ABSTRACT
Cryptic diversity is common in the red algae and is often discovered when comparing specimens from distant locations or different morphotypes of species with high phenotypic plasticity. The genus Lophurella includes seven species from the cold-temperate coasts of the southern hemisphere. L. periclados is the only species reported from Australia where two morphotypes were identified in relation to levels of wave exposure. In New Zealand, three species of Lophurella have been reported – the endemic L. caespitosa (type locality Parimahu, North Island of New Zealand), L. hookeriana (type locality Cape Horn, South America) and L. periclados. We reassessed species diversity of Lophurella in Australia and New Zealand with the aim of determining (1) whether New Zealand and South American specimens of L. hookeriana actually represent a single species, and (2) if the morphotypes of L. periclados mask cryptic diversity. We studied rbcL sequences and morphological features of 36 specimens identified as L. periclados, one specimen of L. caespitosa, and five samples of L. hookeriana, three from New Zealand and two from Cape Horn. Molecular analyses revealed that L. hookeriana from New Zealand and South America are distinct species and the new species L. pauciramulosa is described from New Zealand. L. periclados is a complex involving four species and we propose three new species, L. mutabilis, L. nigra and L. tasmanica. Cryptic diversity in L. periclados did not align with the previously defined ecotypes and several species were often found at the same site. L. periclados, L. nigra and L. tasmanica can be distinguished by morphological characters. Conversely, L. mutabilis has high morphological plasticity, with characters that overlap with L. periclados and L. nigra, and can only be distinguished by DNA sequences.
Introduction
The genus Lophurella F.Schmitz (in Schmitz & Falkenberg, Citation1897) includes seven recognized species (Guiry & Guiry, Citation2019). It differs from other genera in the Rhodomelaceae by the following combination of characters: thalli consist of prostrate and erect terete axes, with axes having four or seven pericentral cells that are completely corticated from close to the apices, bearing radially arranged determinate branches (Falkenberg, Citation1901; Womersley, Citation2003). Based on these features, Lophurella was originally placed in the tribe Polysiphonieae (Falkenberg, Citation1901; Hommersand, Citation1963; Womersley, Citation2003). However, it was recently transferred to the Pterosiphonieae using molecular and morphological evidence (Díaz-Tapia et al., Citation2017). The rhizoids of Lophurella have multicellular haptera and differ from the unicellular haptera characteristic of the Polysiphonieae and Streblocladieae (Díaz-Tapia et al., Citation2017).
Lophurella is restricted to the cold-temperate southern hemisphere, with species reported from Australia, New Zealand, South America and Tristan da Cunha (Guiry & Guiry, Citation2019). L. periclados (Sonder) F.Schmitz, the generitype, is common in the low intertidal in Southern Australia, Victoria and Tasmania and also found in New South Wales (Millar & Kraft, Citation1993; Womersley, Citation2003). It is the only member of the genus in Australia, its type locality (Port Phillip Bay, Victoria), and is easily distinguished from other members of the Rhodomelaceae (Womersley, Citation2003). It has also been reported in New Zealand where it differs from congeners by having scarcely branched main erect axes that bear abundant determinate branches (Adams, Citation1994; Womersley, Citation2003). L. caespitosa (Harvey) Falkenberg is endemic to New Zealand and is characterized by its emerald green colour and being smaller (up to 5 cm) than its congeners in the region (Adams, Citation1994; Nelson, Citation2013). The third member of the genus recorded in New Zealand is L. hookeriana (J.Agardh) Falkenberg (type locality Cape Horn, South America), characterized by long erect axes (up to 15 cm) that are more profusely branched and with fewer determinate branches than other species (Adams, Citation1994). Three species have been recorded only in South America: L. patula (J.D.Hooker & Harvey) De Toni, L. gaimardii (Gaudichaud ex C.Agardh) De Toni and L. comosa (J.D.Hooker & Harvey) Falkenberg. Finally, L. christosphersenii Baardseth is only known in Tristan da Cunha (Baardseth, Citation1941).
Species delimitation based on morphological characters is often difficult in marine macroalgae that exhibit high morphological plasticity or converge to present similar morphologies (Verbruggen, Citation2014). As a result, cryptic diversity is commonly discovered when molecular assisted taxonomy is used for species diversity assessments (e.g. Guillemin et al., Citation2016; Saunders et al., Citation2017), including in the family Rhodomelaceae (Savoie & Saunders, Citation2016, Citation2019; Díaz-Tapia et al., Citation2018a). New cryptic species have been detected as the result of comparing sequence data for specimens of the presumed same species from distant locations (e.g. Bustamante et al., Citation2014; Díaz-Tapia et al., Citation2018a; Schneider et al., Citation2018). This led us to hypothesize that the records of L. hookeriana from New Zealand and South America might actually correspond to different species. Cryptic diversity is also common within a geographical region (e.g. Guillemin et al., Citation2016; Savoie & Saunders, Citation2016, Citation2019). Phenotypic plasticity is often recognized in red algal species with morphological variation in relation to environmental conditions. However, the use of sequence data has shown that this plasticity often masks cryptic species (Milstein & Saunders, Citation2012; Zanolla et al., Citation2014; Barreto de Jesús et al., Citation2019). We suspected that the morphotypes of L. periclados might correspond to different species, because L. periclados is known to exhibit morphological variability in Tasmania associated with different levels of wave exposure (Womersley, Citation2003). The aim of this work is to test these hypotheses, re-assessing species diversity of the genus Lophurella in Australia and New Zealand using rbcL plastid gene sequences and detailed morphological studies of the specimens.
Materials and methods
Material of Lophurella spp. was collected during surveys of the family Rhodomelaceae in Victoria and eastern Tasmania (Australia) and New Zealand (Supplementary table S1). Regions adjacent to the known range of the genus in Australia, the York Peninsula (Southern Australia) and the northern coast of Tasmania were explored without finding Lophurella. We also obtained two samples of L. hookeriana from Cape Horn (Chile), its type locality. Materials for DNA extraction were dried in silica gel desiccant. Plants for morphological study were preserved in 4% formalin seawater at 4°C and stored in the dark. Some specimens were mounted in 20% Karo® Syrup (ACH Foods, Memphis, Tennessee, USA). Sections for microscopic observations were made by hand using a razor blade. Voucher specimens were deposited in the University of Melbourne Herbarium (MELU), the National Herbarium of Victoria (MEL) and Museum of New Zealand Te Papa Tongarewa (WELT).
DNA was extracted from silica gel-dried material following Saunders & McDevit (Citation2012) or an adapted cetyltrimethylammonium bromide (CTAB) protocol (Doyle & Doyle, Citation1987). PCR amplification of rbcL was carried out using primers F57/rbcLrevNEW, F2/R1008, F2/R1464 and F2/R1452 (Saunders & Moore, Citation2013; Díaz-Tapia et al., Citation2018a). Reactions were performed in a total volume of 25 µl, consisting of 5 µl 5× MyTaqTM reaction buffer, 0.7 µl 10 µM of forward and reverse primers, 0.125 µl 1U µl–1 My TaqTM DNA Polymerase (Bioline, London, UK), 17.475 µl MilliQ® water and 1 µl template DNA. The PCR profile consisted of initial denaturation (93°C for 3 min), 35 cycles of denaturation (94°C for 30 s), primer annealing (45°C for 30 s), and extension (74°C for 90 s) and final extension (74°C for 5 min). The PCR products were purified and sequenced by Macrogen (Korea) or the sequencing service of the University of A Coruña.
Thirty-nine new rbcL sequences were analysed together with the four sequences available in GenBank (Supplementary table S1). One of the GenBank sequences (KT825866) was originally misidentified as Womersleyella pacifica Hollenberg. However BLAST searches revealed its close similarity to Lophurella and we included it in our dataset. Sequences were aligned using Muscle in Geneious 6.1.8 (Kearse et al., Citation2012). The alignment was 1424 nucleotides long in total, and sequence lengths were 665–1464 bp.
To obtain a species-level phylogeny of the genus a Maximum likelihood (ML) phylogeny was inferred. This phylogeny includes a single sequence per haplotype, selected according to quality in terms of length (i.e. the longest sequence). The phylogenetic tree for rbcL was estimated with Maximum likelihood using RAxML 8.1.X (Stamatakis, Citation2014). GTR-Gamma was used as the nucleotide model and branch support was estimated with 100 bootstrap replicates. Two species of Echinothamnion were selected as outgroup, as it is the closest sister genus based on phylogenetic analyses of the tribe Pterosiphonieae (Savoie & Saunders, Citation2016).
Results
Molecular identification and phylogeny
RAxML analyses of the 43 sequences of Lophurella specimens resolved seven lineages that we consider to represent species, three previously recognized and four new species (). Sequence divergence within each lineage was 0–0.9% (0–12 bp), and among lineages 1.0–3.4% (15–44 bp) (Supplementary table S2). The previously recognized species L. caespitosa sampled in New Zealand (its type locality) and L. hookeriana from Cape Horn (Chile), also its type locality, were clearly separated from other species by 1.9–3.4% sequence divergence. Two sequences of L. hookeriana from Chile differed by 0.6% from a sequence from the Falkland Islands. Our molecular data showed that all three specimens that we collected and identified as L. hookeriana in New Zealand differed from the topotype specimens by 1.9–2.3% sequence divergence and we propose L. pauciramulosa sp. nov. from New Zealand.
Fig. 1. Maximum likelihood phylogeny of Lophurella based on rbcL sequences. Species names at the branch tips indicate the original identification based on morphological characters; the vertical bars and their corresponding names indicate the reassessed species diversity based on morphological characters and molecular data. Names of new taxa are printed in bold. Values at the nodes represent bootstrap support, only shown if > 80. CH = Chile, FALK = Falkland Islands, NZ = New Zealand, SA = South Australia, TAS = Tasmania, VIC = Victoria.
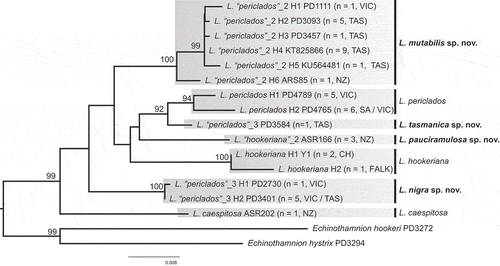
The specimens that we originally identified as L. periclados were resolved in four clades (). All eight specimens collected in Port Phillip Bay, Victoria, the type locality of L. periclados, and nearby areas formed a highly supported clade with two haplotypes that diverged by 0.8% (11 bp). Accordingly, we concluded that our collections of topotype material correspond to L. periclados (). In addition to L. periclados, the only species of the genus previously recorded in Australia, three other species were identified in Australia, one also being present in New Zealand (), and we propose the erection of three new species. L. tasmanica sp. nov. was closely related, with high support, to L. periclados and sequence divergence between them was 1–1.1% (13–15 bp). The clade corresponding to L. nigra sp. nov. included six sequences, five identical and one that diverged by 0.1% (1 bp). The clade corresponding to L. mutabilis sp. nov. consisted of 18 sequences and six haplotypes. The five Australian haplotypes (H1–5) diverged by 0–0.4% (up to 3 bp) and the New Zealand haplotype (H6) diverged by up to 0.9% (12 bp) from Australian haplotypes.
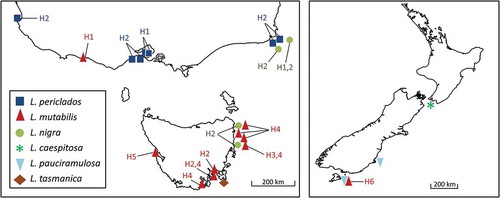
Fig. 2. Distribution of Lophurella spp. and their respective haplotypes (see ) in Australia (left) and New Zealand (right).
Relationships among the species that we identified in the genus Lophurella were not resolved in our phylogenetic analysis (except for grouping the sister species L. periclados and L. tasmanica).
Morphological observations
Of the 40 specimens of Lophurella spp. collected during our sampling surveys of the family Rhodomelaceae in Victoria, Tasmania (Australia) and New Zealand, 36 were morphologically identifiable as L. periclados, three as L. hookeriana and one as L. caespitosa (which is distinctive in colour, thallus length and branching pattern). A description of the characters shared among L. periclados and the four new species recognized in this study is provided, with the details of these characters including their measurements in each species (). We also include a diagnosis of each new species, as well as a summary of the morphological characters that differ among the species here studied (). The description of L. periclados is based on our collections. The only available detailed description of L. periclados was provided by Womersley (Citation2003) but considering the distribution of the selected specimens used by Womersley and our results, his description was most probably based on a mixture of species.
Table 1. Measurements of morphological characters for Lophurella spp. from Australasia (except L. caespitosa).
Table 2. Comparison of selected morphological characters useful for species delineation and distributions between the species of Lophurella from Australia and New Zealand.
Morphology of Lophurella spp. from Australasia (except L. caespitosa)
Vegetative morphology
Thallus formed of prostrate and erect axes (), habit varying among species. Axes consisting of a small axial cell and four pericentral cells, heavily corticated from close to the apices. In cross-section, pericentral cells of young branches covered by a layer of cortical cells (, ). In old parts of thalli, pericentral cells surrounded by 1–4 layers of little-pigmented pseudoparenchymatous cells and a layer of deeply pigmented cortical cells (, ). Cortical cells in surface view rounded to elongate-polygonal. Plastids elliptical to irregular ().
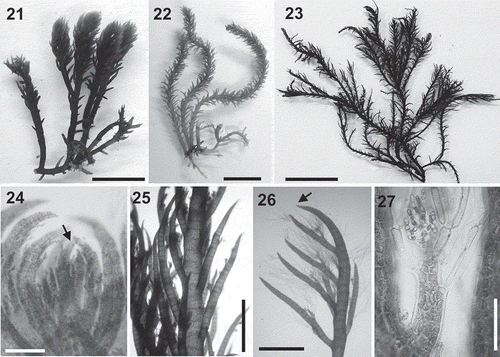
Figs 3–14. Lophurella spp.: vegetative morphology. Fig. 3. Habit of a specimen with prostrate and erect axes (L. nigra). Figs 4–7. Cross-section of an axis in the upper thallus (Figs 4–5) and the mid-thallus (Figs 6–7), with an axial cell (a), four pericentral cells (p), cortical cells (c) and, only in Figs 6–7, pseudoparenchymatous cells (ps). Fig. 8. Cortical cells showing plastids. Fig. 9. Apex of a prostrate axis. Figs 10, 11. Prostrate axes with rhizoids cut off from cortical cells and with multicellular haptera. Fig. 12. Apex of an erect axis with initials on every segment (arrowheads). Fig. 13. Apex of an erect axis bearing trichoblasts on the second-order determinate branches. Fig. 14. Determinate branch bearing spirally arranged trichoblasts. Figs 3, 9, 12, 13, L. nigra; Figs 4, 6, L. tasmanica; Figs 5, 7, 10, 14, L. mutabilis; Fig. 8, L. pauciramulosa, Fig. 11, L. periclados. Scale bars: Fig. 3 = 2.5 mm; Figs 4, 12 = 40 µm; Fig. 5 = 30 µm; Fig. 6 = 400 µm; Fig. 7 = 200 µm; Fig. 8 = 15 µm; Figs 9, 13 = 350 µm; Figs 10, 11 = 100 µm; Fig. 14 = 150 µm.
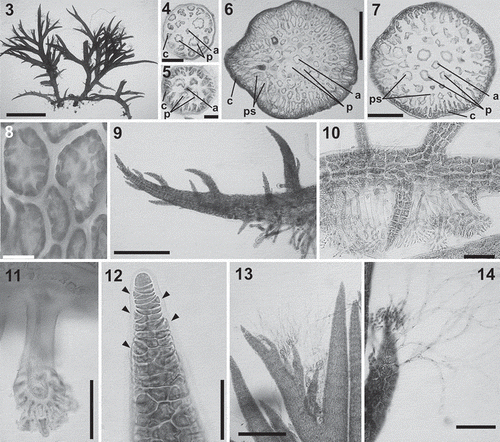
Prostrate axes () growing from a dome-shaped apical cell, increasing in diameter in older parts. Axes lacking trichoblasts, forming a branch initial on every segment or at intervals of several segments; branches spirally arranged, from which endogenous branches arise, also on every segment or at intervals of several segments. Lateral and ventral branches producing further prostrate axes or remaining as short laterals; dorsal branches producing erect axes. Several rhizoids usually formed on every segment, cut off from cortical cells, consisting of a unicellular filament terminating in a multicellular discoid pad (). Haptera initially formed by cells cut off from the basal part of the rhizoidal filament, subsequently branching dichotomously for up to two orders ().
Erect axes growing from a dome-shaped apical cell (), increasing in diameter in mid and basal parts. Branching pattern, abundance and arrangement of determinate branches and trichoblasts varying among species. Trichoblasts, when present, initially short and pigmented, later enlarging and becoming unpigmented, dichotomously branched up to five orders, with uninucleate cells (, ). They were deciduous and left conspicuous scar cells when shed.
Reproductive morphology
Gametophytes dioecious. Spermatangial branches formed on determinate lateral branches, replacing trichoblasts, in dense clusters arranged spirally on every segment (). Spermatangial branches cylindrical, often incurved, with one or two apical sterile cells when mature (). Procarps formed on modified trichoblasts, consisting of a supporting cell bearing a four-celled carpogonial branch, a basal sterile cell and two lateral sterile cells (). Cystocarps formed on determinate branches in mid-parts of the thallus, ovoid and with an apical ostiole (). Carposporangia clavate.
Figs 15–20. Lophurella spp.: reproductive morphology. Fig. 15. Spermatangial branches densely clustered on second-order determinate branches. Fig. 16. Spermatangial branches with one or two sterile apical cells (arrows). Fig. 17. Procarp showing the supporting cell (su), a four-celled carpogonial branch (1–4) and a basal sterile cell (st). Fig. 18. Cystocarp with an apical ostiole (arrow). Fig. 19. Determinate branches with spirally arranged tetrasporangia. Fig. 20. Tetrasporangium with two presporangial (arrows) and a postsporangial (arrowhead) cover cells. Figs 15, 16, L. periclados; Figs 17, 18, 20, L. nigra; Fig. 19, L. mutabilis. Scale bars: Fig. 15 = 300 µm; Figs 16, 18 = 70 µm; Fig. 17 = 10 µm; Fig. 19 = 100 µm; Fig. 20 = 25 µm.
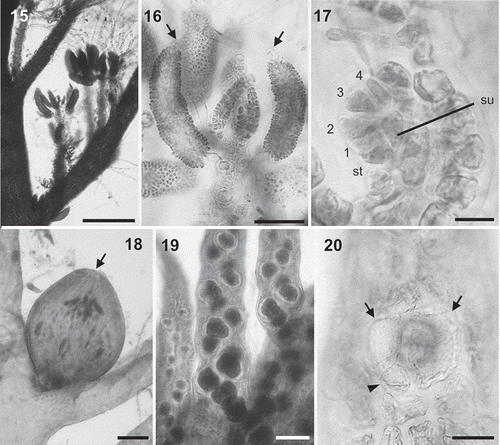
Tetrasporangia formed in mid-parts of the thallus on determinate branches that were more profusely branched than vegetative laterals. One tetrasporangium formed per segment, arranged in densely compacted long spiral series (). Tetrasporangia subspherical, with two presporangial and one postsporangial cover cells that remained ecorticate ().
Lophurella periclados (Sonder) F.Schmitz in Schmitz & Falkenberg, Citation1897: 441(–; Supplementary figs S1–4, S6–10, S33–52)
Figs 21–27. Lophurella periclados. Figs 21–23. Habit of specimens PD2746, PD772, PD4787, respectively. Fig. 24. Apex of the thallus, the arrow showing the apical cell. Fig. 25. Axis with determinate branches. Fig. 26. First-order determinate branch lacking trichoblasts (arrow) and bearing second- and third-order branches with trichoblasts. Fig. 27. Apex of a third-order determinate branch bearing spirally arranged trichoblasts. Scale bars: Figs 21, 22 = 8 mm; Fig. 23 = 15 mm; Fig. 24 = 150 µm; Figs 25, 26 = 850 µm; Fig. 27 = 100 µm.
Basionym: Rhodomela periclados Sonder, Citation1855.
Synonyms: Rhodomela simpliciuscula Harvey nom. nudum.
Lectotype: MEL 612898 (Womersley, Citation2003; Supplementary fig. S1).
Isolectotypes: MEL 612897, 612899, 612900 (Womersley, Citation2003; Supplementary figs S2–4).
Type locality: Port Phillip Bay, Victoria, Australia.
Description
Thallus dorsiventral, consisting of a prostrate system that bears rhizoids ventrally, erect axes dorsally and produces further prostrate axes laterally (–). Erect axes up to 10 cm in length, with main axis unbranched or pseudodichotomously branched up to four orders (–). Axes densely clothed with short determinate branches spirally arranged throughout the length of the main axes, sometimes denuded in basal parts (–). Thalli dark red to black in colour, with a rigid to flaccid texture.
At apices of erect axes, branch initials produced on every segment in a spiral sequence; endogenous determinate lateral branches also developing on every segment (). Determinate branches incurved when young (), soon becoming straight and acquiring a spiny appearance (), 300–500 µm in diameter basally. First-order determinate laterals producing branch initials on every segment; only some initials developing further, producing second and third-orders of endogenous determinate laterals (). Second- and third-order determinate laterals remaining short, often unilaterally arranged (). Basal parts of the erect axes lacking determinate laterals in some specimens, usually unbranched when present. Determinate laterals either overtopping apical cell of the main axis or the apical cell protruding beyond the branches. Trichoblasts absent on main axes and first-order determinate laterals (, ), borne on second- and third-order determinate laterals, on every segment (, ).
Distribution, habitat and morphological variability of our collections and type material
Lophurella periclados was commonly found in Port Phillip Bay and on nearby open coasts and is the only member of the genus that we identified in this region (). It was also collected at Mallacoota, the easternmost reefs in Victoria. It formed turfs in the intertidal zone of wave-exposed reefs, where specimens were robust, with a rigid texture, short (up to 5 cm in length) and scarcely branched (Supplementary figs S6–9). These specimens correspond to haplotype 2 (H2 in ). A sequence from Robe (Southern Australia) that we downloaded from GenBank was identical to H2. The second haplotype (H1 in ) corresponded to specimens collected in the drift or in a marina at Queenscliff, more sheltered locations inside Port Phillip Bay (Victoria). These specimens were more flaccid, more profusely branched and longer (up to 10 cm in length) (Supplementary fig. S10). The morphological variability observed in our specimens is similar to the conspicuous variability in habit of the type material. The type collection of L. periclados is housed at MEL and includes four specimens. Two specimens were short (6 cm) and scarcely branched (Supplementary figs S3, 4), while the remaining two were longer (10 cm) and more profusely branched (Supplementary figs S1, 2). Among them, Womersley (Citation2003) designated MEL612898 as the lectotype (Supplementary fig. S1). The specimens of L. periclados that we collected are in agreement with the type. Lophurella periclados was absent in our collections from Tasmania and New Zealand.
Lophurella mutabilis Díaz-Tapia, sp. nov. (–, Supplementary figs S11–23, S53–69)
Diagnosis: Thalli dorsiventral, with a prostrate system that bears rhizoids ventrally, erect axes dorsally and produces further prostrate axes laterally. Erect axes with main axes unbranched or pseudodichotomously to irregularly branched, clothed with determinate branches, usually on every segment and spirally arranged but occasionally sparse. Axes with four pericentral cells. Erect axes growing by divisions of an apical cell that protrudes above the lateral determinate branches, branch initials forming at apices on every segment or several segments apart. Some or all branch initials developing into determinate branches, 210–400 µm in diameter basally, straight and spine-like when mature. First-order determinate branches producing up to two further orders of branches that remain short. Trichoblasts restricted to determinate branches. rbcL sequence of the holotype: MN149994.
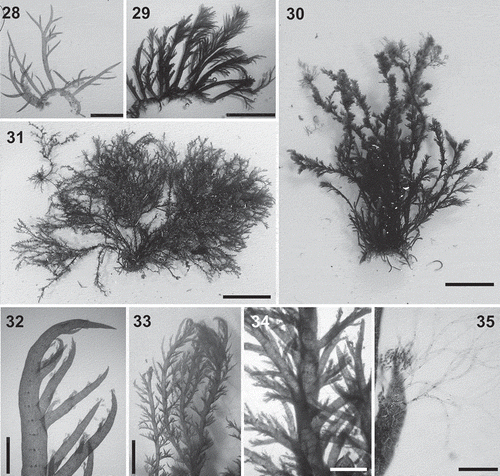
Figs 28–35. Lophurella mutabilis. Figs 28–31. Habit of specimens PD1111, PD3411, PD3483 and PD3106, respectively. Fig. 32. Apical part of an erect axis, forming determinate branches several segments below the apex. Figs 33, 34. Thallus clothed with determinate branches. Fig. 35. Apex of a third-order determinate branch bearing spirally arranged trichoblasts. Scale bars: Fig. 28 = 450 µm; Fig. 29 = 7 mm; Fig. 30 = 2 cm; Fig. 31 = 4 cm; Figs 32, 34 = 700 µm; Fig. 33 = 4 mm; Fig. 35 = 150 µm.
Holotype: MELUA118884a.
Type locality: Blackmans Bay, Tasmania, Australia.
Etymology: ‘mutabilis’ refers to the high variability observed among specimens of this species in habit and other morphological characters.
Description
Thalli dorsiventral, consisting of a prostrate system that bears rhizoids ventrally, erect axes dorsally and produces further prostrate axes laterally (, ). Habit variable, ranging from small (5 mm in length) pseudodichotomously branched specimens with sparse determinate branches () to large specimens (up to 15 cm in length) with main axes branching irregularly alternately or pseudodichotomously, profusely, to up to four orders, with axes clothed by abundant determinate laterals arranged spirally or unilaterally (–). Light to dark red or black in colour, with a rigid to flaccid texture.
Erect axes producing branch initials on every segment in a spiral sequence or, more rarely, several segments apart, all or only some developing into lateral determinate branches (). Determinate branches usually abundant and spirally arranged, clothing the main axes, 210–400 µm in diameter in basal parts and upwardly incurved when young, later becoming straight, spine-like (, ). Determinate laterals producing one or two orders of short determinate branches, arranged spirally or unilaterally. Trichoblasts usually present at the apices of first- and higher order determinate branches, formed on every segment in a spiral arrangement (), absent from the apices of main axes and, in some specimens, also from first-order determinate branches ().
Distribution, habitat and morphological variability
Lophurella mutabilis was abundant in eastern Tasmania (), forming turfs in the low intertidal of moderately to strongly wave-exposed sites. L. mutabilis was highly variable in habit (Supplementary figs S11–23); specimens from sheltered locations (Tinderbox and Southport, Supplementary figs S15–18) were more profusely branched and slender than specimens from exposed sites (Supplementary figs S11–13, S19–23). However, this morphological variability was not reflected in the genetic variability in the rbcL gene, as haplotypes 2 and 4 were found at both types of sites (). L. mutabilis was also collected at a site in western Victoria where a single small (5 mm in length, ) male specimen was found epiphytic on Cystophora sp. Genetically, this specimen corresponded to H1 in . In New Zealand, L. mutabilis H6 () was collected in the low intertidal of a site on Stewart Island.
Lophurella nigra Díaz-Tapia, sp. nov. (–, Supplementary figs S24–28, S70–88)
Diagnosis: Thalli dorsiventral, with a prostrate system that bears rhizoids ventrally, erect axes dorsally and produces further prostrate axes laterally. Erect axes pseudodichotomously or irregularly branched, bearing sparse determinate branches. Axes with four pericentral cells. Branch initials formed on every segment at the apices of the erect axes. Some branch initials developing into determinate branches, 250–400 µm in diameter basally, straight and spine-like when mature. Trichoblasts restricted to second or higher orders of determinate branches. rbcL sequence of the holotype: MN149998.
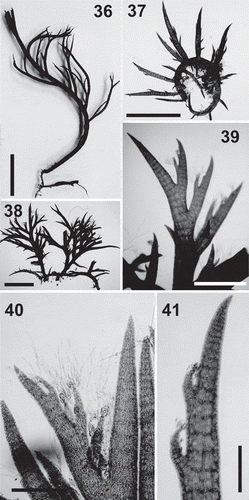
Figs 36–41. Lophurella nigra. Figs 36–38. Habit of specimens PD3555, PD2736 and PD2741, respectively. Figs 39–40. Apex of erect axes bearing two orders of determinate branches, the second-order bearing trichoblasts. Fig. 41. Determinate branch, lacking trichoblasts, bearing an order of determinate branches with young trichoblasts. Scale bars: Fig. 36 = 5 mm; Figs 37, 38 = 2.5 mm; Fig. 39 = 1 mm; Fig. 40 = 350 µm; Fig 41 = 250 µm.
Holotype: MEL2457114.
Type locality: Bastion Point, Mallacoota, Australia.
Etymology: ‘nigra’ refers to the black colour of the thallus.
Description
Thalli dorsiventral, consisting of an extensive prostrate system that bears rhizoids ventrally, erect axes dorsally and produces further prostrate axes laterally (–). Erect axes up to 5 cm in length, irregularly branched up to three orders, either with one main axis and lateral determinate branches or pseudodichotomously branched, with several main axes, that bear sparse and irregularly or unilaterally arranged determinate laterals (–). Thalli dark red to black in colour, with a rigid texture.
Erect axes producing branch initials on every segment, of which only some develop lateral endogenous branches. Determinate laterals unbranched or producing one or two orders of further determinate laterals, often unilaterally arranged (). Determinate laterals 250–400 µm in diameter basally. Trichoblasts formed on second- and third-order determinate laterals, spirally arranged on every segment, but absent from main axes and first-order determinate laterals (, ).
Distribution, habitat and morphological variability
Lophurella nigra was collected in eastern Victoria where it formed turfs in the low intertidal of wave-exposed sites. It was also collected in the same habitat in north-eastern Tasmania, as well as in the subtidal (5 m depth). Victorian specimens were short (up to 7 mm) and robust, while Tasmanian ones were longer (up to 5 cm) and more slender. This variability in habitat and distribution did not correspond with the genetic variability found in the rbcL gene. The two haplotypes were detected at a single sampling site and most specimens, independent of habitat and distribution, corresponded to haplotype 2 (, Supplementary table S1).
Lophurella pauciramulosa Díaz-Tapia, sp. nov. (–, Supplementary figs S29–31, S89–101)
Diagnosis: Thalli predominantly erect, attached by a short prostrate system that bears rhizoids ventrally, erect axes dorsally and produces further prostrate axes laterally. Erect axes pseudodichotomously branched up to seven orders, bearing sparse determinate branches. Axes with four pericentral cells. Branch initials formed on every segment at the apices of the erect axes. Some branch initials developing into determinate branches that are sparse, 250–300 µm in diameter basally. Trichoblasts absent. rbcL sequence of the holotype: MN150002.
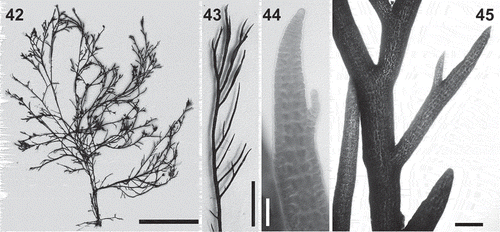
Figs 42–45. Lophurella pauciramulosa. Fig. 42. Habit of the holotype (specimen WELT A033737 (ASR166)). Fig. 43. Apical part of an erect axis with determinate branches. Fig. 44. Apex of an erect axis. Fig. 45. Determinate branch lacking trichoblasts. Scale bars: Fig. 42 = 35 mm; Fig. 43 = 6 mm; Fig. 44 = 60 µm; Fig. 45 = 400 µm.
Holotype: WELT A033737.
Type locality: Green Island, South Island, New Zealand.
Etymology: ‘pauciramulosa’ refers to the scarcity of determinate branches compared with most other members of the genus.
Description
Thalli predominantly erect (), attached to the substratum by a short prostrate system that bears rhizoids ventrally and produces further prostrate axes laterally. Erect axes up to 20 cm in length, branched pseudodichotomously up to seven orders, producing series of unilaterally arranged short determinate laterals at irregular intervals (). Thalli dark dull purple red in colour, drying black, with a firm texture.
Erect axes producing determinate endogenous lateral branches at irregular intervals (, ). Lateral branches 250–300 µm basally, unbranched or once-branched in vegetative thalli. Trichoblasts absent.
Habitat and distribution
This species was collected in the subtidal (2–10 m depth) from the south-east coast of South Island and Stewart Island, New Zealand. L. pauciramulosa is often infected by the parasites Sporoglossum lophurellae Kylin and Colacopsis lophurellae Kylin.
Lophurella tasmanica Díaz-Tapia, sp. nov. (–, Supplementary figs S32, S102–119)
Diagnosis: Thalli dorsiventral, with a prostrate system that bears rhizoids ventrally, erect axes dorsally and produces further prostrate axes laterally. Erect axes with unbranched main axes clothed with spirally arranged determinate branches formed on every segment. Axes with four pericentral cells. Erect axes growing by the division of an apical cell that is overtopped by lateral determinate branches. Branch initials formed at apices of erect axes, on every segment. All branch initials developing into determinate branches, 150–230 µm in diameter basally, upwardly incurved when mature. First-order determinate branches producing up to two further orders of determinate branches. Determinate second-order branches reaching a length similar to the parental determinate branch. Trichoblasts restricted to second- and third-order determinate branches. rbcL sequence of the holotype: MN150004.
Figs 46–50. Lophurella tasmanica. Fig. 46. Habit of specimen PD3584. Fig. 47. Apex of an erect axis with apical cell indicated (arrow). Fig. 48. Apical part of an erect axis densely clothed with determinate branches. Fig. 49. Mid-part of an erect axis with basally branched determinate laterals. Fig. 50. Determinate branch bearing two orders of branches, of which the third-order branches bear trichoblasts. Scale bars: Fig. 46 = 8 mm; Fig. 47 = 100 µm; Fig. 48 = 2.5 mm; Figs 49, 50 = 800 µm.
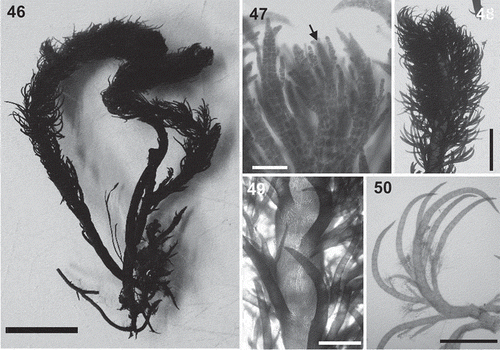
Holotype: MELUA118885a.
Type locality: Port Arthur, Tasmania, Australia.
Etymology: ‘tasmanica’ refers to the type locality of the species.
Description
Thallus dorsiventral, consisting of a prostrate system that bears rhizoids ventrally, erect axes dorsally and produces further prostrate axes laterally (). Erect axes up to 5 cm in length with unbranched main axes clothed by spirally arranged determinate laterals. Thalli dark red in colour, with a rigid texture.
Erect axes producing determinate lateral branches on every segment, spirally arranged and upwardly incurved, overtopping the apical cell of the main axes (–). Lateral branches 150–230 µm diameter in basal parts, producing spirally a second order of determinate branches when young, such branches remaining restricted to basal parts of laterals (). Second-order branches upwardly incurved and reaching a similar length to the parental first-order determinate branch (). A third order of determinate laterals remained as short branches (). Determinate laterals in basal parts of the thalli less profusely branched, probably denuded. Trichoblasts formed on second- and third-order determinate branches in a spiral arrangement on every segment but absent from the apex of the main axes and the first-order determinate branches ().
Habitat and distribution
Only known from the type locality, in south-eastern Tasmania (), where it was collected in the low intertidal of a moderately wave-exposed site.
Discussion
We found that 36 specimens initially identified as Lophurella periclados from Australia and New Zealand represented a complex of four cryptic or semi-cryptic species for which we propose three new species, L. mutabilis, L. nigra and L. tasmanica. Moreover, we found that L. hookeriana from New Zealand differs from specimens from the type locality in Chile, requiring the description of the new species L. pauciramulosa from New Zealand.
The new species are distinguished by their sequence divergence in the rbcL gene relative to the previously recognized species in the genus. Sequence divergence was ≥ 1.8% among species, except between L. tasmanica and L. periclados, which were 1.0–1.1% divergent. Although sequence divergence for this pair of species is less than for the other species here described, they can be morphologically distinguished (see discussion below) and we recognize them as separate species. This contrasts with the recognition of a single species for the six haplotypes we found in L. mutabilis and the two haplotypes of L. periclados. One of the haplotypes of L. mutabilis (H6 in ), the New Zealand specimen, was relatively highly (0.7–0.9%) divergent from Australian specimens. Likewise, the divergence between the two haplotypes of L. periclados (0.8%) was relatively high and they might be considered as separate species. However, in the absence of relevant morphological characters for distinguishing these highly divergent haplotypes, we do not recognize them as distinct species at present. Future work with larger sampling sizes across the distribution range of these lineages as well as additional molecular markers might reveal whether they should be segregated or that they are single lineages with high genetic variability in the rbcL gene. Species boundaries based on sequence data are often based on comparable divergence values among sister species assuming that interspecific divergence is higher than intraspecific variability (Leliaert et al., Citation2014). However, the establishment of boundaries based on sequence divergence is not always straightforward and different species, even if closely related, may have experienced different evolutionary histories resulting in different levels of intraspecific variability (Díaz-Tapia et al., Citation2018a; Phillips et al., Citation2019). In our Lophurella spp. dataset, there was no large difference between intra- and interspecific variability in the rbcL gene, and therefore we also took morphological characters into account when delineating the species.
All the species described here accord with the concept of the tribe Pterosiphonieae, as rhizoids are cut off from pericentral cells and have multicellular haptera (Díaz-Tapia et al., Citation2017). Likewise, they fit the definition of the genus Lophurella (Womersley, Citation2003): the thallus consists of prostrate and erect axes, axes have four pericentral cells and are heavily corticated from close to the apices, spermatangial branches replace trichoblasts and have apical sterile cells, cystocarps are globose and tetrasporangia form spiral series. Moreover, all the studied species had tetrasporangia with two presporangial cover cells and a postsporangial cover cell. Trichoblasts were found abundantly in most species here studied (except L. pauciramulosa) and their arrangement was unusual when compared with other Rhodomelaceae. Trichoblasts in this family are usually produced at the apexes of main axes and branches (Maggs & Hommersand, Citation1993; Womersley, Citation2003; Díaz-Tapia et al., Citation2013). However, in Lophurella, trichoblasts were absent from the main axes and restricted to second or higher order determinate branches. Womersley (Citation2003) noted this particular character in his description of the genus.
Most of the taxonomically informative qualitative morphological characters that are employed for distinguishing species in the Pterosiphonieae were shared among the species studied here (Maggs & Hommersand, Citation1993; Womersley, Citation2003; Savoie & Saunders, Citation2016). Nevertheless, some other detailed morphological features can contribute to species identification. summarizes the main characters that we found useful for distinguishing the species of Lophurella in Australia and New Zealand. They include vegetative morphology, habitat and the presence or absence of parasites (Colacopsis lophurellae and Sporoglossum lophurellae). The reproductive structures when known were virtually uniform among species and were not informative for species delimitation, as is often the case in the Rhodomelaceae (Díaz-Tapia & Bárbara, Citation2011; García-Redondo et al., Citation2016). Morphologically, L. caespitosa, L. pauciramulosa and L. tasmanica can be distinguished from other congeners from Australia and New Zealand. The most conspicuous characters of L. caespitosa are its emerald green colour, the absence of trichoblasts and the branching pattern of erect axes that are denuded below, with abundant branches in upper parts bearing tufts of short determinate laterals at the apices (Adams, Citation1994; Nelson, Citation2013; PD, pers. obs.). The other species, by contrast, are dark red to black in colour, have trichoblasts (except L. pauciramulosa) and the erect axes have a different branching pattern (). L. tasmanica is morphologically similar to L. periclados and some specimens of L. mutabilis which have the main axes clothed with short determinate branches. L. tasmanica differs from this pair of species mainly because its determinate laterals are thinner, more profusely branched, with longer second-order determinate branches, and determinate laterals are incurved at maturity. As a result, the main axes are densely covered by determinate laterals that lack the spiny appearance of L. mutabilis and L. periclados. L. pauciramulosa is mainly distinguished from the other species studied here by having long (up to 20 cm) and predominantly erect thalli, scarce production of determinate branches, complete absence of trichoblasts, as well as the subtidal habitat and the common presence of parasites (C. lophurellae and S. lophurellae). L. pauciramulosa is morphologically distinct from Australian and New Zealand congeners and it can also be distinguished from L. hookeriana, which has trichoblasts on determinate branches (Boraso de Zaixso, Citation2013; EM, pers. obs.).
The other three species here recognized, L. periclados, L. nigra and L. mutabilis, are examples of cryptic species as they cannot be distinguished by morphological characters and DNA sequences are required for their identification. L. periclados and L. nigra are distinct, but L. mutabilis is so variable morphologically that some specimens overlap with the morphological characters of both species. L. periclados has spirally arranged determinate laterals formed on every segment while L. nigra bears sparse determinate laterals in an irregular pattern. We observed specimens of L. mutabilis with both of these habits and other morphological details are also shared with the other two species. Therefore, the high morphological plasticity of L. mutabilis prevents reliable morphological identification of these three species. This scenario is frequently encountered in the red algae and similar problematic morphological delineations have been discussed in other groups (Milstein & Saunders, Citation2012; Carro et al., Citation2014; Verbruggen, Citation2014). However, more commonly, phenotypic plasticity explains the high levels of cryptic diversity detected in the red algae but detailed morphological studies of the species delineated based on DNA data can reveal morphological differences among them (Walker et al., Citation2009; Zanolla et al., Citation2014; Barreto de Jesus et al., Citation2019). The morphological variability described by Womersley (Citation2003) for L. periclados, that he related to different levels of wave exposure, was observed within L. periclados and L. mutabilis. Specimens from sheltered sites are more slender and elongate than those from wave-exposed coasts. Therefore, the cryptic diversity that we detected did not correspond to the morphotypes noted by Womersley (Citation2003).
In addition to the species of Lophurella described here and included in our molecular analyses, another four species are currently recognized. L. comosa, from South America, is clearly distinguished from the rest of the genus by having seven pericentral cells (Hooker & Harvey, Citation1845; Harvey, Citation1847) whereas all other species have four. L. patula and L. gaimardii, both also from South America, resemble L. pauciramulosa in exceeding 10 cm in length and having sparse determinate branches (Hooker & Harvey, Citation1845; De Toni, Citation1905). They differ from the other three species described here, which have shorter erect axes (up to 10 cm, except L. mutabilis) and/or the erect axes are clothed with short determinate branches. L. patula has main axes with alternate branches (Hooker & Harvey, Citation1845; Kylin & Skottsberg, Citation1919), differing from L. pauciramulosa which is pseudodichotomously branched. L. gaimardii has only been reported from the type locality, the Falkland Islands, and according to the original description (Agardh, Citation1822, as Rhodomela), this species has trichoblasts (‘ad apicem ramentorum racemosa, pellucida’) which appear to be shown in the illustration in Bory de Saint-Vincent (Citation1826). Therefore, L. gaimardii differs in this respect from L. pauciramulosa, which lacks trichoblasts. Finally, L. christophersenii from Tristan da Cunha differs from other species because lateral determinate branches are shed from the older parts of the thallus, resulting in a long stem bearing determinate branches only in the upper parts (Baardseth, Citation1941). Moreover, it has spermatangial branches on the first dichotomy of trichoblasts (Baardseth, Citation1941), while spermatangial branches completely replace trichoblasts in other congeners when known. Indeed, this character is uniform in all the genera included at present in the tribe Pterosiphonieae (Womersley, Citation2003; Díaz-Tapia & Bárbara, Citation2011; García-Redondo et al., Citation2016; Díaz-Tapia et al., Citation2017). This leads us to question the placement of L. christophersenii in the genus Lophurella and the tribe Pterosiphonieae, and we suggest that this taxon should be re-evaluated using molecular data.
Species of Lophurella have restricted distributions and our study showed that their range is even narrower than that indicated in previous diversity assessments based on morphological identifications. L. hookeriana was the only species with a transoceanic recorded distribution (Adams, Citation1994; Guiry & Guiry, Citation2019), but our data show that the New Zealand and South American populations represent different species. L. hookeriana and L. pauciramulosa are endemic to South America and New Zealand, respectively. L. periclados was previously reported from south-eastern Australia, Tasmania and New Zealand, but our study showed that it is a complex of four species with different but overlapping distribution patterns (). L. periclados and L. tasmanica were only found in mainland Australia and eastern Tasmania, respectively, while L. nigra was found in both regions. L. periclados has also been recorded in New South Wales (Millar & Kraft, Citation1993) and the identity of these specimens needs to be reassessed using molecular data. L. mutabilis has the widest distribution, including south-eastern Australia, Tasmania and New Zealand. The present distribution of Lophurella spp. suggests that their evolutionary history might have been affected by palaeobiogeographical processes, such as dispersal events between Australasia and South America or the land-bridge formed between Tasmania and mainland Australia during the Pleistocene, in the same way that these processes might have shaped the current distribution of other red algae (Guillemin et al., Citation2014; Díaz-Tapia et al., Citation2018b; Mueller et al., Citation2018). Further studies including wider taxon sampling might contribute to better understanding how these processes have contributed to the evolutionary history of the Australasian red algae.
Given the level of cryptic speciation discovered in the genus in Australia further research in New Zealand is required, including determining the distributional ranges of L. pauciramulosa and L. mutabilis. It is likely that there is further diversity within what has been known as L. hookeriana in New Zealand: this species has been reported from the northern North Island through to the New Zealand subantarctic, and specimens within herbarium collections display considerable morphological variability (WN, pers. obs.). The records of L. periclados in New Zealand also have to be re-examined: this species has been reported from Cook Strait south to Stewart Island as well as from the Chatham Islands, and it is not clear that all of the specimens are correctly referred to L. mutabilis.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2019.1659419
Supplementary table S1. Collection information or publication and GenBank accession numbers of the sequences used in phylogenetic analyses.
Supplementary table S2. Intra- and inter-specific sequence divergence in the rbcL gene in the genus Lophurella.
Supplementary figs S1–32. Habit of specimens of Lophurella spp. from Australasia.
Supplementary figs S33–119. Details of morphological characters of Lophurella spp. from Australasia (except L. caespitosa).
Author contributions
P. Díaz-Tapia: original concept, morphological and molecular analyses, drafting and editing manuscript; C.A. Maggs: original concept, drafting and editing manuscript; W. Nelson: providing specimens, morphological analyses, editing manuscript; E.C. Macaya: providing specimens, morphological analyses; H. Verbruggen: original concept, drafting and editing manuscript.
TEJP-2019-0065-File013.pdf
Download PDF (14.5 MB)TEJP-2019-0065-File012.pdf
Download PDF (36.6 KB)TEJP-2019-0065-File011.pdf
Download PDF (74.7 KB)Acknowledgements
We thank Joana Costa, Kyatt Dixon, Guadalupe Bribiesca-Contreras and the Parks Victoria and Bush Blitz teams for assistance in the field. We thank MELU herbarium, as well as Angharad Johnson and personnel of the National Herbarium of Victoria (MEL) for providing high quality pictures of the type material of Lophurella periclados, and the staff of the herbarium of the Museum of New Zealand Te Papa Tongarewa (WELT).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adams, N.M. (1994). Seaweeds of New Zealand. An Illustrated Guide. Canterbury University Press, Christchurch.
- Agardh, C.A. (1822). Species algarum rite cognitae, cum synonymis, differentiis specificis et descriptionibus succinctis. Volumen primum pars posterior. Ex officina Berlingiana, Lundae.
- Baardseth, E. (1941). The marine algae of Tristan da Cunha. Results of the Norwegian Scientific Expedition to Tristan da Cunha 1937–1938, 9: 1–173.
- Barreto de Jesus, P., Leite Costa, A., de Castro Nunes, J.M., Manghisi, A., Genovese, G., Morabito, M. & Selbach Schnadelbach, A. (2019). Species delimitation methods reveal cryptic diversity in the Hypnea cornuta complex (Cystocloniaceae, Rhodophyta). European Journal of Phycology, 54: 135–153.
- Boraso de Zaixso, A.L. (2013). Elementos para el estudio de las macroalgas de Argentina. Universitaria de la Patagonia, Comodoro Rivadavia.
- Bory de Saint-Vincent, J.B.G.M. (1826). Cryptogamie. In Voyage autour du monde, exécuté par ordre du Roi, sur la corvette de sa majesté, La Coquille, pendant les années 1822, 1823, 1824 et 1825 (Duperrey, L.I., editor), Atlas. Baudouin Frères, Paris.
- Bustamante, D.E., Won, B.Y. & Cho, T.O. (2014). Polysiphonia dokdoensis sp. nov. (Rhodomelaceae, Ceramiales) based on a population previously known as Polysiphonia atlantica sensu Kim & Lee from Korea. Botanica Marina, 57: 281–289.
- Carro, B., López, L., Peña, V., Bárbara, I. & Barreiro, R. (2014). DNA barcoding allows the accurate assessment of European maerl diversity: a proof-of-concept study. Phytotaxa, 190: 176–189.
- De Toni, G.B. (1905). Sylloge algarum omnium hucusque cognitarum. Vol. IV. Florideae. Sectio IV. Privately published, Patavii.
- Díaz-Tapia, P. & Bárbara, I. (2011). Sexual structures in Ptilothamnion sphaericum and Pterosiphonia complanata (Ceramiales, Rhodophyta) from the Atlantic Iberian Peninsula. Botanica Marina, 54: 35–46.
- Díaz-Tapia, P., Bárbara, I. & Berecibar, E. (2013). Vegetative and reproductive morphology of Polysiphonia tripinnata (Rhodomelaceae, Rhodophyta): a new record from the European Atlantic coast. Botanica Marina, 56: 151–160.
- Díaz-Tapia, P., Maggs, C.A., West, J.A. & Verbruggen, H. (2017). Analysis of chloroplast genomes and a supermatrix inform reclassification of the Rhodomelaceae (Rhodophyta). Journal of Phycology, 53: 920–937.
- Díaz-Tapia, P., Maggs, C.A., Macaya, E.C. & Verbruggen, H. (2018a). Widely distributed red algae often represent hidden introductions, complexes of cryptic species or species with strong phylogeographic structure. Journal of Phycology, 54: 829–839.
- Díaz-Tapia, P., Pasella, M. & Verbruggen, H. (2018b). Molecular analyses resolve the phylogenetic position of Polysiphonia adamsiae (Rhodomelaceae, Rhodophyta) and reveal a strong phylogeographic structure in Australia. Phycologia, 57: 593–600.
- Doyle, J.J. & Doyle, J.L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin, 19: 11–15.
- Falkenberg, P. (1901). Die Rhodomelaceen des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. Fauna und Flora des Golfes von Neapel, Monographie 26. Berlin.
- García-Redondo, V., Bárbara, I. & Díaz-Tapia, P. (2016). First record of sexual structures in Pterosiphonia parasitica (Rhodomelaceae, Rhodophyta) from the Iberian Peninsula. Thalassas, 32: 87–90.
- Guillemin, M.-L., Valero, M., Faugeron, S., Nelson, W. & Destombe, C. (2014). Tracing the trans-Pacific evolutionary history of a domesticated seaweed (Gracilaria chilensis) with archaeological and genetic data. PLoS ONE, 9: e114039.
- Guillemin, M.-L., Contreras-Porcia, L., Ramírez, M.E., Macaya, E.C., Contador, C.B., Woods, H., Wyatt, C. & Brodie, J. (2016). The bladed Bangiales (Rhodophyta) of the South Eastern Pacific: molecular species delimitation reveals extensive diversity. Molecular Phylogenetics and Evolution, 94: 814–826.
- Guiry, M.D. & Guiry, G.M. (2019). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 18 November 2018.
- Harvey, W.H. (1847). Nereis australis, or algae of the southern ocean: being figures and descriptions of marine plants, collected on the shores of the Cape of Good Hope, the extra-tropical Australian colonies, Tasmania, New Zealand, and the Antarctic regions; deposited in the Herbarium of the Dublin University. Reeve Brothers, London.
- Hommersand, M.H. (1963). The morphology and classification of some Ceramiaceae and Rhodomelaceae. University of California Publications in Botany, 35: 165–366.
- Hooker, J.D. & Harvey, W.H. (1845). Algae antarcticae, being characters and descriptions of the hitherto unpublished species of algae, discovered in Lord Auckland’s Group, Campbell’s Island, Kerguelen’s Land, Falkland Islands, Cape Horn and other southern circumpolar regions, during the voyage of H.M. discovery ships “Erebus” and “Terror”. London Journal of Botany, 4: 249–276, 293–298.
- Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S, Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P. & Drummond, A. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28: 1647–1649.
- Kylin, H. & Skottsberg, C. (1919). Zur Kenntnis der subantarktischen und antarktischen Meeresalgen. II. Rhodophyceen. In Wissenschaftliche Ergebnisse der Schwedischen Südpolar-Expedition 1901–1903 (Nordenskjöld, O., editor), vol. 4: 2, 1–88. Litographisches Institut des Generalstabs, Stockholm.
- Leliaert, F., Verbruggen, H., Vanormelingen, P., Steen, F., López-Bautista, J.M., Zuccarello, G.C. & De Clerck, O. (2014). DNA-based species delimitation in algae. European Journal of Phycology, 49: 179–196.
- Maggs, C.A. & Hommersand, M.H. (1993). Seaweeds of the British Isles. Volume 1. Rhodophyta. Part 3A. Ceramiales. HMSO, London.
- Millar, A.J.K. & Kraft, G.T. (1993). Catalogue of marine and freshwater red algae (Rhodophyta) of New South Wales, including Lord Howe Island, South-western Pacific. Australian Systematic Botany, 6: 1–90.
- Milstein, D. & Saunders, G.W. (2012). DNA barcoding of Canadian Ahnfeltiales (Rhodophyta) reveals a new species – Ahnfeltia borealis sp. nov. Phycologia, 51: 247–259.
- Mueller, R., Wright, J.T. & Bolch, C.J.S. (2018). Historical demography and colonization pathways of the widespread intertidal seaweed Hormosira banksii (Phaeophyceae) in southeastern Australia. Journal of Phycology, 54: 56–65.
- Nelson, W.A. (2013). New Zealand Seaweeds. An Illustrated Guide. Te Papa Press, Wellington.
- Phillips, J.D., Gillis, D.J. & Hanner, R.H. (2019). Incomplete estimates of genetic diversity within species: implications for DNA barcoding. Ecology and Evolution, 9: 2996–3010.
- Saunders, G.W. & McDevit, D.C. (2012). Methods for DNA barcoding photosynthetic protists emphasizing the macroalgae and diatoms. Methods in Molecular Biology, 858: 207–222.
- Saunders, G.W. & Moore, T.E. (2013). Refinements for the amplification and sequencing of red algal DNA barcode and RedToL phylogenetic markers: a summary of current primers, profiles and strategies. Algae, 28: 31–43.
- Saunders, G.W., Huisman, J.M., Vergés, A., Kraft, G.T. & Le Gall, L. (2017). Phylogenetic analyses support recognition of ten new genera, ten new species and 16 new combinations in the family Kallymeniaceae (Gigartinales, Rhodophyta). Cryptogamie, Algologie, 38: 79–132.
- Savoie, A.M. & Saunders, G.W. (2016). A molecular phylogenetic and DNA barcode assessment of the tribe Pterosiphonieae (Ceramiales, Rhodophyta) emphasizing the Northeast Pacific. Botany, 94: 917–939.
- Savoie, A.M. & Saunders, G.W. (2019). A molecular assessment of species diversity and generic boundaries in the red algal tribes Polysiphonieae and Streblocladieae (Rhodomelaceae, Rhodophyta) in Canada. European Journal of Phycology, 54: 1–25.
- Schmitz, F. & Falkenberg, P. (1897). Rhodomelaceae. In Die natürlichen Pflanzenfamilien nebst ihren Gattungen und wichtigeren Arten insbesondere den Nutzpflanzen unter Mitwirkung zahlreicher hervorragender Fachgelehrten, Teil 1, Abteilung 2 (Engler, A. & Prantl, K., editors), 421–480. Verlag von Wilhelm Engelmann, Leipzig.
- Schneider, C.W., Hamzeh, B.F., Lane, C.E. & Saunders, G.W. (2018). A new species of Digenea (Rhodomelaceae, Ceramiales) based upon a molecular assessment and morphological observations of plants historically known as D. simplex in Bermuda. Phytotaxa, 338: 90–98.
- Sonder, O.W. (1855). Algae annis 1852 et 1853 collectae. Linnaea, 26: 506–528.
- Stamatakis, A. (2014). RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312–1313.
- Verbruggen, H. (2014). Morphological complexity, plasticity, and species diagnosability in the application of old species names in DNA-based taxonomies. Journal of Phycology, 50: 26–31.
- Walker, R.H., Brodie, J., Russell, S., Irvine, L.M. & Orfanidis, S. (2009). Biodiversity of coralline algae in the northeastern Atlantic including Corallina caespitosa sp. nov. (Corallinoideae, Rhodophyta). Journal of Phycology, 45: 287–297.
- Womersley, H.B.S. (2003). The Marine Benthic Flora of Southern Australia. Rhodophyta. Part IIID. Ceramiales – Delesseriaceae, Sarcomeniaceae, Rhodomelaceae. Australian Biological Resources Study & State Herbarium of South Australia, Canberra & Adelaide.
- Zanolla, M., Carmona, R., De La Rosa, J., Salvador, N., Sherwood, A.R., Andreakis, N. & Altamirano, M. (2014). Morphological differentiation of cryptic lineages within the invasive genus Asparagopsis (Bonnemaisoniales, Rhodophyta). Phycologia, 53: 233–242.
