ABSTRACT
This article reviews the role of microbial biofilms in infection, and the antimicrobial chemical diversity of marine macroalgae and their associated microbiomes. Antimicrobial resistance (AMR) represents one of the major health threats faced by humanity over the next few years. To prevent a global epidemic of antimicrobial-resistant infections, the discovery of new antimicrobials and antibiotics, better anti-infection strategies and diagnostics, and changes to our current use of antibiotics have all become of paramount importance. Numerous studies investigating the bioactivities of seaweed extracts as well as their secondary and primary metabolites highlight the vast biochemical diversity of seaweeds, with new modes of action making them ideal sources for the discovery of novel antimicrobial bioactive compounds of pharmaceutical interest. In recent years, researchers have focused on characterizing the endophytic and epiphytic microbiomes of various algal species in an attempt to elucidate host-microbe interactions as well as to understand the function of microbial communities. Although environmental and host-associated factors crucially shape microbial composition, microbial mutualistic and obligate symbionts are often found to play a fundamental role in regulating many aspects of host fitness involving ecophysiology and metabolism. In particular, algal ‘core’ epiphytic bacterial communities play an important role in the protection of surfaces from biofouling, pathogens and grazers through the production of bioactive metabolites. Together, marine macroalgae and their associated microbiomes represent unique biological systems offering great potential for the isolation and identification of novel compounds and strategies to counteract the rise and dissemination of AMR.
Introduction
The emergence of antimicrobial resistance (AMR) in bacteria is an ancient natural process (D’Costa et al., Citation2011) resulting from the perpetual selection of new traits evolving as a result of mutation (Livermore, Citation2002), gradual increases in tolerance to sub-lethal concentrations of biocides (SCENIHR, Citation2009) and horizontal gene transfer through transformation, transduction, recombination and conjugation events (Furuya & Lowy, Citation2006). Despite the undeniable contribution of antibiotic use to the development of a much healthier modern society, the release of large quantities of antibiotics into the environment as a result of their manufacture at an industrial global scale for use in the clinical setting and for agriculture and animal care has increased the selective pressure on bacterial human pathogens (Busetti et al., Citation2014). As a result, in the clinical setting the link between antibiotic use and the generation and dissemination of resistant and multi-resistant strains is well established (Hawkey, Citation2008; Wellington et al., Citation2013). The world faces an emerging epidemic of antibiotic-resistant infections, the second-leading cause of premature death worldwide (Spellberg et al., Citation2008). Without effective solutions to confront AMR, by 2050, 10 million lives a year and more than US$100 trillion of economic output worldwide could be at risk due to the rise of drug-resistant infections (O’Neill, Citation2014). This article reviews the role of microbial biofilms in infection and the acquisition of resistance, and examines the processes involved in the development and maintenance of microbial biofilms. We focus particularly on the role of quorum sensing, discuss antimicrobial bioactives obtained from marine organisms, and review the current state of knowledge of marine macroalgae and their associated microbiomes as a source of antimicrobial chemical diversity.
Biofilms
Biofilms, consortia of surface-attached microbial cells immersed in a self-secreted extracellular polymeric matrix (Costerton et al., Citation1978; Donlan, Citation2002), constitute the principal form of microbial growth in almost all natural and pathogenic environments and a widespread survival strategy amongst microorganisms (O’Toole, Citation2011; Nett et al., Citation2012). The National Institutes of Health (NIH) estimates that microbial biofilms (Davies, Citation2003; Wu et al., Citation2015) are implicated in up to 80% of all human infections. In fact, biofilm aetiology has been described as the root cause of a majority of chronic and recurrent human infections and in almost all device-associated infections (Justin & Melander, Citation2009; Harrison et al., Citation2010; Hoiby et al., Citation2011; Wu et al., Citation2015). Microbial biofilms favour both spontaneous mutation and vertical evolution of resistance genes (Savage et al., Citation2013) as well as their intra- and inter-specific transmission through horizontal gene transfer mechanisms (transformation, conjugation and transduction; Appelbaum, Citation2007). For example, within biofilms, bacteria have been shown to use transposable elements to acquire resistance and develop multi-resistance (Ready et al., Citation2002). The emergence of multiple-antibiotic-resistant strains amongst pathogens normally present in the hospital environment is of particular concern and over a decade ago hospital-acquired infections were estimated to be responsible for an additional annual health care cost of £986 million in England and Wales alone (Plowman, Citation2000; Plowman et al., Citation2001). In light of the role of bacterial biofilms in infection and dissemination of AMR, the isolation and characterization of novel antibiofilm bioactives as well as the identification of novel therapeutic approaches is crucial.
Within biofilms, both Gram positive and Gram negative bacteria use quorum sensing (QS), a type of cell-to-cell communication based on the release and detection of small signalling compounds, to coordinate multicellular behaviour and control a wide variety of physiological activities (Papenfort & Bassler, Citation2016). Some bacterial species use QS to coordinate the transcription and translation of unrelated genetic loci. For instance, the opportunistic human pathogen Pseudomonas aeruginosa uses two hierarchically organized LuxI/LuxR type homologue pairs generally used by some Gram negative bacteria to produce and respond to acyl homoserine lactones, LasI/LasR and Rh1I/Rh1R, to control 170–400 genes via a complex network (Hentzer et al., Citation2002; Schuster et al., Citation2003; Wagner et al., Citation2003; Parsek & Greenberg, Citation2005). The synchronized synthesis and release of gene products with substantially different functions suggests that QS is an adaptive response evolved to cope with conditions of high population density, for instance those found in associations with plant and metazoan hosts (Swift et al., Citation2001).
Biofilms in the marine environment
In marine environments all unprotected submerged surfaces are rapidly colonized by a succession of marine organisms in a process known as biofouling (Callow & Callow, Citation2002). Biofouling begins with the adsorption of dissolved organic matter by newly available surfaces and these ‘conditioned’ surfaces are then rapidly colonized by prokaryotes and unicellular eukaryotes to form microbial biofilms (microfouling). In the marine environment, this stage is followed by macrofouling, the recruitment of invertebrate larvae and algal spores (Callow & Callow, Citation2002). Marine biofilms typically grow as diverse multi-species communities (Mueller et al., Citation2006). In the photic zone they are usually dominated by phototrophic microalgal consortia (Rao et al., Citation1997) and represent a crucial carbon source for other trophic levels, affecting mass transfer processes at the ecosystem level.
Bacteria are typically the primary colonizers of submerged surfaces. As pioneering bacterial species begin to form a biofilm, they establish and define the physicochemical conditions responsible for a complex yet partly ordered secondary recruitment of microorganisms (Dang & Lovell, Citation2000) such as other marine bacteria, protozoans, flagellates and microalgae, as well as invertebrates and macroalgae, and in some cases might be responsible for inducing cellular metamorphosis in some larval types (Dobretsov et al., Citation2006). For example, tetrabromopyrrole, a compound produced by a Pseudoalteromonas bacterium, causes larval metamorphosis of the coral Acropora millepora (Tebben et al., Citation2011). The complexity of the modulations of these phenomena is paralleled by the extreme diversity in the distribution and composition of biological and chemical species found in marine microbial biofilms. Experiments using monospecific biofilms (Wieczorek & Todd, Citation1998; Dobretsov et al., Citation2006; Qian et al., Citation2007) have shown an influence on the activity of the marine flora ascribable to the synthesis and release of antimicrobial compounds and a range of stimulatory signalling molecules that mostly remain to be isolated and characterized (Bowman, Citation2007).
Bioactive compounds
A ‘bioactive compound’ can be defined as a secondary metabolite which at low concentrations exerts either beneficial or harmful effects on living organisms and is therefore of interest for potential industrial or medical applications (Rangel-Huerta et al., Citation2015). Of the more than 1 million natural products that have been discovered from both terrestrial and marine living organisms, 20–25% have shown antimicrobial, antifungal, anti-protozoan, anti-nematode, anticancer, antiviral or anti-inflammatory properties (Bérdy, Citation2005; Penesyan et al., Citation2010; Newman & Cragg, Citation2016). The diversity of natural compounds can be ascribed to the process of natural selection that has driven the evolution of molecules best suited to perform their biological activities (Koehn & Carter, Citation2005).
Natural products, chemicals produced by living organisms, are a traditional source of pharmacologically active compounds (Molinski et al., Citation2009), and continue to be a major inspiration for the majority of US Food and Drug Administration (FDA)-approved agents and for drug discovery and design. More than 60% of small molecule agents approved for use as drugs can be traced back to natural products such as aspirin (willow/birch), morphine (poppy), penicillin (fungus), Lovastatin (fungus), Adriamycin/dauxorubicin (bacterium) and Taxol™ (yew tree).
Although the first indication of the presence in seawater of bacteria with an inhibitory effect against human pathogens such as Vibrio cholerae and Bacillus anthracis has been attributed to De Giaxa (1889; see Balcazar et al., Citation2007), the ‘modern’ study of bioactives of marine origin emerged more than 70 years ago with the pioneering work of the Italian microbiologist Giuseppe Brotzu (Professor of Hygiene at the University of Cagliari, Italy). In 1945 Brotzu grew cultures from seawater samples collected near a sewage outlet in Sardinia (Mediterranean Sea) and tested isolates for antibiotic activity. Brotzu is credited with having isolated the initial strain of the fungus Cephalosporium acremonium, and reported the strong inhibitory activity displayed by crude culture filtrates against a broad range of human pathogens. Subsequent studies on Brotzu’s marine fungal isolate conducted by E. Abraham and G. Newton (Abraham et al., Citation1953) led to the discovery of the cephalosporin family of antibiotics (Bo, Citation2000). Shortly after Brotzu’s work, in light of the growing realization that the bactericidal activity displayed by seawater was attributable to its containing antibiotic compounds produced by microorganisms, Rosenfield & Zobell (Citation1947) carried out the first large-scale systematic study on the antibiotic activity of marine bacteria against several human pathogens, reporting activities against Bacillus anthracis, Mycobacterium laticola and Staphylococcus citreus amongst others.
In the 1950s, work by Werner Bergman and Robert Feeney led to the discovery of spongothymidine and spongouridine. Both compounds were extracted and identified from the Caribbean sponge Tethya crypta (Bergmann & Feeney, Citation1950, Citation1951) and constitute natural nucleoside analogues, structurally similar to the nucleosides of nucleic acids, but containing arabinose rather than the typical ribose. More importantly, these marine-derived compounds displayed unexpected antiviral activities and became the basis for the synthesis of several antiviral and anticancer drugs including AZT (zidovudine) (Fowler et al., Citation2016), commercially known as Retrovir® (GlaxoSmithKline), the first drug for the treatment of HIV, and acyclovir (sold as Zovirax®; Han et al., Citation2017), used to treat infections caused by the herpes simplex virus. Vidarabine®, also known as Ara-A, is a synthetic purine nucleoside analogue derived from the marine bacterium Streptomyces antibioticus isolated from T. crypta sponges (Agrawal et al., Citation2016), used typically as an ophthalmic ointment for the treatment of acute herpes keratoconjunctivitis (Akkaya & Ozkurt, Citation2016) and recurrent superficial keratitis caused by HSV-1 and HSV-2.
Marine ecosystems still largely constitute an untapped resource for pharmaceutical and biotechnological biodiscovery. In the marine environment, whereas submerged non-living surfaces rapidly become macrofouled, the living surfaces of organisms are comparatively free from macrofouling and are covered with a thin film of epibiotic bacteria (Armstrong et al., Citation2001). This is in part ascribable to metabolites effective as antifouling compounds and to the surface characteristics of marine organisms. Marine macroalgae (seaweeds) are known to utilize a plethora of secondary metabolites to defend themselves from herbivores and bacterial colonization of their exposed surfaces. For example, halogenated furanones produced by the red alga Delisea pulchra display antibiofilm effects against Bacillus subtilis (Ren et al., Citation2002), Escherichia coli (Ren et al., Citation2001) and Pseudomonas aeruginosa (Hentzer et al., Citation2002).
Microbes growing on the surface of a host can also contribute to the host’s overall antifouling strategy. For example, epibiotic bacteria that colonize the surface of some crustacean larvae synthesize simple antimicrobial molecules that can defend the larvae from fungal infections (Gil-Turnes et al., Citation1989). Bacteria isolated from the surface of a tunicate and grown as biofilms hindered the attachment of barnacle and tunicate larvae (Holmstrom et al., Citation1992). Moreover, the presence of epiphytic bacteria on the surface of seaweeds has been shown to be important for proper development, with atypical morphology observed in axenic culture (e.g. Marshall et al., Citation2006; Wichard et al., Citation2015), suggesting that seaweeds and their epiphytic microbiome collaborate as a unified functional entity or holobiont (reviewed by Egan et al., Citation2013).
Quorum sensing inhibition as a novel strategy to attenuate bacterial virulence
An emerging approach designed to attenuate bacterial virulence (i.e. the ability to cause damage to living organisms via the production of virulence factors such as enzymes and toxins) and limit the emergence of pathogenic traits relies on interfering with cell-to-cell communication, processes now commonly termed ‘quorum quenching’ and ‘quorum sensing inhibition’ (QSI). In fact, the inability to coordinate communal behaviours can prevent bacterial pathogens from escaping or overcoming host immune responses and establishing an infection (Hentzer et al., Citation2003; Rasmussen & Givskov, Citation2006). Moreover, the ability to switch off virulence gene expression exogenously (Brackman et al., Citation2011) offers a novel strategy for the treatment or prevention of infection (Camara et al., Citation2002). Overall the use of QSIs represents an ‘antivirulence’ strategy relying on the exploitation of small compounds with the capacity of disarming pathogens thereby rendering them harmless within their host by targeting precise factors (such as toxin function and delivery, virulence gene regulation, or cell adhesion) necessary for the establishment of an infection (Mellbye & Schuster, Citation2011). In certain species of bacteria, disruption of QS has been shown to affect biofilm formation (Irie & Parsek, Citation2008) and differentiation (Hardie & Heurlier, Citation2008), often rendering the biofilm more susceptible to treatment with biocides and antibiotics (Brackman & Coenye, Citation2015). For example, acylated homoserine lactone (AHL) QS mutants of Burkholderia cenocepacia and P. aeruginosa form flatter, less structured biofilm (Diggle et al., Citation2007) and are drastically impaired in their ability to maintain cells within the biofilm (Huber et al., Citation2001; Tomlin et al., Citation2005; Yang et al., Citation2009). Of relevance from a strategic therapeutic perspective, QSI-based treatments have been shown to increase the susceptibility of bacterial biofilms to antibiotics both in vitro and in vivo. For example, a significantly greater percentage of infected wax moth Galleria mellonella larvae and C. elegans survived infection by P. aeruginosa and B. cenocepacia following combined treatment with antibiotic and QS inhibitors, compared with treatment with an antibiotic alone (Brackman et al., Citation2011).
Paradoxically, the strong selective pressure imposed by the use of antibiotics in the clinical setting makes this environment a fertile ground for the generation and spread of resistant and multiresistant strains with a consequent rise in morbidity and mortality due to hospital-acquired infections (Hawkey, Citation2008). Since QS is not directly involved in essential processes such as cell division, one can reason that its inhibition will not generate a severe selective pressure likely to result in the development of resistance (Rasmussen & Givskov, Citation2006; Kendall & Sperandio, Citation2007; Sperandio, Citation2007). In fact, the impairment of QS results in a disruption of the signalling systems responsible for the synthesis and secretion of a number of virulence factors. Although it is reasonable to conclude that resistance to QS would be selected in vivo during infection, when QS is involved in colonization, systemic spread and immune evasion (Defoirdt et al., Citation2010), a broad-spectrum combinatorial approach relying on the use of conventional antibiotics in combination with QSIs as an anti-virulence approach would diminish the chance of this event considerably. In a study investigating the vertical evolution of QSI resistance as well as the fitness conferred during bacterial social interaction, Mellbye & Schuster (Citation2011) co-cultured wild type Pseudomonas aeruginosa together with QS mutants (mimicking a QSI-sensitive phenotype) in minimal medium containing either bovine serum albumin (BSA) or adenosine as a sole carbon source. Whereas BSA degradation requires extracellular proteases thus providing a social benefit, adenosine is metabolized intracellularly providing a benefit for the individual. QSI-sensitive mimics were found to retard the growth of wild-type QSI-resistant mimics when grown in BSA (public nutrient acquisition) indicating QSI resistance is unlikely to spread, especially during infection (Mellbye & Schuster, Citation2011).
QSI targets
Marine organisms have proven to be a rich source of natural compounds exhibiting quorum sensing inhibitory activity (Dobretsov et al., Citation2009, Citation2011; Saurav et al., Citation2017). In a study examining the inhibition of marine biofouling by QSI, of 78 bioactives tested from compound libraries derived from marine organisms including sponges, seaweeds, fungi, bacteria, tunicates and cyanobacteria, more than half of them displayed QSI activity (Dobretsov et al., Citation2011). In particular, the compounds hymenialdisin, demethoxy encecalin, microcolins A and B and kojic acid were found to inhibit the QS responses of the LuxR based reporter strains induced by N-3-oxo-hexanoyl-L-homoserine lactone at micromolar concentrations.
The three components of the Gram negative AHL system are (1) the signal molecule generator, (2) the signal molecule itself and (3) the signal molecule receptor, representing the key targets of QSI for an anti-pathogenic drug approach (Rasmussen & Givskov, Citation2006).
(1) In AHL-based Gram negative QS, an inactivation of the LuxI-type synthase would interrupt the synthesis of the relative AHL signal meaning that a significant threshold concentration could not be reached, with failure to activate the downstream genes responsible for virulence. In vitro, a few substrate analogues have been found to actively block the production of AHL. For example, analogues of S-adenosyl-L-methionine (SAM) have proven to be potent inhibitors of AHL synthase in P. aeruginosa (Rasmussen & Givskov, Citation2006). This has yet to be tested in vivo and remains the least investigated method of interfering with QS.
(2) The signalling molecule itself constitutes another target to inhibit QS. The three principal strategies to de-activate a signalling molecule are metabolic, chemical and enzymatic degradation or inactivation. An alkaline pH causes the homoserine lactone ring () to open (Yates et al., Citation2002). For example, when a plant recognizes colonization by the pathogen Erwinia carotovora, which uses AHL-based QS to regulate the synthesis of virulence factors, the plant actively causes alkalinization at the site of attack resulting in lactonolysis. In addition to pH, several other factors including temperature and the length of the acyl side chain influence the opening of the lactone ring. An increase in temperature will accelerate the rate at which the ring opens, whereas the longer the side chain the slower will be the lactonolysis.
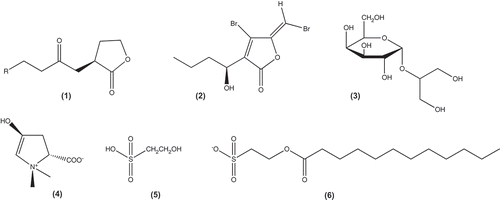
Figs 1–6. Molecular structures of acyl homoserine lactones and quorum sensing inhibitors isolated from marine algae. Fig. 1. General structure of acyl homoserine lactones. Fig. 2. Halogenated furanones. Fig. 3. Floridoside. Fig. 4. Betonicine. Fig. 5. Isethionic acid. Fig. 6. 2-dodecanoyloxyethanesulfonate. Figure adapted from Saurav et al. (Citation2017).
AHL lactonases are enzymes that catalyse the ring opening reaction of the lactone ring (Rasmussen & Givskov, Citation2006). Several Bacillus species are known to produce the lactonase enzyme AiiA (Dong et al., Citation2000), which is specific for the degradation of AHLs. Homologues of AiiA have also been found in other members of the Bacillus genus as well as members of the genera Pseudomonas, Arthrobacter and Klebsiella (Rasmussen & Givskov, Citation2006). This form of inactivation is reversible when the pH is acidic. Moreover, when the AiiA gene was heterologously expressed in P. aeruginosa PAO1 a significant inhibition of virulence gene production and swarming motility was achieved (Reimmann et al., Citation2002). Similarly, when cloned and expressed in Burkholderia species the AiiA gene coding for the lactonase enzyme significantly reduced virulence in this pathogen (Ulrich Citation2004; Wopperer et al., Citation2006). AHL acylases are another class of enzymes that can deactivate the Gram negative signalling molecule by cleaving the N-acyl bond of AHLs. Production of acylases has been reported in numerous genera of bacteria including Ralstonia, pseudomonads, and a Streptomyces (Lin et al., Citation2003). Bacteria such as Variovorax paradoxus and P. aeruginosa produce amino acylases responsible for the cleavage of the peptide bond of the signal molecule (Rasmussen & Givskov, Citation2006) and can use the products of this metabolism as their sole source of energy. It has been hypothesized that P. aeruginosa creates its own AHL-acylases to regulate its own QS system, possibly to evade detection during initial infection of a host (Sio et al., Citation2006).
(3) In AHL-based QS, the LuxR transcription factor responsible for the regulation of downstream QS-dependent pathways represents another valid target for QSI. The use of small AHL analogues to prevent LuxR activation has proven a successful strategy to target LuxR type transcription factors (Suga & Smith, Citation2003). These analogues can displace the original AHL and cause activation of the LuxR-type protein, acting as competitive agonists (Schaefer et al., Citation1996). Synthetic analogues are developed in one of three ways: substitution in the acyl side chain leaving the ring unchanged; substitution and alteration to the lactone ring while the side chain remains unchanged; or extensive modification to both the side chain and lactone ring (Rasmussen & Givskov, Citation2006).
Algal compounds – promising leads for the treatment of biofilm-related infections
Macroalgal bioactives such as sulphated polysaccharides and kahalalides have long been recognized for medical applications (Smit, Citation2004) and interest in them remains high (e.g. Barbosa et al., Citation2014). However, to date, only a few lead compounds and their synthetic derivatives have progressed to animal trials (e.g. Wu et al., Citation2004).
Seaweeds rely on the coating/secretion of secondary metabolites (toxins and broad spectrum antimicrobials and antivirals) for protection against micro- and macro-colonizing organisms (Hentzer et al., Citation2003). For example, as previously noted, several halogenated furanone compounds isolated from the red seaweed Delisea pulchra (Givskov et al., Citation1996) are released at its surface at concentrations capable of inhibiting both prokaryotic and eukaryotic colonization (Steinberg et al., Citation2002). These compounds were shown to be QSI-active against a broad range of bacteria (Givskov et al., Citation1996; Hentzer et al., Citation2002). The furanones produced by Delisea accelerate the turnover of the LuxR transcription factor inhibiting QS-dependent gene expression in Gram negative bacteria (Manefield et al., Citation2002) and the capacity to synthesize such compounds is likely to have evolved as an antifouling strategy to preserve the surface of algal fronds from colonization by Gram negative marine bacteria. However, as they are brominated, their application in humans is limited, making it necessary to search for QSI from other natural sources (Zhu & Sun, Citation2008). Overall, macroalgae have yielded more than 3000 natural products, accounting for approximately 20% of marine natural compounds (Amsler, Citation2008).
Red seaweeds (Rhodophyta)
Research on red seaweeds has discovered that the majority of macroalgal secondary metabolites account for more than 1500 bioactives (Maschek & Baker, Citation2008). With the exception of phlorotannins, which are unique to brown algae, red seaweeds synthesize all major classes of algal natural products (Blunt et al., Citation2016). Red algae primarily synthesize isoprenoid and acetogenin derivatives, as well as amino acid, shikimate and nucleic acid derivatives (Amsler, Citation2008). Halogenated compounds underpin red algal chemistry, with over 90% of compounds reported to contain bromine or chlorine (Cabrita et al., Citation2010).
The genus Laurencia (Rhodomelaceae, Ceramiales), which has been the subject of nearly 50% of the publications on red algal chemistry, produces a plethora of halogenated sesquiterpenes and C15 acetogenins, as well as higher terpenes (Davis & Vasanthi, Citation2011). Laurencia species occur widely on temperate and tropical coasts and are recognized as a rich source of novel secondary metabolites (Cabrita et al., Citation2010). Several of them display promising antimicrobial activity against a range of bacteria. For example, an unidentified species of Laurencia from Malaysia exerted potent antimicrobial activity against a range of marine bacteria; two halogenated C15 acetogenin compounds, elatol and iso-obtusol, were isolated from this alga and structurally elucidated based on spectroscopic data, confirming the potential of these compounds as a source of pharmaceutically relevant bioactives (Vairappan et al., Citation2001). In extracts from L. majuscula, elatol inhibited six bacterial species, with significant antimicrobial activities against Staphylococcus epidermis, Klebsiella pneumonia and Salmonella sp. Iso-obtusol, a polyhalogenated sesquiterpene produced by Laurencia obtusa, was found to display antimicrobial activity against several bacteria, and proved particularly active against K. pneumonia and Salmonella sp. (Vairappan, Citation2003). Interestingly, the antimicrobial activity of elatol and iso-obtusol was found to be equal to or better than conventional antibiotics against K. pneumonia and Salmonella sp. through a bacteriostatic mode of action (Vairappan, Citation2003). Subsequently, Vairappan et al. (Citation2010) discovered a novel brominated diterpene, 10-acetoxyangasiol, as well as four previously known metabolites, aplysidiol, cupalaurenol, 1-methyl-2,3,5-tribromoindole, and chamigrane epoxide in Laurencia sp. These compounds displayed strong antimicrobial activity against clinically relevant bacteria including Staphylococcus aureus, Streptococcus pyogenes, Salmonella sp. and Vibrio cholera.
Members of the order Bonnemaisoniales also produce a diverse array of secondary halogenated metabolites displaying antimicrobial activity (Nash et al., Citation2005). Delisea, Asparagopsis, Bonnemaisonia and Ptilonia all synthesize a group of linear halogenated ketones and branched lactones. Amongst these, the fimbrolides, a group of halogenated furanones () from Delisea pulchra from south-eastern Australia, show QSI activity against a range of bacteria, functioning as an intracellular signal antagonist as well as accelerating LuxR turnover (Rasmussen et al., Citation2000; Manefield et al., Citation2002), and hence providing an antifouling defence (Kjelleberg & Steinberg, Citation2001). From a screen of 39 macroalgae, Asparagopsis taxiformis extracts were shown to inhibit QS in C. violaceum CV026 bioreporter assays (Jha et al., Citation2013). Based on Ion Cyclotron Resonance Fourier Transformation Mass Spectrometry analysis of the QSI-active fraction, the authors proposed that the compound responsible for the QSI activity was 2-dodecanoyloxyethanesulfonate (; Jha et al., Citation2013).
Bonnemaisonia hamifera (, ) is native to Japan, was introduced into the North Atlantic Ocean prior to 1890 (Maggs & Stegenga, Citation1998) and is now widely distributed there. Bonnemaisonia hamifera has a heteromorphic life cycle, alternating between a diploid filamentous ‘Trailliella’ tetrasporophyte and a haploid gametophyte (Breeman et al., Citation1988). Like Delisea pulchra, B. hamifera produces an assortment of mono- and poly-halogenated bioactives including 2-heptanones, 2-heptanols, acetates and acids, some of which display antimicrobial activity (Siuda et al., Citation1975; Jacobsen & Madsen, Citation1978; McConnell & Fenical, Citation1979; Enge et al., Citation2013; Nylund et al., Citation2013).
Figs 7–10. The red algae Bonnemaisonia hamifera and Bonnemaisonia asparagoides display strong antimicrobial activity against AHL quorum sensing bioreporter strain Chromobacterium violaceum. Algal samples were overlaid with C. violaceum in 0.5% agar prior to incubation. Fig. 7. B. hamifera washed in ddH2O. Fig. 8. B. hamifera pre-washed in 70% ethanol; QSI activity not altered by ethanol wash. Fig. 9. B. asparagoides washed in ddH2O. Fig. 10. B. asparagoides washed in 70% ethanol, exhibiting significant loss of QSI activity which has been extracted by the ethanol wash.
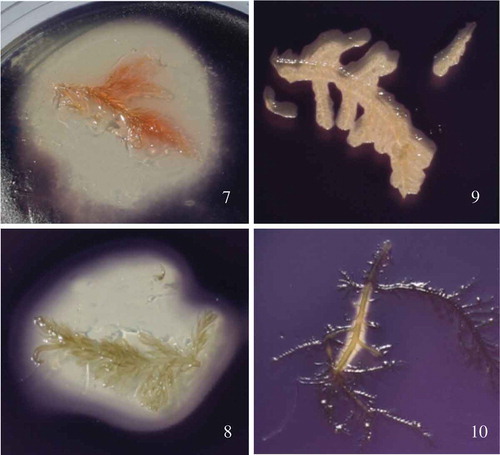
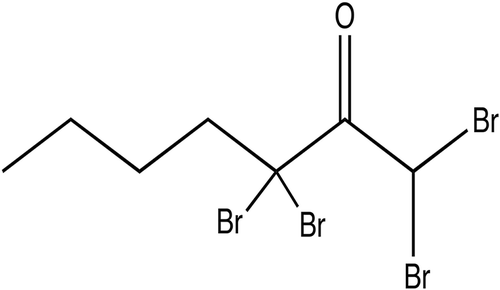
One of the main secondary metabolites, 1,1,3,3-tetrabromo-2-heptanone (), stored in specialized gland cells in the Trailliella phase, has an ecologically relevant role as an antifouling agent against bacterial surface colonization. Natural surface concentrations (3.6 µg cm–2) of 1,1,3,3-tetrabromo-2-heptanone applied to artificial panels significantly reduced the number of settled bacteria (Nylund et al., Citation2008). Moreover, organic extracts of B. hamifera show broad-spectrum antimicrobial activity at ecologically relevant concentrations (Nylund et al., Citation2005, Citation2008, Citation2013) confirming the potential of this species as a novel source of marine-derived antibiofilm compounds active against human pathogens. The compound also acts as a chemical grazing deterrent (Enge et al., Citation2013), which is metabolically expensive to produce but protects the seaweed against bacteria as well as grazers (Nylund et al., Citation2013).
Fig. 11. Structure of 1,1,3,3-tetrabromo-2-heptanone, a poly-brominated 2-heptanone produced by Bonnemaisonia hamifera displaying antifouling properties.
It is interesting to note that several of these members of the Bonnemaisoniales found in Europe and containing halogenated compounds such as bromophenols (Paul et al., Citation2006) are aliens. These compounds undoubtedly contribute to their invasive potential by deterring grazing and allowing the establishment of high biomass (Enge et al., Citation2013). This is a clear indication that alien species are worth targeting in the search for new bioactives. QSI compounds have also been described from a few non-invasive red algae, such as Ahnfeltiopsis flabelliformis (Gigartinales) from Korea which has been shown to produce three AHL inhibitory compounds, floridoside (), betonicine () and isethionic acid () (Kim et al., Citation2007).
Brown seaweeds (Phaeophyceae)
Brown algae have also yielded a rich chemical diversity with more than 1140 reported secondary metabolites. The most studied and representative bioactives of the brown seaweeds comprise diterpenes, phlorotannins and small C11 acetogenins, all with very little halogenation (Blunt et al., Citation2007). Phlorotannins are distinguishing compounds of brown algae, with a wide range of activities of pharmacological interest (Supplementary table 1) including antimicrobial (Eom et al., Citation2012), antiviral (Ahn et al., Citation2004), antidiabetic (Kang et al., Citation2013; Lee & Jeon, Citation2013), anti-inflammatory (Sugiura et al., Citation2013), anti-allergic (Sugiura et al., Citation2009), anti-cancer (Lee et al., Citation2012) and anti-neurodegenerative (Myung et al., Citation2005; Jung et al., Citation2009; Heo et al., Citation2012; Sathya et al., Citation2013) especially against Alzheimer’s disease (Yoon et al., Citation2008, Citation2009; Ahn et al., Citation2012). In addition, phlorotannins have been shown to provide photo-protection in brown seaweeds (Gómez & Huovinen, Citation2010) and studies exploring their cosmeceutical potential have highlighted the capacity to inhibit UV-A-induced oxidative stress associated with photo-ageing in humans (Pallela et al., Citation2010). The ecological role of phlorotannins in brown seaweeds appears to include defence against epiphytes (Nakajima et al., Citation2016), as well as grazing deterrence (McClintock & Baker, Citation2001).
Although many studies examining brown algal chemistry have focused on Dictyota (Dictyotaceae) and its wealth of terpenes (>250) (Munro & Blunt, Citation2005), several other genera display activities of pharmacological relevance. For example carotenoids from several brown algae have a wide range of bioactivities (Peng et al., Citation2011). The meroditerpenoid methoxybifurcarenone isolated from Cystoseira tamariscifolia displays antifungal activity against three plant pathogenic fungi and antibacterial activity against Agrobacterium tumefaciens and E. coli (Bennamara et al., Citation1999).
Halidrys siliquosa (family Sargassaceae) is a large temperate macroalga growing up to 120 cm long in rock pools and sometimes as forests in the shallow subtidal zone. The bioactive potential of H. siliquosa was identified over four decades ago. Hornsey & Hide (Citation1974, Citation1976) screened crude extracts of H. siliquosa against a series of opportunistic human pathogens and discovered antimicrobial activity against Staphylococcus aureus, E. coli, Bacillus subtilis, Streptococcus pyogenes and Proteus. Culioli et al. (Citation2008) reported the antifouling activity of meroditerpenoids isolated from this species and identified nine tetraprenyltoluquinol-related metabolites exhibiting antifouling properties and inhibiting the growth of the marine bacteria Cobetia marina, Marinobacterium stanieri, Vibrio fischeri and Pseudoalteromonas haloplanktis. Non-cytotoxic concentrations of these meroditerpenoids were found to prevent the settlement of cyprids of Balanus amphitrite. Halidrys siliquosa crude extract was active against the parasites Trypanosoma brucei rhodesiense, T. cruzi and Leishmania donovani and the bacterium Mycobacterium tuberculosis (Spavieri et al., Citation2010) highlighting the potential of this alga for the treatment of mycobacterial and protozoal infections.
Busetti et al. (Citation2015) reported antimicrobial and antibiofilm activity of methanolic extracts of H. siliquosa against clinically relevant human pathogens of the genera Staphylococcus, Streptococcus, Enterococcus, Pseudomonas, Proteus, Stenotrophomonas and Chromobacterium. Biofilms of S. aureus MRSA ATCC 33593 and S. aureus MRSA NCTC 10442 were found to be susceptible to H. siliquosa extract which achieved minimum biofilm eradication concentration (MBEC) values of 1.25 mg ml–1 and 5 mg ml–1 respectively. Active extracts showed no toxicity against wax moth (G. mellonella) larvae across a wide range of concentrations (Busetti et al., Citation2015). The activity of H. siliquosa methanolic extracts against the emerging pathogen Stenotrophomonas maltophilia suggests the production of bioactives with the potential to be used in a treatment strategy for cystic fibrosis as well as therapies for Staphylococcus biofilm-related infections. Moreover, the promising range of activities displayed by H. siliquosa organic extracts against clinically relevant, antibiotic-resistant, human pathogens highlights this alga as a candidate for further studies focused on the isolation of antibiofilm compounds and antimicrobials for the treatment of infections involving multi-resistant pathogenic strains.
Macroalgal microbiomes as a source of novel bioactives of pharmaceutical relevance
In recent years, several studies characterizing algal epiphytic bacterial communities (–) have highlighted the presence of ‘core microbial species’ in mutualistic or obligate association with their host (Singh et al., Citation2015). In particular, several bacterial epiphytes have been reported to produce bioactive compounds that can protect macroalgal surfaces from biofouling (Dobretsov & Qian, Citation2002). However, whereas several concerted studies have focused on characterizing the composition of the human microbiomes as well as deciphering the physiological significance of the host-microbe interactions underlying the mutualistic relationships therein, in seaweeds the microbiomes and the significance of their functional relationship with their hosts remain largely unexplored. The advent of culture-independent, DNA-based, metagenomic and transcriptomic methods has provided powerful new tools for the characterization of host-associated microbiomes as well as for the elucidation of the many, complex, yet often fundamental processes involved in host–microbe interactions, providing future studies with the tools to investigate the functional microbiome involved in the often complex life cycles of macroalgae (Singh & Reddy, Citation2014, 2016). The discoveries deriving from such studies could assist in promoting fitness and productivity in macroalgal species of commercial interest through the modulation of a functionally active microbiome as well as providing enormous potential for the discovery of novel antibiofilm or QSI compounds of clinical relevance.
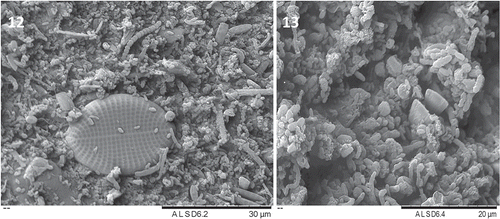
Figs 12–13. SEM of the epiphytic microbial colonization of Halidrys siliquosa algal fronds. Fig. 12. Diatom embedded amongst diverse prokaryotes. Fig. 13. Three-dimensional structure of microbial biofilm.
For example, the epiphytic bacterium Pseudoalteromonas tunicata isolated from the surface of Ulva lactuca can hinder biofilm formation of competing Gram negative microbes through the synthesis of pigmented substances that inhibit LuxR-dependent transcriptional control through a similar mode of action to the furanones (McLean et al., Citation2004). Halobacillus salinus, a marine Gram positive bacterium isolated from a seagrass, synthesizes and releases QSI bioactives active against Gram negative strains (Teasdale et al., Citation2009) through competitive binding (Teasdale et al., Citation2009). These examples indicate that QS inhibition represents a natural, widespread, antifouling strategy evolved by marine organisms making marine ecosystems an ideal source for the discovery of QS inhibitors with potentially clinically relevant antibiofilm activity.
In a recent study, an isolate belonging to the Pseudoalteromonas genus obtained from the algal fronds of the red seaweed Plocamium maggsiae displayed potent QSI activity against acyl homoserine lactone-based reporter strains (Busetti et al., Citation2014). The isolate’s filter-sterilized supernatant significantly diminished biofilm biomass both during biofilm formation as well as in pre-established, mature P. aeruginosa PAO1 biofilms causing a 0.97-log reduction and a 2-log reduction in PAO1 biofilm viable counts in the biofilm formation and eradication assays. The crude organic extract obtained from this isolate displayed a minimum inhibitory concentration (MIC) of 2 mg ml–1 against PAO1 but failed to produce a minimum bactericidal concentration (MBC) confirming the lack of antimicrobial activity in the extract at the concentrations tested. Sub-MIC concentrations of the crude organic extract were found to significantly reduce the quorum sensing (QS)-dependent production of the two virulence factors pyoverdin and pyocyanin in P. aeruginosa PAO1 without affecting growth. A combinatorial approach using tobramycin and the crude organic extract at 1 mg ml–1 against planktonic P. aeruginosa PAO1 increased the effectiveness of tobramycin by 10 times, lowering its MIC against this pathogen from 0.75 to 0.075 mg ml–1 (Busetti et al., Citation2014). The results of this study confirm the efficacy of combinatorial strategies combining current antibiotic treatment with (non-antibiotic) QSI compounds derived from algal microbial epiphytes to improve the efficacy of current antibiotic treatments.
Future perspectives
The imminent global health threat of antimicrobial resistance with the realistic prospect of mankind entering a ‘post-antibiotic era’ has driven research into innovative therapeutic strategies relying on different targets and approaches for the treatment of microbial infections. The gradual elucidation of widespread bacterial communication (QS) systems regulated by small diffusible signal molecules as a means to coordinate group behaviours has revolutionized our classical conception of bacteria as unicellular and thus independent in nature. Targeting complex social behaviours, which include virulence and pathogenicity, regulated by chemical intra- and inter-species signal molecules which allow them to coordinate their behaviour at a community level, represents a novel target for non-antibiotic anti-infective chemotherapy.
Marine organisms are known to produce a variety of QSIs that can thwart biofilm development of competing species (McClean et al., Citation1997; Bauer & Robinson, Citation2002; Saurav et al., Citation2017), representing an important resource for the isolation of novel ‘antipathogenic’ antibiofilm compounds. Bacteria from algal microbiomes remain a relatively untapped source of novel candidate compounds displaying QSI activity with the potential to attenuate biofilm formation, virulence factor production or increase the antimicrobial susceptibility of clinically important pathogenic bacteria in the constant fight against emergence of multi-resistant microorganisms (Saurav et al., Citation2017).
As in many other discovery and development programmes in marine bioactives, there are a multitude of challenges associated with the biodiscovery and commercialization of macroalgal compounds as pharmaceutical agents. These include accessibility to the biodiversity, efficient screening, sustainable supply, variability in the spectrum and quantities of bioactives produced (due to factors such as seasonality and geographic distribution), elucidation of the mechanism of action, suitable pharmacokinetics/pharmacodynamic parameters and ultimately costs associated with sustainable aquaculture and processing. Despite this, a significant body of early-stage biodiscovery research highlights marine macroalgae as promising sources of novel antimicrobials, antibiofilm compounds, antivirals, anticancer, antimicrobial, anti-inflammatory and neuroprotective agents.
Several studies have validated approaches that combine regular antibiotic agents with non-antibiotic compounds, such as QSIs, to enhance the effectiveness of present treatments, but have not yet moved to clinical trials. Drawing inspiration from nature, future studies could focus on evaluating the combinatorial effects of algal secondary metabolites with those produced by the core members of their bacterial microbiomes in an attempt to mimic the complex natural chemical mechanisms underlying the mutualistic symbiotic relationships in their environments.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2017.1376709Supplementary table 1. Pharmacologically relevant activities of phlorotannins from brown algae.
Supplementary_material.docx
Download MS Word (18.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Alessandro Busetti
All authors contributed to the manuscript. A. Busetti prepared the first draft; A. Busetti, C.A. Maggs and B.F. Gilmore reviewed, revised and updated the manuscript.
References
- Abraham, E.P., Newton, G.G., Crawford, K., Burton, H.S. & Hale, C.W. (1953). Cephalosporin N: a new type of penicillin. Nature, 21: 171–343.
- Agrawal, S., Adholeya, A. & Deshmukh, S.K. (2016). The pharmacological potential of non-ribosomal peptides from marine sponge and tunicates. Frontiers in Pharmacology, 7: 10.3389/fphar.2016.00333.
- Ahn, B.R., Moon, H.E., Kim, H.R., Jung, H.A. & Choi, J.S. (2012). Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Archives of Pharmacal Research, 35: 1989–1998.
- Ahn, M.J., Yoon, K.D., Min, S.Y., Lee, J.S., Kim, J.H., Kim, T.G., Kim, S.H., Kim, N.G., Huh, H. & Kim, J. (2004). Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biological and Pharmaceutical Bulletin, 27: 544–547.
- Akkaya, S. & Ozkurt, Y.B. (2016). Persistent symblepharon in an infant following epidemic keratoconjunctivitis. Medical Hypothesis, Discovery and Innovation in Ophthalmology, 5: 74–77.
- Amsler, C.D. (2008). Algal Chemical Ecology. Springer Verlag, Berlin.
- Appelbaum, P.C. (2007). Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). International Journal of Antimicrobial Agents, 30: 398–408.
- Armstrong, E., Yan, L., Boyd, K.G., Wright, P.C. & Burgess, J.G. (2001). The symbiotic role of marine microbes on living surfaces. Hydrobiologia, 461: 37–40.
- Balcazar, J.L., Rojas-Luna, T. & Cunningham, D.P. (2007). Effect of the addition of four potential probiotic strains on the survival of Pacific white shrimp (Litopenaeus vannamei) following immersion challenge with Vibrio parahaemolyticus. Journal of Invertebrate Pathology, 96: 147–150.
- Barbosa, M., Valentão, P. & Andrade, P.B. (2014). Bioactive compounds from macroalgae in the new millennium: implications for neurodegenerative diseases. Marine Drugs, 12: 4935–4972.
- Bauer, W.D., & Robinson, J.B. (2002). Disruption of bacterial quorum sensing by other organisms. Current Opinion In Biotechnology, 13: 234–237.
- Bennamara, A., Abourriche, A., Berrada, M., Charrouf, M., Chaib, N., Boudouma, M. & Garneau, F.X. (1999). Methoxybifurcarenone: an antifungal and antibacterial meroditerpenoid from the brown alga Cystoseira tamariscifolia. Phytochemistry, 52: 37–40.
- Bérdy, J. (2005). Bioactive microbial metabolites. Journal of Antibiotics, 58: 1–26.
- Bergmann, W. & Feeney, R.J. (1950). The isolation of a new thymine pentoside from sponges. Journal of the American Chemical Society, 72: 2809–2810.
- Bergmann, W. & Feeney, R.J. (1951). Contributions to the study of marine products. XXXII. The nucleosides of sponges. I. Journal of Organic Chemistry, 16: 981–987.
- Blunt, J.W., Copp, B.R., Hu, W.P., Munro, M.H., Northcote, P.T. & Prinsep, M.R. (2007). Marine natural products. Natural Product Reports, 24: 31–86.
- Blunt, J.W., Copp, B.R., Keyzers, R.A., Munro, M.H. & Prinsep, M.R. (2016). Marine natural products. Natural Product Reports, 33: 382–431.
- Bo, G. (2000). Giuseppe Brotzu and the discovery of cephalosporins. Clinical Microbiology and Infection, 6 (Suppl 3): 6–9.
- Bowman, J.P. (2007). Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Marine Drugs, 5: 220–241.
- Brackman, G., Celen, S., Hillaert, U., Van Calenbergh, S., Cos, P., Maes, L., Nelis, H.J. & Coenye, T. (2011). Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp. PLoS ONE, 6: e16084.
- Brackman, G. & Coenye, T. (2015). Quorum sensing inhibitors as anti-biofilm agents, Current Pharmaceutical Design, 21: 5–11.
- Brackman, G., Cos, P., Maes, L., Nelis, H.J. & Coenye, T. (2011). Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrobial Agents and Chemotherapy, 55: 2655–2661.
- Breeman, A.M., Meulenhoff, E.J.S. & Guiry, M.D. (1988). Life history regulation and phenology of the red alga Bonnemaisonia hamifera. Helgoländer Meeresuntersuchungen, 42: 535–551.
- Busetti, A., Shaw, G., Megaw, J., Gorman, S.P., Maggs, C.A. & Gilmore, B.F. (2014). Marine-derived quorum-sensing inhibitory activities enhance the antibacterial efficacy of tobramycin against Pseudomonas aeruginosa. Marine Drugs, 13: 1–28.
- Busetti, A., Thompson, T.P., Tegazzini, D., Megaw, J., Maggs, C.A. & Gilmore, B.F. (2015). Antibiofilm activity of the brown alga Halidrys siliquosa against clinically relevant human pathogens. Marine Drugs, 13: 3581–3605.
- Cabrita, M.T., Vale, C. & Rauter, A.P. (2010). Halogenated compounds from marine algae. Marine Drugs, 8: 2301–2317.
- Callow, M.E. & Callow, J.E. (2002). Marine biofouling: a sticky problem. The Biologist, 49: 10–14.
- Camara, M., Williams, P. & Hardman, A. (2002). Controlling infection by tuning in and turning down the volume of bacterial small-talk. The Lancet Infectious Diseases, 2: 667–676.
- Costerton, J.W., Geesey, G.G. & Cheng, K.J. (1978). How bacteria stick. Scientific American, 238: 86–95.
- Culioli, G., Ortalo-Magne, A., Valls, R., Hellio, C., Clare, A.S. & Piovetti, L. (2008). Antifouling activity of meroditerpenoids from the marine brown alga Halidrys siliquosa. Journal of Natural Products, 71: 1121–1126.
- D’Costa, V.M., King, C.E., Kalan, L., Morar, M., Sung, W.W., Schwarz, C., Froese, D., Zazula, G., Calmels, F., Debruyne, R., Golding, G.B., Poinar, H.N. & Wright, G.D. (2011). Antibiotic resistance is ancient. Nature, 477: 457–461.
- Dang, H. & Lovell, C.R. (2000). Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Applied and Environmental Microbiology, 66: 467–475.
- Davies, D. (2003). Understanding biofilm resistance to antibacterial agents. Nature Reviews Drug Discovery, 2: 114–122.
- Davis, G.D. & Vasanthi, A.H. (2011). Seaweed metabolite database (SWMD): a database of natural compounds from marine algae. Bioinformation, 5: 361–364.
- Defoirdt, T., Boon, N. & Bossier, P. (2010). Can bacteria evolve resistance to quorum sensing disruption? PLoS Pathogens, 6: e1000989.
- Diggle, S.P., Crusz, S.A. & Cámara, M. (2007). Quorum sensing. Current Biology, 17: R907–910.
- Dobretsov, S., Dahms, H.U. & Qian, P.Y. (2006). Inhibition of biofouling by marine microorganisms and their metabolites. Biofouling, 22: 43–54.
- Dobretsov, S. & Qian, P.Y. (2002). Effect of bacteria associated with the green alga Ulva reticulata on marine micro- and macrofouling. Biofouling, 18: 217–228.
- Dobretsov, S., Teplitski, M., Bayer, M., Gunasekera, S., Proksch, P. & Paul, V.J. (2011). Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling, 27: 893–905.
- Dobretsov, S., Teplitski, M. & Paul, V. (2009). Mini-review: quorum sensing in the marine environment and its relationship to biofouling. Biofouling, 25: 413–427.
- Dong, Y.H., Xu, J.L., Li, X.Z. & Zhang, L.H. (2000). AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proceedings of the National Academy of Sciences of the United States of America, 97: 3526–3531.
- Donlan, R.M. (2002). Biofilms: microbial life on surfaces. Emerging Infectious Diseases, 8: 881–890.
- Egan, S., Harder, T., Burke, C., Steinberg, P., Kjelleberg, S. & Thomas, T. (2013). The seaweed holobiont: understanding seaweed–bacteria interactions. FEMS Microbiology Reviews, 37: 462–476.
- Enge, S., Nylund, G.M., Harder, T. & Pavia, H. (2013). An exotic chemical weapon explains low herbivore damage in an invasive alga. Ecology, 93: 2736–2745.
- Eom, S., Kim, Y. & Kim, S. (2012). Antimicrobial effect of phlorotannins from marine brown algae. Food and Chemical Toxicology, 50: 3251–3255.
- Fowler, M.G. et al. (2016). Benefits and risks of antiretroviral therapy for perinatal HIV prevention. New England Journal of Medicine, 375: 1726–1737.
- Furuya, E.Y. & Lowy, F.D. (2006). Antimicrobial-resistant bacteria in the community setting. Nature Reviews Microbiology, 4: 36–45.
- Gil-Turnes, M.S., Hay, M.E. & Fenical, W. (1989). Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science, 246: 116–118.
- Givskov, M., de Nys, R., Manefield, M., Gram, L., Maximilien, R., Eberl, L., Molin, S., Steinberg, P.D. & Kjelleberg, S. (1996). Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. Journal of Bacteriology, 178: 6618–6622.
- Gómez, I. & Huovinen, P. (2010). Induction of phlorotannins during UV exposure mitigates inhibition of photosynthesis and DNA damage in the kelp Lessonia nigrescens. Photochemistry and Photobiology, 86: 1056–1063.
- Han, S.B., Kim, S.K., Lee, J.W., Lee, D., Chung, N., Jeong, D.C., Cho, B. & Kang, J. (2017). Varicella zoster virus infection after allogeneic hematopoietic cell transplantation in children using a relatively short duration of acyclovir prophylaxis: a retrospective study. Medicine, 96: e6546.
- Hardie, K.R. & Heurlier, K. (2008). Establishing bacterial communities by ‘word of mouth’: LuxS and autoinducer 2 in biofilm development. Nature Reviews Microbiology, 6: 635–643.
- Harrison, J.J., Stremick, C.A., Turner, R.J., Allan, N.D., Olson, M.E. & Ceri, H. (2010). Microtiter susceptibility testing of microbes growing on peg lids: a miniaturized biofilm model for high-throughput screening. Nature Protocols, 5: 1236–1254.
- Hawkey, P.M. (2008). The growing burden of antimicrobial resistance. Journal of Antimicrobial Chemotherapy, 62 (Suppl. 1): i1–i9.
- Hentzer, M., Riedel, K., Rasmussen, T.B., Heydorn, A., Andersen, J.B., Parsek, M.R., Rice, S.A., Eberl, L., Molin, S., Hoiby, N., Kjelleberg, S. & Givskov, M. (2002). Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology, 148: 87–102.
- Hentzer, M., Wu, H., Andersen, J.B., Riedel, K., Rasmussen, T.B., Bagge, N., Kumar, N., Schembri, M.A., Song, Z., Kristoffersen, P., Manefield, M., Costerton, J.W., Molin, S., Eberl, L., Steinberg, P., Kjelleberg, S., Hoiby, N. & Givskov, M. (2003). Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO Journal, 22: 3803–3815.
- Heo, S., Cha, S., Kim, K., Lee, S., Ahn, G., Kang, D., Oh, C., Choi, Y., Affan, A., Kim, D. & Jeon, Y. (2012). Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2-induced oxidative stress in murine hippocampal neuronal cells, HT22. Applied Biochemistry and Biotechnology, 166: 1520–1532.
- Hoiby, N., Ciofu, O., Johansen, H.K., Song, Z.J., Moser, C., Jensen, P.O., Molin, S., Givskov, M., Tolker-Nielsen, T. & Bjarnsholt, T. (2011). The clinical impact of bacterial biofilms. International Journal of Oral Science, 3: 55–65.
- Holmstrom, C., Rittschof, D. & Kjelleberg, S. (1992). Inhibition of settlement by larvae of Balanus amphitrite and Ciona intestinalis by a surface-colonizing marine bacterium. Applied and Environmental Microbiology, 58: 2111–2115.
- Hornsey, I.S. & Hide, D. (1974). The production of antimicrobial compounds by British marine algae I. Antibiotic-producing marine algae. British Phycological Journal, 9: 353–361.
- Hornsey, I.S. & Hide, D. (1976). The production of antimicrobial compounds by British marine algae II. Seasonal variation in production of antibiotics. British Phycological Journal, 11: 63–67.
- Huber, B., Riedel, K., Hentzer, M., Heydorn, A., Gotschlich, A., Givskov, M., Molin, S. & Eberl, L. (2001). The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology, 147: 2517–2528.
- Irie, Y. & Parsek, M.R. (2008). Quorum sensing and microbial biofilms. Current Topics in Microbiology and Immunology, 322: 67–84.
- Jacobsen, N. & Madsen, J.O. (1978). Halogenated metabolites including brominated 2-heptanols and 2-heptyl acetates from the tetrasporophyte of the red alga Bonnemaisonia hamifera. Tetrahedron Letters, 33: 3065–3068.
- Jha, B., Kavita, K., Westphal, J., Hartmann, A., Schmitt-Kopplin, P. (2013). Quorum sensing inhibition by Asparagopsis taxiformis, a marine macroalga: separation of the compound that interrupts bacterial communication. Marine Drugs, 11: 253–265.
- Jung, W.K., Heo, S.J., Jeon, Y.J., Lee, C.M., Park, Y.M., Byun, H.G., Choi, Y.H., Park, S.G. & Choi, I.W. (2009). Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. Journal of Agricultural and Food Chemistry, 57: 4439–4446.
- Justin, J.R. & Melander, C. (2009). Small molecule approaches toward the non-microbicidal modulation of bacterial biofilm growth and maintenance. Anti-Infective Agents in Medicinal Chemistry, 8: 295–314.
- Kang, M.C., Wijesinghe, W.A., Lee, S.H., Kang, S.M., Ko, S.C., Yang, X., Kang, N., Jeon, B.T., Kim, J., Lee, D.H. & Jeon, Y.J. (2013). Dieckol isolated from brown seaweed Ecklonia cava attenuates type II diabetes in db/db mouse model. Food and Chemical Toxicology, 53: 294–298.
- Kendall, M.M. & Sperandio, V. (2007). Quorum sensing by enteric pathogens. Current Opinion in Gastroenterology, 23: 10–15.
- Kim, J.S., Kim, Y.H., Seo, Y.W. & Park, S. (2007). Quorum sensing inhibitors from the red alga, Ahnfeltiopsis flabelliformis. Biotechnology & Bioprocess Engineering, 12: 308–311.
- Kjelleberg, S., & Steinberg, P. (2001). Surface warfare in the sea. Microbiology Today, 28: 134–135.
- Koehn, F.E. & Carter, G.T. (2005). The evolving role of natural products in drug discovery. Nature Reviews Drug Discovery, 4: 206–220.
- Lee, J.Y., Kim, S.M., Jung, W., Song, D., Um, B., Son, J. & Pan, C. (2012). Phlorofucofuroeckol-A, a potent inhibitor of aldo-keto reductase family 1 member B10, from the edible brown alga Eisenia bicyclis. Journal of the Korean Society for Applied Biological Chemistry, 55: 721–727.
- Lee, S.H. & Jeon, Y.J. (2013). Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia, 86: 129–136.
- Lee, S.J., Park, S.Y., Lee, J.J., Yum, D.Y., Koo, B.T. & Lee, J.K. (2002). Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis. Applied and Environmental Microbiology, 68: 3919–3924.
- Lin, Y.H., Xu, J.L., Hu, J., Wang, L.H., Ong, S.L., Leadbetter, J.R. & Zhang, L.H. (2003). Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Molecular Microbiology, 47: 849–860.
- Livermore, D.M. (2002). Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clinical Infectious Disease, 34: 634–640.
- Maggs, C.A. & Stegenga, H. (1998). Red algal exotics on North Sea coasts. Helgoländer Meeresuntersuchungen, 52: 243–258.
- Manefield, M., Rasmussen, T.B., Henzter, M., Andersen, J.B., Steinberg, P., Kjelleberg, S. & Givskov, M. (2002). Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology, 148: 1119–1127.
- Marshall, K., Joint, I., Callow, M.E., & Callow, J.A. (2006). Effect of marine bacterial isolates on the growth and morphology of axenic plantlets of the green alga Ulva linza. Microbial Ecology, 52: 302–310.
- Maschek, J.A. & Baker, B.J. (2008). The chemistry of algal secondary metabolism. In Algal Chemical Ecology (Amsler, C.D., editor), 1–20. Springer, Berlin.
- McClean, K.H. et al. (1997). Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology, 143: 3703–3711.
- McClintock, J.B. & Baker, B.J. (2001). Marine Chemical Ecology. CRC Press, Boca Raton.
- McConnell, O.J. & Fenical, W. (1979). Antimicrobial agents from the marine red algae of the family Bonnemaisoniaceae, in Marine Algae in Pharmaceutical Science (Hoppe, H.A., editor), 479–500. Walter de Gruyter, Berlin.
- McLean, R.J., Pierson, L.S. 3rd & Fuqua, C. (2004). A simple screening protocol for the identification of quorum signal antagonists. Journal of Microbiological Methods, 58: 351–360.
- Mellbye, B. & Schuster, M. (2011). The sociomicrobiology of antivirulence drug resistance: a proof of concept. mBio, 2: 10.1128/mBio.00131–11.
- Molinski, T.F., Dalisay, D.S., Lievens, S.L. & Saludes, J.P. (2009). Drug development from marine natural products. Nature Reviews Drug Discovery, 8: 69–85.
- Mueller, L.N., de Brouwer, J.F., Almeida, J.S., Stal, L.J. & Xavier, J.B. (2006). Analysis of a marine phototrophic biofilm by confocal laser scanning microscopy using the new image quantification software PHLIP. BMC Ecology, 6 (1). doi: 10.1186/1472-6785-6-1.
- Munro, M.H.G., & Blunt, J.W. (2005). MarineLit, A Marine Chemical Literature Database, Version 12.5. Marine Chemistry Group, University of Canterbury, Christchurch, New Zealand.
- Myung, C., Shin, H., Bao, H.Y., Yeo, S.J., Lee, B.H. & Kang, J.S. (2005). Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: possible involvement of the inhibition of acetylcholinesterase. Archives of Pharmacal Research, 28: 691–698.
- Nakajima, N. et al. (2016). Diversity of phlorotannin profiles among sargassacean species affecting variation and abundance of epiphytes. European Journal of Phycology, 51: 307–316.
- Nash, R., Rindi, F. & Guiry, M.D. (2005). Optimum conditions for cultivation of the Trailliella phase of Bonnemaisonia hamifera Hariot (Bonnemaisoniales, Rhodophyta), a candidate species for secondary metabolite production. Botanica Marina, 48: 257–265.
- Nett, J.E., Marchillo, K., & Andes, D.R. (2012). Modeling of fungal biofilms using a rat central vein catheter. Methods in Molecular Biology, 845: 547–556.
- Newman, D.J. & Cragg, G.M. (2016). Natural products as sources of new drugs from 1981 to 2014. Journal of Natural Products, 79: 629–661.
- Nylund, G.M., Cervin, G., Hermansson, M. & Pavia, H. (2005). Chemical inhibition of bacterial colonization by the red alga Bonnemaisonia hamifera. Marine Ecology Progress Series, 302: 27–36.
- Nylund, G.M., Cervin, G., Persson, F., Hermansson, M., Steinberg, P.D. & Pavia, H. (2008). Seaweed defence against bacteria: a poly-brominated 2-heptanone from the red alga Bonnemaisonia hamifera inhibits bacterial colonisation. Marine Ecology Progress Series, 369: 39–50.
- Nylund, G.M., Enge, S. & Pavia, H. (2013). Costs and benefits of chemical defence in the red alga Bonnemaisonia hamifera. PLoS ONE, 8: e61291.
- O’Neill, J. (2014). Review on antimicrobial resistance, December 2014. Available at http://amr-review.org/.
- O'Toole, G.A. (2011). Microtiter dish biofilm formation assay. Journal of Visualized Experiments, 47: pii: 2437. doi:10.3791/2437.
- Pallela, R., Na-Young, Y. & Kim, S.K. (2010). Anti-photoaging and photoprotective compounds derived from marine organisms. Marine Drugs, 8: 1189–1202.
- Papenfort, K. & Bassler, B.L. (2016). Quorum sensing signal–response systems in Gram-negative bacteria. Nature Reviews Microbiology, 14: 576–588.
- Parsek, M.R. & Greenberg, E.P. (2005). Sociomicrobiology: the connections between quorum sensing and biofilms. Trends in Microbiology, 13: 27–33.
- Paul, N.A., de Nys, R. & Steinberg, P.D. (2006). Chemical defence against bacteria in the red alga Asparagopsis armata: linking structure with function. Marine Ecology Progress Series, 306: 87–101.
- Penesyan, A., Kjelleberg, S. & Egan, S. (2010). Development of novel drugs from marine surface associated microorganisms. Marine Drugs, 8: 438–459.
- Peng, J., Yuan, J., Wu, C. & Wang, J. (2011). Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Marine Drugs, 9: 1806–1828.
- Plowman, R. (2000). The socioeconomic burden of hospital acquired infection. European Communicable Disease Bulletin, 5: 49–50.
- Plowman, R., Graves, N., Griffin, M.A.S., Roberts, J.A., Swan, A.V., Cookson, B. & Taylor, L. (2001). The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. Journal of Hospital Infection, 47: 198–209.
- Qian, P.Y., Lau, S.C.K., Dahms, H.U., Dobretsov, S. & Harder, T. (2007). Marine biofilms as mediators of colonization by marine macroorganisms: implications for antifouling and aquaculture. Marine Biotechnology, 9: 399–410.
- Rangel-Huerta, O.D., Pastor-Villaescusa, B., Aguilera, C.M. & Gil, A. (2015). A systematic review of the efficacy of bioactive compounds in cardiovascular disease: phenolic compounds. Nutrients, 7: 5177–5216.
- Rao, T., Rani, P., Venugopalan, V. & Nair, K. (1997). Biofilm formation in a freshwater environment under photic and aphotic conditions. Biofouling, 11: 265–282.
- Rasmussen, T.B. & Givskov, M. (2006). Quorum-sensing inhibitors as anti-pathogenic drugs. International Journal of Medical Microbiology, 296: 149–161.
- Rasmussen, T.B., Manefield, M., Andersen, J.B., Eberl, L., Anthoni, U., Christophersen, C., Steinberg, P., Kjelleberg, S. & Givskov, M. (2000). How Delisea pulchra furanones affect quorum sensing and swarming motility in Serratia liquefaciens MG1. Microbiology, 146: 3237–3244.
- Ready, D., Roberts, A.P., Pratten, J., Spratt, D.A., Wilson, M. & Mullany, P. (2002). Composition and antibiotic resistance profile of microcosm dental plaques before and after exposure to tetracycline. Journal of Antimicrobial Chemotherapy, 49: 769–775.
- Reimmann, C., Ginet, N., Michel, L., Keel, C., Michaux, P., Krishnapillai, V., Zala, M., Heurlier, K., Triandafillu, K., Harms, H., Defago, G. & Haas, D. (2002). Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology, 148: 923–932.
- Ren, D., Sims, J.J. & Wood, T.K. (2001). Inhibition of biofilm formation and swarming of Escherichia coli by (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Environmental Microbiology, 3: 731–736.
- Ren, D., Sims, J.J. & Wood, T.K. (2002). Inhibition of biofilm formation and swarming of Bacillus subtilis by (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Letters in Applied Microbiology, 34: 293–299.
- Rosenfeld, W.D. & Zobell, C.E. (1947). Antibiotic production by marine microorganisms. Journal of Bacteriology, 54: 393–398.
- Sathya, R., Kanaga, N., Sankar, P. & Jeeva, S. (2013). Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskal) C. Agardh. Arabian Journal of Chemistry. doi:10.1016/j.arabjc.2013.09.039.
- Saurav, K., Costantino, V., Venturi, V., Steindler, L. (2017). Quorum sensing inhibitors from the sea discovered using bacterial N-acyl-homoserine lactone-based biosensors. Marine Drugs, 15: 53.
- Savage, V.J., Chopra, I. & O’Neill, A.J. (2013). Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrobial Agents and Chemotherapy, 57: 1968–1970.
- SCENIHR (2009). Assessment of the Antibiotic Resistance Effects of Biocides. European Commission, Brussels.
- Schaefer, A.L., Hanzelka, B.L., Eberhard, A. & Greenberg, E.P. (1996). Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. Journal of Bacteriology, 178: 2897–2901.
- Schuster, M., Lostroh, C.P., Ogi, T. & Greenberg, E.P. (2003). Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. Journal of Bacteriology, 185: 2066–2079.
- Singh, R.P., Baghel, R.S., Reddy, C.R.K. & Jha, B. (2015). Effect of quorum sensing signals produced by seaweed-associated bacteria on carpospore liberation from Gracilaria dura. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2015.00117.
- Singh, R.P. & Reddy, C.R.K. (2014). Seaweed-microbial interactions: key functions of seaweed-associated bacteria. FEMS Microbiology Ecology, 88: 213–230.
- Singh, R.P. & Reddy, C.R.K. (2015). Unraveling the functions of the macroalgal microbiome. Frontiers in Microbiology, 6: 1488. http://doi.org/10.3389/fmicb.2015.01488.
- Sio, C.F., Otten, L.G., Cool, R.H., Diggle, S.P., Braun, P.G., Bos, R., Daykin, M., Camara, M., Williams, P. & Quax, W.J. (2006). Quorum quenching by an N-acyl-homoserine lactone acylase from Pseudomonas aeruginosa PAO1. Infection and Immunity, 74: 1673–1682.
- Siuda, J.F., Van Blaricom, G.R., Shaw, P.D., Johnson, R.D., White, R.H., Hager, L.P. & Rinehart, K.L. (1975). 1-Iodo-3,3-dibromo-2-heptanone, 1,1,3,3-tetrabromo-2-heptanone, and related compounds from the red alga Bonnemaisonia hamifera. Journal of the American Chemical Society, 97: 937–938.
- Smit, A.J. (2004). Medicinal and pharmaceutical uses of seaweed natural products: a review. Journal of Applied Phycology, 16: 245–262.
- Spavieri, J., Allmendinger, A., Kaiser, M., Casey, R., Hingley-Wilson, S., Lalvani, A., Guiry, M.D., Blunden, G. & Tasdemir, D. (2010). Antimycobacterial, antiprotozoal and cytotoxic potential of twenty-one brown algae (Phaeophyceae) from British and Irish waters. Phytotherapy Research, 24: 1724–1729.
- Spellberg, B., Guidos, R., Gilbert, D., Bradley, J., Boucher, H.W., Scheld, W.M., Bartlett, J.G., Edwards, J., Jr & Infectious Diseases Society of America (2008). The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clinical Infectious Diseases, 46: 155–164.
- Sperandio, V. (2007). Novel approaches to bacterial infection therapy by interfering with bacteria-to-bacteria signaling. Expert Review of Anti-Infective Therapy, 5: 271–276.
- Steinberg, P.D., De Nys, R. & Kjelleberg, S. (2002). Chemical cues for surface colonization. Journal of Chemical Ecology, 28: 1935–1951.
- Suga, H. & Smith, K.M. (2003). Molecular mechanisms of bacterial quorum sensing as a new drug target. Current Opinion in Chemical Biology, 7: 586–591.
- Sugiura, Y., Matsuda, K., Okamoto, T., Yamada, Y., Imai, K., Ito, T., Kakinuma, M. & Amano, H. (2009). The inhibitory effects of components from a brown alga, Eisenia arborea, on degranulation of mast cells and eicosanoid synthesis. Journal of Functional Foods, 1: 387–393.
- Sugiura, Y., Tanaka, R., Katsuzaki, H., Imai, K. & Matsushita, T. (2013). The anti-inflammatory effects of phlorotannins from Eisenia arborea on mouse ear edema by inflammatory inducers. Journal of Functional Foods, 5: 2019–2023.
- Swift, S., Downie, J.A., Whitehead, N.A., Barnard, A.M., Salmond, G.P. & Williams, P. (2001). Quorum sensing as a population-density-dependent determinant of bacterial physiology. Advances in Microbial Physiology, 45: 199–270.
- Teasdale, M.E., Liu, J., Wallace, J., Akhlaghi, F. & Rowley, D.C. (2009). Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in gram-negative bacteria. Applied and Environmental Microbiology, 75: 567–572.
- Tebben, J., Tapiolas, D.M., Motti, C.A., Abrego, D., Negri, A.P., Blackall, L.L., Steinberg, P.D. & Harder, T. (2011). Induction of larval metamorphosis of the coral Acropora millepora by tetrabromopyrrole isolated from a Pseudoalteromonas bacterium. PLoS ONE, 6: e19082.
- Tomlin, K.L., Malott, R.J., Ramage, G., Storey, D.G., Sokol, P.A. & Ceri, H. (2005). Quorum-sensing mutations affect attachment and stability of Burkholderia cenocepacia biofilms. Applied and Environmental Microbiology, 71: 5208–5218.
- Ulrich, R.L. (2004). Quorum quenching: enzymatic disruption of N-acylhomoserine lactone-mediated bacterial communication in Burkholderia thailandensis. Applied and Environmental Microbiology, 70: 6173–6180.
- Vairappan, C.S. (2003). Potent antibacterial activity of halogenated metabolites from Malaysian red algae, Laurencia majuscula (Rhodomelaceae, Ceramiales). Biomolecular Engineering, 20: 255–259.
- Vairappan, C.S., Daitoh, M., Suzuki, M., Abe, T. & Masuda, M. (2001). Antibacterial halogenated metabolites from the Malaysian Laurencia species. Phytochemistry, 58: 291–297.
- Vairappan, C.S., Ishii, T., Lee, T.K., Suzuki, M. & Zhaoqi, Z. (2010). Antibacterial activities of a new brominated diterpene from Borneon Laurencia spp. Marine Drugs, <bold>8</bold>: 1743–1749.
- Wagner, V.E., Bushnell, D., Passador, L., Brooks, A.I. & Iglewski, B.H. (2003). Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. Journal of Bacteriology, <bold>185</bold>: 2080–2095.
- Wellington, E.M., Boxall, A.B., Cross, P., Feil, E.J., Gaze, W.H., Hawkey, P.M., Johnson-Rollings, A.S., Jones, D.L., Lee, N.M., Otten, W., Thomas, C.M. & Williams, A.P. (2013). The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. The Lancet Infectious Diseases, 13: 155–165.
- Wichard, T., Charrier, B., Mineur, F., Bothwell, J.H., De Clerck, O. & Coates, J.C. (2015). The green seaweed Ulva: a model system to study morphogenesis. Frontiers in Plant Science, 6: 72.
- Wieczorek, S.K. & Todd, D.C. (1998). Inhibition and facilitation of settlement of epifaunal marine invertebrate larvae by microbial biofilm cues. Biofouling, 12: 81–118.
- Wopperer, J., Cardona, S.T., Huber, B., Jacobi, C.A., Valvano, M.A., & Eberl, L. (2006). A quorum-quenching approach to investigate the conservation of quorum-sensing-regulated functions within the Burkholderia cepacia complex. Applied and Environmental Microbiology, 72: 1579–1587.
- Wu, H., Moser, C., Wang, H.Z., Hoiby, N. & Song, Z.J. (2015). Strategies for combating bacterial biofilm infections. International Journal of Oral Science, 7: 1–7.
- Wu, H., Song, Z., Hentzer, M., Andersen, J. B., Molin, S., Givskov, M. & Høiby, N. (2004). Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. Journal of Antimicrobial Chemotherapy, 53: 1054–1061.
- Yang, L., Rybtke, M.T., Jakobsen, T.H., Hentzer, M., Bjarnsholt, T., Givskov, M. & Tolker-Nielsen, T. (2009). Computer-aided identification of recognized drugs as Pseudomonas aeruginosa quorum-sensing inhibitors. Antimicrobial Agents and Chemotherapy, 53: 2432–2443.
- Yates, E.A., Philipp, B., Buckley, C., Atkinson, S., Chhabra, S.R., Sockett, R.E., Goldner, M., Dessaux, Y., Camara, M., Smith, H. & Williams, P. (2002). N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infection and Immunity, 70: 5635–5646.
- Yoon, N.Y., Chung, H.Y., Kim, H.R. & Choi, J.E. (2008). Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fisheries Science, 74: 200–207.
- Yoon, N.Y., Lee, S., Yong-Li & Kim, S. (2009). Phlorotannins from Ishige okamurae and their acetyl- and butyrylcholinesterase inhibitory effects. Journal of Functional Foods, 1: 331–335.
- Zhu, H. & Sun, S.J. (2008). Inhibition of bacterial quorum sensing-regulated behaviors by Tremella fuciformis extract. Current Microbiology, 57: 418–422.
