ABSTRACT
Sequence data generated during a Canadian barcode survey (COI-5P) of the tribes Polysiphonieae and Streblocladieae, a large and taxonomically challenging group of red algae, revealed significant taxonomic confusion and hidden species diversity. Polysiphonia pacifica Hollenberg, P. paniculata Montagne, P. stricta (Dillwyn) Greville and Vertebrata fucoides (Hudson) Kuntze were all complexes of two or more genetically distinct yet overlooked species. One variety of P. pacifica was elevated to the rank of species as P. determinata (Hollenberg) Savoie & Saunders, stat. nov. Several new additions to the Canadian flora were recorded including P. kapraunii Stuercke & Freshwater and P. morrowii Harvey. Subsequent multi-gene (COI-5P, LSU and rbcL) phylogenetic analyses confirmed that the genus Polysiphonia Greville was polyphyletic, and currently assigned species resolved with many other genera. Polysiphonia sensu stricto was restricted to a group of species that formed a monophyletic lineage with the type, Polysiphonia stricta. Carradoriella P.C.Silva was resurrected based on the South African species Carradoriella virgata (C.Agardh) P.C.Silva. Species previously attributed to Polysiphonia were transferred to Carradoriella, Leptosiphonia and Vertebrata as well as to three new genera described here: Acanthosiphonia gen. nov., based on A. echinata (Harvey) comb. nov.; Eutrichosiphonia gen. nov. for E. confusa (Hollenberg) comb. nov. and E. sabulosia (B.Kim & M.S.Kim) comb. nov.; and Kapraunia gen. nov., which includes K. schneideri (Stuercke & Freshwater) comb. nov. and three additional species.
Introduction
Recent taxonomic changes in the family Rhodomelaceae resulted in the segregation of the tribe Streblocladieae from the tribe Polysiphonieae, which together contain some 15 genera with over 300 species worldwide (Díaz-Tapia et al., Citation2017a; Guiry & Guiry, Citation2018). Species in these tribes are filamentous and polysiphonous, with terete axes composed of four to 24 pericentral cells surrounding a central axial cell (Womersley, Citation2003). Thalli have a radial or dorsiventral construction, with branches and trichoblasts either in a spiral pattern or arranged unilaterally on the adaxial side of the axes (Hommersand, Citation1963). Some vegetative characters that were traditionally afforded taxonomic importance for genus- and species-level distinction included: the number of pericentral cells, the presence or absence of cortication and trichoblasts, the position of trichoblasts and scar cells, the relationship of lateral branches to trichoblasts, and whether rhizoids were in open connection with pericentral cells or cut off by a cross-wall (Hollenberg, Citation1942a; Kapraun Citation1977; Stuercke & Freshwater, Citation2008; Bárbara et al., Citation2013). Reproductive characters considered important included the arrangement of tetrasporangia, the number of tetrasporangia per segment, the size and shape of cystocarps, the number of cells in the carpogonial branch (three or four), and the shape of spermatangial axes, their arrangement in relation to trichoblasts, and the number of sterile terminal cells (Kim & Lee, Citation1999; Stuercke & Freshwater, Citation2008).
At the core of the tribe Polysiphonieae sensu lato (including the recently segregated Streblocladieae), the genus Polysiphonia Greville has long been in need of a comprehensive molecular-assisted taxonomic revision. It is the largest genus of red algae, including approximately 200 species worldwide, which are widely distributed, as well as morphologically and ecologically diverse (Maggs & Hommersand, Citation1993; Guiry & Guiry, Citation2018). This diversity, combined with its convoluted nomenclatural history, renders Polysiphonia an especially difficult genus for taxonomic and phylogenetic research (Kim et al., Citation2000; Choi et al., Citation2001).
The name Hutchinsia was originally proposed for this group by C. Agardh (Citation1817); however, Polysiphonia was proposed as a replacement by Greville (Citation1823) because Hutchinsia had already been used for a genus of cruciferous plants rendering Hutchinsia C.Agardh illegitimate. In his Flora Edinensis, Greville (Citation1824) included eight species of Polysiphonia, but failed to designate a type. Polysiphonia urceolata (Dillwyn) Greville was designated as the lectotype species by Silva (Citation1952), and was later synonymized with Polysiphonia stricta (Dillwyn) Greville (Maggs & Hommersand, Citation1993). Polysiphonia was conserved against other names for this genus, viz. Grammita Bonnemaison, Gratelupella Bory de St. Vincent and Vertebrata S.F.Gray (Greuter et al., Citation1994). Kylin (Citation1956) and Christensen (Citation1967) both recognized the genus Vertebrata, for V. lanosa (Linnaeus) T.A.Christensen, as distinct from Polysiphonia. However, the segregation of V. lanosa from Polysiphonia was not widely accepted until Choi et al. (Citation2001) presented molecular evidence to support recognition of this genus.
In ongoing efforts to render Polysiphonia monophyletic, Kim & Lee (Citation1999) proposed recognition of the genus Neosiphonia M.S.Kim & I.K.Lee based on Neosiphonia flavimarina M.S.Kim & I.K.Lee from Korea. Several morphological characters were used to distinguish Neosiphonia from Polysiphonia including: rhizoids that are cut off from pericentral cells, procarps bearing a three-celled carpogonial branch, and tetrasporangia arranged in a spiral series (Kim & Lee, Citation1999). Soon after recognition of Neosiphonia, an important re-assessment of the type of Polysiphonia, P. urceolata (= P. stricta), was undertaken by Kim et al. (Citation2000). They examined type material to characterize accurately the vegetative and reproductive characters of this species, and found that P. stricta differs in several key morphological characters from most other species assigned to this genus. Among the defining characters were: four pericentral cells, absence of cortication, rhizoids in open connection with pericentral cells, trichoblasts absent or rare, and tetrasporangia occurring in straight series. Kim et al. (Citation2000) noted that this combination of morphological characters was also shared by a small group of Polysiphonia species including P. atlantica Kapraun & J.N.Norris, P. morrowii Harvey, P. pacifica Hollenberg, P. scopulorum Harvey, and P. subtilissima Montagne, but that most other species assigned to this genus have more than four pericentral cells, cortication, rhizoids that are cut off from pericentral cells by a cross wall, abundant trichoblasts, and tetrasporangia occurring in spiral series. Despite these findings, no formal taxonomic changes were proposed.
Molecular tools such as DNA barcoding and phylogenetic analyses have been critical for resolving outstanding taxonomic issues in this difficult group. In one of the first published phylogenetic analyses to consider Polysiphonia, Choi et al. (Citation2001) used nuclear small-subunit rDNA (SSU) sequence data to show that this genus was polyphyletic. In their analyses, Polysiphonia resolved as three well-supported lineages: the ‘Polysiphonia group’, which included the type species, P. stricta; the ‘Neosiphonia group’ which included species of Neosiphonia, as well as P. elongata (Hudson) Sprengel and P. virgata (C.Agardh) Sprengel; and the ‘multi-pericentral group’, which included species of Boergeseniella Kylin, Enelittosiphonia Segi and Vertebrata S.F.Gray, as well as several species of Polysiphonia with greater than four pericentral cells (Choi et al., Citation2001). A corresponding analysis of anatomical characters showed that members of the Polysiphonia group (i.e. Polysiphonia sensu stricto) shared the morphological characters discussed by Kim et al. (Citation2000), consistent with the proposal that Polysiphonia should be restricted to a small subset of species with morphological characteristics similar to the type. Subsequent phylogenetic analyses based on SSU and other markers such as COI-5P (the 5’ end of the cytochrome c oxidase subunit I gene) and, most commonly in this group, rbcL (the large subunit of RuBisCO), have also resolved Polysiphonia as polyphyletic (Stuercke & Freshwater, Citation2010; Mamoozadeh & Freshwater, Citation2011, Citation2012; Bárbara et al., Citation2013; Kim & Kim, Citation2014). In a recent study of the tribe Pterosiphonieae, Savoie & Saunders (Citation2016) resurrected the genus Polyostea Ruprecht (abbreviated Po. throughout this manuscript) based on three species of Pterosiphonia as well as Polyostea arctica (J.Agardh) Savoie & G.W.Saunders, and demonstrated that Polyostea belonged in the tribe Polysiphonieae sensu lato.
Based on a combination of morphological evidence and molecular phylogenetic analyses of rbcL and SSU sequence data, Díaz-Tapia et al. (Citation2017b) proposed a major overhaul of the taxonomy of Neosiphonia, Polysiphonia, Vertebrata and related genera. They found that the type of Melanothamnus Bornet & Falkenberg, M. somalensis Bornet & Falkenberg, fell into a large clade of Neosiphonia species including the generitype. Since Melanothamnus has taxonomic priority, 46 species of Fernandosiphonia Levring, Neosiphonia and Polysiphonia were transferred to Melanothamnus, including the types of Fernandosiphonia and Neosiphonia. These species are united by the same characters described by Kim & Lee (Citation1999) for Neosiphonia, most importantly the 3-celled carpogonial branch. The species Neosiphonia echinata (Harvey) N.Mamoozadeh & D.W.Freshwater was transferred back to Polysiphonia as it did not join this clade. Furthermore, based on their molecular phylogenetic analyses of the multi-pericentral group described by Choi et al. (Citation2001), Díaz-Tapia et al. (Citation2017b) concluded that species within this clade should be transferred to Vertebrata, the oldest name, resulting in six new combinations and 13 resurrected names. These transfers included the types of Boergeseniella, Brongniartella Bory, Ctenosiphonia Falkenberg and Enelittosiphonia. Species within the Vertebrata clade have more than six pericentral cells, and have multi-nucleate trichoblast cells (Díaz-Tapia et al., Citation2017b). A second, larger analysis of the family Rhodomelaceae by Díaz-Tapia et al. (Citation2017a) resulted in the segregation of the tribe Streblocladieae from the tribe Polysiphonieae for Lampisiphonia, Leptosiphonia, Melanothamnus, Polyostea, Streblocladia, Vertebrata and others. In essence, Díaz-Tapia et al. (Citation2017a) have restricted the Polysiphonieae to include Polysiphonia sensu stricto and a second lineage in need of genus-level assignment. We have adopted these proposed taxonomic changes throughout our study.
There are 23 species of Polysiphonieae and Streblocladieae from five genera reported in Canada (Melanothamnus, Polysiphonia, Polyostea, Pterochondria Hollenberg and Vertebrata). In eastern Canada, there are 12 species listed: Melanothamnus harveyi (Bailey) Díaz-Tapia & Maggs, Polyostea arctica (an Arctic species), Vertebrata fucoides (Hudson) Kuntze, V. lanosa, V. nigra (Hudson) Díaz-Tapia & Maggs and seven species of Polysiphonia (Sears, Citation2002). In British Columbia, there are 13 species listed: Polyostea bipinnata (Postels & Ruprecht) Ruprecht, Po. hamata (E.S.Sinova) Savoie & G.W.Saunders, Po. robusta (N.L.Gardner) Savoie & G.W.Saunders, Pterochondria woodii (Harvey) Hollenberg, and nine species of Polysiphonia (Gabrielson et al., Citation2006; Filloramo et al., Citation2017). Two of these species, Polysiphonia brodiei (Dillwyn) Sprengel and P. stricta, are reported from both Atlantic Canada and British Columbia (Sears, Citation2002; Gabrielson et al., Citation2006).
The objectives of this study were to conduct a COI-5P barcode survey to assess species diversity within the tribes Polysiphonieae and Streblocladieae in Canada, and also to investigate the evolutionary relationships of species within these tribes using phylogenetic analyses of COI-5P, rbcL and nuclear large-subunit rDNA (LSU) sequence data. Molecular phylogenetic analyses were compared to traditional morphological assessments. The main goal of this study was to determine generic boundaries and establish a natural system of classification within this speciose and diverse group. Species-level taxonomy of cryptic and overlooked taxa will be dealt with in future publications.
Methods
Specimens of Polysiphonieae, Streblocladieae, and other rhodomelacean tribes were collected from the intertidal and subtidal (using scuba) from the Arctic, Atlantic and Pacific coasts of North America, with an emphasis on the coasts of Atlantic Canada (New Brunswick, Newfoundland, Nova Scotia and Prince Edward Island), British Columbia and New England (Connecticut, Maine, Massachusetts, New Hampshire and Rhode Island) between 2004 and 2016 (Supplementary table S1). Specimens were also collected from Australia, Norway, South Africa and South Korea, and samples were sent to us by colleagues from Ireland, Oman and Russia (Supplementary table S1). Collections were dried in silica gel to serve as both a voucher and for subsequent DNA extraction; in some cases additional vouchers were pressed on herbarium paper (Saunders & McDevit, Citation2012).
DNA was extracted from dried material and 664 bp near the 5’ end of the cytochrome c oxidase subunit I gene (COI-5P) were amplified following Saunders & McDevit (Citation2012). The primer pair used for each sequence is recorded with their GenBank accession number (Supplementary table S2). In some cases when COI-5P amplification was unsuccessful the alternative barcode rbcL-3P (800 bp of the large subunit of the RuBisCO gene) was used (Saunders & Moore, Citation2013). Subsequently, rbcL (1363 bp) and the large subunit of ribosomal DNA (LSU, 2696–2864 bp) were also amplified for some representative collections as outlined in Saunders & Moore (Citation2013) (Supplementary table S2). Amplified products were sent to Génome Québec for sequencing and data were edited and aligned using Geneious R7 version 7.1.7 (http://www.geneious.com, Kearse et al., Citation2012). Edited COI-5P sequence data were entered into the BOLDSYSTEMS database webpage (Ratnasingham & Hebert, Citation2007) where a barcode gap analysis with a pairwise distance model was used to determine the inter- and intraspecific variation and nearest neighbour for each genetic species group. To expand our analyses, COI-5P and rbcL sequences for relevant rhodomelacean species were downloaded from GenBank (Supplementary table S2).
A multi-gene concatenated alignment was constructed using 28 LSU, 47 rbcL and 43 COI-5P sequences for 47 taxa (28 taxa had all three markers, 15 taxa had rbcL and COI-5P only, and four taxa had rbcL only). Nine of the 47 rbcL sequences and six of the 43 COI-5P sequences included in the multi-gene concatenated alignment were downloaded from GenBank (Supplementary table S2). For phylogenetic inference RAxML version 7.2.8 in Geneious R7 (Stamatakis, Citation2006) was used to run a maximum-likelihood (ML) analysis on the concatenated alignment with a GTR + I + G model, partitioned by gene and codon, with 1000 bootstrap replicates. Single gene alignments were also analysed to ensure that there were no major discordances between the three markers.
An rbcL-only alignment was also constructed for 87 taxa, with 43 out of the 87 rbcL sequences downloaded from GenBank to expand our analysis. The rbcL sequences ranged in length from 950 to 1363 base pairs. For phylogenetic inference the program RAxML in Geneious R7 was again used to run a ML analysis on the alignment with a GTR + I + G model, partitioned by codon, with 1000 bootstrap replicates.
For morphological assessments dried material was rehydrated in seawater and morphological and reproductive features of the specimens were observed using a Leica DM5000B light microscope and photographed with a Leica DFC480 digital camera. Images were edited using Adobe Photoshop CC 2015 and ImageJ 1.49v. If necessary, slides were stained using a 1% aniline blue solution in 6% 5N hydrochloric acid, and permanent slides were prepared with a 50% corn syrup, 4% formalin solution. For the taxonomic results, homotypic and heterotypic synonyms of species names were retrieved from Algaebase (Guiry & Guiry, Citation2018), as well as taxonomic guides for the north-east Atlantic (Maggs & Hommersand, Citation1993), north-west Atlantic (Sears, Citation2002) and north-east Pacific (Abbott & Hollenberg, Citation1976). Herbarium abbreviations listed in this paper follow Thiers (Citation2018).
Results
DNA barcode survey
Atlantic Canada and the Canadian Arctic
We identified 14 genetic species groups in Atlantic Canada and the Arctic using COI-5P and rbcL-3P sequence data (; Supplementary table S1). Five of these genetic groups could be assigned to reported species for this area: Melanothamnus harveyi (n=94), Polyostea arctica (n=17), Polysiphonia (=Leptosiphonia) flexicaulis (Harvey) Collins (n=30), Vertebrata lanosa (n=11) and V. nigra (n=9). We collected specimens that were a morphological match to Polysiphonia(=Leptosiphonia) fibrillosa (Dillwyn) Sprengel (n=5), but these were 1.07% different in rbcL from a collection of P. fibrillosa from Ireland (AF342912) and 4.65% different in COI-5P from collections of P. fibrillosa from Norway (). The Canadian collections were provisionally named P. sp._1fibrillosa.
Table 1. Intra- and interspecific variation in COI-5P sequence data for species of Polysiphonieae and Streblocladieae from Canada and the contiguous United States.
Two genetic groups were a morphological match to Vertebrata fucoides and were referred to as Vertebrata sp._1fucoides (n=55) and V. sp._2fucoides (n=22). These genetic groups had 0.31% and 0.33% intraspecific divergence respectively, and were 1.66% different from one another (). Vertebrata sp._2fucoides was a match (2–3 bp different) to rbcL sequences in GenBank for European collections of V. fucoides (from Ireland, JX828163 and Spain, JX828146), and we also found this group in Norway (n=1).
Three genetic groups in Atlantic Canada were identified as Polysiphonia stricta based on morphology. One group was considered authentic P. stricta (n=21) and was a match in COI-5P and rbcL sequence (identical, or 1–2 base pairs different) to previously published sequences identified as P. stricta from Europe (England, AY958167, Northern Ireland, AY958163, and Spain, KF648521). We also collected this species in Norway (n=7). Polysiphonia stricta had 0.31% intraspecific divergence in COI-5P sequence and was 2.6% divergent from its nearest neighbour (). The other two genetic groups were assigned temporary names: Polysiphonia sp._1stricta (n=38) and P. sp._3stricta (n=31). These two genetic groups had 0.69 and 1.15% intraspecific divergence in COI-5P, respectively; P. sp._1stricta was most closely related to P. morrowii (3.67% different), while P. sp._3stricta was most closely related to P. stricta (2.6% different; ). Two specimens of Polysiphonia sp._1stricta were collected in northern British Columbia (Supplementary table S1).
The final three genetic species groups all represented new additions to the Canadian flora: Polysiphonia(=Acanthosiphonia) echinata Harvey (n=15), P. kapraunii Stuercke & Freshwater (n=1), and an unidentified species provisionally named P. sp._23GWS (n=3). Polysiphonia(=Acanthosiphonia) echinata had no intraspecific variation and was 10.09% different in COI-5P sequence from its nearest neighbour, Polyostea arctica (). Our single Canadian collection of Polysiphonia kapraunii, from southern New Brunswick, was a match (2 bp different) to rbcL sequence data on GenBank from authentic material of this species (EU492920; Stuercke & Freshwater, Citation2010) and was 9.23% different in COI-5P from P. morrowii (). Polysiphonia sp._23GWS was collected from New Brunswick, Canada and Rhode Island, USA, with no intraspecific divergence in COI-5P and 9.79% difference from Polysiphonia sp._3stricta, the nearest genetic group (). We also collected this genetic group in Norway (n=6), and a COI-5P sequence in GenBank identified as P. stricta from Portugal (KF648515) was an exact match to this unidentified species.
Four species reported from Atlantic Canada were not collected in our Canadian survey. Two of these species, Polysiphonia(=Carradoriella) elongata (n=2) and Polysiphonia(=Kapraunia) schneideri Stuercke & Freshwater (n=3), were collected in the eastern USA (Supplementary table S1). The other two, Polysiphonia(=Leptosiphonia) brodiei and P. subtilissima, were not found in either Canada or the north-eastern USA.
British Columbia
We identified 16 genetic species groups of Polysiphonieae and Streblocladieae in British Columbia. Seven of these could be assigned to known species reported for this area: Polyostea bipinnata (n=2), Po. hamata (n=13), Po. robusta (n=74), Polysiphonia(=Melanothamnus) akkeshiensis Segi (n=7), P. (=Vertebrata) hendryi N.L.Gardner (n=56), P. scopulorum var. villum (J.Agardh) Hollenberg (n=21) and Pterochondria(=Vertebrata) woodii (n=12) (). Polysiphonia(=Vertebrata) hendryi (n=55) had 2.45% intraspecific variation in COI-5P sequence data, which is relatively high when compared with the other species included in this study (). Although we were able to assign our collections of P. hendryi to the five varieties reported by Hollenberg (Citation1961), we found that morphological differences between specimens did not correspond to the genetic variation in this group (–). There were no genetic groups closely related to P. hendryi, with Vertebrata lanosa being the nearest (10.3% different in COI-5P, ).
Fig. 1–3. The gross morphology of three genetically verified collections of Vertebrata hendryi (N.L.Gardner) comb. nov. from Haida Gwaii and Prince Rupert, British Columbia corresponding to different varieties. These specimens are 0 to 0.45% different in COI-5P sequence. Fig. 1. A mid intertidal collection matching the described morphology and epiphytic habitat of the type variety (GWS038576). Fig. 2. A low intertidal collection matching the morphology of ‘var. gardneri’ (GWS004980). Fig. 3. A low intertidal collection with lax branching matching the morphology of ‘var. deliquescens’ (GWS031343). Scale bars: Fig. 1 = 1 cm, Fig. 2 = 1.5 cm, Fig. 3 = 2 cm.

We found seven genetic groups that were part of species complexes in British Columbia, including two specimens of Polysiphonia sp._1stricta (n=38) collected in Haida Gwaii. Four genetic groups in British Columbia were a morphological match to Polysiphonia pacifica Hollenberg, two of which could be unequivocally assigned to varieties of this species based on morphology and habitat: P. pacifica var. determinata Hollenberg (n=11), and the type variety P. pacifica Hollenberg var. pacifica (n=29). Polysiphonia pacifica var. determinata had 0.9% variation in COI-5P and was 4.67% different from its nearest neighbours, P. morrowii and P. sp._1stricta (). This variety was elevated to the rank of species, as P. determinata (Hollenberg) stat. nov. (). Collections of P. determinata were more densely branched and compact when compared with collections of P. pacifica var. pacifica (). Furthermore, P. determinata was found only in the mid to low intertidal, while P. pacifica var. pacifica was found almost exclusively in the sublittoral, to a depth of 20 m.
Fig. 4–5. Gross morphology of Polysiphonia determinata (Hollenberg) stat. nov. and P. pacifica var. pacifica Hollenberg. Fig. 4. Polysiphonia determinata in the low intertidal in Bamfield, British Columbia (GWS004015). Fig. 5. A subtidal (5 m) collection of Polysiphonia pacifica var. pacifica from Monterey, California (GWS022117). Scale bars: Fig. 4 = 1 cm, Fig. 5 = 2 cm.

The other three genetic groups identified as Polysiphonia pacifica could not be conclusively assigned to varieties at this time and two were temporarily named P. sp._1pacifica (n=17) and P. sp._2pacifica (n=2). Polysiphonia sp._1pacifica and P. sp._2pacifica had 0.6 and 0.15% intraspecific divergence, respectively, and were 2.86% different from one another (). A fifth genetic group was found only in California, and was tentatively identified as Polysiphonia pacifica var. disticha Hollenberg (n=1, ). Based on the genetic data, this variety of P. pacifica should also be elevated to the rank of species; however, more collections are needed to confirm this identification as it is based on a single specimen.
There were two closely related genetic groups attributable to Polysiphonia paniculata Montagne in British Columbia (). We assigned these genetic groups interim names: Polysiphonia sp._1paniculata (n=41) and P. sp._2paniculata (n=14). These two genetic groups had 0.62% and 0.31% intraspecific variation in COI-5P, respectively, but were only 0.96% different in COI-5P sequence (). Polysiphonia sp._1paniculata was found only in British Columbia while P. sp._2paniculata was found in British Columbia and California.
The remaining two genetic groups were new records for British Columbia. Polysiphonia morrowii (n=9) was collected from Bamfield, Bowen Island and Haida Gwaii (; Supplementary table S1). The type locality of this species is Hakodate, Japan, and our collections were a match in rbcL sequence to collections of P. morrowii from South Korea (AY396027, AY396031). We also collected a single specimen in Bamfield of an unidentified species of Melanothamnus, M. sp._21GWS, which was 2.45% divergent in COI-5P sequence from Polysiphonia(=Melanothamnus) akkeshiensis (). Three species previously reported for this area were not collected during this study: Polysiphonia(=Leptosiphonia) brodiei, P. macounii Hollenberg and P. senticulosa Harvey.
Phylogenetic analyses
A maximum likelihood (RAxML) analysis of the concatenated LSU, rbcL and COI-5P sequence alignment resolved the genus Polysiphonia as polyphyletic relative to most included genera of the Polysiphonieae and Streblocladieae (). The Polysiphonieae and Streblocladieae were sister to one another, and were each resolved as a monophyletic and highly supported clade (). To clarify the relative position for species for which rbcL data were available in GenBank, we ran expanded analyses with this single gene, with each tribe presented separately for clarity (, ).
Fig. 6. RAxML results for the combined LSU, rbcL and COI-5P alignment. Values are ML bootstrap support, an asterisk signifies ≥98% support for that node, a dash indicates ≤50% support for that node, and the scale bar indicates substitutions per site. Bold type indicates the type of a genus and a delta symbol indicates a new combination.
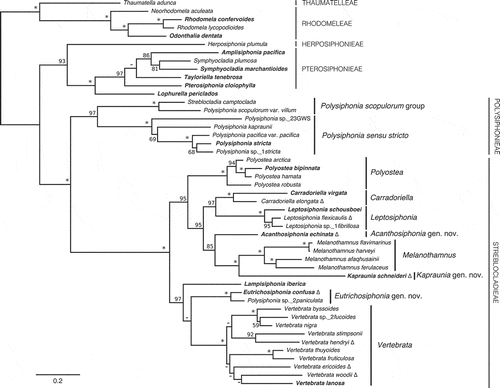
Fig. 7. A portion of the RAxML results for the expanded rbcL-only alignment, showing the tribe Polysiphonieae. Support values are ML bootstrap values, an asterisk signifies ≥98% support for that node, a dash indicates ≤50% support for that node, and the scale bar indicates substitutions per site. Bold type indicates the type of a genus.
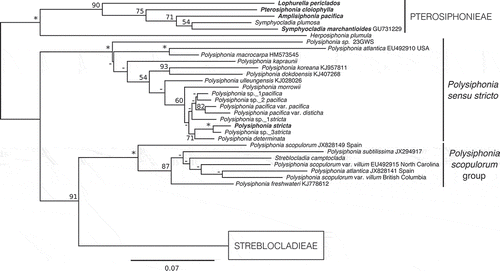
Fig. 8. A portion of the RAxML results for the expanded rbcL-only alignment, showing the tribe Streblocladieae. Support values are ML bootstrap values, an asterisk signifies ≥98% support for that node, a dash indicates ≤50% support for that node, and the scale bar indicates substitutions per site. Bold type indicates the type of a genus and a delta symbol indicates a new combination.
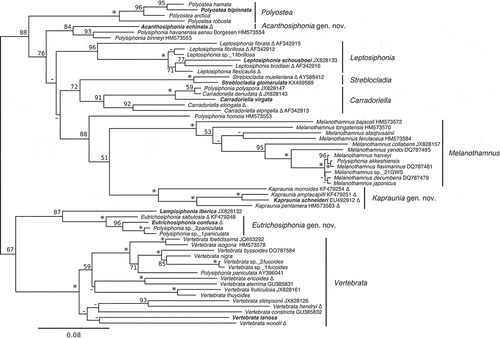
The tribe Polysiphonieae
In the multi-gene RAxML analysis this clade consisted of two distinct and highly supported monophyletic lineages, with the type of Polysiphonia, P. stricta, falling within one of these lineages (). We have restricted the boundaries of Polysiphonia sensu stricto to include only the clade containing the type species (, ).
The generic affinities of Polysiphonia scopulorum var. villum and Streblocladia camptoclada (Montagne) Falkenberg (not the type of the genus) are currently unresolved (). Our collections of P. scopulorum var. villum are from British Columbia, and they also matched a GenBank accession reportedly of this species from Oregon (AY396039), but not GenBank accessions identified as P. scopulorum var. villum from North Carolina (EU492915, ). We have provisionally referred to this clade as the ‘Polysiphonia scopulorum group’ (, ). The type of Streblocladia, S. glomerulata (Montagne) Papenfuss, falls within the second large clade in our analyses, highlighting the incorrect taxonomic placement of S. camptoclada ().
The tribe Streblocladieae
In the multi-gene phylogenetic analysis the tribe Streblocladieae resolved as two large clades (). The first large clade included species of Leptosiphonia, Melanothamnus and Polyostea along with five species of Polysiphonia (). Polysiphonia virgata and P. elongata clustered together in a highly supported group (), and the genus Carradoriella P.C.Silva was resurrected based on the type Carradoriella virgata (C.Agardh) P.C.Silva, with transfer of P. elongata to Carradoriella. An rbcL-only analysis including additional sequence data from GenBank indicated that Polysiphonia denudata (Dillwyn) Greville ex Harvey, Polysiphonia elongella Harvey and Polysiphonia polyspora (C.Agardh) Montagne also fell within the Carradoriella clade (). Polysiphonia denudata and Polysiphonia elongella were transferred to Carradoriella based on the available GenBank sequence data, but considering that cryptic species often occur within this group these species should be studied further using a combination of molecular and morphological tools. Polysiphonia polyspora was not transferred at this time, as there is a lack of information on the morphology of this species in the literature, and the location of the type specimen could not be determined. The Carradoriella clade was highly supported in both analyses (, ). In the rbcL-only analysis the type of Streblocladia, S. glomerulata, clustered in a highly supported clade with Polysiphonia muelleriana J.Agardh, as a moderately supported sister lineage to Carradoriella. Polysiphonia muelleriana was therefore transferred to Streblocladia.
Polysiphonia flexicaulis, P. fibrillosa and P. sp._1fibrillosa clustered with Leptosiphonia schousboei (the type of the genus) in both analyses, and both P. fibrillosa and P. flexicaulis were transferred to Leptosiphonia (, ). Although P. sp._1fibrillosa requires valid description, it should be considered assignable to Leptosiphonia. Polysiphonia brodiei and P. fibrata (Dillwyn) Harvey also fell within the Leptosiphonia clade based on rbcL sequence data from GenBank () and were transferred. Once again these groups should be further investigated using additional markers to investigate the possibility of cryptic species.
Polysiphonia echinata was resolved, with moderate support, as a relatively deep sister to Melanothamnus (). In the rbcL-only analyses it formed a moderately supported clade with Polysiphonia binneyi Harvey and Polysiphonia havanensis sensu Børgesen (). The new genus Acanthosiphonia gen. nov. was described for A. echinata (Harvey) comb. nov., while transfer of P. binneyi and P. havanensis requires further study. The phylogenetic position of Polysiphonia homoia Setchell & N.L.Gardner was unresolved (). The genus Melanothamnus as circumscribed by Díaz-Tapia et al. (Citation2017b) was monophyletic and strongly supported in our analyses, as was the recently resurrected genus Polyostea (, ). In the rbcL-only analysis (), Polysiphonia akkeshiensis fell within the Melanothamnus clade and is here transferred to this genus.
Polysiphonia schneideri was sister to the Melanothamnus clade in the multi-gene analysis (). In the rbcL-only analysis, Polysiphonia amplacapilli B.Kim & M.S.Kim, P. morroides B.Kim & M.S.Kim and P. pentamera Hollenberg clustered with P. schneideri in a highly supported clade (). A new genus, Kapraunia gen. nov., was described for these four species with K. schneideri (Stuercke & Freshwater) comb. nov. designated as the type.
The second large clade within the tribe Streblocladieae included species of Lampisiphonia H.G.Choi, Díaz-Tapia & Bárbara, Perrinia Womersley, Pterochondria Hollenberg, and Vertebrata, as well as three species of Polysiphonia ().
In the multi-gene analyses, Polysiphonia confusa and P. sp._2paniculata clustered together in a highly supported lineage as a relatively deep sister to Vertebrata (). In the rbcL-only analyses P. confusa, P. sp._1paniculata and P. sp._2paniculata were joined by Polysiphonia sabulosia B.Kim & M.S.Kim (). Eutrichosiphonia gen. nov. was described for E. confusa (Hollenberg) comb. nov. and E. sabulosia (B.Kim & M.S.Kim) comb. nov. was also transferred. Eutrichosiphonia was highly supported in both phylogenetic analyses (, ). Transfer of P. paniculata to this genus will require verifying the identity of this species and linking the original species concept to a genetic group.
The recently expanded genus Vertebrata (Díaz-Tapia et al., Citation2017b) was monophyletic and highly supported in our analyses (, ). Polysiphonia hendryi fell within this clade, sister to V. stimpsonii, and was transferred to Vertebrata. The types of two monotypic genera, Perrinia and Pterochondria, also fell within the Vertebrata clade and were placed in Vertebrata as Vertebrata ericoides (Harvey) Kuntze and Vertebrata woodii (Harvey) Kuntze (, ). A collection of P. paniculata from southern Chile (Punta Arenas) within this clade (AY396041) did not match our collections of P. sp._1paniculata and P. sp._2paniculata from British Columbia and California.
Taxonomic results
Acanthosiphonia gen. nov
Description: Thalli radially organized, polysiphonous, composed of erect axes arising from a basal rhizoidal holdfast or from prostrate axes attached to the substrate by unicellular rhizoids; rhizoids cut off from pericentral cells; pericentral cells 4; axes ecorticate or lightly corticated below; trichoblasts present, occurring every segment in a spiral pattern, leaving persistent scar cells; branches forming in the axils of trichoblasts. Tetrasporangia tetrahedral, one per segment, in spiral series in laterals. Gametophytes dioecious, procarps with a 4-celled carpogonial branch, mature cystocarps globose to subglobose; spermatangial axes cylindrical, borne on the first dichotomy of a modified trichoblast, and lacking sterile terminal cells.
Type species: Acanthosiphonia echinata (Harvey) comb. nov.
Etymology: From the Greek for spine or thorn, ‘acantha’, for the short spine-like laterals of the type species, and from the Greek ‘siphon’, for the polysiphonous construction of the species in this genus.
Comments: Acanthosiphonia is currently monotypic; however, further phylogenetic analyses are needed to determine whether Polysiphonia binneyi and P. havanensis sensu Børgesen belong in this genus ().
Acanthosiphonia echinata (Harvey) comb. nov
Basionym: Polysiphonia echinata Harvey 1853, p. 38 (Nereis boreali-americana; or, contributions towards a history of the marine algae of the Atlantic and Pacific coasts of North America. Part II. Rhodospermeae. Smithsonian Contributions to Knowledge, 5(5): [i–ii], [1] –258, pls XIII–XXXVI).
Homotypic synonym: Neosiphonia echinata (Harvey) Mamoozadeh & Freshwater.
Heterotypic synonym: Polysiphonia fracta Harvey.
Holotype: TCD0012799.
Type locality: Key West, Monroe County, Florida, USA.
Distribution: Our collections of A. echinata from New Brunswick and Nova Scotia (Supplementary table S1) were a match (1 bp different in rbcL) to GenBank accessions from Florida (HM573558, HM573559, HM573560). This species is reported from North Carolina to Mexico and the Caribbean (Guiry & Guiry, Citation2018), and was recently found in Indonesia as well (Bustamante et al., Citation2015a).
Carradoriella P.C.Silva
Amended description: Thalli radially organized, polysiphonous, composed of erect axes arising from a basal discoid holdfast made up of aggregated cortical filaments and rhizoids; rhizoids cut off from pericentral cells; pericentral cells 4 to 16; axes with light to heavy cortication; trichoblasts present or absent, when present occurring every segment in a spiral pattern, leaving persistent scar cells. Tetrasporangia tetrahedral, one per segment, in either a straight or spiral series in laterals. Gametophytes dioecious, procarps with a 4-celled carpogonial branch, mature cystocarps globose or ovoid; spermatangial axes conical or cylindrical, borne on the first dichotomy of a modified trichoblast, with 0–4 sterile terminal cells.
Type species: Carradoriella virgata (C.Agardh) P.C.Silva.
Carradoriella denudata (Dillwyn) comb. nov.
Basionym: Conferva denudata Dillwyn 1809, p. 85, pl. G (British Confervae; or coloured figures and descriptions of the British plants referred by botanists to the genus Conferva. London: W. Phillips).
Homotypic synonyms: Polysiphonia denudata (Dillwyn) Greville ex Harvey
Heterotypic synonyms: Hutchinsia biasolettiana C.Agardh, H. variegata C.Agardh, Polysiphonia leptura Kützing, P. variegata (C.Agardh) Zanardini, P. variegata (C.Agardh) J.Agardh, P. vidovichii Meneghini ex Kützing.
Holotype/Lectotype: BM-K (Maggs & Hommersand, Citation1993).
Type locality: Southampton, England.
Distribution: Reported from the Netherlands to Portugal, the Mediterranean, and western Africa (Maggs and Hommersand, Citation1993).
Carradoriella elongata (Hudson) comb. nov.
Basionym: Conferva elongata Hudson Citation1762, p. 484 (Flora anglica; exhibens plantas per regnum angliae sponte crescentes, distributas secundum systema sexuale: cum differentiis specierum, synonymis auctorum, nominibus incolarum, solo locorum, tempore florendi, officinalibus pharmacopoeorum. Prostant venales apud J. Nourse in the Strand, et C. Moran in Covent-Garden, Londini [London]).
Homotypic synonyms: Boryna elongata (Hudson) Bory, Ceramium elongatum (Hudson) Roth, Hutchinsia elongata (Hudson) C.Agardh, Neosiphonia elongata (Hudson) Xiang Si-duan, Polysiphonia elongata (Hudson) Sprengel, Rhodomela elongata (Hudson) Fries.
Heterotypic synonyms: Ceramium brachygonum Lyngbye, Fucus muscoides Forsskål, Hutchinsia brachygona (Lyngbye) S.F.Gray, H. lyngbyei C.Agardh, H. ruchingeri C.Agardh, H. strictoides Lyngbye, Polysiphonia arborescens Kützing, P. chalarophloea Kützing, P. clavigera Kützing, P. commutata Kützing, P. fragilis Spherk, P. delphina De Notaris, P. denudata f. fragilis Woronichin, P. haematites Kützing, P. laxa Kützing, P. leptoclonia Zanardini ex De Toni, P. macroclonia Kützing, P. microdendron J.Agardh, P. robusta Kützing, P. rosea Greville, P. ruchingeri (C.Agardh) Zanardini, P. schuebeleri Foslie, P. stenocarpa Kützing, P. strictoides (Lyngbye) Kützing, P. trichodes Kützing.
Lectotype: BM-K (Maggs & Hommersand, Citation1993).
Type locality: England.
Distribution: Our collections are from New England, as well as Ireland and Norway (Supplementary table S1). This species is reported from Norway to Portugal, the British Isles and the Mediterranean, as well as from Atlantic Canada and New England (Maggs & Hommersand, Citation1993).
Carradoriella elongella (Harvey) comb. nov.
Basionym: Polysiphonia elongella Harvey in W.J.Hooker 1833, p. 334 (The English Flora of Sir James Edward Smith. Class XXIV. Cryptogamia. Vol. V. Part I. Comprising the Mosses, Hepaticae, Lichens, Characeae and Algae. London: Longman, Brown, Green & Longman).
Homotypic synonyms: Neosiphonia elongella (Harvey) M.S.Kim & I.K.Lee.
Lectotype: TCD sine numero (Maggs & Hommersand, Citation1993).
Type locality: Sidmouth, Devon, England.
Distribution: Reported from the British Isles to France, as well as the Mediterranean (Maggs & Hommersand, Citation1993).
Eutrichosiphonia gen. nov
Description: Thalli radially organized, polysiphonous, composed of erect axes arising from prostrate axes attached to the substrate by unicellular rhizoids; rhizoids cut off from pericentral cells; pericentral cells 5 to 12; axes ecorticate; trichoblasts abundant, occurring every segment in a spiral pattern, leaving conspicuous, persistent scar cells; branches forming laterally to trichoblasts by arising from the basal cell of a trichoblast or forming in the axils of trichoblasts. Tetrasporangia tetrahedral, one per segment, in spiral series in laterals. Gametophytes dioecious, procarps with a 4-celled carpogonial branch, mature cystocarps ovoid to globose, 280–430 μm in diameter. Spermatangial axes cylindrical, borne on the first dichotomy of a modified trichoblast, usually lacking sterile terminal cells but occasionally 1 sterile terminal cell present.
Type species: Eutrichosiphonia confusa (Hollenberg) comb. nov.
Etymology: From the Greek for good or well-developed, ‘eu’, as well as the Greek for hairs, ‘trich’, referring to the abundant, well-developed trichoblasts of species in this genus, and from the Greek ‘siphon’ owing to the polysiphonous construction of species in this genus.
Eutrichosiphonia confusa (Hollenberg) comb. nov.
Basionym: Polysiphonia confusa Hollenberg Citation1961, p. 350 (Marine red algae of Pacific Mexico, Part 5: The genus Polysiphonia. Pacific Naturalist, 2: 345–375, 7 plates).
Homotypic synonym: Neosiphonia confusa (Hollenberg) J.N.Norris.
Heterotypic synonym: Polysiphonia inconspicua Hollenberg nom. illeg.
Holotype: US 61222 (Hollenberg 3285).
Type locality: Corona del Mar, California, USA.
Distribution: Our collections are only from Santa Cruz, California (Supplementary table S1), but E. confusa is reported from California and northern Baja California, Mexico (Abbott & Hollenberg, Citation1976).
Eutrichosiphonia sabulosia (B.Kim & M.S.Kim)
comb. nov.
Basionym: Polysiphonia sabulosia B.Kim & M.S.Kim 2014, p. 192, fig. 4 (Three new species of Polysiphonia sensu lato (Rhodophyta) based on the morphology and molecular evidence. Algae, 29(3): 183–195).
Holotype: JNUB-JD120106-1.
Type locality: Jongdal, Jeju Island, South Korea.
Distribution: Reported from Jeju Island, South Korea (Kim & Kim, Citation2014).
Kapraunia gen. nov
Description: Thalli radially organized, polysiphonous, composed of erect axes arising from a discoid rhizoidal holdfast, or from prostrate axes attached to the substrate by unicellular rhizoids, or from both a discoid holdfast and secondary prostrate axes; rhizoids cut off from pericentral cells; pericentral cells 4 to 7; axes ecorticate, or with light cortication at the base of older plants; trichoblasts present, occurring in no distinct pattern, sometimes leaving persistent scar cells, or scar cells may be rare; branches forming laterally to trichoblasts by arising from the basal cell of a trichoblast, or replacing trichoblasts. Tetrasporangia tetrahedral, one per segment, in spiral series in laterals in some species and in straight series in others. Gametophytes dioecious, procarps with 4-celled carpogonial branch, mature cystocarps globose, 300–520 μm in diameter. Spermatangial axes cylindrical, borne on the first dichotomy of a modified trichoblast, either lacking sterile terminal cells or with 1–3 sterile terminal cells.
Type species: Kapraunia schneideri (Stuercke & Freshwater) comb. nov.
Etymology: Named in recognition of Dr Donald F. Kapraun for his significant contributions to research on the genus Polysiphonia.
Kapraunia schneideri (Stuercke & Freshwater)
comb. nov.
Basionym: Polysiphonia schneideri Stuercke & Freshwater Citation2010, p. 302, figs 1–11 (Two new species of Polysiphonia (Ceramiales, Florideophyceae) from the western Atlantic. Botanica Marina, 53(4): 301–311).
Holotype: MICH (WNC-8782).
Type locality: Wrightsville Beach, New Hanover County, North Carolina, USA.
Distribution: Reported from southern New England to Panama (Mamoozadeh & Freshwater, Citation2012). Our collections were from Rhode Island and South Carolina (Supplementary table S1).
Kapraunia amplacapilli (B.Kim & M.S.Kim)
comb. nov.
Basionym: Polysiphonia amplacapilli B.Kim & M.S.Kim 2014, p. 185, fig. 2 (Three new species of Polysiphonia sensu lato (Rhodophyta) based on the morphology and molecular evidence. Algae, 29(3): 183–195).
Holotype: JNUB-UD120705-1.
Type locality: Udo Strait, Jeju Island, South Korea.
Distribution: Reported from Jeju Island, South Korea (Kim & Kim, Citation2014).
Kapraunia morroides (B.Kim & M.S.Kim) comb. nov.
Basionym: Polysiphonia morroides B.Kim & M.S.Kim 2014, p. 190, fig. 3 (Three new species of Polysiphonia sensu lato (Rhodophyta) based on the morphology and molecular evidence. Algae, 29(3): 183–195).
Holotype: JNUB-UD120610-11.
Type locality: Udo Strait, Jeju Island, South Korea.
Distribution: Reported from Jeju Island, South Korea (Kim & Kim, Citation2014).
Kapraunia pentamera (Hollenberg) comb. nov.
Basionym: Polysiphonia pentamera Hollenberg Citation1968, pp. 204–205, fig. 2D (An account of the species of Polysiphonia of the Central and Western Tropical Pacific Ocean I. Oligosiphonia. Pacific Science, 22(1): 56–98).
Holotype: US 48526.
Type locality: Eniwetok Atoll, Marshall Islands.
Distribution: Reported from Panama, Hawaii, the Marshall Islands and Micronesia (Guiry & Guiry, Citation2018).
Leptosiphonia Kylin
Amended description: Thalli radially organized, polysiphonous, composed of erect axes arising from a discoid holdfast or from prostrate axes attached to the substrate by unicellular rhizoids; rhizoids cut off from pericentral cells; pericentral cells 4 to 16; axes with light to heavy cortication; trichoblasts present, occurring every segment in a spiral pattern, leaving persistent scar cells; branches forming in the axils of trichoblasts. Tetrasporangia tetrahedral, with one or two per segment depending on the species. Gametophytes dioecious, procarps with a 4-celled carpogonial branch; spermatangial axes cylindrical, borne on the first dichotomy of a trichoblast, and lacking sterile terminal cells.
Type species: Leptosiphonia schousboei (Thuret) Kylin.
Leptosiphonia brodiei (Dillwyn) comb. nov.
Basionym: Conferva brodiei Dillwyn 1809, p. 81, pl. 107 (as ‘brodiaei’) (British Confervae; or coloured figures and descriptions of the British plants referred by botanists to the genus Conferva. London: W. Phillips).
Homotypic synonyms: Conferva brodiei Dillwyn, Ceramium brodiei (Dillwyn) C.Agardh, Hutchinsia brodiei (Dillwyn) Lyngbye, Polysiphonia brodiei (Dillwyn) Sprengel, Vertebrata brodiei (Dillwyn) Kuntze.
Heterotypic synonyms: Hutchinsia penicillata C.Agardh, Polysiphonia penicillata (C.Agardh) Sprengel, P. brodiei f. densa Holmes & Batters, P. brodiei f. typica Holmes & Batters.
Lectotype: BM-K (Maggs & Hommersand, Citation1993).
Type locality: Bantry Bay, Co. Cork, Ireland.
Distribution: Reported from Europe including the Mediterranean, as well as the Atlantic and Pacific coasts of North America (Maggs & Hommersand, Citation1993). In this study we did not find L. brodiei in North America.
Leptosiphonia fibrata (Dillwyn) comb. nov.
Basionym: Conferva fibrata Dillwyn 1809: 84, pl. G (British Confervae; or coloured figures and descriptions of the British plants referred by botanists to the genus Conferva. London: W. Phillips).
Homotypic synonyms: Hutchinsia allochroa var. fibrata (Dillwyn) C.Agardh, H. fibrata (Dillwyn) C.Agardh, Polysiphonia fibrata (Dillwyn) Harvey.
Lectotype: BM-K (Maggs & Hommersand, Citation1993).
Type locality: Forres, Moray, Scotland.
Distribution: Reported from the British Isles to Spain (Maggs & Hommersand, Citation1993).
Leptosiphonia fibrillosa (Dillwyn) comb. nov.
Basionym: Conferva fibrillosa Dillwyn 1809, p. 86, pl. G (British Confervae; or coloured figures and descriptions of the British plants referred by botanists to the genus Conferva. London: W. Phillips).
Homotypic synonyms: Hutchinsia fibrillosa (Dillwyn) C.Agardh, Polysiphonia fibrillosa (Dillwyn) Sprengel, P. violacea f. fibrillosa (Dillwyn) Rosenvinge, P. violacea var. fibrillosa (Dillwyn) Areschoug.
Heterotypic synonyms: Ceramium violaceum var. tenue Roth, Hutchinsia aculeata C.Agardh, H. divaricata C.Agardh, H. divaricata Hornemann, H. expansa C.Agardh, H. implicata Lyngbye, H. spinulosa (Greville) C.Agardh, H. stricta (Roth) Lyngbye, H. tenuis C.Agardh, Polysiphonia aculeata (C.Agardh) Fries, P. bulbosa Fries, P. carmichaeliana Harvey, P. divaricata (C.Agardh) Sprengel, P. elongata f. expansa (C.Agardh) J.Agardh ex Levring, P. expansa (C.Agardh) Fries, P. grevillei Harvey, P. griffithsiana Harvey, P. myriococca Montagne, P. nutans Montagne, P. richardsonii Hooker ex Harvey, P. spinulosa Greville, P. tenuis (C. Agardh) Fries, P. violacea f. aculeata (C.Agardh) Rosenvinge, P. violacea f. bulbosa (Suhr ex Areschoug) Kylin, P. violacea f. subbrodiei (Areschoug) Kylin, P. violacea f. tenuis (Roth) Rosenvinge, P. violacea var. bulbosa Suhr ex Areschoug, P. violacea var. subbrodiei Areschoug, P. violacea var. tenuissima Areschoug.
Lectotype: BM-K (Maggs & Hommersand, Citation1993).
Type locality: Brighton, England.
Distribution: Reported from Norway (where we have collections (Supplementary table S1) to France and the Baltic Sea (Maggs & Hommersand, Citation1993). Collections previously referred to as L. fibrillosa in Atlantic Canada and New England probably represent an undescribed species, provisionally referred to here as L. sp._1fibrillosa.
Leptosiphonia flexicaulis (Harvey) comb. nov.
Basionym: Polysiphonia violacea var. flexicaulis Harvey 1853, p. 44 (Nereis boreali-americana; or, contributions towards a history of the marine algae of the Atlantic and Pacific coasts of North America. Part II. Rhodospermeae. Smithsonian Contributions to Knowledge, 5(5): [i–ii], [1] –258, pls XIII–XXXVI).
Homotypic synonym: Polysiphonia flexicaulis (Harvey) Collins.
Lectotype, designated here: TCD0018099.
Type locality: Halifax, Nova Scotia, Canada.
Distribution: We collected L. flexicaulis throughout eastern Canada, as far north as northern Labrador, with a few collections from Maine and Massachusetts as well (Supplementary table S1).
Comments: We have viewed an image of TCD0018099 included in Harvey’s collections from Halifax at Trinity College, Dublin. The morphology of this collection matches the genetic group to which we attribute this name and is here designated as the lectotype for this species.
Melanothamnus akkeshiensis (Segi) comb. nov.
Basionym: Polysiphonia akkeshiensis Segi Citation1951, p. 232, figs 23, 24; pl. 9: figs 1–3 (Systematic study of the genus Polysiphonia from Japan and its vicinity. Journal of the Faculty of Fisheries, Prefectural University of Mie, 1: 167–272, 36 figs, 16 plates).
Holotype: SAP (Segi Citation1951).
Type locality: Hokkaido, Japan.
Distribution: Japan (Segi, Citation1951) and British Columbia (Savoie & Saunders, Citation2015).
Polysiphonia determinata (Hollenberg) stat. nov.
()
Basionym: Polysiphonia pacifica var. determinata Hollenberg 1942, p. 778 (An account of the species of Polysiphonia on the Pacific coast of North America. I. Oligosiphonia. American Journal of Botany, 29(9): 772–785).
Holotype: UC 276272 (N.L. Gardner 3327).
Type locality: Promontory Point, Carmel Bay, Monterey, California.
Distribution: Reported from British Columbia to Santa Barbara County, California (Abbott & Hollenberg, Citation1976), which matches our collections here (Supplementary table S1).
Streblocladia muelleriana (J.Agardh) comb. nov.
Basionym: Polysiphonia muelleriana J.Agardh 1870, p. 455 (Om Chatham-öarnes alger. Öfversigt af Kongl. Vetenskaps-Akademiens Förhandlingar, Stockholm 27: 435–456).
Isotype: TCD0012792.
Type locality: Chatham Islands, New Zealand.
Distribution: Reported from New Zealand (Adams, Citation1991).
Vertebrata hendryi (N.L.Gardner) comb. nov.
(–)
Basionym: Polysiphonia hendryi N.L. Gardner 1927, p. 101, pl. 24, figs 1, 2, pl. 25 (New Rhodophyceae from the Pacific coast of North America. VI. University of California Publications in Botany, 14: 99–138, pls 20–36).
Heterotypic synonyms: Polysiphonia collinsii Hollenberg, P. gardneri Kylin.
Holotype: UC 296607.
Type locality: Santo Domingo, Baja California, Mexico.
Distribution: Reported from Alaska to Mexico (Abbott & Hollenberg, Citation1976). Our collections are from British Columbia and California (Supplementary table S1). This entity is a cryptic complex in need of further study.
Discussion
DNA barcode survey
Atlantic Canada and the Canadian Arctic
Five genetic species groups in Atlantic Canada were a straightforward match to a morphological species concept: Leptosiphonia flexicaulis, Melanothamnus harveyi, Polyostea arctica, Vertebrata lanosa and V. nigra (). One Atlantic Canadian genetic group was a morphological match to Leptosiphonia fibrillosa, but differed genetically (4.65% in COI-5P) from European collections of this species (). The type locality of L. fibrillosa is Brighton, England (Maggs & Hommersand, Citation1993, as Polysiphonia fibrillosa), and pending further study we have referred to our Canadian specimens as L. sp._1fibrillosa. In two cases (Polysiphonia stricta and Vertebrata fucoides) two or three genetic species groups were assigned to the same morphological species concept. Leptosiphonia brodiei and Polysiphonia subtilissima were not collected during this study, but three new records for this region were documented: Acanthosiphonia echinata, Polysiphonia kapraunii and P. sp._23GWS.
The distribution of the two genetic groups assigned to Vertebrata fucoides overlapped in the north-west Atlantic, but only one (V. sp._2fucoides) was a match in COI-5P and rbcL sequence to European collections. Vertebrata sp._2fucoides probably represents authentic V. fucoides, as the type locality of this species is Yorkshire, England (Hudson, Citation1762). However, these two groups are relatively close (1.66% in COI-5P) and further study using the internal transcribed spacer of the ribosomal cistron (ITS) could establish whether these two genetic groups represent different species or are previously geographically separated populations of the same biological species (e.g. McDevit & Saunders, Citation2010; Filloramo & Saunders, Citation2016).
There were three genetic groups morphologically attributable to the type of Polysiphonia, P. stricta, in Atlantic Canada (). One genetic group was identified as authentic P. stricta as it matched COI-5P and rbcL accessions in GenBank for European collections. The type locality of this species is Swansea, Wales, with syntype localities in England (Maggs & Hommersand, Citation1993; Guiry & Guiry, Citation2018). While all three genetic groups within the P. stricta complex were found in Atlantic Canada and New England, only Polysiphonia sp._1stricta and P. sp._3stricta were found in the Arctic. Polysiphonia sp._1stricta and P. sp._3stricta were 3.67 and 2.6% different from their nearest neighbours in COI-5P sequence, respectively, and will be assessed in future publications (). A comprehensive molecular-assisted study with further sampling in Europe and the Arctic is needed to accurately understand this species complex.
Acanthosiphonia echinata and Polysiphonia kapraunii were new additions to the Canadian flora in Atlantic Canada. Acanthosiphonia echinata was collected from sheltered sites (mainly seagrass beds and estuaries) along the Northumberland Strait in both New Brunswick and Nova Scotia. The majority of our collections were epiphytic on seagrass, but one was found growing on oysters at an aquaculture site in Cape Breton. A single specimen of P. kapraunii was collected from Sam Orr’s Pond in New Brunswick, a tidally influenced brackish pond in the Bay of Fundy where other non-native marine species have been found (Saunders et al., Citation2013).
The unidentified genetic group Polysiphonia sp._23GWS falls within Polysiphonia sensu stricto as currently circumscribed, however it is genetically distinct from the remainder of the species in this group (, ). Polysiphonia sp._23GWS was morphologically similar to Polysiphonia stricta, as well as other species within the Polysiphonia sensu stricto clade, but had frequent adventitious branchlets, which are not reported for P. stricta (Maggs & Hommersand, Citation1993; Kim et al., Citation2000) and are not found in the genetic groups assigned to P. stricta in Atlantic Canada (data not shown). COI-5P data from our North American collections of Polysiphonia sp._23GWS matched a collection from Portugal identified as P. stricta (Díaz-Tapia & Bárbara, Citation2013), as well as six collections from Norway (Supplementary table S1). Díaz-Tapia & Bárbara (Citation2013) noted that there was genetic diversity amongst their collections of P. stricta from the Iberian Peninsula, but did not describe the new genetic entity or compare the morphology of their two genetic groups. Polysiphonia sp._23GWS may represent a new species, but an assessment of this genetic group in Europe is necessary to assess this possibility.
British Columbia
Seven COI-5P genetic species groups could be assigned to a morphological species concept in British Columbia: Polyostea bipinnata, Po. hamata, Po. robusta, Melanothamnus akkeshiensis, Polysiphonia scopulorum var. villum, Vertebrata hendryi and V. woodii (). There were two cases where multiple genetic species groups were assigned to the same morphospecies concept – four genetic species groups were identified as Polysiphonia pacifica and two as P. paniculata. Three of the species recorded for this area (Leptosiphonia brodiei, Polysiphonia macounii and P. senticulosa) were not collected; however, two new groups were recorded (Polysiphonia morrowii and Melanothamnus sp._21GWS, ).
Hollenberg (Citation1944, Citation1961) described five varieties of V. hendryi; however, the observed genetic variation (2.45% in COI-5P) did not correspond to the observed morphological variation in our collections (–). A single collection from California was 2.45% different from the collections from British Columbia, but when only samples from British Columbia were considered the intraspecific variation was still relatively high (1.2%; ). Our collection from California corresponded to the morphology of the type variety, but we also have collections morphologically assignable to the type variety from British Columbia. Vertebrata hendryi remains a species complex that requires further investigation with a focus on collections from California, and considerable taxonomic work remains.
Five genetic species groups were identified as Polysiphonia pacifica in British Columbia and California based on morphology (). Three of these genetic groups were assigned to varieties of P. pacifica (Hollenberg Citation1942a), including the type variety. Polysiphonia pacifica var. determinata was elevated to the rank of species as it was genetically and morphologically distinct from the type variety (, , ). Our Californian collections of P. determinata (Hollenberg) stat. nov. and P. pacifica var. pacifica are both from Monterey County, near their respective type localities of Pebble Beach and Santa Cruz, California (Supplementary table S1). We also had two genetic groups (P. sp._1pacifica and P. sp._2pacifica) that could be morphologically assigned to P. pacifica, but were not a consistent match to any of the described varieties of this species or to any other species reported in the British Columbia flora. Further study of these groups is needed.
In British Columbia, Polysiphonia paniculata was resolved as a species complex of two closely related genetic species groups (). Similar to the V. fucoides complex discussed above, the relatively low difference in COI-5P between these two groups may be indicative of population-level differences within a species, perhaps due to past geographic barriers, as opposed to indicating two distinct species. Sequence data from the ITS would be helpful to determine whether these two groups represent distinct species (e.g. McDevit & Saunders, Citation2010; Filloramo & Saunders, Citation2016). Furthermore, the type locality of P. paniculata is Peru, and although this species is considered to be present and abundant in British Columbia and California, sequence data from topotype material is necessary to confirm whether either of the North Pacific genetic species groups truly represents P. paniculata (Montagne, Citation1842; Abbott & Hollenberg, Citation1976; Gabrielson et al., Citation2006). There are sequence data from a specimen identified as P. paniculata from Chile in GenBank (AY396041), but there was no associated morphological information in the paper (Kim et al., Citation2004). It is possible that one of the North Pacific P. paniculata genetic groups is assignable to Polysiphonia californica Harvey, a synonym of P. paniculata (Guiry & Guiry, Citation2018). Further study is needed to determine whether these two genetic groups represent distinct species, and which entity (if either) represents ‘true’ Polysiphonia paniculata.
Polysiphonia morrowii was newly recorded for the Canadian flora, with most of our collections of this species from Haida Gwaii in northern British Columbia (Supplementary table S1). Polysiphonia morrowii is considered native to the western North Pacific (Perestenko, Citation1980; Kudo & Masuda, Citation1992), but was reportedly introduced to Argentina (Croce & Parodi, Citation2014), Chile (Kim et al., Citation2004), the Mediterranean (Curiel et al., Citation2002), the North Atlantic (Geoffroy et al., Citation2012), and New Zealand (D’Archino et al., Citation2013). Polysiphonia morrowii is morphologically similar to the native species P. pacifica and P. senticulosa, and its presence in British Columbia could have been easily overlooked. Polysiphonia morrowii and P. senticulosa in particular are very similar in morphology, and the relationship between these two species has been debated in the literature. Kudo & Masuda (Citation1981, Citation1992) found that they were very similar in morphology, but did not formally synonymize the two as they lacked collections from the type locality of P. senticulosa (Washington, USA). D’Archino et al. (Citation2013) discussed the relationship between P. morrowii and P. senticulosa and concluded that a comprehensive molecular and morphological assessment was needed. Whether the two species are distinct entities or not, it is possible that in the past collections of P. morrowii in British Colombia were attributed to P. senticulosa.
The genetic group Melanothamnus sp._21GWS consisted of a single collection from Bamfield on the west coast of Vancouver Island, British Columbia. It was 2.45% divergent in COI-5P from the nearest species, Melanothamnus akkeshiensis. At present the identity of this species is unknown.
Phylogenetic analyses of the tribes Polysiphonieae and Streblocladieae
The genus Polysiphonia has long been problematic for red algal taxonomists. Many vegetative characters historically considered important in this group have proven variable and unreliable for species determination, while reproductive characters are generally highly conserved (Choi et al., Citation2001; Stuercke & Freshwater, Citation2008, Citation2010). For species identification as well as delimitation of genera in the tribe Polysiphonieae sensu lato there has been a traditional focus on characters such as the number of pericentral cells, the presence or absence of trichoblasts and their arrangement, the origin of rhizoids and branches, the arrangement of tetrasporangia and number per segment, the number of cells in the carpogonial branch, and the position of spermatangial axes (Stuercke & Freshwater, Citation2008). Multiple phylogenetic analyses, including this study, have shown that Polysiphonia is not monophyletic and that species generally fall scattered amongst three large clades which comprise the Polysiphonieae and the newly recognized Streblocladieae (Choi et al., Citation2001; Bárbara et al., Citation2013; Díaz-Tapia et al., Citation2017a). For ease of comparison with previous studies and to simplify discussion of these speciose tribes they are presented in turn below. Although some generic-level morphological characters previously considered useful in this group were no longer consistent when compared with the results of molecular phylogenetic analyses, others such as the origin of rhizoids and the number of cells in the carpogonial branch did prove useful for delimitation of genera. We have summarized the morphological characters of key genera included in this study to facilitate comparison between molecular and morphological analyses ().
Table 2. Genus-level vegetative and reproductive characters in Polysiphonia and other selected genera of the Polysiphonieae and Streblocladieae.
The tribe Polysiphonieae
In the multi-gene RAxML analysis () the tribe Polysiphonieae consisted of two distinct and highly supported monophyletic lineages, which have together been commonly referred to as Polysiphonia sensu stricto (or the ‘Polysiphonia group’) in the literature (Mamoozadeh & Freshwater, Citation2012; Bárbara et al., Citation2013; Bustamante et al., Citation2015b). We have restricted the term Polysiphonia sensu stricto to refer only to the group containing the type, and refer to the second clade as the Polysiphonia scopulorum group (, ).
Polysiphonia sensu stricto
Species in Polysiphonia sensu stricto share several important morphological characters: four pericentral cells, no cortication, rhizoids in open connection with pericentral cells, and tetrasporangia in straight series with one tetrasporangium per segment (). Trichoblasts are usually absent or rare. This combination of characters was unique within the members of the Polysiphonieae studied here, although similar to the Polysiphonia scopulorum group discussed below. Polysiphonia sensu stricto was also highly supported in phylogenetic analyses (, ). Species attributed to Polysiphonia falling outside this clade will require transfer to other genera.
The Polysiphonia scopulorum group
The Polysiphonia scopulorum group is a monophyletic lineage (, ) consisting of species that share many morphological characteristics with Polysiphonia sensu stricto. This clade is genetically distinct from Polysiphonia sensu stricto and should be recognized as a separate genus; however, we are reluctant to assign a generic name at this time due to the taxonomic confusion within this group, as well as the possibility of an available genus name applicable to this clade (discussed below). Based on the morphology of species within this group for which we have collections (P. scopulorum var. villum from British Columbia and Streblocladia camptoclada from South Africa) or that have a detailed morphological review associated with their GenBank data (P. freshwateri (Bustamante et al., Citation2015b) and P. subtilissima (Lam et al., Citation2013)), several morphological characters unite these species (). They have ecorticate axes with four pericentral cells, and erect axes arising endogenously from prostrate axes. Rhizoids are unicellular and in open connection with pericentral cells. Trichoblasts and scar cells are abundant and conspicuous in P. freshwateri, P. scopulorum var. villum and P. subtilissima, but are lacking in S. camptoclada. Tetrasporangia are in straight series in apical laterals in most species, but may sometimes be slightly spiralled in P. freshwateri. It is also clear that there is significant taxonomic confusion within this lineage, as there are three distinct rbcL sequences identified as Polysiphonia scopulorum or P. scopulorum var. villum in GenBank (). The type locality of P. scopulorum is Rottnest Island, Western Australia, and the type locality of P. scopulorum var. villum is Mexico (probably the Pacific coast according to Hollenberg (Citation1968)).
The Polysiphonia scopulorum group shares superficial similarities with some rhodomelacean genera thought to be closely related to Polysiphonia, such as Bryocladia F.Schmitz, Lophosiphonia Falkenberg and Streblocladia F.Schmitz. However, the type of Streblocladia, S. glomerulata (Montagne) Papenfuss, fell into the second large clade in our rbcL-only analyses, and along with Streblocladia muelleriana was sister to Carradoriella (). Although S. camptoclada falls within the P. scopulorum group, based on molecular and morphological evidence this species does not belong in Streblocladia. The type of Lophosiphonia, L. obscura (C.Agardh) Falkenberg, was recently included in phylogenetic analyses as well, and although L. obscura was resolved within the tribe Polysiphonieae it did not ally with the Polysiphonia scopulorum group (see Díaz-Tapia et al., Citation2017a).
Unlike Lophosiphonia and Streblocladia, the type species of Bryocladia has never been included in phylogenetic analyses and there are no molecular data available on GenBank, so its relationship with Polysiphonia sensu stricto is unknown. Based on morphology, it is unlikely that the Polysiphonia scopulorum group belongs to Bryocladia because species of Bryocladia have 6 to 12 pericentral cells and abundant simple, spirally arranged determinate axes covering the main axes (Schneider & Searles, Citation1991), whereas species belonging to the Polysiphonia scopulorum group have four pericentral cells and lack spirally arranged determinate axes. Molecular analyses with type species of related rhodomelacean genera, including Bryocladia, will be necessary to resolve the generic affinities of this clade.
The tribe Streblocladieae
Carradoriella P.C.Silva
The genus Carradoriella was resurrected based on the South African type species Carradoriella virgata, which was previously attributed to Polysiphonia. We have proposed the transfer of Polysiphonia denudata, P. elongata and P. elongella to Carradoriella. This clade was highly supported in both analyses (, ), and although there is some variability in morphology within this group, these species share some important characters including the presence of cortication and a discoid holdfast made up of aggregated cortical filaments and rhizoids (). Species in this group form solitary plants with a distinct main axis, often heavily corticated at the base. Carradoriella denudata, C. elongata and C. elongella have trichoblasts or scar cells on every segment, and tetrasporangia in spiral series, whereas C. virgata is lacking trichoblasts and has tetrasporangia in straight series (Maggs & Hommersand, Citation1993; Stegenga et al., Citation1997). In C. virgata, rhizoidal cortication may develop in between the outer pericentral cells and the central axial cell (Stegenga et al., Citation1997). Also, in both C. denudata and C. elongata, secondary pericentral cells can develop. These pericentral cells form outside the primary pericentral cells in an alternating fashion and are likely derived from the rhizoidal cortication covering the main axes (Maggs & Hommersand, Citation1993). Despite the morphological differences between some members of this genus they are strongly allied in phylogenetic analyses ( and ). Carradoriella denudata, C. elongata, C. elongella and P. polyspora are all European species (C. elongata is found in North America as well), while C. virgata, the type, is endemic to southern Africa (Maggs & Hommersand, Citation1993; Stegenga et al., Citation1997).
Streblocladia F.Schmitz
The type of Streblocladia, S. glomerulata, was sister to Carradoriella in our rbcL-only analyses along with Streblocladia muelleriana (). The genus Streblocladia shares many similarities with Polysiphonia, but differs in that its members display primary dorsiventrality, with distichous and unilateral insertion of branches and trichoblasts (Hommersand, Citation1963; Díaz-Tapia & Bárbara, Citation2013). Although some authors report sympodial branching for Streblocladia, Hommersand (Citation1963) argues that the branching is not truly sympodial and rather this appearance is caused by lateral branches overtopping the apices (see also Kylin, Citation1938). The type, S. glomerulata, has 7–10 pericentral cells, spiralled tetrasporangia, and is native to New Zealand (Adams, Citation1994; Díaz-Tapia & Bárbara, Citation2013). Streblocladia muelleriana, also from New Zealand, is morphologically similar to S. glomerulata, with sympodial branching, 9–12 pericentral cells, and spiralled tetrasporangia (Adams, Citation1991), and was transferred to Streblocladia based on the morphological and molecular evidence. Adams (Citation1991) suggested that P. muelleriana belonged in Streblocladia, but did not formally transfer this species. There are four other species in Streblocladia not assessed here (Guiry & Guiry, Citation2018), and although species within Streblocladia share dorsiventral apices they differ in many other important morphological characters (Díaz-Tapia & Bárbara, Citation2013), indicating the need for a thorough molecular phylogenetic assessment of this genus. Streblocladia is the type genus of the tribe Streblocladieae.
Leptosiphonia Kylin
The genus Leptosiphonia was previously monotypic and represented solely by the species Leptosiphonia schousboei. This species was first placed in Polysiphonia, and then transferred to Ophidocladus Falkenberg (Falkenberg, Citation1901), because it shared the character of two tetrasporangia per segment with the sole species of that genus. However, L. schousboei differed from O. simpliciusculus (P.Crouan & H.Crouan) Falkenberg in several important morphological characters including the arrangement of trichoblasts and spermatangial branches (Díaz-Tapia & Bárbara, Citation2013) and therefore the genus Leptosiphonia was described (Kylin, Citation1956). Sequence data for L. schousboei in GenBank were part of a molecular and morphological survey of seaweeds of the Atlantic Iberian Peninsula (Díaz-Tapia & Bárbara, Citation2013). Phylogenetic analyses showed that four species previously attributed to Polysiphonia, P. brodiei, P. flexicaulis, P. fibrata and P. fibrillosa (including P. sp._1fibrillosa), formed a well-supported monophyletic clade with L. schousboei (, ), and we have proposed the transfer of these species to Leptosiphonia. Although these four species have only one tetrasporangium per segment, the Leptosiphonia clade is united by many morphological characters including the presence of cortication, trichoblasts occurring every segment in spiral series leaving conspicuous scar cells, branches forming in the axils of trichoblasts, and rhizoids that are cut off from pericentral cells (). The number of tetrasporangia per segment has traditionally been considered a significant generic-level character in the Rhodomelaceae, but Díaz-Tapia et al. (Citation2017b) recently transferred the type of Ctenosiphonia (C. hypnoides (Welwitsch) Falkenberg) to Vertebrata based on molecular and morphological evidence, even though it has two tetrasporangia per segment unlike most species in Vertebrata. In this case there is molecular and morphological evidence to support the transfer of P. brodiei, P. fibrata, P. fibrillosa and P. flexicaulis to Leptosiphonia, despite similar differences in tetrasporangial arrangement. In addition, we have noticed that L. fibrillosa and L. flexicaulis seem to decay particularly rapidly after collection when compared with other species of Streblocladieae. Maggs & Hommersand (Citation1993) mention this as well for L. fibrata, suggesting that some species within this clade could be particularly sensitive to changes in salinity or temperature. Although the Canadian genetic species group L. sp._1fibrillosa requires further study, it clearly allies with species of Leptosiphonia (, ).
Acanthosiphonia gen. nov.
Acanthosiphonia gen. nov. was described for Acanthosiphonia echinata (Harvey) comb. nov. Acanthosiphonia echinata has previously been placed in Polysiphonia and Neosiphonia (Díaz-Tapia et al., Citation2017b); however, phylogenetic analyses show that it does not ally with the types of either of those genera (, ). At present, Acanthosiphonia is monotypic, but further analyses are needed to determine if Polysiphonia binneyi and P. havanensis sensu Børgesen should be transferred. Polysiphonia binneyi and P. havanensis sensu Børgesen are sister to Acanthosiphonia in phylogenetic analyses, but with only moderate support. Although these three species share some important morphological characters, including four pericentral cells, branches forming in the axils of trichoblasts, and tetrasporangia in a spiral arrangement, they differ in the connection between rhizoids and pericentral cells (Mamoozadeh & Freshwater, Citation2011, Citation2012). In A. echinata, rhizoids are cut off from pericentral cells, whereas in P. binneyi and P. havanensis sensu Børgesen rhizoids are reportedly in open connection with pericentral cells (Mamoozadeh & Freshwater, Citation2011, Citation2012). To our knowledge, P. binneyi and P. havanensis sensu Børgesen are the only species outside of Polysiphonia sensu stricto and the P. scopulorum group to have rhizoids in open connection with pericentral cells (). Further work is needed to examine this character in these two species. Although Acanthosiphonia and Leptosiphonia are distinct in phylogenetic analyses, they share many important morphological characters (, ). These two genera differ mainly in the habit of their respective species. Acanthosiphonia echinata is bushy and lacking distinct main axes, with plants usually attached via prostrate axes with rhizoids. Species of Leptosiphonia (except L. schousboei) generally have distinct main axes and discoidal basal holdfasts.
Melanothamnus Bornet & Falkenberg and Polyostea Ruprecht
The genera Melanothamnus and Polyostea were both highly supported and monophyletic in our phylogenetic analyses (, ). Melanothamnus was recently expanded by Díaz-Tapia et al. (Citation2017b) to include most of the species previously placed in the genus Neosiphonia. Species within Melanothamnus are distinguished from other species of Streblocladieae by having two unique characteristics: 3-celled carpogonial branches, and plastids that are found only on the radial walls of pericentral cells, giving the cells a transparent or ‘glassy’ appearance (Díaz-Tapia et al., Citation2017b).
Species of Polyostea are characterized by having rhizoids that are cut off from pericentral cells, proximal segments of lateral branches fused to main axes over 0.5–1.0 axial segments, and radial development at the apices that becomes flattened and distichous distally (Savoie & Saunders, Citation2016). It is interesting to note that to date this genus is restricted to the North Pacific and Arctic Oceans.
Kapraunia gen. nov.
The genus Kapraunia was described for four species previously attributed to Polysiphonia: K. schneideri (the type), K. amplacapilli, K. morroides and K. pentamera. This group was strongly supported in molecular analyses (, ) and species are united by several morphological characters including: trichoblasts that occur in no particular pattern, branches that form laterally from the basal trichoblast cell or replace trichoblasts, a discoid holdfast with subsequent development of secondary prostrate axes, and rhizoids that are cut off from pericentral cells. Historically, it was thought that lateral branches in Polysiphonia could develop in one of three possible ways: independently of trichoblasts, replacing trichoblasts, or forming in the axils of trichoblasts (Stuercke & Freshwater, Citation2008). Mamoozadeh & Freshwater (Citation2012) first noted that K. pentamera and K. schneideri had a fourth type, where branches develop laterally from the basal trichoblast cell. Kapraunia amplacapilli was described as having branches forming in the axils of trichoblasts; however, if Kim & Kim (Citation2014) were unaware of this fourth possible character state it may have been overlooked. This character needs to be reassessed for K. amplacapilli, but based on the description and photographs of this species (Kim & Kim, Citation2014) it is possible that branches form laterally to trichoblasts. However, in K. morroides branches replace trichoblasts in development (Kim & Kim, Citation2014). Branch development in relation to trichoblasts may be an important genus- and species-level character for the tribe Streblocladieae; however, it requires further study and consideration that there are at least four possible character states, as opposed to the three character states considered previously. Although Kapraunia and Melanothamnus are sister to one another in phylogenetic analyses (), species of Kapraunia do not share the defining characters previously discussed for Melanothamnus. Species of Kapraunia do not have plastids restricted to radial walls of the pericentral cells, and furthermore at least three species in this genus (K. amplacapilli, K. morroides and K. schneideri) have a 4-celled carpogonial branch (Díaz-Tapia et al., Citation2013; Kim & Kim, Citation2014). Sequence data retrieved from GenBank for Kapraunia amplacapilli, K. schneideri and K. morroides were from the original collections of each species, while K. pentamera had a detailed morphological assessment linked to the available sequence data (Stuercke & Freshwater, Citation2010; Mamoozadeh & Freshwater, Citation2012; Kim & Kim, Citation2014).
Eutrichosiphonia gen. nov.
Eutrichosiphonia gen. nov. was described for two species previously attributed to Polysiphonia, Eutrichosiphonia confusa (the type) and E. sabulosia. The two genetic groups identified as Polysiphonia paniculata in British Columbia and California (see barcode results) also fall within this clade (, ). Eutrichosiphonia formed a strongly supported clade in phylogenetic analyses, and along with Lampisiphonia was sister to the recently expanded genus Vertebrata (, ). Species in Eutrichosiphonia were united by several consistent morphological characters including: greater than four pericentral cells, rhizoids that are cut off from pericentral cells; abundant trichoblasts on every segment in a spiral pattern, leaving conspicuous, persistent scar cells; ecorticate axes; and tetrasporangia in spiral series (). There are significant morphological differences between Eutrichosiphonia and Lampisiphonia, as L. iberica Bárbara, Secilla, Díaz-Tapia & H.G.Choi, the sole species of the genus, is heavily corticated, lacks trichoblasts and has tetrasporangia in straight series (Bárbara et al., Citation2013). Although phylogenetic analyses show that both of the genetic species groups attributed to Polysiphonia paniculata in British Columbia (P. sp._1paniculata and P._sp._2paniculata) fall within Eutrichosiphonia, they cannot be transferred at this time, as we are uncertain which (if any) of these groups represents authentic Polysiphonia paniculata. Our collections of E. confusa were from Santa Cruz, California, ~600 km from the type locality of Corona del Mar, California. The sequence data retrieved from GenBank for P. sabulosia were cited in the protologue of the species (Kim & Kim, Citation2014).
Vertebrata S.F.Gray
In our analyses the recently expanded genus Vertebrata (Díaz-Tapia et al., Citation2017b) was monophyletic and highly supported (, ). Based on molecular and morphological evidence, we have proposed the transfer of Polysiphonia hendryi to Vertebrata and reinstated the names V. ericoides and V. woodii for Perrinia ericoides (Harvey) Womersley (an Australian species) and Pterochondria woodii (Harvey) Hollenberg (, ). Perrinia and Pterochondria were monotypic genera. These three species fell unequivocally within the Vertebrata clade (, ), with V. hendryi sister to V. stimpsonii, a species from Japan previously attributed to Enelittosiphonia, V. ericoides, sister to the New Zealand species V. aterrima, and V. woodii sister to the type, V. lanosa. However, relationships within this clade were generally poorly supported in our analyses (). Vertebrata as characterized by Díaz-Tapia et al. (Citation2017b) is a morphologically diverse genus, yet two characters unite its members: greater than six pericentral cells, and well-developed conspicuous trichoblasts with multinucleate basal cells (except for the type, V. lanosa, which lacks trichoblasts altogether). Vertebrata ericoides, V. hendryi and V. woodii all have more than six pericentral cells (Hollenberg, Citation1942b, Citation1944; Womersley, Citation2003). Vertebrata woodii lacks trichoblasts, but V. ericoides and V. hendryi have abundant and conspicuous trichoblasts, although the number of nuclei in the basal trichoblast cell of the latter two species has not been examined at this time. It is interesting to note that in our analyses, V. woodii is most closely related to V. lanosa, which also lacks trichoblasts (although the support for this relationship is low, ). In addition, both of these species grow epiphytically on brown algae with rhizoids penetrating the host (Hollenberg, Citation1942b; Maggs & Hommersand, Citation1993). Vertebrata lanosa is an obligate epiphyte on Ascophyllum (rarely other brown algae), while Vertebrata woodii is usually epiphytic on Cystoseira and Pleurophycus. Vertebrata ericoides was originally placed in the monospecific genus Perrinia partly due to the fact that this species has two tetrasporangia per segment, a relatively unusual feature for species of Streblocladieae, which generally have one tetrasporangium per segment. However, V. hypnoides, which was recently placed in Vertebrata by Díaz-Tapia et al. (Citation2017b), has two tetrasporangia per segment. Leptosiphonia schousboei also has two tetrasporangia per segment, as discussed above. The genus Vertebrata was highly supported in phylogenetic analyses (, ), and although species within this large clade are morphologically variable, they are united by having more than six pericentral cells, in contrast to the four pericentral cells of species of Polysiphonia sensu stricto.
Conclusions
Molecular phylogenetic analyses were critical for resolving evolutionary relationships of genera within the tribes Polysiphonieae and Streblocladieae. Significant taxonomic changes were necessary to resolve the ongoing confusion within these tribes, including the transfer of species from Polysiphonia to the resurrected genus Carradoriella, the previously monotypic genus Leptosiphonia, the recently expanded genus Vertebrata, and the newly described genera Acanthosiphonia gen. nov., Eutrichosiphonia gen. nov. and Kapraunia gen. nov. Expanded phylogenetic analyses with more collections and additional taxa will be necessary to resolve the outstanding uncertainties. We found that using combinations of morphological characters in addition to molecular evidence was the most useful method for defining genera within these tribes. There are also many species-level issues within this group that will require sequencing topotype material as well as detailed morphological assessments. Future publications will address some of the species-level taxonomic issues raised in this study. Although morphological characters can be difficult to interpret and unreliable within this group, they may still be useful when used in combination with molecular analyses.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://doi.org/10.1080/09670262.2018.1483531
Supplementary table S1. Collection information for samples included in this study.
Supplementary table S2. GenBank accession numbers for sequence data included in this study. GenBank accessions in bold type were newly determined for this study.
Author contributions
Amanda M. Savoie: original concept, analysis of molecular data, drafting and editing manuscript; G. Saunders: original concept, analysis of molecular data, editing manuscript.
Acknowledgements
We would like to thank K. Dixon, G. Filloramo, D. McDevit and T. Moore for generating some of the sequence data used in this paper, as well as all of the colleagues involved in collecting (Supplementary table S1). We are grateful to P. Díaz-Tapia and C. Maggs for sharing with us their sequence for Streblocladia glomerulata. We would also like to thank C.W. Schneider and M.J. Wynne for their help with Latin nomenclature, as well as M.S. Kim for her helpful comments on an earlier draft of this manuscript. We also thank N. Sloan for encouraging us to study the seaweeds of Haida Gwaii, as well as Parks Canada and the Haida Nation, especially staff of the Gwaii Haanas National Park Reserve and Haida Heritage Site, for extensive support of our field studies in this unique region.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abbott, I.A. & Hollenberg, G.J. (1976). Marine Algae of California. Stanford University Press, Stanford, CA.
- Adams, N.M. (1991). The New Zealand species of Polysiphonia Greville (Rhodophyta). New Zealand Journal of Botany, 29: 411–427.
- Adams, N.M. (1994). Seaweeds of New Zealand. An Illustrated Guide. Canterbury University Press, Christchurch.
- Agardh, C.A. (1817). Synopsis algarum Scandinaviae adjecta dispositione universali algarum. Berling, Lund.
- Bárbara, I., Choi, H.-G., Secilla, A., Díaz-Tapia, P., Gorostiaga, J. M., Seo, T.-K., Jung, M.-Y. & Bercibar, E. (2013). Lampisiphonia iberica gen. et sp. nov. (Ceramiales, Rhodophyta) based on morphology and molecular evidence. Phycologia, 52: 137–155.
- Bustamante, D.E., Won, B.Y. & Cho, T.O. (2014a). Polysiphonia dokdoensis sp. nov. (Rhodomelaceae, Ceramiales) based on a population previously known as Polysiphonia atlantica sensu Kim & Lee from Korea. Botanica Marina, 57: 281–289.
- Bustamante, D.E., Won, B.Y. & Cho, T.O. (2014b). Polysiphonia ulleungensis sp. nov. (Rhodomelaceae, Rhodophyta): a new diminutive species from Korea belonging to Polysiphonia sensu stricto. Algae, 29: 111–120.
- Bustamante, D.E., Won, B.Y. & Cho, T.O. (2015a). First record of Neosiphonia echinata (Rhodomelaceae, Rhodophyta) in the South Pacific: an introduced species in Southeast Asia. Botanica Marina, 58: 345–354.
- Bustamante, D.E., Won, B.Y. & Cho, T.O. (2015b). Polysiphonia freshwateri sp. nov. and Polysiphonia koreana sp. nov.: two new species of Polysiphonia (Rhodomelaceae, Rhodophyta) from Korea. European Journal of Phycology, 50: 330–342.
- Choi, H.-G., Kim, M.-S., Guiry, M.D. & Saunders, G.W. (2001). Phylogenetic relationships of Polysiphonia (Rhodomelaceae, Rhodophyta) and its relatives based on anatomical and nuclear small-subunit rDNA sequence data. Botany, 79: 1465–1476.
- Christensen, T. (1967). Two new families and some new names and combinations in the algae. Blumea, 15: 91–94.
- Croce, M.E. & Parodi, E.R. (2014). The Japanese alga Polysiphonia morrowii (Rhodomelaceae, Rhodophyta) on the South Atlantic Ocean: first report of an invasive macroalga inhabiting oyster reefs. Helgoland Marine Research, 68: 241–252.
- Curiel, D., Bellemo, G., La Rocca, B., Scattolin, M. & Marzocchi, M. (2002). First report of Polysiphonia morrowii Harvey (Ceramiales, Rhodophyta) in the Mediterranean Sea. Botanica Marina, 45: 66–70.
- D’Archino, R., Neill, K.F. & Nelson, W.A. (2013). Recognition and distribution of Polysiphonia morrowii (Rhodomelaceae, Rhodophyta) in New Zealand. Botanica Marina, 56: 41–47.
- Díaz-Tapia, P. & Bárbara, I. (2013). Seaweeds from sand-covered rocks of the Atlantic Iberian Peninsula. Part 1. The Rhodomelaceae (Ceramiales, Rhodophyta). Cryptogamie Algologie, 34: 325–422.
- Díaz-Tapia, P., Kim, M.-S., Secilla, A., Bárbara, I. & Cremades, J. (2013). Taxonomic reassessment of Polysiphonia foetidissima (Rhodomelaceae, Rhodophyta) and similar species, including P. schneideri, a newly introduced species in Europe. European Journal of Phycology, 48: 345–362.
- Díaz-Tapia, P., Maggs, C.A., West, J.A. & Verbruggen, H. (2017a). Analysis of chloroplast genomes and a supermatrix inform reclassification of the Rhodomelaceae (Rhodophyta). Journal of Phycology, 53: 920–937.
- Díaz-Tapia, P., McIvor, L., Freshwater, D.W., Verbruggen, H., Wynne, M.J. & Maggs, C.A. (2017b). The genera Melanothamnus Bornet & Falkenberg and Vertebrata S.F. Gray constitute well-defined clades of the red algal tribe Polysiphonieae (Rhodomelaceae, Ceramiales). European Journal of Phycology, 52: 1–30.
- Falkenberg, P. (1901). Die Rhodomelaceen des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. Fauna und Flora des Golfes von Neapel, Monographie 26.
- Filloramo, G.V. & Saunders, G.W. (2016). Molecular-assisted alpha taxonomy of the genus Rhodymenia (Rhodymeniaceae, Rhodymeniales) from Australia reveals overlooked species diversity. European Journal of Phycology, 51: 354–367.
- Filloramo, G., Savoie, A.M., Selivanova, O., Wynne, M.J. & Saunders, G.W. (2017). Key Kamchatkan collections provide new taxonomic and distributional insights for reportedly pan-North Pacific species of Rhodymeniophycidae (Rhodophyta). Phycologia, 56: 296–302.
- Gabrielson, P.W., Widdowson, T.B. & Lindstrom, S.C. (2006). Keys to the Benthic Marine Algae and Seagrasses of British Columbia, Southeast Alaska, Washington and Oregon. Dept. of Botany, University of British Columbia, Vancouver.
- Geoffroy, A., Le Gall, L. & Destombe, C. (2012). Cryptic introduction of the red alga Polysiphonia morrowii Harvey (Rhodomelaceae, Rhodophyta) in the North Atlantic Ocean highlighted by a DNA barcoding approach. Aquatic Botany, 100: 67–71.
- Greuter, W., Barrie, F.R., Burdet, H.M., Chaloner, W.G., Demoulin, V., Hawksworth, D.L., Jørgensen, P.M., Nicolson, D.H., Silva, P.C., Trehane, P. & McNeill, J. (1994). International Code of Botanical Nomenclature (Tokyo Code) adopted by the Fifteenth International Botanical Congress, Yokohama, August–September 1993. Koeltz Scientific Books, Königstein.
- Greville, R.K. (1823). Scottish Cryptogamic Flora, or Coloured Figures and Descriptions of Cryptogamic Plants, Belonging Chiefly to the Order Fungi; and Intended to Serve as a Continuation of English Botany. Vol. 2. MacLachlan & Stewart; Baldwin, Craddock & Joy, Edinburgh & London.
- Greville, R.K. (1824). Flora Edinensis: or a Description of Plants Growing near Edinburgh, Arranged According to the Linnean System, with a Concise Introduction to the Natural Orders of the Class Cryptogamia, and Illustrated Plates. Printed for William Blackwood, Edinburgh; and T. Cadell, Strand, London.
- Guiry, M.D. & Guiry, G.M. (2018). AlgaeBase. http://www.algaebase.org.
- Hollenberg, G. (1942a). An account of the species of Polysiphonia on the Pacific coast of North America. I. Oligosiphonia. American Journal of Botany, 29: 772–785.
- Hollenberg, G. (1942b). Phycological notes – I. Bulletin of the Torrey Botanical Club, 69: 528–538.
- Hollenberg, G. (1944). An account of the species of Polysiphonia on the Pacific coast of North America II. Polysiphonia. American Journal of Botany, 31: 474–483.
- Hollenberg, G. (1961). Marine red algae of Pacific Mexico. Part 5: The genus Polysiphonia. Pacific Naturalist, 2: 345–375.
- Hollenberg, G. (1968). An account of the species of Polysiphonia of the Central and Western Tropical Pacific Ocean I. Oligosiphonia. Pacific Science, 22: 56–98.
- Hommersand, M.H. (1963). The morphology and classification of some Ceramiaceae and Rhodomelaceae. University of California Publications in Botany, 35: vii +165–366, 52 figs, 6 pls.
- Hudson, W. (1762). Flora anglica; exhibens plantas per regnum angliae sponte crescentes, distributas secundum systema sexuale: cum differentiis specierum, synonymis auctorum, nominibus incolarum, solo locorum, tempore florendi, ofììcinalibus pharmacopoeorum. Prostant venales apud J. Nourse in the Strand, et C. Moran in Covent-Garden, Londini [London].
- Kapraun, D.F. (1977). The genus Polysiphonia in North Carolina, USA. Botanica Marina, 20: 313–330.
- Kapraun, D.F. & Rueness, J. (1983). The genus Polysiphonia (Ceramiales, Rhodomelaceae) in Scandinavia. Giornale Botanico Italiano, 117: 1–31.
- Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P. and Drummond, A. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28: 1647–1649.
- Kim, B. & Kim, M.-S. (2014). Three new species of Polysiphonia sensu lato (Rhodophyta) based on the morphology and molecular evidence. Algae, 29: 183–195.
- Kim, M.-S. & Lee, I.K. (1999). Neosiphonia flavimarina gen. et sp. nov. with a taxonomic reassessment of the genus Polysiphonia (Rhodomelaceae, Rhodophyta). Phycological Research, 47: 271–281.
- Kim, M.-S., Maggs, C.A., McIvor, L. & Guiry, M.D. (2000). Reappraisal of the type species of Polysiphonia (Rhodomelaceae, Rhodophyta). European Journal of Phycology, 35: 83–92.
- Kim, M.-S., Yang, E.C., Mansilla, A. & Boo, S.M. (2004). Recent introduction of Polysiphonia morrowii (Ceramiales, Rhodophyta) to Punta Arenas, Chile. Botanica Marina, 47: 389–394.
- Kudo, T. & Masuda, M. (1981). A taxonomic study of Polysiphonia morrowii Harvey (Rhodophyta, Ceramiales). Japanese Journal of Phycology, 29: 263–272.
- Kudo, T. & Masuda, M. (1992). Taxonomic features of Polysiphonia morrowii Harvey (Ceramiales, Rhodophyta). Korean Journal of Phycology, 7: 13–26.
- Kylin, H. (1938). Verzeichnis einiger Rhodophyceen von Südafrika. Acta Universitatis Lundensis, 34: 1–26, 10 figs, 8 plates.
- Kylin, H. (1956). Die Gattungen der Rhodophyceen. C.W.K. Gleerups, Lund.
- Lam, D.W., García-Fernández, M.E., Aboal, M. & Vis, M.L. (2013). Polysiphonia subtilissima (Ceramiales, Rhodophyta) from freshwater habitats in North America and Europe is confirmed as conspecific with marine collections. Phycologia, 52: 156–160.
- Lancelot, A. (1966). Quelques Rhodomélacées des genres Polysiphonia et Leptosiphonia a Biarritz. Bulletin du Centre d’Etudes et de Recherches Scientifiques, Biarritz, 6: 95–107.
- Maggs, C.A. & Hommersand, M.H. (1993). Seaweeds of the British Isles. Volume 1. Rhodophyta. Part 3A. Ceramiales. HMSO, London.
- Mamoozadeh, N.R. & Freshwater, D.W. (2011). Taxonomic notes on Caribbean Neosiphonia and Polysiphonia (Ceramiales, Florideophyceae): five species from Florida, USA and Mexico. Botanica Marina, 54: 269–292.
- Mamoozadeh, N.R. & Freshwater, D.W. (2012). Polysiphonia sensu lato (Ceramiales, Florideophyceae) species of Caribbean Panama including Polysiphonia lobophoralis sp. nov. and Polysiphonia nuda sp. nov. Botanica Marina, 55: 317–347.
- Masuda, M., Kudo, T., Kawaguchi, S. & Guiry, M.D. (1995). Lectotypification of some marine red algae described by W. H. Harvey from Japan. Phycological Research, 43: 191–202.
- McDevit, D.C. & Saunders, G.W. (2010). A DNA barcode examination of the Laminariaceae (Phaeophyceae) in Canada reveals novel biogeographical and evolutionary insights. Phycologia, 49: 235–248.
- Montagne, C. (1842). Troisième centurie de plantes cellulaires exotiques nouvelles. Décades V, VI, VII et VIII. Annales des Sciences Naturelles, Botanique, Seconde Série, 18: 241–282.
- Nam, K.W. & Kang, P.J. (2012). Algal flora of Korea. Volume 4, Number 4. Rhodophyta: Ceramiales: Rhodomelaceae: 18 genera including Herposiphonia. National Institute of Biological Resources, Incheon.
- Perestenko, L.P. (1980). Algae of Peter the Great Bay. Nauka, Leningrad.
- Ratnasingham, S. & Hebert, P.D.N. (2007). BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Molecular Ecology Notes, 7: 355–364.
- Saunders, G.W. & McDevit, D.C. (2012). Methods for DNA barcoding photosynthetic protists emphasizing the macroalgae and diatoms. Methods in Molecular Biology, 858: 207–222.
- Saunders, G.W. & Moore, T.E. (2013). Refinements for the amplification and sequencing of red algal DNA barcode and RedToL phylogenetic markers: a summary of current primers, profiles and strategies. Algae, 28: 1–13.
- Saunders, G.W., Hawkins, N. & Wilkin, S. (2013). A survey of Sam Orr’s Pond (New Brunswick, Canada) uncovers the invasive green alga Codium fragile (Chlorophyta) and the orange-striped green anemone Diadumene lineata (Cnidaria), first records for the Bay of Fundy and Canada, respectively. BioInvasions Records, 2: 1–5.
- Savoie, A.M. & Saunders, G.W. (2015). Evidence forthe introduction of the Asian red alga Neosiphonia japonica and its introgression with Neosiphonia harveyi(Ceramiales, Rhodophyta) in the Northwest Atlantic. Molecular Ecology, 24: 5927–5937.
- Savoie, A.M. & Saunders, G.W. (2016). A molecular phylogenetic and DNA barcode assessment of the tribe Pterosiphonieae (Ceramiales, Rhodophyta) emphasizing the Northeast Pacific. Botany, 94: 917–939.
- Schneider, C.W. & Searles, R.B. (1991). Seaweeds of the Southeastern United States. Cape Hatteras to Cape Canaveral. Duke University Press, Durham & London.
- Sears, J. (2002). NEAS Keys to the Benthic Marine Algae of the Northeast Coast of North America from Long Island Sound to the Strait of Belle Isle. UMass Dartmouth, Dartmouth, MA.
- Segi, T. (1951). Systematic study of the genus Polysiphonia from Japan and its vicinity. Journal of the Faculty of Fisheries, Prefectural University of Mie, 1: 167–272, 36 figs, 16 plates.
- Silva, P.C. (1952). A review of nomenclatural conservation in the algae from the point of view of the type method. University of California Publications in Botany, 25: 241–323.
- Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22: 2688–2690.
- Stegenga, H., Bolton, J.J. & Anderson, R.J. (1997). Seaweeds of the South African West Coast. Bolus Herbarium, University of Cape Town, Cape Town.
- Stuercke, B. & Freshwater, D.W. (2008). Consistency of morphological characters used to delimit Polysiphonia sensu lato species (Ceramiales, Florideophyceae): analyses of North Carolina, USA specimens. Phycologia, 47: 541–559.
- Stuercke, B. & Freshwater, D. W. (2010). Two new species of Polysiphonia (Ceramiales, Florideophyceae) from the western Atlantic. Botanica Marina, 53: 301–311.
- Taylor, W.R. (1937). Marine Algae of the Northeastern Coast of North America. The University of Michigan Press, Ann Arbor, MI.
- Thiers, B. (2018). Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih/.
- Womersley, H.B.S. (2003). The marine benthic flora of southern Australia – Part IIID Ceramiales – Delesseriaceae, Sarcomeniaceae, Rhodomelaceae. Australian Biological Resources Study & State Herbarium of South Australia, Canberra & Adelaide.
- Wynne, M.J. (1986). Report on a collection of benthic marine algae from the Namibian coast (southwestern Africa). Nova Hedwigia, 43: 311–355.
- Yamanouchi, S. (1906). The life history of Polysiphonia violacea. Botanical Gazette, 41: 425–433.
