ABSTRACT
To resolve historical misinterpretations of species descriptions and to comprehend the morphological diversity together with the distribution of Ulva compressa Linnaeus in northern Germany, a morphological and molecular study was undertaken of recently collected specimens and herbarium vouchers. Phylogenetic analyses from sequences of the plastid encoded tufA gene confirmed that U. compressa is abundant along the German Baltic Sea and North Sea coasts. We were able to genetically confirm the presence of U. compressa in the Baltic Sea below salinities of 15 PSU. However, we detected morphologies agreeing with the attached and branched tubular type material only in the North Sea, while U. compressa on Baltic Sea coasts indiscriminately exhibited a very distinct morphology of sheet-like thalli that were always unattached, with the exception of one collection site. Drifting forms were also frequently detected in the Wadden Sea, but not on the island of Helgoland. The tufA sequences of attached and tubular forms of U. compressa from the German Wadden Sea were identical to the drifting sheets found in the Wadden and Baltic Seas and the sequence divergence was extremely small at ≤0.9%. The proliferating, blade-like thalli of U. compressa appear as a nuisance ecotype that is able to form massive accumulations associated with oxygen depletion. Mass accumulations were observed to cause severe damage and increased mortality of habitat forming Zostera and Ruppia populations.
Introduction
The order Ulvales encompasses a variety of conspicuous green seaweeds distributed throughout marine, brackish and freshwater environments around the world. Their capacity to tolerate changing biotic and abiotic conditions and highly polluted and nutrient-enriched environments ranks them among the best known species for potential anthropogenic dispersal (Schaffelke et al., Citation2006). These opportunistic traits, as well as a potential for strong proliferation under suitable nutrient supply (Teichberg et al., Citation2010), increase the nuisance potential of some representatives. Several Ulva species have demonstrated the capacity to form large blooms, so called ‘green tides’ (Smetacek & Zingone, Citation2013). Such mass accumulations of drifting algal biomass in sheltered bays and shorelines have increased substantially in number and extent during the last century (Fletcher, Citation1996), mainly due to nutrient supply from agriculture and livestock (Fletcher, Citation1996; Charlier et al., Citation2008; Smetacek & Zingone, Citation2013).
Green tides often cause severe economic losses in tourism, and problems related to the removal and disposal of beached biomass (Dion & Le Bozec, Citation1996; Charlier et al., Citation2008; Smetacek & Zingone, Citation2013). Anoxic decomposition of Ulva produces and releases gaseous hydrogen sulphide, which can be a severe health hazard (Reiffenstein et al., Citation1992). In addition, green tides also hamper ecosystem services provided by coastal environments, as they modify biogeochemistry and biodiversity (Norkko & Bonsdorff, Citation1996a, Citation1996b; Valiela et al., Citation1997; Raffaelli et al., Citation1998; Charlier et al., Citation2008; Arroyo et al., Citation2012). Highly productive seagrass meadows are often impacted by such mass accumulations of organic biomass. Seagrass ecosystems, as represented for example by dense populations of the eelgrass Zostera marina L. and Ruppia cirrhosa (Petagna) Grande play a pivotal role in protection from coastal erosion and provide nurseries for many organisms (Duarte, Citation2000; Marbá et al., Citation2006). Additionally, they play a key role in coastal carbon and nutrient cycling and thus offer important services for a healthy ecosystem (Smith et al., Citation1984; Lee et al., Citation2007). Even though seagrass species were abundant and widely distributed in northern Europe, a strong decline in these important primary producers has been observed over recent decades (Borum et al., Citation2006; Waycott et al., Citation2009). It has also been observed that ‘macroalgal-tides’ noticeably change the abundance of seagrasses and lead to a decline in their populations (Hartog den, Citation1994; Ansell et al., Citation1998; McGlathery, Citation2001).
However, not every Ulva species necessarily has the same potential to cause such negative effects. It seems some taxa within the genus Ulva are rarely found (Bliding, Citation1963; Kornmann & Sahling, Citation1977; Brodie et al., Citation2007). Rare recordings of Ulva species can also be due to the high intraspecific morphological plasticity and interspecific similarity, which makes precise species identification based solely on morphological characters extremely difficult (Bliding, Citation1963; Koeman & Van den Hoek, Citation1982). As an alternative, molecular approaches have been developed during the last two decades, which have resolved many taxonomic problems. One of the most significant changes was the transfer of the former genus Enteromorpha, that contained tubular species only, to the genus Ulva which now comprises tubular and sheet-like species (Tan et al., Citation1999; Hayden et al., Citation2003).
It is generally agreed that due to high plasticity many species within the genus Ulva have been wrongly recorded and listed in inventories. Worldwide more than 402 historic names and synonyms exist for the approximately 132 species that are currently recognized (Guiry & Guiry, Citation2018). A striking example of frequent taxonomic changes due to a highly variable morphology is Ulva compressa Linnaeus. Having been first described as a highly branched species and named Tremella marina tenuissima, compressa by Dillenius (Citation1742), Linnaeus (Citation1753) integrated this species in his binomial system and named it Ulva compressa. For a detailed history of the taxonomic changes and phylogenetic regroupings of U. compressa see Blomster et al. (Citation1998).
A characteristic that has been a subject of debate is the allegedly unique tubular form and the branching pattern of U. compressa. Several studies have stressed that branching of Ulva species may depend on extrinsic factors such as salinity, which excludes thallus branching as a suitable character for taxonomic identification (De Silva & Burrows, Citation1973; Reed & Russell, Citation1978; Blomster et al., Citation1998, Citation2002). The flexibility of the gross morphology of U. compressa was first observed and genetically underlined by Tan et al. (Citation1999) in the British Isles. Also, Blomster et al. (Citation2002) reported that DNA sequences obtained from specimens of U. compressa clustered with a sheet-like individual that, based on morphology, was assigned to the species Ulva pseudocurvata Koeman & Hoek.
Together with such taxonomic observations the phylogeographic distribution of U. compressa has also received some attention. For example, the tubular and highly branched form of U. compressa has originally been reported by several independent authors to occur in inner and outer parts of the Baltic Sea (Bliding, Citation1963; Nielsen et al., Citation1995; Tolstoy & Willén, Citation1997). In a more recent survey, Leskinen et al. (Citation2004) systematically investigated its distribution along the Baltic Sea salinity gradient and concluded that U. compressa is not present in areas where the salinity drops below 15 PSU. However, Leskinen et al. (Citation2004) exclusively focused on specimens that exhibited the branched tubular morphology of the holotype.
The objective of the present study was to assess the morphological diversity, and to re-evaluate the distribution, of U. compressa over a range of distinct, geographically separated habitats in northern Germany which offer different ecological conditions. Sampling sites in the Baltic Sea, Wadden Sea and on the North Sea island of Helgoland were included to examine the nuisance potential of different morphological forms within these areas.
Lacking tides, but being subject to irregular sea level change driven by wind and air pressure dynamics, the SW Baltic Sea is a brackish water body that is mainly characterized by substrata of stones, gravel and sand (Rönnbäck et al., Citation2007). Along approximately 260 km of coastline in the northern German state of Schleswig-Holstein the annual mean salinity of the Baltic drops from approximately 18 to 12 PSU (Gräwe et al., Citation2014), but freshwater inflow and local seasonal fluctuations generate considerably steeper gradients. In contrast, the Wadden Sea is a fully marine and tidal ecosystem in the SE North Sea. It is protected in part by barren sands and small islands and characterized by extended sand and mud flats. Hard substrata for algal attachment are scarce and mostly provided by molluscs or artificial structures. Whereas the Wadden Sea mainly provides soft bottoms, the North Sea offshore island Helgoland represents a unique environment in northern Germany. The island largely lacks protection from waves and with its rock pedestal it provides the only hard substratum formation in fully marine conditions in Germany (Reinke, Citation1889).
All three areas were surveyed for the presence of U. compressa during two consecutive years at different seasons. For species identification a genetic approach was used, based upon use of the plastid encoded genetic marker tufA. Preceding studies mostly used the ITS or rbcL gene markers (Blomster et al., Citation1998, 2002; Tan et al., Citation1999 Hayden et al., Citation2003; Leskinen et al., Citation2004; Heesch et al., Citation2009; Hofmann et al., Citation2010; Kraft et al., Citation2010). However, a more recent study that compared different markers clearly indicated that tufA was a more suitable barcode marker for green algae (Saunders & Kucera, Citation2010). The genetic approach was combined with morphological observations. Observations of mass accumulations of U. compressa were also documented during the survey. In order to reveal potential historical confusions of U. compressa with other species in the study area several herbarium specimens were also examined and genetically characterized.
Materials and methods
Field collection and sample preparation
Samples of Ulva compressa were collected at 121 locations along the northern German state of Schleswig-Holstein. These were distributed along 537 km of Baltic Sea coast and 466 km of North Sea coast (Statistisches Jahrbuch Schleswig-Holstein Citation2016/2017) in such a way that distances between adjacent locations never exceeded 25 km. Seasonal collections were carried out in summer (July–August 2014 and August–September 2015) and spring (April 2015 and March 2016). A limited number of sites were also visited during winter (November 2014/2015–beginning of March 2015), but no substantial green algal growth was observed. In the Wadden Sea and at Helgoland, samples were collected at low tide. Also interior waterbodies directly adjacent to the coast (overflow basins, drain channels, reservoirs) were checked. On the Baltic coast samples were collected from open coasts, in shallow lagoons and in estuaries to ~120 cm below mean water level using waders and an aquascope, when water levels were low.
Representative Ulva specimens of all morphologies that were present at each site were collected. The specimens were pre-identified on the basis of morphological characters such as cell size and form, number of pyrenoids and location of the chloroplast at three parts of the thallus: base, middle and apex. Morphological examinations followed the identification schemes of Koeman and Van den Hoek (Citation1982), taking into account comments by Tan et al. (Citation1999) and Maggs et al. (Citation2007) regarding the variable overall morphology. Aforementioned characters were studied using light microscopy, whereas Lugol’s solution (iodine-potassium iodide) was used to dye pyrenoids. In addition, epiphyte-free material of each specimen was conserved either with silica gel or by lyophilization, for DNA barcoding (see below). Residual thalli were preserved as herbarium vouchers, and voucher specimen were deposited at GEOMAR herbarium. Salinity was measured using a WTW portable conductivity meter (Xylem Analytics, Weilheim, Germany).
Some U. compressa specimens from herbarium collections (Haussknecht Herbar Friedrich-Schiller-University Jena (JE), Herbarium of the Alfred Wegener Institute at List, Sylt and Herbarium of the GEOMAR Helmholtz Centre for Ocean Research, Kiel) were also included in the survey, in order to investigate past distributions and to reveal potential historical misidentifications. For additional information about the herbarium samples used in this study see and .
Table 1. Sample list of genetically processed herbarium material.
DNA extraction, amplification and sequencing
Genomic DNA was isolated from the collected algal tissue with an Invisorb Spin Plant Mini Kit (Stratec, Birkenfeld, Germany) following the manufacturer’s protocol. Herbarium material not older than 35 years and accessible in sufficient amounts (at least 1 × 1 cm) was extracted using the same method. For older herbarium material, a direct PCR method using the Phire Plant Direct PCR kit (Thermo Fisher Scientific) was used. For DNA barcoding the elongation factor tufA gene was amplified by polymerase chain reaction (PCR), using the primers tufGF4 (Saunders & Kucera, Citation2010) and tufAR (Famà et al., Citation2002) and the following temperature profile: initial denaturation at 94°C for 4 min, then 38 cycles of (a) 94°C for 1 min, (b) 55°C for 30 s, (c) 72°C for 1 min and 72°C of final extension for 7 min. The PCR-amplified products were directly sequenced by GATC biotech (Konstanz, Germany). Assemblage of forward and reverse sequences and reciprocal editing was done with Sequencher (v. 4.1.4, Gene Codes Corporation, Ann Arbor, Michigan, USA). Sequences obtained in this way were uploaded in GenBank (accession numbers MF979636–MF979643 for herbarium material and MF979644-MF979661 for fresh material).
Phylogenetic analyses
The sequences obtained were aligned with reference sequences downloaded from GenBank and used for further phylogenetic analysis. Alignments were prepared with MAFFT (Katoh et al., Citation2002) and sequence divergence values were calculated with the uncorrected p distance in MEGA v. 5.0 (Tamura et al., Citation2011). By using MrModeltest software version v. 2.2. (Nylander, Citation2004) the optimal substitution model was determined and found to be GTR+G+I. Phylogenetic analyses were then carried out using the maximum likelihood method (ML) implemented in RAxML version 8 (Stamatakis, Citation2014) and the chosen substitution model with 1000 bootstrap iterations. Bayesian inference (BI) analyses were computed with MrBayes version 3.2.2 (Ronquist et al., Citation2012). Four Monte Carlo Markov chains were run for 5x106 generations and the sampling frequency was set to 1000. The Bayesian analysis ran 1 015 000 generations and was stopped automatically when the average standard deviation for split frequencies dropped below 0.01. With default settings the first 25% of generations were discarded and 1524 trees remained. Of those, 50% consensus trees and posterior probabilities were calculated.
Evaluation of Ulva mass accumulations
Surface area coverage and depth of U. compressa drifting or beached accumulations were quantified with a tape measure. With the aforementioned conductivity meter the state of oxygen depletion within the algal bloom was also recorded at 15 cm intervals to a water depth of 150 cm and a beach distance of 400 cm in six replicates. For the evaluation of anoxic zones within the bloom, sediment cores were punched out by using a 45 × 15 cm transparent PVC cylinder. Unaffected areas of the same site served as controls. Thalli of bloom-forming algae were collected from the study sites and underwent molecular identification by barcoding as described before. Within randomly selected plots in the affected areas (1 × 1 m; n = 6) we evaluated the damage and mortality of Zostera and Ruppia species. Dead specimens and those exhibiting damaged leaves (measured by brown spots and rotted leaves) were counted and correlated with unaffected areas of the same sampling site which functioned as control sites.
Results
Altogether, 128 samples of U. compressa were detected by barcoding in 55 out of 121 different locations: 18 out of 73 locations at the Baltic Sea coast; 30 out of 38 at the Wadden Sea coast; and 7 out of 10 on the island of Helgoland (, Supplementary table 1). At the collection time, salinities at the Baltic Sea locations ranged from 9.0 to 17.4 PSU, while those at the North Sea locations ranged from 30 to 32.5 PSU (Supplementary table 1). Thus, U. compressa was detected over a wide geographic range and a broad range of habitats and salinities.
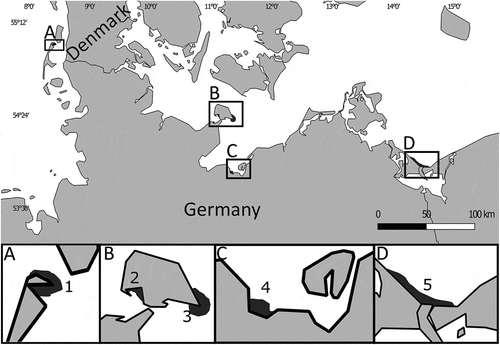
Fig. 2. Sites in northern Germany where Ulva compressa was collected. Overview map about the Baltic Sea with respective sea surface salinity. Visualization of the salinity gradient within the Baltic Sea by isohalines with particular salinity values (PSU) in circles (HELCOM-data) dropping with increasing distance from the North Sea. Dashed box (A) represents sampling area for specimen used in this study. (A) Map of northern Germany with numbered sampling sites and indication of found morphologies (legend) at the Wadden Sea (no. 1–27), on Helgoland (no. 28–33) and in the Baltic Sea (no. 35–47). See insets a–g for a better resolution.
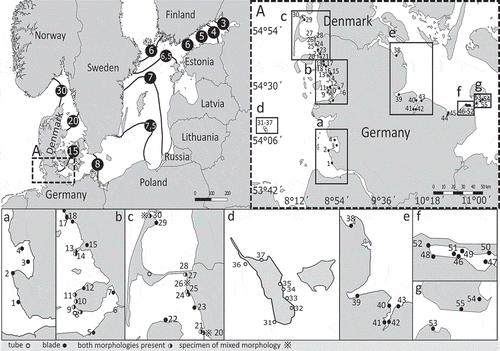
Within the sample set a variation of morphological characters was observed throughout northern Germany (, –) and only 34% of all specimens corresponded to the tubular and highly branched morphology () of the holotype of U. compressa (Linnaeus, Citation1753). The remaining specimens mostly exhibited morphologies with extended blades, but mixed forms combining both morphologies within a single specimen were also found (–). A gradient of gross morphologies was observed throughout the sampling area. On Helgoland we found only tubular specimens of U. compressa. The specimens grew attached and resemble the holotype described by Linnaeus (Citation1753). In the Wadden Sea both morphologies were present, sometimes occurring together in the same location. Tubular forms in the Wadden Sea were mostly attached, while sheet-like thalli were found drifting and only few individuals grew attached. In contrast, in the Baltic Sea only specimens with bladed thalli were observed (, and ). With the exception of one single population detected at Wulfen (N 54°24.535, E 011°10.388), these were all unattached. Notably, at Wulfen, attachment was only observed on artificial substrata (rubber mat, bricks or concrete), despite high abundance of natural stones. In spring (March–April) bladed U. compressa were typically 3 cm to maximally 9 cm in diameter. Individuals then increased in size until July–August. Drifting thalli could become larger than attached individuals and some specimens in very sheltered locations in the Baltic Sea reached diameters of up to 150 cm. Drifting thallus sheets appeared round, lobed or with an amorphous shape and they were often interspersed with holes of various sizes. Attached bladed thalli in the Wadden Sea were often rosette-shaped, while those at Wulfen differed from the drifting representatives by having a more lanceolate form with either no or only sparse holes.
Cells of the apical and middle part of the bladed morphotype were arranged in indistinct, slightly curved rows and had a rectangular or irregularly polygonal shape with rounded corners, 9–27 µm in surface view and 11–45 µm × 4–17 µm in transverse sections (). Most cells of the middle and apical thalli contained a single, lobed, parietal chloroplast and, in most cases, one central pyrenoid, while some cells exhibited two, or rarely three, pyrenoids (). Thalli with cap-like chloroplasts and one or two (rarely three) marginal pyrenoids were less common (). In attached specimens cells of the rhizoidal zone and the basal thallus part were long-drawn-out and club-shaped, exhibiting pyrenoids in variable number, ranging from one to four per cell (). The main body of these rhizoidal cells was 3–15 µm in surface view, while the tail was up to 40 µm. Transverse sections confirmed that blade-like thalli of U. compressa were distromatic and 30–50 µm thick ().There were no micro- or macromorphological differences observed in sheet-like specimens originating from the Baltic Sea or Wadden Sea, except for some specimens exhibiting a rosette shape in the Wadden Sea. Tubular specimens of the Wadden Sea resembled the characters already described for this morphotype of U. compressa (Linnaeus, Citation1753; Blomster et al., Citation1998; Maggs et al., Citation2007). For a comparison of micromorphological characters from tubular and sheet-like specimens of U. compressa, see .
Table 2. Comparison of micromorphological features of the middle and apical thallus parts of the different gross morphologies represented by Ulva compressa.
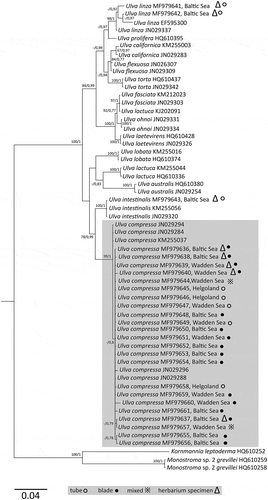
For a selected set of 18 sequences () representing all the detected morphotypes, ML and BI analyses were conducted using 773 aligned characters of tufA. Eight sequences of herbarium material () and 29 reference sequences of Ulva specimens were additionally included in the alignment, and outgroup sequences of Kornmannia leptoderma (Kjelman) Bliding (HQ610252) and Monostroma grevillei (Thuret) Wittrock (HQ610259 & HQ610258) were added to the analyses. The topologies of the BI and ML trees were similar, and the ML tree was used to visualize the topology (). The node separating the genus Ulva from outgroup taxa received strong bootstrap and posterior probability support (100/1). Bootstrap support and Bayesian probability values were higher at the terminal nodes than for internally located ones. The analysis confirmed several well-supported clusters of downloaded Ulva reference sequences that correspond to morphologically distinguishable species and have been described elsewhere (e.g. Saunders & Kucera, Citation2010; Rinkel et al., Citation2012; Kirkendale et al., Citation2013).
Table 3. Sample list of Ulva compressa samples collected in 2014–2016 in northern Germany and used for phylogenetic analyses.
Figs 3–11. Morphotypes of Ulva compressa found in northern Germany and micromorphology of bladed thalli. Fig. 3. Tubular, branched form of U. compressa sampled in the Wadden Sea, Nordstrand (September 2015), referring to the morphology of the holotype. Fig. 4. Specimen exhibiting mixed morphologies of the tubular and blade-like form of U. compressa from the Wadden Sea, Dagebüll (September 2015). Fig. 5. Drifting U. compressa blade without rhizoidal zone and Fig. 6. blade attached to concrete, collected in the Baltic Sea, Wulfen, Fehmarn (August 2015). Figs 7–11 display the micromorphology of blade-like U. compressa collected at Wulfen, Fehmarn in the Baltic Sea (August 2015). Fig. 7. Cells of drifting U. compressa are arranged in indistinct slightly curved rows and Fig. 8. of rectangular or irregularly polygonal shape with rounded corners. The single lobed chloroplast is either parietal and cells containing 1–2(3) central pyrenoids or Fig. 9. chloroplasts appear cap-like and pyrenoids are marginal. Attached thalli provide the same micromorphology in the middle and apical thallus parts and additionally they exhibit strongly elongated cells of a club-shaped form in the rhizoidal area Fig. 10., if present, which contain a variable number of pyrenoids (1–4). Fig. 11. Transections show the distromatic thallus structure of U. compressa.
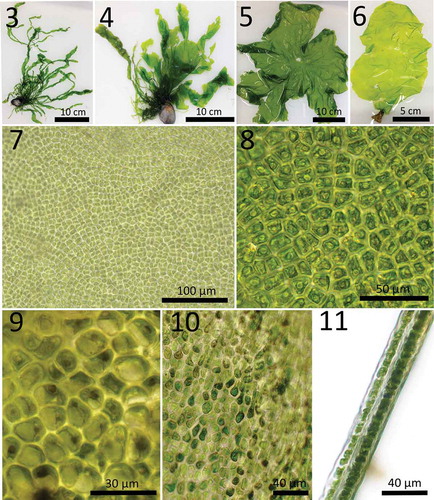
Fig. 12. Maximum likelihood tree inferred from tufA sequences. Numbers at nodes refer to bootstrap values left and Bayesian posterior probabilities (1000 replicates). Nodes with <50% bootstraps and <0.70 Bayesian support are not labelled. Branch lengths are drawn proportional to the amount of sequence change. Collection areas (Baltic Sea, Wadden Sea, Helgoland) are indicated. Samples marked with an unfilled circle are of tubular morphology, those labelled with a black circle are thalli with a blade shape and thalli exhibiting a mixed morphology are labelled with an asterisk. Triangles were used to mark sequences of herbarium material. Specimen of Ulva compressa, regardless of origin or morphology, appearing in a monophyletic clade.
Figs 13–16. Mass accumulation of blade-like Ulva compressa in August 2016 inducing hypoxic conditions at a shallow bay (Wulfen) at the German Baltic Sea island Fehmarn. Fig. 13. Top and Fig. 14. Underwater view of the massive drifting mats of blade-like Ulva compressa. Thalli forming mats of 100–120 cm thickness. Fig. 15. Close-up of strongly affected Zostera marina and Ruppia cirrhosa populations after removing drifting biomass. Fig. 16. Sediment cores show a clear zonation of (a) dense drifting algae material, (b) decomposition zone and (d) black, anoxic zone, emitting a foul odour.

For U. compressa the analyses revealed a well-supported clade (99/1) including all of our samples, regardless of their morphological habit, and a number of reference sequences referring to the tubular morphology of the holotype of U. compressa. Additionally some of the examined herbarium specimens also clustered within this clade. Sequence divergence between recent tubular, blade-like individuals and specimens exhibiting a mix of both morphologies was extremely low within this clade and ranged from 0–0.9% (, branch length drawn proportionally to sequence change). The gross morphology of a sample, tubular or blade-like, was not correlated with its position within the phylogenetic tree nor with a morphological species concept. Both BI and ML supported this morphologically mixed clade and thus confirms U. compressa as a good example of phenotypic plasticity within the genus Ulva.
It was not an objective of our study to identify the minimum salinity in which tubular forms of U. compressa can be found. However, the apparent absence of such forms at the German Baltic Sea coast supports the idea that their distribution must be restricted to salinities above 17 PSU (, Supplementary table 1). Three different historical samples that had been collected between 1957 and 1983 at the German Baltic Sea coast as Enteromorpha compressa, and which exhibited the holotype morphology of U. compressa, consistently belonged to other species, either U. linza L. or U. intestinalis L. (). In contrast, U. compressa exhibiting blade-like morphologies was found at salinities as low as 9 PSU. We also detected five different historical Ulva specimens that had been collected in northern Germany between 1971 and 1984 which all exhibited the blade-shaped morphology of U. compressa and also corresponded genetically with U. compressa. Notably, all of them had been wrongly assigned to U. lactuca Linnaeus (), suggesting that the species have been confused in the area for decades.
On both Baltic Sea and Wadden Sea coasts the blade-like and drifting morphotype of U. compressa occasionally formed local mass accumulations. For example, this was observed in 2016 at a shallow and wave protected beach at Wulfen on the Baltic Sea coast (, ). Ulva compressa thalli formed a loose drifting mat of 100–150 cm thickness, covering 23 × 12 m of a Zostera marina and Ruppia cirrhosa bed in a water depth of 150–200 cm below mean sea surface (). The lowest 5 to 30 cm of these aggregations consisted of decaying biomass. Oxygen concentrations dropped from 9.8 mg l–1 at the sea surface to 0 mg l–1 directly above the seafloor beneath the drifting biomass. An intense odour of H2S was emitted from the surface of sediment cores, which exhibited an anoxic, dark coloured top layer to a depth of 4–16 cm (). Concomitantly, increased mortality of Z. marina and R. cirrhosa was observed and ~70% of the covered seagrass appeared seriously damaged or dead. No anoxic water or sediment and no damaged seagrass were observed in reference areas in the close vicinity that were free of drifting U. compressa. Very similar effects of U. compressa aggregations, i.e. drifting blades closely entangled with filaments of Chaetomorpha melagonium (Weber & Mohr) Kützing, were also seen at Nordstrand (N54°29.163, E 008°49.114) in the Wadden Sea, in summer 2016.
Discussion
Here, we provide a completely revised picture of the distribution and morphological variability of Ulva compressa in Schleswig-Holstein, a study area of limited size that nonetheless includes coastal sections of two important sea areas in Northern Europe, namely the North Sea and the Baltic Sea. The clade obtained by phylogenetic analysis of tufA sequences clearly encompasses specimens both of tubular and blade-like morphologies and occasionally the occurrences of mixed morphotypes. This morphological diversity is not in agreement with the taxonomic concepts proposed for U. compressa in identification keys for the region and adjacent areas (Kornmann & Sahling, Citation1977, Citation1994; Rothmaler, Citation1984; Pankow, Citation1990). As a consequence, it is strongly suggested that virtually all specimens of U. compressa in the German Baltic and many of those in the Wadden Sea have so far been misidentified and this may also be true for adjacent areas. Our observations are at the same time in contradiction and in agreement with the findings of Leskinen et al. (Citation2004). Focusing on the phylogeographic structure and distribution of U. compressa in the Baltic Sea, the authors stated that its distribution was very restricted and that the species was absent at salinities lower than 15 ppt. These observations seem to be in stark contrast with our findings of a relatively wide distribution and even of occasional mass developments of U. compressa at salinities in the range between 9 and 15 PSU. However, the study of Leskinen et al. (Citation2004) was exclusively focused upon branched tubular algal specimens that corresponded morphologically with the holotype of U. compressa. It addressed the issues of branching patterns (Burrows, Citation1959) and the relationship of U. compressa with typically unbranched tubular species like Ulva intestinalis (Linnaeus, Citation1753). The possibility that exclusively sheet-like and nearly exclusively unattached individuals might be present at lower salinities was not considered. Indeed, specimens of U. compressa that resembled the holotype were also in the present study never found at salinities of 17 PSU or less, which confirms the findings of Leskinen et al. (Citation2004).
Ulva compressa with blade-like morphology was first discovered in brackish water in the Ythan Estuary in Scotland (Tan et al., Citation1999), but it is not limited to low salinities. In the present study specimens with extended distromatic blades were found in high abundance along the coastline of the Wadden Sea, where salinity usually ranges from 30 to 33.5 PSU. Here, U. compressa blades were particularly frequent in environments like overflow basins, drain channels and reservoirs, where water temperature can rise temporarily to over 40°C and salinity varies strongly due to desiccation and flooding. In contrast, tubular individuals were relatively rare in such relatively stressful locations. Apparently, the bladed morphology of U. compressa is less limited in its distribution than the allegedly characteristic tubular form, suggesting that it may be able to survive under a broader range of environmental conditions.
Both the wide distribution of blade-like morphotypes and the absence of tubular forms at low salinities are also supported by our investigation of herbarium material from northern Germany. Five different historical samples with blade-like morphology that had been collected in the Wadden and Baltic Sea in the 1970s and 1980s were identified as U. compressa, which confirms that the presence of this morphotype in the whole area is not new. Likewise, our findings for historical tubular specimens, which had all been wrongly assigned to U. compressa (), are in accordance with an absence of this morphotype from the Baltic Sea area in the past. Given this information, and that blade-like forms have so far not been recognized as U. compressa, we predict that all historical records of this species from the German and more easterly Baltic Sea areas were probably based upon confusion with other tubular species, such as Ulva linza Linnaeus Citation1753 (MF979641 & MF979642) or U. intestinalis (MF979643), despite the fact that U. compressa is in the area and has also been there in the past. Further, all historical records of U. compressa with blade-like morphology that we discovered were originally wrongly assigned to U. lactuca. Most historical and more recent identification keys for Germany (Fraude, Citation1882; Rothmaler, Citation1984; Pankow, Citation1990) and species lists (Nielsen et al., Citation1995; Schories et al., Citation2009) include U. lactuca, although we have been unable to detect any genetic evidence for its presence in our sampling sites in the Baltic Sea and Wadden Sea. We therefore speculate that many other historical records of U. lactuca in Schleswig-Holstein and its adjacent areas may have also been incorrect, due to confusion with the blade-like morphotype of U. compressa.
The presence of foliose Ulva compressa in the North Sea has been described before. On the basis of molecular investigation, Tan et al. (Citation1999) revealed a close relationship between tubular U. compressa and foliose Ulva specimens from the Scottish East coast that exhibited the typical morphology of U. pseudocurvata Koeman and Van den Hoek (Citation1981). ITS gene sequences indicated conspecificity of both forms and Tan et al. (Citation1999) suggested that U. pseudocurvata might just be a morphological variation of U. compressa. Based upon specimens collected in the Netherlands, U. pseudocurvata was originally described as a species with distinctly curved, sometimes straight, and more or less symmetrical thalli that were missing a hollow stipe (Koeman & Van den Hoek, Citation1981). According to these authors, the latter character was one of the main traits that distinguished U. pseudocurvata from a highly similar taxon, U. curvata (Kützing) De Toni. Due to the absence of the above mentioned morphological features, specimens collected on Helgoland and previously identified as U. curvata (Kornmann & Sahling, Citation1977) were revised and allocated to U. pseudocurvata (Kornmann & Sahling, Citation1994). Since then, U. pseudocurvata is listed in the species inventories of Helgoland (Bartsch & Kuhlenkamp, Citation2000; Schories et al., Citation2009), while the distribution of U. curvata is assumed to extend over the Wadden Sea coasts of Schleswig-Holstein and the Baltic Sea coastline of eastern Germany (Schories et al., Citation2009). Thalli with strongly curved habit that resembled U. pseudocurvata or U. curvata were occasionally sampled within our field survey on Helgoland and at the Wadden Sea coast. However, material from Helgoland was genetically most similar with U. lactuca (GenBank accession no. HQ610341, data not shown), while material from the mainland coasts always clustered within U. compressa. We were not able to detect genetic evidence of a delimited clade representing specimens resembling the descriptions and characteristics of U. pseudocurvata or U. curvata. Thus U. pseudocurvata and U. curvata are either very rare species or, and perhaps more probable, simply morphological variations within U. compressa and U. lactuca. To clarify the taxonomic status of U. pseudocurvata and U. curvata further genetic investigation of the type material of both species has to be carried out.
Although the sheet-like morphotype of U. compressa has so far not been correctly recognized taxonomically, it appears as one of the more problematic nuisance seaweeds in Germany. In its free-floating form it clearly has the potential to form green tides, and on a local scale we repeatedly observed sediment anoxia and damaged seagrass meadows in the direct vicinity of biomass accumulations. The southern North Sea and, in particular, the Baltic Sea are still among the most eutrophic sea areas worldwide, although nutrient input has been reduced since its peak in the early 1980s (Wernand et al., Citation2013). Given that the bladed morphotype of U. compressa had already been recorded in the area at that time, it may certainly have significantly contributed to the formation of sediment anoxia in the Wadden Sea, which was more prevalent some decades ago than today (Reise et al. Citation2015). Extensive green tides formed by foliose U. compressa were observed by Tan et al. (Citation1999) in Aberdeenshire (Scotland), and by Guidone et al. (Citation2013) and Hofmann et al. (Citation2010) in New England. It remains as an open question why the bladed morphotype of U. compressa has a stronger capacity to form green tides, a wider tolerance of different environmental conditions and thereby probably a more invasive character than the tubular morphotype.
We are also currently unable to explain why U. compressa was detected exclusively unattached in most of the Baltic Sea sites that we surveyed. In Ulva mutabilis Føyn 1958 the formation of rhizoids is controlled by specific bacteria (Wichard et al., Citation2015) and a similar mechanism may act in U. compressa. Under these conditions a lack of specific microorganisms at reduced salinity may lead to a reduced formation of rhizoids. However, attached specimens of U. compressa were repeatedly detected at one site in the Baltic and in this light a genetic basis for the formation or non-formation of rhizoids appears more likely.
We can conclude that two extremely different morphologies exist within the species U. compressa and that transition forms are rarely exhibited in our study area. Consequently they have so far been misinterpreted as different species. Our findings highlight once again that the genus Ulva comprises a complex of species with morphological varieties and forms that are still poorly understood. The number of morphologies a single species can possess is in most cases unknown, and different morphotypes of the same species are still regarded as distinct taxa. This underlines the utmost importance of applying molecular methods for species identification within this morphologically highly variable genus, and it once more points out that species knowledge still is, and should be, an important focus in phycological research.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2018.1513167.
Supplementary table 1. Sample list provides overview of sampling sites at which Ulva compressa was found.
Author contributions
S. Steinhagen: experimental design, field work and algae collection, laboratory work, phylogenetic analysis, drafting and editing manuscript; R. Karez: experimental design, drafting and editing manuscript; F. Weinberger: original concept, specimens collection, drafting and editing manuscript.
Table S1
Download MS Word (37.3 KB)Acknowledgements
We thank our colleagues G. Bonthond and F. Barboza for technical support. We would like to thank T. Sanders for the valuable comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ansell, A., Gibson, R., Barnes, M. & Press, U. (1998). Ecological impact of green macroalgal blooms. Oceanography and Marine Biology: An Annual Review, 36: 97–125.
- Arroyo, N.L., Aarnio, K., Mäensivu, M. & Bonsdorff, E. (2012). Drifting filamentous algal mats disturb sediment fauna: impacts on macro–meiofaunal interactions. Journal of Experimental Marine Biology and Ecology, 420–421: 77–90.
- Bartsch, I. & Kuhlenkamp, R. (2000). The marine macroalgae of Helgoland (North Sea): an annotated list of records between 1845 and 1999. Helgoland Marine Research, 54: 160–189.
- Bliding, C. (1963). A critical survey of European taxa in Ulvales, Part I: Capsosiphon, Percursaria, Blidingia, Enteromorpha. Opera Botanica, 8: 1–160.
- Blomster, J., Maggs, C.A. & Stanhope, M.J. (1998). Molecular and morphological analysis of Enteromorpha intestinalis and E. compressa (Chlorophyta) in the British Isles. Journal of Phycology, 34: 319–340.
- Blomster, J., Back, S., Fewer, D.P., Kiirikki, M., Lehvo, A., Maggs, C.A. & Stanhope, M.J. (2002). Novel morphology in Enteromorpha (Ulvophyceae) forming green tides. American Journal of Botany, 89: 1756–1763.
- Borum, J., Sand-Jensen, K., Binzer, T., Pedersen, O. & Greve, T.M. (2006). Oxygen movement in seagrasses. In Seagrasses: Biology, Ecology and Conservation (Orth, R.J. & Duarte C.M., editors), 255–270. Springer, Dordrecht.
- Brodie, J., Maggs, C.A., Society, B.P. & John, D.M. (2007). The green seaweeds of Britain and Ireland. British Phycological Society.
- Burrows, E.M. (1959). Growth form and environment in Enteromorpha. Journal of the Linnean Society of London, Botany, 56: 204–206.
- Charlier, R.H., Morand, P. & Finkl, C.W. (2008). How Brittany and Florida coasts cope with green tides. International Journal of Environmental Studies, 65: 191–208.
- De Silva, M.W.R.N. & Burrows, E.M. (1973). An experimental assessment of the status of the species Enteromorpha intestinalis (L.) Link and Enteromorpha compressa (L.) Grev. Journal of the Marine Biological Association of the United Kingdom, 53: 895–904.
- Dillenius, J.J. (1742). Historia muscorum. Oxonii: E Theatro Sheldoniano.
- Dion, P. & Le Bozec, S. (1996). The French Atlantic Coasts. In Marine Benthic Vegetation: Recent Changes and the Effects of Eutrophication (Schramm, W. & Nienhuis, P.H., editors), 251–264. Springer Berlin, Heidelberg.
- Duarte, C.M. (2000). Marine biodiversity and ecosystem services: an elusive link. Journal of Experimental Marine Biology and Ecology, 250: 117–131.
- Famà, P., Wysor, B., Kooistra, W.H.C.F. & Zuccarello, G.C. (2002). Molecular phylogeny of the genus Caulerpa (Caulerpales, Chlorophyta) inferred from chloroplast tufA gene. Journal of Phycology, 38: 1040–1050.
- Fletcher, R. (1996). The occurrence of “green tides” – a review. In Marine Benthic Vegetation: Recent Changes and the Effects of Eutrophication (Schramm, W. & Nienhuis, P.H., editors), 7–43. Springer Berlin, Heidelberg.
- Fraude, H. 1882. Grund-und Plankton-Algen der Ostsee. Kessinger Publishing, Greifswald, Germany.
- Gräwe, D., Prange, S., Koch, F., Neumann, T., Wasmund, N., Hirt, U., Gadegast, M., Mahnkopf, J., Czudowski, L., Mischke, U., Venohr, M., Heidecke, C., v. Heinrich, J. & Brockmann, U. (2014). Harmonisierte Hintergrund- und Orientierungswerte für Nährstoffe und Chlorophyll-a in den deutschen Küstengewässern der Ostsee sowie Zielfrachten und Zielkonzentrationen für die Einträge über die Gewässer. Bund/Länder-Ausschuss Nord- und Ostsee (BLANO), Bonn.
- Guidone, M., Thornber, C., Wysor, B. & O´Kelly, C.J. (2013). Molecular and morphological diversity of Narragansett Bay (RI, USA) Ulva (Ulvales, Chlorophyta) populations. Journal of Phycology, 49: 979–995.
- Guiry, M.D. & Guiry, G.M. (2018). AlgaeBase. World-wide electronic publication. National University of Ireland, Galway. http://www.algaebase.org.
- Hartog den, C. (1994). Suffocation of a littoral Zostera bed by Enteromorpha radiata. Aquatic Botany, 47: 21–28.
- Hayden, H.S., Blomster, J., Maggs, C.A., Silva, P.C., Stanhope, M.J. & Waaland, J.R. (2003). Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. European Journal of Phycology, 38: 277–294.
- Heesch, S., Broom, J.E., Neill, K.F., Farr, T.J., Dalen, J.L. & Nelson, W.A. (2009). Ulva, Umbraulva and Gemina: genetic survey of New Zealand taxa reveals diversity and introduced species. European Journal of Phycology, 44: 143–154.
- Hofmann, L.C., Nettleton, J.C., Neefus, C.D. & Mathieson, A.C. (2010). Cryptic diversity of Ulva (Ulvales, Chlorophyta) in the Great Bay Estuarine System (Atlantic USA): introduced and indigenous distromatic species. European Journal of Phycology, 45: 230–239.
- Katoh, K., Misawa, K., Kuma, K.-I. & Miyata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30: 3059–3066.
- Kirkendale, L., Saunders, G.W. & Winberg, P. (2013). A molecular survey of Ulva (Chlorophyta) in temperate Australia reveals enhanced levels of cosmopolitanism. Journal of Phycology, 49: 69–81.
- Koeman, R.P.T. & Van den Hoek, C. (1981). The taxonomy of Ulva (Chlorophyceae) in the Netherlands. British Phycological Journal, 16: 9–53.
- Koeman, R. & Van den Hoek, C. (1982). The taxonomy of Enteromorpha Link, 1820, (Chlorophyceae) in the Netherlands I: The section Enteromorpha. Archiv für Hydrobiologie, 63: 279–330.
- Kornmann, P. & Sahling, P.-H. (1977). Meeresalgen von Helgoland. Helgoländer wissenschaftliche Meeresuntersuchungen, 29: 1–289.
- Kornmann, P. & Sahling, P.-H. (1994). Marine algae of Helgoland – 2nd Supplement. Helgolander Meeresuntersuchungen, 48: 365–406.
- Kraft, L.G.K., Kraft, G.T. & Waller, R.F. (2010). Investigations into southern Australian Ulva (Ulvophyceae, Chlorophyta) taxonomy and molecular phylogeny indicate both cosmopolitanism and endemic cryptic species. Journal of Phycology, 46: 1257–1277.
- Lee, K.S., Park, S.R. & Kim, Y.K. (2007). Effects of irradiance, temperature and nutrients on growth dynamics of seagrasses: a review. Journal of Experimental Marine Biology and Ecology, 350: 144–175.
- Leskinen, E., Alström-Rapaport, C. & Pamilo, P. (2004). Phylogeographical structure, distribution and genetic variation of the green algae Ulva intestinalis and U. compressa (Chlorophyta) in the Baltic Sea area. Molecular Ecology, 13: 2257–2265.
- Linnaeus, C. (1753). Species Plantarum, Exhibentes Plantas Rite Cognitas Ad Genera Relatas: Cum Differentiis Specificis, Nominibus Trivialibus, Synonymis Selectis, Locis Natalibus, Secundum Systema Sexuale Digestas. Trattner, Stockholm.
- Maggs, C.A., Blomster, J., Mineur, F. & Kelly, J. (2007). Ulva. In The Green Seaweeds of Britain and Ireland (Brodie, J., Maggs, C.A. & John, D., editors). British Phycological Society, London.
- Marbá, N., Holmer, M., Gacia, E. & Barrón, C. (2006). Seagrass beds and coastal biogeochemistry. In Seagrasses: Biology, Ecology and Conservation (Orth, R.J. & Duarte C.M., editors), 137–157. Springer, Dordrecht.
- McGlathery, K. (2001). Macroalgal blooms contribute to the decline of seagrass in nutrient-enriched coastal waters. Journal of Phycology, 37: 453–456.
- Nielsen, R., Kristiansen, A., Mathiesen, L. & Mathiesen, H. (1995). Distributional index of the benthic macroalgae of the Baltic Sea area. Acta Botanica Fennica, 155: 1–51.
- Norkko, A. & Bonsdorff, E. (1996a). Population responses of coastal zoobenthos to stress induced by drifting algal mats. Marine Ecology Progress Series, 140: 141–151.
- Norkko, A. & Bonsdorff, E. (1996b). Rapid zoobenthic community responses to accumulations of drifting algae. Marine Ecology Progress Series, 131: 143–157.
- Nylander, J.A.A. (2004). MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University.
- Pankow, H. (1990). Ostsee-Algenflora. G. Fischer.
- Raffaelli, G.G., Raven, J.A. & Poole, L.J. (1998). Ecological impact of green macroalgal blooms. Oceanography and Marine Biology, 125: 37–97.
- Reed, R.H. & Russell, G. (1978). Salinity fluctuations and their influence on bottle brush morphogenesis in Enteromorpha intestinalis (L) Link. British Phycological Journal, 13: 149–153.
- Reiffenstein, R.J., Hulbert, W.C. & Roth, S.H. (1992). Toxicology of hydrogen sulfide. Annual Review of Pharmacology and Toxicology, 32: 109–134.
- Reinke, J. (1889). Notiz über die Vegetationsverhältnisse in der deutschen Bucht der Nordsee. Berichte der Deutschen Botanischen Gesellschaft, 7: 367–369.
- Reise, K., Buschbaum, C. & Dolch, T. (2015). Vorkommen von Grünalgen und Seegras im Nationalpark Schleswig-Holsteinisches Wattenmeer 2014. Report on behalf of Landesamt für Küsten- und Naturschutz (State Agency for coastal protection and nature conservancy), Tönningen, Germany.
- Rinkel, B.E., Hayes, P., Gueidan, C. & Brodie, J. (2012). A molecular phylogeny of Acrochaete and other endophytic green algae (Ulvales, Chlorophyta). Journal of Phycology, 48: 1020–1027.
- Rönnbäck, P., Kautsky, N., Pihl, L., Troell, M., Soerqvist, T. & Wennhage, H. (2007). Ecosystem goods and services from Swedish coastal habitats: identification, valuation, and implications of ecosystem shifts. Ambio, 36: 534–544.
- Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Rothmaler, W. (1984). Exkursionsflora für die Gebiete der DDR und BRD Band 1: Niedere Pflanzen. Volk und Wissen Volkseigener Verlag, Berlin.
- Saunders, G.W. & Kucera, H. (2010). An evaluation of rbcL, tufA, UPA, LSU and ITS as DNA barcode markers for the marine green macroalgae. Cryptogamie Algologie, 31: 487–528.
- Schaffelke, B., Smith, J.E. & Hewitt, C.L. (2006). Introduced macroalgae – a growing concern. Journal of Applied Phycology, 18: 529–541.
- Schories, D., Selig, U. & Schubert, H. (2009). Species and synonym list of the German marine macroalgae based on historical and recent records. Meeresbiologische Beiträge, University Rostock.
- Smetacek, V. & Zingone, A. (2013). Green and golden seaweed tides on the rise. Nature, 504: 84–88.
- Smith, R.D., Dennison, W.C. & Alberts, R.S. (1984). Role of seagrass photosynthesis in root aerobic processes. Plant Physiology, 74: 1055–1058.
- Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312–1313.
- Statistisches Jahrbuch Schleswig-Holstein (2016 /2017). Statistisches Amt für Hamburg und Schleswig-Holstein (Statistikamt Nord), Hamburg, Germany.
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28: 2731–2739.
- Tan, I.H., Blomster, J., Hansen, G., Leskinen, E., Maggs, C.A., Mann, D.G., Sluimam, H.J. & Stanhope, M.J. (1999). Molecular phylogenetic evidence for a reversible morphogenetic switch controlling the gross morphology of two common genera of green seaweeds, Ulva and Enteromorpha. Molecular Biology and Evolution, 16: 1011–1018.
- Teichberg, M., Fox, S.E., Olsen, Y.S., Valiela, I., Martinetto, P., Iribarne, O., Muto, E.Y., Petti, M.a.V., Corbisier, T.N., Soto-Jiménez, M., Páez-Osuna, F., Castro, P., Freitas, H., Zitelli, A., Cardinaletti, M. & Tagliapietra, D. (2010). Eutrophication and macroalgal blooms in temperate and tropical coastal waters: nutrient enrichment experiments with Ulva spp. Global Change Biology, 16: 2624–2637.
- Tolstoy, A. & Willén, T. (1997). Preliminär checklista över makroalger i Sverige. ArtDatabanken.
- Valiela, I., McClelland, J., Hauxwell, J., Behr, P.J., Hersh, D. & Foreman, K. (1997). Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnology and Oceanography, 42: 1105–1118.
- Waycott, M., Duarte, C.M., Carruthers T.J.B., Orth, R.J., Dennison, W.C., Olyarnik, S., Calladine, A., Fourqurean, F.W., Heck Jr., K.L., Hughes, A.R., Kendrick, G.A., Kenworthy, W.J., Short, F.T. & Williams, S.L. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceeding of the National Academy of Sciences of the USA, 106: 2377−12381.
- Wernand, M.R., Van Der Woerd, H.J. & Gieskes, W.W. (2013). Trends in ocean colour and chlorophyll concentration from 1889 to 2000, worldwide. PLoS ONE, 8: e63766.
- Wichard, T., Charrier, B., Mineur, F., Bothwell, J.H., De Clerck, O. & Coates, J.C. (2015). The green seaweed Ulva: a model system to study morphogenesis. Frontiers in Plant Science, 6.