ABSTRACT
Coccomyxa Schmidle (Trebouxiophyceae, Chlorophyta) is a genus of green microalgae that has worldwide distribution, with representatives found in a broad range of terrestrial and aquatic ecosystems. Members of this taxon can be free-living and/or associated with other organisms, establishing different types of relationships (e.g. parasitic, symbiotic). The most frequent organisms forming associations with Coccomyxa are fungi and marine animals, most importantly mussels. Moreover, there is a well-documented association between a Coccomyxa strain and Gingko biloba and some Coccomyxa-like microalgae have been found in the bark of other trees. Here we describe a green algal strain found in association with carnivorous plants of the genus Drosera L. The systematics of Coccomyxa based only on classical methods is problematic, due to the very simple morphology of the genus and its high phenotypic plasticity. Therefore, the microalga associated with Drosera plants was isolated, maintained in culture and subjected to an integrative taxonomy approach, including morphological, ultrastructural and molecular analyses, along with ecological considerations. Phylogenetic reconstructions based on the rbcL and 18S rRNA genes and molecular data obtained from the ITS2 spacer, including both sequence and secondary structure analyses, showed that the investigated microorganism belonged to a lineage distinct from those sequenced so far. An accurate taxonomic comparison between the microalga associated with Drosera and each of the species already described for the genus Coccomyxa was also carried out, considering morphology, ultrastructure and ecology. The results as a whole allowed us to erect a new species inside the genus Coccomyxa: Coccomyxa cimbrica K.Sciuto, B.Baldan & I.Moro
Introduction
The genus Coccomyxa (Trebouxiophyceae, Chlorophyta) was first described by Schmidle (Citation1901) to include green coccoid microalgae characterized by very few diagnostic features: irregular elliptical to globular cells, 6–14 × 3–6 μm in size, with a single parietal chloroplast lacking pyrenoids and absence of flagellate stages.
Members of this genus are found in different habitats worldwide and can adopt various lifestyles: there are marine, freshwater and soil free-living strains, and Coccomyxa associated with other organisms (e.g. epiphytic, endophytic, parasitic, symbiotic) (Darienko et al., Citation2015; Malavasi et al., Citation2016; Gustavs et al., Citation2017). In several cases, the association with other organisms is not obligate, since the Coccomyxa strains could be isolated and grown in culture (e.g. Belzile & Gosselin, Citation2015; Sokolnikova et al., Citation2016; Cao et al., Citation2018). The most-studied associations between Coccomyxa and other organisms concern the species C. parasitica R.N. Stevenson & G.R. South infesting different mollusc species (Stevenson & South, Citation1974; Gray et al., Citation1999; Rodríguez et al., Citation2008; Vázquez et al., Citation2010; Sokolnikova et al., Citation2016); the Coccomyxa phycobionts found in several lichens (e.g. Lohtander et al., Citation2003; Zoller & Lutzoni, Citation2003; Muggia et al., Citation2011; Yahr et al., Citation2015); and the supposed symbiosis between Coccomyxa and Gingko biloba L. (Trémouillaux-Guiller et al., Citation2002; Trémouillaux-Guiller & Huss, Citation2007). Moreover, Coccomyxa is one of the most frequently occurring microalgae in the bark of various tree species (e.g. Kulichová et al., Citation2014; Štifterová & Neustupa, Citation2015) and, recently, the species C. antarctica S. Cao & Q. Zhou was described as an epiphyte of the lichen Usnea aurantiacoatra (Jacq.) Bory (Cao et al., Citation2018).
Coccomyxa systematics based only on morphology is difficult both at genus and species levels, and features considered diagnostic have been recently questioned. For example, mucilage formation, once considered useful to discriminate Coccomyxa (huge mucilage production) from Pseudococcomyxa Korshikov (one-sided mucilage cap) and Choricystis (Skuja) Fott (no mucilage production), was shown not to be a diagnostic feature, since this character depends on environmental conditions (Darienko et al., Citation2015). Experiments have also demonstrated high phenotypic plasticity within the Coccomyxa genus in response to changes in environmental parameters such as salinity, which makes it difficult to discriminate among different species (Darienko et al., Citation2015).
As for other taxa, the use of molecular methods in conjunction with more classical approaches have allowed clarification of Coccomyxa systematics. Darienko et al. (Citation2015) recognized seven species in the genus, using integrative taxonomy and focusing on DNA barcoding and secondary structure analyses of 18S rRNA and ITS loci. Later, Malavasi et al. (Citation2016), based on a larger 18S rRNA + ITS region dataset and with particular attention to the ecological characters, detected 27 Coccomyxa species clusters. Currently, Algaebase (Guiry & Guiry, Citation2018) lists 47 species and four infraspecific names for Coccomyxa, out of which 33 are taxonomically accepted.
Here we describe a Coccomyxa strain that was found in association with carnivorous plants of the genus Drosera L. The organism was studied with an integrative taxonomy approach, including morphological, ultrastructural and molecular data, as well as ecological considerations. Molecular analyses were based on three different loci: the rbcL and 18S rRNA genes and the ITS2 spacer in the 18S–28S rRNA ITS region, with a particular focus on the secondary structures. The obtained results allowed us to describe a new species in the genus Coccomyxa: C. cimbrica K.Sciuto, B.Baldan & I.Moro
Materials and methods
Isolation of the microalgal strain from Drosera plants
Plants of Drosera capensis L. and D. rotundifolia L. were collected in 2012 from Palù di Sotto peat bog, in Marcésina plain, north-east of the Asiago plateau, Veneto, Italy and left to grow in sterile boxes containing solid MS1/2 medium (Murashige & Skoog, Citation1962), with the following composition: 2.2 g l–1 of ‘Murashige and Skoog Medium’ (Duchefa Biochemie), 20 g l–1 of saccharose and 0.8% plant agar (Duchefa Biochemie). Plants were kept in a chamber at a temperature of about 24°C and under cool white fluorescent lamps, with a light intensity of 30–50 μmol photons m–2 s–1 and a photoperiod of 16 h light/8 h darkness.
During the plantlet incubation, a light green biofilm was observed on the solid MS1/2 medium, near the base of both the Drosera species (). With the light microscope (Leica 5000, Wetzlar, Germany), the biofilms sampled from both the carnivorous plant species were seen to be composed of a green coccoid microalga.
Fig. 1. Photograph of a culture box where Drosera capensis plants are grown in sterile conditions. Inset shows magnification of one of the plants, at the base of which a green biofilm can be observed (arrows).
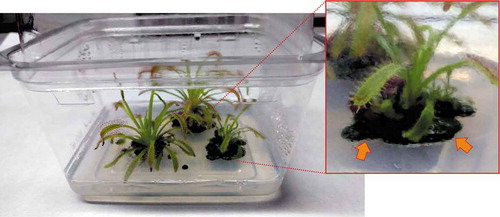
In order to eliminate microorganisms, explants of Drosera stem and leaf tissue (used to propagate plants) were washed using the protocol by Guidolin (Citation2012), including washing steps with ethanol 70% and sodium hypochlorite at different concentrations. The sterilized Drosera explants were incubated in new sterile boxes under the above reported growth conditions, but, after a few weeks, the microalgal biofilm was still observed in the boxes.
The microalga was isolated from the solid growth medium of the plant boxes, and cultures were set up under the same growth conditions as the Drosera plants in order to characterize and identify this entity. For practical purposes the microorganism isolated from D. capensis was named ‘DC isolate’ and the one sampled from D. rotundifolia was tagged as ‘DR isolate’. The isolated microalga was grown in MS1/2 medium, both solid and liquid, but it was also able to grow in Czurda liquid medium (Aaronson, Citation1970) and BG11 liquid medium (Rippka et al., Citation1979).
DNA extraction and amplification of selected molecular markers
Culture aliquots of DC and DR isolates were ground in a mortar with liquid nitrogen and quartz sand (Honeywell Fluka), and total genomic DNA was extracted with Genomic DNA Purification Kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA) following the manufacturer’s recommendations.
For the rbcL gene, an amplicon of about 700 bp was obtained with the primer pair ScenRub_F1 and ScenRub_R1, according to Sciuto et al. (Citation2015).
The 18S rRNA gene was obtained from the amplification of two partially overlapping fragments, using the primers listed in Supplementary table S1; the first part of the 18S rRNA (about 900 bp long) was amplified according to Andreoli et al. (Citation1999), while the second part (about 1200 bp long) was amplified as follows: a first step at 95°C for 5 min; 30 cycles of 95°C for 50 s, 52°C for 50 s, and 72°C for 1 min and 30 s; and a final step at 72°C for 8 min.
An amplicon of about 800 bp was obtained for the ITS region (including part of the 18S rRNA gene, the ITS1 spacer, the 5.8S rRNA gene, the ITS2 spacer and part of the 28S rRNA gene) with the primers ITS1 (Hall et al., Citation2010) and ITS4 (White et al., Citation1990), according to Hall et al. (Citation2010).
PCR protocols were carried out in 50 µl aliquots with the Taq DNA polymerase (Thermo Fisher Scientific, Waltham, Massachusetts, USA) following the producer’s instructions.
Amplification products were verified by agarose gel electrophoresis and purified with HT ExoSAP-IT High-Throughput PCR Product Cleanup (Thermo Fisher Scientific, Waltham, Massachusetts, USA) before sequencing. Sequencing was performed at the BMR Genomics Sequencing Service (University of Padova) with the same primer pairs used for amplification. In the case of the 18S rRNA gene, additional sequencing primers were used to obtain a full-length sequence (Supplementary table S1); of which primers CoccoF1 (5′-GACACAAGGAGGTAGTGA-3′, forward primer) and CoccoR1 (5′-GCGKAKGCCTGCTTTG-3′, reverse primer) were designed in this study.
Final consensus sequences were assembled with the help of the SeqMan II program from the Lasergene software package (DNAStar©, Madison, Wisconsin, USA) and then compared with the sequences available in the INSDC (International Nucleotide Sequence Database Collaboration) archives using the BLAST tool (Altschul et al., Citation1990).
For each of the considered loci, the sequences of the DC and DR isolates were aligned with MUSCLE (Edgar, Citation2004) and showed 100% identity in their overlapping part (they differed by a few bases in length). Therefore, for each molecular marker, only the longest of the obtained sequences (691 bp for the rbcL gene, 1773 bp for the 18S rRNA gene, 773 bp for the ITS region) was deposited in the INSDC repositories, through the ENA (European Nucleotide Archive) platform, with the following accession numbers: LS999579 (rbcL gene), LS999578 (18S rRNA gene), LS999580 (18S–28S rRNA ITS).
Phylogenetic analyses based on rbcL and 18S rRNA genes
Separate datasets were created for the rbcL and 18S rRNA genes, including the sequences obtained in this study and other sequences available for the genus Coccomyxa in the INSDC archives, and used to obtain multiple alignments with MUSCLE (Supplementary figs S1, S2). The sequences of Elliptochloris bilobata SAG 245.80 and Hemichloris antarctica SAG 62.90 were chosen as outgroups. The rbcL dataset included 25 sequences, for a total of 564 aligned positions, out of which 160 were parsimony-informative. The 18S rRNA alignment included 77 sequences and a total of 2613 aligned positions, including the presence of long insertions at different points of the locus for given taxa (Supplementary fig. S2); there were 114 parsimony-informative sites.
Phylogenetic analyses based on Neighbour joining (NJ) and Maximum parsimony (MP) methods were carried out with MEGA version 5 (Tamura et al., Citation2011), while Maximum likelihood (ML) method analyses were performed with the program PHYML version 3.065 (Guindon & Gascuel, Citation2003). The robustness of NJ, MP and ML tree topologies was tested through non-parametric Bootstrap (BT) re-sampling (Felsenstein, Citation1985) with 1000 replicates.
Bayesian inference (BI) analyses were carried out using MrBayes version 3.1.2 (Ronquist & Huelsenbeck, Citation2003), after having generated the nexus files with the program Mesquite version 2.71 (Maddison & Maddison, Citation2009). The BI analyses consisted of two separate concurrent Markov chain Monte Carlo (MCMC) runs, each composed of four chains (three heated and one cold). Each MCMC ran for 1 × 106 generations for the rbcL dataset and 10 × 106 generations for the 18S rRNA dataset, with trees sampled every 100 generations. At the end of each run, the posterior distribution was considered adequately sampled when the average standard deviation of the split frequencies was ≤ 0.01. The first 2500 trees for the rbcL locus and 25 000 trees for the 18S rRNA gene were discarded as burn-in, as determined by the stationarity of the lnL using Tracer version 1.5 (Rambaut & Drummond, Citation2007). Consensus topologies and posterior probabilities (PP) values were derived from the remaining trees.
For ML and BI analyses, the best fit models were found using jModelTest version 0.1.1 (Posada & Crandall, Citation1998; Posada & Buckley, Citation2004; Posada, Citation2008) under the BIC criterion (Schwarz, Citation1978). For the rbcL dataset, the model that best fit the data was TPM3+G and the following parameters were implemented: substitution rate matrix with A-C substitutions = 0.1478, A-G = 2.7188, A-T = 1.0000, C-G = 0.1478, C-T = 2.7188, G-T = 1.0000; gamma shape = 0.2070. For the 18S rRNA dataset, the model that best fit the data was TIM3+G and the following parameters were implemented: nucleotide frequencies as freqA = 0.2408, freqC = 0.2329, freqG = 0.2994, freqT = 0.2269; substitution rate matrix with A-C substitutions = 1.8989, A-G = 2.1988, A-T = 1.0000, C-G = 1.8989, C-T = 4.3783, G-T = 1.000; gamma shape = 0.07.
Final images of the rbcL and 18S rRNA phylogenetic reconstructions (Supplementary fig. S3, ) were prepared for publication with CorelDRAW Graphics Suite X4.
ITS2 analyses
The ITS region sequences of the investigated strain and of C. antarctica Ua6, for which the ITS2 secondary structure had not been determined, were analysed on the ITS2 Database (Schultz et al., Citation2006; Ankenbrand et al., Citation2015); the ‘Annotate’ tool (Keller et al., Citation2009) was used to detect spacer boundaries and ITS2 secondary structures were obtained by ‘Homology modeling’ (Wolf et al., Citation2005), after having excluded the 5.8S and LSU loci. The 5.8S/LSU stem was manually folded by comparing with the ITS2 secondary structures inferred for Coccomyxa by Darienko et al. (Citation2015), and further constraints were added to some parts of the obtained structures to bring them in line with the other published structures of Coccomyxa (Darienko et al., Citation2015). The predicted secondary structures (, Supplementary fig. S4) were visualized and edited with VARNA version 3.9 (Darty et al., Citation2009) and the ITS2 secondary structure picture of the investigated microalga () was depicted with CorelDRAW Graphics Suite X4.
ITS2 spacer boundaries were also determined based on the ITS region sequences available for C. glaronensis CCALA 306 (AY333646) and other unclassified Coccomyxa strains (KN-2011-C2, KN-2011-C3, KN-2011-S2; INSDC accessions: HE586528, HE586529, HE586548, respectively); these ITS2 sequences were compared with those of strains Coccomyxa sp. KN-2011-C4 (HE586508) and UTEX SNO83 (HE586506), for which the ITS2 secondary structures were determined by Darienko et al. (Citation2015), by aligning them with MUSCLE (Supplementary fig. S5).
An ITS2 sequence + structure dataset was created, adding the ITS2 sequences and secondary structures of the investigated microalga and of C. antarctica Ua6 to the other 43 ITS2 sequences + structures reported by Darienko et al. (Citation2015) for Coccomyxa. The ITS2 sequences + structures of E. bilobata SAG 245.80 and H. antarctica SAG 62.90 were used as outgroups. The ITS2 sequence + structure dataset was aligned with 4SALE version 1.7 (Seibel et al., Citation2006, Citation2008). The generated ITS2 sequence + structure alignment (Supplementary fig. S6) was used to infer Coccomyxa phylogeny with the program ProfDistS version 0.9.9 (Wolf et al., Citation2008), by applying the NJ method with the implemented ITS2 specific sequence-structure GTR substitution model; the robustness of the obtained topology was tested by BT re-sampling (1000 replicates). An alignment of only ITS2 sequences was also exported from 4SALE and used for further phylogenetic analyses with MEGA, based on NJ, MP and ML methods. For the ML analysis, the model that best fit the data, found with Modeltest implemented in MEGA under the BIC criterion, was GTR+G. BT re-sampling (1000 replicates) was applied to test the robustness of the obtained trees.
From the ITS2 sequence + structure alignment, the program 4SALE generated a matrix showing the compensatory base changes (CBCs) among the considered Coccomyxa species. Final images of the ITS2 sequence + structure phylogenetic reconstruction () and of the ITS2 CBC matrix (Supplementary fig. S7) were obtained with CorelDRAW Graphics Suite X4.
Following the method proposed by Darienko et al. (Citation2015), the 73 barcode sites of Coccomyxa consensus ITS2 secondary structure (fig. 3 in Darienko et al., Citation2015) were located on the ITS2 secondary structures inferred for the investigated microalga and for C. antarctica Ua6; successively, each barcode site was codified into a number representing the ITS2 sequence + structure information (A-U = 1, U-A = 2, G-C = 3, C-G = 4, G•U = 5, U•G = 6, mismatch = 7, deletion or unpaired or single bases = 8; Supplementary fig. S8). The numeric barcodes generated in this way for the investigated microalga and C. antarctica Ua6 were manually aligned with other Coccomyxa ITS2 numeric barcodes found by Darienko et al. (Citation2015). The obtained numeric barcode alignment (Supplementary fig. S9) was transformed in nexus format with Mesquite and used for NJ, MP and ML analyses with PAUP version 4.0a162 (Swofford, Citation2002), testing the robustness of the obtained topologies by BT re-sampling (1000 replicates). The final image of the ITS2 numeric barcode phylogenetic reconstruction () was prepared for publication with CorelDRAW Graphics Suite X4.
Microscopy
Culture aliquots of the microalga were observed with a Leica 5000 fluorescence/transmitted light microscope (Leica Microsystems GmbH, Wetzlar, Germany), equipped with a digital image acquisition system. Some cell suspension aliquots were incubated in the dark with 8 μg ml–1 4′,6 diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and visualized with epifluorescence microscopy.
Further aliquots of the strain were fixed in 3% glutaraldehyde in 0.1 M cacodylate buffer (pH 6.9) and prepared for electron microscopy analyses as reported in Moro et al. (Citation2010). Scanning electron microscope observations were carried out with a JSM-6490 JEOL (Akishima, Tokyo, Japan) microscope, operating at 25 kV, and transmission electron microscope observations were performed with a FEI Tecnai G2 (Hillsboro, Oregon, USA) microscope, operating at 100 kV.
A few weeks after the sterilization with ethanol 70% + sodium hypochlorite, Drosera explants from the boxes where the microalga biofilm had been present were observed with a Leica MZ16 stereomicroscope (Leica Microsystems GmbH, Wetzlar, Germany), equipped with a digital image acquisition system, and with the scanning electron microscope, using the above reported procedure.
Final pictures were prepared for publication with CorelDRAW Graphics Suite X4.
Results
During experiments aiming to grow and propagate different Drosera L. species in laboratory conditions (Guidolin, Citation2012), green biofilms were observed in the solid artificial medium, near the base of the young D. capensis and D. rotundifolia plants. With the light microscope, both the green biofilms appeared to be composed of a green, oval-shaped microalga, without any particular diagnostic features; the microalga isolated from D. capensis growth medium was temporarily named ‘DC isolate’, while that sampled from D. rotundifolia boxes was tagged as ‘DR isolate’. Molecular analyses based on different loci were carried out to better identify the microorganisms.
Phylogenetic data
For each of the molecular markers (rbcL, 18S rRNA, ITS region), the sequences obtained from the DC and DR isolates were identical; this confirmed that the microalgal biofilms found on D. capensis and D. rotundifolia growth medium were composed of the same microalgal strain, which was named strain DCDR.
Based on the BLAST search, the rbcL gene fragment (691 bp) obtained from the investigated microalga showed the highest identities (90–93%) with different strains ascribed to the genus Coccomyxa Schmidle but not identified at the species level. Only 22 other sequences of organisms attributed to Coccomyxa could be included in the analyses based on the rbcL marker, due to the scarce availability in public databases. The resulting rbcL phylogenetic reconstruction (Supplementary fig. S3) appeared to be divided into three blocks. The upper part of the tree included strains grouping into different clades, which corresponded to already described Coccomyxa species (C. solorinae Chodat, C. simplex Mainx and C. subellipsoidea E. Acton) and to a cluster already reported by Malavasi et al. (Citation2016) as ‘clade E’. The middle part of the tree was represented by C. dispar Schmidle, type species of the genus. The lower part of the rbcL tree included strain DCDR and Coccomyxa strains not identified at the species level, with the exception of strain 078 (INSDC accession: KT253162) which was reported as ‘C. cf. olivacea’. The Coccomyxa from Drosera plants was not strictly related to any of the analysed strains.
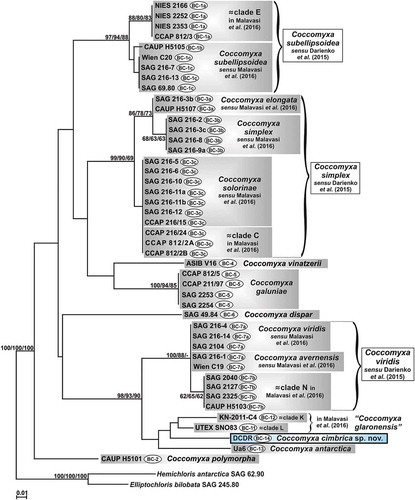
The 18S gene sequence (1773 bp) obtained from the examined microalga was also compared with the sequences available in the INSDC repositories through a BLAST search and the highest identities found (97–99%) were with various Coccomyxa strains, both unidentified at the species level and ascribed to known species (e.g. C. glaronensis Jaag, C. parasitica R.N. Stevenson & G.R. South, C. antarctica S. Cao & Q. Zhou, C. viridis Chodat). In the 18S rRNA phylogenetic reconstruction (), which included many more sequences, 14 clades corresponded to already described species (C. solorinae, C. simplex, C. elongata Chodat & Jagg, C. melkonianii V. Malavasi & P. Skaloud, C. subellipsoidea, C. vinatzerii T. Darienko & T. Pröschold, C. galuniae T. Darienko & T. Pröschold, C. actinabiotis Rivasseau et al., C. polymorpha T. Darienko & T. Pröschold, C. onubensis Garbayo et al. ex J.L. Fuentes et al., C. dispar, C. antarctica, C. avernensis Jaag, C. viridis) and nine clades had been reported in previous papers (clades C, D, E, I, KL, M, N in Malavasi et al. (Citation2016); the clade named as ‘OTU 08’ in Hallmann (Citation2015); the clade containing the two Flensburg fjord strains + strain CPCC 508 + strain CP-01 in Sokolnikova et al. (Citation2016)). Clade KL was temporarily named here as ‘C. glaronensis’ and the clade including the two Flensburg fjord strains, strain CPCC 508, and strain CP-01 as ‘C. parasitica’. A clade constituted by two marine Coccomyxa strains (RCC 891 and RCC 903) was here detected for the first time, and other uncultured Coccomyxa isolates were interspersed in the tree (KN-2011-C10, KN-2011-C15 and an uncultured Coccomyxa from tree bark).
Fig. 2. Phylogenetic reconstruction of the genus Coccomyxa based on 18S rRNA gene analyses. The NJ topology is depicted and the numbers associated with nodes indicate support values for NJ, MP, ML and BI analyses, respectively. Only bootstrap supports ≥50% and posterior probabilities ≥0.95 are reported. Values for nodes that obtained support in only one of the performed phylogenetic analyses were omitted. Horizontal bar represents expected number of nucleotide substitutions per site. Grey boxes represent species according to the most recent classification or OTUs found/described in previous papers. Coccomyxa cimbrica sp. nov. is highlighted with a light blue box. Brackets plus white boxes report the classification by Darienko et al. (Citation2015).
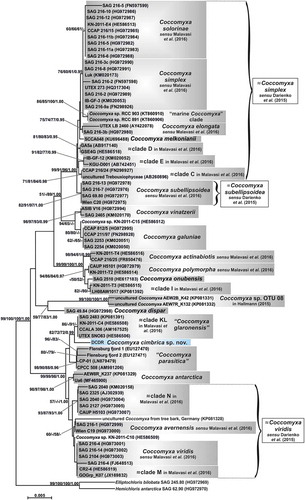
As in the rbcL gene reconstruction, the 18S rRNA phylogeny appeared to be divided into three blocks: the upper part of the tree included most of the lineages and the middle part was represented by C. dispar plus the clade named as ‘Coccomyxa sp. OTU 08’. The lower part of the tree consisted of a well-supported (99/99/98/1.00) group of taxa: ‘C. glaronensis’, ‘C. parasitica’, C. antarctica, C. avernensis, C. viridis, clades M and N, Coccomyxa sp. KN-2011-C10, the uncultured Coccomyxa from tree bark and strain DCDR. This last was sister taxon to clade KL, even if it did not appear strictly related to this lineage nor any other clade of the phylogenetic reconstruction.
ITS2 data
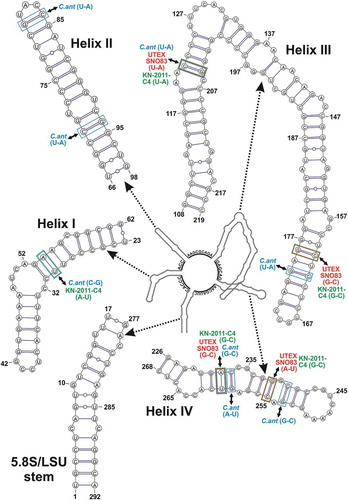
The ITS2 spacer secondary structure obtained for strain DCDR was 292 residues long and in line with the published ITS2 secondary structures of Coccomyxa (Darienko et al., Citation2015), including the conserved spacers among the four helices ().
Fig. 3. Hypothetical secondary structure of the ITS2 spacer for Coccomyxa cimbrica sp. nov. The whole secondary structure is represented on a small scale in the centre of the figure; the nucleotide sequences of the spacers between the four main helices are reported on the structure. The 5.8S/LSU stem and the four main helices, indicated by dotted arrows, are depicted in detail at higher magnification; in paired regions, A-U pairings are represented with a single line, G-C pairings with a double line, and unconventional pairings with a line interrupted by a circle. Compensatory base changes (CBCs) between C. cimbrica and some focus taxa are represented by coloured squares (light blue for C. antarctica Ua6, red for strain UTEX SNO83, light green for strain KN-2011-C4) connected to the name of the interested taxon by double-headed black arrows.
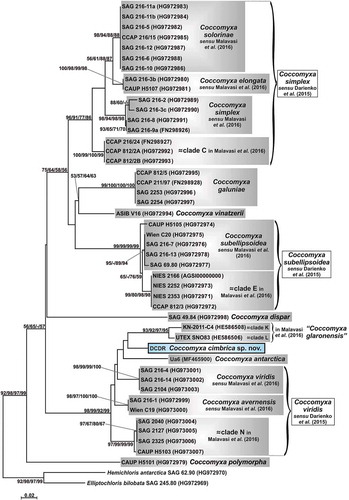
The ITS2 sequences and secondary structures of the investigated microalga () and of C. antarctica Ua6 (Supplementary fig. S4) were compared with the others available for the genus Coccomyxa in the phylogenetic reconstruction depicted in . The resulting ITS2 sequence + structure tree appeared again subdivided into blocks: most of the Coccomyxa lineages grouped in the upper part of the reconstruction and C. dispar was placed in the middle part, as sister taxon to them. In contrast to the 18S rRNA tree, C. polymorpha was not placed in the upper part, but at the base of the genus Coccomyxa. The lower part of the tree was represented by a well-supported (98/99/92/99) cluster of taxa: ‘C. glaronensis’ (= clades K + L), C. antarctica, C. viridis, C. avernensis, clade N and the investigated microalga. In particular, strain DCDR was placed as sister taxon to clades K + L, here represented by the two strains KN-2011-C4 and UTEX SNO83, respectively, and C. antarctica Ua6 was at the base of this group; however, the phylogenetic relationships among the four lineages were not statistically supported, except for the grouping of clades K and L (93/92/97/95).
Fig. 4. Phylogenetic reconstruction of the genus Coccomyxa based on ITS2 spacer (sequences + structures) analyses. The NJ topology is depicted and the numbers associated with nodes indicate support values for NJ with ProfDistS, NJ with MEGA, MP and ML analyses, respectively. Only bootstrap supports ≥50% are reported. Values for nodes that obtained support in only one of the performed phylogenetic analyses were omitted. Horizontal bar represents expected number of nucleotide substitutions per site. Grey boxes represent species according to the most recent classification or OTUs found/described in previous papers. Coccomyxa cimbrica sp. nov. is highlighted with a black outline. Brackets plus white boxes report the classification by Darienko et al. (Citation2015).
Looking at the CBC matrix (Supplementary fig. S7), the investigated strain showed two CBCs with strain UTEX SNO83, three with strain KN-2011-C4 and eight with C. antarctica Ua6. More precisely, the CBCs between strain DCDR and strain KN-2011-C4 fell in helices I and III, while those between the investigated organism and strain UTEX SNO83 were only in helix III (). The CBCs between strain DCDR and C. antarctica Ua6 involved all four helices (). One CBC was observed also between strain KN-2011-C4 and strain UTEX SNO83 (it fell in helix I) and five CBCs were present between each of these organisms and C. antarctica Ua6 (Supplementary fig. S7).
Focusing on the ITS2 secondary structure, that of the investigated microalga was most similar to those of C. antarctica Ua6, KN-2011-C4 and UTEX SNO83, even though some differences were observed (Supplementary table S2). Helices I and IV were the most diverse among the considered strains, and helices II and III appeared the most conserved; in particular, helix II showed differences only in the terminal part, while helix III differences involved the terminus and the major central left bulge, including the stem region just upstream of it (Supplementary table S2).
The large central left bulge of helix III, producing an angle of about 270° that caused the helix to fold up, was observed only in the Coccomyxa strains grouping with high support in the lower part of all the phylogenetic reconstructions (i.e. C. avernensis, C. viridis, C. antarctica, clades N, K, L and strain DCDR) (data not shown).
Following the method proposed by Darienko et al. (Citation2015), two new ITS2 numeric barcodes were obtained for C. antarctica Ua6 (barcode BC-13) and the investigated microalga (barcode BC-14) (Supplementary fig. S9). In the ITS2 numeric barcode phylogenetic reconstruction () most of the lineages (clade E, C. subellipsoidea, C. elongata, C. simplex, C. solorinae, clade C, C. vinatzerii and C. galuniae) were in the upper part of the tree, the lineage of C. dispar was in the central part and the remaining linages (C. viridis, C. avernensis, clade N, clade KL, C. antarctica and strain DCDR) constituted a well-supported group (98/93/90) in the lower part. C. polymorpha was at the base of the genus Coccomyxa. Again the investigated microalga was sister taxon to clades K + L, and C. antarctica Ua6 was placed at the base of them, but without any statistical support.
Fig. 5. Phylogenetic reconstruction of the genus Coccomyxa based on ITS2 numeric barcode analyses. The NJ topology is depicted and the numbers associated with nodes indicate support values for NJ, MP and ML analyses, respectively. Only bootstrap supports ≥50% are reported. Values for nodes that obtained support in only one of the performed phylogenetic analyses were omitted. Horizontal bar represents number of changes. Grey boxes represent species according to the most recent classification or OTUs found/described in previous papers. Coccomyxa cimbrica sp. nov. is highlighted with a black outline. Brackets plus white boxes show the classification by Darienko et al. (Citation2015). For each Coccomyxa strain, the corresponding ITS2 numeric barcode is reported.
The ITS2 sequence (AY333646) of Coccomyxa glaronensis CCALA 306 was not included in the ITS2 phylogenetic reconstructions because it was too short; however, it was compared with those of strains representing clades K and L and with the sequences of three further Coccomyxa strains (KN-2011-S2, KN-2011-C2, KN-2011-C3) (Supplementary fig. S5). This analysis revealed that the ITS2 sequence of C. glaronensis CCALA 306 had several differences from that of strain UTEX SNO83, while it was almost identical to the sequences of strains KN-2011-C4, KN-2011-S2, KN-2011-C2, KN-2011-C3, with the exception of some residue substitutions that fell in the terminal hairpin of helix II and in the major left bulge of helix III that did not cause structural changes.
Formal description of the new species
Coccomyxa cimbrica K.Sciuto, B.Baldan & I.Moro sp. nov. (–)
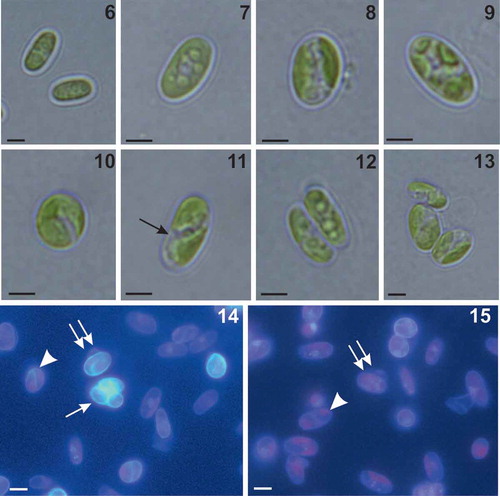
Figs 6–15. Morphology of Coccomyxa cimbrica sp. nov. Figs 6–10. Variable shapes of culture cells. Fig. 11. Cell reproduction by oblique division (arrow). Figs 12–13. Asexual reproduction through cell division with the formation of 2–4 autospores. Figs 14–15. Fluorescence images of culture cells stained with 4′,6 diamidino-2-phenylindole (DAPI). Note small mucilaginous caps (double arrows), reproduction by oblique division (arrow head) and, in Fig. 14, an autosporangium (arrow). Scale bars: Figs 6–13, 2 µm; Figs 14–15, 3 µm.
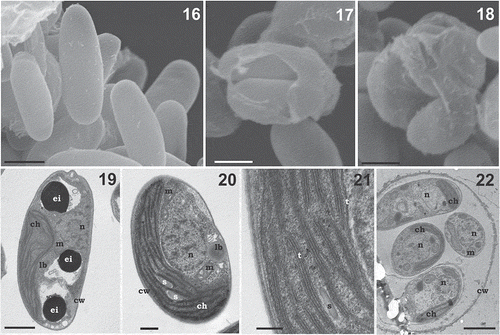
Figs 16–22. Morphology and ultrastructure of Coccomyxa cimbrica sp. nov. Fig. 16. Scanning electron micrograph of elliptical vegetative cells showing smooth cell wall. Figs 17, 18. Scanning electron micrographs of asexual reproduction by cell division through the rupture of mother cell wall and the formation of 2 (Fig. 17) and 3 (Fig. 18) autospores. Figs 19–20. Ultrastructure of cells showing thin and smooth cell wall (cw), nucleus (n), mitochondria (m), and a parietal chloroplast (ch) lacking pyrenoid, with some starch granules (s). Note in the cytoplasm the presence of some electron dense bodies (ei) and lipid bodies (lb). Fig. 21. Detail of the thylakoid membranes (t), single or piled up forming grana, and a starch granule (s) located among the thylakoids. Fig. 22. Micrograph of an autosporangium with four mature vegetative daughter cells enclosed by the mother cell wall (cw). Note in the autospores the presence of chloroplast (ch) and nucleus (n). Scale bars: Figs 16, 17, 18, 2 µm; Fig. 19, 1 µm; Fig. 20, 500 nm; Fig. 21, 200 nm; Fig. 22, 2 µm.
Diagnosis: Vegetative cells single, ellipsoidal to ovoid, variable in size, 4.5–7.0 µm long and 2.0–3.0 µm wide. Cells with thin and smooth wall, occasionally with small mucilaginous caps. Parietal, single, cup-shaped chloroplast, without a pyrenoid. Thylakoids single or stacked to form grana. Oblique cell division. Asexual reproduction by 2–4 autospores released through rupture of the autosporangium cell wall. Detected by molecular and phylogenetic analyses based on the 18S rRNA, rbcL and ITS2 loci. ITS2 secondary structure with distinctive features.
Holotype (designated here): resin-embedded sample deposited at the Herbarium Patavinum (PAD), the herbarium of the Botanical Garden – University of Padova, with the deposition number A000629.
Type locality: Palù di Sotto peat bog, Marcésina plain, Asiago plateau, Veneto, Italy.
Etymology: The specific epithet ‘cimbrica’ (fem. adj.) means ‘of Cimbri’, an ancient Germanic tribe that, in the past, lived in the Asiago plateau.
Living culture (ex-holotype): a living culture of strain DCDR, from which the holotype was derived, is available at the Culture Collection of Algae at Göttingen University (SAG), under the code SAG 2589, and at the Culture Collection of Cryophilic Algae (CCCryo), Fraunhofer, Germany, under the code CCCryo 506-18.
DNA sequences: LS999579 (rbcL gene), LS999578 (18S rRNA gene), LS999580 (18S-28S rRNA ITS).
Description
Free-living cells of Coccomyxa cimbrica were single, with variable shapes: mainly ellipsoidal, 4.5–7.0 µm long and 2.0–3.0 µm wide (, and ), but also ovoid ( and ). In the scanning electron microscope, cells showed a smooth cell wall (). Cells had a single parietal chloroplast (), occupying most of the total cell volume (–). Occasionally, small mucilaginous caps were observed in a few cells (, ). Asexual reproduction occurred by cell division with the formation of 2–4 autospores, released through the rupture of the mother cell wall (–, , ). The reproduction was always by oblique cell division (, , ). Ultrastructural observations carried out in the transmission electron microscope highlighted a thin and smooth cell wall (–); inside the cells, the nucleus, mitochondria, and a single, parietal and cup-shaped chloroplast were evident. Many starch granules were observed among the thylakoid membranes, but without a pyrenoid (, ). Thylakoids were either single or paired to form grana stacks (). The nucleus was often located in the middle of the cell, in a chloroplast depression (, ). In the cytoplasm, lipid bodies were present (, ), associated () or not () with electron dense bodies. Autosporangia with mature vegetative daughter cells, surrounded by the mother cell wall, were also observed ().
Association of strain DCDR with Drosera plants
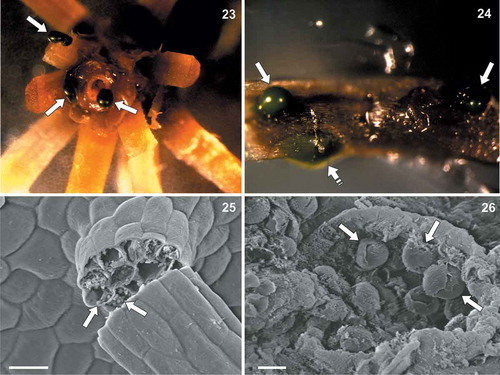
Explants of Drosera stem and leaf tissue, sterilized with 70% ethanol + sodium hypochlorite washings, were observed with the stereomicroscope and scanning electron microscope a few weeks after the treatment (–). In both stem () and leaf () sterilized portions, green masses corresponding to living colonies of strain DCDR were observed. In particular, some microalgal masses appeared to be just below the Drosera leaf epidermis and some others were emerging from leaf tissue lacerations (). With the scanning electron microscope, globular structures resembling strain DCDR were observed emerging from leaf tissue lacerations ( and ).
Figs 23–26. Coccomyxa cimbrica sp. nov. associated with Drosera capensis plants. Fig. 23. Stereomicroscope magnification of a sterilized D. capensis stem fragment after 70% ethanol + sodium hypochlorite washings. Green masses corresponding to C. cimbrica colonies are observed on the plant surface and coming out from the stem (white arrows). Fig. 24. Stereomicroscope magnification of a sterilized D. capensis leaf portion. Green C. cimbrica colonies are observed just below the plant epidermis (white dashed arrow) and coming out from tissue lacerations (white arrows). Fig. 25. Scanning electron micrograph of a D. capensis leaf sticky glandular hair (‘tentacle’). Inside the broken tentacle, globular structures resembling C. cimbrica cells are present (white arrows). Fig. 26. Scanning electron micrograph of D. capensis leaf surface. From a tissue laceration, globular structures similar to C. cimbrica cells are observed (white arrows). Scale bars: Fig. 16, 20 µm; Fig. 17, 5 µm.
Discussion
A green coccoid microalga was observed in the solid artificial growth medium used to propagate different Drosera L. species in vitro, and it was impossible to eradicate with ethanol + sodium hypochlorite washings (Guidolin, Citation2012). The microorganism was subsequently isolated in culture and subjected to an integrative taxonomic approach in order to identify it.
The molecular and phylogenetic analyses, carried out using rbcL, 18S rRNA and ITS2 loci, revealed that the microalga (tagged as ‘strain DCDR’) belongs to the genus Coccomyxa Schmidle (Trebouxiophyceae, Chlorophyta). Indeed, the simple morphological features of strain DCDR, in particular the small elliptical cells, presence of a single parietal chloroplast lacking pyrenoids, absence of flagellate stages, oblique cell division, and reproduction through releasing of 2–4 autospores from mature autosporangia, also fit with the description of the genus (Schmidle, Citation1901; Darienko et al., Citation2015; Malavasi et al., Citation2016).
Based on all the obtained trees (Supplementary fig. S3, , , ), the investigated organism represents a lineage distinct from all the other Coccomyxa clades and it does not coincide with any sequenced Coccomyxa species, considering both the classifications proposed by Darienko et al. (Citation2015) and Malavasi et al. (Citation2016).
In all the phylogenetic reconstructions, strain DCDR is placed inside a well-supported group including C. viridis, C. avernensis, C. antarctica, and the clades N, K, L; in the 18S rRNA tree, the species C. parasitica also belongs to this cluster. All the entities forming this highly supported clade in the lower part of the trees have a unique signature in their ITS2 secondary structure: a big central left bulge in the first half of helix III that generates an angle of about 270° causing the helix to almost bend back on itself.
Also, the ITS2 secondary structure analyses highlight the uniqueness of the investigated microalga with respect to with all the other sequenced lineages of the genus Coccomyxa. Indeed, following the method proposed by Darienko et al. (Citation2015), a new Coccomyxa ITS2 numeric barcode (BC-14) was obtained for strain DCDR (Supplementary fig. S9), and its ITS2 secondary structure (), even if very similar to those of C. antarctica Ua6 and of strains KN-2011-C4 and UTEX SNO83, shows distinctive features. In particular, helices I and IV of strain DCDR are very different from those of the mentioned taxa, and also the terminal part of the most conserved helices, II and III (Supplementary table S2). Based on Coleman (Citation2007), who reports that in Eukaryotes helices I and IV are the most variable and useful to distinguish species and subspecies, this finding is further evidence that the microalga represents a new entity among the sequenced species of the genus Coccomyxa. In addition, there is a feature unique to strain DCDR in the region upstream of the big central left bulge in helix III: all the focus taxa show a single base left bulge followed by a 4–5 residue stem region, while DCDR has a 3-residue asymmetrical internal loop plus a 4-residue stem region (Supplementary table S2). Since helices II and III are very conserved inside a given genus (Coleman, Citation2007, Citation2009), structural changes in these helices strongly suggest that the microalga found in association with Drosera plants is a Coccomyxa species different from the others sequenced so far.
The CBC analysis also confirms that strain DCDR represents an entity different from those for which ITS2 data are available, since it has CBCs with all the other Coccomyxa taxa, including the most related strains Ua6, KN-2011-C4 and UTEX SNO83 (, Supplementary fig. S7). The CBCs between the investigated organism and each of the focus taxa also include the very conserved helices II and III; in particular, two CBCs fall in the terminal part of helix III, involving two of the 30 most conserved nucleotides in the 5’ side near the tip (Coleman, Citation2007, Citation2009). Müller et al. (Citation2007) demonstrated that when a CBC occurs between two members of the same genus, they belong to different species with a reliability of 93.11%. In addition, Coleman (Citation2009) states that when two organisms have a CBC involving the critical 30 nucleotide stretch in the 5’ terminus of helix III they cannot interbreed.
In the 18S rRNA tree, strain DCDR is sister taxon to a phylogenetic lineage that corresponds to the clade tagged as ‘clade KL’ by Malavasi et al. (Citation2016). With respect to this previous paper we included in our analyses two further organisms: Coccomyxa sp. SAG 2483 and C. glaronensis CCALA 306. The ITS2 phylogenetic reconstructions could include only two strains of clade KL (KN-2011-C4 and UTEX SNO83), since the available ITS2 sequence for strain CCALA 306 was too short (from the 5.8S rRNA to just the beginning of helix IV) and the ITS2 sequence of strain SAG 2483 was not available. In the ITS2 trees, clade KL splits into two distinct clades, K (represented by strain KN-2011-C4) and L (coinciding with strain UTEX SNO83), as found previously by Malavasi et al. (Citation2016); moreover, strains KN-2011-C4 and UTEX SNO83 also show a CBC in helix I of their ITS2 locus, suggesting that they probably belong to distinct species. In order to discern if clades K or L coincided with the species C. glaronensis, the incomplete ITS2 sequence of strain CCALA 306 was aligned with those of strains belonging to clades K and L and those of further Coccomyxa strains found through a BLAST search (KN-2011-S2, KN-2011-C2, KN-2011-C3). This analysis highlighted that the ITS2 sequences of strains CCALA 306, KN-2011-S2, KN-2011-C2 and KN-2011-C3 are almost identical (no CBCs and few differences, causing no structural changes in the ITS2 secondary structures) to that of strain KN-2011-C4 in the overlapping part and, thus, clade K coincides with the species C. glaronensis. Interestingly, strain CCALA 306 is a lichen photobiont and all the other clade K strains were found in tree bark or spruce needles.
In all the phylogenetic reconstructions, clade N included the organism Coccomyxa sp. SAG 2325, which was found in association with Ginkgo biloba L. (Trémouillaux-Guiller et al., Citation2002). In the classification by Darienko et al. (Citation2015), clade N is included in the C. viridis lineage, and thus the Coccomyxa isolated from Ginkgo would also belong to this species. Based on the work by Malavasi et al. (Citation2016), clade N is a new lineage, distinct from C. viridis, and thus Coccomyxa sp. SAG 2325 would represent a new species. Our molecular data, in particular the analyses based on the ITS2 locus, support the second hypothesis. Therefore, clade N members represent a new Coccomyxa species that can live both freely in different environments (strains SAG 2040, SAG 2127, CAUP H5103) and in association with plants (strain SAG 2325). The formal description of this new species, which seems to have a worldwide distribution (Trémouillaux-Guiller & Huss, Citation2007), will require further investigation.
In the 18S rRNA phylogenetic reconstruction, a well-supported clade, here tagged as ‘C. parasitica’, was observed in the lower part of the tree. Unfortunately, ITS2 data were not available for the strains forming this cluster. The two Flensburg fjord strains (18S rRNA INSDC accessions: EU127470 and EU127471) are parasites of the blue mussel Mytilus edulis (Rodríguez et al., Citation2008) and strain CP-01 infests the horse mussel Modiolus kurilensis (Sokolnikova et al., Citation2016), while strain CPCC 508 was found free-living in a metal contaminated lake in Canada (Verma et al., Citation2009). However, the mollusc-infesting strains also seem able to live freely in the environment. Based on the infection mechanism and on some morphological similarities, Rodríguez et al. (Citation2008) attributed the two Flensburg fjord strains to the species C. parasitica R.N.Stevenson & G.R.South, even though the authors observed some differences from the original description of this species (Stevenson & South, Citation1974). Unfortunately, the reference strain of C. parasitica, CCAP 216/18, seems to have been overgrown by a different organism (Rodríguez et al., Citation2008). The 18S rRNA sequences of the strains clustering in the clade tagged as ‘C. parasitica’ share identities of 99.36–99.88% in their overlapping part (1719 aligned positions; data not shown). Given the high conservation of the 18S rRNA gene, analysis of the ITS2 locus will be necessary to confirm that these strains belong to the same species, even if the morphological and ecological data suggest that they represent the species C. parasitica.
Overall, the two phylogenetic reconstructions based on the ITS2 locus are congruent, even if in some cases the ITS2 sequence + structure tree () seems to detect more clades than the ITS2 numeric barcode reconstruction (). This is due to the methodology used; in the case of the ITS2 sequence + structure analysis the whole ITS2 locus is considered, while for the ITS2 numeric barcode reconstruction only 73 sites (= barcode sites) are used (Darienko et al., Citation2015). These sites coincide with the conserved regions identified by Coleman (Citation2007, Citation2009), which are: the 14 base pairs of the 5.8S-LSU stem; the first 5 and 11 pairings of helix I and helix II, respectively; and all the paired regions of helix III. For example, in the case of C. subellipsoidea sensu Malavasi et al. and clade E strains (together representing C. subellipsoidea sensu Darienko et al.), no CBCs are shown among their ITS2 structures, even if there are some differences in the secondary structure conformation of the second half of helix I, the first half of helix III and at the base of helix IV (see supplementary fig. S2 in Darienko et al., Citation2015). The numeric barcode method (Darienko et al., Citation2015) leads to the detection of a unique main numeric barcode, even if three sub-barcodes (= haplotypes) are recognized: BC-1a for clade E strains and BC-1b and BC-1c for the remaining C. subellipsoidea sensu Darienko et al. strains. BC-1a differs from BC-1b and BC-1c in three and two numeric barcode positions, respectively; BC-1b and BC-1c are distinguished by just one numeric barcode position. All the numeric barcode differences among BC-1a, BC-1b and BC-1c fall in the second part of helix III (Supplementary fig. S9). Mirroring this situation, both the ITS2 sequence + structure and the ITS2 numeric barcode trees show little distance among the strains corresponding to the numeric barcodes BC-1a, BC-1b and BC-1c, with the BC-1a strains (= clade E) diverging more from the others (= C. subellipsoidea sensu Malavasi et al.). Malavasi et al. (Citation2016) suggest the distinction between clade E and the other C. subellipsoidea strains based on ecological data: clade E organisms are all free living and epilithic, while the C. subellipsoidea sensu Malavasi et al. cluster is mainly composed of photobionts of soil lichens. Indeed, Darienko et al. (Citation2015) also notice that, inside C. subellipsoidea, the BC-1a strains (= clade E) are more sensitive to high salinities than the BC-1c strains. For other Coccomyxa lineages, the results obtained with the ITS2 sequence + structure analysis and the ITS2 numeric barcode method are less symmetrical. This is the case for C. viridis sensu Malavasi et al., C. avernensis and clade N, which in the ITS2 numeric barcode tree () cluster with high support (suggesting the attribution to a single species, as proposed by Darienko et al., Citation2015); conversely, in the ITS2 sequence + structure reconstruction () they are not grouped, even though placed very close. Again, these results can be explained by focusing on the ITS2 portions included in the analyses. In fact, C. viridis sensu Malavasi et al. and C. avernensis show the same ITS2 numeric barcode (= BC-7a) and differ from clade N (= BC-7b) in just one numeric barcode position falling in helix III (Supplementary fig. S9). Vice versa, looking at the whole ITS2 secondary structures, four CBCs are found between C. viridis sensu Malavasi et al. and C. avernensis (Supplementary fig. S7), but they fall outside the considered numeric barcode regions. In particular, two CBCs are in the second half of helix I and the other two in the distal part of helix IV (Supplementary fig. S6). Also, three CBCs (one in the second half of helix I and two in the distal part of helix IV) are observed between the ITS2 secondary structures of clade N and C. viridis sensu Malavasi et al. and four CBCs (all falling in the second half of helix IV) are present between the ITS2 secondary structures of clade N and C. avernensis. Therefore, in order to obtain phylogenies more congruent with those based on the ITS2 sequence + structure analysis, the numeric barcode method should be extended to all the paired sites of the ITS2 locus.
For 13 Coccomyxa taxa (at specific or infraspecific taxonomic rank) listed as ‘taxonomically accepted’ in Algaebase (Guiry & Guiry, Citation2018), molecular data are not available and, thus, their sequences could not be included in the present study. Of these, C. flava Jaag is not listed in the Index Nominum Algarum (INA), while C. subglobosa Pascher and C. subglobosa f. scabra S. Watanabe are indicated as homotypic synonyms of Neocystis subglobosa (Pascher) Hindák and N. subglobosa f. scabra (S. Watanabe) I. Kostikov et al., respectively, in the INA. C. astericola Rosenvinge and C. ophiurae Rosenvinge are parasites of echinoderms (Mortensen & Rosenvinge, Citation1910, Citation1933). C. corbierei Wille was found on wood in thermal springs at Cherbourg, France (Wille, Citation1910). C. icmadophilae Jaag and C. ovalis Jaag are phycobionts in lichens, while C. pallescens Chodat was found on the lichen C. gracilis (L.) Willd., but as an epiphyte (Chodat, Citation1913; Jaag, Citation1933). C. subsphaerica var. terrestris A.K. Mitra is a soil species sampled from Allahabad, India (Mitra, Citation1951). There is not much information about the morphology and ecology of C. naegeliana (Artari) Wille, C. subsphaerica Chodat & Jaag and C. thallosa Chodat, even though C. naegeliana and C. subsphaerica seem to have been sampled from Switzerland and C. naegeliana seems to be cosmopolitan (Wille, Citation1909; Chodat, Citation1913; Jaag, Citation1933).
The molecular and phylogenetic evidence obtained, as a whole, along with the morphological and ecological considerations, justify the erection of the new species C. cimbrica K.Sciuto, B.Baldan & I.Moro for the microalga found in association with Drosera L. plants. C. cimbrica is able to live freely, but certain microscope images (–) and its resistance to repeated 70% ethanol + sodium hypochlorite washings suggest that the microalga can penetrate and grow inside the carnivorous plants, probably just below the epidermis. Further investigations will be necessary to confirm this hypothesis and, if verified, to clarify the nature of the facultative relationship between C. cimbrica and Drosera.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at accssed https://doi.org/10.1080/09670262.2019.1618920
Supplementary table S1. Primers used to amplify and sequence the 18S rRNA gene.
Supplementary table S2. Comparison among the ITS2 secondary structures of the investigated microalga (strain DCDR), C. antarctica Ua6, and strains KN-2011-C4 and UTEX SNO83. For each strain, the total length of the ITS2 structure is given in parentheses near strain designation, each of the four helices is described from the base to the terminal hairpin, and lengths and sequences of the five spacers are reported. The ITS2 secondary structures of strains KN-2011-C4 and UTEX SNO83 are described as reported by Darienko et al. (Citation2015). The differences found in the ITS2 structures of the focus strains compared with the ITS2 structure of the strain DCDR are underlined.
Supplementary fig. S1. Sequence alignment of the rbcL gene in FASTA format.
Supplementary fig. S2. Sequence alignment of the 18S rRNA gene in FASTA format.
Supplementary fig. S3. Phylogenetic reconstruction of the genus Coccomyxa based on rbcL gene analyses. The NJ topology is depicted and the numbers associated with nodes indicate support values for NJ, MP, ML and BI analyses, respectively. Only bootstrap supports ≥50% and posterior probabilities ≥0.95 are reported. Values for nodes that obtained support in only one of the performed phylogenetic analyses were omitted. Horizontal bar represents expected number of nucleotide substitutions per site. Grey boxes represent species according to the most recent classification or OTUs found/described in previous papers. Coccomyxa cimbrica sp. nov. is highlighted with a light blue box. Brackets plus white boxes report the classification by Darienko et al. (Citation2015).
Supplementary fig. S4. ITS2 secondary structure of Coccomyxa antarctica Ua6.
Supplementary fig. S5. Alignment of the ITS2 sequences of Coccomyxa glaronensis CCALA 306 and of strains UTEX SNO83 (= clades L), KN-2011-C4 (= clade K), KN-2011-S2, KN-2011-C2 and KN-2011-C3.
Supplementary fig. S6. Sequence + structure alignment of the ITS2 spacer in FASTA format.
Supplementary fig. S7. Compensatory base changes (CBCs) matrix among the ITS2 of Coccomyxa taxa considered in the ITS2 phylogenetic reconstruction. The outgroups Elliptochloris bilobata SAG 245.80 and Hemichloris antarctica SAG 62.90 are included. Strain identifiers are reported in the first left column and species/clades are indicated above the matrix. Grey rectangles highlight CBCs among members of a same species/clade; that of C. cimbrica sp. nov. is in light blue colour.
Supplementary fig. S8. Determination of the ITS2 numeric barcode of Coccomyxa cimbrica sp. nov.
Supplementary fig. S9. ITS2 numeric barcodes of Coccomyxa species. Numeric barcodes from BC-1a to BC-12 are those reported in Darienko et al. (Citation2015). Numeric barcodes BC-13 and BC-14 were determined in this work. Numeric barcodes of the outgroups Elliptochloris bilobata SAG 245.80 and Hemichloris antarctica SAG 62.90 are also included. For Coccomyxa genus, strain identifiers and both taxonomic classifications by Darienko et al. (Citation2015) and Malavasi et al. (Citation2016) are reported.
Author contributions
K. Sciuto: original concept, production of molecular data, molecular and phylogenetic analyses, manuscript drafting and editing; B. Baldan: light and scanning electron microscope analyses, manuscript drafting and editing; S. Marcato: isolation and culturing of the strain; I. Moro: original concept, scanning and transmission electron microscope analyses, manuscript drafting and editing, financial support.
TEJP-2018-0124-File020.jpg
Download JPEG Image (13.2 MB)TEJP-2018-0124-File019.pdf
Download PDF (1.6 MB)TEJP-2018-0124-File018.jpg
Download JPEG Image (6.9 MB)TEJP-2018-0124-File017.txt
Download Text (37.9 KB)TEJP-2018-0124-File016.pdf
Download PDF (70.7 KB)TEJP-2018-0124-File015.pdf
Download PDF (1.5 MB)TEJP-2018-0124-File014.jpg
Download JPEG Image (3 MB)TEJP-2018-0124-File013.txt
Download Text (204 KB)TEJP-2018-0124-File012.txt
Download Text (14.5 KB)TEJP-2018-0124-File011.docx
Download MS Word (16.7 KB)TEJP-2018-0124-File010.docx
Download MS Word (16.5 KB)Acknowledgements
We thank Valerio Guidolin for having collected the Drosera plants from which Coccomyxa cimbrica was isolated.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aaronson, S. (1970). Experimental Microbial Ecology. Academic Press, New York.
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215: 403–410.
- Andreoli, C., Moro, I., La Rocca, I., Rigoni, F., Dalla Valle, L. & Bargelloni, L. (1999). Pseudopleurochloris antarctica gen. et sp. nov., a new coccoid xanthophycean from pack-ice of Wood Bay (Ross Sea, Antarctica): ultrastructure, pigments and 18S rRNA gene sequence. European Journal of Phycology, 34: 149–159.
- Ankenbrand, M.J., Keller, A., Wolf, M., Schultz, J. & Förster, F. (2015). ITS2 database V: twice as much. Molecular Biology and Evolution, 32: 3030–3032.
- Belzile, C. & Gosselin, M. (2015). Free-living stage of the unicellular algae Coccomyxa sp. parasite of the blue mussel (Mytilus edulis): low-light adaptation, capacity for growth at a very wide salinity range and tolerance to low pH. Journal of Invertebrate Pathology, 132: 201–207.
- Cao, S., Zhang, F., Zheng, H., Liu, C., Peng, F. & Zhou, Q. (2018). Coccomyxa antarctica sp. nov. from the Antarctic lichen Usnea aurantiacoatra. PhytoKeys, 98: 107–115.
- Chodat, R. (1913). Monographies d’algues en culture pure. K.J. Wyss, Bern.
- Coleman, A.W. (2007). Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Research, 35: 3322–3329.
- Coleman, A.W. (2009). Is there a molecular key to the level of ‘‘biological species” in eukaryotes? A DNA guide. Molecular Phylogenetics and Evolution, 50: 197–203.
- Darienko, T., Gustavs, L., Eggert, A., Wolf, W. & Pröschold, T. (2015). Evaluating the species boundaries of green microalgae (Coccomyxa, Trebouxiophyceae, Chlorophyta) using integrative taxonomy and DNA barcoding with further implications for the species identification in environmental samples. PLoS ONE, 10: e0127838.
- Darty, K., Denise, A. & Ponty, Y. (2009). VARNA: interactive drawing and editing of the RNA secondary structure. Bioinformatics, 25: 1974–1975.
- Edgar, R.C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32: 1792–1797.
- Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using bootstrap. Evolution, 39: 783–791.
- Gray, A.P., Lucas, I.A.N., Seed, R. & Richardson, C.A. (1999). Mytilus edulis chilensis infested with Coccomyxa parasitica (Chlorococcales, Coccomyxaceae). Journal of Molluscan Studies, 65: 289–294.
- Guidolin, V. (2012). Propagazione in vitro di Drosera capensis, Drosera rotundifolia e Dionea muscipula. Bachelor thesis. University of Padova, Italy.
- Guindon, S. & Gascuel, O. (2003). A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52: 696–704.
- Guiry, M.D. & Guiry, G.M. (2018). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Available at: http://www.algaebase.org.
- Gustavs, L., Schiefelbein, U., Darienko, T. & Pröschold, T. (2017). Symbioses of the green algal genera Coccomyxa and Elliptochloris (Trebouxiophyceae, Chlorophyta). In Algal and Cyanobacteria Symbioses (Grube, M., Seckbach, J. & Muggia, L., editors), 169–208. World Scientific (Europe).
- Hall, J.D., Fucikova, K., Lo, C., Lewis, L.A. & Karol, K.G. (2010). An assessment of proposed DNA barcodes in freshwater green algae. Cryptogamie, Algologie, 31: 529–555.
- Hallmann, C. (2015). Biodiversity of terrestrial algal communities from soil and air-exposed substrates using a molecular approach. PhD Thesis. Georg-August University School of Science, Göttingen, Germany.
- Jaag, O. (1933). Coccomyxa Schmidle Monographie einer Algengattung. Gebrüder Fretz, Bern.
- Keller, A., Schleicher, T., Schultz, J., Müller, T., Dandekar, T. & Wolf, M. (2009). 5.8S-28S rRNA interaction and HMM-based ITS2 annotation. Gene, 430: 50–57.
- Kulichová, J., Škaloud, P. & Neustupa, J. (2014). Molecular diversity of green corticolous microalgae from two sub-Mediterranean European localities. European Journal of Phycology, 49: 345–355.
- Lohtander, K., Oksanen, I. & Rikkinen, J. (2003). Genetic diversity of green algal and cyanobacterial photobionts in Nephroma (Peltigerales). Lichenologist, 35: 325–339.
- Maddison, W.P. & Maddison, D.R. (2009). Mesquite: A modular system for evolutionary analysis, version 2.71. Available at: http://mesquiteproject.org.
- Malavasi, V., Škaloud, P., Rindi, F., Tempesta, S., Paoletti, M. & Pasqualetti, M. (2016). DNA-based taxonomy in ecologically versatile microalgae: a re-evaluation of the species concept within the coccoid green algal genus Coccomyxa (Trebouxiophyceae, Chlorophyta). PLoS ONE, 11: e0151137.
- Mitra, A.K. (1951). Certain new members of Volvocales from Indian soils. Phytomorphology, 1: 58–64.
- Moro, I., Rascio, N., La Rocca, N., Sciuto, K., Albertano, P., Bruno, L. & Andreoli, C. (2010) Polyphasic characterization of a thermo-tolerant filamentous cyanobacterium isolated from the Euganean thermal muds (Padova, Italy). European Journal of Phycology, 45: 143–154.
- Mortensen, T. & Rosenvinge, L.K. (1910). Sur quelques plantes parasites dans des echinoderms. Bulletin de l’Académie Royale des Sciences et des Lettres de Danemark, 1910: 339–354.
- Mortensen, T. & Rosenvinge, L.K. (1933). Sur une nouvelle algue, Coccomyxa astericola, parasite dans une astérie. Kongelige Danske Videnskabernes Selskab Biologiske Skrifter, 10: 1–8.
- Muggia, L., Baloch, E., Stabentheiner, E., Grube, M. & Wedin, M. (2011). Photobiont association and genetic diversity of the optionally lichenized fungus Schizoxylon albescens. FEMS Microbiology Ecology, 75: 255–272.
- Müller, T., Philippi, N., Dandekar, T., Schultz, J. & Wolf, M. (2007). Distinguishing species. RNA, 13: 1469–1472.
- Murashige, T. & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15: 473–497.
- Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25: 1253–1256.
- Posada, D. & Buckley, T.R. (2004). Model selection and model averaging in phylogenetics: advantages of the AIC and Bayesian approaches over likelihood ratio tests. Systematic Biology, 53: 793–808.
- Posada, D. & Crandall, K.A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics, 14: 817–818.
- Rambaut, A. & Drummond, A.J. (2007). Tracer, version 1.5. Available at: http://beast.bio.ed.ac.uk/Tracer.
- Rippka, R., Deruelles, J., Waterbury, J.B., Herdman, M. & Stanier, R.Y. (1979). Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. Journal of General Microbiology, 111: 1–61.
- Rodríguez, F., Feist, S.W., Guillou, L., Harkestad, L.S., Bateman, K., Renault, T. & Mortensen, S. (2008). Phylogenetic and morphological characterization of the green algae infesting blue mussel Mytilus edulis in the North and South Atlantic oceans. Diseases of Aquatic Organisms, 81: 231–240.
- Ronquist, F. & Huelsenbeck, J.P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572–1574.
- Schmidle, W. (1901). Über drei Algengenera. Berichte der Deutschen Botanischen Gessellschaft, 19: 10–24.
- Schultz, J., Müller, T., Achtziger, M., Seibel, P.N., Dandekar, T. & Wolf, M. (2006). The internal transcribed spacer 2 database – a web server for (not only) low level phylogenetic analyses. Nucleic Acids Research, 34: W704–707.
- Schwarz, G. (1978). Estimating the dimension of a model. Annals of Statistics, 6: 461–464.
- Sciuto, K., Lewis, L.A., Verleyen, E., Moro, I. & La Rocca, N. (2015). Chodatodesmus australis sp. nov. (Scenedesmaceae, Chlorophyta) from Antarctica, with the emended description of the genus Chodatodesmus, and circumscription of Flechtneria rotunda gen. nov., sp. nov. Journal of Phycology, 51: 1172–1188.
- Seibel, P.N., Müller, T., Dandekar, T., Schultz, J. & Wolf, M. (2006). 4SALE – a tool for synchronous RNA sequence and secondary structure alignment and editing. BMC Bioinformatics, 7: 498.
- Seibel, P.N., Müller, T., Dandekar, T. & Wolf, M. (2008). Synchronous visual analysis and editing of RNA sequence and secondary structure alignments using 4SALE. BMC Research Notes, 1: 91.
- Sokolnikova, Y., Magarlamov, T., Stenkova, A. & Kumeiko, V. (2016). Permanent culture and parasitic impact of the microalga Coccomyxa parasitica, isolated from horse mussel Modiolus kurilensis. Journal of Invertebrate Pathology, 140: 25–34.
- Stevenson, R.N. & South, G.R. (1974). Coccomyxa parasitica sp. nov. (Coccomyxaceae, Chlorococcales), a parasite of giant scallops in Newfoundland. British Phycological Journal, 9: 319–329.
- Štifterová, A. & Neustupa, J. (2015). Community structure of corticolous microalgae within a single forest stand: evaluating the effects of bark surface pH and tree species. Fottea, Olomouc, 15: 113–122.
- Swofford, D.L. (2002). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, MA.
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011). MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28: 2731–2739.
- Trémouillaux-Guiller, J. & Huss, V.A.R. (2007). A cryptic intracellular green alga in Ginkgo biloba: ribosomal DNA markers reveal worldwide distribution. Planta, 226: 553–557.
- Trémouillaux-Guiller, J., Rohr, T., Rohr, R. & Huss, V.A.R. (2002). Discovery of an endophytic alga in Ginkgo biloba. American Journal of Botany, 89: 727–733.
- Vázquez, N., Rodríguez, F., Ituarte, C., Klaich, J. & Cremonte, F. (2010). Host-parasite relationship of the geoduck Panopea abbreviata and the green alga Coccomyxa parasitica in the Argentinean Patagonian coast. Journal of Invertebrate Pathology, 105: 254–260.
- Verma, V., Bhatti, S., Huss, V.A.R. & Colman, B. (2009). Photosynthetic inorganic carbon acquisition in an acid-tolerant free-living species of Coccomyxa (Chlorophyta). Journal of Phycology, 45: 847–854.
- White, T.J., Bruns, T., Lee, S. & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications (Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J., editors), 315–322. Academic Press, New York.
- Wille, N. (1909). Conjugatae und Chlorophyceae. In Die natürlichen Pflanzenfamilien Nachträge zum I. Teil, Abteilung 2 über die Jahre 1890 bis 1910. (Engler, A. & Prantl, K., editors), 1–96. Verlag von Wilhelm Engelmann, Leipzig.
- Wille, N. (1910). Über Coccomyxa corbierei n. sp. Nyt Magazin for Naturvidensk, 48: 298–302.
- Wolf, M., Achtziger, M., Schultz, J., Dandekar, T. & Müller, T. (2005). Homology modeling revealed more than 20,000 rRNA internal transcribed spacer 2 (ITS2) secondary structures. RNA, 11: 1616–1623.
- Wolf, M., Ruderisch, B., Dandekar, T. & Müller, T. (2008). ProfdistS: (Profile-) distance based phylogeny on sequence-structure alignments. Bioinformatics, 24: 2401–2402.
- Yahr, R., Florence, A., Škaloud, P. & Voytsekhovich, A. (2015). Molecular and morphological diversity in photobionts associated with Micarea s. str. (Lecanorales, Ascomycota). The Lichenologist, 47: 403–414.
- Zoller, S. & Lutzoni, F. (2003). Slow algae, fast fungi: exceptionally high nucleotide substitution rate differences between lichenized fungi Omphalina and their symbiotic green algae Coccomyxa. Molecular Phylogenetics and Evolution, 29: 629–640.
