ABSTRACT
The Chaetophorales (Chlorophyceae) consist of filamentous green algae that were mostly described directly from natural samples by light microscopy. However, the descriptions were often based on morphological characters that are either homoplasious or sensitive to environmental factors. This clearly prevents proper identification of several chaetophoralean taxa and highlights the need for taxonomic revision at different taxonomic levels. In the present study, we focused on revision of the Chaetophorales at the family level. We used a well-resolved molecular phylogeny to re-evaluate morphological characters in the Chaetophorales under clearly defined laboratory conditions. We identified five morphological characters that permitted unambiguous identification of most chaetophoralean families, and used these characters to establish a new family, the Fritschiellaceae (fam. nov.), and emend the Chaetophoraceae Greville 1824. Knowledge gained from this study lends additional weight to the current classification of the Chaetophorales and may serve as an example for taxonomic revisions of other complex filamentous algae.
Introduction
Green plants (Viridiplantae) comprise two lineages: (1) The Streptophyta that colonized land and (2) the Chlorophyta that have adapted to land but remained mostly aquatic. Chlorophyta (= Chlorophytes) comprise green algae that can be anatomically very simple (= unicellular) as well as relatively complex (= multicellular) organisms. Whereas unicellular chlorophytes are extensively studied (e.g. Chlamydomonas, Chlorella, Coccomyxa, Micromonas, Ostreococcus), anatomically complex ones are far less-known.
One example of the lesser-known chlorophytes are the Chaetophorales (Chlorophyceae). These are semi-terrestrial and freshwater filamentous algae with different degrees of morphological complexity in terms of development of an upright and a prostrate system and development of branching. They are distributed worldwide and can be found either as individual plants or as a part of biofilms (Saxena, Citation1962; Islam, Citation1963; Tupa, Citation1974; Sarma, Citation1986; Skinner & Entwisle, Citation2004; Bonaventura et al., Citation2006; Caisová et al., Citation2015). The Chaetophorales were mostly described during the 19th and early 20th century by directly observing the thalli in natural samples using light microscopy (e.g. Bory de Saint-Vincent, Citation1808; Kützing, Citation1843; Hazen, Citation1902; Cook, Citation1970). However, their descriptions were based on morphological characters that are often highly homoplasious (Caisová et al., Citation2011) and sensitive to environmental factors (e.g. Tupa, Citation1974). As a result, several previously well-accepted genera were found to be polyphyletic (Chaetophora, Stigeoclonium; Caisová et al., Citation2011) and the number of recognized chaetophoralean species varies considerably (from 3 to 40 in the genus Stigeoclonium; compare Simons et al., Citation1986 with Printz, Citation1964). These inconsistencies clearly indicate that taxonomic revisions are needed in the Chaetophorales at different taxonomic levels.
The first step towards a meaningful taxonomic revision of chaetophoralean species is to understand which characters define higher taxonomic levels. Recently, we presented (i) a comprehensive phylogeny recovered from species that represent all described chaetophoralean families and genera (Caisová et al., Citation2015) and (ii) a general protocol for morphological analyses of multi- and unicellular chlorophytes based on cultures grown under controlled experimental conditions (Caisová et al., Citation2015; Caisová, Citation2016). Here, our aim is to use these two resources to investigate taxonomically informative morphological characters in the Chaetophorales at the family level.
Materials and methods
Strain selection
A meaningful taxonomic revision of the Chaetophorales rests upon knowledge of their phylogenetic relationships. Here, we used a recently published chaetophoralean phylogeny (Caisová et al., Citation2015) to examine taxonomically informative morphological characters at the family level. In brief, this phylogeny consisted of 35 strains of Chaetophorales and 23 strains of their closest relatives (Chaetopeltidales, Oedogoniales, Sphaeropleales). It was based on the nuclear-encoded rRNA operon (3498 positions) and constructed using maximum likelihood (RAxML); for details see Supplementary fig. 1 and Caisová et al. (Citation2015). Based on this phylogeny, a total of 14 chaetophoralean strains were selected among the Schizomeridaceae, Aphanochaetaceae, Uronemataceae and Chaetophoraceae sensu Caisová et al., Citation2015 (Supplementary fig. 1) and subjected to detailed morphological analyses (see below). Note that the family Barrancaceae was not included in this study as it has previously been investigated by Caisová et al. (Citation2015).
Fig. 1. Schematic presentation of important morphological characters for delimitation of chaetophoralean families. Five taxonomically important morphological characters at family level exist in the Chaetophorales. These characters are: (1) Type of zoospore germination, (2) Formation of the first cross wall, (3) Nature of apical cell of germlings, (4) Nature of prostrate system (= type of attachment) and (5) Nature of upright system. Taxonomically important characters are labelled with arrows and encircled numbers. Character no. 4 (= prostrate system/type of attachment) is shaded in grey. The individual families (except the Barrancaceae published already in Caisová et al., Citation2015) are labelled with capital letters ranging from A to E. Schematic drawings reflect typical development of strains within each family; early developmental stages (attached zoospores) are on the left and later developmental stages (mature filaments) are on the right. The cladogram showing relationships among chaetophoralean families is based on fig. 4 in Caisová et al. (Citation2015).
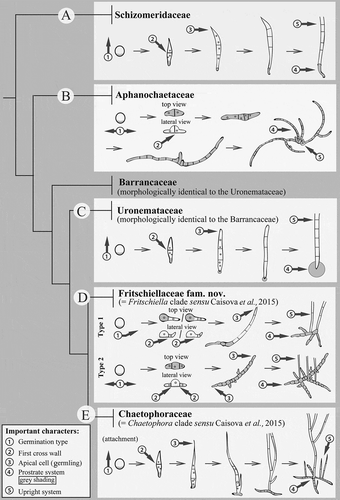
Figs 2–19. Microphotographs showing taxonomically important morphological characters within the Schizomeridaceae and the Aphanochaetaceae. Figs 2–7. Development of Schizomeris leibleinii SAG 44.84. Fig. 2. Zoospore release. Fig. 3. Erect type of germination (encircled number 1). Fig 4. First cross wall located in the centre of the germling (encircled number 2). Fig. 5. Pointed shape of the apical cell in the germling (encircled number 3). Fig. 6. Epifluorescence micrographs of a development of the single-celled prostrate system, r: a ring-like structure. Fig. 7. One upright filament (encircled number 5) per one prostrate system (here the single-celled prostrate system, encircled number 4); dashed lines: the single-celled prostrate system attached to the substrate. Figs 8–19. Development of Aphanochaete repens SAG 31.85. Fig. 8. Attached zoospore. Fig. 9. Prostrate type of germination – bipolar (encircled number 1), m: mucilage around attached zoospore. Figs 10–11. First cross wall located in the centre of the germling (encircled number 2). Fig. 12. Rounded shape of apical cells, germling (encircled number 3). Figs 13–17. Development of the prostrate system over five days in a single filament. Figs 18–19. Mature thallus with extensive prostrate system (encircled number 4, dashed line) and reduced upright system (encircled number 5), two different optical planes. Scale bars: 10 µm.
Growth of strains
To compare morphological characters between all chaetophoralean families (including the previously investigated Barrancaceae), selected strains were grown under the same conditions as used by Caisová et al. (Citation2015). These conditions were: Bold-Basal Medium (BBM), 23°C, 14 h light: 10 h dark cycle with 10–15 μmol photons m−2 s−1 photon flux rate with fluorescent lamps, and transfer every 4 weeks into fresh medium. Strains from the present study are available through the Coimbra Collection of Algae (ACOI), Portugal; the Culture Collection of Algae at the University of Cologne (CCAC), Germany; and Culture Collection of Algae at Göttingen University (SAG), Germany.
Morphological observations
Long-term cultures were observed and documented with an inverted cell culture microscope CK X 41 (Olympus, Tokyo, Japan). For high resolution imaging, inverted microscopes Zeiss IM (Oberkochen, Germany) and BZ-8000, Biozero, Keyence (Osaka, Japan) were used. Fluorescence microscopy observations were made with a Nikon Eclipse E800 light microscope with a UV-2E/C* epifluorescence filter (excitation 340–380, dichroic 400, emission 435–485).
All Chaetophorales grow attached to the substrate to some extent and cannot be removed without damage. Thus, for this study, algae were grown directly on the glass slide that was used for microscopy. Depending on the complexity of the upright system of the alga two different strategies were applied. (1) Algae with a reduced upright system (the Aphanochaetaceae) were grown in a thin aluminium/glass microscope slide chamber filled with liquid medium (fig. 1 in Reize & Melkonian, Citation1989) and were observed directly from above using Zeiss IM and Keyence microscopes. Ten individuals were monitored per day. (2) Algae with a well-developed upright system (all Chaetophorales with exclusion of the Aphanochaetaceae) were grown in two different growing chambers. Each of them allowed monitoring of different developmental stages of the alga: to observe young stages (from day 1 to day 3), a large plastic Petri dish with glass cover slips on the bottom was used. Prior to each observation, one coverslip with biomass was taken out of the Petri dish, attached to a microscope slide and observed using the Zeiss IM. To monitor older stages with a well-developed upright system (from day 4 on), a 35 mm imaging dish with integrated glass-bottom was used (IBIDI cells in focus, Planegg, Germany; Cat.No: 81158; https://ibidi.com/dishes/176–dish-35-mm-high-glass-bottom.html). Here, a circular coverslip of 10 mm diameter was placed on the bottom of each IBIDI Petri dish prior to light microscopy. This caused flattening of the upright filaments without any damage and thus allowed a more precise observation of a complete alga. In this case, algae were observed directly in the IBIDI Petri dish using Olympus CK X 41 and Keyence microscopes. For all strains with a well-developed upright system (Chaetophorales with exclusion of the Aphanochaetaceae), 20 organisms were monitored per strain/per day. Note that in all investigated algae only one type of reproduction – zoospore formation – was observed. Zoospores were induced on selected substrates by mixing 4 weeks old suspensions of algal filaments and fresh medium (1/4 ‘old’ culture and 3/4 fresh medium), and were released within 24–48 hours. After zoospore settlement, the remaining biomass (original filaments + unattached zoospores) was removed and chambers were filled with fresh culture medium. Medium was exchanged daily. Germlings of all strains were observed daily over a period of 2 weeks. Such a strategy allowed monitoring of the development from a single cell stage (here a zoospore) to a mature filament, and thus comparison of morphological features throughout the life cycles of all 14 chaetophoralean strains investigated. A schematic drawing of the workflow is shown in Supplementary fig. 2.
Calcofluor white (Calcofluor White M2R 1 g l−1, Evans blue 0.5 g l−1; Sigma-Aldrich, Seelze, Germany; protocol of the manufacturer) was used to visualize the prostrate system of the Schizomeridaceae.
Analysis of morphological traits
A total of 20 morphological characters were used in the analyses. A complete list of characters is provided in Supplementary table 1. To identify taxonomically important morphological characters at the family level, we applied a similar approach as described in Caisová et al. (Citation2013). In brief, examination of morphological characters was guided by phylogenetic reconstruction: (1) Morphological characters were compared among species within one family → identification of shared morphological characters of a family. (2) Shared morphological characters among families were compared within the Chaetophorales → identification of taxonomically important morphological characters of individual chaetophoralean families. These important morphological characters were used for taxonomic revision of the Chaetophorales at the family level (for details see Results).
Table 1. Chaetophoralean families and their characteristic morphological features
Results
Evaluation of morphological data using the phylogenetic/comparative approach revealed five taxonomically important morphological characters at the family level. These characters are: (1) Type of zoospore germination, (2) Formation of the first cross wall, (3) Nature of the apical cell of germlings/young filaments, (4) Nature of the prostrate system (= type of attachment) and (5) Nature of the upright system. In the following paragraphs, these characters are at first described and illustrated for each chaetophoralean family ( – schematic drawings, – – microphotographs), and subsequently used for the taxonomic revision.
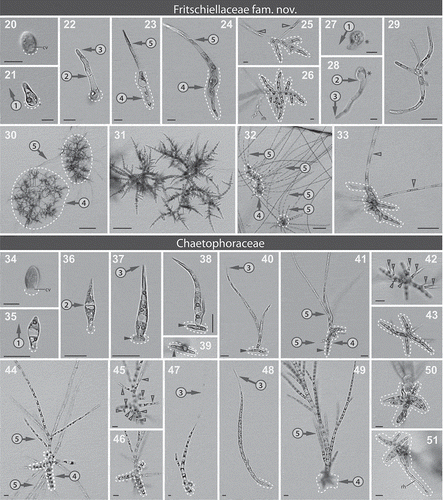
Figs 20–51. Microphotographs showing important morphological characters within the Fritschiellaceae fam. nov. (Figs 20–33) and the Chaetophoraceae (Figs 34–51). Figs 20–26. Development of ‘Chaetophora elegans’ ACOI 457. Fig. 20. Attached zoospore, CV: contractile vacuole. Fig. 21. Pseudo-erect type of germination (encircled number 1). Fig. 22. First cross wall located in the lateral outgrowth of the germling (encircled number 2), apical cell slightly rounded (encircled number 3). Figs 23–24. Young filament with the prostrate (encircled number 4, dashed line) and upright system (encircled number 5). Fig. 25. Mature thallus with two upright filaments (empty arrowheads) per one prostrate system (dashed line). Fig. 26. Fully developed prostrate system, rh: rhizoid. Figs 27–29. Fritschiella tuberosa SAG 112.80. Fig. 27. Germination – note that the type of germination cannot be clearly recognized. Fig. 28. First cross wall located close the lateral outgrowth of the germling (encircled number 2), apical cell with slightly acute shape (encircled number 3). Fig. 29. Mature thalli, the upright and the prostrate system cannot be distinguished. Asterisks: identical cell. Figs 30–31. ‘Stigeoclonium tenue’ CCAC 3492 B. Fig. 30. Mature thalli with several upright filaments (encircled number 5) per one prostrate system (encircled number 4, dashed line). Fig. 31. Prostrate system. Figs 32–33. ‘Stigeoclonium sp.’ CCAC 1901 B. Fig. 32. Mature thalli with the upright (encircled number 5) and the prostrate system (encircled number 4, dashed line). Fig. 33. Mature thallus with two upright filaments (empty arrowheads) per one prostrate system (dashed line). Figs 34–43. Development of Stigeoclonium variabile CCAP 477/13. Fig. 34. Attached zoospore, CV: contractile vacuole. Fig. 35. Erect type of germination (encircled number 1). Fig 36. First cross wall situated in the centre of the germling (encircled number 2). Figs 37–39. Formation of the prostrate system (dashed line) over two days, full arrowheads: older outgrowth. Figs 37–38. Side view. Fig. 39. View from the bottom. Fig. 40. Apical cell of the main filament extended into a hair, young thallus (encircled number 3), full arrowheads: older outgrowth of the prostrate system. Figs 41–43. Mature thallus with developed upright (encircled number 5) and prostrate system (encircled number 4, dashed line). Fig. 41. Side view, full arrowheads: older outgrowth of the prostrate system. Figs 42–43. Bottom view, two optical planes of one thallus showing several upright filaments (empty arrowheads) per one prostrate system. Figs 44–47. Chaetophora lobata ACOI 447. Fig. 44. Mature thallus with several upright filaments (encircled number 5) per one prostrate system (encircled number 4, dashed line), side view. Figs 45–46. Bottom view, two optical planes of one thallus showing several upright filaments (empty arrowheads) per one prostrate system (dashed line). Fig. 47. Apical cell of the main filament extended into a hair, germling (encircled number 3). Figs 48–51. Draparnaldia plumosa ACOI 629. Fig. 48. Apical cell of the main filament extended into a hair, germling (encircled number 3). Fig. 49. Mature thallus with upright (encircled number 5) and prostrate system (encircled number 4, dashed line), side view. Fig. 50. Detail of prostrate system (dashed line), bottom view. Fig. 51. Prostrate system with rhizoid (rh), bottom view. Dashed lines: prostrate system/site of attachment. Scale bars: 10 µm.
(1) Type of zoospore germination (labelled with encircled number 1 in all Figures). In the Chaetophorales three types of zoospore germination can be distinguished. The most common is the erect germination that leads to a simultaneous differentiation of a prostrate and an upright system. The erect germination is typical for the Schizomeridaceae, Barrancaceae, Uronemataceae and the Chaetophoraceae emended here (= the Chaetophora clade sensu Caisová et al., Citation2015), (, , , –, –). By contrast, the Aphanochaetaceae and some members of the Fritschiellaceae fam. nov. described here (= the Fritschiella clade sensu Caisová et al., Citation2015) display the prostrate type of germination (, -Type 2, –). They initially produce a prostrate system from which an upright system may later develop. Sporadically, a pseudo-erect germination occurs in ‘Chaetophora elegans’ ACOI 457 (Fritschiellaceae fam. nov.) (-Type 1, –). This type of germination gives rise to an erect filament before initiating a prostrate system. Note that in Fritschiella the type of germination is unclear (–).
(2) Formation of first cross walls (labelled with encircled number 2 in all Figures). First cross walls arise in two ways: (1) The attached germling divides parallel to the substrate with the first cross wall located in the centre of the germling. This division seems to be associated with taxa having the erect type of germination, i.e. with the Schizomeridaceae, Barrancaceae, Uronemataceae and the Chaetophoraceae emend. (, , , , ). (2) The attached germling divides perpendicular to the substrate and the first division takes place either in the centre (Aphanochaetaceae, , –) or in the lateral outgrowth(s) of the germling (Fritschiellaceae fam. nov., , , ). Perpendicular division occurs in taxa with the pseudo-erect (Fritschiellaceae fam. nov.) and the prostrate (Aphanochaetaceae, Fritschiellaceae fam. nov.) type of germination.
(3) Nature of the apical cell of germlings/young filaments (labelled with encircled number 3 in all Figures). The morphology of apical cells of the germling/young filament is an important character in the early development of several chaetophoralean families. In the Schizomeridaceae the apical cell has a pointed tip that is often slightly curved (, ). In the Chaetophoraceae emend. the apical cell of a main axis of the erect filament is extended into a hair (, , , , ). In others, the apical cell has either a rounded (Aphanochaetaceae, Fritschiellaceae fam. nov., , , ) or slightly acute or acuminate tip (Barrancaceae, Uronemataceae, Fritschiellaceae fam. nov., , , , ).
(4) Nature of prostrate system (= type of attachment) (labelled with encircled number 4 and grey shading or dashed line in all Figures). The prostrate system defines the polarity of the alga (-grey background, –-dashed line), and is determined early in development: The zoospore attaches by its anterior end (where the flagella are inserted), which contains the hyaline protoplasm and contractile vacuoles. After flagella retraction, the hyaline protoplasm with contractile vacuoles either divides and moves to opposite parts of the germling (prostrate germination) or remains at the anterior end (erect and pseudo-erect germination). The hyaline part(s) with protoplasm represent(s) the origin and direction of elongation of the prostrate system. Three types of the prostrate system/attachment can be recognized based on the pattern of zoospore germination:
The holdfast is typical for the Barrancaceae (fig. 1G in Caisová et al., Citation2015) and the Uronemataceae (), both with erect zoospore germination. The holdfast has been studied previously by Caisová et al. (Citation2015). It consists of a circular, hyaline, extracellular polysaccharide-rich matrix that is firmly attached to the substrate. It also includes a ring-like structure that is built from cell wall material (either cellulose or chitin).
The single-celled prostrate system occurs in the Schizomeridaceae, another family with erect type of zoospore germination (, –). It is initially defined by a ring-like structure attached to the substrate. Later, a single-celled filament is formed as an outgrowth from the ring, and is connected to the substrate by its end. Both the ring-like structure and the single-celled outgrowth stain positively with Calcofluor White (). Occasionally the ring-like structure and the single-celled outgrowth produce a reduced holdfast (not shown).
The multi-celled filamentous prostrate system occurs in algae with prostrate, and pseudo-erect zoospore germination (Aphanochaetaceae, Fritschiellaceae fam. nov.), as well as in one family with erect type of zoospore germination (Chaetophoraceae emend). It arises in one of two ways: (1) Simultaneously – simultaneous outgrowths from one, two or four hyaline area(s) of the zoospore/germling, see Aphanochaetaceae for bipolar (, –) and cruciate germlings (fig. 18 in Tupa, Citation1974), and Fritschiellaceae fam. nov. for unipolar and bipolar germlings ( – Type 1 and Type 2, –, –, –). (2) Progressively – two to four outgrowths emerge one after another from a single hyaline area of the zoospore/germling, see Chaetophoraceae emend. (, –, , , –). In all cases the prostrate filaments are attached to the surface along their length, and grow by elongation and subsequent divisions of their apical cells. Each cell of the filament except the apical cell can give rise to a new branch. New branches are initiated from the central part of the parental cell and oriented at right angles relative to the main axis (–, , , , , ). In the adult stages the main axis is often no longer manifest. Occasionally rhizoid-like structures can occur ().
(5) Nature of upright system (labelled with encircled number 5 in all Figures). The complexity of the upright system correlates with the complexity of prostrate system (= type of attachment):
The Chaetophorales with simple attachment (i.e. holdfast, single-celled prostrate system) possess only one erect filament per attachment. This erect filament is unbranched or with ‘false branching’ (Caisová et al., Citation2015), displays intercalary growth, and is typical for the Schizomeridaceae, Barrancaceae and Uronemataceae (, , ).
By contrast, the Chaetophorales with complex attachment (i.e. a multi-celled filamentous prostrate system) possess several erect filaments per attachment. Erect filaments generally develop from the centre towards the margin of the prostrate system. The first erect filament is formed from the original zoospore cell, others from any other cell of the prostrate system except the most apical ones (e.g. –, ). Erect filaments grow by elongation and transverse divisions of apical (Aphanochaetaceae, Fritschiellaceae fam. nov.) or intercalary cells (Chaetophoraceae emend). They can be branched or unbranched: poorly developed unbranched filaments occur in the Aphanochaetaceae (, ), well developed unbranched filaments in the Fritschiellaceae fam. nov. (, , ), and branched filaments occur in the Chaetophoraceae emend. (, , ). Note that in Fritschiella (Fritschiellaceae fam. nov.) the upright and the prostrate systems cannot be distinguished (). Branched filaments consist of main axes, side branches (growing upwards) and occasionally rhizoids (growing downwards from the basal cells of main axes) (e.g. , , , respectively). Whereas the main axis and side branches show intercalary growth, rhizoids grow by apical divisions. Both side branches and rhizoids grow out from the apical parts of square/rectangular parental cells, forming an acute angle with the main axis (, , ). In older developmental stages, a main axis is often no longer manifest. An exception is Draparnaldia, in which a main axis can often be clearly distinguished (). In addition, Draparnaldia is the only known member of the Chaetophoraceae emend. with only one (very rarely two) erect filament per one complex attachment (–).
Another potentially important character is the number of flagella per zoospore. Whereas most of the Chaetophorales (Aphanochaetaceae, Fritschiellaceae fam. nov. and Chaetophoraceae emend) have exclusively 4 flagella per zoospore, the Barrancaceae and Schizomeridaceae display up to 24 (multiples of 4) flagella per zoospore (Prasad & Srivastava, Citation1963; Caisová et al., Citation2015; present study). However, in the Uronemataceae, the situation seems to be more complicated. Uronema trentonense CCAP 386/5 displays 4, 8 and 12 flagella per zoospore, but in Uronema sp. CCAC 3491 only quadriflagellate zoospores were observed (present study). To clarify the number of flagella per zoospore in the Uronemataceae, further studies using other strains are necessary. Therefore, we did not include the number of flagella per zoospore in the list of taxonomically important morphological characters. A summary of above listed principal morphological characters for the systematics and taxonomic revision of the Chaetophorales at the family level is provided in .
Taxonomic revision
The Chaetophorales Wille Citation1901 as circumscribed by Caisová et al. (Citation2015) currently consists of five families: Schizomeridaceae, Aphanochaetaceae, Barrancaceae, Uronemataceae and Chaetophoraceae. The Chaetophoraceae further splits into two molecularly well-supported clades, the Fritschiella clade and the Chaetophora clade. Our present study also revealed that the Fritschiella and Chaetophora clades differ by several morphological characters, summarized in . Thus, on the basis of these morphological (present study) and molecular (Caisová et al., Citation2011, Citation2015) differences, we here erect a new family, Fritschiellaceae fam. nov. and emend the Chaetophoraceae (Greville Citation1824) emend. Caisová et al. Citation2015.
Fritschiellaceae fam. nov
With the general characteristics of the order Chaetophorales
Description: Filaments with apical–basal polarity defined by a multicellular prostrate system at the end of the filament attached to the substrate. Zoospore germination prostrate or pseudo-erect. The first cross wall of the attached germling perpendicular to the substrate, in the (lateral) outgrowth(s) of the germling. Apical cell of the germling slightly acute or acuminate. Young and mature filaments consist of the prostrate and the upright system. The prostrate system multicelled, developed by unilateral outgrowth from the original zoospore cell (taxa with pseudo-erect germination) or simultaneously by bilateral outgrowths from the original zoospore cell (taxa with prostrate germination). The upright system consists of several well-developed mostly unbranched filaments per one prostrate system. Upright and prostrate system with apical growth.
Fritschiellaceae fam. nov. is the most inclusive clade containing Fritschiella tuberosa SAG 112.80 but not Chaetophora lobata ACOI 447.
Type genus: Fritschiella Iyengar (Citation1932), p. 335.
Type species: Fritschiella tuberosa Iyengar (Citation1932), p. 335, figs 1, 9.
Lectotype (designated here): fig. 1 in Iyengar (Citation1932).
Chaetophoraceae Greville (Citation1824), p. xix, 321 (named as Chaetophoroideae) emend.
With the general characteristics of the order Chaetophorales
Emended description: Zoospore germination is erect. The first cross wall of the attached germling is parallel to the substrate and located in the centre of the germling. The apical cell of the germling extends into a hair. The prostrate system develops successively by usually two to four outgrowths from the original zoospore cell. The prostrate system consists either of filaments with ± square cells, which are firmly attached to the substrate along their whole length (firm substrate), or of filaments with long thin cells (rhizoids) penetrating into the substrate (soft substrate). Prostrate system with apical growth. The upright system consists of one/two (Draparnaldia) or mostly several erect branched filaments per one prostrate system. Branches forming an acute angle with the main axis, sometimes terminating with gradually tapering, multicellular hairs. Upright system with intercalary growth. As a non-homoplasious molecular synapomorphy, the first pair of Helix 18 [H441] in the nuclear-encoded 18S rRNA is U-A instead of G-C (unique within Chaetophorales), see Caisová et al. (Citation2011).
Type genus: Chaetophora Schrank (Citation1783), pp. 124, 125.
Type species (for lectotype, see Caisová et al., Citation2011): Chaetophora lobata Schrank (Citation1783), pp. 125, 126; figs. 2, 4.
Discussion
Classification of the Chaetophorales follows the ICN (International Code of Nomenclature for algae, fungi, and plants; Melbourne Code; http://www.iapt-taxon.org/nomen/main.php). It was originally based on morphological data (e.g. Islam, Citation1963; Cox & Bold, Citation1966; Tupa, Citation1974) and more recently phylogenetic information was added (Caisová et al., Citation2011, Citation2015). Here, for the first time, we have analysed and interpreted morphological data of the Chaetophorales in a phylogenetic context. We have identified five morphological traits/characters, which can be used to classify families in the Chaetophorales, except for discriminating Barrancaceae and Uronemataceae. However, in these two families, morphological stasis is combined with considerable genetic diversity at the rDNA level (as often occurs in unicellular algae). Thus, we think it is justified to separate taxa (genera) of the Chaetophorales into families as these are monophyletic and differ both molecularly and for the most part morphologically using the characters established in this study. For distinguishing Barrancaceae (there is currently only one taxon known) from Uronemataceae, we consider molecular non-homoplasious synapomorphies and zoospore formation (as described in Caisová et al., Citation2015) as diagnostic characters.
In order to ensure that our results are fully reproducible, this study was solely based on cultures and defined experimental conditions. In fact, the taxonomic significance of two of five characters identified here (type of zoospore germination and prostrate system morphology) had already been recognized previously (Cox & Bold, Citation1966; Tupa, Citation1974; Van Beem & Simons, Citation1988; Michetti et al., Citation2004; Guo-Hui et al., Citation2006). However, these characters were assigned to species-level instead of family-level classification, see for example investigations of the genus Stigeoclonium in Cox & Bold (Citation1966), Francke & Simons (Citation1984), Simons et al. (Citation1986) and Michetti et al. (Citation2010). This wrong-level assignment can be explained by a lack of phylogenetic information: Chaetophoralean classification is traditionally based on morphology and Stigeoclonium had been accepted as a morphologically well-defined genus (Islam, Citation1963; Cox & Bold, Citation1966; John, Citation1984). However, with the first molecular data it became clear that genus Stigeoclonium is polyphyletic and its species are dispersed over two well-supported clades (the Fritschiella and Chaetophora clade) in Caisová et al. (Citation2011). Because these two clades are now recognized as two different families (Fritschiellaceae fam. nov. and Chaetophoraceae emend., present study), characters that were originally assigned to species-level now are associated with family-level classification. This example clearly highlights the importance of interpretation of morphological data in their phylogenetic context.
The Chaetophorales are organisms that are not only taxonomically difficult, but also widespread, environmentally important and fascinatingly complex with patterns of differentiation and specialization that rival the situation seen in basal land plants. Therefore, our morphological analyses, in addition to their taxonomic implications, also provide a framework for studying developmental processes and responses of these algae to environmental cues. In the following, we discuss three morphological processes with relevance to development and environmental adaptations.
(1) Zoospore germination – In the Chaetophorales three main types of zoospore germination are observed – erect, pseudo-erect and prostrate. Which type is favoured can already be recognized during zoospore settling. The zoospore either ‘walks’ on the substrate with its flagella (typical for the prostrate germination) or ‘spins’ against the substrate (typical for erect and pseudo-erect germination). We assume that these different modes of behaviour might relate to the specific development (substrate space requirements) of chaetophoralean zoospores after their attachment: The ‘walking’ zoospore becomes amoeboid, flattens and germinates horizontally (–). – The zoospore is attached by a large area of its cell body, which requires a certain space (without epiphytes) on the substrate. → The ‘walking’ mode of behaviour is probably needed to screen the substrate before settling. In contrast, the ‘spinning’ zoospore keeps its original ± ellipsoid shape (species with erect germination) or becomes slightly rounded (species with the pseudo-erect germination) and germinates to form an upright (vertical) filament (–, –). The zoospore is attached only by its anterior end and does not require so much ‘empty’ substrate for growth. The different space requirements proposed for zoospore attachment are also relevant during later developmental stages in the Chaetophorales. As already noticed by Cox & Bold (Citation1966) and Michetti et al. (Citation2004), species with a pseudo-erect or erect type of germination, in general, have a less developed prostrate system than those with a prostrate type of germination. A special case is the genus Fritschiella (Fritschiellaceae fam. nov.), in which distinction between the prostrate and upright system or the types of germination cannot be made (Singh, Citation1941; Cox & Bold, Citation1966; present study). A better taxon sampling in the Fritschiellaceae would be needed to understand developmental processes in Fritschiella.
(2) Angle of branching – The upright and prostrate system of the Chaetophorales differ fundamentally with respect to filament branching. In the prostrate system branching is oriented at right angles relative to the main axis, whereas in the upright system branching angles are acute. These different branching angles in the upright and prostrate systems may be related to where in the parental cell the branch is initiated. In the case of the prostrate systems the branching angle (around 90°) is probably a function of the branch being generated from the middle of a square/rectangular cell which then presumably extends across a 2-D surface to maximize contact with, and thus adherence to, the substrate. The upright system is somewhat more interesting because the polarity of the branching event within the parental cell seems to be important (branches with acute angles, growing upwards, originate from the apical part of a parental cell). Whether, in this case, the positioning of the branching site within the cell is determined by endogenous signals/processes or by gravity is not clear and further research is needed to address this question. It can even be speculated that there is some analogy to branching angles in the shoots of flowering plants in terms of enhancing light capture. In the Chaetophorales, the acute angle of branching allows lateral spread of the thallus, optimizing light utilization in three dimensions but in a streamlined way with respect to how the alga would be orientated in a flow of water.
(3) Morphological plasticity – From the literature it is known that the derived members of the Chaetophorales (in present study defined as the Fritschiellaceae fam. nov. and Chaetophoraceae emend.) show considerable plasticity in a series of morphological characters, such as the prostrate system (Islam, Citation1963; Simons et al., Citation1986). Our observations revealed that the morphological differentiations of the prostrate system may relate to different types of substrate. For example, in liquid medium with a firm substrate (glass, plastic), the prostrate system of some strains consists of filaments (with ± squared cells) that are firmly attached to the substrate along their whole length. While in biphasic medium (agar covered with a thin layer of liquid) the prostrate system of the same strains consists of rhizoids (long thin cells) growing into the agar. This morphological shift (1) explains difficulties in the identification of several chaetophoralean species that were solely based on the presence/absence of rhizoids (mainly in Stigeoclonium), and (2) it highlights a remarkable ability of chaetophoralean algae to adapt to different environments. However, not all morphological characters are plastic. Since the five different morphological characters (identified in this study) correlate with phylogeny (and thus systematics), selection of the characters must have operated on long-term environmental adaptations. For example, according to our observations, a combination of a less developed prostrate system and a well-developed upright system with branched filaments (= Chaetophoraceae emend.) is presumably favoured in water with currents (i.e. rivers, streams, inlets and outlets of a pond/pool, shores of the larger lakes). In contrast, a combination of a well-developed prostrate system and a less developed upright system with unbranched filaments (= Fritschiellaceae fam. nov.) is favoured in calm water bodies (ponds, rain-water puddles) and spray zones of waterfalls and around springs.
Clearly, the discovery of stable morphological characters to differentiate families provides additional support for the current classification of the Chaetophorales. According to our present knowledge the morphological characters determined here to define families can be applied for the Chaetophorales in general, except for discriminating Barrancaceae and Uronemataceae. Nevertheless, these two families show a considerable genetic diversity at the rDNA level. In addition, Barrancaceae have so far only been found in a very special habitat (volcanic canyon) and display several developmental traits (zoospore and aplanospore formation, fragmentation through ‘filament splitting’) that are correlated with a highly fluctuating environment (Caisová et al., Citation2015). In contrast, we know next to nothing about developmental adaptations to habitats in the Uronemataceae. Therefore, it is difficult to discuss the Barrancaceae/Uronemataceae issue in further detail here. Perhaps future biodiversity and genome studies can shed light on this question.
Also, it should be stressed that the set of morphological characters identified in this study is based exclusively on cultures, and that these characters are stable and reproducible under the experimental conditions chosen. We do not imply that all Chaetophorales can be identified at family level by inspection of natural samples nor can we exclude that additional phylogenetic lineages will be discovered in the Chaetophorales, which do not differ in the morphological traits from the families identified here (as in Barrancaceae and Uronemataceae). In order to avoid such ambiguities, we have included in the diagnosis the boundaries of the families by exclusion criteria (Fritschiellaceae fam. nov. is the most inclusive clade containing Fritschiella tuberosa SAG 112.80 but not Chaetophora lobata ACOI 447, present study) and non-homoplasious molecular synapomorphies (i.e. molecular signatures unique for the clades/families within the Chaetophorales, see Chaetophoraceae emend. in the present study and Barrancaceae in Caisová et al., Citation2015).
In conclusion, the present study demonstrated the value of using molecular phylogeny to probe morphological traits that could support classification. Knowledge gained from this study is important not only for chaetophoralean systematics but also for understanding developmental processes and behavioural and morphological adaptations to various environmental factors in this group of algae.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https:doi.org/10.1080/09670262.2018.1453090
Supplementary table 1. A complete list of morphological characters included in the analyses. A total of 20 morphological characters were analysed. Characters which were identified as taxonomically important are in bold. The presence and absence of the individual characters in 14 investigated strains is indicated by + and −, respectively.
Supplementary fig 1. Chaetophoralean strains investigated in this study. Strains investigated are highlighted in a blue background. Strain selection was made based on the recently published chaetophoralean RAxML phylogeny (Caisová et al., Citation2015). This phylogeny is based on 3498 positions of the nuclear-encoded rRNA operon. Node support values are shown as follows: RAxML/NJ/MP/MrBayes. Branches with maximally supported nodes (100/100/100/1.00) are in boldface. Several strains of the Chaetophorales are with two accession numbers. The first accession number refers to the 18S rRNA gene and the second to the 5.8S rRNA gene, ITS2 and partial 28S rRNA gene.
Supplementary fig. 2. Morphological observations – Experimental setup. A schematic workflow from zoospore induction to microscopic observation is presented. Two different strategies were applied: (1) The Aphanochaetaceae (= algae with reduced upright system) were grown and directly observed in a thin aluminium slide chamber. (2) All Chaetophorales with exclusion of Aphanochaetaceae (= algae with well-developed upright system) were grown on coverslips in a large Petri dish and in the IBIDI glass bottom Petri dish. Coverslips were attached to the microscope slides (→ the upright system gets flattened) and used for the observation of young stages. The IBIDI Petri dish was used for observation of older developmental stages. In this case, the upright system was flattened by using a circular coverslip.
TEJP-2017-0078-File008.pdf
Download PDF (361.8 KB)TEJP-2017-0078-File007.pdf
Download PDF (381 KB)TEJP-2017-0078-File006.pdf
Download PDF (37.3 KB)Acknowledgements
We thank two anonymous reviewers for their valuable comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Lenka Caisová
L. Caisová: original concept and study design, experiments and data analyses, writing the manuscript. M. Melkonian: original concept and study design, editing the manuscript.
References
- Bonaventura, S.M., Vinocur, A., Allende, L. & Pizarro, H. (2006). Algal structure of the littoral epilithon in lentic water bodies at Hope Bay, Antarctic Peninsula. Polar Biology, 29: 668–680.
- Bory de Saint-Vincent M. (1808). Mémoire sur le genre Draparnaldia, de la famille des Conferves (Voyez la planche 35). Annales du Muséum d’histoire naturelle, 12: 399–409.
- Caisová, L. (2016). Dicranochaete – an enigmatic green alga with surprising adaptive capabilities. Phycologia, 55: 219–229.
- Caisová, L., Marin, B., Sausen, N., Pröschold, T. & Melkonian, M. (2011). Polyphyly of Chaetophora and Stigeoclonium within the Chaetophorales (Chlorophyceae), revealed by sequence comparisons of nuclear-encoded SSU rRNA genes. Journal of Phycology, 47: 164–177.
- Caisová, L., Marin, B. & Melkonian, M. (2013). A consensus secondary structure of ITS2 in the Chlorophyta identified by phylogenetic reconstruction. Protist, 164: 482–496.
- Caisová, L., Pérez Reyes, C., Cruz Álamo, V., Martel Quintana, A., Surek, B. & Melkonian, M. (2015). Barrancaceae – a new green algal lineage with structural and behavioral adaptations to a fluctuating environment. American Journal of Botany, 102: 1482–1492.
- Cook, P.W. (1970). An unusual new species of Draparnaldia from Lake Champlain. Journal of Phycology, 6: 62–67.
- Cox, E.R. & Bold, H.C. (1966). Taxonomic investigations of Stigeoclonium. Phycological Studies, 7: 1–167.
- Francke, J.A. & Simons, J. (1984). Morphology and systematics of Stigeoclonium Kützing (Chaetophorales). In Systematics of the Green Algae (Irvine, D.E.G. & John, D.M., editors), 363–377. Academic Press, London.
- Greville, R.K. (1824). Flora Edinensis: or a description of plants growing near Edinburgh, arranged according to the Linnean System, with a concise introduction to the natural orders of the Class Cryptogamia, and illustrative plates. W. Blackwood, Edinburgh.
- Guo-Hui, Z., Wen-Mei, B., Quan-Xi, W. & Jian-Guo, C. (2006). A culture study of heterotrichy and its characteristics in three Stigeoclonium species. Acta Phytotaxonomica Sinica, 44: 654–669.
- Hazen, T.E. (1902). The Ulotrichaceae and Chaetophoraceae of the United States. Memoirs of the Torrey Botanical Club, 11: 135–250.
- Islam, A.K.M.N. (1963). A revision of the genus Stigeoclonium. Nova Hedwigia Beihefte, 10: 1–164.
- Iyengar, M.O.P. (1932). Fritschiella, a new terrestrial member of the Chaetophoraceae. New Phytologist, 31: 329–335.
- John, D.M. (1984). On the systematic of the Chaetophorales. In Systematics of the Green Algae (Irvine, D.E.G. & John, D.M., editors), 207–232. Academic Press, London.
- Kützing, F.T. (1843). Phycologia Generalis oder Anatomie, Physiologie und Systemkunde der Tange. F.A. Brockhaus, Leipzig.
- Michetti, K.M., Leonardi, P.I. & Cáceres, E.J. (2004). Zoospore germination and germling development in Chaetophora elegans (Chaetophorales, Chlorophyta). Algological Studies, 111: 115–126.
- Michetti, K.M., Leonardi, P.I. & Cáceres, E.J. (2010). Morphology, cytology and taxonomic remarks of four species of Stigeoclonium (Chaetophorales, Chlorophyceae) from Argentina. Phycological Research, 58: 35–43.
- Prasad, B.N. & Srivastava, P.N. (1963). Observations on the morphology, cytology, and asexual reproduction of Schizomeris leibleinii. Phycologia, 2: 148–156.
- Printz, H. (1964). Die Chaetophoralean der Binnengewässer (eine systematische Übersicht). Hydrobiologia, 24: 1–376.
- Reize, I.B. & Melkonian, M. (1989). A new way to investigate living flagellated/ciliated cells in the light microscope: immobilization of cells in agarose. Botanica Acta, 102: 145–151.
- Sarma, P. (1986). The freshwater Chaetophorales of New Zealand. Nova Hedwigia Beihefte, 58: 1–163.
- Saxena, P.N. (1962). Algae of India-1, Chaetophorales. Bulletin of the National Botanic Gardens, 57: 1–59.
- Schrank, F.v.P. (1783). Botanische Rhapsodien. Der Naturforscher (Halle), 19: 116–126.
- Simons, J., Van Beem, A.P. & De Vries, P.J.R. (1986). Morphology of the prostrate thallus of Stigeoclonium (Chlorophyceae, Chaetophorales) and its taxonomic implications. Phycologia, 25: 210–220.
- Singh, R.N. (1941). On some phases in the life history of the terrestrial alga Fritschiella tuberosa Iyeng., and its autecology. New Phytologist, 40: 170–182.
- Skinner, S. & Entwisle, T.J. (2004). Non-marine algae of Australia: 5. Macroscopic Chaetophoraceae (Chaetophorales, Chlorophyta). Telopea, 10: 613–633.
- Tupa, D.D. (1974). An investigation of certain chaetophoralean algae. Nova Hedwigia Beihefte, 46: 1–155.
- Van Beem, A.P. & Simons, J. (1988). Growth and morphology of Draparnaldia mutabilis (Chlorophyceae, Chaetophorales) in synthetic medium. British Phycological Journal, 23: 143–151.
- Wille, N. (1901). Algologische Notizen. 7, 8. Nyt Magazin for Naturvidenskaberne 39: 1–24.
