bangiales, Rhodophyta). Journal of Phycology, 41: 294–304.
- Pareek, M., Mishra, A. & Jha, B. (2010). Molecular phylogeny of Gracilaria species inferred from molecular markers belonging to three different genomes. Journal of Phycology, 46: 1322–1328.
- Payo, D.A., Leliaert, F., Verbruggen, H., D’hondt, S., Calumpong, H.P. & De Clerck, O. (2013). Extensive cryptic species diversity and fine-scale endemism in the marine red alga Portieria in the Philippines. Proceedings of the Royal Society B: Biological Science, 280: 20122660.
- Phang, S-M., Yeong, H-Y., Lim, P-E., Nor, A.R.M. & Gan, K.T. (2010). Commercial varieties of Kappaphycus and Eucheuma in Malaysia. Malaysian Journal of Science, 29: 214–224.
- Porse, H. & Rudolph, B. (2017). The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. Journal of Applied Phycology. doi:10.1007/s10811-017-1144-0.
- Ramírez, M.E., Contreras-Porcia, L., Guillemin, M.-L., Brodie, J., Valdivia, C., Flores-Molina, M.R., Nuñez, A., Bulboa Contador, C. & Lovazzano, C. (2014). Pyropia orbicularis sp. nov. (Rhodophyta, Bangiaceae) based on a population previously known as Porphyra columbina from the central coast of Chile. Phytotaxa, 158: 133–153.
- Rice, E.L. & Bird, C.J. (1990). Relationships among geographically distant populations of Gracilaria verrucosa (Gracilariales, Rhodophyta) and related species. Phycologia, 29: 501–510.
- Robba, L., Russell, S.J., Barker, G.L. & Brodie, J. (2006). Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta). American Journal of Botany, 93: 1101–1108.
- Rodgers, S.K. & Cox, E.F. (1999). The distributions of the introduced rhodophytes Kappaphycus alvarezii, Kappaphycus striatum and Gracilaria salicornia in relation to various physical and biological factors in Kane’ohe Bay, O’ahu, Hawai’i. Pacific Science, 53: 232–241.
- Saengkaew, J., Muangmai, N. & Zuccarello, G.C. (2016). Cryptic diversity of the mangrove-associated alga Bostrychia (Rhodomelaceae, Rhodophyta) from Thailand. Botanica Marina, 59: 363–371.
- Safari, S. & Dardak, R.A. (2015). Case study on seaweed production system in Indonesia. Economic and Technology Management Review, 10b: 107–120.
- Sánchez, N., Vergés, A., Peteiro, C., Sutherland, J.E. & Brodie, J. (2014). Diversity of bladed Bangiales (Rhodophyta) in western Mediterranean: recognition of the genus Themis and descriptions of T. ballesterosii sp. nov., T. iberica sp. nov., and Pyropia parva sp. nov. Journal of Phycology, 50: 908–929. [Corrigendum: Journal of Phycology, 51: 401 (2015)]
- Sellers, A.J., Saltonstall, K. & Davidson, T.M. (2014). The introduced alga Kappaphycus alvarezii (Doty ex PC Silva, 1996) in abandoned cultivation sites in Bocas del Toro, Panama. BioInvasions Records, 4(1). doi: 10.3391/bir.2015.4.1.01.
- Silva, P.C., Basson, P.W. & Moe, R.L. (1996). Catalogue of the benthic marine algae of the Indian Ocean. University of California Publications in Botany, 79: 1–1259.
- Song, W.L. (2016). Study on high-temperature-resistance of the new varieties “Shenfu No. 1” and “Shenfu No. 2” of Porphyra haitanensis by cultivation at sea area. Journal of Shanghai Ocean University, 25: 522–527. (In Chinese with English abstract)
- Steentoft, M., Irvine, L.M. & Farnham, W.F. (1995). Two terete species of Gracilaria and Gracilariopsis (Gracilariales, Rhodophyta) in Britain. Phycologia, 34: 113–127.
- Sutherland, J.E., Lindstrom, S., Nelson, W., Brodie, J., Lynch, M., Hwang, M.S., Choi, H.-G., Miyata, M., Kikuchi, N., Oliveira, M., Farr, T., Neefus, C., Mortensen, A., Milstein, D. & Müller, K. (2011). A new look at an ancient order: generic revision of the Bangiales. Journal of Phycology, 47: 1131–1151.
- Tan, J., Lim, P.E., Phang, S.M., Hong, D.D., Sunarpi, H. & Hurtado, A.Q. (2012). Assessment of four molecular markers as potential DNA barcodes for red algae Kappaphycus Doty and Eucheuma J. Agardh (Solieriaceae, Rhodophyta). PloS ONE, 7: e52905.
- Tan, J., Lim, P.E. & Phang, S.M. (2013). Phylogenetic relationship of Kappaphycus Doty and Eucheuma J. Agardh (Solieraceae, Rhodophyta) in Malaysia. Journal of Applied Phycology, 25: 13–29.
- Tan, J., Lim, P.E., Phang, S.M., Rahiman, A.A., Nikmatullah, A., Sunarpi, H. & Hurtado, A.Q. (2014). Kappaphycus malesianus sp. nov.: a new species of Kappaphycus (Gigartinales, Rhodophyta) from Southeast Asia. Journal of Applied Phycology, 26: 1273–1285.
- Tseng, C.K. (1935). Economic seaweeds of Kuangtung Province, China. Lingnan Science Journal, 14: 93–104.
- Ueda, S. (1932). Systematic study of the genus Porphyra in Japan. Suiko-Kenkyu-Kokoku, 28: 1–45.
- Valderrama, D. et al. (2015). The economics of Kappaphycus seaweed cultivation in developing countries: a comparative analysis of farming systems. Aquaculture Economics and Management, 19: 251–277. https://doi.org/10.1080/13657305.2015.1024348.
- Veeragurunathan, V., Eswaran, K., Malarvizhi, J. & Gobalakrishnan, M. (2015). Cultivation of Gracilaria dura in the open sea along the southeast coast of India. Journal of Applied Phycology, 27: 2353–2365.
- Wang, T., Xu, Y., Xie, C.T., Ji, D.H. & Chen, C.S. (2013). Construction of multiplex PCR in variety identification of Porphyra haitanensis “Z-26” based on SCAR marker. Journal of Fisheries of China, 37: 688–695. (In Chinese with English abstract)
- West, A.L., Mathieson, A.C., Klein, A.S., Neefus, C.D. & Bray, T.L. (2005). Molecular ecological studies of New England species of Porphyra (Rhodophyta, Bangiales). Nova Hedwigia, 80: 1–24.
- Williams, S.L. & Smith, J.E. (2007). A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annual Review of Ecology, Evolution and Systematics, 38: 327–359.
- Xie, X.X., Chen, C.S., Xu, Y., Ji, D.H. & Xie, C.T. (2013). Comparative analysis on applicability of DNA sequence markers for identification of Pyropia haitanensis germplasm. Journal of Applied Oceanography, 32: 404–410. (In Chinese with English abstract)
- Yamamoto, H. (1978). Systematical and anatomical study of the genus Gracilaria in Japan. Memoirs of the Faculty of Fisheries of Hokkaido University, 25: 97–152.
- Yang, L.E., Han, X.L., Zhou, W., Deng, Y.Y., Xu, G.P., Hu, C.M., Zhu, J.Y., Lu, Q.Q. & Xu, J.G. (2016). Genetic diversity of different cultivars of Pyropia yezoensis. Guangxi Sciences, 23: 241–247. (In Chinese with English abstract)
- Yang, L-E., Lu, Q.Q. & Brodie, J. (2017). A review of the bladed Bangiales (Rhodophyta) in China: history, culture and taxonomy. European Journal of Phycology, 52: 251–263.
- Yang, M.Y. & Kim, M.S. (2015). Molecular analyses for identification of the Gracilariaceae (Rhodophyta) from the Asia–Pacific region. Journal of Applied Phycology, 37: 775–787.
- Yow, Y.Y., Lim, P.E. & Phang, S.M. (2013). Assessing the use of mitochondrial cox1 gene and cox2-3 spacer for genetic diversity study of Malaysian Gracilaria changii (Gracilariaceae, Rhodophyta) from Peninsular Malaysia. Journal of Applied Phycology, 25: 831–838.
- Zhao, S. & He, P. (2011). Molecular identification based on ITS sequences for Kappaphycus and Eucheuma cultivated in China. Chinese Journal of Oceanology and Limnology, 29: 1287–1296.
- Zheng, B.F. & Li, J. (2009). Flora Algarum Marinarum Sinicarum (中国海藻志). Tomus II. Rhodophyta, 65–107. Science Press, Beijing. (In Chinese)
- Zuccarello, G.C., Critchley, A.T., Smith, J., Sieber, V., Lhonneur, G.B. & West, J.A. (2006). Systematics and genetic variation in commercial Kappaphycus and Eucheuma (Solieriaceae, Rhodophyta). Journal of Applied Phycology, 18: 643–651. https://doi.org/10.1007/s10811-006-9066-2.
