ABSTRACT
Cryptonemia specimens collected in Bermuda over the past two decades were analysed using gene sequences encoding the large subunit of the nuclear ribosomal DNA and the large subunit of RuBisCO as genetic markers to elucidate their phylogenetic positions. They were additionally subjected to morphological assessment and compared with historical collections from the islands. Six species are presently found in the flora including C. bermudensis comb. nov., based on Halymenia bermudensis, and the following five new species: C. abyssalis, C. antricola, C. atrocostalis, C. lacunicola and C. perparva. Of the eight species known in the western Atlantic flora prior to this study, none is found in Bermuda. Specimens reported in the islands in the 1900s attributed to C. crenulata and C. luxurians are representative of the new species, C. antricola and C. atrocostalis, respectively.
Introduction
At the time W.R. Taylor (Citation1960) published his flora of the western Atlantic tropical and subtropical waters, two species in the red algal genus Cryptonemia (Halymeniaceae) had been reported for Bermuda, and only three for the entire region. The earliest record of Cryptonemia in Bermuda was Rein’s (Citation1873) report of C. crenulata (J. Agardh) J. Agardh, a species later listed in the flora by Collins & Hervey (Citation1917) and Howe (Citation1918). The other species reported in these early 20th century floras was C. luxurians (C. Agardh) J. Agardh which had been discovered earlier in Bermuda and misidentified as the delesseriaceous Botryoglossum platycarpum (Turner) Kützing by Kemp (Citation1857). Over the past two decades, we have made numerous collections of Cryptonemia in Bermuda which form the basis of this report. At present, there are 45 Cryptonemia species listed as ‘current’ in AlgaeBase (Guiry & Guiry, Citation2017), only eight of which are found in the western Atlantic (Wynne, Citation2017). In the last 15 years little molecular work has been done on the genus, consisting of a few sequences from around the world (Gavio & Fredericq, Citation2002; Kim et al., Citation2012; Calderon et al., Citation2014; D’Archino et al., Citation2014; Yang & Kim, Citation2014). Using molecular barcoding and phylogenetics, we have identified six species in the genus for Bermuda, including five new to science, and have investigated the early records of Cryptonemia from the last century.
Materials and methods
Sample collection and processing
Deepwater collections were made on the 2016 cruise of the R/V Baseline Explorer by technical divers and the other samples were collected by scuba diving or snorkelling. Voucher specimens are deposited in KIRI, MICH, NY, the Bermuda Natural History Museum, UNB, US and CWS’s personal herbarium. Herbarium abbreviations follow the online Index Herbariorum (http://sweetgum.nybg.org/ih/) and standard author initials of taxa follow Brummitt & Powell (Citation1992). Site locations for all collections were taken using a Garmin™ eTrex H GPS (Olathe, Kansas, USA).
Specimens were pressed fresh on herbarium paper and many were photographed live. Fragments or entire individuals were dried in silica gel for DNA extraction and, when possible, additional fragments were preserved in 4–5% formalin in filtered seawater for anatomical study. Thin sections were made with an American Optical freezing microtome model 880 (San Diego, California, USA). The sections were mounted in 30% corn syrup with acidified 1% aniline blue in a ratio of 20:1 with a few drops of formalin as a medium preservative. Herbarium specimens were scanned on an HP 309a Photosmart Premium scanner (Hewlett-Packard Company, Palo Alto, California, USA), and photomicrographs were taken using a Zeiss Axioskop 40 microscope (Oberkochen, Germany) equipped with a model 11.2 Spot InSight 2 digital camera (Diagnostic Instruments, Sterling Heights, Michigan, USA). The digital images were composed in Adobe Photoshop™CS6 v. 13.0.1 (Adobe Systems, San Jose, California, USA). Unless otherwise mentioned, the Phycotheca Boreali-Americana (P.B.-A.) exsiccata referred to represents the set belonging to the first author. Data for some species were collected from archived specimens and labels digitized on the online Macroalgal Herbarium Portal (http://macroalgae.org/portal/index.php).
Molecular methods
Specimens used in molecular analyses are recorded in . After collection, specimens were silica-dried and total DNA was extracted following Saunders & McDevit (Citation2012). For the assignment of specimens to genetic groups, data for the rbcL-3P (3’ end of the plastid ribulose-1,5-bisphosphate carboxylase large subunit) and in some cases the UPA (universal plastid amplicon: Sherwood & Presting (Citation2007), as a secondary marker) were amplified as outlined in Saunders & Moore (Citation2013). These data were aligned in Geneious v.10.1.3 (http://www.geneious.com; Kearse et al., Citation2012) with the former subjected to barcode gap analyses by calculating the uncorrected distances between specimens (n=33 (including 3P region for specimens for which the longer rbcL fragment was determined; ); 800 bp (Milton et al., Citation2013)). For phylogenetic analyses, partial LSU (large subunit of the nuclear ribosomal cistron – owing to a conflict for LSU and rbcL for one specimen, additional LSU data were generated for other specimens of the C. bermudensis/C. lacunicola complex; ) and a longer region of the rbcL was amplified for each of the genetic groups identified previously (Saunders & Moore, Citation2013). Sequence data were generated with the Big Dye Terminator Cycle Sequencing Ready Reactions BDA sequencing kit (Applied Biosystems, Foster City, California, USA) and bidirectional reads assembled (excluding the 5’ and 3’ primer regions) using Geneious for all markers with additional data sourced from GenBank (). Individual LSU (n=20; 2796 bp) and rbcL (n=38; 1269 bp) alignments, as well as a concatenated (n=13; 4064 bp) alignment, were generated for phylogenetic analyses.
Table 1. Collection details for isolates included in the molecular analyses of this study with newly generated GenBank accession numbers in bold type.
For phylogenetic analyses, the best models for the individual gene regions LSU rDNA, and rbcL were first estimated (AIC) in Modeltest (v 3.06; Posada & Crandall, Citation1998) as implemented in PAUP* (Swofford, Citation2003) through Geneious. Bayesian Inference analysis was performed using MrBayes v3.2.2 (Ronquist et al. Citation2012). Two Metropolis-coupled Markov chain Monte Carlo (MCMCMC) runs consisting of one cold chain and three hot chains were performed with each run being sampled every 100 generations for 1 010 000 generations. After confirming that the average standard deviation of split frequencies between runs was below 0.01, the trees were merged. The resulting tree and posterior probabilities were calculated from the 20 202 trees generated. Maximum likelihood phylogenetic analysis was conducted under the GTR+I+G model using the raxmlGUI (Silvestro & Michalak, Citation2011). Node support was calculated using 1000 replicates of bootstrap resampling.
Results
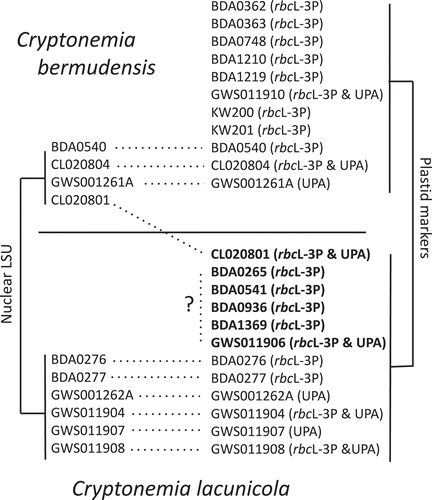
Our rbcL-3P gap analyses have revealed seven genetic groups for Cryptonemia in Bermuda (). These are assigned in the taxonomic section below to C. abyssalis, C. antricola, C. atrocostalis, C. bermudensis, C. lancunicola, C. perparva and C. sp. 1_Bda. There was almost no within-group genetic variation, with only C. antricola having a single variable site (). In contrast, between-group genetic variation was typically high, with the exception of C. bermudensis and C. lacunicola having only four fixed differences () which nonetheless displayed a clear barcode gap (). Data generated for UPA added specimens to some of these previous groups, and were consistent with rbcL-3P when data were available for both markers, but no additional genetic species were uncovered.
Table 2. Barcode gap comparisons (rbcL-3’) for specimens assignable to the genus Cryptonemia from Bermuda ().
Fig. 1. Maximum likelihood phylogenetic trees of (A) rbcL and (B) LSU data from Cryptonemia and related taxa of Halymeniaceae. Support values represent bootstrap proportion/and Bayesian posterior probability. Bootstrap values below 50 and posterior probabilities below 0.60 are not shown.
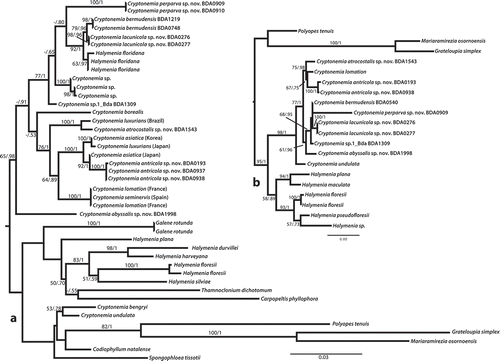
Full rbcL () and LSU () analyses were largely congruent in their relative placement of novel Cryptonemia species. The nuclear LSU and plastid rbcL analyses for the initial specimens surveyed from the Cryptonemia bermudensis/C. lacunicola complex (CL020801; ) were non-concordant. Consequently, LSU was sequenced for additional specimens for comparison with the plastid data (; ). Ultimately, only the initial specimen for which both of these markers was generated showed signs of introgression or incomplete lineage sorting, although the lack of LSU data for five additional collections of putative C. lacunicola leaves uncertainty as to the true identity for these specimens (BDA0265, BDA0541, BDA0936, BDA1369, GWS011906; ). Using samples that did not show signs of introgression, a concatenated tree of LSU and rbcL data improved support values within Cryptonemia (), although the placement of C. abyssalis and C. sp.1_Bda within the genus was not resolved with certainty.
Fig. 2. Specimens of Cryptonemia bermudensis and C. lancunicola for which nuclear (LSU) and plastid (rbcL and or UPA) data were available. At least one specimen (CL020801) shows clear evidence of introgression or incomplete lineage sorting, while five others (BDA0265, BDA0541, BDA0936, BDA1369, GWS011906) have only plastid data and may be bona fide C. lacunicola or C. bermudensis introgressed with the plastid of the former.
As discussed below, one of the Cryptonemia genetic groups is represented by a well-known species previously assigned to Halymenia with a type locality in the islands. Five other taxa are newly discovered species and some of these were formerly misassigned to other species of Cryptonemia. These are described herein as new species. One genetic group, C. sp.1_Bda, had insufficient material to be described as a new species at this time.
Cryptonemia abyssalis C.W. Schneider & Popolizio, sp. nov. (–)
Description: Plants rosy-red and erect to 11 cm wide and 6 cm tall, composed of a successive series of reniform blades marginally producing multiple orders of blades in an opuntioid pattern (), arising from small discoidal holdfasts on short stipes (4 mm), adhering to paper when dried; oldest blades (pad-like) to 2 cm broad and 1.5 cm tall, inner portions of them 90–100 µm thick, slightly swollen at the margins (), the margins producing triangular pegs many of which eventually develop into stipe-like extensions bearing new proliferous pads, margins at the site of pegs swollen to 200–220 µm thick (), the ultimate (developing) order of blades smaller than those produced earlier; medulla comprised of a compact cavity with dense intertwining, elongate filaments, 2.4–5.0 µm diam. interconnected with slightly enlarged ganglial cells; medulla giving rise to a 3-layered cortex on both surfaces; cortical cells subglobose to elongate, innermost layer of cells 4.5–7.5 µm diam., outer cortical cells 3–6 µm diam., irregularly rounded in surface view; monoecious, auxiliary cells found on plants with spermatangia; spermatangia globose to irregularly ovate and elongate, 2.5–4.5 µm in longest dimension, formed in irregular patches () on both surfaces of blades; carpogonial branches, cystocarps and tetrasporangia not seen.
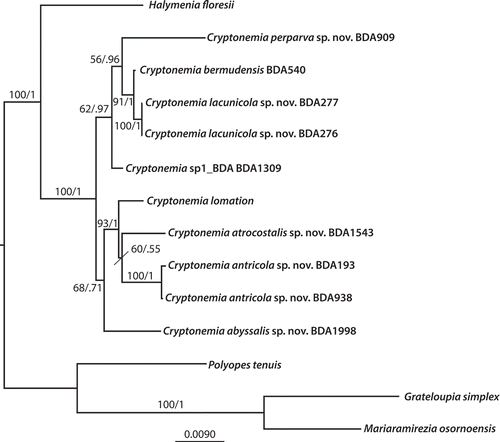
Fig. 3. Maximum likelihood phylogenetic tree of concatenated LSU and rbcL sequences of Cryptonemia and related Halymeniaceae. Support values represent bootstrap proportion/and Bayesian posterior probability. Bootstrap values below 50 and posterior probabilities below 0.60 are not shown.
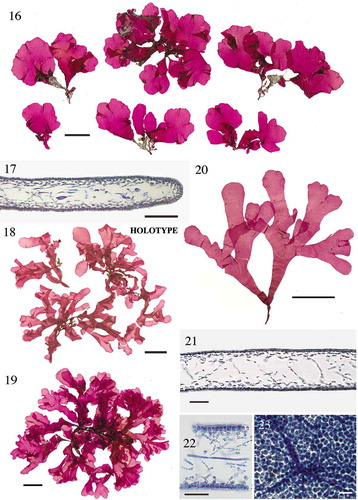
Figs 4–10. Bermuda Cryptonemia species. Figs 4–7. Cryptonemia abyssalis sp. nov., CWS/TRP 16-13-5 [BDA1998]. Fig. 4. Holotype. Fig. 5. Transverse section through blade at swollen margin. Fig. 6. Transverse section through blade at swollen margin at proliferous peg. Fig. 7. Collapsed section of a male sorus showing surface spermatia. Figs 8–10. Cryptonemia antricola sp. nov. Fig. 8. Holotype, CWS/CEL 10-14-33 [BDA0193]. Fig. 9. Habit, CWS/CEL 03-52-9. Fig. 10. Transverse section through blade, CWS/CEL/TRP 12-18-18. Scale = Fig. 4, 2 cm; Figs 5, 6, 100 µm; Fig. 7, 40 µm; Figs 8, 9, 1 cm; Fig. 10, 50 µm.
![Figs 4–10. Bermuda Cryptonemia species. Figs 4–7. Cryptonemia abyssalis sp. nov., CWS/TRP 16-13-5 [BDA1998]. Fig. 4. Holotype. Fig. 5. Transverse section through blade at swollen margin. Fig. 6. Transverse section through blade at swollen margin at proliferous peg. Fig. 7. Collapsed section of a male sorus showing surface spermatia. Figs 8–10. Cryptonemia antricola sp. nov. Fig. 8. Holotype, CWS/CEL 10-14-33 [BDA0193]. Fig. 9. Habit, CWS/CEL 03-52-9. Fig. 10. Transverse section through blade, CWS/CEL/TRP 12-18-18. Scale = Fig. 4, 2 cm; Figs 5, 6, 100 µm; Fig. 7, 40 µm; Figs 8, 9, 1 cm; Fig. 10, 50 µm.](/cms/asset/ce287d01-6980-48f7-b086-fc8910f66b85/tejp_a_1452297_f0004_c.jpg)
Etymology: From a Latinization of the Gr. noun translated as abyss meaning ‘deep’, for the very deep subtidal habitat where it was collected offshore of Bermuda.
Holotype (designated here): Craig W. Schneider (CWS)/Thea R. Popolizio (TRP) 16-13-5 [BDA 1998], 31 Jul. 2016, ledge north north-east of St. George’s I., Bermuda, western Atlantic Ocean, 32°28′58.05′′N, 64°35′04.31′′W, depth 90 m (deposited in MICH; GenBank MF685028 (LSU), MF782465 (rbcL)) (); Isotype – Herb. CWS.
Distribution: At present, endemic to the deep offshore reefs of Bermuda.
Remarks: This new species truly defines the term ‘opuntioid’ () as it mimics a similar marginal production of cactus pads in the prickly-pear Opuntia, and its spectacular habit alone separates it from all of the 45 other currently accepted species in the genus Cryptonemia. Because the most appropriate epithet was already chosen for a species from the Gulf of California, C. opuntioides E.Y. Dawson (Citation1966), we selected the epithet for our new species from its habitat at great depth offshore of Bermuda. Cryptonemia opuntioides is endemic to the northern Gulf of Mexico and ‘poorly known’ (Norris, Citation2014). The type specimen is a small, eroded blade with two larger blades produced from its ‘eroded or grazed margins’ (Dawson, Citation1966, ; Norris, Citation2014, fig. 204C), which have no further orders of branching nor even the marginal pegs developed in C. abyssalis.
Secondary and higher order blades are commonly produced from branched stipes or from the margins of blades in Cryptonemia (Chiang, Citation1970). The generitype, C. lomation (Bertolini) J. Agardh, produces similar small reniform blades like those in C. abyssalis but these are formed from branched stalks, not the margins of blades (Bertolini, Citation1818, Pl. X, , as Fucus lomation Bertolini). Another species with some morphological similarity is the eastern Atlantic/Mediterranean C. tuniformis (Bertolini) Zanardini which produces orders of blades on marginal stipe-like projections, but the blades are elongate and not nearly as regularly produced as the reniform blades of the new species (Kützing, Citation1868, fig. 94). Other species of Cryptonemia produce marginal blades, but none ever appear ‘opuntioid’ as they are large strap-shaped blades with small bladelets (at times only in proximal or distal portions of the first-order blades), e.g. C. atrocostalis sp. nov. (see Schneider & Searles, Citation1991, fig. 323 as C. luxurians; account of the species below) and C. heteronema (M. Howe) Acleto & Zúñiga (Citation2011, ). Other species in the genus produce small blades at the margins but most of these bladelets continue to develop into full-sized blades of 2nd or 3rd orders, e.g. C. angustata (Setchell & N.L. Gardner) E.Y. Dawson, C. chiangii Acleto, C. hibernica Guiry & L.M. Irvine, C. obovata J. Agardh and C. papenfussii Y.-M. Chiang.
Figs 11–15. Cryptonemia atrocostalis sp. nov. Fig. 11. Holotype, TRP/CWS 12-131-3 [BDA1543]. Fig. 12. Habit, TRP/CWS 12-79-7. Fig. 13. Transverse section through blade at swollen margin, TRP/CWS 12-131-3. Fig. 14. Transverse section through blade midrib, TRP/CWS 12-131-3. Fig. 15. Surface layer displacement by subcortical tetrasporangia, CWS 799. Scale = Figs 11, 12, 2 cm; Figs 13, 14, 100 µm; Fig. 15, 10 µm.
![Figs 11–15. Cryptonemia atrocostalis sp. nov. Fig. 11. Holotype, TRP/CWS 12-131-3 [BDA1543]. Fig. 12. Habit, TRP/CWS 12-79-7. Fig. 13. Transverse section through blade at swollen margin, TRP/CWS 12-131-3. Fig. 14. Transverse section through blade midrib, TRP/CWS 12-131-3. Fig. 15. Surface layer displacement by subcortical tetrasporangia, CWS 799. Scale = Figs 11, 12, 2 cm; Figs 13, 14, 100 µm; Fig. 15, 10 µm.](/cms/asset/5f644f46-5814-4d7d-9bb1-f31a4a96e2fd/tejp_a_1452297_f0005_c.jpg)
Figs 16–23. Bermuda Cryptonemia species. Figs 16, 17. Cryptonemia bermudensis, CWS/CEL 09-33-2. Fig. 16. Collection of specimens from the type locality, Tucker’s Town Bay. Fig. 17. Transverse section through blade. Figs 18–23. Cryptonemia lacunicola sp. nov. Fig. 18. Holotype, CWS/CEL 05-9-15. Fig. 19. Specimen from the type locality, Cliff Pool, Walsingham Park. CWS/CEL 08-39-4. Fig. 20. Specimen from the type locality, CWS/CEL 06-16-5. Fig. 21. Transverse section through blade, CWS/CEL 05-4-4. Fig. 22. Transverse section through blade of isotype demonstrating fine and coarse medullary filaments, CWS/CEL 05-9-14. Fig. 23. Surface view of cortex showing medullary ganglion, CWS/CEL 01-13-28. Scale = Fig. 16, 18–20, 2 cm; Figs 17, 21, 100 µm; Fig. 22, 50 µm; Fig. 23, 20 µm.
Cryptonemia antricola C.W. Schneider, C.E. Lane & G.W. Saunders, sp. nov. (–)
Description: Plants dark rosy-red and erect to 2.5 cm tall, composed of narrowly ligulate, irregularly branched, cartilaginous blades (), arising from small discoidal holdfasts on short subterete stipes, often more than one upright from the same holdfast, not adhering well to paper when dried; blades to 2 mm wide and 57–90 µm thick, the margins entire (), proliferous blades re-growing terminally after being grazed by herbivores; medulla comprised of a compact cavity with few to dense intertwining, elongate filaments (), 2.5–3.5 µm diam. and occasional dark staining, swollen ganglial cells; medulla giving rise to a 2-(3-) layered cortex on both surfaces, subsurface cells subglobose to transversely elongate reaching 17 µm diam., outer cortical cells 3–10 µm diam., irregular to transversely elongate in transverse section, irregularly angular to rounded in surface view; gametangia and tetrasporangia unknown.
Etymology: From the noun antrum (L., n.), grotto, for the subtidal cave where it persists at the type locality, and –cola (L. comp., indeclinable) for dweller or inhabitant, thus literally ‘grotto-dweller’.
Holotype (designated here): CWS/Christopher E. Lane (CEL) 10-14-33 [BDA0193], 21 Aug. 2010, rock floor of grotto entrance, Tobacco Bay, St. George’s I., Bermuda, western Atlantic Ocean, 32°23′20.1′′N, 64°40′44.1′′W, depth 2 m (deposited in MICH; GenBank MF685029 (LSU), MF782466 (rbcL)) (); Isotypes – UNB [BDA192], Herb. CWS.
Selected collections: Refer to Supplementary text file, S1.
Misapplied name for Bermuda: Cryptonemia crenulata, P.B.-A. no. 2100 sensu Collins et al. (Citation1916).
Distribution: Thus far, known only from shaded near shore sites and offshore reefs in Bermuda, western Atlantic Ocean.
Remarks: We first noticed a sizable matted population of this attractive species on the floor of a grotto opening in Tobacco Bay, where water surges with great energy. This type locality population of Cryptonemia antricola persists throughout the year (Supplementary text file, S1). The habitat that gives the new species its name, as living on the floors of grottos or caves, is where it was collected in Gravelly Bay by A.B. Hervey in 1913, the specimens later being distributed as part of P.B.-A. exsiccata identified as C. crenulata (Collins et al., Citation1916; Collins & Hervey, Citation1917). These historical specimens are clearly not C. crenulata, being dramatically smaller in all dimensions and bearing smooth, non-crenulate margins. We have not seen specimens of what Rein (Citation1873), followed by subsequent 19th century workers, earlier had called C. crenulata from Bermuda, but the Hervey P.B.-A. Gravelly Bay specimens clearly represent the morphology of C. antricola as described here. Collins & Hervey (Citation1917) and Howe (Citation1918) gave accounts of the same P.B.-A. no. 2100 describing the specimens as reduced, or smaller, forms than normally found for C. crenulata. Neither account mentioned marginal dentations (as typical of C. crenulata) for the Bermuda specimens, confirming the smooth margins of our own collections, and observations of theirs.
There are no species in the genus that compare to the overall habit of Cryptonemia antricola, therefore it represents a species with a unique branching pattern among congeners. It is larger in size than C. perparva (described below), but its blades are narrowly ligulate and much branched off long to short terete stipes and blade margins. Interestingly, the molecular data show that C. antricola is more closely related to sequences of C. asiatica M.Y. Yang & M.S. Kim and C. luxurians from Japan (), rather than its congeners from Bermuda.
One narrow species described with a question as to its generic placement. Cryptonemia subdichotoma Womersley & A. Bailey (Citation1970), is only known from the Solomon Islands and there have been no other accounts since the protologue (Guiry & Guiry, Citation2017). This species is regularly pseudodichotomous, to 12 cm tall and with axes 1.5–3.0 mm broad and 100–130 µm thick (Womersley & Bailey, Citation1970), so it is a larger and thicker-bladed species than C. antricola.
Cryptonemia atrocostalis C.W. Schneider, C.E. Lane & G.W. Saunders, sp. nov. (–)
Description: Plants with new growth membranous and pinkish-red, older portions firm, leathery, and dark rosy-red, to 25 cm tall, attached by small to large discoid holdfasts, giving rise to one or more simple to branched erect axes (, ); axes broadly ligulate above brief to long stipes which extend into the attenuate blade bases as midribs reaching 1/2 the total length of the expanded blades or beyond, often several blades issued from extended older, terete stipes () and holdfasts; blades pseudodichotomously to alternately branched 0.6–2.5 cm wide, with few to many marginal obovate to subcircular bladelets, these at times concentrated on the truncate to rounded apices; margins undulate () and entire to erose; blades 75–300 µm thick in vegetative portions, swollen at the margins (), medulla comprising much of the blade width, composed of densely arranged, narrow filaments 2–5 µm diam., surrounded by swollen ganglial cells with bodies 7–23 µm diam., giving rise to a few-layered cortex (), progressively smaller from medulla outwards, surface cells 3–5 µm diam., irregularly angular to rounded in surface view; midribs more greatly thickened on the ventral surface by anticlinal tiers of cortical cells (); tetrasporangia globose and obovoid to ellipsoid, cruciately divided, 7–10 µm diam., 9–15 µm long, loosely to densely formed in marginal bladelets near the apices, displacing cortical cells at maturity (); dioecious, spermatangia in patches on marginal bladelets, short ellipsoid, 1.5–3.0 µm diam.; carposporophytes immersed in marginal bladelets or blade tips, globose, 100–150 µm diam., with basal placentas, involucral filaments, and small cortical ostioles; carposporangia subquadrate to irregularly angled, 7.5–10.0 µm diam.
Etymology: From atro- (L., comp.) for dark, and costalis (L., f.) for midrib.
Holotype (designated here): TRP/CWS 12-131-3 (BDA1543), 2 Oct. 2012, off Great Head Park, St. David’s I., Bermuda, western Atlantic Ocean, 32°22′06.9′′N, 64°38′30.1′′W, depth 18 m on rock (deposited in MICH; GenBank MF685031 (LSU), MF782469 (rbcL)) ().
Selected collections: Refer to Supplementary text file, S1.
Distribution: From Bermuda and North Carolina, as currently known, but probably more widespread.
Remarks: Originally collected in Bermuda during the mid-19th century and misidentified as the delesseriaceous Botryoglossum platycarpum (Kemp Citation1857). Collins & Hervey (Citation1917) and Howe (Citation1918) later recognized that the specimens belonged in the Cryptonemiales (= Halymeniales) and identified them as Cryptonemia luxurians (C. Agardh) J. Agardh (type locality: Brazil). This taxonomy was followed by subsequent floristic workers until C. luxurians was synonymized with C. seminervis (C. Agardh) J. Agardh (type locality: Cádiz, Spain) by Price et al. (Citation1986). Recently however, Yang & Kim (Citation2014) firmly re-established the two as distinct species using rbcL sequences from the type localities of C. luxurians and C. seminervis that showed a 2.7% sequence divergence between them. During that study, Yang & Kim (Citation2014) described C. asiatica for what had until that time been identified as C. luxurians in Japan. When costate Bermuda specimens were subjected to rbcL analysis (), they showed a difference of 10 base pairs (bp), or 0.8% divergence, from Brazilian sequences (GenBank AF488813) revealing that these two are likewise unique species. There are morphological similarities between the two western Atlantic species with distinct midribs, C. atrocostalis and C. luxurians, both being comprised of ligulate blades with undulate margins and with abundant marginal proliferations on mature blades (Taylor, Citation1960, pl. 58, ; Yoneshigue-Braga, Citation1972; Schneider & Searles, Citation1991, fig. 323), thus representing cryptic sibling species (, ). Tetrasporangia of both western Atlantic species are found in these marginal proliferations (Taylor, Citation1960; Schneider & Searles, Citation1991), while in C. seminervis they are scattered in the cortex of blades (Irvine, Citation1983). Cryptonemia seminervis has broadly expanded fan-like blades and short, basal midribs, features differing from the ligulate blades of C. atrocostalis and C. luxurians, with midribs generally reaching half their lengths or more (; Kützing, Citation1869; Schneider & Searles, Citation1991).
Specimens of Cryptonemia atrocostalis from North Carolina (Schneider & Searles, Citation1973, Citation1991, 'as C. luxurians’) are considerably larger (to 2.5 cm wide and 25 cm tall) and more robust than the ones we have collected in Bermuda from 13–18 m over the last decade, perhaps due to the grazing pressure on Bermuda reefs, a phenomenon we have observed for several ‘reduced forms’ of macroalgal species in the islands (Schneider & Lane, Citation2008; Schneider & Flook, Citation2017). Howe (Citation1918) reported island specimens that were approximately 10 cm tall, a bit larger than those that we have collected, the largest blades being 8 cm tall. Challenger Bank specimens collected from 52–64 m in 1960 off Bermuda are to 2 cm broad and 16 cm long with small marginal proliferations like those in North Carolina. Despite the fact that our recent Bermuda specimens are considerably smaller than those we have collected in North Carolina (to 2.5 cm wide and 25 cm tall; Schneider & Searles, Citation1991), rbcL sequences show a near perfect match for specimens from the two locations. Bermuda blade widths (to 1 cm wide) are more similar to those reported as C. seminervis from Florida, 0.5–1.5 cm (Dawes & Mathieson, Citation2008), but it remains to be determined whether they are correctly identified using molecular sequencing. Yoneshigue-Braga (Citation1972) described Brazilian specimens of C. luxurians to 7 cm in height with blades 115–125 µm thick. Blades of C. atrocostalis can reach nearly three times that height and more than twice the thickness. Furthermore, Yoneshigue-Braga (Citation1972, ) noted midribs often reaching from the base to the apex of many blades, the remainder covering 2/3 of the length of blades, all of these exceeding the costae of C. atrocostalis. Due to the great similarity of these two western Atlantic species with midribs, it is best to use molecular data to identify specimens, especially those in the Caribbean Sea and Gulf of Mexico.
Cryptonemia bermudensis (Collins & M. Howe) C.W. Schneider, C.E. Lane & G.W. Saunders, comb. nov. (, )
Basionym: Halymenia bermudensis Collins & M. Howe Citation1916, Proc. Amer. Acad. Arts & Sci., vol. 53, p. 180.
Type locality: Castle Harbour, near Tucker’s Town, Bermuda I., Bermuda, western Atlantic Ocean (Holotype: Halymenia bermudensis, leg. F.S. Collins no. 7074, 25 Apr. 1912 [NY]; isotypes distributed as Phycotheca Boreali-Americana [1915], Fasc. XLI, no. 2050).
Selected collections: Refer to Supplementary text file, S1.
Distribution: Bermuda, Florida, and historically more widespread from the Caribbean and Gulf of Mexico to Brazil, but genetic comparisons from these regions are required.
Misapplied name for Bermuda: Halymenia floridana J. Agardh (Guimarães & Fujii, Citation1998; Schneider, Citation2003; see remarks below).
Remarks: The most common, widespread and broadest of the Cryptonemia species in Bermuda (4–30 cm; Collins & Howe, Citation1916), this taxon was originally designated Halymenia bermudensis by Collins & Howe (Citation1916), and as many as 80 isotypes were distributed as P.B.-A. no. 2050 (Collins et al., Citation1915) to herbaria throughout the world. This species is found in a variety of inshore and offshore subtidal locations, and much of the year is particularly abundant in Walsingham Pond and Harrington Sound. The mature form of Cryptonemia bermudensis is comprised of multiple broad blades that are invariably lobate, these arising from distinct branched stipes that reach 1 cm in length (Collins & Howe, Citation1916). The terete stipes occasionally extend into the blade as short basal midribs. The youngest specimens are originally obovate to suborbicular in shape, but once they reach a few cm high, they form deep lobes or branches, these extending to 2–3 orders.
In a morphological study of the foliose species of Halymenia in Brazil, H. bermudensis was moved by Guimarães & Fujii (Citation1998) into synonymy with H. floridana J. Agardh (type locality = Florida, USA). It is clear from our molecular work that the highly variable Bermuda specimens from throughout the islands, including the type locality, fall in a well-supported clade (, ) along with other species of Cryptonemia. Thus, this taxon needs reassignment to the genus as we propose above. Based upon observations of type material, the species to which H. bermudensis had been reassigned, H. floridana, has larger and less dissected blades (Guimarães & Fujii, Citation1998, ) than the highly branched species from Bermuda (). Although Collins & Howe (Citation1916) mentioned that Halymenia bermudensis had some similarities with H. floridana, they distinguished the two species using a suite of characters. In our rbcL analysis (), three specimens from Brazil identified as H. floridana are shown to represent a genetic species in Cryptonemia, distinct from a sister clade including C. bermudensis from the type locality and C. lacunicola (see account below). At this time, given the taxonomic assignment of H. floridana to Halymenia, we suggest that prior to moving this species to Cryptonemia that specimens from its type locality in Florida need to be sequenced and analysed to demonstrate their genetic relationship with Brazilian isolates identified as the same species. It is possible, especially at a time when geographically cryptic species are being discovered locally in Bermuda and elsewhere around the globe (e.g. Saunders et al., Citation2006; Schneider & Lane, Citation2008; Schneider et al., Citation2010), that H. floridana and the Brazilian isolates are distinct species, possibly in different genera.
Cryptonemia bermudensis is most closely related to another species in Bermuda, C. lacunicola (, ), a taxon with which it is found growing sympatrically in Walsingham Pond. In early stages of growth, these two species are impossible to distinguish morphologically. We also collected several small, branched specimens from the Dry Rocks Reef off Key West, Florida, that were a perfect genetic match to C. bermudensis from its type locality, and we suspect that it may be shown to be more widespread in warm western Atlantic waters with further molecular studies.
Cryptonemia lacunicola C.W. Schneider, C.E. Lane & G.W. Saunders, sp. nov. (–)
Description: Plants light to dark rosy-red and erect to 15 cm tall, composed of broadly ligulate, pseudodichotomously to irregularly branched blades; single, or occasionally a few, proximally tapering blades on simple to branched stipes to 1 cm long, arising from small discoidal holdfasts or eroded axes, the stipes at times extending into proximal portions of the blades as short midribs; blades increasing in breadth distally, to 3 cm wide and 80–150 µm thick below branching nodes in upper portions, the margins entire, often undulate (–), but appearing crenate distally due to an abundance of incipient branching; medulla comprised of a loosely woven, mucilaginous matrix with fine intertwining, elongate filaments (, ), 1.5–3.5 µm diam., some running from one cortex to another, all interspersed with coarse, darkly staining filaments generally 4–8 µm diam. directly connected to large densely staining, stellate ganglial cells (); medulla giving rise to a 2-(3-) layered cortex composed of cells 4.5–9.0 µm diam., transversely elongate in transverse section, irregularly rounded angular in surface view; gametangia and tetrasporangia unknown.
Etymology: From lacuna (L., f.), pool, for the sink-hole pools where it persists throughout the year in Walsingham Park, and –cola (L. comp., indecl.) for dweller or inhabitant, thus literally ‘pool-dweller’.
Holotype (designated here): CWS/CEL 05-9-15, 19 Jul. 2005, Cliff Pool, Walsingham Park, Bermuda I., Bermuda, western Atlantic Ocean, 32°20′50.4′′N, 64°42′41.3′′W, depth 0–2 m on rock (deposited in MICH; GenBank MG191639 (UPA), MG191579F782469 (LSU)) (); Isotypes – NY, Herb. CWS.
Selected collections: Refer to Supplementary text file, S1.
Distribution: Thus far, known only from Bermuda.
Remarks: Our numerous collections of the new species from shaded inland sinkholes and cave mouths in Walsingham Park have some features in common with its congener and genetic sibling, Cryptonemia bermudensis. When we first collected it, it was considered a “stretched out” shade morphology of the more broadly bladed C. bermudensis. Our genetic sequences, however, show them to be distinct species. Cryptonemia lacunicola has narrower blades (generally 2 cm or less in width as compared with the significantly wider blades of C. bermudensis, to 12 cm), appearing strap-like and more open in habit (–), somewhat similar in gross morphology to congeneric species known in the Caribbean and Brazil (Guiry & Guiry, Citation2017), C. bengryi W.R. Taylor (type locality = Jamaica) and C. limensis (Kütz.) J.A. Lewis (type locality = Peru). Genetic material of these species collected in the Caribbean and Brazil is unavailable at present, but there are morphological differences. Cryptonemia bengryi has blades borne on long, terete stipes and is deeply cleft to palmately lobed (Taylor, Citation1960; Díaz-Piferrer, Citation1965). Cryptonemia limensis has palmately organized blades lacking stipes with broadly ovate lobes and retuse apices (Acleto, Citation1973, as C. chiangii Acleto and C. peruviana Acleto; Lewis, Citation1990), features not seen in the new species. One Pacific species presently considered a synonym of C. limensis, C. guaymasensis (E.Y. Dawson) E.Y. Dawson from Baja California, has narrower lobes than the more typical plants of the species from South America, but it too has retuse apices causing Lewis (Citation1990) to synonymize them.
Cryptonemia lacunicola has a pseudodichotomous branching pattern that sets it apart from C. bermudensis, branching to 4–5 orders of magnitude at maturity, the blades increasing in width from the base to the apex (). Cryptonemia bermudensis produces broadly ovate to cuneate blades significantly broader (to 12 cm) than the new species (to 3 cm) and remain tightly branched to just a few orders (). Anatomically, the two species’ cell sizes and arrangements are mostly indistinguishable. Both have coarse, darkly staining medullary filaments networking between substellate ganglia as well as dimorphic finer medullary filaments traversing the central core of the axes. Collins & Howe (Citation1916) reported the coarser medullary filaments from 6–20 µm diam. for C. bermudensis, whereas those in C. lacunicola range from 4–8 µm diam., but these hardly represent a character warranting species level discussions. In short, the genetic differences and overall habit are the only way to differentiate these sympatric species in Bermuda, particularly as they appear able to occasionally hybridize ().
Cryptonemia lacunicola is found in a robust population throughout the entire year at the type locality and other nearby sinkholes in Walsingham Park on Bermuda I. Many of these inland areas lack the large herbivorous fish that would reduce individuals to a smaller cropped habit on coastal reefs, a pattern seen contrasting many other species in sinkholes and nearshore boiler reefs (e.g. Botryocladia spp., see Schneider & Lane, Citation2008). Interestingly, hundreds of collected specimens from throughout the entire year have not turned up reproductive characteristics. Collins & Howe (Citation1916, p. 172) were equally frustrated by the lack of reproduction in their hundreds of specimens of C. bermudensis, and we too have discovered none in our collections.
Cryptonemia perparva C.W. Schneider, C.E. Lane & G.W. Saunders sp. nov. (–)
Description: Single, or occasionally a few, blades arising from often long stipes on small discoidal holdfasts (), blades not adhering well to paper; rosy-red blades simple, to 1.3 cm tall, obovate, elliptical to subspherical in outline, margins entire; blades 40–100 µm thick in vegetative portions, medulla comprised of a compact cavity with few to many intertwining, elongate filaments (), 1.5–4.5 µm diam. and large, oddly lobate to stellate swollen ganglial cells (); medulla giving rise to a 2-(3-) layered cortex, subsurface cells small, subglobose to flattened reaching 10 µm diam., outer cortical cells 3–6 µm diam., irregularly globose to elongate in transverse section, irregularly angular to rounded in surface view; dioecious, subglobose auxiliary cells in ampullae, 10–17 µm diam. (), produced from medullary filaments; carposporophytes immersed in blades, globose, 100–250 µm in diam., with stretched involucral filaments, dramatically swelling both blade surfaces to 270 µm thick (, ); carposporangia subglobose to irregularly elongate, 9.5–14.5 µm diam.; spermatangia formed in extensive sori on both blade surface from elongated spermatangial mother cells produced directly or on one-celled pedicels produced from outer cortical cells, spermatia ovoid to ellipsoidal, 1 µm diam. (); tetrasporangia ellipsoid (), cruciately divided, 10–12 µm diam., 9–18 µm long, clustered over the surface of both blade surfaces and compressing adjacent cortical cells ().
Figs 24–31. Cryptonemia perparva sp. nov. Fig. 24. Holotype, CWS/CEL 05-19-12. Fig. 25. Transverse section near margin showing enlarged ganglial cells in medulla, CWS/CEL 03-4-30. Fig. 26. Ampullary filaments surrounding the auxiliary cell prior to fertilization, CWS/CEL 03-4-30. Fig. 27. Cystocarp in transverse section with mature carposporangia, CWS/CEL 03-4-30. Fig. 28. Contiguous cystocarps swelling the thallus, CWS/CEL 03-4-30. Fig. 29. Elongate spermatangial mother cells in a dense sorus produced from outer cortical cells, CWS/CEL 06-4-13. Fig. 30. Tetrasporangia in section and surface view through cortex demonstrating cortical cell displacement between swollen sporangia, CWS/CEL 01-13-28. Fig. 31. Cruciate tetrasporangia formed at the interface of the inner cortex and medulla, CWS/CEL/TRP 12-20-7. Scale = Fig. 24, 1 cm; Figs 25, 26, 29–31, 25 µm; Figs 27, 50 µm; Fig. 28, 150 µm.
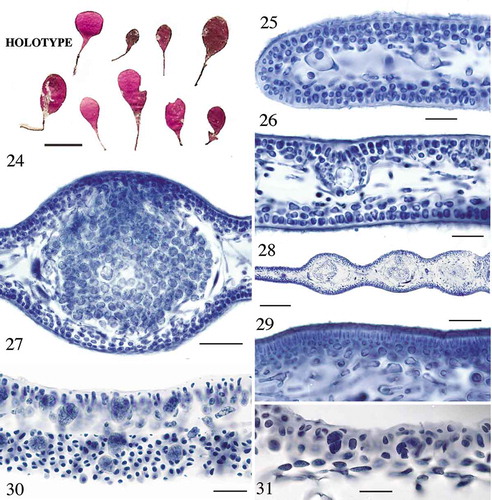
Etymology: From perparva (L., adj., f.) = very small, for the overall size of the species.
Holotype (designated here): CWS/CEL 05-19-12, 22 July 2005, outer reef, John Smith’s Bay, off Canton Pt., south shore of Bermuda I., Bermuda, western Atlantic Ocean, 32°19′09.6′′N, 64°42′46.6′′W, depth 10 m on coral (deposited in MICH; GenBank MG191585 (LSU)) (); Isotypes – UNB, Herb. CWS.
Selected collections: Refer to Supplementary text file, S1.
Distribution: Endemic to Bermuda, western Atlantic Ocean, as presently known.
Remarks: Cryptonemia perparva is a common summer species on the south shore boiler reefs off Bermuda Island. Thalli are found along with Botryocladia bermudana C.W. Schneider & C.E. Lane as understorey plants somewhat protected from grazing fish by a number of larger macroalgae in this high-energy environment including species of Dictyota, Padina, Laurencia and Dasya spinuligera Collins & Hervey. The small blades of C. perparva could have been overlooked as germlings of a number of other Cryptonemia species, but their reproductive state in blades less than 1 cm tall demonstrates that they are mature despite their small stature. Furthermore, rbcL and LSU sequences confirmed that they are distinct from the other species in the genus in Bermuda (, ).
Although carpogonial branch ampullae have not been found in our specimens, female plants of Cryptonemia perparva produce great quantities of small auxiliary cell ampullae () typical of the genus (Kylin, Citation1925; Sjöstedt, Citation1926; Chiang, Citation1970), often in positions adjacent to others such that after presumed fertilization the cross-sections look like well-stuffed sausages (). Auxiliary cells are produced from ampullary filaments on short stalk cells, and are large and subglobose surrounded by ampullary filaments following the pattern described for other Cryptonemia species (Kylin, Citation1925; Sjöstedt, Citation1926; Chiang, Citation1970). Small ellipsoidal spermatia are produced in dense, slightly raised sori on both surfaces of male blades from elongate spermatangial mother cells (). Tetrasporangia are formed within the thallus at the interface of the filamentous medulla and the inner row of cortical cells (). When they expand in size and divide cruciately, the sporangia compress the surrounding cortical cells into arcing elongate cells, leaving themselves uncovered by outer cortical cells at the surface ().
The new species bears comparison with other small and diminutive Cryptonemia species worldwide. Although the epithet for the western Atlantic C. delicatula Joly & Cordeiro (type locality = São Paulo, Brazil) suggests a small and delicate species, in reality it extends to 9 cm tall when proliferous, and 6 cm tall when not (Joly, Citation1965; Joly et al., Citation1966), whereas C. perparva barely exceeds 1 cm tall even at reproductive maturity. A subspecies from South America, C. delicatula ssp. venezuelensis Ganesan (type locality = Venezuela) is smaller than the nominate variety (1.2–3.0 cm tall) and differs from C. perparva due to the presence of its long terete stalks giving rise to the broadening simple blades above (Ganesan, Citation1975). Cryptonemia taylorii I.A. Abbott (type locality = Socorro I., Revillagigedo Archipelago, Mexico) is 4–8 cm tall and 4–6 cm wide, dimensions that dwarf the new species. This eastern Pacific species is very thin, 45–60 µm, accounting for its torn and fragmented habit at maturity (Abbott, Citation1967). Cryptonemia veleroae (E.Y. Dawson) E.Y. Dawson (type locality = Gulf of California, Mexico) is as tall as 4 cm at maturity (Dawson, Citation1944, as Callymenia veleroae E.Y. Dawson; Norris, Citation2014), but has several blades arising from a common holdfast and the blades are split by lacerations as they increase in height. Another small species, C. papenfussii Chiang (type locality = Natal, South Africa), reaches only 3.5 cm high and produces multiple blades from short filiform stalks (Chiang, Citation1970) unlike the new species. One Cryptonemia species that is truly diminutive, C. parva Zhang & B.M. Xia (type locality = Xisha I., China), reaches only 1.4 cm in height, but this species becomes subdichotomously lobed or irregularly palmate at maturity and produces tetrasporangia in nemathecial sori (Zhang & Xia, Citation1983; Xia, Citation2004), whereas the similar-sized C. perparva mostly remains as simple blades at maturity and bears scattered, non-nemathecial tetrasporangia.
Discussion
Prior to this study, only eight species of Cryptonemia were known from the tropical western Atlantic Ocean (Wynne, Citation2017). The description of five new species from Bermuda represents a significant addition for the region and suggests a biodiversity hotspot for the genus in this area. With the limitations of collecting in deep water well beyond the limits of scuba, we only turned up a single spectacular specimen of C. abyssalis in two weeks of mesophotic collecting. All of the other species found in Bermuda are abundant and seen throughout fairly long growing seasons, some representing misidentifications made by earlier workers in the islands. With molecular sequencing available as a tool, many such problems can now be eliminated, and the result is a more accurate assessment of the flora of these Atlantic islands.
Key to Bermuda species of Cryptonemia
1a. Plants with midribs extending to one half the length of the blade C. atrocostalis
1b. Plants lacking midribs or with only basal midribs entering the blades2
2a. Plants less than 3 cm tall at maturity3
2b. Plants greater than 3 cm tall at maturity 4
3a. Simple obovate to subspherical blades, occasionally a few rising from terete stipes C. perparva
3b. Branched ligulate blades to 2 mm wide………………………………C. antricola
4a. Plants with blades growing from marginal projections of other blades in an opuntioid pattern.C. abyssalis
4b. Plants not opuntioid5
5a. Plants pseudodichotomously branched, composed of strap-like blades generally 2 cm or less wideC. lacunicola
5b. Plants with broad obovate lobed blades arising from terete branched axes, blades well over 2 cm at maturityC. bermudensis
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2018.1452297
Supplementary text file S1. Selected collection records of species used for this study.
tejp-2017-0083-File011.docx
Download MS Word (17.8 KB)Acknowledgements
We gratefully acknowledge Dr Thea Popolizio for collecting some of the specimens, as well as her and Tanya Moore for generating many of the sequences used in this study. Ali Parpal, Maura Griffith, Bilal Hamzeh and Dylan Spagnuolo produced some of the sections used in this study. We thank Drs Michael Wynne (MICH) for a loan of specimens, Wilson Freshwater for a sequence of Cryptonemia atrocostalis from North Carolina, Roy Tsuda for information on Cryptonemia holdings in BISH, and Chris Maggs and Tony Manghisi for constructive criticisms of the manuscript. Dr Robbie Smith of the Bermuda Natural History Museum and Chris Flook of the Bermuda Institute of Ocean Sciences (BIOS) provided logistical support in Bermuda. This is contribution no. 258 to the Bermuda Biodiversity Project (BBP) of the Bermuda Aquarium, Natural History Museum and Zoo (BAMZ), Department of Environment and Natural Resources, and Nekton contribution no. 3.
Disclosure statement
No potential conflict of interest was disclosed by the authors.
Additional information
Funding
Notes on contributors
Craig W. Schneider
All of the authors conceived the project and wrote/edited the manuscript. The morphological work was carried out by C.W. Schneider and the genetic analyses were conducted in the laboratories of G.W. Saunders and C.E. Lane.
References
- Abbott, I.A. (1967). Studies in some foliose red algae of the Pacific coast. I. Cryptonemiaceae. Journal of Phycology, 3: 139–149.
- Acleto O., C. (1973). Las algas marinas del Perú. Boletin de la Sociedad Peruana de Botanica, 6: 1–164.
- Acleto O., C. & Zúñiga A., R. (2011). Revisión de las especies Peruanas de Sebdenia (Sebdeniales, Rhodophyta) y descripcíon de Cryptonemia anconensis sp. nov. (Halymeniales, Rhodophyta). Revista Peruana de Biología, 18: 97–111.
- Bertolini, A. (1818). Lettera del dottore Antonio Bertolini Professore di Botanica nell’Università di Bologna al signor Lamouroux Professore di Storia natural nell’Accademia di Caen. Opuscoli Scientifici [Bologna], 2: 286–292, pls. X, XI.
- Brummitt, R.K. & Powell, C.E. (eds) (1992). Authors of Plant Names. Royal Botanic Gardens, Kew, London.
- Calderon, M.S., Boo, H.G. & Boo, S.M. (2014). Morphology and phylogeny of Ramirezia osornoensis gen. & sp. nov. and Phyllymenia acletoi sp. nov. (Halymeniales, Rhodophyta) from South America. Phycologia, 53: 23–26.
- Chiang, Y.-M. (1970). Morphological studies of the red algae of the family Cryptonemiaceae. University of California Publications in Botany, 58: 1–95.
- Collins, F.S. & Hervey, A.B. (1917). The algae of Bermuda. Proceedings of the American Academy of Arts & Sciences, 53: 1–195.
- Collins, F.S. & Howe, M.A. (1916). Notes on species of Halymenia. Bulletin of the Torrey Botanical Club, 43: 169–182.
- Collins, F.S., Holden, I. & Setchell, W.A. (1915). Phycotheca Boreali-Americana (Exsiccata), Algae of North America. Fascicle XLI. Algae of Bermuda. Nos. 2001–2050. Malden, Massachusetts.
- Collins, F.S., Holden, I. & Setchell, W.A. (1916). Phycotheca Boreali-Americana (Exsiccata),, Algae of North America. Fascicle XLII. Algae of Bermuda Nos. 2051–2100. Malden, Massachusetts.
- D’Archino, R. & Nelson, W.A. & Zuccarello, G.C. (2014). Amalthea and Galene, two new genera of Halymeniaceae (Rhodophyta) from New Zealand. Botanica Marina, 57: 185–201.
- Dawes, C.J. & Mathieson, A.C. (2008). The Seaweeds of Florida. University Press of Florida, Gainesville, Florida.
- Dawson, E.Y. (1944). The marine algae of the Gulf of California. Allan Hancock Pacific Expeditions, 3: 189–453.
- Dawson, E.Y. (1966). New records of marine algae from the Gulf of California. Journal of the Arizona Academy of Science, 4: 55–66.
- Díaz-Piferrer, M. (1965). Notas sobre el genero Cryptonemia (Rhodophyta) en Puerto Rico. Caribbean Journal of Science, 5: 1–7.
- Ganesan, E.K. (1975). Studies on the marine algal flora of Venezuela. VI. Cryptonemia delicatula subsp. venezuelensis subsp. nov. (Rhodophyta, Cryptonemiales). Phycologia, 14: 139–143.
- Gavio, B. & Fredericq, S. (2002). Grateloupia turuturu (Halymeniaceae, Rhodophyta) is the correct name of the non-native species in the Atlantic known as Grateloupia doryphora. European Journal of Phycology, 37: 349–359.
- Guiry, M.D. & Guiry, G.M. (2017). AlgaeBase. National University of Ireland, Galway. http://www.algaebase.org, searched on 23 May 2017.
- Guimarães, S.M.P.B. & Fujii, M.T. (1998). Two species of foliose Halymenia (Halymeniaceae, Rhodophyta) from Brazil. Botanica Marina, 41: 495–504.
- Howe, M.A. (1918). Algae. In Flora of Bermuda (Britton, N.L., editor), 489–540. Charles Scribner’s Sons, New York.
- Irvine, L.M. (1983). Seaweeds of the British Isles. Vol. 1. Rhodophyta, Part 2A. Cryptonemiales (sensu stricto), Palmariales, Rhodymeniales. British Museum (Natural History).
- Joly, A.B. (1965). Flora marinha do litoral norte do Estado de São Paulo e regiões circunvizinhas. Boletim de Faculdade de Filosofia, Ciências e Letras, Universidade de São Paulo, Botânica, 21: 1–393.
- Joly, A.B., Cordeiro, M., Yamaguishi, N. & Ugadim, Y. (1966) [‘1965’]. New marine algae from southern Brazil. Rickia, 2: 159–181.
- Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P. & Drummond, A. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28: 1647–1649.
- Kemp, A.F. (1857). Notes on the Bermudas and their natural history, with special reference to their marine algae. Canadian Naturalist and Geologist, 2: 145–156.
- Kim, S.Y., Seo, T.H., Park, J.K., Boo, G.H., Kim, K.M. & Boo, S.M. (2012). Cryptonemia rotunda (Halymeniales) and Schizymenia apoda (Nemastomatales), two new records of red algae from Korea. Algae, 27: 1–8.
- Kützing, F.T. (1868). Tabulae phycologicae. Vol. 18. Nordhausen.
- Kützing, F.T. (1869). Tabulae phycologicae. Vol. 19. Nordhausen.
- Kylin, H. (1925). The marine red algae in the vicinity of the biological station at Friday Harbor, Wash. Lunds Universitets Årsskrift, NF, Avd. 2, 21: 1–87.
- Lewis, J.A. (1990). Cryptonemia limensis (Kützing) Lewis, comb. nov. (Halymeniaceae, Rhodophyta) from Peru. Taxon, 39: 98–104.
- Milton, M., Pierossi, P. & Ratnasingham, S. (2013). Barcode of Life Data Systems Handbook. Biodiversity Institute of Ontario, Canada (http://www.boldsystems.org/libhtml_v3/static/BOLD_Handbook_Oct2013.pdf).
- Norris, J.N. (2014). Marine algae of the northern Gulf of California II. Rhodophyta. Smithsonian Contributions to Botany, 96: [i]-xvi + 1–555.
- Posada, D. & Crandall, K.A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics, 14: 817–818.
- Price, J.H., John, D.M. & Lawson, G.W. (1986). Seaweeds of the western coast of tropical Africa and adjacent islands: a critical assessment. IV. Rhodophyta (Florideae) 1. Genera A–F. Bulletin of the British Museum of Natural History (Botany), 15: 1–122.
- Rein, J.J. (1873). Über die vegetations- verhältnisse der Bermudas-Inseln. Bericht uber die Senckenbergische Naturforschende Gesellschaft in Frankfurt am Main, 1872–1873: 131–153.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D., Darling A., Hohna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Saunders, G.W. & McDevit, D.C. (2012). Methods for DNA barcoding photosynthetic protists emphasizing the macroalgae and diatoms. Methods in Molecular Biology, 858: 207–222.
- Saunders, G.W. & Moore, T.E. (2013). Refinements for the amplification and sequencing of red algal DNA barcode and RedToL phylogenetic markers: a summary of current primers, profiles and strategies. Algae, 28: 31–43.
- Saunders, G.W., Lane, C.E., Schneider, C.W. & Kraft, G.T. (2006). Unraveling the Asteromenia peltata species complex with clarification of the genera Halichrysis and Drouetia (Rhodymeniaceae, Rhodophyta). Canadian Journal of Botany, 84: 1581–1607.
- Schneider, C.W. (2003). An annotated checklist and bibliography of the marine macroalgae of the Bermuda islands. Nova Hedwigia, 76: 275–361.
- Schneider, C.W. & Flook, C.T. (2017). Could marine animal conservation laws be responsible for the decline or extirpation of macroalgal populations in Bermuda over the past century? Botanica Marina, 60: 591–602.
- Schneider, C.W. & Lane, C.E. (2008). Notes on the marine algae of the Bermudas. 9. The genus Botryocladia (Rhodophyta, Rhodymeniaceae), including B. bermudana, B. exquisita and B. flookii spp. nov. Phycologia, 47: 614–629.
- Schneider, C.W. & Searles, R.B. (1973). North Carolina marine algae. II. New records and observations of the benthic offshore flora Phycologia, 12: 201–211
- Schneider, C.W. & Searles, R.B. (1991). Seaweeds of the Southeastern United States. Cape Hatteras to Cape Canaveral. Duke University Press, Durham, North Carolina.
- Schneider, C.W., Lane, C.E. & Saunders, G.W. (2010). Notes on the marine algae of the Bermudas. 11. More additions to the benthic flora and a phylogenetic assessment of Halymenia pseudofloresii (Halymeniales, Rhodophyta) from its type locality. Phycologia, 49: 154–168.
- Sherwood, A.R. & Presting, G.G. (2007). Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and cyanobacteria. Journal of Phycology, 43: 605–608.
- Silvestro, D. & Michalak, I. (2011) [2012]. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity and Evolution, 12: 335–337.
- Sjöstedt, L.G. (1926). Floridean studies. Lunds Universitets Årsskrift, NF, Avd. 2, 22: 1–96.
- Swofford, D.L. (2003). PAUP*. Phylogenetic analyses using parsimony (* and other methods). Sinauer Associates, Sunderland, Massachusetts.
- Taylor, W.R. (1960). Marine Algae of the Eastern Tropical and Subtropical Coasts of the Americas. University of Michigan Press, Ann Arbor, Michigan.
- Womersley, H.B.S. & Bailey, A. (1970). Marine algae of the Solomon Islands. Philosophical Transactions of the Royal Society of London [B. Biological Sciences], 259: 257–352.
- Wynne, M.J. (2017). A checklist of benthic marine algae of the tropical and subtropical western Atlantic: fourth revision. Nova Hedwigia, Beiheft, 145: 1–202.
- Xia, B. [ed.]. (2004). Flora algarum marinarum Sinicarum tomus II Rhodophyta, No. III. Gelidiales, Cryptonemiales, Hildenbrandiales. Consilio Florarum Cryptogamarum Sinicarum, Academiae Sinicae Edita. Science Press, Beijing [in Chinese].
- Yang, M.Y. & Kim, M.S. (2014). Cryptonemia asiatica sp. nov. (Halymeniaceae, Rhodophyta), a new marine macroalgal species from Korea and Japan. Journal of Ecology and Environment, 37: 387–393.
- Yoneshigue-Braga, Y. (1972). Flore marinha bentonica de Baía de Guanabara e Cercantas III. Rhodophyta 2. Cryptonemiales, Gigartinales e Rhodymeniales. Publicacão do Instituto de Pesquisas da Marinha, 62: 1–39, 6 pls.
- Zhang, J. & Xia, B. (1983). Studies on some marine red algae of the Xisha Islands, Guangdong Province, China. IV. Studia Marina Sinica, 20: 123–140.
