ABSTRACT
Scaling up algal cultures to the very large volumes required for commercial production is a complex task and requires skilled and experienced personnel. First it is necessary to consider how to optimize the process of producing enough inoculum for the large ponds or photobioreactors in order to minimize the time and cost required. In order to minimize the need for re-inoculation from stock cultures it is also essential to manage the large-scale cultures to avoid significant contamination or collapse. The maintenance of long-term, stable, high-productivity, large-scale cultures, usually under prevailing outdoor conditions of variable irradiance, temperature and rainfall, presents additional challenges most of which are not seen in the constant environment experienced by small-scale laboratory cultures. Methods and protocols to deal with these can only be developed at the large-scale and they will mostly be specific for the alga being cultured, the culture system being used and the location of the production plant. A common feature of all large-scale operations known to us is that, over time (years), both productivity and reliability of the cultures improve as the operators gather experience in managing their cultures.
Introduction
The potential of microalgae for the production of a range of commercially valuable bioproducts as well as for other applications such as wastewater treatment is well documented (Spolaore et al., Citation2006; Borowitzka, Citation2013b) and in the last decades interest in this field has increased enormously, driven by the desire to use microalgae as an environmentally friendly and renewable source of biofuels. More recently, since the recognition that the production of algal biofuels is not economically viable (Stephens et al., Citation2010; Davis et al., Citation2011), attention has been refocused on microalgae as sources of higher value products. However, most published studies are at the laboratory scale and there has been relatively little work on scaling up to the level required for commercial production. Unfortunately most research on purported commercial applications has considered neither the scale required to produce commercially relevant amounts of algal biomass, nor the key characteristics of such large-scale cultures. Furthermore, many papers deal with downstream processing of the algal biomass, but not with how to produce enough biomass to feed the processing step. As pointed out by V. Bush in the foreword to the Carnegie Institution publication (Burlew, Citation1953): ‘it is desirable to discover whether any new problems would be introduced by a large increase in the size of a culture unit’. In this paper we will try to point out the major issues that have to be considered and overcome in order to scale up algal biomass to commercial production scale (> 10 tonnes (t) dry biomass per annum) with special emphasis on those issues that are revealed only once scaling up is attempted. We will also try and point out some of the solutions adopted in large-scale operations that are usually held as proprietary information not made public by the large-scale producers. We will not deal with the processes of harvesting, drying or extraction, which are well addressed in other papers (e.g. Molina Grima et al., Citation2013; Pahl et al., Citation2013; Chatsungnoen & Chisti, Citation2016).
Few papers have been published on the process and experience of scaling of algal production to a commercial level. The major ones concern the production of Spirulina in the USA (Shimamatsu, Citation1987, Citation2004; Belay et al., Citation1994; Belay, Citation1997, Citation2013) and South Africa (Grobbelaar, Citation2009), the production of Dunaliella salina in Australia (Borowitzka et al., Citation1984, Citation1985; Curtain et al., Citation1987; Moulton et al., Citation1987; Borowitzka & Borowitzka, Citation1989, Citation1990; Borowitzka, Citation1991, Citation1992, Citation1994; Schlipalius, Citation1991) and Israel (Ben-Amotz & Avron, Citation1989, Citation1990; Ben-Amotz et al., Citation1991; Ben-Amotz, Citation2004), and the experience of Sapphire Energy Inc. in the USA (White & Ryan, Citation2015). However, many of these do not provide an in-depth analysis of the limitations and solutions, probably because this information is proprietary, making it difficult to adapt their experience to new facilities.
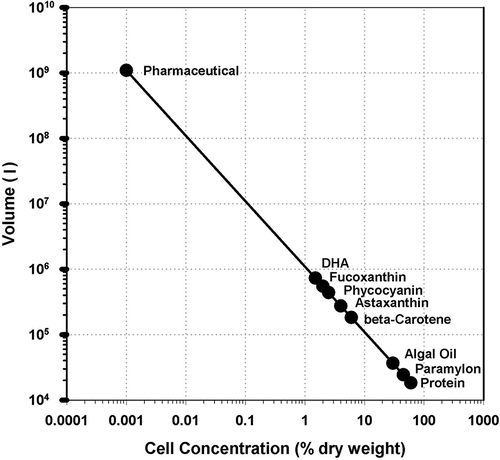
Some appreciation of the scale of production required to be commercially relevant can be gained by a simple calculation which considers the cell content of the desired product and the biomass doubling time. shows the volume required to produce 1 as in 1 tonne of product per year, assuming an algal cell concentration of 0.5 g dwt l–1, doubling time of 2 days and an annual operation time of 365 days for a range of products of current interest. These assumptions are very optimistic and few, if any, commercial operations have achieved such values. The assumptions also do not include other factors such as harvesting efficiency (i.e. the percentage of the biomass actually recovered), losses during extraction and purification of the product, or the fact that the continued operation for 365 days is unlikely due to equipment failures, pond/reactor cleaning requirements, contamination, etc. Furthermore, in many locations seasonal climatic changes, particularly in temperature, do not allow 365 days of production (e.g. Arthrospira cultivation in California, USA; Belay, Citation2013). It is also important to note that for almost all products significantly greater quantities than 1 t are required to be commercially relevant and viable. Despite these limitations this basic calculation provides a useful insight into the volumes required. For example to produce 1 t year–1 of protein about 18 300 l of culture are required, whereas production of β-carotene requires about 182 600 l of culture and fucoxanthin would require about 548 000 l. It should also be appreciated that the actual water requirement is significantly larger due to water loss from the pond/photobioreactor by evaporation (e.g. Borowitzka & Moheimani, Citation2013b; White & Ryan, Citation2015). Of course, 1 t of product is not commercial, and commercial plants need to be able to produce well in excess of 10 t product per annum for high value products and >1000 t for commodity products.
Fig. 1. Volume of algal culture required to produce 1 t of product per year assuming a cell density of 0.5 g dry wt l–1, a doubling time of 2 days and 365 days of production. Product cell contents used in the calculation: protein 60%; paramylon 45%; algal oil (triacylglycerols) 30%; β-carotene 6%; astaxanthin 4%; phycocyanin 2.5%; fucoxanthin 2%; docosahexaenoic acid (DHA) 1.5%; pharmaceutical 0.001%.
Current commercial culture system units range in volume from about 1000 l for the big bag systems used to produce microalgae as feed in aquaculture, to about 3 ×104 to 1 ×108 l for raceway ponds for production of algal biomass or wastewater treatment, respectively, and about 1 ×104 l for tubular photobioreactors to 1 ×109 l for the extensive ponds used for the culture of the green alga Dunaliella salina in Australia (Borowitzka, Citation2016). These volumes are for single units, be it bags, ponds or photobioreactors and a production facility requires a number of these to produce the quantity of algal biomass required.
Problems in scaling up
Scaling up of algal cultures is generally done by a factor of 10 per step, i.e. from 10 ml to 100 ml to 1 l to 10 l etc. For some high-light-sensitive species such as some strains of the cyanobacterium Arthrospira the scale-up factor may need to be reduced to 5 to minimize photoinhibition immediately after inoculation. Again, assuming that it takes about 5 days for the culture to have increased sufficiently to be subcultured at the next step, it takes at least 1 month to progress from a 10 ml stock culture to having enough inoculum for a 10 000 l culture (assuming a scale-up factor of 10). The many steps and the extended time required not only increase costs, but also increase the risk of contamination. The process of producing the inoculum for large-scale commercial cultures is not trivial and is a critical factor to the successful operation of a production facility. It is therefore not surprising that most commercial producers generally guard the exact details of their process. One factor that may help in the process of scaling up and shortening the period from laboratory scale to the production units is modification of the culture depth so that the areal density of the culture is high even when the biomass concentration is relatively low due to the dilution. Another ‘trick’ is using shaded ponds or greenhouse cultures to better control the daily fluctuation in light and temperature.
One option to reduce the time and cost of inoculum scale-up is to operate the large-scale cultures as semi-continuous cultures rather than as batch cultures. Here, rather than harvesting the whole culture volume once the desired biomass concentration (g l–1) is reached, only part of the culture volume is harvested and the harvested volume is replaced either with fresh or recycled medium. The remaining culture in the pond/bioreactor thus acts as the inoculum for the next culture cycle. The proportion of culture harvested and the time interval between harvests is based on the growth rate (doubling time) and the lowest to highest cell density range at which one wishes to operate in order to maintain the culture at the optimal biomass concentration and in exponential growth phase at all times thus maximizing productivity. Long-term (> 6 months) semi-continuous culture in raceway ponds requires the ability to manage contamination of the culture and this has been shown to be possible for a number of species such as Arthrospira spp. (Jiménez et al., Citation2003; Belay, Citation2013), Dunaliella salina (Borowitzka, Citation2013a), Tetraselmis spp. (Moheimani, Citation2013; Fon Sing et al., Citation2014), Botryococcus braunii (Zhang, Citation2013), Pleurochrysis carterae (Moheimani & Borowitzka, Citation2006), Acutodesmus obliquus (Hulatt & Thomas, Citation2011) and Demodesmus armatus (White & Ryan, Citation2015) but as yet only a few of those have actually reached the stage of a viable commercial product. Nutrient addition to replace the nutrients consumed by the harvested algae and other losses also needs to be carefully managed to avoid either the depletion of a critical nutrient or gradual build-up of a particular nutrient to concentrations which will inhibit growth.
Where semi-continuous culture is not possible because the desired metabolite is only produced in sufficient quantity in the stationary growth phase (e.g. astaxanthin in Haematococcus pluvialis), it is still possible to grow the inoculum in semi-continuous mode. In some cases it is also possible to grow the inoculum heterotrophically in fermenters in the dark on an organic carbon substrate such as glucose, achieving high cell concentrations in a short time (Doucha & Lívanský, Citation2012). For some algae such as Chlorella spp. this can be cheaper and produces a higher density inoculum in a shorter time (Zheng et al., Citation2012).
Large-scale outdoor cultures
For all algal cultures the key factors affecting growth and productivity are light, temperature and nutrients. Furthermore, almost all commercial-scale algal biomass production is carried out outdoors in natural light, exposing the algae to diurnal changes in irradiation as well as shorter term changes due to clouds. Detailed reviews of the biology, physiology and management of large-scale outdoor cultures can be found in Richmond (Citation2013), Torzillo & Vonshak (Citation2013), Borowitzka & Moheimani (Citation2013a) and Borowitzka (Citation2016). Here we discuss only some of the most important factors.
Flow rate, turbulence and mixing
As the culture scale increases, the environment, especially the hydrodynamic conditions and the light environment, the algae experience changes. It is easy to induce mixing in small-scale cultures, providing a uniform light and chemical environment for the algal cells and to prevent settling of the cells. However, in large raceway ponds or photobioreactors uniform mixing is difficult, and almost impossible, to achieve. One outcome of this is a reduction in productivity and an increase in the risk of culture collapse as the scale of the culture system increases. Productivities in large production raceway ponds rarely exceed 20 g m–2 day–1 and annual average production is less than that (Belay, Citation1997; Weissman et al., Citation1989; Olaizola, Citation2000; Jiménez et al., Citation2003), although high productivities of up to about 50 g m–2 day–1 can be achieved in small indoor cultures under optimal conditions, and productivities > 30 g m–2 day–1 in small (1–5 m2) raceway ponds outdoors are achievable over very short periods (Moheimani & Borowitzka, Citation2006).
Flow and mixing in large-scale culture units differ markedly from small laboratory-scale mini-ponds and photobioreactors. For example, in large raceway ponds the flow rate varies along the channels and vertical mixing occurs mainly in the vicinity of the paddle wheel where water flow is turbulent, whereas in the long channels flow is predominantly laminar (Hadiyanto et al., Citation2013; Prussi et al., Citation2014). The longer the channels, the longer the region of laminar flow where little vertical mixing occurs. This means that cells lower in the water column receive little or no light for longer periods especially at higher cell densities, whereas the cells at the pond surface are exposed to high irradiances. At the ends of the ponds the flow rate is much higher on the inside of the 180° bends than at the outside and this can lead to settling and accumulation of algal cells at the outside of the bends. The unequal mixing can easily be seen in the ponds by the distribution of foam on the water surface (). In order to minimize this, flow rectifiers are often installed in an attempt to even out the flow around the bends (Borowitzka, Citation2005), but these are only effective in evening out horizontal flow and have little effect on vertical mixing. Alternatively, the pond design can include an eccentrically placed curved wall and/or baffles at the end of the pond furthest from the paddle wheel (Dodd, Citation1986; Shimamatsu, Citation1987), but this also is only of limited effectiveness. It is interesting that the effect on productivity of those additions has never been quantified in large-scale systems.
Fig. 2. Variation in mixing indicated by both the distribution of foam patches at the water surface and wave distribution in a small 200 m2 raceway pond. The distribution of the foam indicates the region of highest surface water flow.

In closed photobioreactors, especially the commonly used tubular photobioreactors with tube diameters of 5–10 cm, similar problems occur with scale-up. In the long straight tube runs of large reactors (10 m and longer) laminar flow occurs and, at high cell densities, little or no light penetrates to the cells at the centre of the tubes for much of the tube length. In helical photobioreactors such as the Biocoil, the tube curvature induces a gradual flow from the centre of the tube to the outside due to the Coriolis effect, with the time taken for a particle to move from the tube periphery to the centre of the tube dependent on the diameter of the reactor helix, the tube diameter and the flow rate. However, large helical photobioreactors cannot be constructed of glass tubing.
It is generally accepted that the two main limiting factors for growth of outdoor algal cultures, that are nutrient sufficient, are light and temperature. In relatively warm regions, where most of the production sites of algal biomass are located, light has been assumed to be the main limiting factor for growth (Richmond & Vonshak, Citation1978). Many studies have been carried out in an attempt to increase light availability to algal cells grown outdoors. Richmond & Vonshak (Citation1978) demonstrated an increase in the productivity of outdoor cultures of A. platensis by increasing the turbulent flow. This observation was confirmed by later studies (Laws et al., Citation1988; Grobbelaar, Citation1994; Hu et al., Citation1998).
The explanation for the observed increase in productivity as a result of the increased mixing speed is still under debate. Some of the explanations attributed the effect to improvement of mass transfer of nutrients, a flashing light effect (Kok, Citation1956), as well as to the removal of excess oxygen (Torzillo et al., Citation1984). However, Grobbelaar et al. (Citation1996) showed that the stimulatory effect of intermittent light/dark cycles only becomes pronounced at cycles shorter than 10 s, which is much shorter than that found in raceway ponds. Thus the improvement in productivity observed at higher flow rates in outdoor algal cultures is attributed to an improved light/dark regime which increases the average irradiance received by the algal cells (Richmond, Citation2004). A further effect of greater turbulence at higher flow rates is that the diffusive boundary layer around the algal cells is reduced (Lazier & Mann, Citation1989), thus increasing exchange rates of nutrients and metabolites between the cells and their growth medium (Grobbelaar, Citation1994).
As noted above, achieving consistent high turbulent flow throughout a large raceway pond or in tubular photobioreactors is not possible. For raceway ponds flow rates of 20–30 cm s–1 are used. In terms of design, the maximum width of the channel can be up to 10 m and the length of 100 m. This means that the optimum size of a pond operating at 20–30 cm depth is about 2000 m2 (Borowitzka, Citation2005). Higher flow rates are possible, but the increased productivity is not offset by the markedly higher energy cost. For tubular photobioreactors with a diameter varying from 3–5 cm the minimum flow rate recommended is in the range of 0.6–1 m s–1. However, the flow rate is also limited by the shear sensitivity of the particular algal species (Moheimani et al., Citation2011; Sung et al., Citation2014). The major limitation is the need to have an event of oxygen removal which limits the photobioreactor tube length to about 50 m depending on the cell density, photosynthetic oxygen evolution rates and tube diameter (Camacho Rubio et al., Citation1999).
Light
Algal cultures grown outdoors in raceway ponds are exposed to two different light/dark regimes. The first, a relatively fast one, is a result of the mixing device (usually a paddle wheel) inducing a turbulent flow of the culture, the rate of which dictates the frequency of the light/dark cycle (Laws et al., Citation1988). Thus, in raceway ponds the algal cells are shifted between being fully exposed to solar radiation, when present at the upper culture surface, all the way to complete darkness, when reaching the bottom of the culture, usually at a depth of 15–20 cm. The second regime, a relatively slower one, is a result of the changes in solar radiation during the day from sunrise to sunset. These two light regimes impose a unique physiological condition, in terms of acclimation of the outdoor grown algal cells to light, from a highly light-limited situation up to a light-saturated stage that may even result in photoinhibition (Grobbelaar, Citation1991, Citation1994; Vonshak & Guy, Citation1992; Lu & Vonshak, Citation1999).
An additional fluctuation in light availability is the one that is seasonally based and is less documented due to the lack of long-term outdoor experiments. Yet seasonal variations may have a significant impact on the annual productivity of a large-scale production facility. This issue is a major consideration when selecting a site for a production facility and may have an impact on its economic viability (Venteris et al., Citation2014; Boruff et al., Citation2015).
Biomass density influences the photosynthetic productivity of algal cultures at any given irradiance due to self-shading of the algal cells affecting the actual irradiance received by the cells (Myers et al., Citation1951; Richmond & Vonshak, Citation1978; Hu & Richmond, Citation1994). Thus, in relatively dense cultures, there is an optimum cell density (OCD) where productivity is at its maximum and this is a function of the average irradiance the algal cells receive. The influence of cell density on productivity is shown by Borowitzka (Citation2016). As pointed out earlier the algal cells in large ponds or photobioreactors are exposed to alternating high and low irradiance depending on the flow rate (mixing) and the design of the culture system. However, the frequency of these changes in irradiance in large-scale cultures is relatively slow so that, for practical purposes, it is the average irradiance that the algal cells are exposed to which affects their photosynthetic rate. The average irradiance can be modified to some extent by changing culture depth in the ponds, changing flow rate and/or changing the frequency of harvesting and the amount harvested. The pond depth strategy is particularly useful to compensate for seasonal changes in irradiance and temperature, thus maximizing the annual productivity of the system. During harvesting it is important not to reduce the cell density too much as this can then expose the cells to high irradiances which can cause photoinhibition and thus decrease productivity. One practical approach will be to carry out intensive harvesting which results in a significant dilution in cell density in the late afternoon, thus minimizing photoinhibition during the day.
The importance of OCD as a practical tool for maximizing outdoor algal culture productivity has been discussed extensively by Richmond (Richmond & Zou, Citation1999; Richmond, Citation2000, Citation2013). Other authors use similar terms i.e. the optimum chlorophyll concentration (g chlorophyll a m–3) or the optimum areal density (g dry weight m–2) (Soeder, Citation1980; Hartig et al., Citation1988).
Temperature
Probably the first key characteristic for selecting a strain is its temperature tolerance and optimum temperature (Borowitzka, Citation2013c). Since temperature is impossible to control in large raceway ponds and controlling it is expensive for closed photobioreactors, it is highly desirable to select a strain which can grow well under the temperature conditions prevailing in the culture system at the location of the production plant. The upper lethal temperature is also important if the strain is to survive overheating resulting from equipment or power failures, or due to extremely hot days.
Temperature is a critical factor affecting algal metabolism and growth and algae grow best at their optimum temperature and therefore are better able to cope with any environmental or other stresses. In a well-mixed culture outdoors the temperature fluctuations a culture experiences include diurnal as well as seasonal changes together with changes in irradiance. Beside the direct impact of temperature on growth and productivity, temperature also has very important effects on the overall productivity of the system and these effects are often overlooked. The first of these is due to the de-synchronization between the rate of increase in light in the early morning with the rate of warming of the cultures. While light intensity may increase very rapidly in the morning and reach almost 80% of its maximum within 2–3 h after sunrise, culture temperature will increase more slowly and will peak at noon or even later. As a result cultures are exposed to sub-optimal temperatures in the morning leading to temperature-induced photoinhibitory damage that may result in a loss of potential productivity of more than 25% (Vonshak et al., Citation2001, Citation2014). The impact of sub-optimal morning pond temperature is clearly shown in an experiment where the temperature in one pond was increased by 3–5°C in the morning just before sunrise and compared with a parallel unheated pond (Moheimani & Borowitzka, Citation2007). This early morning temperature increase resulted in an 11–21% increase in biomass productivity. Finding ways to minimize this damage resulting from suboptimal temperatures is not easy at a large scale. Potential options are to install a heating system that uses solar energy, or shading devices that reduce the light impinging on the culture in the early morning, however such options are not economical on a large scale.
An additional impact of temperature is associated with the night temperature and dark respiration. This often underappreciated phenomenon has not received enough attention, mainly because few large-scale facilities measure biomass concentration at least twice a day in their ponds. In the few cases where such measurements have been made, a reduction in cell dry weight of more than 8% was observed between the morning measurement and the evening of the previous day (Torzillo et al., Citation1991). In many regions where night temperature does not drop below 25°C intensive dark respiration may take place and this is due to the need to repair the damage to the photosystems which occurred during the high irradiances experienced during the previous day (Raven & Beardall, Citation2016). One of the ways to reduce the impact of these respiratory biomass losses is to change the practice of harvesting and schedule it for late afternoon rather than early morning. However, this may not always be possible as in very large-scale facilities the capital and operating costs of the harvesting system require that harvesting occurs for most of the day and even at night.
Last but not least, temperature has an effect on the solubility of nutrients and, more importantly, on solubility of gases and thus will impact the amount of CO2 introduced via the paddlewheel from the atmosphere as well as the efficiency of oxygen removal and the prevention of oversaturation by oxygen in intensively growing cultures.
Carbon and oxygen
Another important role of mixing and flow rate is the removal of oxygen during the day in order to minimize photo-oxidation, as well as introducing oxygen during the night in order to prevent development of anaerobic conditions that may promote denitrification. However, oxygen removal in raceway ponds is limited and during the day the oxygen concentration in the pond reaches very high levels due to algal photosynthesis. Similarly, in tubular photobioreactors photosynthetically produced O2 will build up and the inorganic carbon concentration will decline along the tube (Camacho Rubio et al., Citation1999). The high O2 concentration leads to inhibition of photosynthetic carbon fixation by ribulose bis-phosphate carboxylase/oxygenase (RuBisCo; Beardall & Raven, Citation2013) and thus results in a marked decline in productivity. Since the relative oxidase vs. carboxylase function of RuBisCo depends on the relative concentrations of O2 and CO2 the addition of CO2 to ponds will increase productivity by reducing inorganic C limitation as well as O2 inhibition. However, supplying CO2 to large ponds is rather inefficient due to the slow absorption of CO2. Various types of devices to supply CO2 have been designed and they provide some improvement over direct bubbling of CO2 (Becker, Citation1994). Whichever device is used, it is best to add the CO2 ‘on demand’ by using a pH-stat system (Moheimani & Borowitzka, Citation2006; Grobbelaar, Citation2009). This has the advantage of maintaining the culture pH near the optimum for the particular algal strain. Alternatively, inorganic C can be added in the form of bicarbonate, but this has the disadvantage of slowly increasing the ionic concentration (either Na+ or K+ depending on whether NaHCO3 or KHCO3 is added) ultimately leading to a reduction in algal growth (Kim et al., Citation2014). In the case of Arthrospira, a combination of both bicarbonate and carbon dioxide addition is used so as to maintain a high alkalinity favouring the growth of Arthrospira as well as providing a source of inorganic carbon for photosynthesis, i.e. starting with a high concentration of NaHCO3 and then adding CO2 as required with the process controlled by following the changes in pH.
Nutrient source and supply
Nutrients are a major expense for large-scale culture and their source and composition need to be considered once scaling up of cultivation systems is desired. The normal nutrients used in laboratory cultures are generally too expensive for commercial algal biomass production. Aside from cost, the following factors for nutrients need consideration: (1) their influence on the ability to recycle the medium; (2) the chemical composition and potential impurities due to regulatory requirements when the product is aimed at the feed and food markets; and (3) if the aim is to achieve ‘organic certification’, some of the relatively cheap and easy to handle nitrogen and phosphorus sources are not allowed to be used (Barminski et al., Citation2016).
Maintaining optimal concentration of nutrient levels is considered an easy task in algal cultivation and yet it may serve as an excellent example of problems facing industrial algal mass culture when moving from small-scale to large-scale continuous operation. Aside from the obvious issues of monitoring the level of nutrients and the frequency of monitoring, there are the questions of the mode of supply and the solubility of the nutrient.
There are additional problems that are faced only when scaling up. These include local availability and cost of the nutrients as well as the cost of transportation. Furthermore there is the question of storage and ease of handling, raising issues such as the use of liquid nutrients like phosphoric acid or ammonia versus the use of dry powder. Unfortunately there is no simple answer and in many cases the solution will be very much site-dependent.
One example of the factors to be considered is the source of nitrogen. Many facilities tend to use urea because it is the cheapest nitrogen source. However, some algal species are not able to take up urea and it is the bacteria in the medium which use the urea as a carbon source, releasing ammonia which is then taken up and used by the algae. Nitrate is an alternative, but the metabolic reduction of nitrate to ammonia requires an investment of energy (ATP) before it can be used in the biosynthesis of protein thus affecting productivity (Raven & Giodano, Citation2016). Ideally one would prefer to use ammonia as a nitrogen source, but high ammonia concentrations can be toxic to the algal culture (Azov & Goldman, Citation1982) and may also affect the production of certain secondary metabolites such as β-carotene. Furthermore, rapid uptake of ammonia in dense cultures can result in significant acidification of the medium leading to the death of the algae, especially under conditions of rapid growth (Borowitzka & Borowitzka, Citation1988).
Recycling of the culture medium
Recycling of the medium after harvesting is essential for large-scale algal culture for economic and environmental reasons. Recycling of the medium reduces water costs and also the cost of nutrients, as they are still present in the medium in substantial amounts after harvesting. Furthermore, recycling the medium reduces the costs and potential environmental impacts associated with disposing of the very large volumes of water used in algal culture (Borowitzka & Moheimani, Citation2013b).
When considering and testing harvesting methods the ability to recycle the medium must be an important consideration. Potential issues with the recycling of media are (1) selective enrichment of culture-contaminating organisms or unwanted cell morphologies (Belay et al., Citation1994); (2) build-up of specific ions which negatively affects the growth of the algae (Hadj-Romdhane et al., Citation2012); (3) build-up of autoinhibitors (Lívanský et al., Citation1996; Rodolfi et al., Citation2003); (4) an increase in dissolved organic matter due to cell breakage during harvesting (Fon Sing et al., Citation2014); and (5) carry-over of flocculant leading to flocculation of the cells in the culture. Experience with existing commercial and research culture systems has shown that long-term culture with recycling of the medium is possible (Hadj-Romdhane et al., Citation2013; White & Ryan, Citation2015; Moheimani, Citation2016) and sometimes actually increases the productivity (Fon Sing et al., Citation2014), although the medium to be recycled may need some pre-treatment or conditioning before it is returned to the culture ponds. Medium recycling is successfully practiced by most large-scale commercial algal producers.
Strain selection
Although the importance of selection of strains suitable for high productivity outdoor cultivation is acknowledged in many reviews and papers only very few studies actually deal with this issue. Out of more than 300 publications dealing with strain selection or improvement fewer than 10% actually address this issue in detail and almost none evaluate the strains in terms of outdoor performance and their suitability for large-scale year-round cultivation. Rather, many studies have focused only on laboratory optimization of growth rate and/or the content of the desired compound such as lipids or carotenoids. Productivity, which is critical for the economic production of algae and algal products (Griffiths & Harrison, Citation2009), also is often not considered or evaluated thus making such studies largely irrelevant to commercial-scale culture. A detailed assessment of the screening and selection of algal species and strains suitable for large-scale commercial culture can be found in Borowitzka (Citation2013c). One important recommendation made in this article is that potential algal strains should be tested under outdoor conditions at a very early stage of the strain selection and evaluation process. Outdoor cultures can also be used as part of the strain-selection process by exploiting in-pond evolution of algal strains resulting from genetic and associated phenotypic changes which commonly occur in algal cultures (Lakeman & Cattolico, Citation2007; Lakeman et al., Citation2009; Borowitzka, Citation2016) to isolate those strains most suited for high productivity and reliable outdoor cultures.
Aside from random mutagenesis, advances in the manipulation of the genetic materials of algal cells also provide opportunities to improve algal strains. These technologies include genetic engineering, homologous recombination and CRISPR/Cas9 genome editing (see Gan & Maggs, Citationin press). Unlike for higher plants, the development of genetically modified/genetically engineered algae is still in its early stages and restricted to a small number of species (Radakovits et al., Citation2010; Kilian et al., Citation2011; Guihéneuf et al., Citation2016; Nymark et al., Citation2016). Little is known about the stability of these modified algal strains in large-scale cultures or whether they can potentially pose any environmental risks. Further, it remains to be seen how existing legislation and regulations can be, or will be, applied to genetically modified algae (Beacham et al., Citation2017). Recently the first test of phenotype stability and ecological risk of a genetically engineered microalga has been carried out in California, USA, using a strain of the chlorophyte Acutodesmus dimorphus which had been genetically engineered by the addition of two genes, one for enhanced fatty acid biosynthesis, and one for recombinant green fluorescence protein (GFP) expression. This strain was field tested in 800 l outdoor ponds for a period of 50 days (Szyjka et al., Citation2017). Both genes and their associated phenotypes were maintained over this time. Dispersion of the genetically engineered algae was observed, but they were not able to outcompete native algal strains. Although this study was carried out for only a short time and at a small scale with only one species, the results are encouraging. Longer-term studies with other species which have different life cycles will be needed to develop suitable guidelines and regulations for the large-scale culture of genetically modified microalgae. Another drawback is that most of the genetic modification techniques were developed for strains used in laboratory studies that were chosen for the ease by which they can be manipulated and not because they have a commercial value. Thus the need to develop more reliable methodologies for strains that are currently mass produced is of high priority. A recent publication in this direction reports the development of an efficient transformation system in Arthrospira platensis (Jeamton et al., Citation2017).
Contaminants and disease
All large-scale cultures, irrespective of whether they are ‘open’ systems such as raceway ponds or ‘closed’ systems such as tubular photobioreactors are susceptible to contamination by other algal species, predatory protozoa, fungi and bacteria. Growing species with highly selective environmental requirements, such as D. salina (high salinity), Arthrospira (high alkalinity) or Chlorella (high nutrients), is one option to reduce the risk of contamination, but most algal species of interest are not extremophiles. It also needs to be recognized that all large-scale algal cultures, with the exception of heterotrophic cultures, will not be axenic and will have an associated bacterial flora (microbiome). This bacterial flora is not a problem normally, but can be a major factor in culture collapse if the algae are stressed and in poorly mixed systems. Similarly, protozoan and invertebrate predators (e.g. amoebae, ciliates and even rotifers and crustaceans) can be managed in most cases although the management method(s) tend to be very species- and culture system-specific. Fungal infections, especially the parasitic chytrid fungi, present a more difficult management problem in both open and closed systems (Gutman et al., Citation2009; Carney & Lane, Citation2014). The infectivity of these parasites appears to be influenced by factors such as irradiance, temperature, nutrient status of the algal cultures and the population density of the algal cultures (Bruning, Citation1991a, b; Gsell et al., Citation2013). The apparent differential susceptibility of different strains of the same species which has been observed in some cases (De Bruin et al., Citation2004) suggests that by judicious strain selection this property may be used to reduce the risk of infection.
It is our experience that algal cultures become more susceptible to infection and predation if they are not growing under their optimum conditions. Thus, the key factor for minimizing the risks and impacts of these contaminants is to maintain optimum conditions in the variable environment they are exposed to (Flynn et al., Citation2017). This again highlights the importance of selecting strains whose temperature optima match the temperatures prevailing at the site of production.
On-line and daily monitoring
Large-scale microalgal culture is really a type of intensive farming where the aim is to maintain a reliable monoculture at a high productivity. This requires a good understanding of the physiology of the alga being cultured and the development of culture management protocols. There is also the requirement to have an effective culture monitoring system which provides early warning of any problems so that early remedial actions can be taken before the culture collapses. This monitoring system must be robust and reliable, and able to be applied to the many ponds or photobioreactors of a production plant at relatively low cost. Visual observation and experience of the operator are important. For example a change in the colour of the culture can indicate either contamination or some deficiency. Similarly, if the cultures begin to ‘stick’ to the ponds or photobioreactor walls or there is excessive foaming then this indicates that the culture is under some stress and culture collapse is likely (). However, visual observation can only detect major changes in the culture and is not a reliable early warning method. The most common parameters for monitoring beside temperature and light and the easiest parameters to monitor are pH and O2 concentration, both of which will change diurnally in a predictable manner in actively growing cultures. Any deviation from a normal daily pattern will indicate some problem with the culture. If the cultures are grown using a pH-stat system for CO2 supply then the rate of CO2 supply can be used for monitoring. This monitoring can easily be automated. Another methodology that gained a lot of interest in laboratory studies and is increasingly attracting attention in agriculture is the use of variable fluorescence parameters (Masojidek et al., Citation2011). A simple measurement like Fv/Fm which represents the maximal or potential photochemical efficiency of photosystem II (PSII) may serve as an early indicator for a stress-induced process that is developing in the culture and may result in a significant reduction in productivity. Further studies need to be done using this parameter on-line in outdoor cultures to gain a better understanding and interpretation. Growth and productivity are followed in most of the sites by estimation of a few parameters like turbidity, chlorophyll content and dry weight. Each of these has advantages and disadvantages in terms of labour and cost. Developing a reliable monitoring and interpolation protocol is almost a prerequisite for a successful scale up and commercial success of a large-scale algal operation.
Fig. 3. Very high level of foaming in a raceway pond of Arthrospira, suggesting that there is a high level of protein dissolved in the culture as well as over-saturation of oxygen.

Scaling up algal cultures to commercial volumes and maintaining these cultures is not a trivial task and requires skilled and experienced staff underpinned by good science. The key operational requirements for large-scale commercial microalgal culture are: (1) the efficient and reliable production of the inoculum in the shortest time possible; (2) effective monitoring of the cultures for early detection of any potential problems which may affect the stability of the culture so that remedial action can be taken; (3) the development of operating protocols for the management of the cultures under the changing environment under outdoor conditions to maintain reliability, high productivity and product quality for long periods; (4) strategies for avoiding culture collapse in the case of equipment breakdown interrupting normal operations. All of these considerations and protocols will need to be both site- and species-specific and must be cognisant of the fact that the entire operation must be profitable.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Michael A. Borowitzka
The authors contributed equally to this paper.
References
- Azov, Y. & Goldman, J.C. (1982). Free ammonia inhibition of algal photosynthesis in intensive cultures. Applied and Environmental Microbiology, 43: 735–739.
- Barminski, R., Storteboom, H. & Davis, J.G. (2016). Development and evaluation of an organically certifiable growth medium for cultivation of cyanobacteria. Journal of Applied Phycology, 28: 2623–2630.
- Beacham, T.A., Sweet, J.B. & Allen, M.J. (2017). Large scale cultivation of genetically modified microalgae: a new era for environmental risk assessment. Algal Research, 25: 90–100.
- Beardall, J. & Raven, J.A. (2013). Limits to phototrophic growth in dense culture: CO2 supply and light. In Algae for Biofuels and Energy (Borowitzka, M.A. & Moheimani, N.R., editors), 91–97. Springer, Dordrecht.
- Becker, E.W. (1994). Microalgae: Biotechnology and Microbiology. Cambridge University Press, Cambridge.
- Belay, A. (1997). Mass culture of Spirulina outdoors – The Earthrise Farms experience. In Spirulina platensis (Arthrospira): Physiology, Cell-biology and Biochemistry (Vonshak, A., editor), 131–158. Taylor & Francis, London.
- Belay, A. (2013). Biology and industrial production of Arthrospira (Spirulina). In Handbook of Microalgal Culture: Applied Phycology and Biotechnology (Richmond, A. & Hu, Q., editors), 339–358. Blackwell, Oxford.
- Belay, A., Ota, Y., Miyakawa, K. & Shimamatsu, H. (1994). Production of high quality Spirulina at Earthrise Farms. In Algal Biotechnology in the Asia-Pacific Region (Phang, S.M., Lee, K., Borowitzka, M.A. & Whitton, B., editors), 92–102. Institute of Advanced Studies, University of Malaya, Kuala Lumpur.
- Ben-Amotz, A. (2004). Industrial production of microalgal cell-mass and secondary products – major industrial species: Dunaliella. In Microalgal Culture: Biotechnology and Applied Phycology (Richmond, A., editor), 273–280. Blackwell Science, Oxford.
- Ben-Amotz, A. & Avron, M. (1989). The biotechnology of mass culturing Dunaliella for products of commercial interest. In Algal and Cyanobacterial Biotechnology (Cresswell, R.C., Rees, T.A.V. & Shah, N., editors), 91–114. Longman Scientific & Technical, Harlow.
- Ben-Amotz, A. & Avron, M. (1990). The biotechnology of cultivating the halotolerant alga Dunaliella. Trends in Biotechnology, 8: 121–126.
- Ben-Amotz, A., Shaish, A. & Avron, M. (1991). The biotechnology of cultivating Dunaliella for production of ß-carotene rich algae. Bioresource Technology, 38: 233–235.
- Borowitzka, L.J. (1991). Development of Western Biotechnology algal beta-Carotene plant. Bioresource Technology, 38: 251–252.
- Borowitzka, L.J. (1992). Commercial Dunaliella production: history of development. In Profiles on Biotechnology (Villa, T.G. & Abalde, J., editors), 233–245. Universidade de Compostela, Santiago de Compostela.
- Borowitzka, L.J. (1994). Commercial pigment production from algae. In Algal Biotechnology in the Asia-Pacific region (Phang, S.M., Lee, K., Borowitzka, M.A. & Whitton, B., editors), 82–84. Institute of Advanced Studies, University of Malaya, Kuala Lumpur.
- Borowitzka, L.J. & Borowitzka, M.A. (1989). Industrial production: methods and economics. In Algal and Cyanobacterial Biotechnology (Cresswell, R.C., Rees, T.A.V. & Shah, N., editors), 294–316. Longman Scientific, London.
- Borowitzka, L.J. & Borowitzka, M.A. (1990). Commercial production of ß-carotene by Dunaliella salina in open ponds. Bulletin of Marine Science, 47: 244–252.
- Borowitzka, L.J., Borowitzka, M.A. & Moulton, T. (1984). The mass culture of Dunaliella: from laboratory to pilot plant. Hydrobiologia, 116/117: 115–121.
- Borowitzka, L.J., Moulton, T.P. & Borowitzka, M.A. (1985). Salinity and the commercial production of beta-carotene from Dunaliella salina. Nova Hedwigia, Beihefte, 81: 217–222.
- Borowitzka, M.A. (2005). Culturing microalgae in outdoor ponds. In Algal Culturing Techniques (Anderson, R.A., editor), 205–218. Elsevier Academic Press, London.
- Borowitzka, M.A. (2013a). Dunaliella: biology, production, and markets. In Handbook of Microalgal Culture (Richmond, A. & Hu, Q., editors), 359–368. John Wiley & Sons, Chichester.
- Borowitzka, M.A. (2013b). High-value products from microalgae – their development and commercialisation. Journal of Applied Phycology, 25: 743–756.
- Borowitzka, M.A. (2013c). Species and strain selection. In Algae for Biofuels and Energy (Borowitzka, M.A. & Moheimani, N.R., editors), 77–89. Springer, Dordrecht.
- Borowitzka, M.A. (2016). Algal physiology and large-scale outdoor cultures of microalgae. In The Physiology of Microalgae (Borowitzka, M.A., Beardall, J. & Raven, J.A., editors), 601–652. Springer, Dordrecht.
- Borowitzka, M.A. & Borowitzka, L.J. (1988). Limits to growth and carotenogenesis in laboratory and large-scale outdoor cultures of Dunaliella salina. In Algal Biotechnology (Stadler, T., Mollion, J., Verdus, M.C., Karamanos, Y., Morvan, H. & Christiaen, D., editors), 371–381. Elsevier Applied Science, Barking.
- Borowitzka, M.A. & Moheimani, N.R. (2013a). Open pond culture systems. In Algae for Biofuels and Energy (Borowitzka, M.A. & Moheimani, N.R., editors), 133–152. Springer, Dordrecht.
- Borowitzka, M.A. & Moheimani, N.R. (2013b). Sustainable biofuels from algae. Mitigation and Adaptation Strategies for Global Change, 18: 13–25.
- Boruff, B.J., Moheimani, N.R. & Borowitzka, M.A. (2015). Identifying locations for large-scale microalgae cultivation in Western Australia: a GIS approach. Applied Energy, 149: 379–391.
- Bruning, K. (1991a). Effects of phosphorus limitation on the epidemiology of a chytrid phytoplankton parasite. Freshwater Biology, 25: 409–417.
- Bruning, K. (1991b). Infection of the diatom Asterionella by a chytrid. 1. Effects of light on reproduction and infectivity of the parasite. Journal of Plankton Research, 13: 103–117.
- Burlew, J.S. (1953). Algae Culture. From Laboratory to Pilot Plant. Carnegie Institution of Washington, Washington, DC.
- Camacho Rubio, F., Acien Fernandez, F.G., Sanchez Perez, J.A., Garcia Camacho, F. & Molina Grima, E. (1999). Prediction of dissolved oxygen and carbon dioxide concentration profiles in tubular photobioreactors for microalgal culture. Biotechnology and Bioengineering, 62: 71–86.
- Carney, L.T. & Lane, T.W. (2014). Parasites in algae mass culture. Frontiers in Microbiology, 5: 278.
- Chatsungnoen, T. & Chisti, Y. (2016). Oil production by six microalgae: impact of flocculants and drying on oil recovery from the biomass. Journal of Applied Phycology, 28: 2697–2705.
- Curtain, C.C., West, S.M. & Schlipalius, L. (1987). Manufacture of ß-carotene from the salt lake alga Dunaliella salina; the scientific and technical background. Australian Journal of Biotechnology, 1: 51–57.
- Davis, R., Aden, A. & Pienkos, P. (2011). Techno-economic analysis of microalgae for fuel production. Applied Energy, 88: 3524–3531.
- De Bruin, A., Ibelings, B.W., Rijkeboer, M., Brehm, M. & Van Donk, E. (2004). Genetic variation in Asterionella formosa (Bacillariophyceae): is it linked to frequent epidemics of host-specific parasitic fungi? Journal of Phycology, 40: 823–830.
- Dodd, J.C. (1986). Elements of pond design and construction. In CRC Handbook of Microalgal Mass Culture (Richmond, A., editor), 265–283. CRC Press, Boca Raton, FL.
- Doucha, J. & Lívanský, K. (2012). Production of high-density Chlorella culture grown in fermenters. Journal of Applied Phycology, 24: 35–43.
- Flynn, K.J., Kenny, P. & Mitra, A. (2017). Minimising losses to predation during microalgae cultivation. Journal of Applied Phycology, 29: 1829–1840.
- Fon Sing, S., Isdepsky, A., Borowitzka, M.A. & Lewis, D.M. (2014). Pilot-scale continuous recycling of growth medium for the mass culture of a halotolerant Tetraselmis sp. in raceway ponds under increasing salinity: a novel protocol for commercial microalgal biomass production. Bioresource Technology, 161: 47–54.
- Gan, S.Y. & Maggs, C.A. (in press). Random mutagenesis and precise gene editing technologies: applications in algal crop improvement and functional genomics. European Journal of Phycology.
- Griffiths, M.J. & Harrison, S.T.L. (2009). Lipid productivity as a key characteristic for choosing algal species for biodiesel production. Journal of Applied Phycology, 21: 493–507.
- Grobbelaar, J.U. (1991). The influence of light dark cycles in mixed algal cultures on their productivity. Bioresource Technology, 38: 189–194.
- Grobbelaar, J.U. (1994). Turbulence in mass algal cultures and the role of light dark fluctuations. Journal of Applied Phycology, 6: 331–335.
- Grobbelaar, J.U. (2009). From laboratory to commercial production: a case study of a Spirulina (Arthrospira) facility in Musina, South Africa. Journal of Applied Phycology, 21: 523–527.
- Grobbelaar, J.U., Nedbal, L. & Tichý, V. (1996). Influence of high frequency light/dark fluctuations on photosynthetic characteristics of microalgae photoacclimated to different light intensities and implications for mass algal cultivation. Journal of Applied Phycology, 8: 335–343.
- Gsell, A.S., de Senerpont Domis, L.N., van Donk, E. & Ibelings, B.W. (2013). Temperature alters host genotype-specific susceptibility to chytrid infection. PLoS ONE, 8: e71737.
- Guihéneuf, F., Khan, A. & Tran, L.-S.P. (2016). Genetic engineering: a promising tool to engender physiological, biochemical, and molecular stress resilience in green microalgae. Frontiers in Plant Science, 7: 400.
- Gutman, J., Zarka, A. & Boussiba, S. (2009). The host-range of Paraphysoderma sedebokerensis, a chytrid that infects Haematococcus pluvialis. European Journal of Phycology, 44: 509–514.
- Hadiyanto, H., Elmore, S., Van Gerven, T. & Stankiewicz, A. (2013). Hydrodynamic evaluations in high rate algae pond (HRAP) design. Chemical Engineering Journal, 217: 231–239.
- Hadj-Romdhane, F., Jaouen, P., Pruvost, J., Grizeau, D., Van Vooren, G. & Bourseau, P. (2012). Development and validation of a minimal growth medium for recycling Chlorella vulgaris culture. Bioresource Technology, 123: 366–374.
- Hadj-Romdhane, F., Zheng, X., Jaouen, P., Pruvost, J., Grizeau, D., Croué, J.P. & Bourseau, P. (2013). The culture of Chlorella vulgaris in a recycled supernatant: effects on biomass production and medium quality. Bioresource Technology, 132: 285–292.
- Hartig, P., Grobbelaar, J.U., Soeder, C.J. & Groeneweg, J. (1988). On the mass culture of microalgae: areal density as an important factor for achieving maximal productivity. Biomass, 15: 211–221.
- Hu, Q. & Richmond, A. (1994). Optimising the population density in Isochrysis galbana grown outdoors in a glass column photobioreactor. Journal of Applied Phycology, 6: 391–396.
- Hu, Q., Zarmi, Y. & Richmond, A. (1998). Combined effects of light intensity, light-path, and culture density on output rate of Spirulina platensis (Cyanobacteria). European Journal of Phycology, 32: 165–171.
- Hulatt, C.J. & Thomas, D.N. (2011). Energy efficiency of an outdoor microalgal photobioreactor sited at mid-temperate latitude. Bioresource Technology, 102: 6687–6695.
- Jeamton, W., Dulsawat, S., Tanticharoen, M., Vonshak, A. & Cheevadharanak, S. (2017). Overcoming intrinsic restriction enzyme barriers enhances transformation efficiency in Arthrospira platensis. Plant and Cell Physiology, 54: 822–830.
- Jiménez, C., Cossío, B.R., Labella, D. & Niell, F.X. (2003). The feasibility of industrial production of Spirulina (Arthrospira) in southern Spain. Aquaculture, 217: 179–190.
- Kilian, O., Benemann, C.S.E., Niyogi, K.K. & Vick, B. (2011). High-efficiency homologous transformation in the oil-producing alga Nannochloropsis sp. Proceedings of the National Academy of Sciences USA, 108: 21265–21269.
- Kim, J., Lee, J.-Y. & Lu, T. (2014). Effects of dissolved inorganic carbon and mixing on autotrophic growth of Chlorella vulgaris. Biochemical Engineering Journal, 82: 34–40.
- Kok, B. (1956). Photosynthesis in flashing light. Biochimica et Biophysica Acta, 21: 245–258.
- Lakeman, M.B. & Cattolico, R.A. (2007). Cryptic diversity in phytoplankton cultures is revealed using a simple plating technique. Journal of Phycology, 43: 662–647.
- Lakeman, M.B., von Dassow, P. & Cattolico, R.A. (2009). The strain concept in phytoplankton ecology. Harmful Algae, 8: 746–758.
- Laws, E.A., Taguchi, S., Hirata, J. & Pang, L. (1988). Mass culture optimization studies with four marine microalgae. Biomass, 16: 19–32.
- Lazier, J.R.N. & Mann, K.H. (1989). Turbulence and the diffusive layers around small organisms. Deep Sea Research Part A. Oceanographic Research Papers, 36: 1721–1733.
- Lívanský, K., Dedic, K., Binova, J., Tichy, V., Novotny, P. & Doucha, J. (1996). Influence of the nutrient solution recycling on the productivity of Scenedesmus obliquus, utilization of nutrients and water in outdoor cultures. Algological Studies, 81: 105–113.
- Lu, C. & Vonshak, A. (1999). Photoinhibition in outdoor Spirulina platensis cultures assessed by polyphasic chlorophyll fluorescence transients. Journal of Applied Phycology, 11: 355–359.
- Masojidek, J., Vonshak, A. & Torzillo, G. (2011). Chlorophyll fluorescence applications in microalgal mass cultures. In Chlorophyll a Fluorescence in Aquatic Science: Methods and Applications (Suggett, D.J., Prásil, O. & Borowitzka, M.A., editors), 277–292. Springer, Dordrecht.
- Moheimani, N.R. (2013). Long-term outdoor growth and lipid productivity of Tetraselmis suecica, Dunaliella tertiolecta and Chlorella sp (Chlorophyta) in bag photobioreactors. Journal of Applied Phycology, 25: 167–176.
- Moheimani, N.R. (2016). Tetraselmis suecica culture for CO2 bioremediation of untreated flue gas from a coal-fired power station. Journal of Applied Phycology, 28: 2139–2146.
- Moheimani, N.R. & Borowitzka, M.A. (2006). The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. Journal of Applied Phycology, 18: 703–712.
- Moheimani, N.R. & Borowitzka, M.A. (2007). Limits to growth of Pleurochrysis carterae (Haptophyta) grown in outdoor raceway ponds. Biotechnology and Bioengineering, 96: 27–36.
- Moheimani, N.R., Isdepsky, A., Lisec, J., Raes, E. & Borowitzka, M.A. (2011). Coccolithophorid algae culture in closed photobioreactors. Biotechnology and Bioengineering, 108: 2078–2087.
- Molina Grima, E., Acién Fernández, F.G. & Robles Medina, A. (2013). Downstream processing of cell mass and products. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology (Richmond, A. & Hu, Q., editors), 267–309. Wiley Blackwell, Chichester.
- Moulton, T.P., Borowitzka, L.J. & Vincent, D.J. (1987). The mass culture of Dunaliella salina for ß-carotene: from pilot plant to production plant. Hydrobiologia, 151/152: 99–105.
- Myers, J., Phillips, J.N. & Graham, J. (1951). On the mass culture of algae. Plant Physiology, 26: 539–548.
- Nymark, M., Sharma, A.K., Sparstad, T., Bones, A.M. & Winge, P. (2016). A CRISPR/Cas9 system adapted for gene editing in marine algae. Scientific Reports, 6: 24951.
- Olaizola, M. (2000). Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. Journal of Applied Phycology, 12: 499–506.
- Pahl, S., Lee, A., Kalaitzidis, T., Ashman, P., Sathe, S. & Lewis, D. (2013). Harvesting, thickening and dewatering microalgae biomass. In Algae for Biofuels and Energy (Borowitzka, M.A. & Moheimani, N.R., editors), 165–185. Springer, Dordrecht.
- Prussi, M., Buffi, M., Casini, D., Chiaramonti, D., Martelli, F., Carnevale, M., Tredici, M.R. & Rodolfi, L. (2014). Experimental and numerical investigations of mixing in raceway ponds for algae cultivation. Biomass and Bioenergy, 67: 390–400.
- Radakovits, R., Jinkerson, R.E., Darzins, A. & Posewitz, M.C. (2010). Genetic engineering of algae for enhanced biofuel production. Eukaryotic Cell, 9: 486–501.
- Raven, J.A. & Beardall, J. (2016). Dark respiration and organic carbon loss. In The Physiology of Microalgae (Borowitzka, M.A., Beardall, J. & Raven, J.A., editors), 129–140. Springer, Dordrecht.
- Raven, J.A. & Giodano, M. (2016). Combined nitrogen. In The Physiology of Microalgae (Borowitzka, M.A., Beardall, J. & Raven, J.A., editors), 143–154. Springer, Dordrecht.
- Richmond, A. (2000). Microalgal biotechnology at the turn of the millennium: a personal view. Journal of Applied Phycology, 12: 441–451.
- Richmond, A. (2004). Biological principles of mass cultivation. In Handbook of Microalgal Cultures. Biotechnology and Applied Phycology (Richmond, A., editor), 125–177. Blackwell Science, Oxford.
- Richmond, A. (2013). Biological principles of mass cultivation of photoautotrophic microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology (Richmond, A. & Hu, Q., editors), 171–204. John Wiley & Sons, Chichester.
- Richmond, A. & Vonshak, A. (1978). Spirulina culture in Israel. Archiv für Hydrobiologie, 11: 274–280.
- Richmond, A. & Zou, N. (1999). Efficient utilisation of high photon irradiance for mass production of photoautotrophic micro-organisms. Journal of Applied Phycology, 11: 123–127.
- Rodolfi, L., Zittelli, G.C., Barsanti, L., Rosati, C. & Tredeci, M.R. (2003). Growth medium recycling in Nannochloropsis sp. mass culture. Biomolecular Engineering, 20: 243–248.
- Schlipalius, L. (1991). The extensive commercial cultivation of Dunaliella salina. Bioresource Technology, 38: 241–243.
- Shimamatsu, H. (1987). A pond for edible Spirulina production and its hydraulic studies. Hydrobiologia, 151/152: 83–89.
- Shimamatsu, H. (2004). Mass production of Spirulina, an edible alga. Hydrobiologia, 512: 39–44.
- Soeder, C.J. (1980). Massive cultivation of microalgae: results and prospects. Hydrobiologia, 72: 197–209.
- Spolaore, P., Joannis-Cassan, C., Duran, E. & Isambert, A. (2006). Commercial applications of microalgae. Journal of Bioscience and Bioengineering, 101: 87–96.
- Stephens, E., Ross, I.L., King, Z., Mussgnug, J.H., Kruse, O., Posten, C., Borowitzka, M.A. & Hankamer, B. (2010). An economic and technical evaluation of microalgal biofuels. Nature Biotechnology, 28: 126–128.
- Sung, M.-G., Shin, W.-S., Kim, W., Kwon, J.-H., & Yang, J.-W. (2014). Effect of shear stress on the growth of continuous culture of Synechocystis PCC 6803 in a flat-panel photobioreactor. Korean Journal of Chemical Engineering, 31: 1233–1236.
- Szyjka, S.J., Mandal, S., Schoepp, N.G., Tyler, B.M., Yohn, C.B., Poon, Y.S., Villareal, S., Burkart, M.D., Shurin, J.B. & Mayfield, S.P. (2017). Evaluation of phenotype stability and ecological risk of a genetically engineered alga in open pond production. Algal Research, 24: 378–386.
- Torzillo, G. & Vonshak, A. (2013). Environmental stress physiology with reference to mass cultures. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology (Richmond, A. & Hu, Q., editors), 90–111. John Wiley & Sons, Chichester.
- Torzillo, G., Giovannetti, L., Bocci, F. & Materassi, R. (1984). Effect of oxygen concentration on the protein content of Spirulina biomass. Biotechnology and Bioengineering, 26: 1134–1135.
- Torzillo, G., Sacchi, A. & Materassi, R. (1991). Temperature as an important factor affecting productivity and night biomass loss in Spirulina platensis grown outdoors in tubular photobioreactors. Bioresource Technology, 38: 95–100.
- Venteris, E.R., McBride, R.C., Coleman, A.M., Skaggs, R.L., & Wigmosta, M.S. (2014). Siting algae cultivation facilities for biofuel production in the United States: trade-offs between growth rate, site constructability, water availability, and infrastructure. Environmental Science and Technology, 48: 3559–3566.
- Vonshak, A. & Guy, R. (1992). Photoadaptation, photoinhibition and productivity in the blue-green alga, Spirulina platensis grown outdoors. Plant Cell and Environment, 15: 613–616.
- Vonshak, A., Torzillo, G., Masojidek, J. & Boussiba, S. (2001). Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta). Plant Cell and Environment, 24: 1113–1118.
- Vonshak, A., Laorawat, S., Bunnag, B. & Tanticharoen, M. (2014). The effect of light availability on the photosynthetic activity and productivity of outdoor cultures of Arthrospira platensis (Spirulina). Journal of Applied Phycology, 26: 1309–1315.
- Weissman, J.C., Tillett, D.M. & Goebel, R.P. (1989). Design and operation of an outdoor microalgae test facility. Final subcontractors report. SERI/STR-232-3569, 1–56. Solar Energy Research Institute, Golden, Colorado.
- White, R.L. & Ryan, R.A. (2015). Long-term cultivation of algae in open-raceway ponds: lessons from the field. Industrial Biotechnology, 11: 213–220.
- Zhang, J. (2013). Culture of Botryococcus braunii. M.Sc. thesis, Murdoch University, Murdoch, Western Australia.
- Zheng, Y., Chi, Z., Lucker, B. & Chen, S. (2012). Two-stage heterotrophic and phototrophic culture strategy for algal biomass and lipid production. Bioresource Technology, 103: 484–488.
