ABSTRACT
Female reproductive structures, especially ampullae, play a significant role in defining generic boundaries in the family Halymeniaceae, despite recent advances in phylogenetic analyses aided by molecular data. Surveys of red algae from the Korean subtidal zone have revealed new evolutionary lineages within this family. We here propose a new genus in the Halymeniaceae, Nesoia H.W.Lee & M.S.Kim, gen. nov., with a description of N. delicatula sp. nov., N. pulchella sp. nov. and N. latifolia (P. Crouan & H. Crouan ex Kützing) comb. nov., based on Halymenia latifolia. Nesoia, which constitutes a distinct lineage of the Halymeniaceae in rbcL phylogenetic trees, has a foliose thallus with a single-layered cortex and medulla of anticlinal filaments. Carpogonial ampullae bear two-celled carpogonial branches and auxiliary cell ampullae have multiple orders of branching, mostly up to three or four. The auxiliary cell ampullary system of Nesoia is a unique type previously undescribed in the Halymeniaceae: each cell of the first-order ampullary filament produces bilateral second-order filaments, and the auxiliary cell is the basal cell of one of the two second-order filaments borne on the second or third cell of the first-order filament. Discovery of the new genus Nesoia suggests the need for more careful and systematic exploration of species diversity in this group of red algae.
Introduction
The red algal family Halymeniaceae Bory is composed of 34 genera and 321 species (Guiry & Guiry, Citation2017). Members are characterized by multiaxial thalli with slender to robust blades or branches, a cortex composed of spherical to ovoid cells and an anticlinal filamentous or pseudoparenchymatous medulla, with or without stellate or refractive ganglioid cells (Womersley & Lewis, Citation1994). In the Halymeniaceae, Grateloupia C. Agardh, Halymenia C. Agardh and Cryptonemia J. Agardh are the most speciose genera comprising ~70% of all species in the family (Guiry & Guiry, Citation2017).
Features of female reproductive structures, such as the type of carpogonial branch, presence or absence of a procarp and type of gonimoblast development, have provided important diagnostic characteristics for distinguishing higher-level taxa of red algae (Min-Thein & Womersley, Citation1976; Hommersand & Fredericq, Citation1990). Taxonomic studies of the Halymeniaceae have relied particularly on unique organs like carpogonial ampullae and auxiliary cell ampullae, which support the carpogonial branches and auxiliary cells, respectively. Chiang (Citation1970) showed that the Halymeniaceae could be divided into five types based on auxiliary cell ampullary structure: Grateloupia-type, Thamnoclonium-type, Halymenia-type, Aeodes-type and Cryptonemia-type.
Although molecular analyses using the rbcL gene have discovered many unrecognized taxa and contributed to red algal phylogenetic reappraisal (Boo et al., Citation2016; Díaz-Tapia et al., Citation2017; Rousseau et al., Citation2017), detailed morphological studies of female reproductive structure anatomy should still be recognized as important (Gurgel & Fredericq, Citation2004; Kang & Kim, Citation2016; Yang & Kim, Citation2017). Systematics of the Halymeniaceae have undergone various revisions. Kawaguchi et al. (Citation2002) transferred two members of the genus Carpopeltis F. Schmitz, C. affinis (Harvey) Okamura and C. prolifera (Hariot) Kawaguchi & Masuda, to Polyopes J. Agardh because of their Aeodes-type auxiliary cell ampullae and proximity to the Polyopes clade in the rbcL analysis. Lin et al. (Citation2008) revealed two new kinds of auxiliary cell ampullae in the genus Grateloupia, the G. taiwanensis-type with three orders of unbranched filaments, and the G. orientalis-type with two orders of unbranched filaments. Gargiulo et al. (Citation2013) transferred Grateloupia dichotoma J. Agardh and G. horrida Kützing to Dermocorynus P. Crouan & H. Crouan based on female reproductive characteristics, 5-celled carpogonial branches and 4-celled auxiliary branches.
Foliose species of Halymenia have few morphological characteristics that enable easy species recognition due to their simple blade shape with few externally discernible features (Abbott, Citation1967; De Smedt et al., Citation2001). This results in difficulties in delimiting H. elongata C. Agardh, H. floridana J. Agardh (Azevedo et al., Citation2016b) and H. latifolia P. Crouan & H. Crouan ex Kützing (D’Archino et al., Citation2014), for example. However, several new foliose species have recently been revealed with the help of molecular analyses (Tan et al., Citation2015, Citation2017; Azevedo et al., Citation2016b). Discovery of the genus Amalthea D’Archino & W. A. Nelson through in-depth investigation of female reproductive development provided further evidence for the need to resolve the taxonomic position of Halymenia within the Halymeniaceae (D’Archino et al., Citation2014).
Our survey of the Korean subtidal zone has provided opportunities to discover novel Halymeniaceae. We obtained foliose red algal specimens, including mature female samples, and carried out both morphological observations and sequence analyses using the rbcL gene. The aims of this study were (1) to determine the taxonomic positions of unrecognized specimens based on detailed examination of vegetative and reproductive structures, and (2) to describe a new auxiliary cell ampullary type for the Halymeniaceae observed in Korean specimens.
Materials and methods
Samples were collected using scuba from depths of 20–25 m at Jeju Island, Korea and photographed using a Canon Powershot G7X camera (Canon, Tokyo, Japan) to assist description of the external morphology. Samples for DNA analyses were removed from the thallus and dried in silica gel. Samples for anatomical investigation were preserved in 2.5% formalin in seawater. Sections were manually prepared using a razor or a bench-top freezing microtome (NK-101-II; Nippon Optical Works Co., Ltd, Tokyo, Japan). In addition, some specimens were squashed to observe female reproductive structures. Sectioned material was stained either with 1% aniline blue acidified with 1% HCl and mounted in 35% custom-made corn syrup, or stained with Wittmann’s aceto-iron-haematoxylin-chloral hydrate (Wittmann, Citation1965) and mounted in 50% Hoyer’s mounting medium (Lin et al., Citation2012). Photomicrographs were obtained using a BX43 microscope (Olympus, Tokyo, Japan) with an EOS 600D digital camera (Canon, Tokyo, Japan). Digitized images were adjusted for clarity using Adobe Photoshop software (Adobe Systems Inc., San Jose, California, USA; ver. 6.1). Pressed herbarium sheets were deposited as voucher specimens in the herbaria of Jeju National University (JNUB), Korea and the National Institute of Biological Resources (NIBR), Incheon, Korea.
DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Following DNA extraction PCR was performed to amplify the plastid-encoded large subunit of the rbcL gene using the primer combinations F145–R898 and F762–R1442 (Kim et al., Citation2010). Amplification was performed in a 20 μl reaction mixture using AccuPower PCR PreMix (Bioneer, Daejeon, Korea) and Swift MaxPro thermal cyclers (ESCO, Singapore). The PCR parameters were as follows: initial denaturation at 96°C for 4 min, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 2 min with a final extension at 72°C for 7 min. PCR products were purified using the AccuPrep Purification Kit (Bioneer) and sequenced commercially at Macrogen (Seoul, Korea). Sequencing electropherograms were edited using Chromas software (Queensland, Australia; version 1.45). All available rbcL sequence data of 53 selected Halymeniaceae species and three outgroup species (Plocamium maggsiae G.W. Saunders & Lehmkuhl, Sebdenia flabellata (J. Agardh) P.G. Parkinson, S. integra Gavio, Hickerson & Fredericq) were obtained from GenBank. Multiple sequence alignment was performed using the BioEdit software (Hall, Citation1999) under visual inspection.
Uncorrected pairwise distance between two rbcL sequences was estimated using MEGA 5.0 software (Tamura et al., Citation2011). To construct the rbcL phylogenetic tree, maximum likelihood (ML) analysis was performed using RAxML software (Stamatakis, Citation2006). RAxML was performed with all three codons partitioned under the GTR + Γ + I model. To identify the best tree, 200 independent tree inferences were performed using the −# option with default –I (automatically optimized subtree pruning-regrafting rearrangement) and –c (25 distinct rate categories) software options. To generate bootstrap support values for the phylogeny, 1000 bootstrap replicates were performed. Bayesian phylogenetic inference (BI) was generated using MrBayes software (Ronquist & Huelsenbeck, Citation2003; ver. 3.1.2). ML and BI trees were edited with the program FigTree (v1.4.0).
Results
Molecular phylogeny
A set of 60 rbcL gene sequences from representative taxa, especially the genus Halymenia, was analysed to resolve generic relationships among Halymeniaceae and outgroup species (). The alignment of rbcL sequences included 1440 sites in total with 538 variable sites (32.4%) and 437 parsimony-informative sites (25.6%). The phylogenetic tree () provides bootstrap values for ML and BI posterior probability values.
Fig. 1. Phylogenetic tree of the Halymeniaceae including the new genus Nesoia with outgroup taxa based on ML analysis of rbcL sequences. Support values on each branch are ML bootstrap (>60%, left) and Bayesian posterior probability (>0.60, right). Scale bar represents substitutions per site.
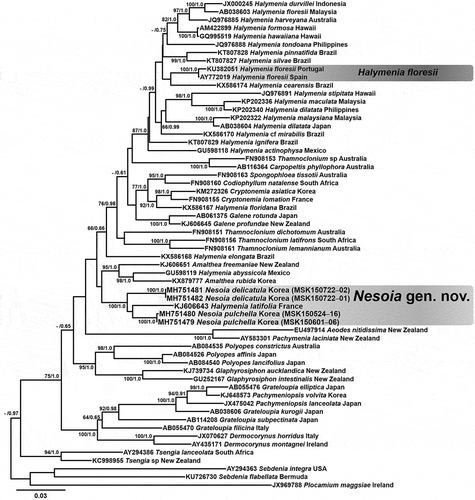
Figs 2‒15. Nesoia delicatula sp. nov. Fig. 2. Holotype, MSK150722-01, Gwideok, Jeju Island, Korea, monoecious gametophyte. Fig. 3. Isotype, MSK150722-02, vegetative. Fig. 4. Isotype, MSK150722-03, tetrasporophyte. Fig. 5. Isotype, MSK150722-04, monoecious gametophyte. Fig. 6. Isotype, MSK150722-05, tetrasporophyte. Fig. 7. Cortical cells in surface view. Fig. 8. Subcortical stellate cells and filaments. Fig. 9. Cross-section of blade margin showing single-layered cortex. Fig. 10. Entire margin of blade. Fig. 11. Cross-section of a blade showing the medulla composed of anticlinal filaments. Fig. 12. A subcortical stellate cell connected to anticlinal medullary filaments. Fig. 13. Tetrasporangia among cortical cells showing cruciate division (arrows). Fig. 14. Surface view of monoecious gametophyte showing spermatangia and carposporophyte (cs). Fig. 15. Spermatangia (sp) cut off from the spermatangial mother cells (smc). Scale bars: Figs 2‒6 = 2 cm; Figs 7, 12‒14 = 20 μm; Figs 9‒11 = 40 μm; Fig. 15 = 10 μm.
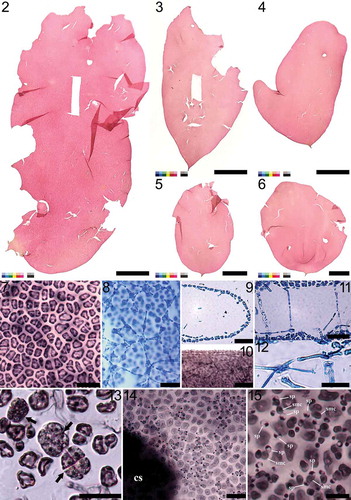
Figs 16‒24. Nesoia delicatula sp. nov. Fig. 16. Early stage of auxiliary cell ampulla borne on subcortical stellate cell (scc) showing the auxiliary cell (aux) as one of the first cells of second-order filament (labelled 1’–4’) borne on third cell of first-order filament (labelled 1–7). Fig. 17. Further development of the auxiliary cell ampulla. The first- (labelled 1–7), second- (labelled 1’–5’), and third-order filaments (labelled 1”–3”) are marked for clarity. Fig. 18. Mature auxiliary cell ampulla with a subcortical stellate cell (scc) and an auxiliary cell (aux). Fig. 19. Early stage of post-fertilization showing a fusion cell complex (fcc) bearing a gonimoblast initial (gbi) surrounded by ampullary filaments. Fig. 20. Gonimolobe (g) division from the gonimoblast initial (gbi). Funnel-shaped fusion cell complex (fcc) is linked to a connecting filament (cf). Fig. 21. Further division of gonimolobes (g) from the gonimoblast initial and the development of involucral filaments (if) from the ampullary filaments (af). A connecting filament is visible. Fig. 22. Formation of pericarp by several ampullary filaments that have developed into involucral filaments (if). Connecting filaments (cf) are still visible. Fig. 23. Mature pericarp wholly formed of involucral filaments encasing carposporangia. Fig. 24. Mature cystocarp. Scale bars = 10 μm.
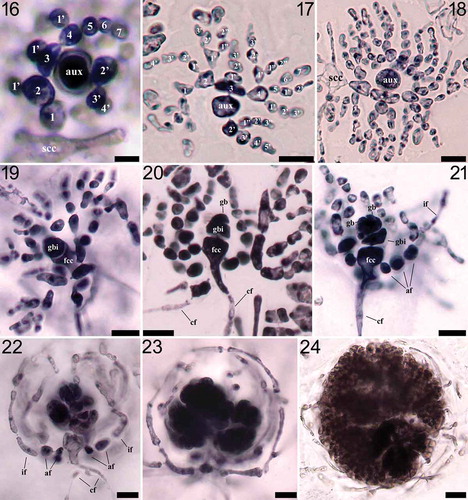
Figs 25‒31. Nesoia pulchella sp. nov. Fig. 25. Holotype, MSK150524-16, Dodu, Jeju Island, Korea, vegetative. Fig. 26. Topotype, MSK150601-06, vegetative. Fig. 27. Cortical cells in surface view. Fig. 28. Entire margin of blade. Fig. 29. Subcortical stellate cells and filaments. Fig. 30. A subcortical stellate cell. Fig. 31. Cross-section of a blade showing the medulla composed of anticlinal filaments. Scale bars: Figs 25‒26 = 1 cm; Figs 27‒29, 31 = 30 μm; Fig. 30 = 10 μm.
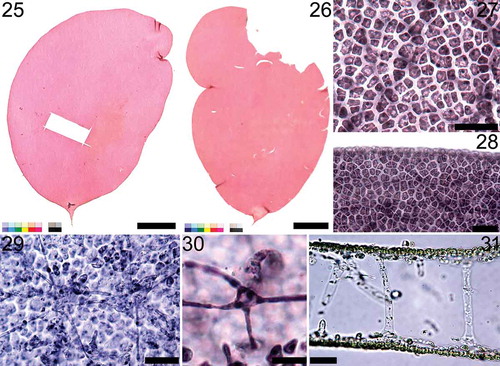
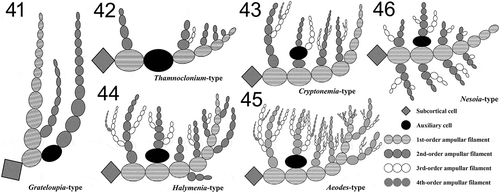
Figs 32‒40. Nesoia pulchella sp. nov. Fig. 32. Carpogonial branch ampulla with a two-celled carpogonial branch with a hypogynous cell (hy), carpogonium (cp) and a trichogyne (tr). Fig. 33. Early stage of auxiliary cell ampulla borne on subcortical stellate cell (scc). The auxiliary cell (aux), first-order (labelled 1–5) and second-order filaments (labelled 1’–2’) are marked for clarity. Fig. 34. Further development of the auxiliary cell ampullae. First- (labelled 1–7), second- (labelled 1’–5’) and third-order filaments (labelled 1”–2”) are marked. Fig. 35. Mature auxiliary cell ampulla. Fig. 36. Enlarged auxiliary cell (aux) during maturation. Fig. 37. A fully mature auxiliary cell (aux) and connecting filaments (cf). Fig. 38. Early post-fertilization stage showing a fusion cell complex (fcc) with distinct connecting filaments (cf) and a gonimoblast initial (gbi) surrounded by the ampullary filaments. Fig. 39. A gonimolobe (g) has segregated from a gonimoblast initial (gbi). Fig. 40. Young cystocarp wrapped in involucral ampullary filaments (if). Scale bars = 10 μm.
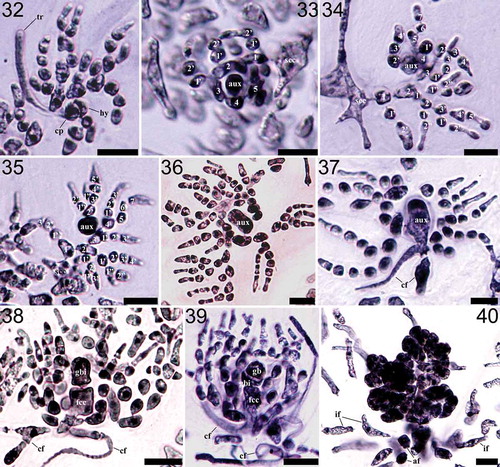
Figs 41‒46. Schematic illustrations of the representative auxiliary cell ampullary types of the family Halymeniaceae including the novel Nesoia-type. Fig. 41. Grateloupia-type characterized by the simplest ampullary filament structure of up to three orders of ampullary filaments with only one filament of each order (Kawaguchi et al., Citation2001; De Clerck et al., Citation2005). Fig. 42. Thamnoclonium-type characterized by the auxiliary cell in the middle of the first-order filament (Chiang, Citation1970; D’Archino et al., Citation2014). Fig. 43. Cryptonemia-type characterized by sparser ampullary filaments than Aeodes- and Halymenia-types and the non-basal type auxiliary cell in the middle of a second-order ampullary filament (Chiang, Citation1970). Fig. 44. Halymenia-type characterized by unilateral second-order ampullary filaments borne on each cell of a first-order ampullary filament (Womersley & Lewis, Citation1994; De Smedt et al., Citation2001). Fig. 45. Aeodes-type characterized by bushy branched ampullary filaments of up to five orders from a first-order ampullary filament (Chiang, Citation1970; Womersley & Lewis, Citation1994). Fig. 46. Nesoia-type characterized by first-order ampullary filament borne on a subcortical cell, two second-order ampullary filaments grown bilaterally from each cell of the first-order ampullary filament, and the auxiliary cell, an initial cell of the second-order ampullary filaments (Maggs & Guiry, Citation1982; this study).
In the rbcL sequence analysis, two species from Korea and Halymenia latifolia from France (KJ606643) formed a monophyletic group with strong support (BS = 100%, PP = 1.00; ). Sequences for the two Korean species diverged by only 3.6–3.8% from H. latifolia from France. In the ML analysis, this clade was positioned near the Amalthea clade including A. freemaniae D’Archino & W.A. Nelson from New Zealand (KJ606651), A. rubida H.W. Lee & M.S. Kim from Korea (KX879777) and H. abyssicola E.Y. Dawson from Mexico (GU598119) with 5.2–8.0% intergeneric divergence, but had very weak BS and PP support (). However, this clade was separate and distant from both the clade including the type species of Halymenia, H. floresii (Clemente) C.Agardh and the Cryptonemia clade in the phylogenetic tree. (). These results suggest that the clade containing two Korean species and H. latifolia from France should be recognized as an independent genus of foliose Halymeniaceae. We propose the new genus Nesoia H.W.Lee & M.S.Kim, gen. nov. to accommodate these species.
Morphological observations
Nesoia H.W. Lee & M.S. Kim, gen. nov.
Diagnosis: Plant erect, foliose, entire. Cortex composed of small polygonal cells in surface view, with a subcortex composed of larger stellate and short filamentous cells. Medulla composed of sparsely distributed anticlinal filaments. Tetrasporangia cruciately divided. Tetrasporophytes and gametophytes isomorphic. Gametophytes monoecious. Spermatangia distributed over the thallus surface. Female gametophytes non-procarpic. Carpogonial branch two-celled. Auxiliary cell ampullae growing into three to four orders, first-order ampullary filament producing bilateral second-order filaments. Auxiliary cell the basal cell of one of bilateral second-order ampullary filaments. Pericarps formed with pole-shaped and colourless involucral filaments originating from ampullary filaments of fusion cell complex. Cystocarps usually spherical, immersed in medulla.
Type species: Nesoia delicatula H.W.Lee & M.S.Kim, sp. nov.
EEtymology: The genus name, Nesoia, is derived from Nῆσοι (Nesoi), the goddesses of islands in Greek mythology.
Nesoia delicatula H.W. Lee & M.S. Kim, sp. nov. (Figs 2–24)
Holotype: MSK150722-01 (), monoecious gametophyte, Jeju Island in Korea, 22 July 2015, deposited in Jeju National University Herbarium (JNUB).
Isotype: MSK150722–02 (), MSK150722–03 (), MSK150722–04 () and MSK150722–05 (), deposited in JNUB; NIBRRD0000001645 (MSK150722–iso01), deposited in the National Institute of Biological Resources (NIBR), Incheon, Korea.
Type locality: Gwideok 1-ri, Jeju Island, Korea (33°27ʹ05.19ʺ N, 126°17ʹ21.09ʺ E).
Etymology: The specific epithet (delicatula) was chosen to describe the thin, soft and delicate thallus.
Habitat: Nesoia delicatula sp. nov. grows in the subtidal zone in areas with strong tidal currents, at a depth of 25 m attached to rhodoliths, shells or pebbles. The benthic habitat is covered with sandy sediment.
Other specimens examined: MSK150722–sp07, sp09 and sp11, gametophyte, Gwideok 1-ri, Jeju Island, Korea, 1 June 2015; MSK150722–sp08 and sp12, tetrasporophyte, Gwideok 1-ri, Jeju Island, Korea, 1 June 2015; MSK150722–sp10, vegetative, Gwideok 1-ri, Jeju Island, Korea, 1 June 2015.
Habit and anatomy: Thallus is erect, solitary, foliose and entire (), growing as oblong ( or circular blade () with obtuse to rounded apex. When fully grown, thalli are 7–11 cm high, up to 19 cm, and 5–6 (–10) cm wide. Blades are usually symmetrical with occasional slits or punctures in lamina () but sometimes slightly asymmetric or irregular (‒). Blades are thin, 145–174 μm thick, with lubricous, soft and delicate texture. Blade margin is entire (). Blade arises from a discoid holdfast attached to rhodoliths, shells or pebbles. Cortical cells are polygonal and compact, 13–18 μm × 6–11 μm in surface view (). Cortex is single-layered, 11–17 μm thick, and composed of small, deeply pigmented, angular to roundish cells (). Subcortex is a single-layered coarse mesh composed of larger, less-pigmented stellate cells connected with short filamentous cells (). Subcortical stellate cells are 28–38 μm × 30–45 μm. Medulla is composed of sparsely distributed anticlinal filaments 10–16 μm in diameter (). Medullary filaments are composed of two to three nodes jointed by pit connections () and connected to subcortical stellate cells ().
Reproductive structures: Tetrasporangia are round to ellipsoid, 12–18 μm × 13–19 μm in size, and divided cruciately during maturation (). They are formed among cortical cells over the whole surface of the thallus (). Tetrasporophytes and gametophytes are isomorphic. Gametophytes are monoecious (). Spermatangia are tear-shaped to spherical and distributed over the surface of the thallus (). Spermatangia of 5.1–6.3 μm × 5.2–6.2 μm size are generally colourless but appear dark when stained due to nucleus (). Cut off from roundish spermatangial mother cells, spermatangia usually grow in pairs and are stained darker than cortical cells (). Spermatangial mother cells are borne, usually in pairs, from cortical cells and become 5.1–6.8 μm × 6.6–8.2 μm size when fully matured (). Female gametophytes are non-procarpic. Carpogonial ampullae were not observed but auxiliary cell ampullae are abundant. First-order ampullary filaments are borne directly on subcortical cells and grow laterally. On each of the first-order ampullary filament cells, two second-order ampullary filaments grow bilaterally (). The first cell of one of the two second-order ampullary filaments, arising from the second or third cell of the first-order ampullary filament, becomes an auxiliary cell (). When fully mature, auxiliary cells are 13.8–17.0 μm × 15.4–18.4 μm in size, and appear dark with thickened cell walls when stained (). Third-order ampullary filaments grow unilaterally only from the first cells of second-order ampullary filaments, except for the filament bearing the auxiliary cell (). At maturity, auxiliary cell ampullae are usually composed of three orders of ampullary filaments (). After diploidization with connecting filaments, fusion complexes are formed. The funnel-shaped fusion cell complex is concave toward the base and is 24–30 μm × 17–19 μm (). A gonimoblast initial 14–17 μm × 11–14 μm is cut off transversely from the fusion cell complex. Initially oval gonimoblast initials become cap-shaped and obtuse as they mature (). After a series of transverse and vertical divisions, gonimoblast initials produce gonimolobes (). Disorganized ampullary filaments and their lateral filaments organize themselves around a fusion complex into a layered globe of involucre filaments and enclose the fusion complex and its offspring gonimoblast initial and gonimolobes (). Ampullary filaments become transparent involucral filaments in due course () and connecting filaments become invisible (). Involucral filaments later become 2–4 layered pericarps encasing carposporangia and eventually form cystocarps (). A fully grown cystocarp is spherical, 178–244 μm × 184–273 μm, up to 325 × 326 μm ().
Nesoia pulchella H.W. Lee & M.S. Kim, sp. nov. (Figs 25–40)
Holotype: NIBRRD0000001670 (), vegetative, Jeju Island, Korea, 24 May 2015, deposited in NIBR.
Topotype: MSK150601–06 (), June 2015, deposited in JNUB.
Type locality: Dodu, Jeju Island, Korea (33°30ʹ27.75ʺ N, 126°56ʹ08.49ʺ E).
Etymology: The specific epithet (pulchella = pretty) was chosen to reflect the appearance of the thallus.
Habitat: Nesoia pulchella sp. nov. grows in the subtidal zone in areas with strong tidal currents, at a depth of 25 m, attached to pebbles, rhodoliths or shells, in areas of sandy sediment.
Other specimens examined: MSK150524–16–sp01, Dodu, Jeju Island, Korea, 24 May 2015, vegetative; MSK150601–06–sp01, Dodu, Jeju Island, Korea, 1 June 2015, gametophyte; MSK150601–06–sp02, Dodu, Jeju Island, Korea, 1 June 2015, tetrasporophyte; MSK150601–06–sp03, Dodu, Jeju Island, Korea, 1 June 2015, vegetative.
Habit and anatomy: Thallus grows to an obovate shape with rounded apex and acute base (). Fully grown thallus is about 7 cm high and 4 cm wide. Blade is usually symmetrical () but occasionally extended tears, slits and punctures also appear (). Blade is 189–198 μm thick with lubricous and delicate texture. Blade margin is entire (). Cortical cells are polygonal and compact in surface view, 9–15 μm × 8–11 μm in size (). Cortex is single-layered, 11–13 μm thick (). Subcortex is also single-layered and composed of stellate cells connected with short filamentous cells (). Subcortical stellate cells are 13–17 μm × 14–17 μm in size (). Medullary anticlinal filaments are sparsely arranged, and 10–13 μm in diameter ().
Reproductive structures: Female gametophytes are non-procarpic. Carpogonial branch is a two-celled monocarpogonial structure composed of a funnel-shaped hypogynous cell and a conical carpogonium (). The cylindrical trichogyne, 50–80 (100) μm in length and about 2 μm in diameter, grows from a carpogonium (). Carpogonial ampullae are observed less frequently than auxiliary cell ampullae. Auxiliary cell ampullae are composed of multiple-order ampullary filaments. First-order ampullary filaments grow directly from a subcortical cell () and each cell produces two second-order ampullary filaments bilaterally (). The basal cell of one of the two second-order ampullary filaments becomes an auxiliary cell () and enlarges to 12.8–19.2 μm × 9.5–12.6 μm when fully grown (). At maturity, auxiliary cell ampullae are usually composed of three to four orders of ampullary filaments () and connecting filaments are connected to the auxiliary cell (). After diploidization, fusion complexes are formed. A fusion complex is cup-shaped, 10.9–15.8 μm × 9.9–12.7 μm, and concave toward the base (). A gonimoblast initial is cut off from a fusion complex transversely () and produces gonimolobes through transverse divisions (). Ampullary filaments and lateral filaments organize themselves into a globe of involucre filaments and enclose the fusion complex, a gonimoblast initial and gonimolobes together (). Involucral filaments develop into a pericarp tightly encasing carposporangia (). Mature pericarps and cystocarps were not observed.
Nesoia latifolia (P. Crouan & H. Crouan ex Kützing) H.W. Lee & M.S. Kim, comb. nov.
Basionym: Halymenia latifolia P. Crouan & H. Crouan ex Kützing. Kützing, F.T. Citation1866: Tabulae phycologicae; oder, Abbildungen der Tange. Vol. XVI, 34, pl. 96, figs a‒c, Nordhausen: Gedruckt auf kosten des Verfassers (in commission bei W. Köhne).
Heterotypic synonyms: Halymenia trabeculata Ercegovic Citation1949: Sur quelques algues rouges, rares ou nouvelles, de I’Adriatique, 65, figs 9‒10, Acta Adriatica 4, 91‒121 [reprint 1‒81]; Halymenia latifolia var. trabeculata (Ercegovic) Codomier Citation1973: Tableau de détermination des Rhodophycées non calcifies, à thalle comportant une medulla filamenteuse, de la côte des Albères (France), 136, Bulletin de la Société Botanique de France.
Discussion
The Halymeniaceae is a well-studied and species-rich family of florideophytes with diverse frond morphology (Guiry & Guiry, Citation2017). Recent taxonomic studies have resolved the phylogenetic position of several foliose species based on the rbcL region (D’Archino et al. Citation2014; Azevedo et al., Citation2016b; Lee et al., Citation2016; Tan et al., Citation2017). In the present study, careful observations of female reproductive morphology combined with rbcL sequence analysis led to the discovery of a new genus including two new species, and the identification of an undescribed type of auxiliary cell ampulla. These two new species from Korea were resolved in the same clade with Halymenia latifolia (KJ606643) from France (), representing the new genus Nesoia gen. nov. Previous phylogenetic analyses of Halymenia showed that the genus is paraphyletic. Use of rbcL sequence data demonstrated that H. latifolia and other species, such as H. abyssicola, H. elongata and H. floridana, are resolved in a clade of Halymenia excluding the type, H. floresii (D’Archino et al. Citation2014; Azevedo et al., Citation2016b; Lee et al., Citation2016; Tan et al., Citation2017).
Our morphological observations of the reproductive structures in Nesoia correspond well to a previous description of the female reproductive structure of N. latifolia (Maggs & Guiry, Citation1982, as Halymenia latifolia). It was characterized by a two-celled carpogonial branch, densely branched auxiliary cell ampullae, the auxiliary cell being the initial of one of the second-order ampullary filaments, and pericarps originating from ampullary filaments. Hence, the new genus Nesoia with a unique type of auxiliary cell ampulla can be considered as tying up one loose end in the taxonomy of the Halymeniaceae.
The interpretation of phylogenetic relationships within the Halymeniaceae prior to molecular analyses had predominantly relied on morphological characteristics, especially vegetative features (Guiry & Irvine, Citation1974; Kraft, Citation1977). However, this was particularly problematic because foliose species of Halymeniaceae have insufficient morphological characters to determine their generic position accurately (D’Archino et al., Citation2014). In New Zealand, species now placed in the genus Amalthea were previously considered to belong to Halymenia (Adams, Citation1994) due to their vegetative morphology, with a smooth thallus exhibiting sparse anticlinal medullary filaments (Tan et al., Citation2015, Citation2017; Azevedo et al., Citation2016b). However, Amalthea is clearly distinguished from Halymenia by female reproductive structures: Amalthea has five-celled carpogonial branches and Thamnoclonium-type auxiliary cell ampullae (D’Archino et al., Citation2014; ) while Halymenia has two-celled carpogonial branches (Chiang, Citation1970; Womersley & Lewis, Citation1994) and Halymenia-type auxiliary cell ampullae (Chiang, Citation1970; Azevedo et al., Citation2016b; Tan et al., Citation2017; ). This clearly demonstrates that detailed observations of female reproductive structures provide crucial characteristics for establishing generic boundaries in the Halymeniaceae (Gargiulo et al., Citation2013).
Despite the widespread adoption of molecular analyses, there is still inadequate resolution of the phylogenetic relationships among Halymeniaceae species (Manghisi et al., Citation2014). For example, the Amalthea clade, consisting of A. freemaniae (KJ606651) and H. abbysicola (GU598119), was moderately supported (77% BS in rbcL phylogeny) in D’Archino et al. (Citation2014). This support increased when more taxa were included, to 86% in Azevedo et al. (Citation2016a, Citationb) and 87% in Lee et al. (Citation2016) who included a new Amalthea species, A. rubida (KX879777). Our addition of N. delicatula and N. pulchella sequences has improved the resolution of the Amalthea clade, with 95% support in ML analysis, and defining the generic position of N. latifolia accurately has strengthened phylogenetic construction of the Halymeniaceae ().
The auxiliary cell ampulla of Nesoia is of a distinctive evolutionary type compared with the other five types reported in the Halymeniaceae and summarized by Chiang (Citation1970) (). As shown schematically in and described in the morphology of N. delicatula and N. pulchella, each cell of first-order ampullary filaments produces two second-order filaments bilaterally in the Nesoia-type auxiliary cell ampullae. In other types, each cell of the first-order ampullary filament usually produces unilateral second-order filaments, not only in the sparsely branched Grateloupia-type () and Thamnoclonium-type () but also in densely branched types such as the Halymenia-type () and Aeodes-type ().
In Nesoia species, auxiliary cells are the basal cell of one of the two secondary ampullary filaments borne on the second or third cells of first-order filaments. This is also observed in Aeodes-, Halymenia- and Grateloupia-types except that each of the first-order filament cells produces at most one second-order filament unilaterally (Chiang, Citation1970). On the contrary, in the Cryptonemia-type (), the auxiliary cell is a non-basal cell of secondary ampullary filaments and in the Thamnoclonium-type the auxiliary cells are among the first-order filaments (Chiang, Citation1970). If we disregard the bilateral branching pattern, the Nesoia-type auxiliary cell ampullary system () may be considered as an intergrade between the Aeodes- and Halymenia-types due to the auxiliary cell location and dense filament branching pattern (; Womersley & Lewis, Citation1994). In the Thamnoclonium-type, the auxiliary cell is in the first-order ampullary filament (D’Archino et al., Citation2014; ) and that of Cryptonemia-type is a non-basal cell of a second-order filament (Chiang, Citation1970; ). Although the auxiliary cell in the Grateloupia-type is the basal cell of a second-order filament, there is only one ampullary filament in each order (Kawaguchi et al., Citation2001; De Clerck et al., Citation2005; ).
Kawaguchi et al. (Citation2004) considered that the carpogonial ampulla is as useful as the auxiliary cell ampulla in phylogenetic classification of the Halymeniaceae. Carpogonial branch ampullae of Nesoia are branched to three orders with two-celled carpogonial branches (), similar to Carpopeltis, Cryptomenia and Halymenia (Womersley & Lewis, Citation1994; Kawaguchi et al., Citation2004). Chiang (Citation1970) distinguished the Grateloupia- and the Halymenia-types based on the pericarp origin. However, pericarps of Nesoia are made up of the involucral filaments derived from the auxiliary ampullary filaments only (), differing from the Halymenia-type pericarp origin from auxiliary ampullae and a small number of neighbouring medullary filaments (Chiang, Citation1970; Tan et al., Citation2017). Nesoia has monoecious gametophytes (), like Carpopeltis, Codiophyllum, Dermocorynus and some Grateloupia species (Guiry & Maggs, Citation1982; Womersley & Lewis, Citation1994), while most known Halymenia species are dioecious (Womersley & Lewis, Citation1994), as is Amalthea, placed close to Nesoia in the rbcL phylogeny (D’Archino et al. Citation2014). The female reproductive structures in this group can provide diagnostic criteria to reassess the phylogenetic relationships within the Halymeniaceae (Manghisi et al., Citation2014). The conclusions of recent phylogenetic revisions in Halymeniaceae have shown the value of systematics based on efforts to investigate specimens from diverse areas and to carefully characterize reproductive anatomy as well as molecular signatures of members of the Halymeniaceae.
Acknowledgements
We thank all members of the molecular phylogeny team of the marine algal laboratory at Jeju National University. We appreciate Dr Wendy Nelson’s editing and Dr D’Archino’s instruction in the staining method. We acknowledge support from the National Institute of Biological Resources (NIBR).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Hyung Woo Lee
H.W. Lee: framed the experimental design, laboratory and fieldwork, data analysis and interpretation, and manuscript preparation and editing. M.S. Kim contributed to experimental design, data interpretation, manuscript preparation and editing.
References
- Abbott, I.A. (1967). Studies in some foliose red algae of the Pacific coast. Ⅰ. Cryptonemiaceae. Journal of Phycology, 3: 139–149.
- Adams, N.M. (1994). Seaweeds of New Zealand. Canterbury University Press, Christchurch, New Zealand.
- Azevedo, C.A.A., Cassano, V. & Oliveira, M.C. (2016a). Diversity of branched Halymenia (Halymeniales, Rhodophyta) species on the Brazilian coast: molecular and morphological analyses reveal three new species. Phycologia, 55: 431–444.
- Azevedo, C.A.A., Cassano, V. & Oliveira, M.C. (2016b). Phylogenetic relationships among Halymenia (Halymeniaceae, Rhodophyta) species on the Brazilian coast with description of Halymenia cearensis sp. nov. Phytotaxa, 280: 241–258.
- Boo, G.H., Cai, Y. & Boo, S.M. (2016). Molecular identification of gelidioid algae (Gelidiales, Rhodophyta) from Singapore with a description of Gelidiun sentosaense sp. nov. Phycologia, 55: 247–256.
- Chiang, Y.-M. (1970). Morphological studies of red algae of the family Cryptonemiaceae. University of California Publications in Botany, 58: 1–95.
- D’Archino, R., Nelson, W.A. & Zuccarello, G.C. (2014). Amalthea and Galene, two genera of Halymeniaceae (Rhodophyta) from New Zealand. Botanica Marina, 57: 185–201.
- Codomier, L. (1973). Tableau de détermination des Rhodophycées non calcifies, à thalle comportant une medulla filamenteuse, de la côte des Albères (France). Bulletin de la Société Botanique de France, 120: 3–4, 133–140.
- De Clerck, O., Gavio, B., Fredericq, S., Bárbara, I. & Coppejans, E. (2005). Systematics of Grateloupia filicina (Halymeniaceae, Rhodophyta), based on rbcL sequences and morphological evidence, including the reinstatement of G. minima and the description of G. capensis sp. nov. Journal of Phycology, 41: 391–410.
- De Smedt, G., De Clerck, O., Leliaert, F., Coppejans, E. & Liao, L.M. (2001). Morphology and systematics of the genus Halymenia C. Agardh (Halymeniales, Rhodophyta) in the Philippines. Nova Hedwigia, 73: 293–322.
- Díaz-Tapia, P., McIvor, L., Freshwater, D.W., Verbruggen, H., Wynne, M.J. & Maggs, C.A. (2017). The genera Melanothamnus Bornet & Falkenberg and Vertebrata S.F. Gray constitute well-defined clades of the red algal tribe Polysiphonieae (Rhodomelaceae, Ceramiales). European Journal of Phycology, 52: 1–30.
- Ercegovic, A. (1949). Sur quelques algues rouges, rares ou nouvelles, de l’Adriatique (On some red algae, rare or new, from the Adriatic). Acta Adriatica, 8: 1–81.
- Gargiulo, G.M., Morabito, M. & Manghisi, A. (2013). A re-assessment of reproductive anatomy and postfertilization development in the systematics of Grateloupia (Halymeniales, Rhodophyta). Cryptogamie, Algologie, 34: 3–35.
- Guiry, M.D. & Guiry, G.M. (2017). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org.
- Guiry, M.D. & Irvine, L.M. (1974). A species of Cryptonemia new to Europe. British Phycological Journal, 9: 225–237.
- Guiry, M.D. & Maggs, C.A. (1982). The morphology and life history of Dermocorynus montagnei Crouan frat. (Halymeniaceae; Rhodophyta) from Ireland. British Phycological Journal, 17: 215–228.
- Gurgel, C.F.D. & Fredericq, S. (2004). Systematics of the Gracilariaceae (Gracilariales, Rhodophyta): a critical assessment based on rbcL sequence analyses. Journal of Phycology, 40: 138–159.
- Hall, T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41: 95–98.
- Hommersand, M.H. & Fredericq, S. (1990). Sexual reproduction and cystocarp development. In Biology of the Red Algae (Cole, K.M. & Sheath, R.G., editors), 305–345. Cambridge University Press, Cambridge.
- Kang, J.C. & Kim, M.S. (2016). Transfer of Papenfussia japonica (Delesseriaceae, Rhodophyta) from Korea to the genus Augophyllum. Botanica Marina, 59: 405–415.
- Kawaguchi, S., Shimada, S., Wang, H.W. & Masuda, M. (2004). The new genus Yonagunia Kawaguchi et Masuda (Halymeniaceae, Rhodophyta), based on Y. tenuifolia Kawaguchi et Masuda sp. nov. from Southern Japan and including Y. formosana (Okamura) Kawaguchi et Masuda comb. nov. from Southeast Asia. Journal of Phycology, 40: 180–192.
- Kawaguchi, S., Wang, H.W., Horiguchi, T., Lewis, J.A. & Masuda, M. (2002). Rejection of Sinkoraena and transfer of some species of Carpopeltis and Sinkoraena to Polyopes (Rhodophyta, Halymeniaceae). Phycologia, 41: 619–635.
- Kawaguchi, S., Wang, H.W., Horiguchi, T., Sartoni, G. & Masuda, M. (2001). A comparative study of the red alga Grateloupia filicina (Halymeniaceae) from the northwestern Pacific and Mediterranean with the description of Grateloupia asiatica sp. nov. Journal of Phycology, 37: 433–442.
- Kim, M.S., Yang, M.Y. & Cho, G.Y. (2010). Applying DNA barcoding to Korean Gracilariaceae (Rhodophyta). Cryptogamie, Algologie, 31: 387–401.
- Kraft, G.T. (1977). The morphology of Grateloupia intestinalis from New Zealand, with some thoughts on generic criteria within the family Cryptonemiaceae (Rhodophyta). Phycologia, 16: 43–51.
- Kützing, F.T. (1866). Tabulae phycologicae oder Abbildungen der Tange. Published by the author, Nordhause. Vol. 16, pp. 35, 100 pls.
- Lee, H.W., Yang, M.Y. & Kim, M.S. (2016). Verifying a new distribution of the genus Amalthea (Halymeniales, Rhodophyta) with description of A. rubida sp. nov. from Korea. Algae, 31: 341–349.
- Lin, S-.M., D’Archino, R. & Hommersand, M.H. (2012). A new method of cystocarp development in the red algal genus Callophyllis (Kallymeniaceae) from Chile. Journal of Phycology, 48: 784–792.
- Lin, S-.M., Liang, H-.Y. & Hommersand, M.H. (2008). Two types of auxiliary cell ampullae in Grateloupia (Halymeniaceae, Rhodophyta), including G. taiwanensis sp. nov. and G. orientalis sp. nov. from Taiwan based on rbcL gene sequence analysis and cystocarp development. Journal of Phycology, 44: 196–214.
- Maggs, C.A. & Guiry, M.D. (1982). Morphology, phenology and photoperiodism in Halymenia latifolia Kütz. (Rhodophyta) from Ireland. Botanica Marina, 25: 589–599.
- Manghisi, A., Le Gall, L., Ribera, M.A., Bonillo, C., Gargiulo, G.M. & Morabito, M. (2014). The Mediterranean endemic new genus Felicinia (Halymeniales, Rhodophyta) recognized by a morphological and phylogenetic integrative approach. Cryptogamie, Algologie, 35: 221–243.
- Min-Thein, U. & Womersley, H.B.S. (1976). Studies on southern Australian taxa of Solieriaceae, Rhabdoniaceae and Rhodophyllidaceae (Rhodophyta). Australian Journal of Botany, 24: 1–166.
- Ronquist, F. & Huelsenbeck, J.P. (2003). MrBayes3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572–1574.
- Rousseau, F., Gey, D., Kurihara, A., Maggs, C.A., Martin-Lescanne, J., Payri, C., de Reviers, B., Sherwood, A.R. & Le Gall, L. (2017). Molecular phylogenies support taxonomic revision of three species of Laurencia (Rhodomelaceae, Rhodophyta), with the description of a new genus. European Journal of Taxonomy, 269: 1–19.
- Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22: 2688–2690.
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evoultion, 28: 2731–2739.
- Tan, P-.L., Lim, P-.E., Lin, S-.M. & Phang, S-.M. (2017). Halymenia johorensis sp. nov. (Halymeniaceae, Rhodophyta), a new foliose red algal species from Malaysia. Journal of Applied Phycology, 30: 187–195.
- Tan, P-.L., Lim, P-.E., Lin, S-.M., Phang, S-.M., Draisma, S.G.A. & Liao, L.M. (2015). Foliose Halymenia species (Halymeniaceae, Rhodophyta) from Southeast Asia, including a new species, Halymenia malaysiana sp. nov. Botanica Marina, 58: 203–217.
- Wittmann, W. (1965). Aceto-iron-haematoxylin-chloral hydrate for chromosome staining. Stain Technology, 40: 161–164.
- Womersley, H.B.S. & Lewis, J.A. (1994). The Marine Benthic Flora of Southern Australia. Rhodophyta. Part ⅢA. Bangiophyceae and Florideophyceae (Acrochaetiales, Nemaliales, Gelidiales, Hilenbrandiales and Gigartinales sensu lato). Australian Biological Resources Study, Canberra.
- Yang, M.Y. & Kim, M.S. (2017). Molecular analyses and reproductive structure to verify the generic relationships of Hypnea and Calliblepharis (Cystocloniaceae, Gigartinales), with proposal of C. saidana comb. nov. Algae, 32: 87–100.
