ABSTRACT
The cosmopolitan genus Ceramium Roth (Ceramiaceae), with over 200 currently accepted species, is one of the largest in the Rhodophyta. The genus is characterized by cylindrical or slightly compressed thalli, with axial cells incompletely to completely covered by cortical cells, alternate to pseudo-dichotomous branching and straight to inrolled apices. Despite recent studies, the nomenclature and taxonomy of this genus are still very problematic, linked to the high degree of variation in morphological characters, small thallus sizes, epiphytic habit and the existence of cryptic species. The Mediterranean Sea is reported to have a particularly high diversity of Ceramium species: the North Adriatic Sea, in particular Venice and nearby coasts, was a favourite classical collecting area from where several species were described. In this study we characterize Ceramium sampled from transitional waters of the Venice Lagoon (Italy) by molecular and morphological approaches. Through phylogenetic analyses, using the plastid ribulose-1,5-bisphosphate carboxylase/oxygenase gene (rbcL) as molecular marker, we identify six distinct taxonomic entities, of which four represent taxa not currently recognized in the Venice Lagoon or the wider Mediterranean Sea. One of these is the poorly known species Ceramium connivens Zanardini, which is non-spiny and fully corticated when mature with short naked internodes developing in older axes. Two entities with erect partially corticated thalli without spines, probably misidentified up to now as Ceramium diaphanum (Lightfoot) Roth, correspond to the new species Ceramium rothianum Wolf et al. and to the forgotten species Ceramium nodosum (Kützing) A.W. Griffiths & Harvey. The last entity, characterized by prostrate axes giving rise to narrow erect incompletely corticated, non-spiny axes, is identified as the rare, poorly known species Ceramium incospicuum Zanardini.
Introduction
With 211 currently accepted species (Guiry & Guiry, Citation2019), the genus Ceramium established by Roth (Citation1797) is not only one of the largest genera in the Rhodophyta, but also one of the most systematically complex groups (Maggs et al., Citation2002; Barros-Barreto et al., Citation2006; Wolf et al., Citation2011a). It typically occurs in eulittoral or shallow subtidal habitats and the wide distribution of some species may be associated with their occurrence in hull fouling communities (Boudouresque & Verlaque, Citation2002; Mineur et al., Citation2007; Grosholz et al., Citation2015).
Members of this genus are characterized by cylindrical or slightly compressed thalli with axial cells that are incompletely to fully covered by cortical cells. Branching pattern varies from alternate to pseudo-dichotomous, and the apices can be straight or incurved. In general, tetrasporangia are produced from periaxial or cortical cells, spermatangia occur on cortical nodes and cystocarps are spherical and surrounded by involucral branchlets (Dixon, Citation1960; Hommersand, Citation1963; Womersley, Citation1978; Cho et al., Citation2001).
Traditionally, various vegetative and reproductive features have been used for the classification of Ceramium species, such as branching pattern, degree of apex curvature, presence/absence of cortical spines, number of periaxial cells, diameter of main axes, degree of cortication and tetrasporangial arrangement (Dixon, Citation1960; Womersley, Citation1978; Maggs & Hommersand, Citation1993; South & Skelton, Citation2000; Cho et al., Citation2001).
Taxonomic problems within the genus Ceramium are mostly related to the high degree of variation in these morphological characters, which are often strongly influenced by environmental conditions. Therefore, determining whether different phenotypes represent environmentally influenced growth forms or indicate genetic differences is very difficult (Maggs et al., Citation2002). For example, the names C. diaphanum (Lightfoot) Roth, C. rubrum C. Agardh and C. strictum Roth have been applied erroneously to a wide range of taxa in the genus Ceramium over the past two centuries (Feldmann-Mazoyer, Citation1941; Garbary et al., Citation1978; Rueness, Citation1978; Maggs & Hommersand, Citation1993; Maggs et al., Citation2002; Gabrielsen et al., Citation2003). Moreover, the identification of new species is often complicated by their small size, epiphytic habit and by the existence of cryptic taxa. Another common problem is that studies combining morphological and molecular investigations have been mostly carried out along the Atlantic and Pacific coasts (Maggs et al., Citation2002; Yang & Boo, Citation2004; Skage et al., Citation2005; Barros-Barreto et al., Citation2006; Won & Cho, Citation2011; Hughey & Boo, Citation2016), whereas several valid species names are based on Mediterranean types for which molecular data are not available. Wolf et al. (Citation2011a) revealed that Adriatic samples morphologically ascribable to Atlantic species actually represented phylogenetically distinct taxa.
Moreover, the lack of type material and of detailed morphological descriptions for some species as well as inaccuracies in the original taxonomic treatments make the comparison of newly collected specimens with historical samples very demanding and further complicate their correct identification (Silva et al., Citation1996). Nomenclatural and taxonomic problems are still ongoing, and undescribed taxa are continuously being found in different regions of the world (Maggs et al., Citation2002; Sartoni & Boddi, Citation2002; Cho et al., Citation2003a, Citation2003b; Serio et al., Citation2011; Won & Cho, Citation2011; Hughey & Boo, Citation2016). In particular, in the Northern Adriatic Sea, a well-studied area for algal biodiversity, new species are recorded every year (e.g., Sfriso et al., Citation2010, Citation2012, Citation2014a; Sfriso & Facca, Citation2013; Wolf et al., Citation2011b, Citation2012, Citation2014; Marchini et al., Citation2015). Indeed, this area is very interesting for systematic and ecological investigations, because of the high natural biodiversity of the Mediterranean Sea (Coll et al., Citation2010) and the extensive anthropogenic impacts (e.g. shipping, aquaculture activities, environmental pollution) that favour the introduction and settlement of allochthonous species (Sfriso et al., Citation2010, Citation2012; Sfriso & Facca, Citation2013; Wolf et al., Citation2012, Citation2014). Unfortunately, there have been few studies combining morphological and molecular analyses in this region and the genus Ceramium was investigated only in 2011, when a taxonomic revision compared some Venetian species with Atlantic representatives of the same presumed species (Wolf et al., Citation2011a).
Here we characterize specimens collected at different sites in the Venice Lagoon (Italy) in order to re-examine the presence and biodiversity of Ceramium species in this well-studied area using comparative molecular and morphological data. Through phylogenetic analyses, based on the plastid ribulose-1,5-bisphosphate carboxylase/oxygenase gene (rbcL) as the molecular marker, we identify six distinct taxonomic entities. Two of these entities correspond to C. derbesii and C. nudiusculum, species already recorded for this area (Wolf et al., Citation2011a), while the other four lineages represent taxa new to the Venice Lagoon according to the currently available checklists. The identity of these four entities is discussed and a comparison is made between them and other morphologically similar Ceramium species. In particular, the new species C. rothianum sp. nov. Wolf et al. is recognized for the first time and here described.
Materials and methods
Sampling
Ceramium samples were collected at six sites in the Venice Lagoon () from May 2017 to May 2018. Macrophytes were collected in the framework of programmes assessing the ecological status of the lagoon by applying the Macrophytes Quality Index (MaQI; Sfriso et al., Citation2014b), according to the requirements of the European Water Directive 2000/60/EC. Detailed information for localities and geographic coordinates is provided in Supplementary table S1. Ceramium thalli were found attached to rocks at ~1–2 m depth and epiphytic on Zostera marina L. at 0.5 m depth; samples were collected by scuba divers. For each sample, part was silica gel-dried for molecular analyses and part was preserved in 4% formaldehyde/seawater solution for morphological observations.
Molecular analyses
Genomic DNA was extracted using the Genomic DNA purification kit (Thermo Scientific™). The rbcL gene was obtained by amplification of two overlapping fragments with the primer pairs F57-R753 and F577-RrbcSstart (Freshwater & Rueness, Citation1994), following the PCR conditions listed in Wolf et al. (Citation2011a). The obtained PCR products (about 1200 bp long) were cleaned using the HT ExoSAP-IT (Applied Biosystems™) and sequencing was carried out at the BMR Genomics Sequencing Service (University of Padova, Italy) with the same primers used in the amplification reactions. The SeqMan II program from the Lasergene software package (DNAStar©, Madison, Wisconsin, USA) was used to assemble the final consensus sequences, which were then compared with other publicly available sequences using BLAST v.2.0 software (Altschul et al., Citation1990). The new sequences were deposited in the International Nucleotide Sequence Database Collaboration (INSDC) repositories through the European Nucleotide Archive (ENA) platform with the GenBank accession numbers reported in Supplementary table S1.
To infer the phylogenetic position of the collected specimens, a dataset of 63 rbcL sequences of different Ceramium species was constructed; the most similar sequences according to BLAST and all the sequences of adequate length were included. Spyridia filamentosa (DQ787579) was chosen as outgroup according to Wolf et al. (Citation2011a). Sequences for comparison were downloaded from the USA National Center for Biotechnology Information (NCBI) web server (http://www.ncbi.nlm.nih.gov). A multiple sequence alignment (1200 bp) was obtained using MUSCLE (Edgar, Citation2004; www.ebi.ac.uk/Tools/msa/muscle/).
Phylogenetic analyses were performed with MEGA v. 5.1 (Tamura et al., Citation2011) using Neighbour joining (NJ), Maximum parsimony (MP) and Maximum likelihood (ML) methods. For ML, the model that best fit the data, according to the ModelTest software implemented in MEGA v. 5.1, under the BIC criterion (Schwarz, Citation1978), was TN93 + G. Non-parametric bootstrap re-sampling (Felsenstein, Citation1985) was performed to test the robustness of the tree topologies (1000 replicates).
Bayesian inference (BI) analyses were carried out with MrBayes v.3.1.2 (Ronquist & Huelsenbeck, Citation2003); the nexus files for BI were generated with Mesquite v.2.71 (Maddison & Maddison, Citation2009). The analyses included two separate concurrent MCMC runs, each composed of four chains (three heated and one cold). Each Markov chain ran for 10×106 generations, sampling trees every 100 generations. The sampling of the posterior distribution was considered to be acceptable when the average standard deviation of the split frequencies was ≤0.01. The first 25 000 trees were discarded as burn-in, as determined by the stationarity of the lnL evaluated with Tracer v. 1.5 (Rambaut & Drummond, Citation2007). The consensus topology and posterior probabilities (PP) values were calculated from the remaining trees. The final phylogenetic reconstruction was prepared for publication with CorelDRAW X5. Nucleotide divergences were calculated for each new taxonomic entity in comparison with the sequences of the most closely related species using MUSCLE.
Morphological analyses
Specimens were preserved in 4% formaldehyde/seawater solution, stained when necessary with 0.2% toluidine blue (aqueous solution), and observed using a light microscope (Leica 5000B, Wetzlar, Germany) equipped with a digital image acquisition system.
For cytological observations, thalli were fixed for two hours in 6% glutaraldehyde in 0.1 M cacodylate buffer (pH 6.9), post-fixed in 1% osmium tetroxide in the same buffer for 2 hours, and dehydrated in a graded ethanol series followed by propylene oxide. Samples were then embedded in Araldite resin (Moro et al., Citation2003). 1 µm thick sections were cut using a Reichert Ultracut S ultramicrotome (New York, USA), stained with basic toluidine blue (1% toluidine blue and 1% sodium tetraborate, 1:1 by volume), washed with distilled water, dried, and then observed with a light microscope (Leica 5000B).
To determine if these taxonomic entities represented new species for the Venice Lagoon and the Mediterranean Sea or if they could be attributed to known taxa for which rbcL sequences had not yet been produced, we made a morphological comparison between our samples and those Venetian and Mediterranean species lacking molecular data. The species of Ceramium included in the comparison have been chosen following the latest available check-lists and studies: for the Venice Lagoon, we referred to Sfriso & Curiel (Citation2007) and Wolf et al. (Citation2011a); for the Mediterranean Sea, we followed Gómez Garreta et al. (Citation2001), Sartoni & Boddi (Citation2002), Serio et al. (Citation2006, Citation2011), and Hassoun et al. (Citation2018).
Results
Molecular analyses
The phylogenetic reconstruction based on the rbcL gene () identified a total of 29 samples as belonging to two species already reported from the Venice Lagoon, C. derbesii Solier ex Kützing (four specimens) and C. nudiusculum (Kützing) Rabenhorst (25 specimens). The other 37 specimens were attributed to four other distinct taxonomic entities (tagged as ‘Ceramium sp.#1’, ‘Ceramium sp.#2’, ‘Ceramium sp.#3’ and ‘Ceramium sp.#4’) that could not be identified by comparison with publicly available sequences.
Fig. 2. Phylogenetic reconstruction based on the rbcL gene. Bootstrap values ≥50% and posterior probabilities ≥ 0.70 are indicated at nodes (NJ/MP/ML/BI). Names of Ceramium species reported to be present in the Mediterranean Sea according to the available check-lists are shown in blue. Sequences obtained in this study are highlighted in bold. For each species one sequence per station was included in the analyses. Sampling station and number of samples per site are given in parentheses. The four new species are marked with grey boxes.
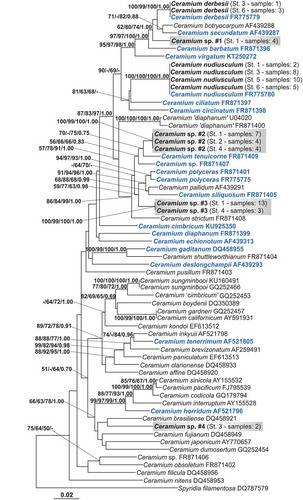
Ceramium sp.#1 (represented by four specimens) was placed in a clade with several fully corticated non-spiny species: C. derbesii, C. botryocarpum A.W.Griffiths ex Harvey, C. secundatum Lyngbye, C. barbatum (J.E.Smith) Duby and C. virgatum Roth. Ceramium sp.#2 (15 specimens) and Ceramium sp.#3 (16 specimens) grouped with some non-spiny partially corticated species: C. tenuicorne (Kützing) Waern, C. polyceras (Kützing) Zanardini, C. pallidum (Kützing) Maggs & Hommersand, C. siliquosum (Kützing) Maggs & Hommersand and C. strictum sensu Harvey (Citation1851, pl. 344). Ceramium sp.#4 (two specimens) was not positioned in a supported clade. The species phylogenetically closest to this entity were two Atlantic taxa, C. brasiliense A.B. Joly and C. fujianum Barros-Barreto & Maggs, and two Pacific ones, C. dumosertum R.E. Norris & Abbott and C. japonicum Okamura.
Nucleotide divergences among the most closely related species were: 2.67% Ceramium sp.#1-C. secundatum; 2.00% Ceramium sp.#2-C. tenuicorne; 3.25% Ceramium sp.#3-C. tenuicorne; 6.08% Ceramium sp.#4-C. brasiliense. The intraspecific divergences calculated for all the sequences included in the analyses ranged from 0% (C. derbesii, C. nudiusculum, Ceramium sp.#2, sp.#3) to 0.83% (C. polyceras).
Morphological characterization of Ceramium spp. #1, #3 and #4
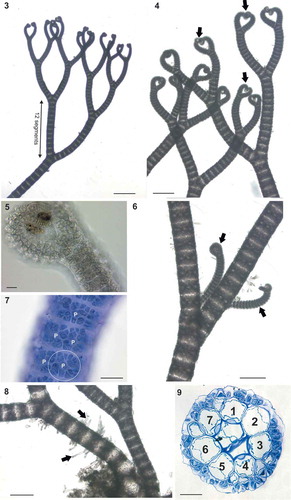
Ceramium sp.#1 (–) thalli were fully corticated, 2–3 cm high, red-brown in colour () and attached to the substratum by a dense mass of multicellular rhizoids (). Axes, 125–250 µm in diameter, were pseudodichotomously branched at intervals of 12–19 segments, with irregular adventitious branches (). Apices were tightly inrolled (, , ) and young axes had narrow cortical gaps visible after staining (); cortical gaps widened in the basal parts due to elongation of the axial cells (). The nodes consisted of 7 periaxial cells, each giving rise to 2 ascending (acropetal) and 2–3 descending (basipetal) filaments including initial cortical cells. In transverse section, cortication resulted in 1–2 layers, 25 µm thick (). In surface view, the cortical cells appeared to be arranged in rosettes around each periaxial cell (). Reproductive structures of Ceramium sp.#1 were not found.
Figs 3–9. Ceramium sp.#1 (identified here as Ceramium connivens). Fig. 3. Axes branching pseudodichotomously at regular intervals of 12 segments. Fig. 4. Axes with tightly inrolled apices (arrows). Fig. 5. Detail of apices. Fig. 6. Young adventitious lateral branches (arrows). Fig. 7. Detail of nodal cortication showing periaxial cells (P), each with two ascending and three descending filaments (see circled example). Fig. 8. Multicellular rhizoids (arrows). Fig. 9. Section of node with 7 periaxial cells. Scale bars: Fig. 3, 500 µm; Figs 4, 6 and 8, 250 µm; Figs 9 and 7, 50 µm; Figs 5, 20 µm.
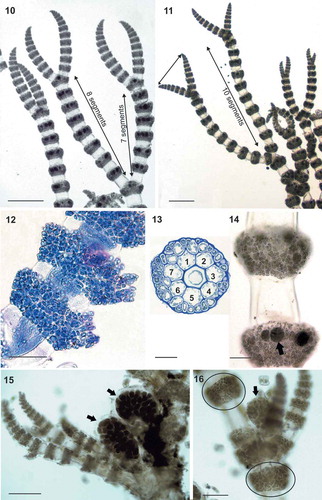
Figs 10–16. Ceramium sp.#3 (identified here as Ceramium nodosum). Figs 10–11. Axes with slightly curved apices, branching pseudodichotomously at intervals of 8 to 10 segments. Fig. 12. Multi-layered nodal cortication in a stained tetrasporangial axis. Fig. 13. Section of a node with 7 periaxial cells. Fig. 14. Tetrasporangia (arrow) entirely covered by cortical filaments. Figs 15–16. Female thalli showing gonimolobes (arrows) surrounded by incurved involucral branchlets. Non-reproductive nodes show periaxial cells giving rise to 2 ascending and 2 descending filaments (circled). Scale bars: Figs 10 and 11, 500 µm; Fig. 15, 200 µm; Fig. 14, 125 µm; Figs 12 and 16, 100 µm; Fig. 13, 50 µm.
Figs 17–24. Ceramium sp.#4 (identified here as Ceramium incospicuum). Fig. 17. Prostrate axes giving rise to erect axes (arrows). Fig. 18. Main axes with abundant multicellular rhizoids (arrows). Fig. 19. Detail of rhizoids showing multicellular pads (arrows). Fig. 20. Section of a node with 7 rounded-quadrangular periaxial cells. Fig. 21. Nodal cortication of young axes showing periaxial cells (P), two acropetal cortical initials (A1) and two basipetal cortical initials (B1). Fig. 22. Nodal cortication of mature axes showing periaxial cell (P) and 2-celled basipetal cortical filaments (arrows). Fig. 23. Exserted tetrasporangia (arrows) protruding from the cortication and covered by a hyaline membrane. Fig. 24. Detail of tetrasporangium contents (arrow) detached from node. Scale bars: Fig. 17, 500 µm; Fig. 18, 250 µm; Fig. 23 and 19, 100 µm; Figs 20, 22 and 24, 50 µm; Fig. 21, 25 µm.
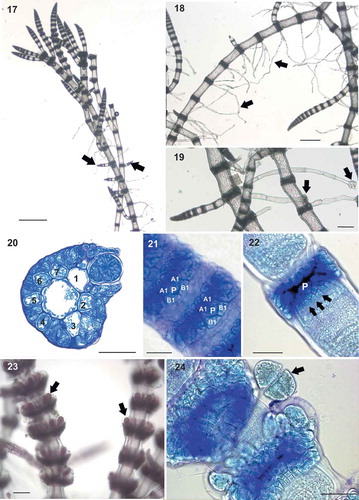
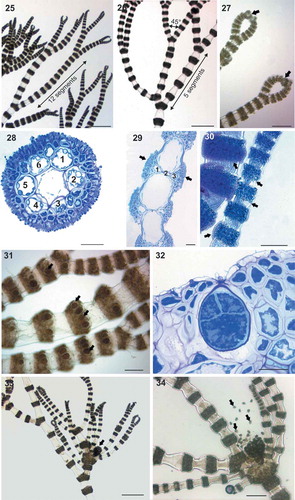
Figs 25–34. Ceramium sp.#2 (identified here as Ceramium rothianum sp. nov.). Figs 25–26. Axes branching pseudodichotomously at intervals of 5 to 12 segments with branch angles of 45°. Fig. 27. Incurved apices (arrows). Fig. 28. Transverse section of the node of male thallus with 6 periaxial cells, cortication and a continuous layer of spermatangia. Fig. 29. Longitudinal section of the male axes showing spermatangial sori (arrows). Fig. 30. Detail of young and mature spermatangial nodes (arrows). Fig. 31. Tetrasporangia (arrows) covered by cortical filaments. Fig. 32. Detail of cruciately divided tetrasporangium. Fig. 33. Gonimolobes (arrows) surrounded by long involucral branchlets. Fig. 34. Gonimolobe detaching carpospores (arrows). Scale bars: Figs 25, 26 and 33, 500 µm; Fig. 34, 250 µm; Figs 27 and 31, 125 µm; Fig. 30, 100 µm; Figs 28, 29 and 32, 30 µm.
Ceramium sp.#3 (–) thalli were small, 0.5–1 cm high, red-brown in colour, with straight to slightly incurved apices (, ). The incompletely corticated axes had a diameter of 100–200 µm and were pseudodichotomously branched at intervals of 7 to 10 segments (, ). Nodes consisted of 7 periaxial cells; although details of nodal construction were difficult to observe due to the reproductive status of the samples and the extensive cortical development, each periaxial cell appeared to give rise to 2 ascending and 2 descending filaments (). Cortication was 20–30 µm thick, composed of 1–3 cell layers (), completely covering the periaxial cells () and resulting in protruding nodes (–). Tetrasporangia, 40–50 µm in diameter, spherical to ovoid, were borne in whorls in every node of fertile axes and were entirely covered by cortical filaments (). Cystocarps consisted of 1–3 globular gonimolobes, 200–300 µm in diameter, and were surrounded by 3 to 4 incurved involucral branchlets (, ). Carposporangia were 20–30 μm in diameter ().
Ceramium sp.#4 (–) thalli were small, consisting of prostrate axes giving rise to erect axes, 1–2 cm high, red-brown in colour and with straight apices (). Axes were very thin, about 80–100 µm in diameter, incompletely corticated, with irregular branching and abundant adventitious branchlets (). Multicellular rhizoids, 300–500 µm long, were cut off downwards from cortical bands of main axes and ended in multicellular pads (, ). Nodes consisted of 7 rounded-quadrangular periaxial cells (), cutting off obliquely two basipetal and two acropetal initial cortical cells (). Each initial acropetal cortical cell produced a small row of elongate cells (). Initial basipetal cortical cells remained undivided except in more mature axes, where basipetal cells divided and formed 1 or 2 rows of cortical cells (). Naked tetrasporangia, 30 × 50 µm in diameter, were borne in whorls in every node of fertile axes, exserted, protruding markedly from the cortication, and covered by a hyaline membrane (, ). No male or female reproductive structures of Ceramium sp.#4 were found.
Description of a new species for Ceramium sp.#2
Ceramium rothianum M.A.Wolf, K.Sciuto, I.Moro, Maggs & A.Sfriso, sp. nov.
Diagnosis: Pseudodichotomously branched thalli, 1–3 cm high, red-brown in colour. Axes 100–200 µm in diameter, incompletely corticated, branching at intervals of 5–12 segments, with branch angles of 30–45° and abundant adventitious branchlets. Apices incurved. 6 periaxial cells per node, each giving rise to 2 acropetal filaments and 2 basipetal filaments, including initial cortical cells. Tetrasporangia borne in whorls, entirely covered by cortical filaments. Tetrasporangia ovate, 40–50 µm in diameter. Spermatangia covering nodes of male thalli. Cystocarps consisting of globular gonimolobes, 200–300 µm in diameter, with a whorl of 5–7 incurved involucral branchlets at maturity. Carposporangia angular, 20–30 μm in diameter. Unique rbcL gene sequences.
Holotype: Voucher A000628 (tetrasporophytic plant), PAD Herbarium, Botanical Garden Padova (Italy)
Type locality: Venice, North Adriatic Sea, Italy.
Etymology: rothianum (neut., adj.) in honour of the German botanist Albrecht Wilhelm Roth, who first described the genus Ceramium.
DNA sequence: LR031258 (rbcL gene).
Description
Ceramium sp.#2 (–) samples were found epiphytic on marine angiosperms (Zostera marina). Thalli were bushy, 1–3 cm high, and red-brown in colour (). Axes were pseudodichotomously branched and distinctly banded (, ). The incompletely corticated axes branched at intervals of 5 to 12 segments, with branch angles of 30° to 45° and abundant adventitious branchlets (, ). Axes were about 100–200 µm in diameter (, ) with strongly incurved apices (). Transverse sections of nodes () showed 6 periaxial cells, which each gave rise to 2 acropetal filaments and 2 basipetal filaments, including initial cortical cells (). Nodes of spermatangial axes had cortication 15–20 µm thick composed of 1–2 internal layers of rounded cortical cells and, externally, by spermatangial sori (, ). The spermatangial sori completely covered the nodes (), with elongate spermatangia cut off obliquely from elongate spermatangial parent cells (, ). Tetrasporangia were borne in whorls in every node of fertile axes and were entirely covered by cortical filaments (). Tetrasporangia were ovate and 40–50 µm in diameter (). Cystocarps consisted of globular gonimolobes, 200–300 µm in diameter, which were surrounded when mature by a whorl of 5 to 7 long incurved involucral branchlets (, ). Gonimolobes contained numerous angular carposporangia, 20–30 μm in diameter ().
Discussion
During this study we analysed 66 samples of Ceramium collected at six sampling sites in the Venice Lagoon. Our molecular analyses, based on the plastid rbcL gene, attributed four of these specimens to C. derbesii Solier ex Kützing and 25 specimens to C. nudiusculum (Kützing) Rabenhorst. These species were reported for the first time from the Venice Lagoon in 2011. C. derbesii is characterized by a fully corticated thallus, while C. nudiusculum is a spiny partially corticated Ceramium (Wolf et al., Citation2011a). Both species are difficult to identify on the basis of morphology due to the lack of clearly distinctive characters. In fact, up to 2002, all the fully corticated species were attributed to C. rubrum Agardh, and only molecular analyses by Maggs et al. (Citation2002) began to separate out some of the different taxonomic entities included in this group, such as C. virgatum Roth, C. secundatum Lyngbye and C. botryocarpum A.W. Griffiths ex Harvey. Until 2011, only one fully corticated species of Ceramium, C. virgatum, had been reported for the Venice Lagoon, although this record has not yet been confirmed by molecular evidence. Molecular analyses have identified fully corticated Ceramium samples from Venice as C. derbesii rather than C. virgatum (Wolf et al., Citation2011a; present study) and it is highly probable that Venetian specimens reported as C. virgatum were (and are) misidentified due to the morphological similarities between these two species.
Classically, spiny partially corticated specimens from the Venice Lagoon were attributed to C. ciliatum (J. Ellis) Ducluzeau, but in 2011 Wolf et al. highlighted the presence of an additional partially corticated spiny species, C. nudiusculum. As in the case of C. virgatum, to date, there are no molecular data confirming the presence of C. ciliatum in the Venice Lagoon; thus, again, we can assume that this species was never present or rare in this area of the North Adriatic Sea and that the spiny species of the lagoon is C. nudiusculum. This hypothesis is also supported by an historical checklist of ‘Istituto Veneto di Scienze, Lettere ed Arti’ that reported specimens of C. nudiusculum in the Venice Lagoon (S. Nicolò dyke, Lido of Venice) from 1847.
The other 37 samples analysed in this study were not identifiable as any previously sequenced species of Ceramium. As shown in the phylogenetic reconstruction (), the specimens divided into four lineages that represent distinct species (Ceramium sp.#1, Ceramium sp.#2, Ceramium sp.#3, Ceramium sp.#4). In fact, the rbcL nucleotide divergences calculated between the sequences of each of these Ceramium entities and the sequences of the most closely related species are much higher than the maximum Ceramium intraspecific divergence (0.83%), and higher than or comparable to the divergences we found between other species of Ceramium: C. secundatum-C. botryocarpum (1.83%), C. derbesii-C. secundatum (2.00%), C. polyceras-C. pallidum (4.75%).
Ceramium sp.#1 (–) is characterized by a non-spiny fully corticated thallus, and it is phylogenetically close to other species with similar morphology: C. secundatum, C. barbatum, C. derbesii, C. virgatum and C. botryocarpum (). C. virgatum, C. derbesii, C. secundatum and C. barbatum are all present in the Mediterranean Sea, while C. botryocarpum is an Atlantic species not reported from the Mediterranean. Although normally fully corticated, some of these species (C. secundatum, C. virgatum and C. botryocarpum) can display short internodal gaps in young axes and longer internodes in older axes (Maggs & Hommersand, Citation1993; Maggs et al., Citation2002). In addition to the above cited species, another fully corticated taxon is reported for the Mediterranean, C. giacconei Cormaci & G. Furnari (Cormaci & Furnari, Citation1991), for which nucleotide sequences are not available. Based on the description reported by Cho et al. (Citation2003b), C. giacconei differs from Ceramium sp.#1 in its branching pattern (irregular in C. giacconei and regularly pseudodichotomous in Ceramium sp.#1), the degree of apex curvature (straight in C. giacconei and inrolled in Ceramium sp.#1) and the number of periaxial cells (9 in C. giacconei and 7 in Ceramium sp.#1).
In particular, Ceramium sp.#1 groups with the other species that have closely inrolled apices and abundant adventitious branching (C. secundatum, C. botryocarpum, C. barbatum and C. derbesii). This clade is sister to C. virgatum, which lacks these features. Moreover, the presence of short naked internodes in basal axes strongly resembles the morphology of specimens of C. secundatum, C. virgatum and C. botryocarpum (Maggs & Hommersand, Citation1993; Maggs et al., Citation2002). The clade formed by Ceramium sp.#1, C. secundatum, C. botryocarpum, C. barbatum, C. derbesii and C. virgatum appears to be Atlantic/Mediterranean, suggesting that rather than representing an introduced taxon Ceramium sp.#1 is a cryptic native species.
Istituto Veneto (Citation1847) published a species checklist for the Venice Lagoon in which Zanardini reported the presence of a new species morphologically very close to C. barbatum: C. connivens Zanardini. This is a poorly known species for which there are no other literature reports. The description of this entity is not exhaustive and resembles specimens previously attributed to C. barbatum. Indeed, Zanardini observed the similarity between these two species and thought that C. connivens could represent a variety of C. barbatum rather than a distinct species. No figures of C. connivens were shown by Zanardini, but some interesting features that he reported for this entity have also been observed in Ceramium #sp.1. In particular, C. connivens had the apices so tightly inrolled that, when put on a herbarium sheet, they repeatedly formed the number ‘8’ “La particolare disposizione delle biforcazioni di questa specie fa sì che il cespuglio disteso sulla carta spesso rappresenti in qualche modo ripetutamente la cifra 8”, as Ceramium #sp.1 does. Also, the terminal dichotomous branching was described by Zanardini as markedly converging “dichotomiis superioribus cancellato-conniventibus” in C. connivens (the basis of the specific epithet). We have observed this also in Ceramium sp.#1, where the final dichotomies converge so much that they form characteristic heart-shaped figures on the herbarium sheet. In addition, the type locality of C. connivens is Venice (North Adriatic, Italy), the same sampling site as our study. This entity appears to be less widespread than the others, as only four specimens of Ceramium sp.#1 were found during our survey. For all these reasons, we hypothesize that Ceramium sp.#1 is identifiable as C. connivens, and report this species in the Venice Lagoon for the first time since 1847. Moreover, we confirm that this entity represents a distinct species and not a variety of C. barbatum, in light of the nucleotide divergence found between these two taxa which is greater than the maximum observed intraspecific divergence for Ceramium.
Ceramium sp.#2 (–) and Ceramium sp.#3 (–) are characterized by erect partially corticated thalli without spines and, together with C. nudiusculum, they were the most abundant Ceramium forms collected during the present survey, found as epiphytes of Zostera marina. Ceramium sp.#2 and Ceramium sp.#3 are phylogenetically related to other Mediterranean species with very similar morphological features: C. tenuicorne (Kützing) Waern, C. polyceras (Kützing) Zanardini and C. siliquosum (Kützing) Maggs & Hommersand (). Because of their overlapping habits, for most of the past two centuries all these species were erroneously assigned to C. diaphanum (Lightfoot) Roth together with other taxa, constituting the C. diaphanum complex sensu Feldmann-Mazoyer (Citation1941).
Up to now, the following non-spiny partially corticated species have been reported for the Venice Lagoon: C. cimbricum H.E. Petersen, C. circinatum (Kützing) J. Agardh, C. codii (H.Richards) Mazoyer, C. deslongchampsii Chauvin ex Duby, C. diaphanum, C. polyceras, C. siliquosum and C. tenerrimum (G.Martens) Okamura (Sfriso & Curiel, Citation2007; Wolf et al., Citation2011a). These taxa were all included in our molecular analyses with the exception of C. codii due to the lack of rbcL sequences for this species. Based on the description of South & Skelton (Citation2000), C. codii differs from Ceramium sp.#2 and Ceramium sp.#3 in the number of periaxial cells (4 in C. codii, 6–7 in Ceramium sp.#2 and Ceramium sp.#3) and the disposition of tetrasporangia (2 for each node in C. codii; 3 or more in whorls in Ceramium sp.#2 and Ceramium sp.#3). For this reason, we can affirm that Ceramium sp.#2 and Ceramium sp.#3 are distinct from all the currently known species of the Venice Lagoon with a similar morphology.
In addition to the Venetian taxa, in the wider Mediterranean area the following additional non-spiny incompletely corticated species with erect thalli have been reported: C. bertholdii Funk, C. graecum Lazaridou & Boudouresque, C. cormacii Serio, Catra, Collodoro & Nisi and C. strobiliforme G.W. Lawson & D.M. John. Based on the description by Funk (Citation1922), C. bertholdii differs from our samples in the diameter of axes (300–400 µm in C. bertholdii, maximum 200 µm in Ceramium sp.#2 and Ceramium sp.#3) and the characteristic form of the apices (rounded to truncate and never incurved in C. bertholdii, strongly inrolled in Ceramium sp.#2 and straight to slightly incurved in Ceramium sp.#3). Based on Serio et al. (Citation2011), C. cormacii differs from Ceramium sp.#2 and Ceramium sp.#3 in the tetrasporangial arrangement (1–2 tetrasporangia per node, partially protruding from the cortex in C. cormacii; 3 or more tetraporangia in whorls completely covered by cortical cells in Ceramium sp.#2 and Ceramium sp.#3). C. graecum can be distinguished from our species by the larger number of periaxial cells (about 10) (Lazaridou & Boudouresque, Citation1992), while C. strobiliforme is smaller than our species (1–3 mm) and is characterized by tetrasporangia borne apically (Cormaci et al., Citation1992).
Ceramium sp.#2 and Ceramium sp.#3 cannot be attributed to any valid species included in recent checklists from the Mediterranean Sea and, up to now, they have probably been misidentified as C. diaphanum. This complex represents a taxonomic nightmare for phycologists and in the past many species were placed in synonymy with C. diaphanum. In this context, we here describe the new species C. rothianum Wolf et al. for Ceramium sp.#2, an entity that is morphologically very similar to C. diaphanum, but phylogenetically clearly separated ().
Species not known from the Mediterranean and not included in the phylogenetic analyses but showing morphological characters similar to C. rothianum sp. nov. are: C. aduncum Nakamura, C. arenarium Simons, C. australe Sonder, C. floridanum J. Agardh, C. macilentum Agardh, C. marshallense Dawson and C. tasmanicum (Kützing) Womersley. Morphological details of these species are listed in .
Table 1. Diagnostic characters of Ceramium rothianum sp. nov. compared with morphologically similar extra-Mediterranean species.
C. aduncum is present over a wide geographical range comprising Asia, Western Australia and Pacific islands (Guiry & Guiry, Citation2019). This species (Lagan & Trono, Citation2017) differs from C. rothianum in the number of periaxial cells and in the tetrasporangial arrangement. C. arenarium is a small species endemic to Southern African coasts (Anderson et al., Citation2016). It differs from C. rothianum in the thallus length and the number of periaxial cells. Australian C. australe (Womersley, Citation1998) differs from C. rothianum in the presence of pseudoperiaxial cells and in the tetrasporangial arrangement. Another species morphologically similar to C. rothianum is C. floridanum from Florida (North America). This taxon is present also in the Caribbean Islands and in South America (Guiry & Guiry, Citation2019). The main difference between C. floridanum and C. rothianum is the branching pattern (Taylor, Citation1960; Stegenga & Vroman, Citation1987). C. macilentum is native to South Australia and widely distributed in America, Asia, Africa, and the Pacific and Indian islands (Guiry & Guiry, Citation2019). According to the description of South & Skelton (Citation2000), it differs from C. rothianum in the tetrasporangial arrangement. Another species widely distributed in these areas is C. marshallense. This taxon (Lagan & Trono, Citation2017) differs from C. rothianum in the thallus length and in the periaxial cells. The last species considered in this comparison is C. tasmanicum, distributed in Australia and New Zealand (Guiry & Guiry, Citation2019). The major differences between C. tasmanicum (Womersley, Citation1998) and C. rothianum are the diameter of the main branches, the number of periaxial cells and the presence of long slender hairs formed profusely from the terminal acropetal and outer cortical cells of young branches ().
C. rothianum sp. nov. is phylogenetically related to the other erect partially corticated species evaluated in this study (). Ceramium sp.#3 can morphologically be attributed to the C. diaphanum complex and, in particular, it resembles a species currently placed in synonymy with C. diaphanum, C. nodosum (Kützing) A.W. Griffiths & Harvey. This species was described by Kützing (Citation1842) as Hormoceras nodosum, epiphytic on other algae in the Gulfs of Genova and Trieste, and it is characterized by protruding nodes, from which the specific epithet (nodosum = knotted, knobbly) derives. Harvey (Citation1847: pl. 90) transferred it to Ceramium, including several specimens from the Mediterranean and Atlantic coasts. Funk (Citation1922) reported the presence of C. nodosum in the Gulf of Naples together with C. bertholdii and distinguished it from C. bertholdii by the shape of the apices (rounded to truncate and never incurved in C. bertholdii, whereas they are straight to slightly incurved in C. nodosum) and by the cortical bands (typically protruding beyond the axial cells in C. nodosum, while nodes have the same diameter as the internodes in C. bertholdii). Moreover, in the historical Venetian checklist (Istituto Veneto, Citation1847), C. nodosum was reported for the first time from the Venice Lagoon as an epiphyte of Zostera.
Up to now, C. nodosum has been treated as a synonym of C. diaphanum (Guiry & Guiry, Citation2019) given the high morphological similarity between the two species. However, looking at the original description by Kützing (Citation1842), C. nodosum is clearly distinguishable from C. diaphanum because the apices are ‘not clearly forcipate’ “apicibus haud clare forcipatis”. Indeed, Ceramium sp. #3 has straight to slightly incurved apices, while C. diaphanum is recognized to have strongly inrolled forcipate apices (Maggs & Hommersand, Citation1993). Moreover, in our phylogenetic reconstruction, Ceramium sp. #3 is placed far from the true C. diaphanum (, sequence accession: FR871399). In addition, while the type locality of C. diaphanum is Scotland (Maggs & Hommersand, Citation1993), C. nodosum was found for the first time in the Gulfs of Genova and Trieste and later reported also from the Venice Lagoon, where we sampled our Ceramium sp. #3 specimens. For all these reasons, we propose that the name Ceramium nodosum (Kützing) A.W. Griffiths & Harvey should be resurrected and applied to our Ceramium sp.#3 samples.
Ceramium sp.#4 (–) is characterized by a partially prostrate thallus from which thin incompletely corticated, erect non-spiny axes originate. In our phylogenetic reconstruction (), this entity represents a lineage clearly distinct from the other Ceramium species included; moreover, the four phylogenetically closest taxa exhibit contrasting morphologies. C. brasiliense A.B. Joly and C. fujianum Barros-Barreto & Maggs are partially corticated taxa, without spines (Barros-Barreto et al., Citation2006); C. dumosertum R.E. Norris & Abbott, described from Hawaii, is a spiny species (Norris & Abbott, Citation1992); and C. japonicum Okamura is fully corticated (Nakamura, Citation1965).
Four species with incompletely corticated prostrate axes have been reported in the Mediterranean: C. bisporum D.L. Ballantine, C. comptum Børgesen, C. cormacii Serio, Catra, Collodoro & Nisi and C. incospicuum Zanardini (Cormaci et al., Citation1994; Sartoni & Boddi, Citation2002; Serio et al., Citation2006, Citation2011). The first three differ from Ceramium sp.#4 in the number of periaxial cells (4 in C. bisporum and C. comptum; 5–6 in C. cormacii; 7 in Ceramium sp.#4). C. bisporum and C. comptum differ, also, in sporangial features (naked bisporangia in C. bisporum; tetrasporangia tetrahedral and completely involucrate in C. comptum; tetrasporangia in whorls, partially involucrate in Ceramium sp.#4) (Sartoni & Boddi, Citation2002; Serio et al., Citation2011). C. cormacii is reported to have unicellular rhizoids while Ceramium sp. #4 is characterized by multicellular rhizoids.
C. incospicuum is rare and poorly known, described by Zanardini (Citation1839) as “Ceramium filis simplicibus tenuissimis apice rectis, articulis nudis roseis, geniculis cellulosis saturate purpureis”. Cormaci et al. (Citation1994) examined the type material of C. incospicuum and their description corresponds to the morphological characters that we observed in Ceramium sp.#4: thin prostrate axes (80–100 µm in diameter), incompletely corticated, with abundant multicellular rhizoids and straight apices; nodes composed by 2–3 transverse cell rows with pericentral cells that cut off obliquely two basipetal and two acropetal cells. The only difference is that, in our samples, in the cortication of mature axes an additional layer of cortical cells is formed basipetally ().
The type locality for C. incospicuum is Dalmatia, on the eastern coast of the Adriatic Sea, very close to the Venice Lagoon. In addition, this species was reported from the Venice Lagoon by Istituto Veneto (Citation1847), together with C. nudiusculum and C. nodosum. During our survey only two specimens were found, making Ceramium sp.#4 the least abundant entity in the present investigation. For all these reasons, we think that Ceramium sp.#4 represents the rare species C. incospicuum. In the present study, for the first time, we were able to observe and describe the periaxial cells in transverse section () and study tetrasporophytes (, ), adding further diagnostic information to the description of this poorly known species.
The genus Ceramium is a challenging group from a systematic point of view and species are very difficult to distinguish based only on morphology, due to the small sizes and the high phenotypic plasticity. As with other macroalgal genera, molecular data have helped to throw light on the taxonomy of this genus. However, up to now, most molecular studies have been outside the Mediterranean, despite many valid Ceramium species names being based on entities described from this area.
While molecular studies have undoubtedly improved our knowledge not only of Ceramium, but also of other macroalgal taxa with complex systematics, this approach often tends to erect new species for newly found lineages, rather than to look for neglected, but already described species. By contrast, in the present study molecular analyses and morphological/ecological observations were combined with a thorough and accurate literature survey in order to verify if Mediterranean species described in the past, for which molecular data were not previously available, could coincide with four taxonomic lineages distinct from all the other sequenced Ceramium taxa. This investigation allowed the re-recognition of C. connivens, C. nodosum and C. incospicuum in the Mediterranean Sea and the description of C. rothianum Wolf et al., sp. nov., thus updating our knowledge of Ceramium biodiversity in the Venice Lagoon. We hope that further studies will adopt a similar method, reconciling the modern molecular approach and the morphological/ecological comparison with old species descriptions to investigate the biodiversity of Ceramium and other taxonomically complex taxa in the Mediterranean area.
Supplementary Information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2019.1618919
Supplementary table S1. Collection information and GenBank accession numbers of rbcL sequences corresponding to the taxonomic entities identified in this study.
Author contributions
M.A. Wolf: original concept, field sampling, molecular and phylogenetic analyses, morphological observations, manuscript drafting and editing; K. Sciuto: Bayesian Inference analyses, manuscript drafting and editing; V.M. Betto: field sampling, molecular laboratory work; I. Moro: microscopy, financial support; C.A. Maggs: manuscript revision and editing; A. Sfriso: ecological expertise, field sampling planning, and financial support.
TEJP-2018-0122-File008.doc
Download MS Word (42.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Agardh, J.G. (1851). Species genera et ordines algarum, seu descriptiones succinctae specierum, generum et ordinum, quibus algarum regnum constituitur. Volumen secundum: algas florideas complectens. Part 1. C.W.K. Gleerup, Lund.
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215: 403–410.
- Anderson, R.J., Stegenga, H. & Bolton, J.J. (2016). Seaweeds of the South African South Coast. World Wide Web electronic publication, University of Cape Town.
- Barros-Barreto, M.B., McIvor, L., Maggs, C.A. & Ferreira, P.C.G. (2006). Molecular systematics of Ceramium and Centroceras (Ceramiaceae, Rhodophyta) from Brazil. Journal of Phycology, 42: 905–921.
- Boudouresque, C.F. & Verlaque, M. (2002). Assessing scale and impact of ship-transported alien macrophytes in the Mediterranean Sea: alien marine organisms introduced by ships in the Mediterranean and Black Seas. CIESM Workshop Monographs, 20: 53–62.
- Cho, T.O., Boo, S.M. & Hansen, G.I. (2001). Structure and reproduction of the genus Ceramium (Ceramiales, Rhodophyta) from Oregon, USA. Phycologia, 40: 547–571.
- Cho, T.O., Fredericq, S. & Boo, S.M. (2003a). Ceramium inkyuii sp. nov. (Ceramiaceae, Rhodophyta) from Korea: a new species based on morphological and molecular evidence. Journal of Phycology, 39: 236–247.
- Cho, T.O., Riosmena-Rodriguez, R. & Boo, S.M. (2003b). First record of Ceramium giacconei (Ceramiaceae, Rhodophyta) in the North Pacific: developmental morphology of vegetative and reproductive structures. Botanica Marina, 46: 548–554.
- Coll, M., Piroddi, C., Steenbeek, J., Kaschner, K., Ben Rais Lasram, F., Aguzzi, J., Ballesteros, E., Bianchi, C.N., Corbera, J., Dailianis, T., Danovaro, R., Estrada, M., Froglia, C., Galil, B.S., Gasol, J.M., Gertwagen, R., Gil, J., Guilhaumon, F., Kesner-Reyes, K., Kitsos, M. S., Koukouras, A., Lampadariou, N., Laxamana, E., López-Fé de la Cuadra, C.M., Lotze, H.K., Martin, D., Mouillot, D., Oro, D., Raicevich, S., Rius-Barile, J., Saiz-Salinas, J.I., San Vicente, C., Somot, S., Templado, J., Turon, X., Vafidis, D., Villanueva, R. & Voultsiadou, E. (2010). The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS ONE, 5: e11842.
- Cormaci, M. & Furnari, G. (1991). The distinction of Ceramium giacconei sp. nov. (Ceramiales, Rhodophyta) in the Mediterranean Sea from Ceramium cingulatum. Cryptogamie, Algologie, 12: 43–53.
- Cormaci, M., Furnari, G., Alongi, G., Dinaro, R. & Pizzuto, F. (1992). On the occurrence in Sicily of three Florideophyceae new to the Mediterranean Sea. Botanica Marina, 35: 447–449.
- Cormaci, M., Furnari, G., Alongi, G. & Serio, D. (1994). On three interesting marine red algae (Ceramiales, Rhodophyta) from the Mediterranean Sea. Giornale Botanico Italiano, 128: 1001–1006.
- Dixon, P.S. (1960). Studies on marine algae of the British Isles: the genus Ceramium. Journal of the Marine Biological Association of the United Kingdom, 39: 331–374.
- Edgar, R.C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32: 1792–1797.
- Feldmann-Mazoyer, G. (1941). Recherches sur les Céramiacées de la Mediterranée occidentale. Imprimerie Minerva, Paris.
- Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39: 783–791.
- Freshwater, D.W. & Rueness, J. (1994). Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia, 33: 187–94.
- Funk, G. (1922). Über einige Ceramiaceen aus dem Golf von Neapel. Beihefte zum botanischen Centralblatt, 39: 223–247.
- Gabrielsen, T.M., Brochmann, C. & Rueness, J. (2003). Phylogeny and interfertility of North Atlantic populations of ‘Ceramium strictum’ (Ceramiales, Rhodophyta): how many species? European Journal of Phycology, 38: 1–13.
- Garbary, D.J., Grund, D. & McLachlan, J. (1978). The taxonomic status of Ceramium rubrum (Huds.) C. Ag. (Ceramiales, Rhodophyceae) based on culture experiments. Phycologia, 17: 85–94.
- Gómez Garreta, A., Gallardo, T., Ribera, M.A., Cormaci, M., Furnari, G., Giaccone, G. & Boudouresque, C.F. (2001). Checklist of Mediterranean Seaweeds. III. Rhodophyceae Rabenh. 1. Ceramiales Oltm. Botanica Marina, 44: 425–460.
- Grosholz, E.D., Crafton, R.E., Fontana, R.E., Pasari, J.R., Williams, S.L. & Zabin, C.J. (2015). Aquaculture as a vector for marine invasions in California. Biological Invasions, 17: 1471–1484.
- Guiry, M.D. & Guiry, G.M. (2019). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway.
- Harvey, W.H. (1847). Phycologia britannica, or, a history of British sea-weeds: containing coloured figures, generic and specific characters, synonymes, and descriptions of all the species of algae inhabiting the shores of the British Islands Plates 73–144. Reeve & Benham, London.
- Harvey, W.H. (1851). Phycologia britannica, or, a history of British sea-weeds: containing coloured figures, generic and specific characters, synonymes, and descriptions of all the species of algae inhabiting the shores of the British Islands Plates 307–360. Reeve & Benham, London.
- Hassoun, M., Wynne, J.W., Moussa, H., Salhi, G., Zbakh, H., Riadi, H. & Kazzaz, M. (2018). An investigation of members of the tribe Ceramieae (Ceramiaceae, Rhodophyta) occurring on both the Mediterranean and Atlantic shores of Morocco. Algae. An International Journal of Algal Research, 33: 243–267.
- Hauck, F. (1875). Verzeichniss der im Golfe von Triest gesammelten Meeralgen. Österreichische Botanische Zeitschrift, 25: 245–248, 283–287, 316–318, 348–352, 386–390.
- Hommersand, M.H. (1963). The morphology and classification of some Ceramiaceae and Rhodomelaceae. University of California Publications in Botany, 35: 165–366.
- Hughey, J. & Boo, G. (2016). Genomic and phylogenetic analysis of Ceramium cimbricum (Ceramiales, Rhodophyta) from the Atlantic and Pacific Oceans supports the naming of a new invasive Pacific entity Ceramium sungminbooi sp. nov. Botanica Marina, 59: 211–222.
- Istituto Veneto di Scienze, Lettere ed Arti (1847). Atti del Reale Istituto Veneto di Scienze, Lettere ed Arti. II Istituto, Italy.
- Kützing, F.T. (1842). Ueber Ceramium Ag. Linnaea, 15: 727–746.
- Lagan, E.J.C. & Trono, G.C. (2017). Notes on Ceramium Roth and Gayliella TO Cho, LJ McIvor et SM Boo (Rhodophyta, Ceramiaceae) from the Philippines. Philippine Science Letters, 10: 38–49.
- Lazaridou, E. & Boudouresque, C.F. (1992). On a new species of Ceramium, C. graecum (Rhodophyta: Ceramiaceae) from Greece. Botanica Marina, 35: 561–565.
- Maddison, W.P. & Maddison, D.R. (2009). Mesquite: A Modular System for Evolutionary Analysis, Version 2.71. http://mesquiteproject.org.
- Maggs, C.A. & Hommersand, M.H. (1993). Seaweeds of the British Isles, vol. 1, Rhodophyta, part 3A, Ceramiales. HMSO, London.
- Maggs, C.A., Ward, B.A., McIvor, L.M., Evans, C.M., Rueness J. & Stanhope M.J. (2002). Molecular analyses elucidate the taxonomy of fully corticated, nonspiny species of Ceramium (Ceramiaceae, Rhodophyta) in the British Isles. Phycologia, 41: 409–420.
- Marchini, A., Ferrario, J., Sfriso, A. & Occhipinti-Ambrogi, A. (2015). Current status and trends of biological invasions in the Lagoon of Venice, a hotspot of marine NIS introductions in the Mediterranean Sea. Biological Invasions, 17: 2943–2962.
- Mineur, F., Johnson, M.P., Maggs, C.A. & Stegenga, H. (2007). Hull fouling on commercial ships as a vector of macroalgal introduction. Marine Biology, 151: 1299–1307.
- Moro, I., Dalla Vecchia, F., La Rocca, N., Rascio, N. & Andreoli, C. (2003). Ultrastructural and cytochemical study on Plocamium cartilagineum (Plocamiales, Rhodophyta) from Ross Sea (Antarctica). Journal of the Royal Society of New Zealand, 41: 359–371.
- Nakamura, Y. (1965). Species of the genera Ceramium and Campylaephora, especially those of northern Japan. Scientific Papers of the Institute of Algological Research of the Faculty of Sciences of the Hokkaido Imperial University, Sapporo, 5: 119–180.
- Norris, R.E. & Abbott, I.A. (1992). New taxa of Ceramieae (Rhodophyta) from Hawaii. Pacific Science, 46: 453–465.
- Rambaut, A. & Drummond, A.J. (2007). Tracer, version 1.5. http://beast.bio.ed.ac.uk/Tracer.
- Ronquist, F. & Huelsenbeck, J.P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572–1574.
- Roth, A.W. (1797). Catalecta botanica quibus plantae novae et minus cognitae describuntur atque illustrantur. Bibliopolo I.G. Mülleriano, Lipsia.
- Rueness, J. (1978). Hybridization in red alga. In Modern Approaches to the Taxonomy of Red and Brown Algae (Irvine, D.E.G. & Price, J.H., editors), 247–262. Academic Press, London.
- Sartoni, G. & Boddi, S. (2002). Ceramium bisporum (Ceramiaceae, Rhodophyta), a new record for the Mediterranean algal flora. Botanica Marina, 45: 566–570.
- Schwarz, G. (1978). Estimating the dimension of a model. Annals of Statistics, 6: 461–464.
- Serio, D., Alongi, G., Catra, M., Cormaci, M. & Furnari, G. (2006). Changes in the benthic algal flora of Linosa Island (Straits of Sicily, Mediterranean Sea). Botanica Marina, 49: 135–144.
- Serio, D., Catra, M., Collodoro, D. & Nisi, A. (2011). Ceramium cormacii sp. nov. (Ceramiaceae, Rhodophyta), a new Mediterranean species epizoic on loggerhead sea turtles (Caretta caretta). Botanica Marina, 54: 545–550.
- Sfriso, A. & Curiel, D. (2007). Check-list of marine seaweeds recorded in the last 20 years in Venice lagoon and a comparison with the previous records. Botanica Marina, 50: 22–58.
- Sfriso, A. & Facca, C. (2013). Annual growth and environmental relationships of the invasive species Sargassum muticum and Undaria pinnatifida in the lagoon of Venice. Estuarine, Coastal and Shelf Science, 129: 162–172.
- Sfriso, A., Maistro, S., Andreoli, C. & Moro, I. (2010). First record of Gracilaria vermiculophylla (Gracilariales, Rhodophyta) in the Po Delta Lagoons, Mediterranean Sea (Italy). Journal of Phycology, 46: 1024–1027.
- Sfriso, A., Wolf, M.A., Maistro, S., Sciuto, K. & Moro, I. (2012). Spreading and autoecology of the invasive species Gracilaria vermiculophylla (Gracilariales, Rhodophyta) in the lagoons of the north-western Adriatic Sea (Mediterranean Sea, Italy). Estuarine, Coastal and Shelf Science, 114: 192–198.
- Sfriso, A., Buosi, A. & Sfriso A.A. (2014a). On the occurrence of Uronema marinum Womersley (Chaetophorales, Chlorophyta) in the north-western lagoons of the Adriatic Sea, Mediterranean Sea (Italy). Mediterranean Marine Science, 15: 101–105.
- Sfriso, A., Facca, C., Bonometto, A. & Boscolo, R. (2014b). Compliance of the Macrophyte Quality index (MaQI) with the WFD (2000/60/EC) and ecological status assessment in transitional areas: the Venice lagoon as study case. Ecological Indicators, 46: 536–547.
- Silva, P.C., Basson, P.W. & Moe, R.L. (1996). Catalogue of the benthic marine algae of the Indian Ocean. University of California Publications in Botany, 79: 1–1259.
- Skage, M., Gabrielsen, T.M. & Rueness, J. (2005). A molecular approach to investigate the phylogenetic basis of three widely used species groups in the red algal genus Ceramium (Ceramiales, Rhodophyta). Phycologia, 44: 353–360.
- South, G.R. & Skelton, P.A. (2000). A review of Ceramium (Rhodophyceae, Ceramiales) from Fiji and Samoa, South Pacific. Micronesica, 33: 45–98.
- Stegenga, H. & Vroman, M. (1987). Notes on some Ceramiaceae (Rhodophyta) from Curaçao, especially those from the exposed northeast coast. Blumea, 32: 397–426.
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28: 2731–2739.
- Taylor, W.R. (1960). Marine algae of the eastern tropical and subtropical coasts of the Americas. University of Michigan Press, Ann Arbor, MI.
- Wolf, M.A., Sciuto, K., Maggs, C.A., Barros-Barreto, M.B., Andreoli, C. & Moro, I. (2011a). Ceramium Roth (Ceramiales, Rhodophyta) from Venice lagoon (Adriatic Sea, Italy): comparative studies of Mediterranean and Atlantic taxa. Taxon, 60: 1584–1595.
- Wolf, M.A., Sfriso, A., Andreoli, C. & Moro, I. (2011b). The presence of exotic Hypnea flexicaulis (Rhodophyta) in the Mediterranean Sea as indicated by morphology, rbcL and cox1 analyses. Aquatic Botany, 95: 55–58.
- Wolf, M.A., Sciuto, K., Andreoli, C. & Moro, I. (2012). Ulva (Chlorophyta, Ulvales) biodiversity in the North Adriatic Sea (Mediterranean, Italy): cryptic species and new introductions. Journal of Phycology, 48: 1510–1521.
- Wolf, M.A., Sfriso, A. & Moro, I. (2014). Thermal pollution and settlement of new tropical alien species: the case of Grateloupia yinggehaiensis (Rhodophyta) in the Venice Lagoon. Estuarine, Coastal and Shelf Science, 147: 11–16.
- Womersley, H.B.S. (1978). Southern Australian species of Ceramium Roth (Rhodophyta). Australian Journal of Marine and Freshwater Research, 29: 205–257.
- Womersley, H.B.S. (1998). The Marine Benthic Flora of Southern Australia. Rhodophyta. Part IIIC. Ceramiales – Ceramiaceae, Dasyaceae. State Herbarium of South Australia, Government of South Australia.
- Won, B.Y. & Cho, T.O. (2011). Ceramium riosmenae sp. nov. (Ceramiaceae, Rhodophyta): a new complete corticated species on Gracilaria from Baja California Sur, Mexico. Algae, 26: 289–297.
- Yang, E.C. & Boo, S.M. (2004). Evidence for two independent lineages of Griffithsia (Ceramiaceae, Rhodophyta) based on plastid protein-coding psaA, psbA and rbcL gene sequences. Molecular Phylogenetics and Evolution, 31: 680–688.
- Zanardini, G. (1839). Sulle alghe. Lettera alla Direzione della Biblioteca italiana. Biblioteca Italiana, Milano.