ABSTRACT
The two-celled colonial synurophyte genus Chrysodidymus was originally distinguished from its close relative Synura on the basis of the colonies always being of two cells, the shape of the cells and the odd swimming nature of the colony. Recently, based on gene sequence data, Chrysodidymus was found to be deeply nested within the Synura clade. As a result Chrysodidymus was placed in synonymy with Synura and a new combination, S. synuroidea, was made for the basionym C. synuroideus. Based on our observations and findings of two-celled colonies originally described under the genus Chrysodidymus, we propose descriptions of two new species Synura papillosa and Synura prowsei and an emended description for Synura synuroidea. Scales of S. synuroidea are significantly smaller than those of the two proposed species and possess an exceptionally wide posterior rim that is perforated with large holes of uneven diameters. Scales of the new species are significantly larger with narrow posterior rims that lack holes. Scales of S. prowsei possess anterior ribs that connect the ends of the posterior rim to the base of the spine and lack surface papillae, whereas scales of S. papillosa have surface papillae, but lack the anterior ribs. In addition, the base plate pore patterns are distinctive for each species. We further designate a neotype for S. synuroidea since the original holotype has been lost. All three species favour dilute, shallow, highly acidic water bodies often with highly coloured dissolved organic matter (CDOM).
Introduction
The two-celled colonial genus Chrysodidymus was originally described from an acidic swamp locality in Malaya, today part of Malaysia (Prowse, Citation1962). Prowse (Citation1962) distinguished the genus from its close relative Synura on the basis of the colonies always being composed of two cells, the shape of the cells and the swimming nature of the colony. Prowse (Citation1962) noted that the posterior ends of the cells that attach the two cells within the colony were wider than the anterior flagellated end, a pattern opposite to that observed in Synura. He further detailed the odd swimming style of the colony as ‘forward and backwards in a straight line’ more or less resembling a tug-of-war match, but distinctly different from the rolling and tumbling pattern observed in Synura. The back and forth oscillating movement, along the longitudinal axes of the colony, has also been observed by others including Nicholls & Gerrath (Citation1985), Graham et al. (Citation1993) and Pusztai et al. (Citation2016). Details of the two flagella, parietal plastids and covering of scales were similar to those of Synura. Although not discussed by Prowse, he does illustrate what is likely a basal contractile vacuole similar to what is also observed in Synura (Prowse, Citation1962, Plate IV, figs m–n).
Prowse (Citation1962) further described two species of Chrysodidymus, C. synuroideus and C. gracilis, differing in the size and shape of the cells. Cells of C. synuroideus, the type for the genus, were distinctly trapezoid-shaped with a wider posterior end, as compared with the longer and narrow cells described for C. gracilis. Unfortunately, Prowse did not comment on the nature of the siliceous scales and no drawings of these structures are given. His illustrations do note the forward projecting nature of the spines on the cell. It is also of interest that the type locality was apparently the same acid swamp for both species, and the holotypes were deposited by Prowse (Citation1962) at the Tropical Fish Culture Research Institute in Malacca. This institution is no longer in existence, and materials are believed to have been moved to the University of Hawaii in 1972. Unfortunately, the types for the two Chrysodidymus species have not been located and are apparently no longer available (Alison Sherwood, personal communication).
Based on observations of living collections made from localities in Michigan and Minnesota, Wujek & Wee (Citation1983) noted that cells within colonies were polymorphic, often changing shape from long and narrow to shorter and more spherical. The sizes and shapes of cells observed by Wujek & Wee (Citation1983) were similar to those reported by Prowse (Citation1962) for both C. synuroideus and C. gracilis, prompting these authors to synonymize the two species as C. synuroideus on the basis of priority. Wujek & Wee (Citation1983) further synonymized Synura microcrepis Nygaard (Nygaard, Citation1978) with C. synuroideus. Graham et al. (Citation1993) reported that in culture cells of recently formed colonies of Chrysodidymus were globose or spherical, but as colonies aged the cells become more elongated and vase-like, with the posterior portion being distinctly wider than the flagellated end. These observations supported the conclusions made by Wujek & Wee (Citation1983), and all reports of Chrysodidymus made since have been as C. synuroideus.
On the basis of a multi-gene study including SSU rDNA, LSU rDNA and rbcL sequences, Pusztai et al. (Citation2016) clearly demonstrated that a strain of C. synuroideus nested deep within the Synura clade, closest to Synura sphagnicola. As a result, they placed Chrysodidymus in synonymy with the genus Synura and proposed the new combination, Synura synuroidea (Prowse) Pusztai, Čertnerová, Škaloudová & Škaloud.
In our study, we examined 78 collections of two-celled colonies of Chrysodidymus (now Synura) over broad geographic regions of North America, Europe and Southeast Asia. We found consistent differences in the ultrastructure of the siliceous scales that imply at least three distinct species. The objectives of this paper are to describe two new species of Synura, and establish a neotype for S. synuroidea.
Materials and methods
Chrysodidymus populations examined as part of this study included 51 localities from North America, 25 from Vietnam and two from Europe (). The 51 North American localities were part of a larger survey of synurophytes that included 264 water bodies in nine regions spanning the east coast of the continent from Florida to Newfoundland (Siver & Lott, Citation2012). The North American observations were made prior to the discovery that Chrysodidymus belonged in Synura, and before specimens were separated according to scale type. We had photographic records and sufficient material to further study 31 of the 51 collections in order to identify and separate specimens into the three taxa. One of the European sites is in the Ukraine and the other one represents a culture isolated from a site in Scotland (Pusztai et al., Citation2016). The 25 Vietnam localities were part of a hydrobiological survey of lakes and reservoirs under the project Ecolan 3.2 of the Russian-Vietnam Tropical Centre and the joint VAST-RFBR project on synurophyte diversity in the tropics. These studies were conducted in the central and southern parts of the country in 2008–2015.
Table 1. Locations and ecological characteristics of sites harbouring Synura synuroidea (n=18), S. papillosa (n=33) or S. prowsei (n=6) used in this study.
A sediment core and a phytoplankton sample taken at the time of collection were examined for Chrysodidymus remains in each of the North American water bodies. Sediment cores were taken from the deep basin of each site with a Glew gravity corer (Glew, Citation1988) and sectioned into 1 cm units using a mechanical extruder (Glew, Citation1989). The 0–1 cm surface section of each core was used in this study. A 10 µm mesh net was used to retrieve a phytoplankton sample from each North American site, and used in conjunction with the surface sediment sample to both establish the presence of Chrysodidymus and image all scale types. Samples from the Vietnam and Ukraine sites were collected from the surface water layer using a 20 μm mesh plankton net and fixed with Lugol’s solution. For the North American sites, the pH was measured with a Fisher Acument 640-A pH meter, and water temperature and specific conductance with a Hydrolab DataSonde 4A. Water colour, largely reflecting the concentration of coloured dissolved humic material (CDOM), was estimated using the platinum-cobalt method (APHA, Citation1985). Additional physical and chemical data for each North American site is available at http://silicasecchidisk.conncoll.edu. For the Vietnam and Ukraine sites, specific conductance, pH and temperature measurements were performed using Hanna Combo (HI 98129 and HI 9828) devices.
For the North American samples, approximately 0.5–1.0 g of sediment from the 0–1 cm section of each core was oxidized with a sulphuric acid-potassium dichromate solution according to Marsicano & Siver (Citation1993) and washed with distilled water. Aliquots of each sediment slurry were dried onto aluminium foil. Aliquots of each phytoplankton sample were also dried directly onto aluminium foil strips immediately after collection. The aluminium foil samples were trimmed, attached onto aluminium stubs using Apiezon wax, coated with a mixture of gold and palladium with a Polaron model E5 100 sputter coater and observed with a Leo (Zeiss) 982 FESEM, a Leo 435V SEM or an FEI Nova FESEM. For the Vietnam and Ukraine samples, an aliquot of each plankton sample was initially washed by repeated centrifugation in deionized water to remove the Lugol’s fixative. Drops of the washed sample were used directly, or digested 4–5 minutes in sulphuric acid with potassium dichromate and washed a second time. Samples were placed on aluminium stubs, coated with gold for 10 min, and observed with either a JEOL 6510 LV or a LEO-1420 SEM, or dried onto formvar-coated grids (EMS FF200-Cu-50, Electron Microscopy Sciences) and observed with a JEM-1011 TEM.
Results
Taxonomy
Based on our observations and findings of two-celled colonies originally identified under the genus Chrysodidymus, we propose an emended description for Synura synuroidea and descriptions of two new species, Synura papillosa and Synura prowsei. Our observations of colony and cell shape of S. synuroidea agree with those recently published by Pusztai et al. (Citation2016). Here we provide additional observations on the ultrastructure of the siliceous scales, and propose a neotype for S. synuroidea.
Synura synuroidea (Prowse) Pusztai, Čertnerová, Škaloudová & Škaloud (–)
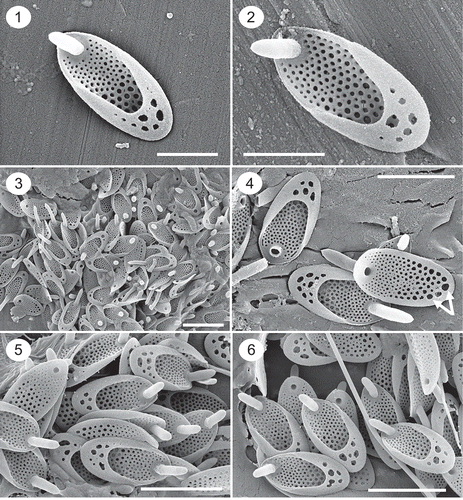
Figs 1–6. SEM images of Synura synuroidea. Figs 1–2. Individual scales illustrating the large posterior rim bearing holes, details of the base plate pores and forward projecting spine. Figs 3–6. Groups of scales from individual cells depicting different numbers and sizes of holes penetrating the posterior rim, and lengths of spines. The undersurface of the scale in Fig. 4 illustrates the differences in the sizes of the base plate pores, and the posterior-most row of large pores situated immediately below the posterior rim (white arrows). Scale: 1 µm (Figs 1–2) and 2 µm (Figs 3–6).
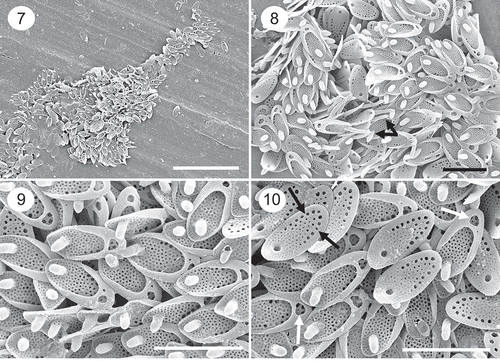
Figs 7–10. SEM images of a population of Synura synuroidea from Orchid Bog, Newfoundland, Canada, where cells of the two-celled colonies were club-shaped and attached by the tapered ends. Fig. 7. Remains of a whole cell showing the club-shape morphology. Figs 8–10. Groups of scales depicting the large posterior rim, base plate pores and forward projecting spine. Scales with longer spines representing the anterior-most portion of the cell are depicted in Fig. 8 (black arrows). The majority of scales on cells in this population have a single large hole in the posterior rim (Fig. 10, white arrows). The row of large base plate pores aligning the posterior marginal are illustrated in Fig. 10 (black arrows). Scale: 2 µm (Figs 8–10) and 10 µm (Fig. 7).
Basionym: Chrysodidymus synuroideus Prowse Citation1962. In: Further Malayan freshwater flagellata, Garden Bull., Singapore, 19(1): 128–129 (Plate IV, Figure n). Type locality: Malaya, Malacca, acid swamps.
Synonym: Synura microcrepis Nygaard Citation1978.
Description
Colonies consisting of two cells attached by their bases at 180°. The shape of cells is highly variable, displays a high degree of phenotypic plasticity, and commonly ranges from elongate to ovoid, trapezoid or pyriform. The basal width of the cells at the point of attachment is equal to or wider than that of the distal end. Scales are elongate-oval in shape, range in size from 1.6–2.8 × 0.7–1.6 µm with a mean of 2.1 × 1.0 µm, and possess a posterior rim and anterior spine. The posterior rim encircles two-thirds to three-quarters of the scale and forms a broad hood that extends over approximately one-third of the scale (, , –). The basal portion of the hood is perforated with one to ten or more holes of variable diameters. Spines are cylindrical in shape, range in length from 0.45 to 1.3 µm, and bend slightly forward extending a short distance beyond the base plate (–). The diameter of the spine is remarkably similar over most of its length, terminating in a blunt, sometimes pointed, apex. The majority of scales possess short spines about one-quarter to one-third the length of the scale, however spines on the anterior end of the cell are often longer (, black arrows). Base plate pores cover the scale, but are often lacking along the very anterior end distal to the spine perforation (). There is a large difference in diameter of the base plate pores. Pores on the anterior one-third to one-half of the scale are small, increase in diameter in the posterior half of the scale, and with the largest pores situated under the rim often forming a single row along the margin (, white arrows; , black arrows). The diameters of the pores situated under the rim are usually 5–10 times as wide as those around the base of the spine. A uniform secondary layer encircles each base plate pore (–).
Neotype: Portion of a single gathering of cells from strain CAUP B712 on SEM stub #NEOTYPE-B712 deposited at the Culture Collection of Algae of the Charles University in Prague (CAUP). Strain S95.E4 was isolated from a small unnamed lake near Loch Garten in the Grampian Mountains, Scotland (57°13′32.55′′N, 3°43′20.71′′W), and is deposited in the Culture Collection of Algae of Charles University, Prague, Czech Republic, CAUP B712. Original reference: Pusztai et al. (Citation2016). Cryptogamie, Algologie 37: 297–307.
Neotype locality: Strain S95.E4 was isolated from a small unnamed lake near Loch Garten in the Grampian Mountains, Scotland (57°13′32.55′′N, 3°43′20.71′′W).
Additional information
We observed cells from living populations (not cultures) with variable shapes ranging from spherical, elongated, oval, trapezoid to pyriform, similar to findings by Graham et al. (Citation1993) and Pusztai et al. (Citation2016). In the vast majority of populations studied, the posterior ends of the cell are wider than the anterior end. However, we did observe one population in a small bog pond (Orchid Bog) in Newfoundland, Canada, where the cells were club-shaped and attached by the tapered ends as is common among species of Synura (–). To our knowledge, this morphology has not been reported for Synura synuroidea, and it differs from all previous findings where the basal portion of the cell is wider than the distal flagellated end. Scales from these club-shaped cells were similar in size, shape and morphology to those from all other populations of S. synuroidea, including the row of large base plate pores aligning the posterior margin (, black arrows). However, most scales had a single large hole in the posterior rim (, , , white arrows), instead of a collection of holes.
Synura papillosa Kapustin, Gusev & Siver, sp. nov. (–)
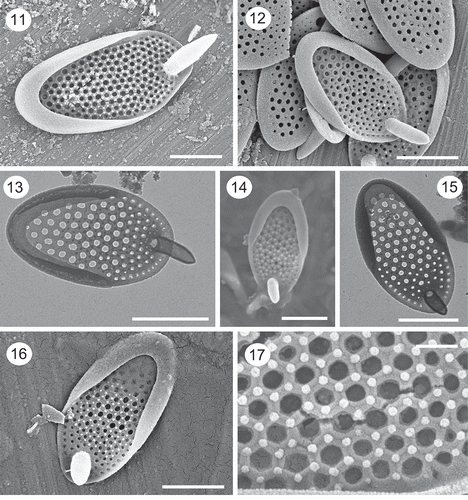
Figs 11–17. SEM and TEM images of Synura papillosa. Images of isolated scales observed with SEM (Figs 11, 14, 16) and TEM (Figs 13, 15) depict the shallow posterior rim, large base plate pores, the stout anterior spines and the arrangement of surface papillae. Figs 14, 16, 17. Note the hexagonal-shaped thickenings, and corresponding surface papillae, surrounding the base plate pores. Figs 13–15 are from the holotype specimen. Scale: 200 nm (Fig. 17), 1 µm (Figs 11–16).
Description
Colonies consisting of two cells attached by their bases at 180°. The shape of cells is highly variable, ranging from elongate to ovoid, trapezoid or pyriform. The basal width of the cells at the point of attachment is equal to or wider than that of the distal end. Scales are elongate-oval in shape, range in size from 2.2–3.4 × 1.2–1.8 with a mean of 2.6 × 1.4 µm, and possess a posterior rim and anterior spine (–). The posterior rim encircles two-thirds to three-quarters of the scale, extends a short distance over the base plate, and lacks perforations. Spines are cylindrical in shape, range in length from 0.41 to 1.2 µm, bend slightly forward extending a short distance beyond the base plate (–), and are larger on the apical-most portion of the cell. The diameter of the spine is similar over most of its length, terminating in a blunt, sometimes pointed, apex. Base plate pores over most of the scale are large, evenly spaced and of similar diameter (–). The diameter of the base plate pores decreases slightly on the anterior end of the scale (, ). The base plate is thickened around each pore, often forming a pentagonal or hexagonal pattern (, ). Surface papillae are present, positioned at the corners of the polygon patterns ().
holotype: Portion of a single gathering of cells on SEM stub #DL2/3 deposited at the Herbarium of the I.D. Papanin Institute for Biology of Inland Waters RAS, Borok (IBIW). Material is from a phytoplankton sample collected by E.S. Gusev on 10 March 2014. – are representative scales from the specimen.
Type locality: Dak Lua swamp, situated at 11°30′43′′N, 107°22′56′′E in Cat Tien National Park, Dong Nai Province, Vietnam.
Etymology: The name papillosa reflects the presence of surface papillae on the scale surface.
Synura prowsei Siver, Kapustin & Gusev, sp. nov. (–)
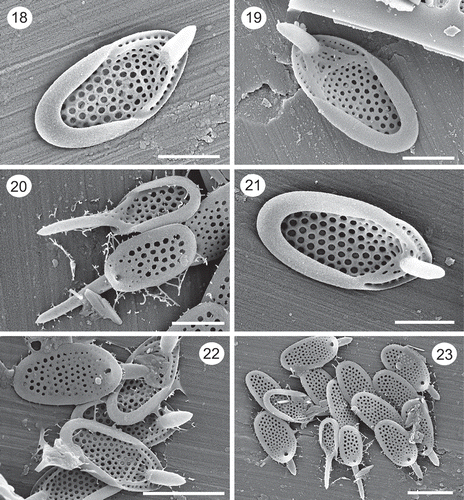
Figs 18–23. SEM images of Synura prowsei. Figs 18–19, 21. Note the shallow posterior rim lacking holes, large and reinforced base plate pores, the stout nature of the forward projecting spines, and the ribs connecting the ends of the posterior rim with the base of the spine. Fig. 20. Two scales with long spines from the anterior portion of the scale (also shown in Fig. 23). Note the large size of the base plate pores on the undersurface of the lower scale. Figs 22–23. Groups of scales. Note the smaller size of the two anterior scales with longer spines (Fig. 23). Figs 20, 22 and 23 are from the holotype specimen. Scale: 1 µm (Figs 18–21) and 2 µm (Figs 22–23).
Description
Colonies consisting of two cells attached by their bases at 180°. The shape of cells is highly variable, ranging from elongate to trapezoid to pyriform, and where the width of the cells at the point of attachment is equal to or wider than that of the distal end. Scales are elongate-oval in shape, range in size from 1.9–3.3 × 1.1–1.7 with a mean of 2.8 × 1.5 µm, and possess a posterior rim and anterior spine (, , ). The posterior rim is narrow, encircles approximately three-quarters of the scale margin, and lacks perforations. Spines are cylindrical in shape, range in length from 0.74 to 1.70 µm, with a mean of 1.09 µm, and bend slightly forward extending a short distance beyond the base plate (, , , ). The diameter of the spine is similar over most of its length, terminating in a blunt, sometimes pointed, apex. Apical-most scales are smaller and with longer spines that can be as long as the base plate (). Base plate pores over most of the scale are large, evenly spaced, and of similar diameter (–). The diameter of the base plate pores decreases slightly on the anterior end of the scale. The base plate is thickened around each pore, often forming a pentagonal or hexagonal pattern (, ). Thick anterior submarginal ribs originate at the ends of the posterior rim and connect to the base of the spine (–). Additional thickenings often cross the base plate and connect the anterior submarginal ribs (, ). A single row of base plate pores is found between the anterior submarginal ribs and the scale margin (, , ). Surface papillae are lacking.
Holotype: Portion of a single gathering of cells on SEM stub deposited at the Canadian Museum of Nature, CANA (127506). Material is from a phytoplankton sample collected by P.A. Siver on 7 June 2001. , and are representative scales from the specimen.
Type locality: White Lake, a shallow Carolina Bay water body situated at 35°39′18′′N, 76°30′20′′W in the Bladen Lakes State Forest, North Carolina, USA. The pond is located along Route 701 in the Town of White Lake.
Etymology: Synura prowsei is in honour of G.A. Prowse who originally described the genus Chrysodidymus.
Ecological and distribution findings
Siver & Lott (Citation2012) examined scaled chrysophyte remains in 262 water bodies along the east coast of North America, ranging from Florida to Newfoundland. A total of 51 of those sites were found to have populations of Chrysodidymus that at the time were all included under the epithet, C. synuroideus. Chrysodidymus populations were found in each of the major regions examined by Siver & Lott (Citation2012), were most common in water bodies from the Pine Barrens of southern New Jersey, coastal Maine and Newfoundland, and rarest in localities in Connecticut and on Cape Cod. In fact, populations were found in all of the water bodies examined from the New Jersey Pine Barrens.
As noted above, we had sufficient material from 31 of the 51 North American sites to further study and determine which species were present (). Synura synuroidea was the most common species, found in 17 of the localities, while S. prowsei and S. papillosa were present in six and eight sites, respectively. Interestingly, none of these collections contained more than one of the three species. Populations of S. synuroidea and S. papillosa were widespread, found from Florida to Newfoundland. In contrast, populations of S. prowsei represented a more southern distribution being found only in the three warmest regions, Ocala (Florida), coastal North Carolina and the New Jersey Pine Barrens ().
All of the 31 North American localities, representing all three species, were low in dissolved salt content with specific conductance values ranging from 20 to 176 µS cm−1 (). All but two sites had specific conductance values less than 74 µS cm−1, and 21 of the localities had values below 50 µS cm−1. The two sites with the highest specific conductance, Debbie’s Pond and Moon Lake, are situated close to the ocean and are directly influenced by sea spray that results in elevated NaCl concentrations. All of the localities harbouring the three species were acidic with pH values ranging from 3.9 to 6.7, and 50% of the sites below pH 5. Synura prowsei had the smallest and more acidic, range in pH, from 4.1 to 5.8, relative to the other two taxa. Even though seven of the sites containing these species were clearwater localities low in CDOM, 66% had water colour values >20 Pt-Co units and half of the localities were darkly stained with values above 50 Pt-Co units (). All three species were found in water bodies with low as well as high dissolved humic matter.
In addition to the ecological records from North America, we also had similar data from 23 sites in Vietnam for Synura papillosa (). In general, at the Vietnam sites, this species was found over a broader pH gradient ranging from 4.6–8.0. Still, 12 of the 23 sites had pH ≤6. Except for three sites, the distribution of S. papillosa along a specific conductance gradient in the Vietnam sites was similar to that observed in North America where 20 sites had a range from 10–117 µS cm−1 (). Isolated scales of S. papillosa were also found in two mangrove swamps with high specific conductance values of 1240 and 3000 µS cm−1. At the time of collection, these two sites had a freshwater layer on top of a more saline layer. It is also possible that S. papillosa grew during the rainy season or were possibly washed in from a neighbouring locality.
Based on a review of the literature, we found 29 records of Chrysodidymus synuroideus that represent either S. synuroidea (n=12) or S. papillosa (n=17) (). The literature records for S. synuroidea represent sites primarily in North America, Europe and Greenland. Records of S. papillosa are more widespread, including sites from North and South America, Europe, Asia and New Zealand.
Table 2. Previous literature records based on EM of Chrysodidymus synuroideus that can be assigned to either Synura synuroidea or S. papillosa. See text for details.
Discussion
Our findings confirm that there are at least three species of Synura with two-celled colonies, suggesting that previous records of Chrysodidymus synuroideus probably represent more than one taxon (). The differences in scale ultrastructure between the three taxa are distinct and were observed consistently across all populations examined in this study. Details of the posterior rim, the pore pattern on the base plate, the presence of surface papillae, and presence of anterior ribs serve to easily separate the three species. The posterior rim on S. synuroidea is significantly wider and bears one or more large holes compared with the posterior rims on S. prowsei and S. papillosa scales. The degree to which the posterior rim overlaps the base plate on S. synuroidea scales is often twice that of S. prowsei and S. papillosa, and the posterior rims on the latter two species lack holes. The difference in the diameters of the base plate pores is greatest on S. synuroidea scales and more similar on S. prowsei and S. papillosa. On S. synuroidea, the base plate pores are large in the posterior region of the scale, especially under the rim, and become very small on the distal end near the spine. In contrast, the base plate pores on both S. prowsei and S. papillosa scales are large and of similar diameter over most of the scale, reducing in size only around the base of the spine. Scales of S. papillosa possess surface papillae that often match the thickened pentagonal or hexagonal pattern around each base plate pore, and lack anterior ribs connecting the posterior rim to the base of the spine. In contrast, surface papillae are lacking on S. prowsei scales, but these scales have thick secondary ribs connecting the posterior rim to the base of the spine. Although scales of S. prowsei and S. papillosa are similar in size, those of S. synuroidea are significantly smaller. The differences observed in the scales between the three species are of similar magnitude and character used to describe and distinguish other Synura species (Nicholls & Gerrath, Citation1985; Siver, Citation1987; Kristiansen & Preisig, Citation2007).
As noted by previous workers (e.g. Graham et al., Citation1993; Pusztai et al., Citation2016) and further documented in our study, cell shape is highly polymorphic within these two-celled Synura taxa making this character not useful for distinguishing between species which was the primary reason for Wujek & Wee (Citation1983) proposing synonymy of C. synuroideus and C. gracilis. Interestingly, these authors noted scales that had posterior rims with and without holes (perforations). Indeed, the TEM images of scales illustrated in Wujek & Wee (Citation1983) represent both S. synuroidea and S. papillosa. In our opinion, the scales in Wujek & Wee (Citation1983, figs 3, 4) clearly have posterior rims and base plate pore patterns that represent S. synuroidea and S. papillosa, respectively. Based on our findings, these scale types are never found on the same cell and clearly represent two distinct species.
These findings beg the question as to whether Prowse (Citation1962) did indeed observe two distinct species when he originally described Chrysodidymus. Unfortunately, although Prowse (Citation1962) mentioned the covering of siliceous scales, he did not discuss or illustrate them, so we do not know if his observations represent one or more species. Further, we do not know which scale type really represents the type, C. synuroideus. It could be any of the three species discussed in this paper, or possibly a different one altogether. The culture used by Pusztai et al. (Citation2016) to establish that Chrysodidymus belongs in Synura clearly represents S. synuroidea as discussed in our paper, and further represents the only strain to date with detailed molecular data. Given that we will never know what species Prowse (Citation1962) originally observed, coupled with the fact that his types have been lost, we designated the neotype for S. synuroidea based on the culture used by Pusztai et al. (Citation2016) and originally isolated from a lake in Scotland as noted above.
One population of Synura synuroidea reported from Orchid Bog, Newfoundland, Canada, possessed two traits that are not common for this species. First, cells within the two-celled colony were attached by their tapered ends, meaning that the cells were wider at the anterior ends. Second, cells of this population had scales with a single large hole in the posterior rim, instead of multiple holes. Although this combination of characters has only been observed on cells from the Orchid Bog site, it is interesting that this site is also the type locality for three new species of Mallomonas and Synura (Siver & Lott, Citation2016, Citation2017). Perhaps this form of S. synuroidea will also prove to be a separate and new species.
All three of the species clearly favour habitats that are dilute and acidic, in agreement with previous findings for Chrysodidymus (Siver, Citation1987; Charles & Smol, Citation1988). That said, S. papillosa was found over a wider pH gradient than the other two species, while S. prowsei was reported only from very acidic localities. Although each species was recorded from a relatively clear-water site, the vast majority of records were from localities with elevated CDOM concentrations. Based on our findings, S. synuroidea and S. papillosa were widespread and found in warm climates as well as in temperate and more northern localities. However, S. papillosa is the most widely distributed of the three taxa, and the only one found in both hemispheres. To date, S. synuroidea is also widely distributed in, but restricted to, the northern hemisphere. In contrast, S. prowsei was restricted to the warmer localities along the Atlantic Coastal Plain in North America. As more records are added for the three species, differences in habitat preferences may emerge that would improve their use as bioindicators (Siver, Citation2015).
In summary, previous records for the genus Chrysodidymus represent at least three different species, all of which represent two-celled colonies where the cells attach and align at 180°, but possess very different scale types. The differences in scale morphology were consistently observed in collections examined from three continents, and are consistent with characteristics used to distinguish between species of Synura.
Author contributions
All authors contributed equally to the findings presented in this paper.
Acknowledgements
We thank Anne Marie Lizarralde for assistance in fieldwork and sample preparation, and James Romanow, Xuanhao Sun and Marie Cantino for assistance with use of SEM facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- American Public Health Association (APHA). (1985). Standard methods. 20th ed. American Water Works Association, Water Pollution Control Federation, Washington, DC.
- Carty, S. & Wujek, D.E. (2003). A new species of Peridinium and new records of dinoflagellates and silica-scaled chrysophytes from Belize. Caribbean Journal of Science, 39: 136–139.
- Charles, D.F. & Smol, J.P. (1988). New methods for using diatoms and chrysophytes to reconstruct past lakewater pH. Limnology and Oceanography, 33: 1451–1462.
- Couté, A. & Franceschini, I.M. (1998). Scale-bearing chrysophytes from acid waters of Florianopolis, Santa Caterina Island, South Brazil. Algological Studies, 88: 37–61.
- Croome, R. & Tyler, P. (1988). Further observations of silica-scaled Chrysophyceae (Paraphysomonadaceae and Mallomonadaceae) from Australian freshwaters. Nova Hedwigia, 46: 481–489.
- Dürrschmidt, M. (1982). Studies on the Chrysophyceae from South Chilean inland waters by means of scanning and transmission electron microscopy. II. Archiv für Hydrobiologie Supplementband (Algological Studies), 63: 121–163.
- Dürrschmidt, M. & Croome, R. (1985). Mallomonadaceae (Chrysophyceae) from Malaysia and Australia. Nordic Journal of Botany, 5: 285–298.
- Glew, J.R. (1988). A new trigger mechanism for sediment samplers. Journal of Paleolimnology, 2: 241–243.
- Glew, J.R. (1989). A portable extruding device for close interval sectioning of unconsolidated core samples. Journal of Paleolimnology, 1: 235–239.
- Graham, L.E., Graham, J.M. & Wujek, D.E. (1993). Ultrastructure of Chrysodidymus synuroideus (Synurophyceae). Journal of Phycology, 29: 330–341.
- Gusev, E.S. (2013). Studies on synurophycean algae from mangrove wetlands (Vietnam). Nova Hedwigia, Beiheft, 142: 87–95.
- Gusev, E.S. Doan-Nhu, H. & Nguyen-Ngoc, L. (2017). Silica-scaled chrysophytes from Cat Tien National Park (Dong Nai Province, Vietnam). Nova Hedwigia, 105: 347–364.
- Hansen, P. (1996). Silica-scaled Chrysophyceae and Synurophyceae from Madagascar. Archiv für Protistenkunde, 147: 145–172.
- Kapustin, D.A. & Gusev, E.S. (2016). Chrysodidymus Prowse (Chrysophyceae, Synurales), a new genus Chrysophyta for the Ukrainian algal flora. International Journal on Algae, 18: 105–110.
- Kristiansen, J. & Menezes, M. (1998). Silica-scaled chrysophytes from an Amazonian flood-plain lake, Mussurá Lake, northern Brazil. Algological Studies, 90: 97–118.
- Kristiansen, J. & Preisig, H.R. (2007). Chrysophyte and Haptophyte Algae, 2: Teil/Part 2: Synurophyceae. In Süsswasserflora von Mitteleuropa (Büdel, B., Gärtner, G., Krienitz, L., Preisig, H.R. & Schagerl, M., editors), vol. 1/2. Springer-Verlag, Berlin.
- Kristiansen, J. & Vigna, M.S. (2002). Chrysophyceae y Synurophyceae de Tierra del Fuego (Argentina). Monografias del Museo Argentino de Ciencias Naturales, 3: 1–45.
- Marsicano, L.J. & Siver, P.A. (1993). A paleolimnological assessment of lake acidification in five Connecticut lakes. Journal of Paleolimnology, 9: 209–221.
- Němcová, Y., Kreidlová, J., Kosová, A. & Neustupa, J. (2012). Lakes and pools of Aquitaine region (France) – a biodiversity hotspot of Synurales in Europe. Nova Hedwigia, 95: 1–24.
- Nicholls, K.H. & Gerrath, J.F. (1985). The taxonomy of Synura (Chrysophyceae) in Ontario with special reference to taste and odour in water supplies. Canadian Journal of Botany, 63: 1482–1493.
- Nygaard, G. (1978). Freshwater phytoplankton from Narssaq area, South Greenland. Botanisk Tidsskrift, 73: 191–238.
- Pichrtová, M. & Veselá, J. (2009). The silica-scaled chrysophytes of the Elbe Sandstone Region, Czech Republic. Fottea, 9: 101–106.
- Prowse, G.A. (1962). Further Malayan freshwater Flagellata. Gardens Bulletin (Singapore), 19: 105–146.
- Pusztai, M., Čertnerová, D., Škaloudová, M. & Škaloud P. (2016). Elucidating the phylogeny and taxonomic position of the genus Chrysodidymus Prowse (Chrysophyceae, Synurales). Cryptogamie, Algologie, 37: 297–307.
- Puytorac, P. de, Mignot J.P., Grain J., Groliere C.A., Bonnet L. & Couillard P. (1972). Premier relevé de certains de protozoaires libres sure le territoire de la station de biologie de l’Université de Montreal (Saint-Hippolyte, Comte de Terrebonne, Québec). Le Naturaliste Canadien, 99: 417–440.
- Roijackers, R.M.M. & Kessels, H. (1986). Ecological characteristics of scale-bearing Chrysophyceae from the Netherlands. Nordic Journal of Botany, 6: 373–385.
- Siver, P.A. (1987). The distribution and variation of Synura species (Chrysophyceae) in Connecticut, USA. Nordic Journal of Botany, 7: 107–116.
- Siver, P.A. (2015). The Synurophyceae. In Freshwater Algae of North America: Ecology and Classification (Wehr, J.D., Sheath, R.G. & Kociolek, J.P.), 605–650. Academic Press, San Diego, CA.
- Siver, P.A. & Lott, A.M. (2000). Preliminary investigations on the distribution of scaled chrysophytes in Vermont and New Hampshire (USA) lakes and their utility to infer lake water chemistry. Nordic Journal of Botany, 20: 233–246.
- Siver, P.A. & Lott A.M. (2012). Biogeographic patterns in scaled chrysophytes from the east coast of North America. Freshwater Biology, 57: 451–467.
- Siver P.A. & Lott A.M. (2016). Descriptions of two new species of Synurophyceae from a bog in Newfoundland, Canada: Mallomonas baskettii sp. nov. and Synura kristiansenii sp. nov. Nova Hedwigia, 102: 501–511.
- Siver P.A. & Lott A.M. (2017). The scaled chrysophyte flora in freshwater ponds and lakes from Newfoundland, Canada, and their relationship to environmental variables. Cryptogamie, Algologie, 38: 1–23.
- Takahashi, E. (1967). Studies on genera Mallomonas, Synura and other plankton in freshwater with the electron microscope. VI. Morphological and ecological observations on genus Synura in ponds and lakes in Yamagata Prefecture. Bulletin of the Yamagata University (Agricultural Science), 5: 99–118.
- Vigna, M.S. & Kristiansen, J. (1996). Biogeographical implications of new records of scale bearing chrysophytes from Tierra del Fuego, Argentina. Archiv für Protistenkunde, 147: 137–144.
- Vigna, M.S. & Munari, C. (2001). Seasonal occurrence of silica-scaled chrysophytes in a Buenos Aires lake (Argentina). Nova Hedwigia, Beiheft, 122: 195–209.
- Vigna, M.S. & Siver, P.A. (2003). Biodiversity and biogeographical implications of silica-scaled chrysophytes (Chrysophyceae and Synurophyceae) of the northeast wetlands of Argentina. Archiv für Hydrobiologie, 158: 359–372.
- Wei Y.-X., Yuan, X.-P., Kristiansen, J. (2014). Silica-scaled chrysophytes from Hainan, Guangdong Provinces and Hong Kong Special Administrative Region, China. Nordic Journal of Botany, 32: 881–896.
- Wujek, D.E. (2013). Silica-scaled chrysophytes (Chrysophyceae and Synurophyceae) from New Zealand freshwaters. II. Additions to the flora. Pacific Science, 67: 113–118.
- Wujek, D.E. & Dziedzic, R.M. (2005). Silica-scaled chrysophytes from Ecuador. Gayana Botanica, 62: 1–8.
- Wujek, D.E. & Wee, J.L. (1983). Chrysodidymus in the United States. Transactions of the American Microscopical Society, 102: 77–80.
