ABSTRACT
A new species in the genus Prasionema (Prasiolales, Trebouxiophyceae) is described from Campbell Island, in the New Zealand subantarctic region, the first record of this genus in the southern hemisphere. Prasionema heeschiae sp. nov. is filamentous, uni- to predominantly biseriate, with disc-shaped cells, and an axial plastid with a central pyrenoid. It is anchored by an enlarged pigmented basal cell. There is evidence of reproduction by both spores and fragmentation. This species was found growing on a timber wharf above the high tide level. The only other species in the genus, P. payeri Heesch, M.Pazoutová & Rindi, was described from Spitzbergen growing on soil in a high nutrient environment. The bipolar distribution of Prasionema is discussed. Based on phylogenetic analyses that included sequence data from holotype material of southern filamentous Prasiolales, we reduce Rosenvingiella australis to synonymy with R. tasmanica.
Introduction
Representatives of the order Prasiolales (Chlorophyta) are found globally from the tropics to the poles and can be found in freshwater, marine and terrestrial habitats (e.g. Broady, Citation1989; Rindi et al., Citation1999; Rindi & Guiry, Citation2004; Moniz et al., Citation2014; Heesch et al., Citation2016; Klochkova et al., Citation2017). Over the past decade there has been a very significant increase in research activity on members of this order, with new discoveries, range extensions for known taxa, and a greatly improved understanding of relationships within the Prasiolales (e.g. Sherwood et al., Citation2000; Rindi et al., Citation2004, Citation2007; Rindi, Citation2010; Moniz et al., 2012a, b, Citation2014; Heesch et al., Citation2012, Citation2016; Sutherland et al., Citation2016; Garrido-Benavent et al., Citation2017; Klochkova et al., Citation2017). These advances have been informed by molecular sequence data and phylogenetic analyses as well as culture studies, field observations and investigations of relationships with endophytes. An important component to the recent progress in this area of research has been collections of specimens from regions and habitats previously unexplored for members of this order.
This paper reports on the discovery of a filamentous member of the Prasiolales on Campbell Island, which is part of the southernmost island group in the New Zealand archipelago. Campbell Island lies south-east of New Zealand on the Campbell Plateau, and is part of the submerged Zealandia microcontinent (Grobys et al., Citation2008; Mortimer et al., Citation2017). Along with the Falkland Islands, the Auckland and Campbell Islands lie within the subantarctic waters, between the subtropical front and the subantarctic front to the north of the Antarctic circumpolar current (Carter et al., Citation2008). The New Zealand subantarctic islands are distant from mainland New Zealand, and are collectively listed as a UNESCO World Heritage site, representing some of the world’s least-modified islands (http://whc.unesco.org/en/list/877/).
This paper reports on both molecular and morphological investigations of a new species of Prasionema Heesch, M.Pazoutová & Rindi, collected from Campbell Island in 2013, and is the first record of the genus from the southern hemisphere. Prasionema was recently described as a monotypic genus from material collected in Spitzbergen (Heesch et al., Citation2016).
Materials and methods
Collections from Campbell Island were carried out (by WAN) in February 2013 with the assistance of the Department of Conservation, with transport to the subantarctic provided by the New Zealand Navy and the HMNZS Otago. On landing at Beeman Point on Campbell Island (52.5504°S, 169.1523°E), material of a green, finely filamentous alga was collected from the surface of the wooden wharf (). The wharf, which had been assessed as structurally unsound, was subsequently demolished over the following few days as part of the Department of Conservation maintenance programme for the southern islands.
Fig. 1. Prasionema heeschiae sp. nov. type locality, wharf at Beeman Point, Campbell Island. Arrow indicates patches of Prasionema on the surface of the wooden wharf.

The majority of the material collected was air dried and has been deposited in the herbarium of the Museum of New Zealand Te Papa Tongarewa (WELT, Thiers, Citation2017). A small subsample was separated at the time of collection and dried in silica gel. Samples from the air-dried material were rehydrated and examined by light microscopy. Photomicrographs were taken using an Olympus BX53 (Tokyo, Japan) with a SC100 (Olympus, Münster, Germany) digital camera.
DNA extraction, amplification and dataset assembly
DNA was extracted from the Campbell Island specimen (WELT A033555), and the rbcL and tufA markers were amplified as in Sutherland et al. (Citation2016). We also amplified and sequenced the tufA marker from DNA extracted from the holotype of Rosenvingiella australis (Heesch et al., Citation2012) using the same methods and primers. Sequences were aligned in Geneious R9 9.0.5 (Biomatters, Auckland). We created separate alignments of rbcL and tufA sequences from our specimen, with sequences available in GenBank from representatives of the Prasiolaceae, including published rbcL and tufA sequence data from the holotype of Rosenvingiella tasmanica, and the published rbcL sequence of the holotype of R. australis (). A third alignment was created from the concatenation of the rbcL and tufA alignments. We restricted the concatenated dataset to taxa for which both markers had been sequenced from a single specimen to avoid the possibility of creating chimeric sequences in the alignment, with the exception of Prasionella wendyae Heesch, M.Pazoutová & Rindi. We included Prasionella wendyae in the concatenated dataset as it is the only representative of that genus, even though only rbcL sequence data are available for it. We included four green algae (Koliella longiseta (Vischer) Hindák, Koliellaceae, Prasiolales; Neocystis brevis (W.Vischer) I.Kostikov & L.Hoffmann, Radiococcaceae, Sphaeropleales; Pabia signiensis T.Friedl & O’Kelly, Trebouxiaceae, Trebouxiales and ‘Chlorella’ mirabilis) as outgroups for each dataset, based on availability of both rbcL and tufA sequences and homology to Prasiolalean sequences by BLAST (Altschul et al., Citation1990). GenBank accession numbers, location and voucher details for sequences used in the analyses are given in .
Table 1. Specimen information and GenBank accession numbers for sequences used in the phylogenetic analyses.
Phylogenetic analyses
We estimated Maximum likelihood trees using PhyML v3.0 (Guindon et al., Citation2010), after identifying appropriate models of sequence evolution using jModeltest 2 (rbcL: TIM1+I+G; tufA: GTR+I+G; concatenated dataset: TIM3+I+G; Darriba et al., Citation2012). Analyses used nearest-neighbour interchange and subtree pruning and regrafting for maximum search space coverage. Support for ML analyses was assessed using the approximate likelihood ratio test (aLRT, Anisimova & Gascuel, Citation2006) and bootstrapping (1000 replicates).
For Bayesian analyses, we used PartitionFinder 1.1.1 (Lanfear et al., Citation2012) to estimate appropriate partitioning schemes and models of sequence evolution for each dataset. Partition schemes and models estimated are shown in . Using MrBayes v3.2.6 x64 (Ronquist et al., Citation2012), Markov chain Monte Carlo analyses were run for 4 000 000 generations for each dataset, sampling every 1000 generations, with parameters free to vary between partitions. Burnin was assessed by inspection of log-likelihood plots and average parameter values using Tracer V1.5 (http://tree.bio.ed.ac.uk/software/tracer/), and was confirmed by inspection of potential scale reduction factor scores calculated by the sump function of MrBayes. Trees were visualized using FigTree 1.4.0 (http://tree.bio.ed.ac.uk/software/figtree).
Table 2. Partitioning strategies and models of sequence evolution estimated by Partitionfinder 1.1.1 for each dataset.
Results
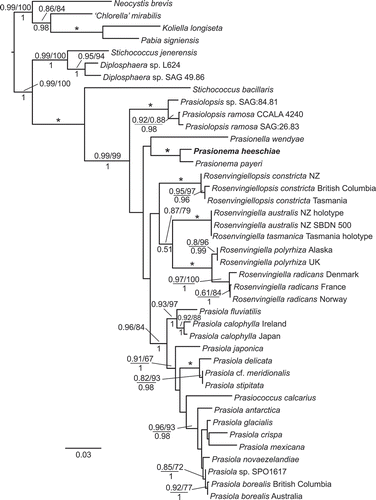
Sequence electropherograms showed no evidence of the material being part of a mixed collection, an issue that might occur with members of the Prasiolales where multiple species can exist in close proximity. Results of the analysis of the rbcL dataset, consisting of 41 taxa and 1098 characters, are shown in . The specimen from Campbell Island was resolved as a sister to Prasionema payeri with support of 1.0 for both approximate likelihood ratio test (aLRT) and posterior probability (PP), and 100% bootstrap support (BS) under maximum likelihood. The position of these two taxa within the Prasiolaceae relative to Prasionella, Rosenvingiella, Rosenvingiellopsis, Prasiococcus and Prasiola was not resolved. Although a sister relationship with Prasionella was recovered in the analysis, this received no significant support under any method. Prasiola (with Prasiococcus nested within it), and Rosenvingiellopsis were both resolved as monophyletic with strong support. Support for Rosenvingiella was only moderate, and there was no support for a sister relationship between the filamentous genera Rosenvingiella and Rosenvingiellopsis. Supplementary fig. 1 is a maximum likelihood phylogram estimated from the tufA dataset.
Fig. 2. Maximum likelihood phylogram estimated from the rbcL dataset. Support values are shown on each branch: approximate Likelihood Ratio Test (aLRT) values and ML bootstrap (%) above, and Bayesian PP values below. An asterisk indicates support of 1/100/1. Only values greater than 0.8 (aLRT), 70% (bootstrap) and 0.9 (PP) are shown. Support values are only shown if two support methods reach the cut-off value. Collection locations are appended to names when two or more representatives of a single taxon are included. Scale bar represents substitutions per site.
The maximum likelihood phylogram derived from the concatenated rbcL and tufA dataset is shown in . The Campbell Island specimen was again resolved as a sister taxon to Prasionema payeri, and clearly distinguished from it. Support for a monophyletic Rosenvingiella was much higher in this analysis (ML aLRT 0.99/ML Bootstrap 95/Bayesian PP 0.94), and a sister relationship between Rosenvingiella and Rosenvingiellopsis received some support under ML aLRT (0.92), but no significant support under either ML bootstrap (58%) or Bayesian analyses (0.67).
Fig. 3. Maximum likelihood phylogram estimated from the concatenated rbcL and tufA dataset. Support values and naming are as for . Scale bar represents substitutions per site.
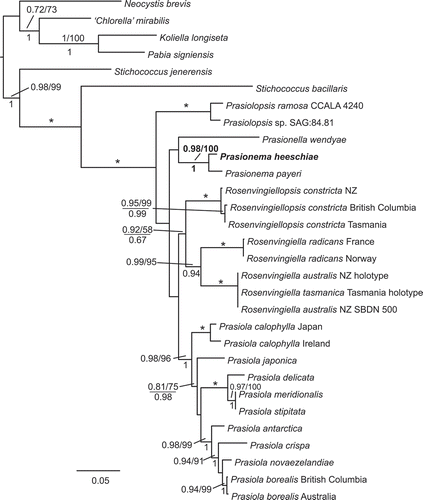
The holotype of Rosenvingiella australis Heesch & W.A.Nelson was resolved with the holotype of R. tasmanica M.B.J.Moniz, Rindi & Guiry: both the rbcL and tufA sequences for these taxa were identical.
Morphology and taxonomic treatment
Prasionema heeschiae W.A.Nelson & J.E.Sutherland, sp. nov. (–)
Diagnosis: Thallus of predominantly biseriate, ribbon-like filaments, occasionally uniseriate; filaments 10–38 µm wide, composed of disc-shaped cells c. 8–20 µm wide, and 4–6 µm high, containing a single axial chloroplast with a central pyrenoid. Filaments anchored by enlarged pigmented cells. Putative gametangia/sporangia in mature multiseriate filaments with gametes/spores released by breakdown of cell walls. Vegetative reproduction by fragmentation of short pieces of filaments.
Figs 4–19. Prasionema heeschiae type material. Fig. 4. Type specimen – growing on wood, surface view. Fig. 5. Type specimen – growing on wood, side view. Fig. 6. View of biseriate filaments at low magnification. Fig. 7. Filaments attached to wood fragment. Fig. 8. Base of filament showing large basal cells. Figs 9–11. Examples of biseriate filaments of increasing maturity with spore development in Fig. 11. Fig. 12. Filaments showing curvature with break in inside filament. Fig. 13. Curved biseriate filament showing independent cell division in each filament. Fig. 14. Filament showing uniseriate and biseriate sections. Fig. 15. Hooked filament. Fig. 16. Helical filament. Figs 17–18. Filaments showing uniseriate and biseriate sections, with possible vegetative reproduction. Fig. 19. Spore release. Scale: Figs 4–5, 5 mm; Fig. 6, 100 µm; Figs 7, 13, 15–15, 19, 50 µm. Figs 8, 9–12, 14, 17–18, 20 µm.

Holotype: WELT A033555, W.A.Nelson, 12 February 2013.
Type locality: New Zealand, Campbell Island, Beeman Point (52.5504°S, 169.1523°E), growing on wooden wharf above high tide line. (The wharf was demolished after the type collection was made.)
Etymology: The specific epithet honours Svenja Heesch, our colleague who has made significant contributions to the study of the Prasiolales.
Description
When Prasionema heeschiae was collected it was mistaken for robust material of Rosenvingiella. It was bright green in colour, growing in dense patches on the decaying wooden wharf (–) about 1.5 m above high tide level, with Prasiola, small clumps of grass and mosses, and among guano deposits. When viewed with a microscope the thalli were found to have a ribbon-like form () with most consisting of biseriate filaments, with intercalary divisions. The filaments were attached to the wood surface by enlarged, pigmented basal cells (–). The cells in the filaments were found to be wider than tall, with the cells within each column of the filament dividing independently (–). Along some filaments there were distinct twists, bends, coils and helices (–). Some of the bends and coils coincided with breaks in one, occasionally both, of the filaments (, , , ).
Vegetative reproduction appeared to be enabled by short pieces of the filament with rounded ends breaking away from the thallus (–). In a number of thalli, there were multiple cell divisions, resulting in pseudoparenchymatous filaments and the production of sporangia or gametangia (, , ). It is not known whether these are sexual or asexual, nor how the spores/gametes develop. The release of spores/gametes occurred through the breakdown of the cell walls () (similar to the disintegration of gametangial cell walls in Rosenvingiella polyrhiza illustrated by Rindi et al., Citation2004), and large clusters were found around the upper portions of filaments.
Distinguishing features
Prasionema heeschiae is almost entirely biseriate, except in fertile regions where the filaments are pluriseriate, and thus differs from P. payeri which is almost entirely uniseriate except (as described by Heesch et al., Citation2016) for a ‘few short biseriate portions’. The width of the filaments in P. payeri is 18–22 μm whereas in P. heeschiae the individual filaments are 8–22 μm wide and the biseriate portions 20–38 μm wide. The material of P. payeri has been found growing on soil without any attachment, so it is not possible to compare the basal cell structure with that reported here for P. heeschiae. Evidence of vegetative fragmentation was observed in P. payeri as well as in P. heeschiae, but spore production was not observed in P. payeri (Heesch et al., Citation2016), either in field or laboratory cultured material. The two taxa are genetically distinct, differing by 17/695 (2.4%) substitutions in tufA sequence data and by 10/1028 (2.1%) in rbcL. This is comparable to sequence differences between other closely related taxa in the Prasiolales datasets: Prasiola borealis from Australia and P. novaezelandiae from New Zealand also differ by 17/695 substitutions in their tufA sequence data, and by only 8/918 substitutions (0.9%) in their rbcL sequence data.
We consider that P. payeri and P. heeschiae are distinct species based on morphological characters, genetic data and the distributions of these two species in different hemispheres and ocean basins.
Nomenclature
Rosenvingiella tasmanica M.B.J.Moniz, Rindi & Guiry, Phycologia 51(1): 93 (2012a)
Synonymy: = Rosenvingiella australis Heesch & W.A.Nelson, Phycologia 51(2): 222–223, Heesch et al. (Citation2012).
Rosenvingiella tasmanica and Rosenvingiella australis were both described in 2012 from collections of filamentous algae made in Tasmania, Australia and Otago, New Zealand, respectively (Moniz et al., 2012a; Heesch et al., Citation2012). Both were described as uniseriate filaments with rhizoids, with the ranges of filament proportions and cell sizes overlapping in the material described. Multiseriate filaments were present in both collections, but were more common in the Otago collections. No sexual spores were seen in the Tasmanian collections, while Heesch et al. (Citation2012) commented that in the Otago collections ‘some multiseriate regions may be gametangia’. In view of the identical sequences obtained at the rbcL and the tufA loci by us and others (Heesch et al., Citation2016) we believe these to be a single taxon with a distribution that includes Tasmania and southern New Zealand. Further collections of R. tasmanica (as R. australis) have been made in New Zealand at sites between latitudes 42–48° (Sutherland and Nelson, unpublished data). The descriptions of both species were published in 2012, with R. tasmanica in an earlier issue of the same journal, and thus that name has priority over R. australis, which is here reduced to synonymy with R. tasmanica. Further collections of filamentous Prasiolales are needed to get a better understanding of the evolution and relationships of this species, as well as more collections from high latitude southern hemisphere sites including southern islands to determine the true extent of its distribution.
Discussion
Taxa belonging to the Prasiolales have very simple morphology and for many years the taxonomy of this group was confused. Insights from culture studies and phylogenetic analyses enabled by molecular data (e.g. Kornmann & Sahling, Citation1974; Hanic, Citation1979; Sherwood et al., Citation2000; Rindi et al., Citation2004, Citation2007; Moniz et al., Citation2012a, b; Heesch et al., Citation2016; Klochkova et al., Citation2017) have led to greatly improved understanding of the genera and species within the order. However, there are aspects of the biology, ecology and systematics of members of the Prasiolales where further research is required, including determining the correct application of names, reliability of morphological characters in species recognition, life history and reproduction, relationships between genera, and the need for further collections from relatively unexplored areas. Recent papers, particularly those from remote or infrequently explored regions, have greatly advanced our understanding of geographic distributions as well as revealed cryptic genetic diversity in these simple green algae, but more work is required (Moniz et al., Citation2012b; Heesch et al., Citation2016; Klochkova et al., Citation2017).
The discovery of a second species in the genus Prasionema raises some interesting questions. Within the Prasiolales a range of distributional patterns are exhibited, with some species apparently globally distributed, and other species with highly restricted distributions (Rindi, Citation2010; Heesch et al., Citation2016). The two species of Prasionema have been found in different hemispheres and different oceans, with the two sites about as distant from each other as is possible. Both species occur in high latitude locations (P. payeri, 78.6°N; P. heeschiae, 52.5°S) growing subaerially. The environment where P. heeschiae was discovered is maritime, influenced by marine conditions although growing above the littoral zone, whereas P. payeri was found on soil in nutrient-rich sites. Both species have been recorded from habitats modified by human activities. While P. heeschiae was found on a wharf, there is no reason to suspect it is an introduced species. Prasionema heeschiae was not found at other sites that have been sampled on Campbell Island, and neither has it been found in collections of Prasiolales from Auckland, Antipodes, Snares and Bounty Islands. However, there have been very few targeted collections of Prasiolales from this remote region. The distribution of P. heeschiae in the New Zealand subantarctic remains unknown. Unfortunately, the type locality of P. heeschiae no longer exists as the wharf was demolished after the collection of the samples was made.
There are other species of Prasiolales distributed in both hemispheres that are found in subaerial habitats similar to those occupied by Prasionema (e.g. Prasiola crispa, present in Antarctica and in Europe) (Moniz et al., Citation2012b). Garrido-Benavent et al. (Citation2017), when discussing the bipolar distribution of Prasiola borealis along the Alaska–Tierra del Fuego axis, considered that the data ‘argue against recurrent genetic exchange, and rather suggest a single historical dispersal event’. The bipolar distribution of the two species of Prasionema may be explained by the genus originating at lower latitudes and colonizing higher latitude sites, or originating at high latitudes in either the north or south with later dispersal. However, without more information about these species and any other as yet undiscovered congeners, their distributions and possible genetic variability, further speculation is not warranted.
The decision to synonymize Rosenvingiella tasmanica and R. australis is based on their morphological similarity, genetic identity at rbcL and tufA loci, and distribution in southern New Zealand and Tasmania. We consider that the situation with these two species of Rosenvingiella differs from that found in the species Prasiola meridionalis and P. stipitata, which are also very similar genetically. Northern hemisphere members of these two taxa have identical rbcL sequences, and differ by only a single substitution over 682 bp at the tufA locus, but show differences in the arrangement of reproductive tissue (Moniz et al., Citation2014). Prasiola meridionalis and P. stipitata were described from the North Pacific and North Atlantic respectively, and Moniz et al. (Citation2014) suggested that the genetic similarity of these species might be due to recent plastid introgression between two sibling species, which have only recently separated, reflecting an ‘Arctic radiation related to the climatic history of the northern Atlantic and northern Pacific Oceans in recent evolutionary times’. Discrimination of these taxa relies on morphological and biogeographic criteria (Heesch et al., Citation2012; Sutherland et al., Citation2016).
The position of Prasionema within the Prasiolaceae relative to Prasionella, Rosenvingiella, Rosenvingiellopsis, Prasiococcus and Prasiola remains unresolved. While the structures of our phylogenetic trees differ from the data presented by Heesch et al. (Citation2016), likely a consequence of differences in taxon sampling, our findings are not in conflict with theirs. In our single gene analyses (rbcL, tufA), Prasionella is resolved with Prasionema, and Rosenvingiella is resolved with Rosenvingiellopsis, but without support. In the concatenated analysis, there is only moderate support under aLRT for the relationship between Rosenvingiella and Rosenvingiellopsis and no support under bootstrap or Bayesian analyses.
Further collections of filamentous Prasiolales are needed to develop a better understanding of the evolution and relationships of these taxa, including the two Prasionema species. More collections from high latitude southern hemisphere sites, including the subantarctic islands, are needed to document these unique and under-studied taxa.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2018.1423577Supplementary fig. 1. Maximum likelihood phylogram estimated from the tufA dataset.
Supplementary_material.pdf
Download PDF (72.6 KB)Acknowledgements
We acknowledge Sarah Wilcox and the Our Far South expedition for material collected in 2012; Pete McClelland and Di Morris of the Department of Conservation, and the Captain and crew of the HMNZS Otago for enabling WAN to collect at the subantarctic islands. Erika MacKay (NIWA) is thanked for assistance with illustrations.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Wendy A. Nelson
W. Nelson: field collections, morphological and anatomical observations, drafting and editing manuscript; J. Sutherland: generating and analysing sequence data, drafting and editing manuscript.
References
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215: 403–410.
- Anisimova, M. & Gascuel, O. (2006). Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Systematic Biology, 55: 539–552.
- Broady, P.A. (1989). The distribution of Prasiola calophylla (Carmich.) Menegh, (Chlorophyta) in Antarctic freshwater and terrestrial habitats. Antarctic Science, 1: 109–118.
- Carter, L., McCave, I.N. & Williams, M.J.M. (2008). Circulation and water masses of the Southern Ocean: a review. In Antarctic Climate Evolution (Florindo, F. & Siegert, M., editors), Developments in Earth and Environmental Sciences, 8: 85–114. Elsevier, Amsterdam.
- Darriba, D., Taboada, G.L., Doallo, R. & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9: 772.
- Fučíková, K., Leliaert, F., Cooper, E.D., Škaloud, P., DHondt, S., de Clerck, O., Gurgel, C.F.D., Lewis, L.A., Lewis, P.O., López-Bautista, J.M., Delwiche, C.F. & Verbruggen, H. (2014). New phylogenetic hypotheses for the core Chlorophyta based on chloroplast sequence data. Frontiers in Ecology and Evolution, 2: 1–12.
- Garrido-Benavent, I., Pérez-Ortega, S. & de los Ríos, A. (2017). From Alaska to Antarctica: species boundaries and genetic diversity of Prasiola (Trebouxiophyceae), a foliose chlorophyte associated with the bipolar lichen-forming fungus Mastodia tessellata. Molecular Phylogenetics and Evolution, 107: 117–131.
- Grobys, J.W.G., Gohl, K. & Eagles, G. (2008). Quantitative tectonic reconstructions of Zealandia based on crustal thickness estimates. Geochemistry Geophysics Geosystems, 9: Q01005. doi: 10.1029/2007GC001691.
- Guindon, S., Dufayard, J.-F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59: 307–321.
- Hanic, L.A. (1979). Observations on Prasiola meridionalis (S. & G.) and Rosenvingiella constricta (S. & G.) Silva (Chlorophyta, Prasiolales) from Galiano Island, British Columbia. Phycologia, 18: 71–76.
- Heesch, S., Sutherland, J.E. & Nelson, W.A. (2012). Marine Prasiolales (Trebouxiophyceae, Chlorophyta) from New Zealand and the Balleny Islands, with descriptions of Prasiola novaezelandiae sp. nov. and Rosenvingiella australis sp. nov. Phycologia, 51: 217–227.
- Heesch, S., Pažoutová, M., Moniz, M.B.J. & Rindi, F. (2016). Prasiolales (Trebouxiophyceae, Chlorophyta) of the Svalbard Archipelago: diversity, biogeography and description of the new genera Prasionella and Prasionema. European Journal of Phycology, 51: 1–17.
- Klochkova, T.A., Klochkova N.G. & Kim, G.H. (2017). Molecular phylogeny of the marine Prasiola and Rosenvingiella species (Chlorophyta: Prasiolales) from Southeastern Kamchatka. Russian Journal of Marine Biology, 43: 34–41.
- Kornmann, P. & Sahling, P.-H. (1974). Prasiolales (Chlorophyta) von Helgoland. Helgoländer wissenschaftliche Meeresuntersuchungen, 26: 99–133.
- Lanfear, R., Calcott, B., Ho, S.Y.W. & Guindon, S. (2012). PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution, 29: 1695–1701.
- Lemieux, C., Otis, C. & Turmel, M. (2014). Chloroplast phylogenomic analysis resolves deep-level relationships within the green algal class Trebouxiophyceae. BMC Evolutionary Biology, 14: 211.
- Moniz, M.B.J., Rindi, F., & Guiry, M.D. (2012a). Phylogeny and taxonomy of Prasiolales (Trebouxiophyceae, Chlorophyta). from Tasmania, including Rosenvingiella tasmanica, sp. nov. Phycologia, 51: 86–97.
- Moniz, M.B.J., Rindi, F., Novis, P.M., Broady, P.A. & Guiry, M.D. (2012b). Molecular phylogeny of Antarctic Prasiola (Prasiolales, Trebouxiophyceae) reveals extensive cryptic diversity. Journal of Phycology, 48: 940–955.
- Moniz, M.B.J., Guiry, M.D. & Rindi, F. (2014). TufA phylogeny and species boundaries in the green algal order Prasiolales (Trebouxiophyceae, Chlorophyta). Phycologia, 53: 396–406.
- Mortimer, N., Campbell, H.J., Tulloch, A.J., King, P.R., Stagpoole, V.M., Wood, R.A., Rattenbury, M.S., Sutherland, R., Adams, C.J., Collot, J. & Seton, M. (2017). Zealandia: Earth’s hidden continent. GSAT Today, 27: 27–35.
- Nyati, S., Beck, A. & Honegger, R. (2007). Fine structure and phylogeny of green algal photobionts in the microfilamentous genus Psoroglaena (Verrucariaceae, lichen-forming Ascomycetes). Plant Biology, 9: 390–399.
- Rindi, F. (2010). Reproduction and life history of the green alga Prasiola linearis Jao (Trebouxiophyceae, Chlorophyta). Botanica Marina, 53: 1–7.
- Rindi, F. & Guiry, M.D. (2004). Composition and spatial variability of terrestrial algal assemblages occurring at the bases of urban walls in Europe. Phycologia, 43: 225–235.
- Rindi, F., Guiry, M.D., Barbiero, R.P. & Cinelli F. (1999). The marine and terrestrial Prasiolales (Chlorophyta) of Galway City, Ireland: a morphological and ecological study. Journal of Phycology, 35: 469–482.
- Rindi, F., McIvor, L. & Guiry, M.D. (2004). The Prasiolales (Chlorophyta) of Atlantic Europe: an assessment based on morphological, molecular and ecological data, including the characterization of Rosenvingiella radicans (Kützing) comb. nov. Journal of Phycology, 40: 977–997.
- Rindi, F., McIvor, L., Sherwood, A., Friedl, T. & Guiry, M. (2007). Molecular phylogeny of the green algal order Prasiolales (Trebouxiophyceae, Chlorophyta). Journal of Phycology, 43: 811–822.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Sherwood, A.R., Garbary, D.J. & Sheath, R.G. (2000). Assessing the phylogenetic position of the Prasiolales (Chlorophyta). using rbcL and 18S rRNA gene sequence data. Phycologia, 39: 139–146.
- Sutherland, J., Miyata, M., Ishikawa, M. & Nelson, W. (2016). Prasiola (Prasiolales, Trebouxiophyceae) in Japan: a survey of freshwater populations and new records of marine taxa. Phycological Research, 64: 110–117.
- Thiers, B. (2017). [Continuously updated] Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Available at: http://sweetgum.nybg.org/ih/
- Thüs, H., Muggia, L., Pérez-Ortega, S., Favero-Longo, S.E., Joneson, S., O’Brien, H., Nelsen, M.P., Duque-Thüs, R., Grube, M., Friedl, T., Brodie, J.A., Andrew, C.J., Lücking, R., Lutzoni, F. & Gueidan, C. (2011). Revisiting photobiont diversity in the lichen family Verrucariaceae (Ascomycota). European Journal of Phycology, 46: 399–415.
