ABSTRACT
The morphology and molecular phylogenetic relationships of Coolia species isolated from Brazil, including C. malayensis, C. tropicalis and a new lineage of the Coolia canariensis complex, are presented. Morphology was investigated by light and scanning electron microscopy. No significant morphological differences between the Brazilian strains and other geographically distant strains of C. malayensis and C. tropicalis were observed. In LSU and ITS rDNA phylogenies C. malayensis and C. tropicalis sequences from Brazil branched within the clade of these species with strains isolated from elsewhere. Phylogenetic relationships (LSU rDNA) within the C. canariensis species complex support the existence of three cryptic species. No morphological difference was discernible among species in this complex. Additional molecular, morphological and toxin data from globally distributed isolates should be acquired to strengthen our knowledge of the C. canariensis species complex.
Introduction
Species in the genus Coolia Meunier are epibenthic dinoflagellates with a broad distribution from tropical to temperate latitudes (Leaw et al., Citation2016). Species of this genus are known to occur in an assemblage that may include other toxigenic epibenthic species from genera such as Ostreopsis Schmidt, Prorocentrum Ehrenberg and Gambierdiscus Adachi & Fukuyo (Faust, Citation1992, Citation1995; Tindall & Morton, Citation1998; Fraga et al., Citation2008; Leaw et al., Citation2016). Species of Coolia occur on diverse substrata including macroalgae, sands (Faust, Citation1992, Citation1995; Ten-Hage et al., Citation2000; Penna et al., Citation2005; Fraga et al., Citation2008; Leaw et al., Citation2010; Laza-Martinez et al., Citation2011; Rhodes et al., Citation2014; Karafas et al., Citation2015), rocks and dead corals (Leung et al., Citation2017).
Currently seven species of Coolia have been described. The type species C. monotis Meunier was described from Belgium (Meunier, Citation1919) and it was the only species in the genus for many years. In 1995 Coolia tropicalis Faust was described from Belize (Faust, Citation1995) and the description of Coolia areolata Ten-Hage, Turquet, Quod and Couté (Ten-Hage et al., Citation2000), Coolia canariensis Fraga (Fraga et al., Citation2008), Coolia malayensis Leaw, P.-T Lim and Usup (Leaw et al., Citation2010) and more recently Coolia palmyrensis Karafas, Tomas and York and Coolia santacroce Karafas, Tomas and York (Karafas et al., Citation2015) followed. Coolia areolata is the only species in the genus that currently lacks molecular characterization.
Sequence data of the internal transcribed spacer (ITS) and D1/D2 region of the large subunit (LSU) of the ribosomal DNA clearly distinguishes the six species that have been genetically characterized (Karafas et al., Citation2015; Gómez et al., Citation2016; Leaw et al., Citation2016; Leung et al., Citation2017). A number of cryptic/pseudocryptic species have been recognized in the genus and more recently, based on increased availability of molecular data and subtle morphological differences, the identities of some species of Coolia have been revised (e.g. some strains previously designated as C. monotis were reassigned to C. malayensis; Leaw et al., Citation2016). Coolia monotis shows a temperate distribution, in the North Atlantic Ocean and the Mediterranean Sea (Lewis et al., Citation2018), while C. malayensis is a warm water species. The two species co-occur only at the subtropical Canary Islands (David et al., Citation2018). Coolia canariensis is phylogenetically divided into two clades, suggesting the existence of cryptic diversity and these were differentiated as C. canariensis and C. cf. canariensis by David et al. (Citation2014).
Like other benthic dinoflagellates, Coolia may produce natural products, some of which have been shown to be toxic (Holmes et al., Citation1995; Rhodes & Tomas, Citation1997; Rhodes et al., Citation2010; Karafas et al., Citation2015; Wakeman et al., Citation2015). Biotoxins and bioactive compounds produced by Coolia species have not been well characterized. Cooliatoxin, a mono-sulphated polyether similar to yessotoxin was the first toxin isolated from a strain of Coolia tropicalis (reported as C. monotis) from Australia (Holmes et al., Citation1995). Wakeman et al. (Citation2015) analysed a strain of C. malayensis from Japan and identified new yessotoxin analogues suggesting unique metabolites in this species. The toxicity of Coolia species is thought to be species-specific and toxicity levels differ among species (Karafas et al., Citation2015). Coolia malayensis, C. tropicalis, C. palmyrensis and C. santacroce have been shown to be toxic in biological assays involving cytotoxicity tests on human cell lines and/or bioassays involving intraperitoneal injections on mice and/or exposure to Artemia nauplii (Rhodes et al., Citation2000, Citation2014; Laza-Martinez et al., Citation2011; Mohammad-Noor et al., Citation2013; Karafas et al., Citation2015). Little is known about the toxicity of Coolia species to various marine organisms, but a recent study has shown the sensitivity of sea urchin larvae (Heliocidaris crassispina) to species of Coolia, particularly to C. malayensis (Leung et al., Citation2017).
In Brazil strains of Coolia malayensis have been isolated from São Paulo (Gómez et al., Citation2016) and the genus has been observed epiphytically on macroalgae at several locations from latitude 22°S to 3°S (Nascimento et al., data not published). The aim of this study was to describe the morphology and phylogenetic relationships of Coolia species isolated from Brazil, including Coolia malayensis, Coolia tropicalis and a new lineage of the Coolia canariensis complex.
Materials and methods
Sample collection and treatment
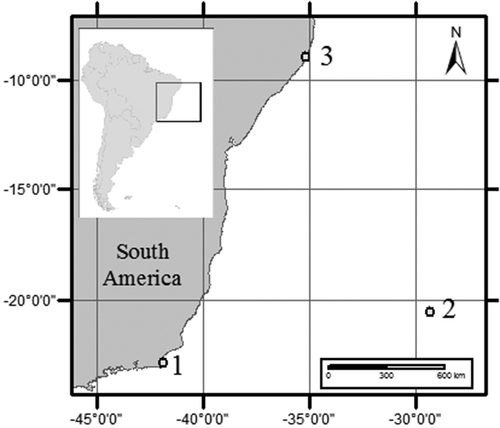
Macroalgal samples were collected from a depth of 1–2 m by snorkel diving from Tartaruga Beach, Armação dos Búzios, Rio de Janeiro (22°45'18''S, 41°54'07''W; ), from Maragogi, Alagoas (8°55'18''S, 35°09'06''W; ) and from a tidal pool in Trindade Island (20°29'22.2''S, 29°20'04.2''W; ). Specimens of macroalgae were placed in sealable plastic bags and vigorously shaken for 2 min to detach the associated epiphytic cells. Live cells of Coolia were isolated from the epiphytic suspension using a micropipette, and were sequentially transferred through four to five drops of sterile and filtered (glass-fibre filter, Millipore AP-40, Millipore Brazil) local seawater. After each transfer, the drop was examined to ensure a single cell was present. After the final transfer, each isolated cell was placed into a separate well of a sterile 96-well tissue culture plate with 90 µl of L2 culture medium (Guillard, Citation1995) prepared with seawater which had been filtered (glass-fibre filter, Millipore AP-40, Millipore Brazil), autoclaved and the salinity adjusted to 34 with deionized water (dH2O). When sufficient cell density was achieved, cells were transferred to a separate well of a sterile 6-well tissue culture plate containing L2 medium and were eventually transferred to 250 ml glass Erlenmeyer flasks. All stock cultures were maintained in a temperature-controlled cabinet at 24 ± 2°C, with a 12 h light:12 h dark cycle and a photon flux density of 60 µmol m–2 s–1 provided by cool-white fluorescent tubes. Photosynthetically active radiation was measured with a QSL-100 quantum sensor (Biospherical Instruments, San Diego, California, USA).
Fig. 1. Map of South America showing the three locations where species of Coolia were isolated from. 1 – Armação dos Búzios, Rio de Janeiro, 2 – Trindade Island, Espírito Santo, 3 – Maragogi, Alagoas, Brazil.
Morphological study
Live cells of C. canariensis species complex (strain UNR-25), C. malayensis (strain UNR-02) and C. tropicalis (strains UNR-14, UNR-22, UNR-23, UNR-24, UNR-27 and UNR-28) were observed using light microscopy (LM) and scanning electron microscopy (SEM). Images were collected using an AxiocamICc1 digital camera (Zeiss, Germany) in LM. For SEM observation, cells of Coolia spp. were fixed with 2% glutaraldehyde SEM grade (Merck, Darmstadt, Germany), filtered through Durapore membrane filters (Millipore, USA), rinsed twice with distilled water and dehydrated in a series of 30, 50, 70, 80, 95 and 100% EtOH followed by hexamethyldisilazane. After being air-dried overnight, they were coated with platinum with a sputter coater (Leica EM ACE 600, Germany) and observed with a Micro Quanta FEG 250 scanning electron microscope (FEI Company, Hillsboro, Oregon, USA) at Instituto Militar de Engenharia (IME), Rio de Janeiro, Brazil. Cell and plate measurements were obtained from SEM images. The dorso-ventral (DV) depth, anteroposterior (AP) length and width of cells were measured, as well as the size of the apical pore complex (APC) and the 6'' plate.
In this study, a modified Kofoid tabulation system (Kofoid, Citation1909) as described in Besada et al. (Citation1982) was followed to name the plates and enable comparisons with other genera.
Phylogenetic analysis
Exponentially growing cells of C. canariensis species complex (strain UNR-25), C. malayensis (strain UNR-02) and C. tropicalis (strains UNR-14, UNR-22, UNR-23, UNR-24, UNR-27 and UNR-28) were harvested in 15 ml centrifuge tubes by centrifugation at 5000 × g for 15 min for DNA extraction. Subsequently, the cells were transferred to 1.5 ml microtubes and centrifuged again at 5000 × g for 15 min to settle the cells into pellets. The supernatant was discarded and the cell pellets were stored at −80°C for further analysis. Genomic DNA was extracted from the pellets using the Qiagen DNeasy Plant Mini Kit (Qiagen Inc., USA) following the manufacturer’s instructions and then stored at −20°C.
Two rDNA loci were analysed in the present study: the D1/D3 region of the large subunit (LSU) and the Internal Transcribed Spacer (ITS = ITS1-5.8S-ITS2) of the ribosomal DNA (rDNA). These two loci have been successfully used in several phylogenetic studies of Coolia species (Jeong et al., Citation2012; David et al., Citation2014; Karafas et al., Citation2015). The LSU D1/D3 region was amplified using the pair of primers D1R/LSUB (5′-ACCCGCTGAATTTAAGCATA-3′/5′-ACGAACGATTTGCACGTCAG-3′) (Scholin et al., Citation1994; Litaker et al., Citation2003). The ITS region was amplified using the pair of primers ITSA/ITSB (5’-GTAACAAGGTHTCCGTAGGT–3’/5’-AKATGCTTAARTTCAGCRGG-3’) (Sato et al., Citation2011). The amplification reaction mixture of 25 µl contained: 1 unit (U) Taq DNA polymerase (Thermo Scientific Inc, USA), 1× reaction buffer with NH4SO4, 2.5 mM MgCl2, 0.16 mM dNTPs (Thermo Scientific, USA), 8 pmol of each primer and 15 ng of genomic DNA. Reactions with LSU and ITS primers comprised an initial 5 min heating step at 94°C, followed by 40 cycles of 94°C for 30 s, 45°C for 30 s, 72°C for 1 min and a final extension at 72°C for 5 min. Sequencing of samples was performed by Macrogen Inc. using both forward and reverse primers. Sequence reads were manually checked and edited using the software BioEdit v7.2.5 (Hall, Citation1999). The new sequences obtained in the present study were BLAST searched against the GenBank database (www.ncbi.nlm.nih.gov/blast) to test for sequence homology with non-target taxa. The sequences obtained in the present study were aligned with other Coolia sequences retrieved from GenBank using MAFFT v7 (Katoh & Standley, Citation2013). Phylogenetic analyses were conducted separately for each molecular marker. Sequences of Ostreopsis ovata were used as outgroup.
The MEGA 7.0 software (Kumar et al., Citation2016) was used to select the best-fit model of nucleotide substitution (T92+G+I for both markers) and construct maximum likelihood (ML) phylogenetic trees with 1000 bootstrap (BS) replications. The phylogenetic relationships were also examined using Bayesian inference (BI) with MrBayes v3.2.6 (Ronquist et al., Citation2012). To sample across nucleotide substitution models, the command 'lset nst=mixed’ was used before running the analysis. Markov Chain Monte Carlo procedure consisted of two independent trials with four chains each. Each chain was run for 1 000 000 generations and sampled every 100th cycle. Posterior probability (PB) values for the resulting 50% majority rule consensus tree were estimated after discarding the first 10% of trees as burn-in.
Average genetic distances (p-distance) within and between sequences of each species was calculated using the software MEGA 7.0 (Kumar et al., Citation2016). All positions containing gaps and missing data were eliminated.
Results
Morphology
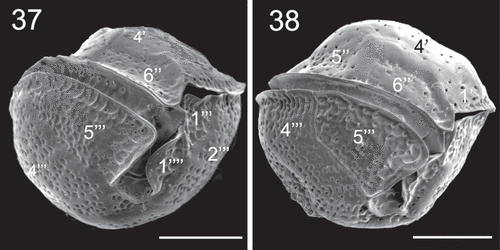
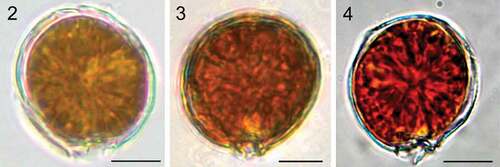
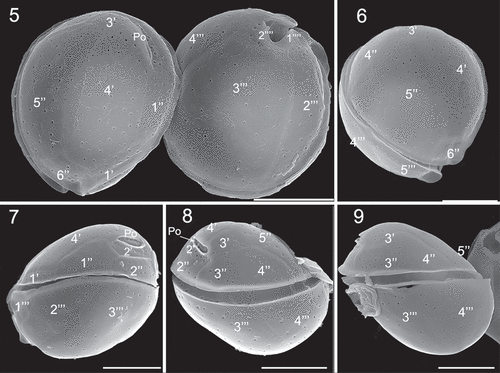
Coolia malayensis, C. tropicalis and C. canariensis species complex contained many golden-brown chloroplasts (). Plate formula of C. malayensis (strain UNR-02) was Po, 4', 6'', 6c, ?s, 5''', 2'''' (). Cells were obliquely roundish when observed in lateral view (). The anterior-posterior axis was oblique and the hypotheca was slightly larger than the epitheca (). Cells were 24.5 ± 2.1 µm in length and DV depth was 25.4 ± 2.2 µm (). The thecal surface was smooth with round pores irregularly scattered (–) that may present poroids or fine perforations (). Individual thecal plates were delineated by faint sutures and smooth growth bands (–). The apical pore plate Po was short and slightly curved (, ), contiguous to plates 2', 3' and 4' and was dorsally and left displaced (), measuring 4.9 ± 1.2 µm (). Plate 1' was small, rectangular shaped and connected to plates 6'', 1'' and 4' (, ). Plate 2' was the smallest in the apical series, and harboured the Po in most of its area (, ). Plate 3' was pentagonal and bordered plates Po, 2', 3'', 4'', 5'' and 4' (). Plate 4' was oblong, hexagonal and situated on the left side of the epitheca, touching plates 1', 1'', 2', Po, 3', 5'' and 6'' ().
Figs 2–4. Light microscopy images. Fig. 2. Coolia malayensis (strain UNR-02). Fig. 3. Coolia tropicalis (strain UNR-28). Fig. 4. Coolia cf. canariensis phylogroup II (strain UNR-25). Scale bars: 10 µm.
Figs 5–9. Scanning electron microscopy (SEM) micrographs of Coolia malayensis (strain UNR-02). Fig. 5. Apical and antapical view. Fig. 6. Oblique view of the epitheca. Fig. 7. Left lateral view. Fig. 8. Right lateral view. Fig. 9. Left-dorsal thecal plate view. Scale bars: 10 µm.
Figs 10–13. SEM micrographs of Coolia malayensis (strain UNR-02). Fig. 10. Detail of the apical pore complex showing the Po. Fig. 11. Detail of pores showing poroids. Fig. 12. Oblique view showing dorsal hypothecal plates. Fig. 13. Ventral view. Scale bars: (10–11) 2 µm, (12–13) 10 µm.
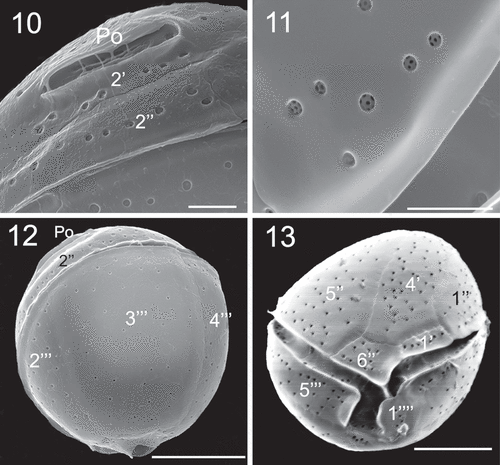
Table 1. Comparison of the morphological features of Coolia malayensis (strain UNR-02), C. tropicalis (strain UNR-28) and C. canariensis complex, including strain UNR-25
In the precingular series, plate 1'' was pentagonal and plate 2'' was rectangular (). Plate 3'' was quadrangular and touched 2'', 2', 3' and 4'', while plate 4'' was quadrangular (). The pentagonal plate 5'' was the largest plate of the epitheca and occupied the most part of the left side of the cell, and was surrounded by plates 6'', 4', 3', 4'' (). Plate 6'' was pentagonal (, ) and was 6.4 µm wide and 4.3 µm long, with a W/L ratio of 1.5 (). The cingulum was equatorial and the sulcus was short and narrow and did not reach the antapex of the cell (). Sulcal plates were not observed. In the hypotheca, there were five plates in the postcingular series, where plates 1''' and 5''' were small and triangular while plates 3''' and 4''' were the largest plates of the hypotheca (, ). The antapical plates 1'''' and 2'''' were small and surrounded the posterior part of the sulcus ().
The morphology of the six studied C. tropicalis strains was the same, and therefore only the morphology of strain UNR-28 was described. Cells of C. tropicalis strain UNR-28 were almost spherical in ventral view () and spherical in antapical view (). The plate formula was Po, 4', 6'', 6c, ?s, 5''', 2''''. Cells were 27.4 ± 2.7 µm long and dorso-ventral (DV) depth was 29.7 ± 2.6 µm (). Cell surface was smooth with scattered pores (–). The Po was slightly curved and situated in the left dorsal side of the epitheca and was in contact with plates 2', 3', 4' (, ). It was 7.0 ± 0.9 µm long (). The apical plate 1' was narrow and about twice as long as wide (). Plate 2' was the smallest in the apical series and was contiguous to the Po (, ). Plate 3' bordered plates 2', Po, 4', 3'', 4'', 5'' (Figs 16, 22). The fourth apical plate (4') was the largest plate of the epitheca and was in contact with 1', 1'', 2', Po, 3', 5'' and 6'' (). The lateral sides of plate 4' were almost parallel and the plate was wider towards the ventral side. In the pre-cingular series, plate 5'' was the largest (). Plate 6'' was wide (), 14.4 µm wide and 3.7 µm long with a W/L ratio of 3.9 (). In the hypotheca, the postcingular plates 3''' and 4''' were large and occupied all of the dorsal side of the cell (). The two antapical plates 1'''' and 2'''' were small and in contact with the sulcal area ().
Figs 14–19. SEM micrographs of Coolia tropicalis (strain UNR-28). Fig. 14. Ventral view. Fig. 15. Oblique view of the epitheca. Fig. 16. Oblique-dorsal view of left side. Figs 17–18. Right lateral view. Fig. 19. Left lateral view. Scale bars: 10 µm.
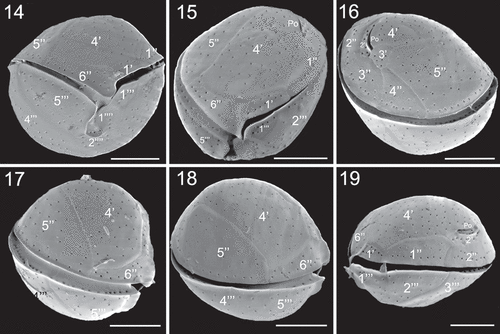
Figs 20–23. SEM micrographs of Coolia tropicalis (strain UNR-28). Fig. 20. Dorsal view of hypotheca. Fig. 21. Antapical view. Fig. 22. Left part of the epitheca in apical view. Fig. 23. Detail of pores on plate 6′′. Scale bars (20–21): 10 µm, (22–23): 3 µm.
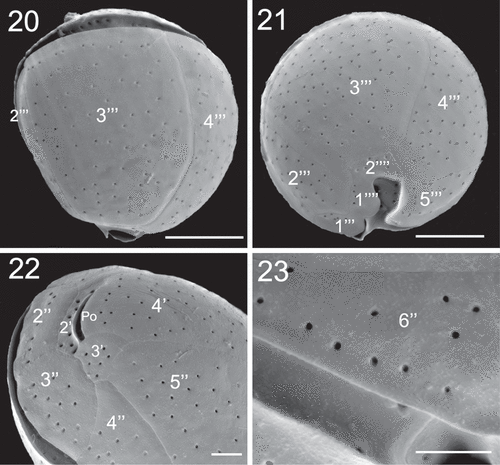
Cells of C. canariensis complex (strain UNR-25) were almost spherical in apical or antapical view with slight anterioposterior compression in lateral view (–). Cells were 24.9 ± 2.3 µm long with a DV depth of 27.5 ± 2.4 µm (). The plate formula was Po, 4', 6'', 6c, ?s, 5''', 2''''. It was not possible to observe all sulcal plates. The apical pore plate Po was elongated (, ) and was 7.8 ± 1.1 µm long (). Plate 4' was hexagonal and was the largest plate of the epitheca; it was surrounded by plates 1', 1'', 2', Po, 3', 5'' and 6'' (–). Plate 2' was short, elongated, hexagonal and in contact with Po along its left and dorsal sides (, ). Plate 3' was pentagonal and in contact with plates 4', Po, 2', 3'', 4'' and 5'' (). Plates 1', 1'', 4'' and 6'' were four sided while plates 1'', 3'' and 5'' were pentagonal (–). Plate 5'' was the largest precingular plate followed by plate 6'' (, ). Plate 6'' was on average 12.2 ± 1.7 µm in width and 6.2 ± 1.1 µm in length, with a W/L ratio of 2.1 ± 0.6 ().
Figs 24–26. SEM micrographs of Coolia cf. canariensis phylogroup II. Fig. 24. Oblique-lateral view. Fig. 25. Left lateral view. Fig. 26. Right lateral view. Scale bars: 10 µm.
Figs 27–30. SEM micrographs of Coolia cf. canariensis phylogroup II. Fig. 27. Apical view. Fig. 28. Detail of the apical pore complex showing the Po. Figs 29–30. Inside view of the epitheca. Scale bars: (27, 29, 30): 10 µm; (28): 4 µm.
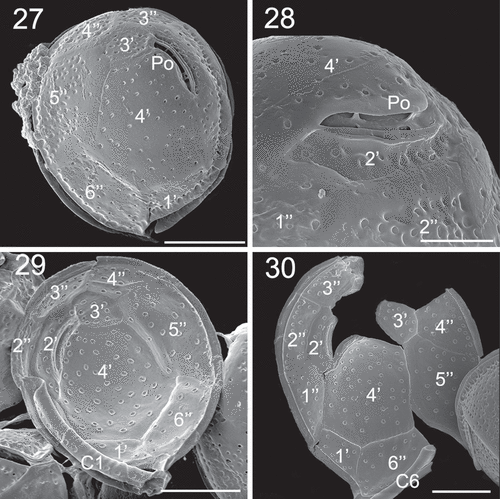
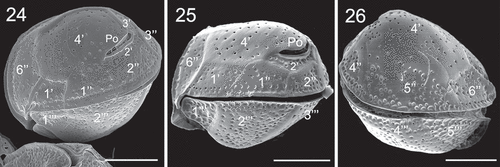
The first postcingular plate 1''' was the smallest of this series and was triangular in shape (, ). Plates 2''', 3''', 4''' and 5''' were large and elongated towards the antapex of the cell (). The ventral edges of plates 1'''', 2'''' and 5''' extended to form sulcal lists (, ). Plate 1'''' looked like a rounded wing and was larger than 2'''' (, , ). Plate 2'''' was small and five sided but with a general triangular appearance ().
Figs 31–34. SEM micrographs of Coolia cf. canariensis phylogroup II. Figs 31–32. Oblique-dorsal view. Fig. 33. Antapical view. Fig. 34. Detail of the surface of plate 2′′′. Arrows: extensions of the thecal surface towards the cingulum. Scale bars: (31–33): 10 µm; (34): 4 µm.
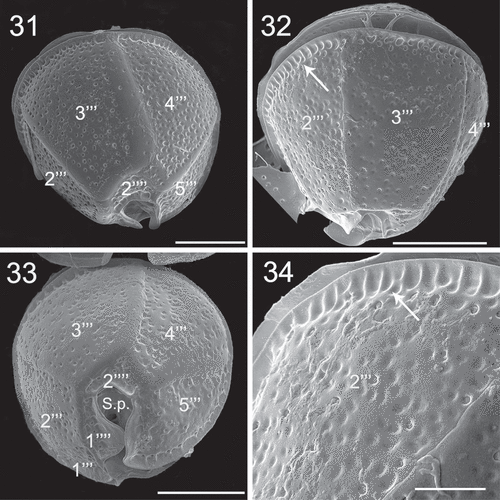
Figs 35–36. SEM micrographs of Coolia cf. canariensis phylogroup II. Fig. 35. Detail of plates surrounding the antapical part of the sulcal region. Fig. 36. Oblique-ventral view. Scale bars: 5 µm.
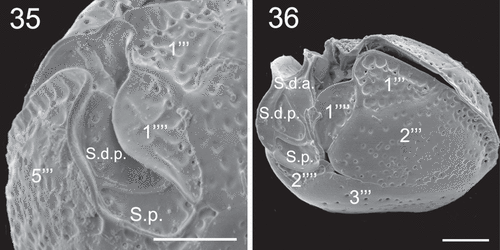
Epithecal plates were smooth with numerous round or oblong pores, and hypothecal plates irregularly bumpy (–, –). On the post-cingular plates there were extensions of the thecal surface, towards the cingulum, that looked like large and shallow pores alongside the cingulum (, ). These extensions were also found beside the sulcus on the edge of plates 1''' () and 5''' () and on the edges of the antapical plates 1'''' and 2'''' (). The sides of contiguous plates that overlap may show wide growth bands that may have pores (, ). The cingulum was deep and descended about one cingular width (). The sulcus was deep and short, with the anterior part partially hidden by plate 1'''' (). Some sulcal plates were identified ().
Ecology
The C. canariensis complex and C. tropicalis were found associated with Gambierdiscus sp., Prorocentrum lima and Amphidinium sp. as epiphytic on macroalgae in a large rocky tidal pool on the north coast of the oceanic Trindade Island. Coolia tropicalis was also found at a macroalgae reef at Maragogi, together with P. lima, Prorocentrum emarginatum, Ostreopsis cf. ovata and Gambierdiscus sp. C. malayensis was found associated with O. cf. ovata, P. lima, Gambierdiscus excentricus and Amphidinium sp. as epiphytic on macroalgae at Armação dos Búzios, Rio de Janeiro.
Phylogenetic analysis
The alignment of the LSU region comparing 71 sequences of Coolia comprised 678 aligned nucleotides. The final alignment of the ITS region based on 45 sequences included 409 aligned nucleotides. Phylogenetic trees based on LSU and ITS sequences presented six well supported clades that represent the six currently described Coolia species for which there are sequences available on GenBank: C. canariensis complex, C. malayensis, C. monotis, C. palmyrensis, C. santacroce and C. tropicalis. For both markers (LSU and ITS rDNA) strains UNR-02, UNR-25 and UNR-28 grouped with C. malayensis, C. canariensis and C. tropicalis sequences retrieved from GenBank, respectively (–). In addition, LSU sequences obtained for the strains UNR-14, UNR-22, UNR-23, UNR-24 and UNR-27 grouped in the C. tropicalis clade ().
Fig. 39. Phylogenetic tree presenting consensus topology from Maximum likelihood analysis and Bayesian inference based on LSU sequences. Operational taxonomic units (OTUs) are identified by: GenBank acession number/species name/strain code/place of origin (when available). Numbers at nodes are bootstrap values from ML analysis and posterior probabilities from BI, respectively (cut-off = 50% for both analysis). New sequences published in this study are displayed in bold.
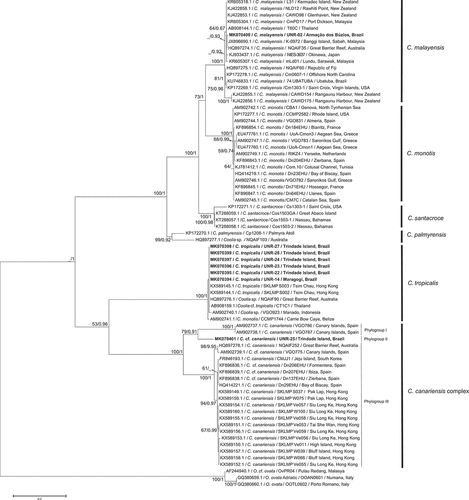
Fig. 40. Phylogenetic tree presenting consensus topology from Maximum likelihood analysis and Bayesian inference based on ITS sequences. Operational taxonomic units (OTUs) are identified by: GenBank acession number/species name/strain code/place of origin (when available). Numbers at nodes are bootstrap values from ML analysis and posterior probabilities from BI, respectively (cut-off = 50% for both analysis). New sequences published in this study are displayed in bold.
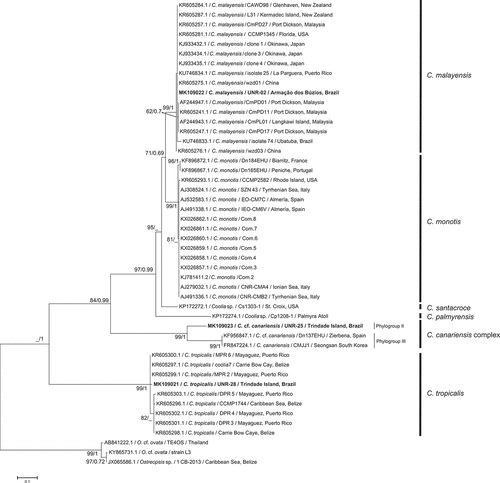
Based on LSU analysis, high bootstrap values (ML) and posterior probabilities (BI) supported the existence of three distinct phylogroups within the C. canariensis complex. Phylogroup I (BS = 100/PB = 1) comprised two C. canariensis sequences from the Canary Islands, Spain: the holotype of the species (strain VGO787), plus one other sequence from the same location (strain VGO786). Phylogroup II comprised strain UNR-25 from Trindade Island, Brazil (BS = 79/PB = 0.91), while phylogroup III (BS = 100/PB = 1) encompassed sequences of C. cf. canariensis from Australia, Hong Kong, South Korea and Spain (). Based on the ITS locus, only two phylogroups of the C. canariensis complex were discernible. Phylogroup II included strain UNR-25 (BS = 99/PB = 1) and phylogroup III encompassed sequences from C. cf. canariensis (BS = 99/PB = 1) (). Phylogroup I from the LSU tree was not present in the ITS tree as there are no sequences available for strains of this clade (strains VGO786 and VGO787).
The genetic distances (p-distance) between Coolia species ranged from 0.1473 (C. malayensis × C. santacroce) to 0.4506 (C. santacroce × C. tropicalis) based on LSU sequences (Supplementary table S1) and from 0.1281 (C. malayensis × C. monotis) to 0.4297 (C. tropicalis × C. canariensis complex) based on ITS locus (Supplementary table S2). The average genetic distance among sequences of the same species ranged from 0.0055 (C. monotis) to 0.1284 (C. palmyrensis) for LSU sequences and from 0.0038 (C. monotis) to 0.1164 (C. canariensis species complex) based on the ITS locus (Supplementary table S3). Genetic distances among ITS sequences of C. palmyrensis and C. santacroce were not calculated because there was only one sequence for each of these species in GenBank.
Concerning the C. canariensis complex, based on the LSU locus, the genetic distance varied between 0.1585 (phylogroup I & II) and 0.2163 (phylogroup I & III). Based on the ITS locus, p-distance was 0.1725 between phylogroups II & III (Supplementary tables S1 and S2). It was not possible to calculate the p-distance between phylogroup I and the other two because there are no ITS sequences available on GenBank for phylogroup I.
Discussion
The topology of the ML and BI trees obtained in the present study agreed with previous studies (Jeong et al., Citation2012; David et al., Citation2014; Karafas et al., Citation2015) for both LSU () and ITS () markers. Based on LSU and ITS sequences, five strains from Trindade Island and one strain from Maragogi were confirmed as C. tropicalis and one strain from Armação dos Búzios was confirmed as C. malayensis. One strain (UNR-25) from Trindade Island represents a new lineage of the C. canariensis complex. The molecular analysis confirmed for the first time the occurrence of C. canariensis and C. tropicalis in the South Atlantic Ocean. Coolia malayensis was reported previously at Ubatuba, Brazil by Gómez et al. (Citation2016).
The LSU and ITS sequences of C. malayensis from Armação dos Búzios (strain UNR-02) are identical to some strains of C. malayensis isolated from elsewhere and slightly different (LSU = 10/762 nt and ITS = 27/468 nt) from the rDNA sequence (GenBank KU746833) of the C. malayensis strain from Ubatuba, which is 335 km away from the site of origin of strain UNR-02. Thus a great molecular diversity of C. malayensis may still be unveiled in the South Atlantic Ocean.
Coolia tropicalis strains isolated from Brazil formed a well supported clade with other strains of C. tropicalis from Australia, Belize, Indonesia, Hong Kong, Thailand and Puerto Rico. The LSU sequences of five strains of C. tropicalis from Trindade Island were identical and were slightly different (10/562 nt) from the strain isolated from Maragogi (UNR-14).
Morphology and genetics (not available for Coolia areolata) have proved to be consistent and clear in differentiating species of Coolia (Fraga et al., Citation2008; Leaw et al., Citation2010, Citation2016; David et al., Citation2014; Karafas et al., Citation2015). Molecular data show that the species C. monotis, C. malayensis, C. palmyrensis and C. santacroce are closely related (C. monotis species complex) and form a sister group to the clade comprising C. tropicalis and C. canariensis complex (Fraga et al., Citation2008; Leaw et al., Citation2010, Citation2016; David et al., Citation2014; Karafas et al., Citation2015). Coolia santacroce appears to be a cryptic species within the C. monotis complex. It is genetically distinct but morphologically very similar to C. monotis and C. malayensis (Karafas & Tomas, Citation2015). In C. santacroce morphological characteristics which were initially presumed to be unique for the species, such as the ratio of APC size to cell size and bimodal pore sizes, have since been shown to be subject to plasticity and geographic variation (Karafas & Tomas, Citation2015).
Notably, C. monotis, C. malayensis, C. palmyrensis and C. santacroce share common general morphological features, the fourth apical plate (4') is oblong and narrow and positioned on the left side of the epitheca (Karafas et al., Citation2015; Leaw et al., Citation2016). Moreover, the width:length (W:L) ratio of the 6'' plate in these species is similar (mean 1.5–1.7, Karafas et al., Citation2015) and the 5'' plate is the largest of the epitheca (Fraga et al., Citation2008; Leaw et al., Citation2010, Citation2016; David et al., Citation2014; Karafas et al., Citation2015; Gómez et al., Citation2016; Leung et al., Citation2017). In the species C. tropicalis, C. areolata and C. canariensis complex, plate 4' is the largest of the epitheca and is centrally positioned (Fraga et al., Citation2008; Jeong et al., Citation2012; David et al., Citation2014) and plate 6'' has a more rectangular shape in these species (Fraga et al., Citation2008; Leaw et al., Citation2010; Jeong et al., Citation2012; David et al., Citation2014; Karafas et al., Citation2015).
In the group comprising the closely related C. monotis, C. malayensis, C. palmyrensis and C. santacroce, cell size, apical pore size and the size and density of pores are the primary characteristics that distinguish C. palmyrensis and C. santacroce from C. monotis and C. malayensis (Karafas et al., Citation2015). Karafas & Tomas (Citation2015) later concluded that C. santacroce is to be referred to as a cryptic species within the C. monotis complex.
The thecal plate ornamentation is the main trait separating C. tropicalis, C. areolata and the C. canariensis complex. While the cell surface is smooth in C. tropicalis, it is heavily areolated in C. areolata and ornamentation is restricted to the hypotheca in the C. canariensis complex (Ten-Hage et al., Citation2000; Fraga et al., Citation2008; David et al., Citation2014; Karafas et al., Citation2015). Considering the C. canariensis complex, the three phylogroups present the same morphology.
Fraga et al. (Citation2008) described the thecal plates surface ornamentation of C. canariensis as thick and smooth and covered with scattered pores, although some postcingular plates, especially 1''' and 5''' may be ornamented with small pits, in the centre of which there is sometimes a pore. The authors also mentioned that plates 3''' and 4' are smoother and apparently thicker than the other plates. The epitheca surface of C. cf. canariensis from Korea was smooth and non-perforated dents were observed in the hypotheca and cingulum and in the bottom of the precingular plates, but the round dents were absent from most parts of the epitheca (Jeong et al., Citation2012). The C. cf. canariensis cells observed by David et al. (Citation2014) had a hypotheca with a rugged texture with irregular bumpy areas, while the epitheca was smaller and smoother.
Cells of C. cf. canariensis phylogroup II (strain UNR-25) had surface ornamentation similar to the other strains of the C. canariensis complex. The epitheca was smooth and the plates in the hypotheca were bumpy and rugged. The edges of the post-cingular plates had ‘extensions’ of the thecal surface towards the cingulum as did the edges of plates 1''', 5''', 1'''' and 2'''' alongside the sulcus. These extensions were also evident along the edges of the plates in the precingular series, and plate 6'' was the least ornamented plate in this series. These extensions are similar to the round non-perforated dents described by Jeong et al. (Citation2012) in the hypotheca, cingulum and in the bottom of some precingular plates of C. cf. canariensis. This ornamentation is also evident in the C. cf. canariensis cells examined by David et al. (Citation2014, see –). In C. areolata, which presents an areolated surface, extensions of the walls separating the areolae were observed in the lists alongside the cingulum and on plate 1''' along the sulcus (see figs 14–15 of Ten-Hage et al., Citation2000). In C. cf. canariensis phylogroup II (strain UNR-25) plates 4' and 6'' seemed to be smooth relative to the other epithecal plates, while plate 3''' seems to be the least rugged plate of the hypotheca, a pattern that is shared with other strains from the C. canariensis complex.
Fraga et al. (Citation2008) described the pores of plate 4' as oval while those of other plates were round in C. canariensis (holotype = strain VGO787). Jeong et al. (Citation2012) and David et al. (Citation2014) reported both round and oval pores in C. cf. canariensis; however, Laza-Martinez et al. (Citation2011) described only round pores in plate 4' of their specimens of C. cf. canariensis. In C.cf. canariensis strain UNR-25 (phylogroup II) plate 4' presents predominantly round pores, but also elongated ones that may be the result of the merging of two pores. It is not clear if the pore shape is a conserved character in the species of Coolia (David et al., Citation2014), and more strains and species need to be evaluated. In some species of the genus Prorocentrum the shape of the pores is probably a variable character and lacks taxonomic relevance (Nascimento et al., Citation2017).
Poroids within the large pores have been observed only in the Korean strain of C. cf. canariensis (Jeong et al., Citation2012). The presence of poroids has been reported in C. malayensis (Leaw et al., Citation2010; Jeong et al., Citation2012 and current study), C. monotis (Laza-Martinez et al., Citation2011) and C. santacroce (Karafas & Tomas, Citation2015). Poroids were not seen in C. cf. canariensis strain UNR-25 (phylogroup II), but this may be due to the presence of mucilage that covers the pores.
Morphological differences among species of Coolia, and other benthic dinoflagellate genera such as Gambierdiscus and Ostreopsis, is often subtle, making species identification challenging without molecular confirmation. The C. canariensis complex comprises three phylogroups that currently cannot be differentiated by morphology. Genetic divergence between phylogroups of the C. canariensis complex are compatible with genetic distances between some pairs of Coolia species. For example, based on the LSU locus, the p-distance values between phylogroups varied from 0.1585 (phylogroup I & II) and 0.2163 (phylogroup I & III) (Supplementary table S1). These values were in the same order, or slightly higher than those observed between C. malayensis & C. santacroce (0.1473), C. malayensis & C. monotis (0.1599) and C. monotis & C. santacroce (0.1519) (Supplementary table S1).
Similar results were observed when ITS sequences were compared. The p-distance between phylogroup II & III was 0.1725, higher than those observed between C. malayensis & C. monotis (0.1281), C. malayensis & C. santacroce (0.1520) and C. monotis & C. santacroce (0.1668) (Supplementary table S2). Litaker et al. (Citation2007) compared the genetic distance (p) between 81 dinoflagellate species belonging to 14 genera and verified that it ranges from 0.042 to 0.580 substitutions per site. They proposed that a between-species genetic distance of ≥ 0.04 could be used to delimit most dinoflagellate species. Considering the ITS sequences, the p-distance observed between phylogroups II & III (0.1725) far exceeds this value. There are no ITS sequences available for phylogroup I, however, the LSU locus revealed that the divergence between phylogroup I & III is greater than the genetic distance between phylogroup II & III.
There is some agreement in the literature that C. canariensis (phylogroup I, –) and C. cf. canariensis (phylogroup III, –) are probably different taxa barring further morphological investigation (Jeong et al., Citation2012) and that the two clades represent two cryptic species (David et al., Citation2014).
In the C. canariensis complex there is enough evolutionary divergence between phylogroups I, II & III to consider them as separate taxa, especially between phylogroup I + II vs phylogroup III, although no morphological difference was discernible among species in this complex. They probably represent cryptic species that have recently diverged. Coolia canariensis (phylogroup I) has been reported only from its type locality, the Canary Islands, while C. cf. canariensis (phylogroup III) apparently has a broader distribution and has been found at the Canary Islands (Fraga et al., Citation2008), Jeju Island in Korea (Jeong et al., Citation2012), the Iberian Peninsula (David et al., Citation2014), Hong Kong (Leung et al., Citation2017), and sequences isolated from Australia and Mexico align with strains in this clade (Leung et al., Citation2017). Coolia cf. canariensis from Brazil (phylogroup II) has been found so far solely at Trindade Island. The only representatives of the C. canariensis complex that have been found in sympatry were C. canariensis (phylogroup I) and C. cf. canariensis (phylogroup III) at the Canary Islands (Fraga et al., Citation2008).
The original description of C. monotis, the type species in the genus, encompasses a number of cryptic/pseudo-cryptic species with their own distinct biogeographies (Momigliano et al., Citation2013; Rhodes et al., Citation2014; Wakeman et al., Citation2015, Leaw et al., Citation2016). Based on molecular data and subtle morphological differences, strains previously designated as C. monotis were reassigned to C. malayensis, C. santacroce and C. palmyrensis (Karafas et al., Citation2015). Leaw et al. (Citation2016) confirmed C. malayensis as a distinct species from C. monotis based on genetics (LSU rDNA and ITS2 phylogenies, and compensatory base changes analyses) and subtle morphological features such as cell size, APC length and plate 3''' size, and proposed the use of the ITS2 rRNA transcript as a potential mini barcode for Coolia species to aid in species identification.
Recently John et al. (Citation2014) reviewed the taxonomic status of species in the Alexandrium tamarense species complex, that are assigned to A. tamarense, A. fundyense or A. catenella based on morphological characters that are not consistent and/or distinctive. These authors combined data on morphology, ITS genetic distances, ITS2 compensatory base changes, mating incompatibilities, toxicity, sxtA toxin synthesis gene and rDNA phylogenies, and concluded that these are five distinct cryptic species according to the ribosomal phylogeny. Phylogenies based on multiple regions in the rDNA operon indicate that the sequences from morphologically indistinguishable isolates of the A. tamarense species complex partition into five clades (John et al., Citation2014). The A. tamarense species complex is among the most widely distributed HAB-causing taxa globally and has been extensively studied.
Fewer studies have addressed the species in the C. canariensis complex. The genetic differences between C. canariensis phylogroups are higher than that for currently accepted species in the genus, but their morphology is similar. Additional data regarding subtle morphological differences, phylogenetic assessment based on ITS sequences (currently lacking for the original C. canariensis – phylogroup I in this study) and secondary structure modelling of compensatory base changes (CBCs) within the ITS2 domain, along with the analysis of cell toxicity from globally distributed isolates, should be acquired to strengthen the taxonomic knowledge of these cryptic species.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2019.1599449
Supplementary table S1. p-distances between Coolia species based on LSU sequences.
Supplementary table S2. p-distances between Coolia species based on ITS sequences.
Supplementary table S3. p-distances within Coolia species based on LSU and ITS sequences.
Author contributions
S.M. Nascimento: isolation and cultivation of strains, scanning electron microscopy, original concept, drafting and editing manuscript; R.A.F. Silva: optical and electron microscopy; F.A. Oliveira: analysis of molecular data, F. Salgueiro: analysis of molecular data, drafting and editing manuscript; S. Fraga: editing manuscript.
TEJP-2018-0125-File017.docx
Download MS Word (13.3 KB)TEJP-2018-0125-File016.docx
Download MS Word (14.2 KB)TEJP-2018-0125-File015.docx
Download MS Word (14.7 KB)Acknowledgements
Carlos E.L. Ferreira, Bianca Del B. Sahm and Livia P. Azevedo are acknowledged for sampling of benthic dinoflagellates at Trindade Island. Joel F. dos Santos and Flávio J.H.T.V. Ramos from Instituto Militar de Engenharia are acknowledged for assistance with SEM. F.A.O. and R.F.A.S. acknowledge Universidade Federal do Estado do Rio de Janeiro for undergraduate research scholarships.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Besada, E.G., Loeblich, L.A. & Loeblich, I.A.R. (1982). Observations on tropical, benthic dinoflagellates from ciguatera-endemic areas: Coolia, Gambierdiscus, and Ostreopsis. Bulletin of Marine Science, 32: 723–735.
- David, H., Laza-Martinez, A., Miguel, I. & Orive, E. (2014). Broad distribution of Coolia monotis and restricted distribution of Coolia cf. canariensis (Dinophyceae) on the Atlantic coast of the Iberian Peninsula. Phycologia, 53: 342–352.
- David, H., Laza-Martinez, Rodríguez F., Fraga, S. & Orive, E. (2018). Coolia spp. (Dinophyceae) in the Canary Islands (NE Atlantic Ocean): the highest species richness observed worldwide. In 18th International Conference on Harmful Algae, Nantes, France.
- Faust, M.A. (1992). Observations on the morphology and sexual reproduction of Coolia monotis (Dinophyceae). Journal of Phycology, 28: 94–104.
- Faust, M.A. (1995). Observation of sand-dwelling toxic dinoflagellates (Dinophyceae) from widely differing sites, including two new species. Journal of Phycology, 31: 996–1003.
- Fraga, S., Penna, A., Bianconi, I., Paz, B. & Zapata, M. (2008). Coolia canariensis sp. nov. (Dinophyceae), a new nontoxic epiphytic benthic dinoflagellate from the Canary Islands. Journal of Phycology, 44: 1060–1070.
- Gómez, F., Qiu, D., Otero-Morales, E., Lopes Rubens, M. & Lin, S. (2016). Circumtropical distribution of the epiphytic dinoflagellate Coolia malayensis (Dinophyceae): morphology and molecular phylogeny from Puerto Rico and Brazil. Phycological Research, 64: 194–199.
- Guillard, R.R.J. (1995). Culture methods. In Manual on Harmful Marine Microalgae. IOC Manual and Guides No. 33 (Hallegraeff, G.M., Anderson, D.M. & Cembella, A.D., editors), 45–56. UNESCO.
- Hall, T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41: 95–98.
- Holmes, M.J., Lewis, R.J., Jones, A. & Wong Hoy, A.W. (1995). Cooliatoxin, the first toxin from Coolia monotis (Dinophyceae). Natural Toxins, 3: 355–362.
- Jeong, H.J., Yih, W.H., Kang, N.S., Lee, S.Y., Yoon, E.Y., Yoo, Y.D., Kim, H.S. & Kim, J.H. (2012). First report of the epiphytic benthic dinoflagellates Coolia canariensis and Coolia malayensis in the waters off Jeju Island, Korea: morphology and rDNA sequences. Journal of Eukaryotic Microbiology, 59: 114–133.
- John, U., Litaker, W., Montresor, M., Murray, S., Brosnahan, M.L. & Anderson, D.M. (2014). Formal revision of the Alexandrium tamarense species complex (Dinophyceae) taxonomy: the introduction of five species with emphasis on molecular-based (rDNA) classification. Protist, 165: 779–804.
- Karafas, S.J. & Tomas, C.R. (2015). Further observations on the genetics and morphometrics of Coolia santacroce (Dinophyceae). Algae, 30: 275–280.
- Karafas, S.J., York, R. & Tomas, C.R. (2015). Morphological and genetic analysis of the Coolia monotis species complex with the introduction of two new species, Coolia santacroce sp. nov. and Coolia palmyrensis sp. nov. (Dinophyceae). Harmful Algae, 46: 18–33.
- Katoh, K. & Standley, D.M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30: 772–780.
- Kofoid, C.A. (1909). On Peridinium steini Jörgensen, with a note on the nomenclature of the skeleton of the Peridinidae. Archiv für Protistenkunde, 16: 25–47.
- Kumar, S., Stecher, G. & Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33: 1870–1874.
- Laza-Martinez, A., Orive, E. & Miguel, I. (2011). Morphological and genetic characterization of benthic dinoflagellates of the genera Coolia, Ostreopsis and Prorocentrum from the south-eastern Bay of Biscay. European Journal of Phycology, 46: 45–65.
- Leaw, C.P., Lim, P.T., Cheng, K.W., Ng, B.K. & Usup, G. (2010). Morphology and molecular characterization of a new species of thecate benthic dinoflagellate, Coolia malayensis sp. nov. (Dinophyceae). Journal of Phycology, 46: 162–171.
- Leaw, C.P., Tan, T.H., Lim, H.C., Teng, S.T., Yong, H.L., Smith, K.F., Rhodes, L., Wolf, M., Holland, W.C., Vandersea, M.W., Litaker, R.W., Tester, P.A., Gu, H., Usup, G. & Lim, P.T. (2016). New scenario for speciation in the benthic dinoflagellate genus Coolia (Dinophyceae). Harmful Algae, 55: 137–149.
- Leung, P.T.Y., Yan, M., Yiu, S.K.F., Lam, V.T.T., Ip, J.C.H., Au, M.W.Y., Chen, C.-Y., Wai, T.-C. & Lam, P.K.S. (2017). Molecular phylogeny and toxicity of harmful benthic dinoflagellates Coolia (Ostreopsidaceae, Dinophyceae) in a sub-tropical marine ecosystem: the first record from Hong Kong. Marine Pollution Bulletin, 124: 878–889.
- Lewis, N. I., Wolny, J.L., Achenbach, J.C., Ellis, L., Pitula, J.S., Rafuse, C., Rosales, D.S. & McCarron, P. (2018). Identification, growth and toxicity assessment of Coolia Meunier (Dinophyceae) from Nova Scotia, Canada. Harmful Algae, 75: 45–56.
- Litaker, R.W., Vandersea, M.W., Kibler, S.R., Reece, K.S., Stokes, N.A., Steidinger, K.A., Millie, D.F., Bendis, B.J., Pigg, R.J. & Tester, P.A. (2003). Identification of Pfiesteria piscicida (Dinophyceae) and Pfiesteria-like organisms using internal transcribed spacer-specific PCR assays. Journal of Phycology, 39: 754–761.
- Litaker, R.W, Vandersea, M.W., Kibler, S.R., Reece, K.S., Stokes, N.A., Lutzoni, F.M., Yonish, B.A., West, M.A., Black, M.N.D. & Tester, P.A. (2007). Recognizing dinoflagellate species using its rDNA sequences. Journal of Phycology, 43: 344–355.
- Meunier, A. (1919). Microplankton de la mer Flamande. 3 Les Peridiniens. Memoirs du Museum Royale d'Hystoire Naturelle Bruxelles, 8: 3–116.
- Mohammad-Noor, N., Moestrup, O., Lundholm, N., Fraga, S., Adam, A., Holmes Michael, J. & Saleh, E. (2013). Autecology and phylogeny of Coolia tropicalis and Coolia malayensis (Dinophyceae), with emphasis on taxonomy of C. tropicalis based on light microscopy, scanning electron microscopy and LSU rDNA1. Journal of Phycology, 49: 536–545.
- Momigliano, P., Sparrow, L., Blair, D. & Heimann, K. (2013). The diversity of Coolia spp. (Dinophyceae Ostreopsidaceae) in the Central Great Barrier Reef Region. PLOS ONE, 8: e79278.
- Nascimento, S.M., Mendes, M.C.Q., Menezes, M., Rodríguez, F., Alves-De-Souza, C., Branco, S., Riobó, P., Franco, J., Nunes, J.M.C., Huk, M., Morris, S. & Fraga, S. (2017). Morphology and phylogeny of Prorocentrum caipirignum sp. nov. (Dinophyceae), a new tropical toxic benthic dinoflagellate. Harmful Algae, 70: 73–89.
- Penna, A., Vila, M., Fraga, S., Giacobbe, M.G., Andreoni, F., Riobó, P. & Vernesi, C. (2005). Characterization of Ostreopsis and Coolia (Dinophyceae) isolates in the western Mediterranean Sea based on morphology, toxicity and internal transcribed spacer 5.88 rDNA sequences. Journal of Phycology, 41: 212–225.
- Rhodes, L. & Tomas, A.E. (1997). Coolia monotis (Dinophyceae): a toxic epiphyitic microalgal species found in New Zealand. New Zealand Journal of Marine and Freshwater Research, 31: 139–141.
- Rhodes, L., Adamson, J., Suzuki, T., Briggs, L. & Garthwaite, I. (2000). Toxic marine epiphytic dinoflagellates Ostreopsis siamensis and Coolia monotis (Dinophyceae) in New Zealand. New Zealand Journal of Marine and Freshwater Research, 34: 371–383.
- Rhodes, L., Smith, K.F., Munday, R., Selwood, A.I., McNabb, P.S., Holland, P.T. & Bottein, M.-Y. (2010). Toxic dinoflagellates (Dinophyceae) from Rarotonga, Cook Islands. Toxicon, 56: 751–758.
- Rhodes, L., Smith, K., Papiol, G.G., Adamson, J., Harwood, T. & Munday, R. (2014). Epiphytic dinoflagellates in sub-tropical New Zealand, in particular the genus Coolia Meunier. Harmful Algae, 34: 36–41.
- Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Sato, S., Nishimura, T., Uehara, K., Sakanari, H., Tawong, W., Hariganeya, N., Smith, K., Rhodes, L., Yasumoto, T., Taira, Y., Suda, S., Yamaguchi, H. & Adachi, M. (2011). Phylogeography of Ostreopsis along West Pacific Coast, with special reference to a novel clade from Japan. PLoS ONE, 6: e27983.
- Scholin, C.A., Villac, M.C., Buck, K.R., Krupp, J.M., Powers, D.A., Fryxell, G.A. & Chavez, F.P. (1994). Ribosomal DNA sequences discriminate among toxic and non-toxic Pseudonitzschia species. Natural Toxins, 2: 155–165.
- Ten-Hage, L., Turquet, J., Quod, J. P. & Couté, A. (2000). Coolia areolata sp. nov. (Dinophyceae), a new sand-dwelling dinoflagellate from the southwestern Indian Ocean. Phycologia, 39: 377–383.
- Tindall, D.R. & Morton, S.L. (1998). Community dynamics and physiology of epiphytic/benthic dinoflagellates associated with ciguatera. In Physiological Ecology of Harmful Algal Blooms (Anderson, D. M., Cembella, A. D. & Hallegraeff, G. M., editors), 293–313. Springer, Heidelberg.
- Wakeman, K.C., Yamaguchi, A., Roy, M.C. & Jenke-Kodama, H. (2015). Morphology, phylogeny and novel chemical compounds from Coolia malayensis (Dinophyceae) from Okinawa, Japan. Harmful Algae, 44: 8–19.