ABSTRACT
The Synurophyceae is a well-supported clade of ecologically important heterokont algae found largely in freshwater planktonic habitats worldwide, whose members have cell coverings consisting of species-specific siliceous scales overlapped in a highly organized manner. Many synurophytes have been described as endemic and are found only in specific regions of the world. A thriving population of the European endemic, Mallomonas intermedia, was discovered in a remote desert pond situated in the Virgin Valley, Nevada, USA and in a stratigraphic sequence from the middle Eocene fossil locality known as Horsefly in British Columbia, Canada. Both North American finds were closely compared with populations from Europe, confirming the identifications. Before these discoveries, this species was recorded from numerous waterbodies exclusively in Europe, but was lacking from hundreds of sites examined from other continents. Its presence in western North America during the warm middle Eocene confirms that historically this species had a significantly wider distribution and may be best classified as a palaeoendemic. Additional species uncovered from a second fossil locality that are closely related to M. intermedia further support the presence of this lineage in North America during the Eocene. The living population in northern Nevada presents an enigma. Does this remote desert population represent a remnant population that has gone undetected until now, or is it a recent arrival from an unknown region by an unknown vector?
Introduction
The Synurophyceae is a well-supported clade of ecologically successful heterokont algae that are found almost exclusively in freshwater ecosystems (Kristiansen, Citation2005; Siver, Citation2015a). The group is characterized by formation of a highly organized cell covering consisting of precisely overlapped siliceous scales. Mallomonas, the largest genus within the synurophytes, is a unicellular motile organism, with one or two flagella, that forms a second type of siliceous structure known as the bristle. Bristles are thin, elongate structures that attach to the apical ends of scales and radiate outward from the cell. The designs of scales and bristles, many of which are highly ornamented and sculptured, are species-specific and used in the formal description of taxa. Given the species-specific nature of the siliceous components, coupled with the fact that numerous species grow under specific environmental conditions, synurophyte remains from sediments have been successfully used in reconstructing past environments (Siver, Citation1993; Stevenson & Smol, Citation2015; Arseneau et al., Citation2016).
Scales of synurophytes all have a base plate perforated with pores and a posterior rim that bends up and over a portion of the base plate (Siver, Citation1991; Kristiansen, Citation2005). Many species of Mallomonas have what are referred to as tripartite scales where the structure is divided into three parts, the dome, shield and flange (Harris, Citation1953). The dome is a raised cavity found along the distal end of the scale into which the proximal end of the bristle is positioned. The shield and posterior flange are delineated by a prominent and largely V-shaped ridge of silica, the V-rib. The base of the V-rib sits on the base plate in the proximal end of the scale and the two arms that form the ‘V’ extend towards the distal end of the scale, often terminating near the margins. The portion of the base plate between the V-rib and dome forms the shield and the portion between the V-rib and posterior rim the flange. Additional structures such as ribs and papillae, referred to as secondary features, can be found on the flange, shield and dome. Species with tripartite scales often form the most highly organized cell coverings (Siver & Glew, Citation1990; Siver, Citation2018). Bristles associated with tripartite scales are elongate, ribbed structures with a flattened proximal end called the foot, which secures the bristle within the dome, and a ribbed shaft that extends outward from the scale. The shaft is typically serrated along one rib and the tip can be pointed or highly modified.
Compared with their close heterokont relatives, the diatoms, the geological history of the synurophytes is poorly known. Although fossil remains of siliceous diatom frustules are relatively common in the geological record, in contrast, scales and bristles are very rare. In fact, until discovery of numerous well-preserved synurophyte remains in the middle Eocene Giraffe Pipe locality located in the North Western Territories, Canada, the oldest records were only several thousand years old (Siver & Wolfe, Citation2005). The Giraffe Pipe fossil locality has yielded much evolutionary history about the clade, especially with respect to the siliceous structures. Species of Mallomonas are well represented at the Giraffe locality and include both extinct and modern lineages (Siver et al., Citation2015). Many of the extinct species had large and robust scales, often four times larger than most modern species (Siver, Citation2015b). In contrast, remains of other species show clear evolutionary stasis with respect to scale and bristle morphology (Siver et al., Citation2009) and findings further illustrate that formation of the highly organized cell covering was already a hallmark of the synurophytes by the middle Eocene (Siver, Citation2018). Still, knowledge of historical biogeographic patterns for synurophyte taxa and use of the group in evaluating ancient aquatic environments, has been largely precluded given the sparse fossil record.
Endemic status implies that an organism is restricted to a specific geographic region or location. Regarding the synurophyte genus Mallomonas, Kristiansen (Citation2002) designated one-third of the 172 known species as endemics, citing poor dispersal mechanisms as a cause for the high rate of endemism among these microscopic organisms (Kristiansen & Lind, Citation2005; Kristiansen, Citation2008). In the case of Mallomonas, endemics are found in multiple sections of the genus, (Kristiansen, Citation2002). The fact that many synurophytes display distinctive biogeographic distributions (Siver & Lott, Citation2012) appears to be in direct conflict with the ubiquity hypothesis which argues that microscopic organisms are widely dispersed and lack such patterns (Finlay, Citation2002). In some cases, synurophyte species are only known from one or a handful of closely situated sites (e.g. M. connensis). Others are much more widespread, but restricted to specific continents (Kristiansen, Citation2008). For example, M. marsupialis, M. fenestrata, M. duerrschmidtiae and M. intermedia are listed as endemic to Australia, tropical South America, North America and Europe, respectively (Kristiansen, Citation2002). In addition, a large number of species are reported as ‘northern temperate-subarctic-Arctic’, spread over northern regions of North America, Europe and northern Asia, but lacking in the southern hemisphere (Kristiansen, Citation2008). Other species, such as M. bangladeschica, are widespread over warm, largely tropical, regions of the world (Kristiansen, Citation2002; Siver & Wolfe, Citation2009).
The objective of this paper is to report on and describe populations of the European endemic species, Mallomonas intermedia, from a small waterbody in a remote desert region of Nevada, USA, as well as from a middle Eocene fossil locality from western Canada known as Horsefly. The North American populations are compared to all available literature records and to specimens studied from collections of active populations in Norway and Romania. The biogeography of M. intermedia, both from modern and historical perspectives, and its status as an endemic versus palaeoendemic organism, are discussed.
Materials and methods
Specimens used in this investigation were collected from five localities, Dufurrena Pond 19 (Nevada, USA), Røertjernet Pond and Skoklefalltjernet Pond (Norway), Lake Cocor (Romania) and the Horsefly Fossil locality in British Columbia, Canada. Plankton net samples were retrieved from Dufurrena Pond 19 and Lake Cocor in October 2017 and March 2018, respectively. Gravity cores were retrieved from both Røertjernet Pond and Skoklefalltjernet Pond in February 2018, cut into 3 cm sections and the top two sections (0–3 cm and 3–6 cm) were examined as part of this study. Four successive subsamples of mudstone rock, each representing ~25 years of geological history, were taken from the Horsefly H2 stratigraphic sequence and examined as part of our investigation. The plankton net, gravity core and rock samples were all examined with scanning electron microscopy (SEM). In addition, observations from plankton net samples taken from Røertjernet Pond in November 1978 and from Skoklefalltjernet Pond in August 1979, and examined at that time with transmission electron microscopy (TEM), were also included in our analyses.
Plankton samples from Dufurrena Pond 19 were air dried onto heavy duty aluminium foil, while those from Lake Cocor were dried onto Formvar-coated grids for initial screening with TEM. Sediment samples from the Røertjernet Pond and Skoklefalltjernet Pond cores were oxidized with a mixture of H2SO4 and potassium dichromate according to Marsicano & Siver (Citation1993). Mudstone rock fragments (50–100 mg) from the Horsefly H2 sequence were oxidized using 30% H2O2 under low heat for 3–4 hours and rinsed with distilled water. Aliquots of each sediment and mudstone sample were also air dried onto heavy duty aluminium foil. The aluminium foil samples were trimmed and attached to aluminium stubs with Apiezon® wax and coated with a mixture of gold and palladium for 2 min with a Polaron Model 5100E sputter coater. TEM grids from Lake Cocor were mounted onto aluminium stubs with double-sided adhesive carbon tape and coated with platinum for 45 s using a Leica ACE600 sputter coater. Samples from Dufurrena Pond 19, both Norwegian Pond cores and the Horsefly sequence were examined with a FEI Nova NanoSEM 450 field emission SEM, and those from Lake Cocor with an FEI Helios NanoLab 660 G3 UC scanning electron microscope.
Small drops of the net samples taken from the Norwegian ponds in 1978–1979 were applied onto Formvar supported and carbon coated Cu grids, fixed in OsO4 vapour and air-dried, washed with distilled water and re-dried. The material was shadow-casted with Pt at a 30° angle with an Edwards Speedivac Coating Unit 12E6/1629, and observed with a Siemens Elmiscope 1A transmission electron microscope. Measurements of scales were made directly from the SEM and TEM micrographs. All samples were thoroughly studied and provided similar results, but only a representative subsample of images are included in the plates.
Site Descriptions
Dufurrena Pond 19 is located in the Sheldon National Wildlife Refuge in the Virgin Valley region of north-western Nevada (41°52′16′′N, 119°1′31.5′′W). The small pond is on the western side of the unimproved road leading south from Route 140 to the Royal Peacock Opal Mine, across from Gooch Table. The area is part of the Great Basin Desert, a cold and arid desert located in the northern portion of the Great Basin (www.sangres.com). The surrounding terrain is largely volcanic with numerous outcrops of silica-rich minerals, including thick diatomite remains from an ancient Miocene lake and opal-bearing rocks. Vegetation is dominated by Artemisia tridentata (big sagebrush), and herds of mule deer and pronghorn antelope are found throughout the region. Dufurrena Pond 19 is a man-made structure originally built as a reservoir for irrigation, but is now owned by the US Fish and Wildlife Service and managed for migrating waterfowl (www.fws.gov/refuge/sheldon; www.sangres.com). The pond is ~3 m deep, surrounded by emergent aquatic plants, has healthy stands of the submergent perennial, Hydrilla verticillata and contains breeding populations of yellow perch and white crappie.
Røertjernet Pond (59°48′38′′N, 10°40′44′′E) and Skoklefalltjernet Pond (59°50′58′′N, 10°40′4′′E), both located in Nesodden municipality, Norway, are small waterbodies with maximum depths of 4 m and 7 m, respectively, impacted to various degrees by human activities, and with contributions of humic substances from the surrounding forests and wetlands. The ponds are also ringed, in part, by Sphagnum and associated bog plants. Røertjernet Pond is situated in a cultural landscape and is influenced by nutrient input from agricultural activity. The surrounding forest is dominated by Salix cinerea, and the emergent macrophyte Calla palustris is common along the shoreline. Skoklefalltjernet Pond is situated in a natural parkland setting composed of a mixed forest of evergreen and deciduous tree species, the pond is surrounded by Myrica gale and Phragmites australis.
Lake Cocor is located in the middle of the Subcarpathian area in the valley of the River Prahova (45°9′2.7′′N, 25°44′54.02′′E). It is a shallow lake with an extended littoral area that partially dries out during the dry months. The lithology of the area varies from conglomerates and sandstones to Miocene marls and clay formation (Rujoiu-Mare et al., Citation2015). Maximum rainfall generally occurs in summer, with an average of 212 mm in June between 2013–2017 (www.worldweatheronline.com), due to penetrating moist Mediterranean air masses from the south, particularly during warm months.
The Horsefly fossil locality (51.5°N, 121°W) is located in east-central British Columbia. Horsefly represents an ancient, warm, monomictic or possibly meromictic, softwater lake that existed during the middle Eocene between 44–52 Ma (Wilson, Citation1977; Wilson & Barton, Citation1996; Wolfe & Edlund, Citation2005) in a region that experienced a warm, wet climate and lacked freezing conditions in winter (Greenwood et al., Citation2005; Archibald et al., Citation2014). Horsefly represents a high resolution record of varved sediments that contain impressive and well-preserved fish, plant, insect, pollen and diatom specimens (Archibald et al., Citation2005). As part of this study we examined a 767-year long sequence (H2 sequence) collected and examined by Wilson & Barton (Citation1996) and archived at the University of Alberta, Canada.
Results
Description of population from Dufurrena Pond 19
Examination using light microscopy of a plankton tow taken on 4 October 2017 from Dufurrena Pond 19 in the Virgin Valley region of northern Nevada revealed a bloom of a species of Mallomonas believed to represent the European endemic, M. intermedia. The population was further studied with SEM and confirmed to indeed be M. intermedia ().
Figs 1–6. Scale and bristle remains from a population of Mallomonas intermedia cells from Dufurrena Pond 19, northern Nevada, USA. Fig. 1. Remains of domed and domeless scales (black arrows), short serrated bristles (double white arrows), and long bristles with lance-shaped apices (single white arrows). Fig. 2. A group of domed body scales. Note the single large transverse rib immediately behind the dome, secondary reticulation on the shield and posterior flange and ribs on the dome. Figs 3–4. Domed body scales denoting the tripartite design, the single transverse rib behind the dome and the ribs originating along the V-rib and extending onto the posterior flange. The scale in Fig. 3 had more ribs on the dome, but less reticulation on the shield than the scale in Fig. 4. Fig. 5. Domeless body scale. Fig. 6. Undersurface of a domed body scale showing the pattern of base plate pores, and close-ups of bristles denoting the lance apex, foot (white arrow) and serration on the shaft (double white arrow). Scale bars = 1 µm (Fig. 5), 2 µm (Figs 3, 4, 6), 4 µm (Fig. 2) and 10 µm (Fig. 1).
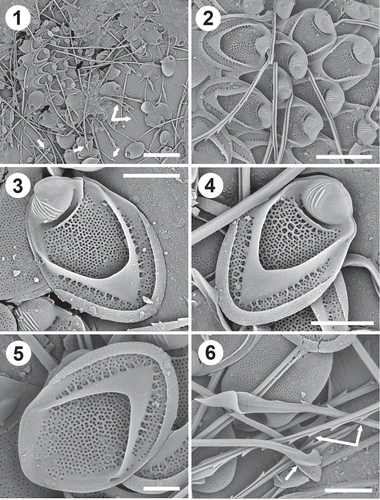
Description: Cells are ovoid to ellipsoidal, ranging in size from 31–42 x 12–16 µm, with tripartite scales and covered with bristles except for the very posterior end. Both domed (–) and domeless () scales are present. Domed body scales are found over the entire cell except for the posterior end. Domeless body scales are fewer in number, and found either scattered among the dome-bearing scales or on the posterior end. Domed scales are broadly oval with slight to no lateral incurvings, ranging in size from 5–6.3 × 3.1–4.2 µm (n=20) with a mean of 5.5 × 3.7, and possess a well-developed V-rib and posterior upturned rim (–). The dome is large, with a series of thin and parallel ribs situated mostly on the left side and oriented more or less parallel with the longitudinal axis of the scale (, ) and an inverted U-shaped opening on the right side from which the bristle emerges. Most scales have 4–6 dome ribs, but the number of ribs ranged from 2–12 with a mean of 5.5 (n=20). The base of the V-rib is situated lower on the shield, close to the posterior rim. The arms of the V-rib are long, curve, become continuous with the anterior submarginal ribs and terminate on the sides of the dome. The base plate is covered with small, evenly spaced pores that form curved transverse rows along the distal portion of the shield, but become more randomly arranged in the proximal end of the scale. Base plate pores are lacking along the anterior flanges. There is a single, prominent, transverse rib on the shield at the base of the dome (, ). On most scales, short ribs radiate from the transverse rib onto the V-rib side of the shield forming a small degree of secondary reticulation. Similar sets of short ribs also originate along the V-rib onto the shield and posterior flange, and are present under the posterior rim. Additional secondary reticulation can be found on the shield. The posterior rim is shallow and encircles approximately half of the scale perimeter. The anterior flanges are smooth. Structure of domeless scales is similar to that of domed scales, except they lack the transverse shield rib.
Two types of bristles were found, varying in length depending on their position on the cell. Bristles associated with the anterior end of the cell are short, slightly curved, ribbed, serrated, range in length from 9–11 µm (n=10), with a pointed apex and a well-formed foot bent at 45–90º relative to the shaft (, double arrow). This bristle type may also be found on other parts of the cell covering. Longer bristles found mostly on the body range in length from 24–31 µm (n=20), possess ribbed shafts that are typically serrated along the distal half and terminate with a lance-shaped apex (,). Each tooth of the serration usually terminates with two small teeth. The bristle expands along two margins at the apex. A triangular-shaped portion of one expanded margin folds over onto the other margin to form the classic lance-shaped apex (,).
Comparing Nevada populations with those from Europe and the Horsefly Locality
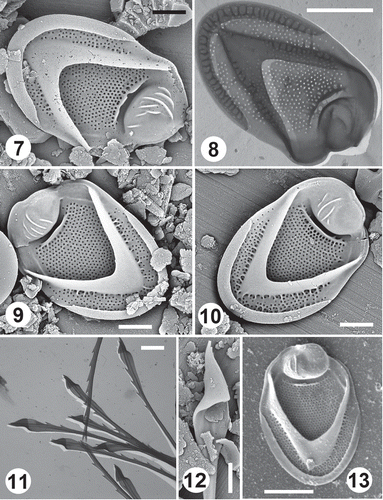
Scales and bristles from the Røertjernet Pond (, , , ) and Skoklefalltjernet Pond (, ) populations matched nicely those from Dufurrena Pond 19. The range and mean size of body scales for the Røertjernet Pond population were 5.2–6.4 × 3.1–3.9 µm and 5.5 × 3.6 µm (n=20), respectively, virtually identical to the population in Dufurrena Pond 19. Details of the dome, V-rib, large transverse shield rib, base plate pores and scale shape are also indistinguishable between both populations. The number of dome ribs overlapped considerably between the two populations, and the mean number of ribs on scales from Røertjernet Pond was 5, close to that for Dufurrena Pond 19. Some scales from Dufurrena Pond 19 had a greater degree of secondary ribbing, but scales could be found from both populations with similar levels of secondary reticulation originating from the transverse rib and V-rib. In addition, both populations had cells bearing smaller serrated bristles as well as longer lance-tipped body bristles. Scale features from the Lake Cocor population () were also similar to those from the Norway and Nevada samples, but some specimens were slightly less silicified and had, on average, fewer dome ribs.
Figs 7–13. Scale and bristle remains of Mallomonas intermedia from Norwegian (Figs 7–12) and Romanian sites (Fig. 13). Figs 7–10. Domed body scales depicting the tripartite nature of the scale, the single prominent transverse rib on the shield and dome ribs with SEM (Figs 7, 9, 10) and TEM (Fig. 8). Figs 11–12. Details of lance-tipped bristles with TEM (Fig. 11) and SEM (Fig. 12). Fig. 13. Domed body scale with fewer dome ribs. Scale bars = 1 µm (Figs 7, 9, 10, 12) and 2 µm (Figs 8, 11, 13).
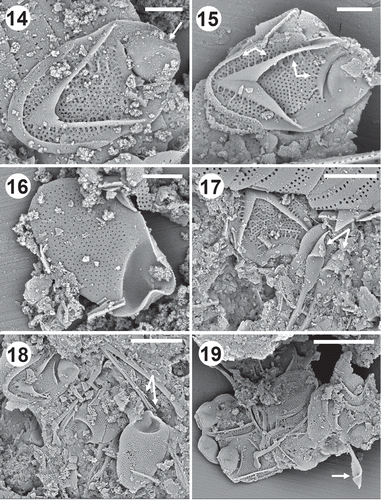
Characteristics of the scales and bristles from the Eocene Horsefly locality (–) also matched those from modern populations. Structure of the large transverse rib, dome, V-rib, secondary reticulation and base plate pores were indistinguishable from modern specimens. In addition, the Horsefly population possessed both bristle types with similar serration patterns and lance-shaped apices (–). Horsefly body scales were slightly smaller than the modern populations examined, ranging in size from 4.5–5.5 × 2.7–3.5 µm, with a mean of 5.0 × 3.1 µm (n=20).
Figs 14–19. Scale and bristle remains of Mallomonas intermedia from the middle Eocene Horsefly locality, British Columbia, Canada. Figs 14, 15. Domed body scales depicting the tripartite nature of the scale and the single prominent transverse rib on the shield just behind the dome. Ribs (arrow) can be seen on the scale in Fig. 14. The short ribs that often originate from the shield-side of transverse rib, along both sides of the V-rib and under the posterior rim are depicted with arrows on Fig. 15. Fig. 16. Undersurface of a domed scale showing the pattern of base plate pores. Note that the pores align in transverse rows on the distal portion of the shield, but are less organized along the proximal end. Fig. 17. Remains of a body scale and the lance tips of two bristles (arrows). Figs 18, 19. Groups of scales and bristles. The serration along the shaft of the longer lance bristles is depicted by the set of arrows in (Fig. 18) and a lance apex is illustrated by the arrow in Fig. 19. Scale bars = 1 µm (Figs 14–16), 2 µm (Fig. 17), 3 µm (Fig. 18) and 4 µm (Fig. 19).
Review of previous records for Mallomonas intermedia from European Sites
M. intermedia has been reported from numerous localities in Europe (), including the UK and the Scandinavian Peninsula, but not from numerous other surveys of synurophytes from other continents (Siver, Citation2015a), resulting in the classification of this species as a ‘European endemic’ (Kristiansen, Citation2002; Voloshko, Citation2010). It has been reported and verified using electron microscopy from waterbodies in Russia (Voloshko, Citation2010), Hungary (Péterfi et al., Citation1998; Barreto, Citation2001, Citation2005), Romania (Péterfi, Citation1967; Péterfi & Momeu, Citation1977; Péterfi et al., Citation2002, Citation2005), Czech Republic (Nemcova et al., Citation2002, Citation2003; Škaloud et al., Citation2013a), Denmark (Asmund, Citation1959; Kristiansen, Citation1988; Hansen et al., Citation1993; Skogstad & Kristiansen, Citation1996), Portugal (Calado & Craveiro, Citation1995; Santos et al., Citation1996), Germany (Dürrschmidt, Citation1984; Gutowski, Citation1997), Finland (Hällfors & Hällfors, Citation1988), UK (Harris & Bradley, Citation1957, Citation1960), the Netherlands (Roijackers, Citation1981; Roijackers & Kessels, Citation1981, Citation1986; Suykerbuyk et al., Citation1995), Norway (Skogstad, Citation1984) and Sweden (Cronberg & Kristiansen, Citation1980). Sites from these records range east to the Russian Polar Urals (Voloshko, Citation2010).
Table 1. Records of Mallomonas intermedia from European localities, including the pH, conductivity and temperature taken at the time of collection. Records from this study are in bold.
In Europe, records of M. intermedia reflect, in part, sampling effort with the species being reported in almost every country where an extensive chrysophyte investigation has occurred (). M. intermedia has been reported in a variety of different types of water bodies, including acidic peat-bogs, natural lakes, artificial eutrophic ponds and shallow temporary ditches and pools. However, the species favours alluvial pools and oxbows episodically connected to rivers (Roijackers & Kessels, Citation1986; Nemcova et al., Citation2003; Škaloud et al., Citation2013a). M. intermedia thrives in a wide range of environmental conditions; pH range: 5.2–8.5 (median value 6.65, n=40), conductivity range 30–530 μS cm–1 (median value 235, n=33). It has been predominantly found in the colder winter and spring months and Roijackers & Kessels (Citation1986) classified M. intermedia as a cold-water species. However, the species has also been found in warmer environments of about 22°C (Santos et al., Citation1996; Škaloud et al., Citation2013a).
Discussion
Based on morphological features used to distinguish the European endemic, M. intermedia, there is no doubt that it is present today in the small Nevada high desert pond, Dufurrena Pond 19. Scale and bristle morphology of the Nevada population match those from European sites, especially in the suite of characters that specifically distinguish the species. The single large transverse rib just behind the dome on the shield is a hallmark feature of M. intermedia (Skogstad, Citation1984; Kristiansen, Citation2002). The presence of long serrated body bristles bearing lance-tipped apices is another distinguishing character (Asmund, Citation1959; Harris & Bradley, Citation1960; Kristiansen, Citation2002). In the case of the Nevada population, other features including scale shape and size, shield and flange ornamentation, size and distribution of base plate pores, presence of domeless scales lacking the transverse shield rib and smaller anterior-placed serrated bristles lacking lance-tips all match the description of M. intermedia. These characters also match the populations of M. intermedia from the Norwegian and Romanian sites. The series of short ribs originating from the prominent transverse rib (Nemcova et al., Citation2002) or the V-rib (Péterfi et al., Citation1998) on scales from European populations is also found on scales from the Dufurrena Pond 19 population. In addition, secondary ribbing on the shield is present on scales from European populations of M. intermedia (Harris & Bradley, Citation1960; Hällfors & Hällfors, Citation1988; Péterfi et al., Citation1998). In summary, ultrastructural characters of scales and bristles of the Dufurrena Pond 19 population clearly confirm the presence of M. intermedia in North America.
The number of dome ribs on M. intermedia scales from Dufurrena Pond 19 was highly variable and some scales had more dome ribs than often reported for this species. Dome ribs are a common feature of M. intermedia (Kristiansen, Citation2002) and easily observed on scales from many populations (e.g. Harris & Bradley, Citation1960; Santos et al., Citation1996). However, the series of parallel dome ribs are thin and the total number can be difficult to discern on the sloped portions of the dome based on TEM images, and even on some SEM images, resulting in the ribs sometimes being described as ‘inconspicuous’ (Kristiansen, Citation2002). Still, European populations with three to five ribs per dome have been previously reported (Calado & Craveiro, Citation1995), and the number of dome ribs on scales from the Røertjernet Pond population ranged from 2–8, with a mean of 5. The number of ribs on the Nevada population was also highly variable, ranging from 2–12, with the majority of scales possessing 4–6 ribs per dome and a mean number of 5.5, similar to the Røertjernet Pond population.
The number of dome ribs is variable on other species of Mallomonas (Siver, Citation1991; Kristiansen, Citation2002). For example, the number of dome ribs on scales of M. striata, M. paludosa, M. sphagniphila and M. duerrschmidtiae is quite variable and these structures can even be lacking on some scales (Siver, Citation1991; Kristiansen, Citation2002). Further, Kristiansen (Citation2002) noted the range of dome ribs for M. asmundiae (Wujek & Van der Veer) Nicholls, another species with lance-tipped bristles (Siver et al., Citation2009), to be highly variable at 6–18. It is possible that this secondary scale feature is related to growth conditions, especially silica availability. Although no data are available, silica concentrations in Dufurrena Pond 19 are likely to be at, or even above, saturating conditions given features of the immediate landscape. The Virgin Valley region of Nevada where the pond is situated is surrounded by numerous outcrops of siliceous-rich minerals, including an extensive freshwater Miocene diatomite deposit. In addition, the Virgin Valley is rich in opal mineraloids and the terrain immediately surrounding the pond contained significant silica-based minerals eroded from the surrounding hills and tables. High silica concentrations are probably responsible for the richly developed scales found within the extensive Nevada population.
The most intriguing aspect of discovering M. intermedia in western North America concerns the biogeography and endemic status of this organism. The distribution and ultimate biogeographic pattern exhibited by a microscopic alga is the result of a complex set of variables, including environmental and habitat constraints, dispersal mechanisms and processes, transportability of the organisms, historical factors and geological considerations (Kristiansen, Citation1996, Citation2001; Potapova & Charles, Citation2002; Vyverman et al., Citation2007; Brodie et al., Citation2009; Verleyen et al., Citation2009; Boo et al., Citation2010; Siver & Lott, Citation2012). According to the Baas–Becking ubiquity hypothesis (Baas-Becking, Citation1934), microscopic algae would be readily dispersed across continents (e.g. everywhere) and found in habitats that support growth and survival (Finlay & Clarke, Citation1999; Finlay, Citation2002). In other words, microbes have cosmopolitan dispersal capabilities and the resulting biogeographic pattern of any microbe should simply reflect the distribution of suitable habitat (Fenchel & Finlay, Citation2004).
Multiple studies have concluded that the ubiquity hypothesis does not apply to many synurophytes (Boo et al., Citation2010; Siver & Lott, Citation2012) and lacustrine diatoms (Vyverman et al., Citation2007; Vanormelingen et al., Citation2008). Numerous species representing these siliceous algal clades display distinct biogeographic distributions that are determined, in part, by dispersal limitations (Vanormelingen et al., Citation2008; Brodie et al., Citation2009; Verleyen et al., Citation2009), historical variables (Siver & Lott, Citation2012) and geographic restrictions (Potapova & Charles, Citation2002). However, the ubiquity hypothesis may apply to some synurophytes with tropical distributions limited by temperature, but is very problematic for species found in temperate regions, especially those restricted to one hemisphere (Řezáčová & Neustupa, Citation2007).
Until now a strong case was made for classifying M. intermedia as endemic to Europe, based on numerous reports of this taxon from European sites (). Findings from the Horsefly locality clearly show that it was present in North America during the middle Eocene, supporting this species as a palaeoendemic. Without further fossil evidence, the extent of the historical distribution of M. intermedia is unknown. It is possible that this taxon was widely distributed in both Europe and North America during the warm Eocene and able to migrate over the ice-free polar region, then retracted to more southern latitudes later in the Cenozoic with cooling temperatures and initiation of permanent ice, eventually becoming, or remaining, well established across Europe. Under this hypothesis, the question is whether M. intermedia also survived in areas of North America, and has been overlooked due to inadequate sampling efforts or simply undetected due to low cell numbers? The question also remains, is the Nevada population a remnant of this historically wider geographic distribution, or is it a recent arrival reintroduced to North America by humans or some other vector? Additional fossil evidence, coupled with a more systematic sampling effort, would aid in solving these questions.
M. intermedia had never been detected in the hundreds of sites previously examined in North America for synurophytes (see studies in Siver, Citation2015a). Its presence in Dufurrena Pond 19 is especially intriguing given its remote location. Although human interaction with the pond is limited, it is a refuge for migrating waterfowl and herds of mule deer and pronghorn antelope. While the deer and antelope herds remain largely restricted to the immediate region, migrating waterfowl could have transported M. intermedia to the pond from significantly further distances. Many waterfowl migrate through this region to and from points as far south as Central and South America as part of the Pacific migration flyway (Mead, Citation1983). Given the fact that Dufurrena Pond 19 is man-made, and assuming M. intermedia was not recently transported to this remote site from Europe, there is a high probability that active populations of this taxon exist in other localities in North America or points further south.
A similar situation was recently reported for the endemic species, M. pseudocoronata, a common element of North American waterbodies (Siver & Lott, Citation2012) recently found proliferating in lakes in Sweden (Cronberg, Citation2010). M. pseudocoronata scales are large, highly distinctive and easily detected with light microscopy (Siver et al., Citation1990; Siver, Citation1991; Kristiansen, Citation2002). The fact that many of the waterbodies where M. pseudocoronata was recently recorded in Sweden had been monitored for years by Cronberg herself, who is a world expert in synurophyte taxonomy, supports the hypothesis that M. pseudocoronata was only recently introduced to this region of Sweden. It is also possible that M. pseudocoronata was historically present in Europe, but rare and undetected until its recent proliferation in Sweden. In fact, the status of M. pseudocoronata as a North American endemic came into question when it was discovered in sediments from an Austrian lake (Smol, Citation1988) and living in a remote pond in Arctic Russia (Siver et al., Citation2005). In a similar fashion, a species considered endemic to Australia, M. alphaphora, was recently discovered in a suite of waterbodies in the Aquitaine region of France (Nemcova et al., Citation2012). Unlike M. intermedia and M. pseudocoronata, both of which were common elements in numerous sites from Europe and North America, respectively, M. alphaphora had only been observed in two Australian waterbodies (Preisig, Citation1989; Furlotte et al., Citation2000) and was perhaps prematurely granted endemic status.
Several fossil species uncovered from the Giraffe Pipe locality, another middle Eocene deposit situated near the Arctic Circle in northern Canada, provide further evidence that additional taxa closely related to M. intermedia were also historically present in North America (Siver et al., Citation2009). Although scales of the fossil species M. lancea differ from those of M. intermedia in a number of characters, they also possess a single, prominent, transverse rib on the shield at the base of the dome, and both species have similar serrated bristles with lance-shaped apices documenting a very close relationship (Siver et al., Citation2009). Scales of another extinct taxon from the Giraffe locality, M. dispar, also possess a single prominent transverse shield rib at the base of the dome, reminiscent of M. intermedia, but the bristles terminate with a structure referred to as an asymmetric helmet. Since the folded portion of an asymmetrical helmet structure is homologous with the fold forming the lance apex, Siver et al. (Citation2009) concluded that M. dispar and M. intermedia probably shared a common ancestor. The presence of M. intermedia, M. lancea and M. dispar in North America during the middle Eocene confirms that historically this lineage was not restricted to Europe.
The fossil remains of M. intermedia found in Horsefly, and other close relatives in Giraffe Pipe, are of particular interest since these fossil localities represent a significant warm period in Earth history. The onset of the Eocene epoch was marked by an abrupt and significant warming event known as the Palaeocene–Eocene thermal maximum at 55 Ma (Schmitz & Paujalte, Citation2007; Zachos et al., Citation2008). Much of the ensuing Eocene, including the time-periods covering the existence of the waterbodies at Horsefly and Giraffe Pipe, experienced very warm temperatures that were significantly higher than today, with maximum values observed during the Early Eocene Climate Optimum (EECO) at 52–50 Ma and another smaller peak near 42 Ma (Zachos et al., Citation2008; Wolfe et al., Citation2017). During the warmest part of the Eocene, the polar regions were ice-free (Zachos et al., Citation2001, Citation2008) and lush forests were found on land masses near the north pole (Hickey et al., Citation1983; McIver & Basinger, Citation1999; Jahren, Citation2007; Greenwood et al., Citation2010). Higher rates of precipitation, coupled with elevated runoff, yielded a significantly fresher Arctic Ocean capable of supporting massive populations of the aquatic fern Azolla and other freshwater organisms, including chrysophytes (Brinkhuis et al., Citation2006). In addition, North America was much closer to Greenland, and Greenland to Europe, than today (Brinkhuis et al., Citation2006). It is highly probable that microbes, including members of the M. intermedia lineage, were more easily transported between North America and Europe during this warm period.
Additional records from the Giraffe Pipe locality support the hypothesis that aquatic organisms were transported long distances during or after the warm Eocene. Siver & Wolfe (Citation2009) demonstrated the presence of other synurophyte and diatom species in Giraffe Pipe whose nearest extant are relatives largely restricted to subtropical and tropical localities, and Pisera et al. (Citation2013) uncovered remains of Potamolaepid sponges that today are distributed almost exclusively in tropical regions of the world. Remains of M. bangladeschica in the Arctic Giraffe Pipe locality, an organism distributed today in warm tropical and subtropical regions, confirm that this taxon was also much more widely distributed, and like M. intermedia is best described as a palaeoendemic (Siver & Wolfe, Citation2009).
Differences in ultrastructural features of siliceous scale and bristle components have formed a solid basis for distinguishing species within the Synurophyceae (Kristiansen, Citation2005; Siver, Citation2015a). Morphologically based concepts for distinguishing between taxa have largely been confirmed using molecular techniques (e.g. Jo et al., Citation2013; Škaloud et al., Citation2013b, Citation2014). Organisms placed into specific sections of the genus, based on similar scale and bristle characteristics, have been confirmed with molecular data. Differences in morphological features, including the presence or absence of a V-rib and bristle type, correspond to the two major clades uncovered within the genus based on molecular data (Jo et al., Citation2013; Siver et al., Citation2015). Some morphospecies have been found to represent suites of cryptic species, but in most cases a re-evaluation of the morphological features usually results in fine-scale differences that corroborate gene-sequence data (Jo et al., Citation2013; Siver et al., Citation2015). For example, Jo et al. (Citation2013) found differences in the patterns of base plate pores on the scale to align with gene data. Thus, organisms with similar suites of ultrastructural characters, including closely related cryptic species, have clustered together in synurophyte-based molecular phylogenies. To date, there are no strains of M. intermedia included in molecular works. Moving forward, it will be interesting to compare gene sequences with morphological features between multiple strains of M. intermedia, including ones from Europe and North America, to better understand fine-scale relationships between taxa. A richer understanding of relationships between morphological and molecular traits within the synurophyte lineage will enhance the use of the fossil record in tracking historical biogeographic and biodiversity patterns and further unravel the evolutionary history of the group.
In summary, given the active population in Nevada, M. intermedia can no longer be considered endemic to Europe. Further, since this organism was present in North America during the Eocene over 44 million years ago, status as a palaeoendemic is more appropriate. Still, the open question is whether additional populations of M. intermedia remain undetected in remote regions of North America, or if the Dufurrena Pond 19 population represents a recent arrival, transported to the pond by an unknown vector.
Author contributions
P.A. Siver: original concept, drafting and editing manuscript, field work, fossil analysis, microscopy; A. Skogstad: editing manuscript, field work, coring, microscopy; Y. Nemcova: editing manuscript, field work, microscopy.
Acknowledgements
We dedicate this paper to our colleague and close friend, Dr Jorgen Kristiansen (University of Copenhagen) for his numerous works on the biodiversity and biogeography of synurophytes. We thank Anne Lott (Connecticut College) for help with sample preparation, Xuanhao Sun (University of Connecticut) for assistance with the SEM facilities, Alex Wolfe (University of Alberta) for securing samples from the Horsefly sequence, Magda Škaloudová (Charles University) for help with the Cocor Lake sample, George Mustoe (Western Washington University) for field collecting advice, and the Dept. of Biosciences, University of Oslo, for use of EM-laboratory facilities and coring equipment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Archibald, S.B., Basinger, J.F., Greenwood, D.R., Mathewes, R.W. & Wilson, M.V.H. (2005). Significance of the Horsefly Fossil Site, British Columbia. British Columbia Paleontological Alliance, British Columbia.
- Archibald, S.B., Morse, G.E., Greenwood, D.R. & Mathewes, R.W. (2014). Fossil palm beetles refine upland winter temperatures in the Early Eocene Climatic Optimum. Proceedings of the National Academy of Sciences USA, 111: 8095–8100.
- Arseneau, K.M.A., Driscoll, C.T., Cummings, C.M., Pope, G. & Cumming, B.F. (2016). Adirondack (NY, USA) reference lakes show a pronounced shift in chrysophyte species composition since ca. 1900. Journal of Paleolimnology, 56: 349–364.
- Asmund, B. (1959). Electron microscope observations on Mallomonas species and remarks on their occurrence in some Danish ponds and lakes III. Dansk Botanisk Arkiv, 18: 1–50.
- Baas-Becking, L.G.M. (1934). Geobiologie of Inleding tot de Milieukunde. W.P. Van Stockum & Zoon N.V., Den Haag.
- Barreto, S. (2001). Three new Mallomonas species (Synurophyceae) from a mountain area in North-Hungary. Nordic Journal of Botany, 21: 437–447.
- Barreto, S. (2005). The silica-scaled chrysophyte flora of Hungary. Nova Hedwigia Beiheft, 128: 11–41.
- Boo, S.M., Kim, H.S., Shin, W., Boo, G.H., Cho, S.M., Jo, B.Y., Siver, P.A., Wolfe, A.P., Bhattacharya, D., Andersen, R.A. & Yoon, H.S. (2010). Complex phylogeographic patterns in the freshwater alga Synura provide new insights into ubiquity vs. endemism in microbial eukaryotes. Molecular Ecology, 19: 4328–4338.
- Brinkhuis, H.S., Schouten, M.E., Collinson, A., Sluijs, A., Sinninghe Damsté, J.S., Dickens, G.R., Huber, M., Cronin, T., Onodera, J., Takahashi, K., Bujak, J.P., Stein, R., van der Burgh, J., Eldrett, J.S., Harding, I.C., Lotter, A.F., Sangiorgi, F., Zijlstra, G., van Konijnenburg-van Cittert, H., de Leeuw, J.W., Matthiessen, J., Backman, J., Moran, K. & the Expedition 302 Scientists. (2006). Episodic fresh surface waters in the Eocene Arctic Ocean. Nature, 441: 606–609.
- Brodie, J., Andersen, R.A., Kawachi, M. & Millar, A.J.K. (2009). Endangered algal species and how to protect them. Phycologia, 48: 423–438.
- Calado, A. & Craveiro, S. (1995). Notes on the ecology of Synurophycean algae found in Portugal. Nordic Journal of Botany, 15: 641–654.
- Cronberg, G. (2010). Mallomonas pseudocoronata Prescott (Synurophyceae), a recent arrival in Scania, southern Sweden? Nova Hedwigia Beiheft, 136: 139–151.
- Cronberg, G. & Kristiansen, J. (1980). Synuraceae and other Chrysophyceae from central Småland, Sweden. Botaniska Notiser, 133: 595–618.
- Dürrschmidt, M. (1984). Studies on scale-bearing Chrysophyceae from the Giessen area, Federal Republic of Germany. Nordic Journal of Botany, 4: 123–143.
- Fenchel, T. & Finlay, B.J. (2004). Here and there or everywhere? Response from Fenchel and Finlay. Bioscience, 54: 884–885.
- Finlay, B.J. (2002). Global dispersal of free-living microbial eukaryote species. Science, 296: 1061–1063.
- Finlay, B.J. & Clarke, K.J. (1999). Ubiquitous dispersal of microbial species. Nature, 400: 828.
- Furlotte, A., Ferguson, J.A. & Wee, J.L. (2000). A floristic and biogeographic survey of the Synurophyceae from Southeastern Australia. Nordic Journal of Botany, 20: 247–256.
- Greenwood, D.R., Archibald, S.B., Mathewes, R.W. & Moss, P.T. (2005). Fossil biotas from the Okanagan Highlands, southern British Columbia and northeastern Washington State: climates and ecosystems across an Eocene landscape. Canadian Journal of Earth Sciences, 42: 167–185.
- Greenwood, D.R., Basinger, J.F. & Smith, R.Y. (2010). How wet was the Arctic Eocene rain forest? Estimates of precipitation from Paleogene Arctic macrofloras. Geology, 38: 15–18.
- Gutowski, A. (1997). Mallomonas species (Synurophyceae) in eutrophic waters of Berlin (Germany). Nova Hedwigia, 65: 299–335.
- Hällfors, G. & Hällfors, S. (1988). Records of chrysophytes with siliceous scales (Mallomonadaceae and Paraphysomonadaceae) from Finnish inland waters. In Flagellates in Freshwater Ecosystems (Jones, R. & Ilmavirta, V., editors), 1–29. Kluwer Academic, Dordrecht.
- Hansen, A.F., Johansen, J.E. & Skovgard, A. (1993). Floristik undersøgelse af skælbærende gulalger i Dyrehaven. Urt, 17: 13–18.
- Harris, K. (1953). A contribution to our knowledge of Mallomonas. Botanical Journal of the Linnean Society, 55: 88–102.
- Harris, K. & Bradley, D.E. (1957). An examination of the scales and bristles of Mallomonas in the electron microscope using carbon replicas. Journal of the Royal Microscopical Society, 76: 37–46.
- Harris, K. & Bradley, D.E. (1960). A taxonomic study of Mallomonas. Journal of General Microbiology, 22: 750–777.
- Hickey, L.J., West, R.M., Dawson, M.R. & Choi, D.K. (1983). Arctic terrestrial biota: paleomagnetic evidence of age disparity with mid-northern latitudes during the Late Cretaceous and early Tertiary. Science, 221: 1153–1156.
- Jahren, A.H. (2007). The Arctic forest of the Middle Eocene. Annual Review of Earth and Planetary Sciences, 35: 509–540.
- Jo, B.Y., Shin, W., Kim, H.S., Siver, P.A. & Andersen, R.A. (2013). Phylogeny of the genus Mallomonas (Synurophyceae) and descriptions of five new species on the basis of morphological evidence. Phycologia, 52: 266–278.
- Kristiansen, J. (1988). Seasonal occurrence of silica-scaled chrysophytes under eutrophic conditions. Hydrobiologia, 161: 171–184.
- Kristiansen, J. (1996). Dispersal of freshwater algae – a review. Hydrobiologia, 336: 151–157.
- Kristiansen, J. (2001). Biogeography of silica-scaled chrysophytes. Nova Hedwigia Beiheft, 122: 23–39.
- Kristiansen, J. (2002). The genus Mallomonas (Synurophyceae): a taxonomic survey based on the ultrastructure of silica scales and bristles. Opera Botanica, 139: 1–218.
- Kristiansen, J. (2005). Golden Algae: A Biology of Chrysophytes. A. R. G. Gantner Verlag, K.G., Ruggell.
- Kristiansen, J. (2008). Dispersal and biogeography of silica-scaled chrysophytes. Biodiversity and Conservation, 17: 419–426.
- Kristiansen, J. & Lind, J.F. (2005). Endemicity in silica-scaled chrysophytes. Nova Hedwigia Beiheft, 128: 65–83.
- Marsicano, L.J. & Siver, P.A. (1993). A paleolimnological assessment of lake acidification in five Connecticut lakes. Journal of Paleolimnology, 9: 209–221.
- McIver, E.E. & Basinger, J.F. (1999). Early Tertiary floral evolution in the Canadian high arctic. Annals of the Missouri Botanical Garden, 86: 523–545.
- Mead, C. (1983). Bird Migration. Country Life Books, Feltham.
- Nemcova, Y., Neustupa, J., Novakova, S. & Kalina, T. (2002). Silica-scaled chrysophytes of the Sumava National Park and the Trebonsko UNESCO Biosphere Reserve (Southern Bohemia, Czech Republic). Nordic Journal of Botany, 22: 375–383.
- Nemcova, Y., Neustupa, J., Novakova, S. & Kalina, T. (2003). Silica-scaled chrysophytes of the Czech Republic. Acta Universitatis Carolinae. Biologica, 47: 285–346.
- Nemcova, Y., Kreidlova, J., Kosova, A. & Neustupa, J. (2012). Lakes and pools of Aquitaine region (France) – a biodiversity hotspot of Synurales in Europe. Nova Hedwigia, 95: 1–24.
- Péterfi, L.S. (1967). Studies on the Rumanian Chrysophyceae (I). Nova Hedwigia, 13: 117–137.
- Péterfi, L.S. & Momeu, L. (1977). Observations on some Mallomonas species from Romania in light and transmission electron microscopes. Nova Hedwigia, 28: 155–202.
- Péterfi, L.S., Momeu, L., Padisák, J. & Varga, V. (1998). Silica-scaled chrysophytes from permanent and temporary waters of Hortobágy, Eastern Hungary. Hydrobiologia, 369/370: 339–351.
- Péterfi, L.S., Momeu, L., Padisák, J. & Torok, A.R. (2002). Silica-scaled flagellates (Synurophyceae) of the “Mestecănişu de la Reci”. Covasna County, Transylvania, Romania. Contribuţii Botanice, 37: 113–118.
- Péterfi, L.S., Momeu, L., Kiss, K.T. & Acs, E. (2005). Recent occurrence of Mallomonas intermedia Kisselev (Synurophyceae, Chrysophyta) in Transylvania (Romania), based on scanning electron microscopy. Acta Botanica Hungarica, 47: 145–149.
- Pisera, A., Siver, P.A. & Wolfe, A.P. (2013). A first account of freshwater potamolepid sponges (Demospongiae, Spongillina, Potamolepidae) from the middle Eocene: biogeographic and paleoclimatic implications. Journal of Paleontology, 87: 373–378.
- Potapova, M.G. & Charles, D.F. (2002). Benthic diatoms in USA rivers: distributions along spatial and environmental gradients. Journal of Biogeography, 29: 167–187.
- Preisig, H.R. (1989). Mallomonas alphaphora (Chrysophyceae): a new species from Western Australia. Plant Systematics and Evolution, 164: 209–214.
- Řezáčová, M. & Neustupa, J. (2007). Distribution of the genus Mallomonas (Synurophyceae) – ubiquitous dispersal in microorganisms evaluated. Protist, 158: 29–37.
- Roijackers, R.M.M. (1981). Chrysophyceae from freshwater localities near Nijmegen, the Netherlands. Hydrobiologia, 76: 179–189.
- Roijackers, R.M.M. & Kessels, H. (1981). Chrysophyceae from freshwater localities near Nijmegen, the Netherlands. II. Hydrobiologia, 80: 231–239.
- Roijackers, R.M.M. & Kessels, H. (1986). Ecological characteristics of scale-bearing Chrysophyceae from the Netherlands. Nordic Journal of Botany, 6: 373–385.
- Rujoiu-Mare, M.R., Olariu, B., Mihai, B.A. (2015). Space planning in Prahova Subcarpathians, Romania. Landslides – land cover relationship analysis. Cinq Continents, 6: 21–41.
- Santos, L.M.A., Calado, A. & Craveiro, S. (1996). Silica-scaled chrysophytes from three alpha-mesosaprobic water-bodies of central Portugal. Nova Hedwigia Beiheft, 114: 171–191.
- Schmitz, B. & Paujalte, V. (2007). Abrupt increase in seasonal extreme precipitation at the Paleocene-Eocene boundary. Geology, 35: 215–218.
- Siver, P.A. (1991). The Biology of Mallomonas: Morphology, Taxonomy and Ecology. Kluwer Academic Publishers, Dordrecht.
- Siver, P.A. (1993). Inferring lakewater specific conductivity with scaled chrysophytes. Limnology and Oceanography, 38: 1480–1492.
- Siver, P.A. (2015a). Synurophyte algae. In Freshwater Algae of North America. 2nd ed. (Wehr, J.D., Sheath, R.G. & Kociolek, J.P., editors), 607–655. Academic Press, San Diego.
- Siver, P.A. (2015b). Mallomonas schumachii sp. nov., a fossil synurophyte bearing large scales described from an Eocene maar lake in Northern Canada. Nova Hedwigia, 101: 285–298.
- Siver, P.A. (2018). Mallomonas aperturae sp. nov. (Synurophyceae) reveals that the complex cell architecture observed on modern synurophytes was well established by the middle Eocene. Phycologia, 57: 273–279.
- Siver, P.A. & Glew, J.R. (1990). The arrangement of scales and bristles on Mallomonas (Chrysophyceae), a proposed mechanism for the formation of the cell covering. Canadian Journal of Botany, 68: 374–380.
- Siver, P.A. & Lott, A.M. (2012). Biogeographic patterns in scaled chrysophytes from the east coast of North America. Freshwater Biology, 57: 451–467.
- Siver, P.A. & Wolfe, A.P. (2005). Eocene scaled chrysophytes with pronounced modern affinities. International Journal of Plant Sciences, 166: 533–536.
- Siver, P.A. & Wolfe, A.P. (2009). Tropical ochrophyte algae from the Eocene of northern Canada: a biogeographic response to past global warming. Palaios, 24: 192–198.
- Siver, P.A., Hamer, J. & Kling, H. (1990). Separation of Mallomonas duerrschmidtiae sp. nov. from M. crassisquama and M. pseudocoronata: implications for paleolimnological research. Journal of Phycology, 26: 728–740.
- Siver, P.A., Voloshko, L.N., Gavrilova, O.V. & Getsen, M.V. (2005). The scaled chrysophyte flora of the Bolshezemelskaya tundra (Russia). Nova Hedwigia Beiheft, 128: 125–150.
- Siver, P.A., Lott, A.M. & Wolfe, A.P. (2009). Taxonomic significance of asymmetrical helmet and lance bristles in the genus Mallomonas (Synurophyceae) and their discovery in Eocene lake sediments. European Journal of Phycology, 44: 447–460.
- Siver, P.A., Jo, B.Y., Kim, J.I., Shin, W., Lott, A.M. & Wolfe, A.P. (2015). Assessing the evolutionary history of the class Synurophyceae (Heterokonta) using molecular, morphometric, and paleobiological approaches. American Journal of Botany, 102: 1–21.
- Škaloud, P., Škaloudová, M., Pichrtová, M., Němcová, Y., Kreidlová, J. & Puzstai, M. (2013a). www.chrysophytes.eu – a database on distribution and ecology of silica-scaled chrysophytes in Europe. Nova Hedwigia Beiheft, 142: 141–146.
- Škaloud, P., Kristiansen, J. & Škaloudová, M. (2013b). Developments in the taxonomy of silica-scaled chrysophytes – from morphological and ultrastructural to molecular approaches. Nordic Journal of Botany, 31: 385–402.
- Škaloud. P., Škaloudová, M., Procházková, A. & Němcová, Y. (2014). Morphological delineation and distribution patterns of four newly described species within the Synura petersenii species complex (Chrysophyceae, Stramenopiles). European Journal of Phycology, 49: 213–229.
- Skogstad, A. (1984). Vegetative cells and cysts of Mallomonas intermedia (Mallomonadaceae, Chrysophyceae. Nordic Journal of Botany, 4: 275–278.
- Skogstad, A. & Kristiansen, J. (1996). Mallomonas lychenensis f. symposiaca f. nov. (Mallomonadaceae, Synurophyceae). Nova Hedwigia Beiheft, 114: 81–89.
- Smol, J.P. (1988). The North American endemic Mallomonas pseudocoronata (Mallomonadaceae, Chrysophyta) in an Austrian lake. Phycologia, 27: 427–429.
- Stevenson, R.J. & Smol, J.P. (2015). Use of algae in ecological assessments. In Freshwater Algae of North America. 2nd ed. (Wehr, J.D., Sheath, R.G. & Kociolek, J.P., editors), 921–962. Academic Press, San Diego.
- Suykerbuyk, R.E.M., Roijackers, R.M.M. & Houtman, S.S.J. (1995). Ecological characteristics of scale-bearing chrysophytes in a small eutrophic pond in the Netherlands. Nordic Journal of Botany, 15: 665–676.
- Vanormelingen, P., Verleyen, E. & Vyverman, W. (2008). The diversity and distribution of diatoms: from cosmopolitanism to narrow endemism. Biodiversity and Conservation, 17: 393–405.
- Verleyen, E., Vyverman, W., Sterken, M., Hodgson, D.A., De Wever, A., Juggins, S., Van de Vijver, B., Jones, V., Vanormelingen, P., Roberts, D., Flower, R., Kilroy, C., Souffreau, C. & Sabbe, K. (2009). The importance of dispersal related and local factors in shaping the taxonomic structure of diatom metacommunities. Oikos, 118: 1239–1249.
- Voloshko, L.N. (2010). The chrysophycean algae from glacial lakes of Polar Ural (Russia). Nova Hedwigia Beiheft, 136: 191–211.
- Vyverman, W., Verleyen, E., Sabbe, K., Vanhoutte, K., Sterken, M., Hodgson, D. A., Mann, D.G., Juggins, S., Van de Vijver, B., Jones, V., Flower, R., Roberts, D., Chepurnov, V.A., Kilroy, C., Vanormelingen, P. & De Wever, A. (2007). Historical processes constrain patterns in global diatom diversity. Ecology, 88: 1924–1931.
- Wilson, M.V.H. (1977). Paleoecology of Eocene lacustrine varves at Horsefly, British Columbia. Canadian Journal of Earth Sciences, 14: 953–962.
- Wilson, M.V.H. & Barton, D.G. (1996). Seven centuries of taphonomic variation in Eocene freshwater fishes preserved in varves: paleoenvironments and temporal averaging. Paleobiology, 22: 535–542.
- Wolfe, A.P. & Edlund, M.B. (2005). Taxonomy, phylogeny, and paleoecology of Eoseira wilsonii gen. et sp. nov., a middle Eocene diatom (Bacillariophyceae: Aulacoseiraceae) from lake sediments at Horsefly, British Columbia, Canada. Canadian Journal of Earth Sciences, 42: 243–257.
- Wolfe, A.P., Reyes, A.V., Royer, D.L., Greenwood, D.R., Doria, G., Gagen, M., Siver, P.A. & Westgate, J.A. (2017). Middle Eocene CO2 and climate reconstructed from the sediment fill of a subarctic kimberlite maar. Geology, 45: 619–622.
- Zachos, J.C., Pagani, M., Sloan, L., Thomas, E. & Billups, K. (2001). Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292: 686–693.
- Zachos, J.C., Dickens, G.R. & Zeebe, R.E. (2008). An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature, 451: 279–283.
