ABSTRACT
Antioxidant enzymes are essential proteins that maintain cell proliferation potential by protecting against oxidative stress. They are present in many organisms including harmful algal bloom (HAB) species. We previously identified the antioxidant enzyme 2-Cys peroxiredoxin (PRX) in the raphidophyte Chattonella marina. This enzyme specifically decomposes a hydrogen peroxide (H2O2). PRX is the only antioxidant enzyme so far identified in C. marina. This study used mRNA-seq, using Trinity assemble and blastx for annotation, to identify a further five antioxidant enzymes from C. marina: Cu Zn superoxide dismutase (Cu/Zn-SOD), glutathione peroxidase (GPX), catalase (CAT), ascorbate peroxidase (APX) and thioredoxin (TRX). In the gene expression analysis of six enzymes (Cu/Zn-SOD, GPX, CAT, APX, TRX and PRX) using light-acclimated (100 μmol photons m−2 s−1) C. marina cells, only PRX gene expression levels were significantly increased by strong light irradiation (1000 μmol photons m−2 s−1). H2O2 concentration and scavenging activity were also increased and significantly positively correlated with PRX gene expression levels. In dark-acclimated cells, expression levels of all antioxidant enzymes except APX were significantly increased by light irradiation (100 μmol photons m−2 s−1). Expression decreased the following day, with the exception of PRX expression. With the exception of CAT, gene expression of antioxidant enzymes was not significantly induced by artificial H2O2 treatment, although average gene expression levels were slightly increased in some enzymes. Thus, we suggest that light is the main trigger of gene expression, but the resultant oxidative stress is also a possible factor affecting the gene expression of antioxidant enzymes in C. marina.
Introduction
The raphidophyte Chattonella marina (Subrahmanyan) Hara & Chihara is a harmful algal bloom (HAB) species, which is distributed widely in coastal areas of temperate and subtropical zones and is responsible for mass mortality of aquaculture fish. Over the last few decades, Chattonella spp. have caused serious economic losses: approximately 7.1 billion yen in 1972 in Harima-nada, Japan (Okaichi & Yanagi, Citation1997), and 2.9 billion yen in 2009 and 5.3 billion yen in 2010 in the Yatsushiro Sea (Onitsuka et al., Citation2011).
Bloom formation of C. marina is determined by factors such as algal growth, predation pressure and physical transport processes (Giddings et al., Citation2014; Pinto et al., Citation2016). Among these factors, algal growth is fundamental to bloom formation, therefore many laboratory (e.g. Amano et al., Citation1998; Marshall & Hallegraeff, Citation1999) and field (e.g. Katano et al., Citation2014; Satta et al., Citation2017) studies have investigated environmental influences on C. marina growth. Recently, Qiu et al. (Citation2013) reported that C. marina can grow under severe environmental conditions, such as strong irradiance of around 1000 µmol photons m–2 s–1. In general, excessive irradiance is harmful to photosynthetic organisms as it causes the production of high levels of reactive oxygen species (ROS). This characteristic of C. marina suggests that it has a high physiological ability to resist light-induced oxidative stress during growth, allowing it to form frequent dense blooms in midsummer in western Japan. However, to date, there are few molecular biological studies (e.g. Kevin et al., Citation2010) relating to antioxidant activities in HAB species including C. marina.
Recently, the link between antioxidant enzymes and stress tolerance has attracted attention in a broad range of organisms including photosynthetic species. Miao et al. (Citation2006) reported that, relative to the wild-type strain, a glutathione peroxidase (GPX) mutant in Arabidopsis thaliana exhibited a higher rate of water loss under drought stress, higher sensitivity to hydrogen peroxide (H2O2) treatment during seed germination and seedling development, and enhanced production of H2O2 in guard cells. When inserted into yeast, Chlorella vulgaris genes encoding the antioxidant enzymes peroxiredoxin and NADPH-dependent thioredoxin reductase increased tolerance to freezing, heat and oxidative stresses (Machida et al., Citation2012). Knock-down strains of rice that were missing functional ascorbate peroxidase (APX) isoforms exhibited early senescence in comparison with wild-type strains (Ribeiro et al., Citation2017).
Relative to other unicellular algae, C. marina generates higher amounts of ROS such as the superoxide anion (O2−) and H2O2 (Oda et al., Citation1997; Kevin et al., Citation2010). These higher amounts of ROS attack various cellular molecules, such as DNA, RNA, proteins and lipids (Johnson et al., Citation1981; Halliwell & Gutteridge, Citation1984). It is possible that C. marina has a more advanced system to protect itself from ROS, using antioxidants. Recently, using proteomic analysis, Qiu et al. (Citation2013) found highly expressed 2-cysteine peroxiredoxin (PRX) in C. marina. The abundance of this protein positively correlated with photosynthetic activity and daily growth rate in cultured strains and field samples (Qiu et al., Citation2017). Kevin et al. (Citation2010) reported that the activity of superoxide dismutase (SOD) and catalase (CAT), two other antioxidant enzymes, was highest in early exponential phase. Our previous study also revealed that gene expression of C. marina PRX increased under strong light intensity; cellular H2O2 concentrations also increased under these conditions (Mukai et al., Citation2018). Generally, PRX has a H2O2 scavenging function and is present in organisms from all biological kingdoms (Yamamoto et al., Citation1999; Dietz, Citation2011; Rhee et al., Citation2012). Thus, in C. marina PRX is thought to assist cell proliferation via the elimination of the H2O2 generated during photosynthesis. It may be a key enzyme for the maintenance of growth potential and cell tolerance to oxidative stress.
Antioxidant enzymes found in photosynthetic organisms include SOD, glutathione peroxidase (GPX), CAT, ascorbate peroxidase (APX) and thioredoxin (TRX) (Schurmann & Jacquot, Citation2000; Rodriguez Milla et al., Citation2003; Navrot et al., Citation2006; Mhamdi et al., Citation2010; Miller Citation2012; Ozyigit et al., Citation2016). These antioxidant enzymes have not been identified in C. marina. In addition, with the exception of PRX, changes in expression of antioxidant enzyme genes in response to environmental stress have not been studied in C. marina. This study aimed to identify antioxidant enzyme gene sequences and analyse gene expression responses to environmental stress to evaluate the contribution of antioxidants to the cell physiology of C. marina.
Next-generation sequencing (NGS) is a powerful tool for gene analysis. The technology is used not only in mammals and plants but also in microalgae (Kim et al., Citation2014; Yoshida et al., Citation2016; Poong et al., Citation2018). Furthermore, mRNA-sequencing (mRNA-seq) is applicable for species with no reference sequence using de novo assembly analysis. The present study newly searched for the cDNA sequence of antioxidant enzymes such as Cu/Zn-SOD, GPX, CAT, APX and TRX in C. marina using NGS data. Gene expression responses to light irradiation and H2O2 treatment were analysed using quantitative-PCR (QPCR) to investigate how those antioxidant enzymes increase tolerance to light-induced oxidative stress in C. marina.
Materials and methods
Chattonella marina strains culture
Axenic strains of Chattonella marina (Subrahmanyan) Hara & Chihara var. antiqua (Hada) Demura & Kawachi (NIES-1) were purchased from the National Institute of Environmental Studies (NIES, Tsukuba, Japan) and cultured using modified SWM-3 medium (Yamasaki et al., Citation2007) (pH 7.9, salinity 30 PSU). Strains were cultured at 25°C under 110 µmol photons m–2 s–1 of cool-white fluorescent illumination (NEC, Tokyo, Japan), using a 14-h light:10-h dark (L:D) photoperiod. Light intensity was measured using a Quantum Scalar Laboratory Irradiance Sensor (QSL-2101, Biospherical Instruments, San Diego, California, USA).
To make the modified SWM-3 medium, seawater was collected from the Tsushima Current around Okinoshima Island, Fukuoka Prefecture, Japan. After filtering through a 0.45-µm polycarbonate filter (Merck Millipore Corporation, Darmstadt, Germany), the seawater was stored for more than 1 year at room temperature in the dark. Nutrients were added, then the modified SWM-3 medium was filtered through a 0.45-µm polycarbonate filter and autoclaved at 121°C for 15 min.
mRNA sequencing
Chattonella marina cells in stationary growth phase were collected by centrifugation (810 × g) at room temperature for 10 min. Total RNA was extracted from the algal pellet using the Agilent Plant RNA Isolation Mini Kit (Agilent Technologies, Palo Alto, California, USA). Then, DNase treatment was conducted using an On-Spin Column DNase I Kit (MO BIO, California, USA). Total RNA was converted to double-strand DNA using TruSeq RNA Sample Preparation Kit v3 (Illumina, California, USA). The sequences were read by paired-end sequencing (maximum lead length 2 × 100 bp) using an Illumina HiSeq2500.
Antioxidant enzymes search, prediction of protein localization and phylogenetic analysis
De novo transcriptome assembly was performed using mRNA-seq data according to the protocol of Haas et al. (Citation2013) with no reference sequence of antioxidant enzymes in C. marina. Quality-check of mRNA-seq data was performed using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). To remove the sequence adapter and low quality reads (Q score < 30), trimmomatic (http://www.usadellab.org/cms/page=trimmomatic) was used. Purified reads were assembled using Trinity software v1.3 (https://github.com/trinityrnaseq/trinityrnaseq/wiki). The sequences assembled by Trinity are henceforth called unigenes (Yoshida et al., Citation2016). We used amino acid sequences of antioxidant enzymes (Cu/Zn-SOD, CAT, GPX, APX and TRX) from other photosynthetic organisms, obtained from the National Center for Biotechnology Information search database (NCBI, https://www.ncbi.nlm.nih.gov/), as reference sequences. The reference sequences were: one higher plant (Arabidopsis thaliana), four Chlorophyceae (Chlamydomonas reinhardtii, Chlorella vulgaris, Volvox carteri and Ulva fasciata), one Prasinophyceae (Micromonas pusilla), one Rhodophyceae (Pyropia haitanensis), one diatom (Thalassiosira pseudonana) and three cyanobacteria (Microcystis aeruginosa, Oscillatoriales cyanobacterium and Synechococcus sp.). Homologues of each unigene were searched by blastx in BLAST (https://ftp://ftp.ncbi.nlm.nih.gov/blast/executables/blast+/LATEST/) with an E-value cut-off of 1e–10. Only unigenes having more than 40% homology were selected, and Cu/Zn-SOD, GPX, CAT, APX and TRX were searched. Protein localization of each antioxidant enzyme was predicted using the specialized prediction server HECTAR for heterokonts (Gschloessl et al., Citation2008), ASAFind for stramenopiles (Gruber et al., Citation2015) and Plant-mPLos for plants (Chou & Shen, Citation2010).
Next, each antioxidant enzyme of C. marina was compared with that of other species through molecular phylogenetic analysis using deduced amino acid sequences by a maximum likelihood method, after alignment by CLUSTAL W in MEGA7 (http://www.megasoftware.net/). The reference species were: three higher plants (A. thaliana, Ozyza sativa and Zea mays), three Chlorophyceae (C. reinhardtii, C. vulgaris and V. carteri), three Rhodophyceae (P. haitanensis, Pyropia yezoensis and Porphyra umbilicalis), three diatoms (T. pseudonana, Thalassiosira oceanica and Phaeodactylum tricornutum), one Phaeophyceae (Ectocarpus siliculosus) and Caenorhabditis elegans or Hydra sinensis as out-groups.
Expression levels of antioxidant enzymes under different light intensities by quantitative PCR
Chattonella marina cells were cultured until early stationary phase (15180 cells ml–1) at 25°C under 14-h light:10-h dark (L:D) photoperiod with irradiance of 100 µmol photons m–2 s–1. About 1000 ml cell suspension was divided among 16 flasks (60 ml each, Thermo Fisher Scientific) and cultured under the same light and temperature conditions for 24 h. Then, 12 of the 16 flasks were divided into three groups (4 flasks per group) and transferred to three light conditions: dark, moderate light and strong light (0, 100 and 1000 µmol photons m–2 s–1, respectively), and incubated for 7 days at 25°C under 14-h light:10-h dark (L:D) photoperiod (irradiation time was from 05:00 to 19:00). The remaining four flasks were used as day 0 samples.
During the exposure period, 13.5 ml cell suspension was sampled from each flask between 10:00 and 11:00 on days 0, 1, 3, 5 and 7. Soon after sampling, a portion of each cell suspension sample was used to count cells and determine H2O2 concentration and H2O2 scavenging activity. The cell density was counted using microscopy. The remaining portion of the cell suspension was centrifuged (1800 × g for 10 min), and the cell pellet was stored at –80°C until RNA extraction for antioxidant enzyme analysis by QPCR.
cDNA was synthesized using random primers in the PrimeScriptII 1st strand cDNA Synthesis Kit (TAKARA BIO Inc, Shiga, Japan). Expression level of each antioxidant enzyme was measured using a Mx3000P Multiplex QPCR System (Stratagene, Tokyo, Japan). Primers and TaqMan probes were designed based on obtained cDNA sequence (). We selected a partial region of 18S ribosomal RNA (18S rRNA) as a reference gene as it showed the most stable expression levels among commonly used reference genes (18S rRNA, cytochrome c oxidase subunit 2 (cox2), glyceraldehyde-3-phosphate dehydrogenase and tubulin) under our experimental conditions. Reaction mixture and thermal conditions followed the method of Mukai et al. (Citation2018). The PCR amplification efficiency of antioxidant enzymes and 18S rRNA ranged from 80.4 to 100.0% and the correlation coefficient of the standard curve ranged from 0.984 to 0.996 in all target genes.
Table 1. Primers and probes used in this experiment
H2O2 concentration and H2O2 scavenging activity measurement
The H2O2 concentration was measured by the luminol reaction-based method using a Lumat LB 9507 instrument (Berthold Technologies, Tokyo, Japan) and was calculated using a standard curve of 0 to 0.1 mM H2O2. We measured H2O2 scavenging activity as catalase activity equivalents using a Lumat LB 9507 instrument. Then, we calculated H2O2 scavenging activity based on the decrease in luminescence of standard samples (0, 10, 20, 30, 40 and 50 units catalase in 50 µl medium). The composition of the reaction mixture, and the measurement times, were calculated following Mukai et al. (Citation2018).
Expression of antioxidant enzymes after H2O2 treatment in dark acclimated cells
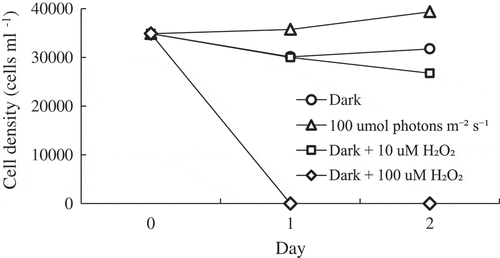
To investigate the influences of light and oxidative stress on the expression of antioxidant enzymes, we subjected dark-acclimated cells to acute exposure to light or H2O2. Twenty flasks (40 ml each, Thermo Fisher) of C. marina cells were cultured until the early stationary phase (~33 000 cells ml–1) at 25°C under 14-h light:10-h dark (L:D) photoperiod with irradiance of 100 µmol photons m–2 s–1. After that, all cell suspensions were mixed, then again divided into 20 flasks and maintained under the same conditions (25°C under 14-h light:10-h dark (L:D) photoperiod with 100 µmol photons m–2 s–1) for 24 h. After that, the 20 flasks were cultured under dark conditions for 24 h, then four flasks were sampled ‘day 0’ at 10:00. At the same time, the remaining 16 flasks were divided into four groups (4 flasks per group) and transferred to four different culture conditions: dark only, dark + 10 µM H2O2, dark + 100 µM H2O2, and moderate light (100 µmol photons m–2 s–1). These groups were sampled (10 ml from 1 flask) from the next day (day 1) at between 10:00 and 11:00. The cell density in each flask was counted using microscopy.
Antioxidant enzyme gene expression levels were quantified according to the method described in the previous section. The PCR amplification efficiency of antioxidant enzymes and 18S rRNA ranged from 98.0% to 111.4%, and the correlation coefficient of the standard curve ranged from 0.992 to 0.999 in all target genes.
Statistical analysis
Statistical analysis was performed using Statcel 3 with Excel (OMS, Saitama, Japan). Two-way ANOVA, Tukey-Kramer and Scheffe’s F test were used to analyse significant differences among treatment groups. Also, correlation analysis was performed between each antioxidant enzyme’s gene expression level and H2O2 concentration and H2O2 scavenging activity, based on Pearson’s correlation coefficient value. H2O2 concentrations and scavenging activity were calculated following Mukai et al. (Citation2018).
Results
De novo assembly and search for antioxidant enzymes
Approximately 20 million reads were obtained from the total RNA of C. marina by mRNA sequencing. Finally, 69 729 unigenes were constructed by Trinity program v1.3. A blastx homology search identified five unigenes with more than 40% homology with reference antioxidant enzymes (). Predicted localizations of antioxidant enzymes were chloroplast (plastid) or cytoplasm for Cu/Zn-SOD, chloroplast (plastid) or mitochondria for GPX, mitochondria or peroxisome for CAT, peroxisome for APX and chloroplast (plastid) for TRX. These cDNA sequences have been registered in the DNA Data Bank of Japan (DDBJ) (accession nos: LC337662 (Cu/Zn-SOD), LC337663 (GPX), LC337664 (CAT), LC337665 (APX) and LC337666 (TRX)).
Table 2. Results of homology search using blastx
Motifs, consensus sequences and phylogenetic analysis of five antioxidant enzymes
In C. marina, one predicted gene was found containing the amino acid sequence motif (WNF) and consensus sequences (NVA and FPCNQF), common to GPX in other photosynthetic organisms (Navrot et al., Citation2006). Similarly, the predicted CAT sequence contained the three consensus amino acid sequences (IPER, RGFA and VGNNTP) common to other photosynthetic organisms (Vera-Cabrera et al., Citation1999). The predicted APX sequence contained an amino acid sequence motif (RLAW), two histidines and three potassium ion binding sites involved in redox activity (Dąbrowska et al., Citation2007). The WCGPC motif, known as an active centre in redox of TRX (Kamo et al., Citation1989; Rivera-Madrid et al., Citation1995), was present in the predicted TRX. The specific motif and consensus sequence of Cu/Zn-SOD common to other photosynthetic organisms (Priya et al., Citation2007; Zhang et al., Citation2015) were not found in the predicted Cu/Zn-SOD sequence. However, part of the copper and zinc binding sites (His46, His48) and the part of the zinc binding site (His71, Asp83 and Asp124) necessary for constructing a secondary bridge of hydrogen bonding by binding with His46 (a copper ligand) and His71 (a zinc ligand) (Rakhit & Chakrabartty, Citation2006) were found. Molecular phylogenetic analysis showed that the majority of C. marina antioxidant enzymes were located in the same node as E. siliculosus in the same phylum (Ochrophyta; Supplementary fig. 1).
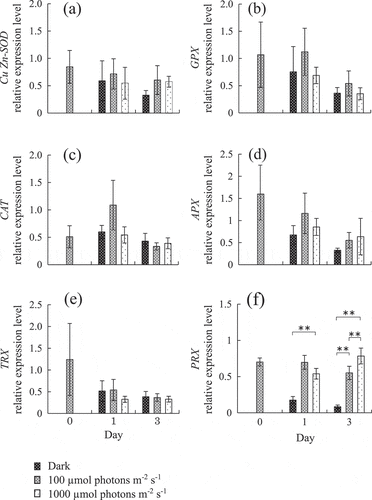
Fig. 1. Changes in expression level of (a) Cu/Zn-SOD, (b) GPX, (c) CAT, (d) APX, (e) TRX and (f) PRX in Chattonella marina cells grown under three different light intensities for 3 days. Values shown are mean ± SD (N = 4). **, p < 0.01 (two-way ANOVA and Tukey-Kramer and Scheffe’s F test comparing different conditions on each day).
Expression levels of antioxidant enzymes under different light intensity
Antioxidant enzyme gene expression levels under different light intensities are shown in . C. marina grown in the continuous dark condition died by day 5. Thus, gene expression analysis was conducted using cell samples over three days. Duration of exposure significantly changed expression levels of all antioxidant enzymes (p < 0.05, two-way ANOVA, data not shown). Light intensity-dependent change of expression levels was only observed in PRX. PRX expression levels were significantly decreased under dark conditions, compared with moderate and strong light, on day 1. In addition, PRX expression level was highest under strong light intensity on day 3 and a significant difference was observed among all treatment groups. The results for the PRX gene are in accordance with our previous study (Mukai et al., Citation2018), which analysed PRX expression levels using cox2 as a reference gene. Interaction between duration of exposure and light intensity was confirmed only for CAT and PRX expression levels (p < 0.05, two-way ANOVA, data not shown).
H2O2 concentration and H2O2 scavenging activity under different light intensities
H2O2 concentration and H2O2 scavenging activity are shown in Supplementary fig. 2. Significant positive correlations were observed between PRX expression levels and H2O2 concentration (R = 0.97, p < 0.01) and H2O2 scavenging activity (R = 0.64, p < 0.05). For all other antioxidant enzymes, there was no significant positive correlation between expression levels and H2O2 concentration or H2O2 scavenging activity (data not shown).
Expression levels of antioxidant enzymes following H2O2 treatment and acute light irradiation in dark acclimated cells
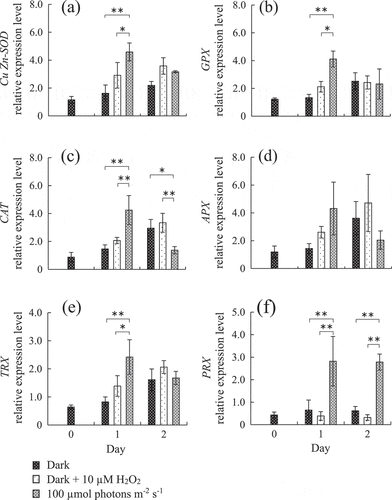
Mean C. marina cell densities were stable or slightly decreased after dark and dark + 10 µM H2O2 treatment, and drastically decreased by dark + 100 µM H2O2 exposure from day 1 onward (). With the exception of APX, expression levels of all antioxidant enzymes were significantly increased by light exposure compared with dark and dark + 10 µM H2O2 conditions on day 1 (). On day 2, a continuation of significant gene induction by light was observed only in PRX. Conversely, CAT expression level under the light condition was significantly decreased compared with dark and dark + 10 µM H2O2.
Fig. 3. Changes of gene expression in Chattonella marina antioxidant enzymes over 2 days under different oxidative stress conditions after dark acclimation. Values shown are mean ± SD (N = 4). **, p < 0.01 (two-way ANOVA, Tukey-Kramer and Scheffe’s F test comparing different conditions on each day).
Discussion
Our previous study reported the molecular structure and light-induced gene expression of the antioxidant enzyme PRX, which is encoded in the chloroplast genome of C. marina (Mukai et al., Citation2018). In this study, we newly identified cDNA homologues and predicted amino acid sequences of five major antioxidant enzymes (Cu/Zn-SOD, GPX, CAT, APX and TRX) in C. marina using mRNA-seq analysis. These predicted antioxidant genes might contribute to the degradation of harmful ROS including O2−, H2O2 and hydroxyl radicals (•OH), which are generated via physiological processes, such as electron flow in chloroplasts and some redox reactions (Fridovich, Citation1978). In photosynthetic organisms, O2− is rapidly converted to H2O2 by SOD (Sharma et al., Citation2012). H2O2 is also generated by oxidation reactions in the peroxisome (Ribeiro et al., Citation2017). Intracellularly generated H2O2 is converted to H2O and O2 by GPX, CAT and APX. PRX also converts an H2O2 to H2O and O2 by being oxidized itself (Dietz, Citation2011; Rhee et al., Citation2012). Then, the oxidized PRX is reduced, with TRX as the electron donor (Schurmann & Jacquot, Citation2000). TRX also reduces oxidized GPX (Navrot et al., Citation2006). These antioxidant enzyme activities are physiologically essential for all organisms, including photosynthetic organisms such as HAB species.
This study describes the light-induced gene expression response of some antioxidant enzymes in C. marina, suggesting their function for removing the oxidative stress produced through photosynthesis or related metabolic activity. However, different expression patterns were observed among antioxidant enzymes, especially between PRX and the other five antioxidant enzymes. In cells acclimated to light (100 µmol photons m–2 s–1), only PRX expression was significantly increased by subsequent strong light irradiation (1000 µmol photons m–2 s–1), indicating light intensity-dependent induction of the PRX gene (). This tendency is in accordance with Mukai et al. (Citation2018) who analysed PRX gene expression using cox2 as a reference gene.
On the other hand, PRX expression level was significantly decreased by dark treatment in light-acclimated C. marina. In our previous study (Mukai et al., Citation2018), there was no significant change in PRX expression with dark treatment, measured by QPCR using cox2 as a reference gene, although mean expression level was slightly decreased. In the present study, we selected 18S rRNA as a reference gene from four candidates because it showed more stable expression than other genes including cox2, throughout the present treatments. As a result, a significant decrease in PRX was detected under dark conditions in this study. Goyer et al. (Citation2002) reported that the gene expression level of PRX in the chloroplast was increased by light irradiation and decreased by dark treatment in the green alga, Chlamydomonas reinhardtii. Thus, the results of this study probably reflect a similar regulation of the C. marina chloroplast-encoded PRX gene under different light conditions to that observed in C. reinhardtii.
Cu/Zn-SOD, GPX, CAT, APX and TRX expression levels were not significantly changed in any light intensity group on the same sampling day (). In higher plants, changes in gene expression levels of antioxidant enzymes such as SOD, GPX, CAT, APX, TRX and PRX in response to abiotic stress have been well studied (Mittler et al., Citation2004; Hernandez et al., Citation2006). Mittler et al. (Citation2004) reported that gene expression levels in all Cu/Zn-SOD and TRX isoforms were decreased, or unchanged, under high light conditions in Arabidopsis thaliana. Furthermore, they also reported that gene expression levels in GPX, CAT and APX isoforms were not increased except for one isoform. In contrast, PRX expression levels are increased by high light intensity in higher plants and cyanobacteria (Mowla et al., Citation2002; Horling et al., Citation2003; Pérez-Pérez et al., Citation2009). This is similar to the response of antioxidant enzymes to light in C. marina. PRX gene expression appears to be more responsive to light intensity than other antioxidant enzymes, as part of the organism’s reaction to oxidative stress within the chloroplast.
However, in dark acclimated C. marina significant transient gene induction in Cu/Zn-SOD, GPX, CAT, TRX and PRX was observed in light irradiation at 100 µmol photons m–2 s–1 on day 1 (). The induction by light irradiation rapidly decreased on day two for all enzymes except for PRX. A similar tendency in response was also observed in APX, although this enzyme did not show significant induction. The temporal induction of expression of these gene may have been caused by deficiency of synthesis of each enzyme during dark acclimation. Thus, almost all genes of the antioxidant enzymes identified in this study are thought to be involved in avoiding photosynthesis-induced oxidative stress.
The control of gene expression of antioxidant enzymes is not well understood. Thus, the present study investigated which factors (oxidative stress or light itself) affect gene expression of antioxidant enzymes (). Our results showed significant induction of gene expression in response to light irradiation for almost all enzymes. However, significant increase of gene expression was not observed in response to 10 µM H2O2 treatment, although average expression levels were slightly increased in all enzymes except for PRX. In another study, gene expression and enzymatic activity in SOD, GPX, CAT, APX and PRX in higher plants were induced by H2O2 (Horling et al., Citation2003; Zhang et al., Citation2006; Zhai et al., Citation2013). Heavy metal induced oxidative stress is also known to increase antioxidant-related gene expression in green algae (Jamers et al., Citation2006; Elbaz et al., Citation2010). On the other hand, Chen et al. (Citation2016) reported that SOD and CAT activity were inhibited depending on the H2O2 concentration cyanobacteria were exposed to. The difference in response to oxidative stress by H2O2 may be due to species-specific variation and/or translational regulation. Our result suggests that light is a main trigger of expression of five of our antioxidant genes, while the resultant oxidative stress is also a possible factor affecting gene expression.
In general, the redox state of the photosynthetic electron transport chain, trans-thylakoid potential and ROS generate plastid-to-nucleus retrograde signaling (Nott et al., Citation2006). In light acclimated cells, four nucleus-encoded enzymes in C. marina (SOD, GPX, CAT, TRX) might similarly be under expression control from a retrograde signal. In cells grown in the light, the expression of the genes encoding these four enzymes did not increase following high light irradiation of 1000 µmol photons m–2 s–1 (). This might be due to sufficient reduction of the electron acceptors in the chloroplast electron transport chain under the acclimating light intensity (100 µmol photons m–2 s–1), which is close to the light intensity (110 µmol photons m–2 s–1) observed to be saturating to growth in C. marina (Yamaguchi et al., Citation1991). This speculation is supported by the increased expression levels of all enzymes except APX by light irradiation in dark acclimated cells, in which the chloroplast electron transport chain might be expected to be oxidized (). Thus, we propose that the expression of the genes encoding these enzymes is subject to retrograde control from the chloroplast, dependent on light availability.
The present study showed a different expression pattern of PRX compared with the five other enzymes. In our previous study, the predicted C. marina PRX gene encoded chloroplast and promoter sequences recognized by plastid-encoded RNA polymerase (PEP) and nuclear-encoded RNA polymerase (NEP) (Mukai et al., Citation2018) in an observed upstream region of the PRX gene, although the NEP gene (rpoT;3) remains undiscovered in marine algae (Smith & Purton, Citation2002). On the other hand, five other enzyme genes were encoded by nuclear DNA (Frugoli et al., Citation1996; Kliebenstein et al., Citation1998; Rodriguez Milla et al., Citation2003; Najami et al., Citation2008; Nuruzzaman et al., Citation2008), therefore expression mechanism of these enzymes is thought to be different from PRX. Expression of photosynthesis-related genes in the chloroplast is regulated by complexes with PEP, some subunit proteins (α, ß, ß′, ß′′) and sigma factors (Kremnev & Strand, Citation2014, Börner et al., Citation2015). These complexes are essential for gene expression, but the expression of sigma factor mRNA is suppressed under dark conditions (Mellenthin et al., Citation2014). Thus, C. marina PRX gene expression may be regulated by light rather than oxidative stress.
It has been reported that physiological change in microalgae is regulated by an endogenous clock (Schulze et al., Citation2010; Cohen & Golden, Citation2015). Edgar et al. (Citation2012) reported that oxidation–reduction cycles of PRX constitute a universal marker for circadian rhythms in all domains of life. In this study, there was upregulation of antioxidant enzyme genes including PRX by irradiation of light against dark-acclimated C. marina. Shikata et al. (Citation2013) reported that the rhythm of diurnal vertical migration in C. marina was regulated by blue light. Therefore, the expression of antioxidant enzymes may be under circadian regulation, dependent on diurnal changes to light intensity, in this species. Further studies are needed to clarify the factors inducing the expression of antioxidant genes and the contribution of these enzymes to physiological process related to the growth and senescence of HAB species.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2019.1576062
Supplementary fig. 1. Molecular phylogenetic trees of antioxidant enzymes of Chattonella marina and other species based on deduced amino acid sequence. (a) Cu/Zn-SOD, (b) GPX, (c) CAT, (d) APX and (e) TRX. Those amino acid sequences were grouped using Maximum likelihood methods after alignment by CLUSTAL W in MEGA7 and a bootstrap analysis using 1000 replicates. Numbers on the branches represent the percentage of 100 bootstrap replicates supporting the given topology.
Supplementary fig. 2. H2O2 concentration and H2O2 scavenging activity under different light intensity.
Author contributions
K. Mukai: original concept, NGS analysis, gene expression analysis, drafting and editing manuscript; Y. Shimasaki: original concept, drafting and editing manuscript; X. Qiu: NGS analysis; K.-U. Yoko: gene expression analysis; K. Chen: NGS analysis; M.R.M. Khanam: photosynthesis measurement; Y. Oshima: original concept, drafting and editing manuscript.
Suuplementary figure 1
Download PDF (432.7 KB)Acknowledgements
This study was partially supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (23780197). We thank Ann Seward, ELS, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Amano, K., Watanabe, M., Kohata, K. & Harada, S. (1998). Conditions necessary for Chattonella antiqua red tide outbreaks. Limnology and Oceanography, 43: 117–128.
- Börner, T., Aleynikova, A.Y., Zubo, Y.O. & Kusnetsov, V.V. (2015). Chloroplast RNA polymerases: role in chloroplast biogenesis. Biochimica et Biophysica Acta (BBA) Bioenergetics, 9: 761–769.
- Chen, C., Yang, Z., Kong, F., Zhang, M., Yu, Y. & Shi, X. (2016). Growth, physiochemical and antioxidant responses of overwintering benthic cyanobacteria to hydrogen peroxide. Environmental Pollution, 219: 649–655.
- Chou, K.C. & Shen, H.B. (2010). Plant-mPLoc: a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE, 5.
- Cohen, S.E. & Golden, S.S. (2015). Circadian rhythms in Cyanobacteria. Microbiology and Molecular Biology Reviews, 79: 373–385.
- Dietz, K.J. (2011). Peroxiredoxins in plants and cyanobacteria. Antioxidants and Redox Signaling, 15: 1129–1159.
- Dąbrowska, G., Kata, A., Goc, A., Szechyńska-Hebda, M. & Skrzypek, E. (2007). Characteristics of the plant ascorbate peroxidase family. Acta Biologica Cracoviensia Series Botanica, 49: 7–17.
- Edgar, R.S., Green, E.W., Zhao, Y., van Ooijen, G., Olmedo, M., Qin, X., Xu, Y., Pan, M., Valekunja, U.K., Feeney, K.A., Maywood, E.S., Hastings, M.H., Baliga, N.S., Merrow, M., Millar, A.J., Johnson, C.H., Kyriacou, C.P., O’Neill, J.S. & Reddy, A.B. (2012). Peroxiredoxins are conserved markers of circadian rhythm. Nature, 485: 459–464.
- Elbaz, A., Wei, Y.Y., Meng, Q., Zheng, Q. & Yang, Z.M. (2010). Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology, 19:1285–1293.
- Fridovich, I. (1978). The biology of oxygen radicals. Science, 201: 875–880.
- Frugoli, J.A., Zhong, H.H., Nuccio, M.L., McCourt, P., McPeek, M.A., Thomas, T.L. & McClung, C.R. (1996). Catalase is encoded by a multigene family in Arabidopsis thaliana (L.) Heynh. Plant Physiology, 112: 327–36.
- Giddings, S. N., MacCready, P., Hickey, B.M., Banas, N.S., Davis, K.A., Siedlecki, S.A., Trainer, V.L., Kudela, R.M., Pelland, N.A. & Connolly, T.P. (2014). Hindcasts of potential harmful algal bloom transport pathways on the Pacific Northwest coast. Journal of Geophysical Research, 4: 2439–2461.
- Goyer, A., Haslekås, C., Miginiac-Maslow, M., Klein, U., Le Marechal, P., Jacquot, J.P. & Decottignies, P. (2002). Isolation and characterization of a thioredoxin-dependent peroxidase from Chlamydomonas reinhardtii. European Journal of Biochemistry, 269: 272–282.
- Gruber, A., Rocap, G., Kroth, P.G., Armbrust, E.V. & Mock T. (2015). Plastid proteome prediction for diatoms and other algae with secondary plastids of the red lineage. Plant Journal for Cell and Molecular Biology, 81: 519–528.
- Gschloessl, B., Guermeur, Y. & Cock, J.M. (2008). HECTAR: a method to predict subcellular targeting in heterokonts. BMC Bioinformatics, 9: 393.
- Haas, B.J., Papanicolaou, A., Yassour, M., Grabherr, M., Blood, P.D., Bowden, J., Couger, M.B., Eccles, D., Li, B., Lieber, M., MacManes, M.D., Ott, M., Orvis, J., Pochet, N., Strozzi, F., Weeks, N., Westerman, R., William, T., Dewey, C.N., Henschel, R., LeDuc, R.D., Friedman, N. & Regev, A. (2013). De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nature Protocols, 8: 1494–1512.
- Halliwell, B. & Gutteridge, J.M. (1984). Oxygen toxicity, oxygen radicals, transition metals and disease. Biochemical Journal, 219: 1–14.
- Hernandez, J.A., Escobar, C., Creissen, G. & Mullineaux, P.M. (2006). Antioxidant enzyme induction in pea plants under high irradiance. Biologia Plantarum, 50: 395–399.
- Horling, F., Lamkemeyer, P., König, J., Finkemeier, I., Kandlbinder, A., Baier, M. & Dietz, K.J. (2003). Divergent light-, ascorbate-, and oxidative stress dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis. Plant Physiology, 131: 317–325.
- Jamers, A., Van der Ven, K., Moens, L., Robbens, J., Potters, G., Guisez, Y., Blust, R. & De Coen, W. (2006). Effect of copper exposure on gene expression profiles in Chlamydomonas reinhardtii based on microarray analysis. Aquatic Toxicology, 80: 249–260.
- Johnson, K.J., Fantone, J.C. & Kaplan, P.A. (1981). In vivo damage of rat lungs by oxygen metabolites. Journal of Clinical Investigation, 67: 983–993.
- Kamo, M., Tsugita, A., Wiessner, C., Wedel, N., Bartling, D., Herrmann, R.G., Aguilar, F., Gardet-Salvi, L. & Schürmann, P. (1989). Primary structure of spinach-chloroplast thioredoxin f. Protein sequencing and analysis of complete cDNA clones for spinach-chloroplast thioredoxin f. European Journal of Biochemistry, 182: 315–322.
- Katano, T., Yoshida, M., Yamaguchi, S., Yoshino, K., Hamada, T., Koriyama, M. & Hayami, Y. (2014). Effect of nutrient concentration and salinity on diel vertical migration of Chattonella marina (Raphidophyceae). Marine Biology Research, 10: 1007–1018.
- Kevin, J.P., Cary, S.C. & Warner, M.E. (2010). Antioxidant enzyme response and reactive oxygen species production in marine raphidophytes. Journal of Phycology, 46: 1161–1171.
- Kim, K.M., Park, J.H., Bhattacharya, D. & Yoon, H.S. (2014). Applications of next-generation sequencing to unravelling the evolutionary history of algae. Journal of Systematic and Evolutionary Microbiology, 64: 333–345.
- Kliebenstein, D.J., Monde, R.A. & Last, R.L. (1998). Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiology, 118: 637–650.
- Kremnev, D. & Strand, A. (2014). Plastid encoded RNA polymerase activity and expression of photosynthesis genes required for embryo and seed development in Arabidopsis. Frontiers in Plant Science, 5: 385.
- Machida, T., Ishibashi, A., Kirino, A., Sato, J., Kawasaki, S., Niimura, Y., Honjoh, K. & Miyamoto, T. (2012). Chloroplast NADPH-dependent thioredoxin reductase from Chlorella vulgaris alleviates environmental stresses in yeast together with 2-Cys peroxiredoxin. PLoS ONE, 7: 45988.
- Marshall, J. A. & Hallegraeff, G. M. (1999). Comparative ecophysiology of the harmful alga Chattonella marina (Raphidophyceae) from South Australian and Japanese waters. Journal of Plankton Research, 21: 1809–1822.
- Mellenthin, M., Ellersiek, U., Borger, A. & Baier, M. (2014). Expression of the Arabidopsis sigma factor SIG5 is photoreceptor and photosynthesis controlled. Plants, 3: 359–391.
- Mhamdi, A., Queval, G., Chaouch, S., Vanderauwera, S., Van Breusegem, F. & Noctor, G. (2010). Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. Journal of Experimental Botany, 61: 4197–4220.
- Miao, Y., Lv, D., Wang, P., Wang, X., Chen, J., Miao, C. & Song, C. (2006). An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. The Plant Cell, 18: 2749–2766.
- Miller, A. F. (2012). Superoxide dismutases: ancient enzymes and new insights. FEBS Letters, 586: 585–595.
- Mittler, R., Vanderauwera, S., Gollery, M. & Breusegem, F. V. (2004). Reactive oxygen gene network of plants. Trends in Plant Science, 9: 490–498.
- Mowla, S.B., Thomson, J.A., Farrant, J.M. & Mundree, S.G. (2002). A novel stress-inducible antioxidant enzyme identified from the resurrection plant Xerophyta viscosa Baker. Planta, 215: 716–726.
- Mukai, K., Teramoto, A., Qiu, X., Shimasaki, Y., Kato-Unoki, Y., Lee, J.M., Mizoguchi, M., Khanam, M.R.M., Satone, H., Tatsuke, T., Kusakabe T. & Oshima, Y. (2018). Gene structure and cDNA sequence of 2-Cys peroxiredoxin in the harmful algal bloom species Chattonella marina and its gene transcription under different light intensities. European Journal of Phycology, 53: 29–38.
- Najami, N., Janda, T., Barriah, W., Kayam, G., Tal, M., Guy, M. & Volokita, M. (2008). Ascorbate peroxidase gene family in tomato: its identification and characterization. Molecular Genetics and Genomics: MGG, 279: 171–182.
- Navrot, N., Collin, V., Gualberto, J., Gelhaye, E., Hirasawa, M., Rey, P., Knaff, D.B., Issakidis, E., Jacquot, J.P. & Rouhier, N. (2006). Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiology, 142: 1364–1379.
- Nott, A., Jung, H.S., Koussevitzky, S. & Chory, J. (2006). Plastid-to-nucleus retrograde signaling. Annual Review of Plant Biology, 57: 739–759.
- Nuruzzaman, M., Gupta, M., Zhang, C., Wang, L., Xie, W., Xiong, L., Zhang, Q. & Lian, X. (2008). Sequence and expression analysis of the thioredoxin protein gene family in rice. Molecular Genetics and Genomics, 280: 139–151.
- Oda, T., Nakamura, A., Shikayama, M., Kawano, I., Ishimatsu, A. & Muramatsu, T. (1997). Generation of reactive oxygen species by raphidophycean phytoplankton. Bioscience Biotechnology and Biochemistry, 61: 1658–1662.
- Okaichi, T. & Yanagi, T. (1997). Sustainable development in the Seto Inland Sea, Japan. Terra Scientific Publishing Company (TERRAPUB), 251–304.
- Onitsuka, G., Aoki, K., Matsuyama, Y., Kimoto, K., Matsuo, H., Kitadai, Y., Nishi, H., Tahara, Y. & Sakurada, K. (2011). Short-term dynamics of a Chattonella antiqua bloom in the Yatsushiro Sea, Japan, in summer 2010: characteristics of its appearance in the southern area. Bulletin of the Japanese Society of Fisheries and Oceanography, 75: 143–153.
- Ozyigit, I.I., Filiz, E., Vatansever, R., Kurtoglu, K.Y., Koc, I., Öztürk, M.X. & Anjum, N.A. (2016). Identification and comparative analysis of H2O2-scavenging enzymes (ascorbate peroxidase and glutathione peroxidase) in selected plants employing bioinformatics approaches. Frontiers in Plant Science, 7: 301.
- Pérez-Pérez, M.E., Mata-Cabana, A., Sánchez-Riego, A.M., Lindahl, M. & Florencio, F.J. (2009). A comprehensive analysis of the peroxiredoxin reduction system in the cyanobacterium Synechocystis sp. strain PCC 6803 reveals that all five peroxiredoxins are thioredoxin dependent. Journal of Bacteriology, 191: 7477–7489.
- Pinto, L., Mateus, M. & Silva, A. (2016). Modeling the transport pathways of harmful algal blooms in the Iberian coast. Harmful Algae, 53: 8–16.
- Poong, S.W., Lim, P.E., Phang, S.M., Wong, C.Y., Pai, T.W., Chen, C.M., Yang, C.H. & Liu, C.C. (2018). Transcriptome sequencing of an Antarctic microalga, Chlorella sp. (Trebouxiophyceae, Chlorophyta) subjected to short-term ultraviolet radiation stress. Journal of Applied Phycology, 30: 87–99.
- Priya, B., Premanandh, J., Dhanalakshmi, R.T., Seethalakshmi, T., Uma, L., Prabaharan, D. & Subramanian, G. (2007). Comparative analysis of cyanobacterial superoxide dismutases to discriminate canonical forms. BioMed Central Genomics, 8: 435.
- Qiu, X., Shimasaki, Y., Tsuyama, M., Yamada, T., Kuwahara, R., Kawaguchi, M., Honda, M., Gunjikake, H., Tasmin, R., Shimizu, M., Sato, Y., Kato-Unoki, Y., Nakashima, T., Matsubara, T., Yamasaki, Y., Ichinose, H., Wariishi, H., Honjo, T. & Oshima, Y. (2013). Growth phase dependent variation of photosynthetic activity and cellular protein expression profile in harmful raphidophyte Chattonella antiqua. Bioscience Biotechnology and Biochemistry, 77: 46–52.
- Qiu, X., Mukai, K., Shimasaki, Y., Tsuyama, M., Matsubara, T., Nakashima, T., Ichinose, H., Nakazima, Y., Honjo, T., & Oshima, Y. (2017). Variations in the expression of photosynthesis–related proteins in field Chattonella marina cells during a harmful algal bloom. Journal of the Faculty of Agriculture, Kyushu University, 62: 373–380.
- Rakhit, R. & Chakrabartty, A. (2006). Structure, folding, and misfolding of Cu,Zn superoxide dismutase in amyotrophic lateral sclerosis. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease, 1762: 1025–1037.
- Rhee, S.G., Woo, H.A., Kil, I.S. & Bae, S.H. (2012). Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. Journal of Biological Chemistry, 287: 4403–4410.
- Ribeiro, C.W., Korbes, A.P., Garighan, J.A., Jardim-Messeder, D., Carvalho, F.E.L., Sousa, R.H.V., Caverzan, A., Teixeira, F.K., Silveira, J.A.G. & Margis-Pinheiro, M. (2017). Rice peroxisomal ascorbate peroxidase knockdown affects ROS signaling and triggers early leaf senescence. Plant Science, 263: 55–65.
- Rivera-Madrid, R., Mestres, D., Marinho, P., Jacquot, J.P., Decottignies, P., Miginiac-Maslow, M. & Meyer, Y. (1995). Evidence for five divergent thioredoxin h sequences in Arabidopsis thaliana. Proceedings of the National Academy of Sciences USA, 92: 5620–5624.
- Rodriguez Milla, M.A, Maurer, A., Rodriguez Huete, A. & Gustafson, J.P. (2003). Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signaling pathways. The Plant Journal, 36: 602–615.
- Satta, C.T., Padedda B.M., Sechi, N., Pulina, S., Loria, A. & Lugliè A. (2017). Multiannual Chattonella subsalsa Biecheler (Raphidophyceae) blooms in a Mediterranean lagoon (Santa Giusta Lagoon, Sardinia Island, Italy). Harmful Algae, 67: 61–73.
- Schulze, T., Prager, K., Dathe, H., Kelm, J., Kiessling, P. & Mittag, M. (2010). How the green alga Chlamydomonas reinhardtii keeps time. Protoplasma, 244: 3–14.
- Schurmann, P. & Jacqout, J. P. (2000). Plant thioredoxin system revisited. Annual Review of Plant Physiology and Plant Molecular Biology, 51: 371–400.
- Sharma, P., Jha, A.B., Dubey, R.S. & Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, Article ID: 217037.
- Shikata, T., Matsunaga, S., Iseki, M., Nishide, H., Higashi, S., Kamei, Y., Yamaguchi, M., Jenkinson, I.R. & Watanabe, M. (2013). Blue light regulates the rhythm of diurnal vertical migration in the raphidophyte red-tide alga Chattonella antiqua. Journal of Plankton Research, 35: 542–552.
- Smith, A. & Purton, S. (2002). The transcriptional apparatus of algal plastids. European Journal of Phycology, 37: 301–311.
- Vera-Cabrera, L., Johnson, W.M., Welsh, O., Resendiz-Uresti, F.L. & Salinas-Carmona, M.C. (1999). Distribution of a Nocardia brasiliensis catalase gene fragment in members of the genera Nocardia, Gordona, and Rhodococcus. Journal of Clinical Microbiology, 37: 1971–1976.
- Yamaguchi, M., Imai, I. & Honjo, T. (1991). Effects of temperature, salinity and irradiance on the growth rates of the noxious red tide flagellates Chattonella antiqua and C. marina (Raphidophyceae). Bulletin of the Japanese Society of Scientific Fisheries, 57: 1277–1284.
- Yamamoto, H., Miyake, C., Dietz, K.J., Tomizawa, K., Murata, N. & Yokota, A. (1999). Thioredoxin peroxidase in the cyanobacterium Synechocystis sp. PCC6803. FEBS Letters, 477: 269–273.
- Yamasaki, Y., Nagasoe, S., Matsubara, T., Shikata, T., Shimasaki, Y., Oshima, Y. & Honjo, T. (2007). Allelopathic interactions between the bacillariophyte Skeletonema costatum and the raphidophyte Heterosigma akashiwo. Marine Ecology Progress Series, 339: 83–92.
- Yoshida, Y., Tomiyama, T., Maruta, T., Tomita, M., Ishikawa, T. & Arakawa, K. (2016). De novo assembly and comparative transcriptome analysis of Euglena gracilis in response to anaerobic conditions. BMC Genomics, 17: 182.
- Zhai, C.Z., Zhao, L., Yin, L.J., Chen, M., Wang, Q.Y., Li, L.C., Xu, Z.S. & Ma, Y.Z. (2013). Two wheat glutathione peroxidase genes whose products are located in chloroplasts improve salt and H2O2 tolerances in Arabidopsis. PLoS ONE, 8.
- Zhang, A., Jiang. M., Zhang, J., Tan, M. & Hu, X. (2006). Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiology, 141: 475–487.
- Zhang, X., Wan, Q., Liu, F., Zhang, K. Sun, A., Luo, B., Sun, L. & Wan, Y. (2015). Molecular analysis of the chloroplast Cu/Zn-SOD gene (AhCSD2) in peanut. The Crop Journal, 3: 246–257.