ABSTRACT
Thalassiosira Cleve is one of the most species-rich marine diatom genera. Previous studies have mainly focused on polar and temperate areas, but recent studies on material from Asian waters suggested that a high and undescribed species diversity of Thalassiosira occurs in Asia. On the basis of plankton samples collected from the South China Sea, a new species, T. sinica sp. nov. Y. Li & Y. Q. Guo is described. The morphology of the cells was examined by light and scanning electron microscopy. The hypervariable region of the nuclear large-subunit ribosomal DNA and the relatively conserved region of the nuclear small-subunit ribosomal DNA were sequenced for information on phylogenetic relationships. The living cells are usually solitary and drum-shaped. The areolae on the valve are delicate, small and arranged in fascicles. In addition to a regular ring of marginal fultoportulae on the valve edge, T. sinica possesses one central fultoportula and a number of fultoportulae arranged into 2–3 irregular rings on the valve face. A rimoportula located inside the ring of marginal fultoportulae possesses a long and strong external tube. The valvocopula and the copulae have rows of pores, but the pores on the valvocopula are larger than those on the copulae. Thalassiosira sinica appears to be included in subgroup C sensu Gedde because of a rimoportula with a distinct external tube located on the valve face. The molecular phylogeny, inferred from both SSU and LSU sequences, does, however, not support the validity of subgroup C, as the closest allies of T. sinica here turned out to be T. diporocyclus and T. lundiana, species in which the rimoportulae are located on the valve margin.
Introduction
Thalassiosira Cleve is one of the most species-rich and well-studied genera of centric diatoms (Hasle & Syvertsen, Citation1997), and both cell abundance and species diversity are significant characteristics of the group (Round et al., Citation1990; Hasle & Syvertsen, Citation1997). Some Thalassiosira species may dominate the phytoplankton community with very high abundances or even form blooms, with negative impacts on fish farms, coastal tourism, etc. (Takano, Citation1990; Hasle & Syvertsen, Citation1997; Chen et al., Citation2004; Fryxell & Hasle, Citation2004; Xie et al., Citation2008).
Thus far, a total of 254 Thalassiosira taxa have been described, of which 174 are accepted taxonomically (Guiry & Guiry, Citation2017). Many Thalassiosira species were described by Grethe R. Hasle & Greta A. Fryxell, based on ultrastructure as seen by electron microscopy (EM) during the period 1960–1990s (Hasle & Syvertsen, Citation1997). Features of valvar processes, mainly fultoportulae (strutted processes) and rimoportulae (labiate processes), have been proposed as key characteristics for accurate identification (Round et al., Citation1990; Hasle & Syvertsen, Citation1997). Colony type, cell shape and the areolation pattern are also useful.
Most Thalassiosira species have been reported from polar and temperate regions (Hasle & Syvertsen, Citation1997). However, studies from Asian waters are inadequate in number (Park & Lee, Citation2010; Li et al., Citation2013). Recently, Thalassiosira was found to be abundant in Asian waters (Lee & Park, Citation2008; Park et al., Citation2009; Park & Lee, Citation2010; Li et al., Citation2013, Citation2014), and several new species have been described (Gedde, Citation1999; Park & Lee, Citation2014, Citation2015; Samanta & Bhadury, Citation2015).
Presently, nearly 50 Thalassiosira taxa have been reported from Chinese coastal waters (Li et al., Citation2013, Citation2014), but a much higher diversity is expected to occur (Guo & Qian, Citation2003; Li et al., Citation2013, Citation2014). In the present study, we describe a new Thalassiosira species from Chinese tropical waters, and we hope the study may serve as the beginning of a period with new studies on Thalassiosira diversity in China.
Materials and methods
Sampling, isolation and culturing
Live samples were collected in coastal waters of south-east China by hauling a plankton net (10 µm mesh size) horizontally. Single cells or chains of Thalassiosira were isolated into a 96-well cell culture plate (Greiner Bio-One GmbH, Frickenhausen, Germany) using a glass micro-pipette and an inverted light microscope (Mshot MI12, Guangzhou, China), each well containing c. 300 µl L1-medium with a salinity of 30 (Guillard & Hargraves, Citation1993). The plate was incubated at c. 22°C in a 12:12 light:dark (L:D) cycle with illumination provided by cool fluorescent lamps. When cell abundance had reached at least 100 (as counted by the inverted light microscope), the strains were transferred to glass triangular flasks. The culture strains were numbered as MC series; MC meaning marine collection. Clonal cultures of Thalassiosira used in this study are listed in . For DNA sequencing, culture aliquots were concentrated and frozen.
Table 1. Sampling information for Thalassiosira strains.
Morphological observations
Cells of each strain were harvested in the mid-exponential phase from the bottom of the flasks by a glass pipette. The cells were subsequently observed and photographed using an Olympus BX-53 light microscope (LM, Olympus, Tokyo, Japan) equipped with an Olympus DP27 camera. Chloroplast features and presence or absence of colony formation were observed and measured on no less than 20 randomly selected living cells.
Diatom frustules were cleaned by transferring c. 10 ml culture aliquots into 75-ml Erlenmeyer flasks, an equivalent volume of concentrated H2SO4 was added and the material was boiled for c. 15 min in a water bath (Cheng et al., Citation1993). Samples were then rinsed several times with distilled water until a neutral pH was obtained. For scanning electron microscopy (SEM), acid-cleaned material was filtered onto IsoporeTM membrane filters, pore size 5 µm (Merck Millipore Ltd., Cork, Ireland). The filters were attached to stubs with double-sticky carbon tape and sputter-coated with gold in a Polaron E500 sputter coater (Gala, Bad Schwalbach, Germany) before examination in a Zeiss Ultra 55 SEM (Zeiss, Oberkochen, Germany).
Parameters of most morphological characters, such as the valve diameter, areolation pattern on valve and bands, characteristics of fultoportulae and rimoportulae were observed, and measured on SEM micrographs. The terminology used for the siliceous structures follows that of von Stosch (Citation1975), Ross et al. (Citation1979) and Theriot & Serieyssol (Citation1994).
Phylogenetic analyses
DNA extraction was performed as described in Lundholm et al. (Citation2002). The relatively conservative region of the nuclear small subunit ribosomal RNA-encoding gene region (SSU rRNA gene) was amplified using the primers SSU-F and SSU-R (Zhen et al., Citation2008) and 38 cycles, each comprising 94°C for 20 s, 54°C for 30 s and 72°C for 2 min. The hypervariable D1–D3 region of the nuclear large subunit ribosomal RNA-encoding gene (LSU rRNA gene) was amplified using the primers D1R-F (Scholin et al., Citation1994) and D3B-R′ (Nunn et al., Citation1996). PCR conditions included 35 cycles, each comprising 94°C for 35 s, 58°C for 35 s and 72°C for 50 s. PCR products were purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) as recommended by the manufacturer, and sent to BGI Corporation (BGI Co. Ltd, Guangzhou, China) for sequencing, using the same primers as for PCR.
For phylogenetic analysis inferred from SSU rDNA and LSU rDNA sequences, nucleotide sequences of the strains obtained in this study were included in an alignment together with sequences from NCBI GenBank. Sequences were aligned and edited manually in BioEdit (Hall, Citation1999), and Lampriscus kittonii was chosen as outgroup based on previous analyses (Kaczmarska et al., Citation2006; Alverson et al., Citation2007). A total of 1672 and 553 base pair positions were included in the analyses for SSU and LSU, respectively. The alignments of SSU and LSU were subjected to Bayesian inference (BI) analyses for comparison with previous studies (Alverson et al., Citation2007). The optimal model for BI was chosen using MrModeltest version 2.3 (Nylander, Citation2004). For both SSU and LSU analyses, a general time-reversible model with a proportion of invariable sites and gamma distribution (GTR+I+G) was selected using the Akaike information criterion (AIC). Bayesian analyses were performed using MrBayes 3.2 (Ronquist et al., Citation2012). The analyses, using four chains, were run for 1 000 000 generations, the temperature set to 0.2. Sample frequency was set to 100 and the number of burn-in generations was 2500. As strains MC1477 and MC6023 had identical sequences of both SSU and LSU rDNA, the molecular phylogeny was conducted using only MC1477.
Results
Morphology
Thalassiosira sinica Y. Li & Y. Q. Guo sp. nov. (–)
Description: Cells solitary and drum-shaped in girdle view, pervalvar axis 25–37 µm. Numerous lobed chloroplasts present. Valves circular, 30–41 µm in diameter. Areolae loculate, arranged into fascicles, 43–49 in 10 µm on the valve face and 51–58 in 10 µm near the valve margin. A central fultoportula with 4–5 satellite pores was present internally, surrounded by a number of unperforated areas. A ring of fultoportulae, 2–3 in 10 µm, each internally comprising 4 satellite pores, was separated by a distance of 1–2 areolae from the valve margin. On the valve face, many fultoportulae, each internally comprising 4 satellite pores, were found in 2–3 irregular rings. External tubes of marginal fultoportulae longer than other fultoportulae on the valves. All fultoportulae with similar internal tubes. A rimoportula with a marked external tube was located inside the ring of marginal fultoportulae. Epicingulum composed of 4 bands.
Figs 1–6. Thalassiosira sinica, LM. . Single cells. . Valve view showing organic threads (arrows) extending from valve margin. . Valve view indicating lobed chloroplasts. . Drum-shaped cell in girdle view. . Valve views of acidified specimens showing marginal fultoportulae (arrowheads) and rimoportulae (arrows). Scale bars: , 50 µm; , µm.
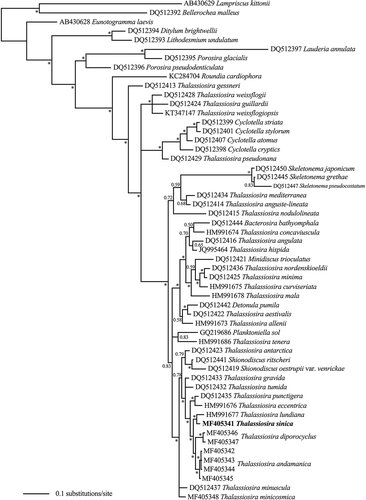
Figs 7, 8. Thalassiosira sinica, SEM. . External valve view. . Internal valve view. Scale bars: , , µm.
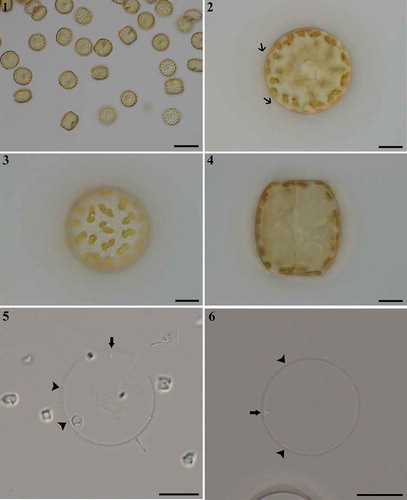
Figs 9–15. Thalassiosira sinica, SEM. –. Central fultoportula without external development of the tube () and four or five satellite pores (, ) each possessing a pore cover (arrowheads in ) internally. . Rimoportula with longer and stronger tube on external valve. . Rimoportula, internal surface of the valve. . Detail of valves, showing marginal fultoportulae with longer tubes externally () than internally (), and valve-face fultoportulae lacking external tubes (), and a short internal tube is visible (). Scale bars: , 1 µm; , 2 µm.
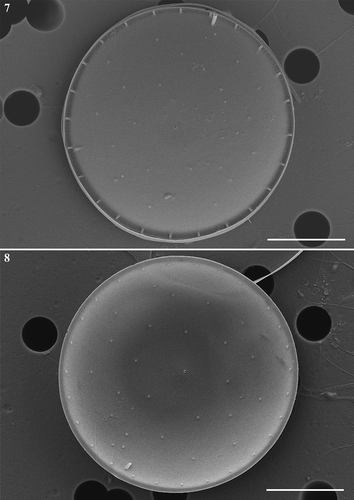
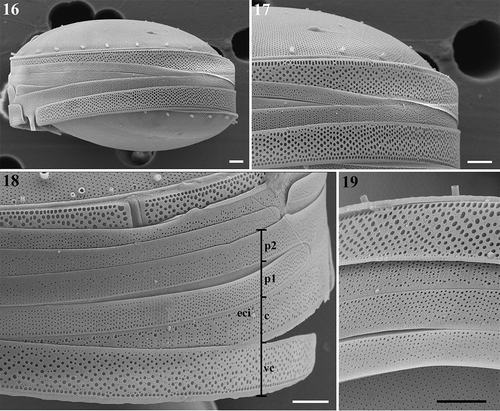
Figs 16–19. Thalassiosira sinica, SEM. . Frustules in girdle views. . Enlargement of frustule part showing the band composition. eci, epicingulum; vc, valvocopula; c, copula; p, pleura, followed by number of pleurae. . Four bands of one valve. Scale bars: , 2 µm.
Holotype: Preserved material C-A-92077 of strain MC1477 was deposited at the Natural History Museum of Denmark and is here illustrated as –.
Isotypes: Permanent slide of strain MC1477 deposited at the Laboratory of Phycology, College of Life Science, South China Normal University, China, catalogue number PS35.
Type locality: Zhanjiang Harbour (21.1267ºN, 110.4015ºE), Guangdong Province, South China Sea, P. R. China.
Molecular characterization: Nucleotide sequences of SSU rDNA and LSU rDNA of strain MC1477 deposited in GenBank (NCBI), with accession numbers MF405349 and MF405341, respectively.
Habitat: Marine, planktonic.
Etymology: The name sinica refers China where the holotype was collected.
Morphological observations: Live cells were solitary () and colonies of chains or mucilage were not observed in the cultures. Long organic threads from the marginal processes were visible in the LM (, arrows). The cells were drum-shaped in girdle view (), with a convex valve 30–41 (34.3±3.4) µm in diameter, and the pervalvar axis 25–37 (31.1±3.6) µm long (n=40). Each cell contained numerous lobed chloroplasts, usually located in the entire periphery of the cell (–). The valve face and the marginal fultoportulae were barely visible in the LM (, arrowheads), and the rimoportulae could be distinguished from the marginal fultoportulae (, arrows). The areolae were not visible in the LM.
In external valve view, the loculate areolae were arranged in a typical fasciculate pattern (Figs 7, ), and distributed uniformly on the valve face, slightly denser near the margin, and with a density of about 43–49 (43.8±3) and 51–58 (56.2±5.2) in 10 µm, respectively (n=20). The foramina of most areolae were usually round (, ), but sometimes fissure-shaped near the valve centre (). One fultoportula was positioned near the valve centre with an external opening pore, but no external tube could be found (, ). At a distance of 1–2 areolae from the valve edge, a ring of marginal fultoportulae was found (, ), with a density of 2–3 fultoportulae in 10 µm, and separated from each other by 14–17 areolae. Rimoportula marginal fultoportula had an open external tube (, , ). The single rimoportula was located with a distance of 4–5 areolae within the marginal ring of the fultoportulae (, ). Its location was somewhat variable, however, usually midway between two marginal fultoportulae or sometimes closer to one fultoportula. Rimoportula opened externally by a distinct tubular extension, which was wider and substantially longer than that of the adjacent marginal fultoportulae (). Besides the above processes, additional fultoportulae were scattered on the valve face, usually arranged into 2–3 irregular rings (). The external tubes of these fultoportulae were very short, or almost absent (, ).
In the internal valve view, the areolae were seen to be cribrate (, ). The single central fultoportula was surrounded by 4–5 satellite pores, each covered by a minute pore cover (, ). Imperforated areas were present around the central fultoportula, this was easier to observe in internal valve view (). Each marginal and scattered valve-face fultoportula was supported by four satellite pores, each with similar pore cover (, ). Internal tubes of all fultoportulae were similar, each with a short extension (, , , ). Internally, the single rimoportula had a thickened labiate opening, obliquely oriented on a very short stalk ().
The epicingulum consisted of four open bands (–). Pores of various size formed irregular plagiotropous rows on the valvocopula, and one regular and denser row of pores was located at the edge of the advalvar part of the valvocopula, with a density of 36.3–39.7 (38.3±1) pores in 10 µm (–) (n=15). On the second copula, striae which each usually comprised 4–6 rows of pores, and distinct interstriae were distributed alternately. One larger-sized pore was located at the abvalvar end of each interstria (). On the third and fourth bands, pores were usually arranged in rows. These pores on the third copula were slightly larger in size than those on the fourth copula and the pores were distributed almost on the complete third band (). On the fourth band, the striae formed by the pores were interrupted by imperforated regions, and the narrow strip near the abvalvar part was imperforated ().
Distribution: This species was sampled and isolated from Zhanjiang Harbour (Nov. 2015) and Daya Bay (Nov. 2016) in the South China Sea.
SSU and LSU phylogeny
In the phylogenetic tree inferred from the SSU sequences (), T. sinica was located into a large cluster with many other Thalassiosira species. It clustered with T. andamanica Gedde and T. diporocyclus Hasle, the three forming a highly supported clade with T. lundiana Fryxell (BPP at each node >90). The clade grouped with two other clades, which mainly comprised Thalassiosira taxa including the type species T. nordenskioeldii Cleve, but also Minidiscus trioculatus (Taylor) Hasle.
Fig. 20. Phylogenetic tree from Bayesian inference (BI) based on SSU rDNA sequences. Lampriscus kittonii makes up the outgroup. Posterior probability values are shown and asterisks indicate a value above 0.9. Thalassiosira sinica is shown in bold.
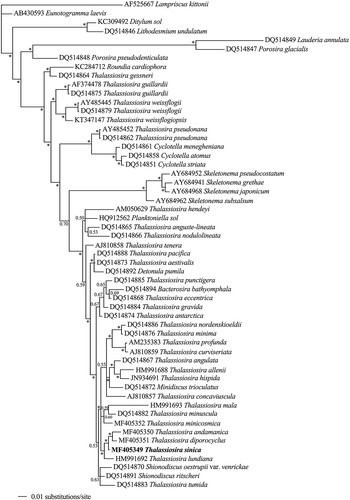
In the LSU tree (), T. andamanica and T. diporocyclus grouped together as sister taxa, and T. sinica grouped with T. lundiana. The four taxa clustered in a group with high supporting value (BPP>90), and they formed a sister clade to a clade comprising T. punctigera (Castracane) Hasle and T. eccentrica (Ehrenberg) Cleve.
Discussion
Comparison among taxa with a rimoportula locating outside the marginal ring of fultoportulae
For identification of Thalassiosira species, key characters are thought to be the location of the rimoportula and presence or absence of external tubes (Hasle, Citation1968; Hasle & Syvertsen, Citation1997). We found the location of the rimoportula at some distance inside the valve edge and the marginal ring of fultoportulae to be the most distinct feature of T. sinica in LM, a feature shared by other Thalassiosira taxa such as T. andamanica, T. angstii (Gran) Makarova, T. licea Fryxell, T. minicosmica Lee & Park, T. minuscula (Hasle) Hasle, T. punctigera (Castracane) Hasle, T. subtilis (Ostenfeld) Gran, T. sundarbana Samanta & Bhadury, T. thailandica Boonyapiwat and T. tubifera Fryxell. Most of these species have drum-shaped cells, except T. licea which has a flat or slight convex valve face (Fryxell, Citation1978). Molecular data are unavailable for most species, except T. andamanica, T. sinica, T. minicosmica, T. minuscula and T. punctigera (, ).
Regarding the position of the rimoportula, three different patterns can be recognized (some shown in ). (1) For most taxa with more than one ring of fultoportulae on the valve face, in addition to the marginal ring of fultoportulae, the rimoportula is usually located in the outermost ring of valve-face fultoportulae, for example in T. andamanica (Gedde, Citation1999; Li et al., Citation2013), T. subtilis (Hasle, Citation1972; Li et al., Citation2014), T. sundarbana (Samanta & Bhadury, Citation2015), T. thailandica (Boonyapiwat & Takano, Citation1986) and T. tubifera (Fryxell, Citation1975). (2) In taxa with only a single marginal ring of fultoportulae, the rimoportulae are located close to valve margin, a short distance inside the marginal fultoportulae ring as in: T. angstii (Fryxell, Citation1978), T. licea (Fryxell, Citation1978), T. minicosmica (Park & Lee, Citation2015), T. minuscula (Hasle, Citation1972; Li et al., Citation2013, Citation2014) and T. punctigera (Takano, Citation1990; Li et al., Citation2013). (3) The location of the rimoportula in T. sinica differs in this respect (, ) by being located some distance inside the marginal ring of fultoportulae and by not joining the outermost ring of fultoportulae. On the other hand, it can be argued that the outermost ring of fultoportulae in T. sinica is positioned at some distance from the valve margin (), not close to the valve margin as in T. andamanica, T. subtilis, T. sundarbana and T. thailandica.
Fig. 22. Stylized drawings of the pattern of processes in Thalassiosira sinica and allied taxa. Fultoportulae are indicated by dots, rimoportulae by black ovals, and occluded processes by hollow ovals. Thalassiosira andamanica redrawn from Gedde (Citation1999), T. subtilis and T. diporocyclus from Hasle (Citation1972), T. sundarbana from Samanta & Bhadury. (Citation2015), T. tubifera and T. lundiana from Fryxell (Citation1975), T. thailandica from Boonyapiwat & Takano (Citation1986), T. sinica from this study.
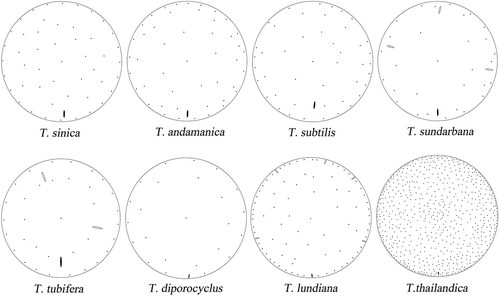
As well as the position, the outer length of the rimoportula may also be used to distinguish the above-mentioned taxa. The longest type occurs in T. andamanica, T. minicosmica and T. minuscula (Hasle, Citation1972; Gedde, Citation1999; Li et al., Citation2013, Citation2014; Park & Lee, Citation2015), T. angstii (Fryxell, Citation1978), T. licea (Fryxell, Citation1978), T. punctigera (Takano, Citation1990; Li et al., Citation2013) and T. tubifera (Fryxell, Citation1975). The shortest tube appears in T. thailandica, when comparing to its valve diameter (Boonyapiwat & Takano, Citation1986; Park et al., Citation2016). In T. sinica (, ) and T. subtilis (Hasle, Citation1972), the length of the tube is intermediate, while detailed information is unclear for T. sundarbana.
Furthermore, different types of fultoportulae are known to occur, not just in different species but even between marginal and valve-face fultoportulae in the same species. In the above taxa, there is typically only a single central fultoportula and it has a short or even missing external tube. Differences between species is therefore normally indicated for marginal and valve-face fultoportulae. In T. subtilis, T. minuscula, T. thailandica, T. tubifera, all fultoportulae appear as round external opening pores, as the external tubes are absent (Hasle, Citation1972; Fryxell, Citation1975; Boonyapiwat & Takano, Citation1986; Li et al., Citation2013, Citation2014; Park et al., Citation2016). In T. andamanica, both the marginal and valve-face fultoportulae possess distinct external tubes which are double-structured and usually tulip shaped (Gedde, Citation1999; Li et al., Citation2013). Similar but small-sized tulip-shaped tubes were reported in marginal fultoportulae of T. angstii (Fryxell, Citation1978) and T. punctigera (Takano, Citation1990; Li et al., Citation2013). Thalassiosira licea is unique in having distinct wings on the marginal fultoportulae (Fryxell, Citation1978). In T. sundarbana, all fultoportulae have short external tubes (Samanta & Bhadury, Citation2015), while in T. minicosmica the length varies (Park & Lee, Citation2015). In the species mentioned above, the marginal and valve-face fultoportulae may have similar external tubes, which may be longer, shorter or even absent. In T. sinica, the external tubes of the marginal fultoportulae were longer, and those of the valve-face fultoportulae were very short or absent (, ), and their internal tubes were similar and short (, ).
A number of other characters have also been found to be useful to distinguish between some of these species. Thalassiosira subtilis, T. minicosmica, T. minuscula and T. tubifera are reported to form mucilaginous colonies (Hasle, Citation1972; Hasle & Syvertsen, Citation1997; Park & Lee, Citation2015), while in T. thailandica, the cells are united in a short chain formed by the valves of neighbouring cells being closely attached (Boonyapiwat & Takano, Citation1986). This has never been recorded in similar taxa. Thalassiosira thailandica is unique due to its large cell size (ranging from 100 to 140 µm) and the great number of valve-face fultoportulae on each valve (Boonyapiwat & Takano, Citation1986; Park et al., Citation2016). Only a single ring of marginal fultoportulae is present in T. angstii, T. licea, T. minicosmica, T. minuscula and T. punctigera, while at least two rings occur in the other taxa. Occluded processes have been reported in T. angstii, T. licea, T. sundarbana, T. punctigera and T. tubifera (Fryxell, Citation1975, Citation1978; Takano, Citation1990; Samanta & Bhadury, Citation2015), not in any of the other taxa. Presence of ribs on the valve margin was used to distinguish T. andamanica and T. punctigera from other taxa (Takano, Citation1990; Gedde, Citation1999), including T. sinica ().
Comparison with closely related taxa
In addition to the position of rimoportula, T. sinica also shares morphological features with species found to be phylogenetically closely related. Thalassiosira sinica is similar to T. diporocyclus and T. lundiana in having drum-shaped frustules and the areolae patterns in fascicles. These species differ in the following features. Occluded processes were reported in T. lundiana (Takano, Citation1990; Hasle & Syvertsen, Citation1997; Park et al., Citation2016), but not in T. sinica and T. diporocyclus. External tubes are totally absent in T. diporocyclus (Hasle, Citation1972; Li et al., Citation2013, Citation2014; Park et al., Citation2016), while external extensions of processes occur in the other two taxa. Moreover, scattered valve-face fultoportulae have been described in T. lundiana (Takano, Citation1990; Li et al., Citation2013). They usually form a single, regular ring in T. diporocyclus (Hasle, Citation1972; Li et al., Citation2013), but they constitute 2–3 irregular rings in T. sinica (). Thalassiosira diporocyclus is also characterized by colonies of many cells embedded in a gelatinous substance, the colonies being spherical, oval or indefinite in shape (Takano, Citation1990; Li et al., Citation2013). Thalassiosira lundiana can be recognized by having two irregular rings of marginal fultoportulae, forming a zig-zag structure, while only one regular marginal ring is present in the other species.
Thalassiosira sinica is similar to Porosira species because of the drum-shape cells, the loculate areolae in a radial or fasciculate pattern, no real mantle, one large rimoportula in the marginal zone, and the remarkable feature of the presence of scattered fultoportulae on the valve face. There are also easily recognizable morphological differences: (1) In Porosira taxa, there is usually an annulus formed by silicified ring near the vale centre and absence of any fultoportulae, similar to Lauderia taxa (Hasle, Citation1973). But in T. sinica, one fultoportula is present near the valve centre (–), which is very common in Thalassiosira species. (2) The areolae, usually irregular in size and outline, form a wavy array in Porosira taxa, and the smallest areolae are normally located near the valve centre (Hasle, Citation1973). In T. sinica, areolae of almost equal size and shape are distributed in a typical fasciculate array (, ). In addition, foramina are round or elongate in Porosira taxa (Hasle, Citation1973; Round et al., Citation1990), but typical circular in T. sinica (, , ). (3) The valve-face fultoportulae are scattered over the whole valve face in Porosira taxa and are usually denser near the valve margin (Hasle, Citation1973), while they are in more uniformly distributed in T. sinica (). The morphological differences are well supported by molecular data. Thalassiosira sinica clustered with the most typical Thalassiosira taxa, and Porosira taxa form a clade outside the clade of the family Thalassiosiraceae (, ). Additional morphological comparisons between T. sinica and allied taxa are shown in .
Table 2. Morphological comparison among Thalassiosira sinica allied taxa.
With regard to the areola structure, several types are known (Park & Lee, Citation2015). Thalassiosira minicosmica, T. andamanica, T. diporocyclus, T. minuscula, T. subtilis, T. lundiana and T. punctigera group together by sharing nearly occluded foramen and small pores. Thalassiosira sinica also shows this structure of the areolae (, , , ), and in the LSU tree, these species clustered together with high support values, except for T. eccentrica. Therefore it is still uncertain whether areolar structure is a useful feature for grouping Thalassiosira taxa into monophyletic groups.
Subgroups to accommodate T. sinica within the genus Thalassiosira
Subgroups within the genus Thalassiosira have been suggested by some diatomists. Based on the length of the external and internal extensions of the fultoportulae, and the presence of a rimoportula near the valve margin or centre, Hasle (Citation1968) introduced two subgroups (A and B). However, an exception, T. andamanica with a valve-face rimoportula and an external fultoportula extension, was discovered by Gedde (Citation1999), who proposed a third subgroup (C) to accommodate this and allied taxa, including T. licea, T. subtilis and T. tubifera. Additional taxa have subsequently been added, such as T. angstii, T. minuscula, T. punctigera and T. sundarbana, and, most recently, T. minicosmica (Park & Lee, Citation2015). Obviously, T. sinica belongs to this subgroup based on morphology. However, the molecular data did not support the validity of subgroup C (, ). While a small clade formed by the four species T. andamanica, T. diporocyclus, T. lundiana and T. sinica is supported by both SSU and LSU data, no synapomorphy could be found.
In recent molecular phylogenetic studies, the known Thalassiosira taxa clustered into several large clades, separated by other genera of the family Thalassiosiraceae (Kaczmarska et al., Citation2006; Alverson et al., Citation2007; Hoppenrath et al., Citation2007; this study). This was taken to indicate that the genus Thalassiosira is not a monophyletic group, but a similar-morphology group. Based on some unusual ultrastructural features, several new genera were therefore established, for example Shionodiscus (Alverson et al., Citation2006). Also T. pseudonana Hasle & Heimdal was transferred back to Cyclotella nana Hustedt (Alverson et al., Citation2011), and T. weissflogii (Grunow) Fryxell & Hasle was renamed Conticribra weissflogii (Stachura-Suchoples & Williams, Citation2009). These proposals were supported by only a limited number of molecular analyses, and they have not been widely accepted.
Acknowledgements
We would like to thank Prof. Øjvind Moestrup and Dr Nina Lundholm for helping us with the English and for the constructive comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Yang Li
Y. Li: original concept, analysis of data, drafting and editing manuscript; Y. Q. Guo: culture isolation, morphological observation, molecular data; X. H. Guo: morphological data.
References
- Alverson, A.J., Kang, S.H. & Theriot, E.C. (2006). Cell wall morphology and systematic importance of Thalassiosira ritscheri (Hustedt) Hasle, with a description of Shionodiscus gen. nov. Diatom Research, 21: 251–262.
- Alverson, A.J., Jansen, R.K. & Theriot, E.C. (2007). Bridging the Rubicon: phylogenetic analysis reveals repeated colonizations of marine and fresh waters by thalassiosiroid diatoms. Molecular Phylogenetics and Evolution, 45: 193–210.
- Alverson, A.J., Beszteri, B., Julius, M.L. & Theriot, E.C. (2011). The model marine diatom Thalassiosira pseudonana likely descended from a freshwater ancestor in the genus Cyclotella. BMC Evolutionary Biology, 11: 125.
- Boonyapiwat, S. & Takano, H. (1986). A new centric diatom from the Gulf of Thailand. Bulletin of Tokai Regional Fisheries Research Laboratory, 119: 41–47.
- Chen, S.W., Gao, Y.H., Du, H., Dong, Q.X. & Huang, C.J. (2004). First recording of Thalassiosira diporocyclus bloom in the southeast China Sea. Chinese Journal of Oceanology and Limnology, 35: 130–137.
- Cheng, Z.D., Gao, Y.H. & Liu, S.C. (1993). Nano-diatoms in Fujian coast. Ocean Press, Beijing. 91 pp.
- Fryxell, G.A. (1975). Three new species of Thalassiosira, with observation on the occluded process, a newly observed structure of diatom valves. Nova Hedwigia, 53: 57–73.
- Fryxell, G.A. (1978). The diatom genus Thalassiosira: T. licea sp. nov. and T. angstii (Gran) Makarova, species with occluded processes. Botanica Marina, 21: 131–141.
- Fryxell, G.A. & Hasle, G.R. (2004). Taxonomy of harmful diatoms. In Manual on Harmful Marine Microalgae (Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., editors), 465–510. Imprimerie Landais, Paris.
- Gedde, A.D. (1999). Thalassiosira andamanica sp. nov. (Bacillariophyceae), a new diatom from the Andaman Sea (Thailand). Journal of Phycology, 35: 198–205.
- Guillard, R.R.L. & Hargraves, P.E. (1993). Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia, 32: 234–236.
- Guiry, M.D. & Guiry, G.M. (2017). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org. Searched on 4 January 2017.
- Guo, Y.J. & Qian, S.B. (2003). Flora Algarum Marinarum Sinicarum, Tomus V. Bacillariophyta, No. 1. Centricae. Science Press, Beijing. 493 pp.
- Hall, T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium, 41: 95–98.
- Hasle, G.R. (1968). The valve processes of the centric diatom genus Thalassiosira. Nytt Magasin for Botanikk, 15: 193–201.
- Hasle, G.R. (1972). Thalassiosira subtilis (Bacillariophyceae) and two allied species. Norwegian Journal of Botany, 19: 111–137.
- Hasle, G.R. (1973). Some marine plankton genera of the diatom family Thalassiosiraceae. In Proceedings of the 2nd international symposium on recent and fossil marine diatoms. Nova Hedwigia, Beihefte, 45: 1–68.
- Hasle, G.R. (1983). Thalassiosira punctigera (Castr.) comb. nov., a widely distributed marine planktonic diatom. Nordic Journal of Botany, 3: 593–608.
- Hasle, G.R. & Syvertsen, E.E. (1997). Marine diatoms. In Identifying Marine Phytoplankton (Tomas C.R., editor). Academic Press, San Diego. pp. 5–385.
- Hoppenrath, M., Beszteri, B., Drebes, G., Halliger, H., Van Beusekom, J.E.E., Janisch, S. & Wiltshire, K.H. (2007). Thalassiosira species (Bacillariophyceae, Thalassiosirales) in the North Sea at Helgoland (German Bight) and Sylt (North Frisian Wadden Sea) – a first approach to assessing diversity. European Journal of Phycology, 42: 271–288.
- Kaczmarska, I., Beaton, M., Benoit, A.C. & Medlin, L.K. (2006). Molecular phylogeny of selected members of the order Thalassiosirales (Bacillariophyta) and evolution of the fultoportula. Journal of Phycology, 42: 121–138.
- Lee, J.H. & Park, J.S. (2008). A study on the fine structure of the marine diatoms of Korean coastal waters: genus Thalassiosira 3. Algae, 23: 187–199.
- Li, Y., Zhao, Q.L. & Lü, S.H. (2013). The genus Thalassiosira off the Guangdong coast, South China Sea. Botanic Marina, 56: 83–110.
- Li, Y., Zhao, Q.L. & Lü, S.H. (2014). Taxonomy and species diversity of the diatom genus Thalassiosira (Bacillariophyceae) in Zhejiang coastal waters, the East China Sea. Nova Hedwigia, 99: 373–402.
- Lundholm, N., Daugbjerg, N. & Moestrup, Ø. (2002). Phylogeny of the Bacillariaceae with emphasis on the genus Pseudo-nitzschia (Bacillariophyceae) based on partial LSU rDNA. European Journal of Phycology, 37: 115–134.
- Nunn, G.B., Theisen, B.F., Christensen, B. & Arctander, P. (1996). Simplicity-correlated size growth of the nuclear 28S ribosomal RNA D3 expansion segment in the crustacean order Isopoda. Journal of Molecular Evolution, 42: 211–223.
- Nylander, J.A.A. (2004). MrModeltest v.2. Program distributed by the author. Evolutionary Biology Center, Uppsala University, Uppsala.
- Park, J.S. & Lee, J.H. (2010). A study on the fine structure of the marine diatoms of Korean coastal waters: genus Thalassiosira 5. Algae, 25: 121–131.
- Park, J.S. & Lee, J.H. (2014). Description of the pseudocryptic species Conticriba weissflogiopsis sp. nov. (Thalassiosirales, Bacillariophyta) isolated from brackish waters in Korea, based on its cingulum structure and molecular analysis. Phytotaxa, 191: 115–128.
- Park, J.S. & Lee, J.H. (2015). A new gelatinous colony-forming Thalassiosira minicosmica sp. nov (Bacillariophyta) from Korean coastal waters and a consideration of the Thalassiosira subtilis group. Diatom Research, 30: 163–173.
- Park, J.S., Jung, S.W. & Lee, J.H. (2009). A study on the fine structure of the marine diatoms of Korean coastal waters: genus Thalassiosira 4. Algae, 24: 67–77.
- Park, J.S., Jung, S.W., Lee, J.H., Yun, S.M. & Lee, J.H. (2016). Species diversity of the genus Thalassiosira (Thalassiosirales, Bacillariophyta) in South Korea and its biogeographical distribution in the world. Phycologia, 55: 403–423.
- Ronquist, F., Teslenko, M. & Mark, P.V. (2012). MrBayes 3.2: efficient Bayesian inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Ross, R., Cox, E.J., Karayeva, N.I., Mann, D.G., Paddock, T.B.B., Simonsen, R. & Sims, P.A. (1979). An amended terminology for the siliceous diatom cell. Nova Hedwigia, Beiheft, 64: 513–533.
- Round, F.E., Crawford, R.M. & Mann, D.G. (1990). The Diatoms: Biology and Morpology of the Genera. 3rd ed. Cambridge University Press, Cambridge. 747 pp.
- Samanta, B. & Bhadury, P. (2015). Thalassiosira sundarbana sp. nov. (Bacillariophyta), an estuarine diatom from Sundarbans mangrove ecoregion based on morphology and molecular phylogeny. Phycological Research, 63: 102–109.
- Scholin, C.A., Herzog, M., Sogin, M. & Anderson, D.M. (1994). Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rDNA. Journal of Phycology, 30: 999–1011.
- Simonsen, R. (1974). The diatom plankton of the Indian Ocean Expedition of RV “Meteor” 1964–1965. “Meteor” Forschungsergebnisse Reihe D, 19: 1–107.
- Stachura-Suchoples, K. & Williams, D.M. (2009). Description of Conticriba tricircularis, a new genus and species of Thalassiosirales, with a discussion on its relationship to other continuous cribra species of Thalassiosira Cleve (Bacillariophyta) and its freshwater origin. European Journal of Phycology, 44: 477–486.
- Takano, H. (1990). Diatoms. In Red Tide Organisms in Japan – An Illustrated Taxonomic Guide (Fukuyo, Y., Takano, H., Chihara, M. & Matsuoka, K., editors), 162–331. Uchida Rokakuho, Tokyo.
- Theriot, E.C. & Serieyssol, K. (1994). Phylogenetic systematics as a guide to understanding features and potential morphological characters of the centric diatom family Thalassiosiraceae. Diatom Research, 9: 429–450.
- Von Stosch H. (1975). An amended terminology of the diatom girdle. Beiheft zur Nova Hedwigia, 53: 1–35.
- Xie, W.L., Li, Y. & Gao, Y.H. (2008). First record of Thalassiosira curviseriata Takano (Bacillariophyceae) and its bloom in the East China Sea. Acta Oceanologica Sinica, 27: 124–132.
- Zhen, Y., Mi, T.Z. & Yu, Z.G. (2008). Detection of Phaeocystis globosa using sandwich hybridization integrated with nuclease protection assay (NPA-SH). Journal of Environmental Science, 20: 1481–1486.