ABSTRACT
Udotea geppiorum is reported to form meadows in deep-water, soft sediment habitats in the Main Hawaiian Islands. The identification of the species, initially published as U. argentea in the archipelago, is revised and confirmed based on morphological assessment and comparative DNA sequencing with western Pacific specimens. Udotea geppiorum is fully corticated and newly shown to display discrete concentric segments arranged in a ‘tongue and groove’ manner, which are connected by medullary siphons running along the entire segment interface. Udotea geppiorum is phylogenetically nested among a cluster of Udotea spp. recently circumscribed within Udotea sensu stricto. This cluster of species is separate from the remainder of the family Udoteaceae, which includes several genera and Udotea spp. sensu lato that lack or exhibit incomplete cortication. Based on field observations conducted in O‘ahu and Maui, U. geppiorum can be found from 20 m to > 90 m depth with a peak in abundance at mesophotic depths of 60–85 m, where it forms extensive meadows that support associated diversity.
Introduction
Although rarely explored, deep-water marine assemblages in the tropics have been shown to possess a remarkable biodiversity, including species new to science and new distributional records of macroalgae (e.g. Agegian & Abbott, Citation1985; Littler et al., Citation1985; Hanisak & Blair, Citation1988; Ballantine, Citation1990; Norris & Olsen, Citation1991; Ballantine & Norris, Citation1994; Ballantine & Aponte, Citation2002; Graham et al., Citation2007). However, because of their richer macroalgal biodiversity in comparison to soft bottom habitats, most studies of macroalgae in deep-water habitats have focused on assemblages attached to hard substratum (such as fore-reefs and pinnacles, e.g. CitationCitationHillis-Colinvaux, Citation1986; Hanisak & Blair, Citation1988; Norris & Olsen, Citation1991; Aponte & Ballantine, Citation2001; Ballantine & Aponte, Citation2005; Graham et al., Citation2007; Leichter et al., Citation2008).
At these mesophotic depths (i.e. from 30–40 m to 150 m; Hinderstein et al., Citation2010), large expanses of sand may provide habitat for the growth of extensive meadows formed by psammophytic siphonous green algae (Bryopsidales) that can anchor themselves in soft bottom substratum via rhizoids, such as Halimeda incrassata (Ellis) Lamouroux (Sangil et al., Citation2018). These meadows may be defined as "relatively flat areas where sizeable and relatively dense upright living algal populations (i.e. rhipsalian Halimeda) occur" (Freile et al., Citation1995: 28), but the definition may also include other bryopsidalean genera, such as the Udoteacean taxon Penicillus capitatus Lamarck (e.g. at depths of 28–50 m in the Canary Islands, Sangil et al., Citation2010). While mesophotic algal meadows are still poorly documented, they may be widespread, especially as they have been reported from a broad geographic range, spanning the Atlantic and the Pacific basin, in the Bahamas (Freile et al., Citation1995), Canary Islands (Sangil et al. Citation2010), the Great Barrier Reef (Drew & Abel, Citation1988), the Marshall Islands (Hillis-Colinvaux, Citation1988) and Hawai‘i (Smith et al., Citation2005; Kahng & Kelley, Citation2007; Spalding, Citation2012).
In the Hawaiian Islands, the photic zone extends to at least 250 m (Agegian & Abbott, Citation1985) and consists of deep-water macroalgal assemblages and coral reefs (Doty et al., Citation1974; Agegian & Abbott, Citation1985; Kahng & Kelley, Citation2007). During the course of baseline surveys conducted between 2000 and 2006 in the deep waters off O‘ahu to explore the distribution of the invasive macroalga Avrainvillea amadelpha (Montagne) A. Gepp & E. Gepp (see Brostoff, Citation1989), a mesophotic Udotea species was discovered forming extensive meadows in sand at depths of 60–85 m. Since these surveys, the taxon was also encountered in 2007 by the City and County of Honolulu in recently dredged areas of Mamala Bay, and reported as a potentially introduced species by local scientists, who identified it as U. argentea Zanardini (Bailey-Brock & Magalhães, Citation2010). However, a pronounced morphological feature of these mesophotic Udotea specimens was the distinct concentric segmentation of the flabellum, a character that does not apply to U. argentea ("obsolete zonata" in Zanardini Citation1858, i.e. zonation scarcely apparent). A search for Pacific Udotea species exhibiting similar habits among the currently accepted Udotea species listed in AlgaeBase (Guiry & Guiry, Citation2019) and a review of the primary literature, both pointed at a rarely reported species known as U. geppiorum Yamada (Citation1930). In congruence with this identification, was the discovery through the Macroalgal Herbarium Portal (macroalgae.org/portal/) of a Hawaiian specimen held at BISH (Thiers, Citation2019) collected in 2005 by Dr R. Okano off Kalaeloa, south-west of O‘ahu, that the late Dr I.A. Abbott had identified as Udotea geppii (subsequently annotated as U. geppiorum by Dr R. Tsuda in 2013, see Supplementary fig. S1).
Udotea geppiorum was originally described as U. geppii Yamada (Citation1930) in honour of Gepps; however according to Guiry & Guiry (Citation2019), the currently accepted epithet with proper genitive plural is Udotea geppiorum. Yamada’s (Citation1930: 141) description was based on type material collected from the Caroline Islands, Palau in SAP (Supplementary fig. S2) and material from the Friendly Islands (Tonga) distributed by Harvey as “Friendly Isl. Alg. No. 94” in BM, and labelled Udotea flabellata, which Yamada considered to be conspecific with his Caroline Islands material. Both of Harvey’s U. flabellata specimens in NY and MICH display similar external features to U. geppiorum (Supplementary figs S3-S4). The name Udotea flabellata was introduced by Lamouroux (Citation1816: 311) who included Corallina flabellum J.Ellis & Solander (Citation1786: 124, pl. 24) in synonymy, rendering Lamouroux’s name superfluous and illegitimate. The correct combination Udotea flabellum (J.Ellis & Solander) M.Howe was later made by Howe (Citation1904: 94, pl. 6). Yamada (Citation1930) did not designate a holotype in the protologue of U. geppiorum and the type specimens from Palau and Tonga thus represent syntypes (see M.J. Wynne annotation, Supplementary fig. S4).
Recently, Lagourgue et al. (Citation2018) explored Udoteaceae diversity in the Caribbean region based on morphometrics and DNA sequencing of chloroplast barcodes (tufA and rbcL) and nuclear markers (18S rRNA). While these authors noted the polyphyly of the genus Udotea (as previously reported by Kooistra, Citation2002), their assessment showed that species exhibiting complete cortication by lateral appendages throughout the thalli (stipe and blade) were monophyletic and included the generitype U. flabellum (see fig. 4, p. 766 in Lagourgue et al., Citation2018). Based on these findings, these authors delimited Udotea sensu stricto in which they included U. dixonii Littler & Littler, U. dotyi Littler & Littler, U. flabellum and U. occidentalis Gepp & Gepp, while proposing that U. argentea, U. geppiorum and U. norrisii Littler & Littler should also be included pending further DNA sequencing for verification. In contrast, the remainder of Udotea spp. sequenced to date (i.e. those phylogenetically remote from Udotea sensu stricto) are incompletely corticated by lateral appendages (i.e. not forming a continuous cortex, e.g. U. spinulosa Howe), or lack cortication on the blade (although cortication may be present in the stipe, e.g. U. conglutinata (J.Ellis & Solander) J.V.Lamouroux and U. cyathiformis Decaisne).
Since Yamada’s (Citation1930) description, U. geppiorum was recorded from a few south-east Asian locations such as Indonesia (e.g. Atmadja & Prud’homme van Reine, Citation2014; Philippines: Silva et al., Citation1987) and Pacific Islands (e.g. Micronesia: Lobban & Tsuda, Citation2003; Fiji: Littler & Littler, Citation2003; Mariana Islands: Tsuda, Citation2003) and may thus be broadly distributed in the western Pacific. In Palau, Ohba et al. (Citation2007) reported that the species was common, growing solitary or in clusters in shallow and deeper lagoons, and outer reefs from 0.5 to 35 m depth. By contrast, in Southern Pacific localities, Littler & Littler (Citation2003) reported that it is not found below 10 m depth. In spite of these records and field observations, the taxon still eludes broader recognition and U. geppiorum may often be misidentified in Pacific localities, i.e. as U. argentea or U. flabellum. This may stem from the fact that the protologue of U. geppiorum is very brief and lacking in morphological details (Yamada, Citation1930), and that the taxon has not previously been sequenced to determine its molecular identity. Reports of U. flabellum in the Pacific probably originate from the use of U. flabellata J.V.Lamouroux nom. illeg. by Harvey (see taxonomic history above), an error that has probably been propagated in the literature. Such records appear biogeographically incongruent because U. flabellum represents a common species in the western Tropical Atlantic (type locality West Indies, Ellis & Solander, Citation1786), and appears to be restricted to this region based on published molecular data (e.g. Kooistra, Citation2002; Lagourgue et al., Citation2018). Similarly, U. argentea, whose type locality is the Red Sea (Zanardini, Citation1858), may represent a misidentification of U. geppiorum in the region, considering that it represents a more commonly known epithet due to its widespread recognition in the Indo-Pacific (Guiry & Guiry, Citation2019).
In the present study, we aim to clarify the molecular identity of U. geppiorum in order to revise the record of Udotea in the Hawaiian archipelago with proper identification (i.e. previously published as U. argentea in Bailey-Brock & Magalhães, Citation2010). We also reassess morphological features of this still poorly documented taxon for overlooked characters. Finally, we report ecological observations on mesophotic meadows discovered in the Main Hawaiian Islands.
Materials and methods
Field observations
Observations were made during several submersible (~50–210 m) and/or remotely operated vehicle (~40–230 m) dives conducted between 2000 and 2006 along the western and southern shores of O‘ahu, Hawai‘i (12.ix.2000, 03.ix.2004, 13.xii.2004, 27.xi.2006), and the vicinity of Maui Island (15.xi.2006 to 19.xi.2006). Submersible dives utilized the Hawai‘i Underwater Research Laboratory’s Pisces IV and Pisces V three-person submersibles and the Remote Operated Vehicle 150 with surface support from the Research Vessel Kaimikai-o-Kanaloa. Submersibles were equipped with two colour video cameras mounted externally that recorded entire dives, allowing image capture and later review of ecological observations. Additional field observations were made via scuba where possible (i.e. patches or meadows at accessible depths).
Collection information and specimens examined
Specimens included a collection by M. Ross via scuba from Barber’s Point, south-west O‘ahu (ARS08534, 36 m depth, 30.ix.2013), another from submersible dives in mesophotic habitats found in the vicinity of Maui Island (ARS01416, > 40 m depth, 16.xi.2006, coll. Terry Kerby), and a third originating from a strain maintained for several months in a marine aquarium in the C. Smith laboratory (TS0398, location of collection in Hawai‘i and collector not available). Two frozen specimens (BBM1 and BBM2) previously collected from Mamala Bay, O‘ahu, and published as ‘U. argentea’ by Bailey-Brock & Magalhães (Citation2010) were also made available for molecular confirmation by Dr Bailey Brock, Biology Department, University of Hawai‘i at Manoa. For comparison with Hawaiian material (see for a typical habit), two western Pacific specimens were also examined and sequenced; these included a silica preserved specimen identified as U. geppii from Fiji collected by D.S and M.M. Littler (DML40191, Great Astrolabe Reef; ~18°51′59.8′′S, 178°30′60.0′′E; 37 m depth, 27.ii.1996), and an unidentified herbarium specimen fitting the habit of U. geppiorum collected from Guam by C. Squair (CS405A, Asan unit of the National Park; 13°28′54.8′′N, 144°42′13.3′′E, 18 m depth, 08.vi.2006, ) (National Park-Assigned Permit Number WAPA-2004-SCI-0001). Anatomical observations (–) were made from a live specimen collected from south-west O‘ahu by H. Spalding (HS174, 35 m, 30.iv.2006). Specimen ARS08534 from southern O‘ahu was deposited at BISH under accession BISH775782 (Supplementary fig. S5).
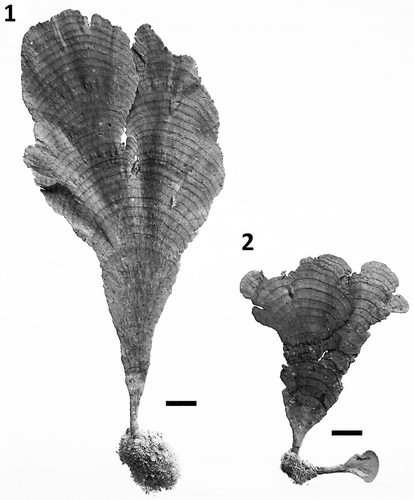
Figs 1–2. Habit of Udotea geppiorum. Fig. 1. Typical habit of a mesophotic specimen collected from south-west O‘ahu Hawai‘i. Fig. 2. Specimen CS405A collected from 18 m depth in Guam. Note the conspicuous concentric segments on both specimens and the more relaxed/elongated holdfast and blade of the mesophotic specimen from Hawai‘i. Specimens display proportional size difference. Scale bars = 1 cm.
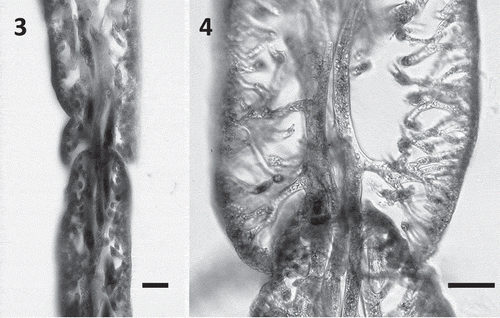
Figs 3–4. Blade anatomy of Udotea geppiorum. Fig. 3. Longitudinal section emphasizing the ‘tongue and groove’ arrangement of concentric segments and bridging medullary siphons between an inferior and a superior segment. Fig. 4. Longitudinal section emphasizing lateral branching of medullary siphons and the dense cortex. Scale bars = 50 μm.
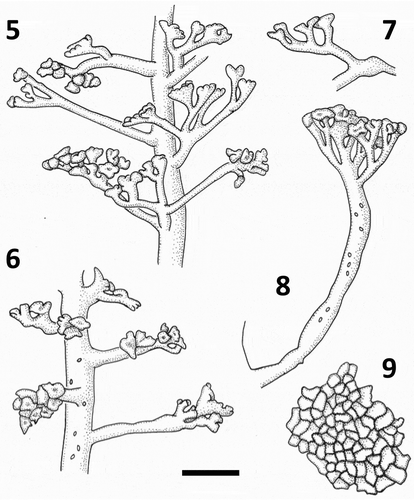
Figs 5–9. Line drawings of Udotea geppiorum medullary siphons. Fig. 5. Blade siphon with lateral branchlets. Fig. 6. Distal blade siphon with lateral branchlets and chloroplasts. Fig. 7. Lateral branchlets from apical region of the blade. Fig. 8. Stipe lateral branchlet with chloroplasts. Fig. 9. Surface view of decalcified blade showing siphon apices. Scale bar = 30 μm.
Molecular sequencing
DNA was extracted from the six U. geppiorum specimens listed in the previous section using a DNeasy Plant Minikit (Qiagen, Valencia, California, USA) and amplified in 25 µl-reactions with MangoTaqTM DNA polymerase (Bioline USA Inc., Taunton, Massachusetts, USA) with previously published primers and cycling conditions for the chloroplast tufA marker (~800 bp, Händeler et al., Citation2009). PCR products were purified using ExoSAP-IT® (Affymetrix, Inc., Cleveland, Ohio, USA) and Sanger sequenced in both directions at LAB (Smithsonian Institution, Washington, DC) or the ASGPB core facility (University of Hawai‘i at Mānoa). Individual chromatograms were assembled into contigs and edited using Sequencher™ v.4.8 (Gene Codes, Ann Arbor, Michigan, USA). For tree building, the newly generated tufA sequences were merged with a comprehensive selection of Udoteacean (ingroup) and Bryopsidalean (outgroup, not shown) taxa downloaded from GenBank (alignment length of 1230 bp); most from Lagourgue et al. (Citation2018) and a few others from Cremen et al. (Citation2018), Sauvage et al. (Citation2016) and Wade & Sherwood (Citation2017) (Supplementary table S1). Udotea geppiorum tufA data was deposited on GenBank under accessions MK460430–MK460432 and MN202614–MN202616. Additional DNA barcodes were generated and deposited for reference on GenBank but not analysed herein, rbcL-5P (MK460427–MK460429) and nuclear 18S rRNA (MK426759).
Phylogenetic analyses
Bayesian and Maximum likelihood analyses were conducted in MrBayes v.3.1.2 (Ronquist & Huelsenbeck, Citation2003) and RAxML-HPC2 on CIPRES computer cluster (http://www.phylo.org, Stamatakis, Citation2014) using a GTR+I+G model of evolution partitioned per codon position as determined by PartitionFinder (Lanfear et al., Citation2012). The Bayesian analysis consisted of two independent runs of four increasingly heated chains run for a maximum of 10 million generations sampled every 1000 generations (resulting in 2×20 000 trees). The runs converged rapidly as demonstrated by the average standard deviation of split frequencies reaching < 0.01 following ~450 000 generations, and large ESS values (⋙ 200) indicating more than sufficient sampling. The first 25% of sampled trees (default) were then discarded as burn-in (n = 10 000 trees) and a majority-rule consensus tree was computed with the remaining trees to obtain node support (posterior probabilities from n = 30 000 trees). The Maximum likelihood analysis consisted of 1000 topological searches from random restarts and 1000 bootstrap replicates for node support estimation (bootstrap).
Results
Molecular analyses
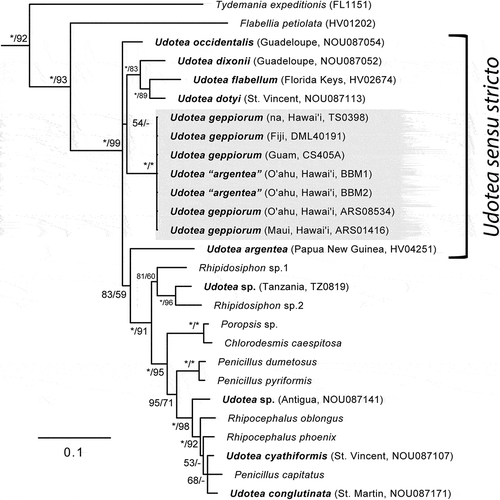
For the U. geppiorum specimens sequenced from Hawai‘i, Fiji and Guam, no genetic variation was observed based on tufA (). Bailey-Brock & Magalhães (Citation2010) specimens from O‘ahu, previously published as ‘U. argentea’ (BBM1 and BBM2), were also identical to those of U. geppiorum and were genetically distinct from a Pacific specimen of U. argentea sequenced from Papua New Guinea by Cremen et al. (Citation2018). Udotea geppiorum was also distinct from U. flabellum from the western Tropical Atlantic. The tufA phylogeny shows that U. geppiorum branches among a poorly resolved cluster (polytomy) of completely corticated Udotea spp. including the generitype U. flabellum, and U. dixonii, U. dotyi and U. occidentalis recently proposed as Udotea sensu stricto by Lagourgue et al. (Citation2018). However, the addition of U. argentea from Cremen et al. (Citation2018) in the phylogeny shows that it branches sister to a large group of partially corticated species (or lacking cortication), which includes Udotea spp. sensu lato (e.g. U. cyathiformis, U. conglutinata) and other genera (Rhipidosiphon Montagne, Rhipocephalus Kützing and Penicillus Lamarck), rendering the topology of Udotea sensu stricto paraphyletic (albeit with low to very low support, BI, 83%, ML, 53%) ().
Fig. 10. Bayesian majority-rule consensus tree depicting the phylogenetic position of Udotea geppiorum in the family Udoteaceae inferred by the chloroplast genes tufA. Values shown at nodes represent posterior probabilities scaled on 100% (before forward slash) and bootstrap support determined via Maximum likelihood analysis (after forward slash). Full support (> 99%) is noted by an asterisk (*) while values below 50% are indicated by an hyphen (-). Udotea spp. are displayed in bold with their sampling locality. Newly sequenced specimens of U. geppiorum are shaded in grey. Note the paraphyly of Udotea sensu stricto caused by the position of U. argentea, and the polyphyly of Udotea spp. sensu lato.
Morphological observations
The thalli of examined Hawaiian specimens of U. geppiorum included a calcified stipe and calcified segmented blade reaching up to 24 cm in height. The holdfast is bulbous to elongate with attached sediment among the rhizoids, and measures up to 6 cm long and 1–2 cm in width. One to several stipes may arise from a single holdfast. Udotea geppiorum is generally anchored in sediment and occasionally on sediment-covered rock. When alive, blades are greyish-olive-green, becoming olive-green with senescence or upon drying. Stipes are variable in length, 0.5–4.0 cm long and occasionally highly reduced (i.e. lacking elongation and thus not forming a clear separation from the blade). The stipe, when present, is subterete, 1.0–4.0 mm in diameter near holdfasts, widening as it joins with the lowermost segment of the blade. The basal subterete segment is generally elongate, up to 40 mm long. Above the basal segment are a series of short concentric (arc-shaped) segments, 1.0–4.0 mm long, each spanning the width of the blade as seen on the type specimens (Supplementary figs S2–S4). Blades are flat and narrowly fan-shaped, to 21 cm long by 1.5–8 cm wide often becoming dissected with overlapping lobes distally, measuring 0.4–1.6 mm thick. All segments possess a proximal groove into which the distally constricted lower segment is inserted, appearing as a ‘tongue and groove’ arrangement (–). The segmentation gives the entire blade a strongly zonate appearance () with new segments being added apically. Despite segmentation, the flabellum is mostly rigid. Medullary siphons pass through the segments’ interface (–) along the entire length of the segment and are emergent (to 50 µm) beyond the apical-most (growing) margin of the distal segment prior to the deposition of calcium carbonate and formation of a new segment.
Medullary siphons in the blade are 20–42.5 µm diameter, and are oriented parallel to one another length-wise in the flabellum. They possess infrequent dichotomies lacking constrictions. The siphons produce abundant lateral branchlets (–). These are irregularly spaced and project mostly alternately from the siphon, being situated perpendicularly to the plane of the blade surface (). Lateral branchlets are 10–17.5 µm in diameter, and range from 50–165 µm long distally to 160–600 µm proximally. Lateral branchlets are simple or dichotomously divided, terminating in stubby, truncated ends (–), as reported for closely related species such as U. dixonii D.S. Littler & Littler, U. dotyi D.S. Littler & Littler, U. flabellum (J. Ellis & Solander) M. Howe and U. occidentalis A. Gepp & E.S. Gepp (Littler & Littler, Citation1990). The distal expanded portions of the lateral siphon branchlets (i.e. their outer surface) are adherent to one another forming a coherent cortex () and in surface view are laterally coherent in a jigsaw fashion (). Reproduction is unknown.
Habitat
Submersible and ROV observations revealed that U. geppiorum formed extensive meadows on sandy bottoms in deep water between 60–85 m (–). These extensive monospecific meadows spread to an estimated 500 m or more in extent. In these dense meadows, the blades of U. geppiorum were tightly spaced, forming a closed canopy with mean ± SE canopy heights of 13.3 ± 0.6 cm (range: 4.9–23.5 cm). At the shallower (from 20–60 m) and deepest edges of its vertical distribution (85 to at least 92 m), U. geppiorum formed sparse patches that range from < 1.0 m to ~15 m in extent. Udotea geppiorum was observed predominately anchored in sand. However, at two locations (at 87 and 50 m depth), the alga was growing on sediment-covered carbonate in assemblages with erect, boring sponges, including Spirastrella spp. (Spirastrellidae). No ecological observations are available for shallow specimens (6 m depth, BISH1053691, Supplementary fig. S1).
Figs 11–12. Submersible views of mesophotic meadows formed by Udotea geppiorum in south-west O‘ahu. Fig. 11. Overlooking an extensive meadow (Dive P4-188). Fig. 12. Edge of a meadow (Dive P5-606). Note that the meadows are so densely vegetated that the sand substrate is not visible among the blades. Picture taken by submersible pilot Terry Kerby in Kaiwi, O‘ahu.

Narrower blades were commonly collected within the denser portions of U. geppiorum meadows, while wider-bladed plants were encountered at the meadow margins. At the deeper and shallower limits of its distribution, U. geppiorum formed round to oval-shaped discrete patches. In some locations it appeared that the patches were coalescing, which may represent the mechanism by which continuous meadows form. On the edge and within occasional gaps in the meadow, other indigenous macroalgae were observed, including Caulerpa mexicana, Halimeda distorta, Distromium flabellatum, Cladophora sp. and Microdictyon sp. At many of the shallower sites visited, the invasive alga Avrainvillea amadelpha was also present. Udotea geppiorum also occurred as discrete patches within deep-water Halophila decipiens seagrass meadows. Assemblages of small fish (Pomacentridae and Labridae) were commonly observed hovering above the Udotea canopy. On submersible approach, these fish sought refuge among the closely spaced blades. On several occasions, the predatory barred jack Carangoides ferdau was observed to aggressively chase and presumably feed on the smaller fish hovering above the algal meadow.
Discussion
Revised record
In the present study, we confirm the revised record of the genus Udotea in Hawai‘i as U. geppiorum and its phylogenetic affinity with Udotea sensu stricto (, Lagourgue et al., Citation2018). The phylogeny shows that U. geppiorum from Hawai‘i is conspecific with specimens sampled from Fiji and Guam that clearly fit the habit of Yamada’s type from Palau (see – vs. the syntype in Supplementary fig. S2), and that the species is separate genetically from other Udotea spp., especially U. argentea and U. flabellum, two names that have potentially been applied to it in the Pacific. Morphologically, U. geppiorum appears to be unique among Udotea spp. in having well-defined concentric segments (Yamada, Citation1930) newly shown to be articulated in a ‘tongue and groove’ manner with medullary siphons running between segments and developing a contiguous cortex (–, ). This conspicuous feature, giving the blade a strong zonation, represents an important morphological character separating U. geppiorum from U. argentea (see Zanardini, Citation1858).
Our phylogeny including all previously sequenced Udotea species that exhibit complete cortication, renders Udotea sensu stricto paraphyletic rather than monophyletic because of the positioning of U. argentea. Although the paraphyletic topology receives overall low to very low support with tufA, a recent plastome-scale phylogenomy including U. argentea and U. flabellum shows a similar topology and has high support (Cremen et al., Citation2018, fig. 1, p. 3). Nonetheless, until similar phylogenomies are re-analysed with increased taxon sampling to further assess the monophyly (or paraphyly) of fully corticated Udotea spp., the genus sensu stricto as currently defined remains valid (Lagourgue et al., Citation2018). In contrast, Udotea spp. lacking or exhibiting incomplete cortication, and clustering remotely in the tree among other Udoteacean genera such as Penicillus, Rhipidosiphon and Rhipocephalus, are in need of taxonomic revision.
In future investigations, continued sequencing of fan-shaped taxa currently included in Udotea spp. will also be important to further resolve/delimit Udotea sensu stricto. For instance, U. norrisii, a completely corticated Udotea species, has not yet been sequenced for tufA or rbcL, although an 18S sequence available on GenBank from Kooistra (Citation2002) suggests that it might be circumscribed within U. flabellum (TS, personal observations). Likewise, the western Atlantic taxon recognized as U. wilsonii Gepp, Gepp & Howe and U. goreaui Littler & Littler, which are heavily calcified could be closely related to Udotea spp. sensu stricto. While in these two species, cortication is not as conspicuous (complete), they display blunt interlocking knobby lateral appendages/papillae throughout the thalli (terminology sensu Littler & Littler, Citation1990 but also referred to as lateral protuberances by Lagourgue et al., Citation2018) that are somewhat reminiscent of those observed in Udotea sensu stricto spp., albeit shorter. Recent morphological illustrations of such appendages/protuberances in U. wilsonii from Yucatan (Acosta-Calderón et al., Citation2018, figs 81–84, p. 209) would also suggest a potential affinity of this taxon with Udotea sensu stricto, as do the morpho-anatomical analyses displayed in Lagourgue et al. (Citation2018, fig. 2, p. 763).
Morphologically, Udotea sensu stricto species have continuous cortication from the stipe to the distal portions of the blade. Notably, after decalcification of U. geppiorum, and others such as U. dixonii and U. flabellum, it is difficult to tease apart the blade’s lateral appendages without breaking many of them in the process. However, in the case of U. flabellum and U. dixonii, this is due to the interwoven or crowded nature of such lateral appendages (Gepp & Gepp, Citation1911; Littler & Littler, Citation1990). In contrast, the blade’s lateral appendages of U. geppiorum terminate in utricle-like flattened tips that firmly adhere to one another at their edges and form a smooth surface, giving this species its distinctive contiguous cortex (, ), reminiscent of the cortex of Halimeda spp. However, as opposed to the mostly polygonal pattern observed in surface view of Halimeda spp., U. geppiorum’s surface shows an irregular, jigsaw-like pattern (). Another unique feature of U. geppiorum is the development of concentric articulated segments (–) joined together in a ‘tongue and groove’ arrangement that spans the entire width of the blade. Comparatively, the somewhat zonate appearance of U. dotyi, U. dixonii and U. flabellum is only superficial rather than due to genuine segmentation. Overall, the morphological similarity of the thalli of western Pacific specimens (including the syntypes in Supplementary figs S2–S4) with those collected from O‘ahu, added to our molecular results (100% identity of Hawaiian and western Pacific specimens) and the paucity of Udotea species exhibiting complete cortication in the Pacific (e.g. U. geppiorum and U. argentea), provide strong evidence for the correct identification of the present revised new record in Hawai‘i as U. geppiorum. Our account of medullary siphon characteristics and measurements closely agreed with those reported in the original description of Udotea geppiorum (Yamada Citation1930, fig. 3, p. 141). Based on the present study and review of the taxonomic history of U. geppiorum, reports of U. flabellum in the Pacific should be interpreted carefully. Likewise, in Pacific herbarium collections, some records of U. argentea may correspond to U. geppiorum.
A meadow-forming Udotea
Although Udoteaceae species have been previously reported as members of the deep-water flora (Gepp & Gepp, Citation1908, Citation1911; Hillis-Colinvaux, Citation1986; Hanisak & Blair, Citation1988; Littler & Littler, Citation1990; Norris & Olsen, Citation1991; Freile et al., Citation1995; Leichter et al., Citation2008) and in some cases have been documented to form meadows (such as Penicillus capitatus in the Canary islands, Sangil et al., Citation2010), to our knowledge, the present study represents the first documented mesophotic meadows formed by a Udotea species, here U. geppiorum (–).
Carbonate and biomass production have been well characterized for other bryopsidalean genera, particularly Halimeda (Hillis-Colinvaux, Citation1980, Citation1991 and references cited therein; Drew, Citation1983; Payri, Citation1988) and Penicillus (Stockman et al., Citation1967), and given the extensive standing crop of U. geppiorum in Hawai‘i, this calcified species is also likely to contribute to aragonitic lime mud production (as seen from the broken calcified remnants of old fronds found in the algal meadow, sediment also agglomerated within the rhizomatous holdfast of the collected specimens; field observations, K. Peyton). The overall contribution of the meadow to lime mud and sediments through thalli growth and death may also play an important role in nutrient cycling and carbonate budget in this mesophotic habitat (i.e. CaCO3 production/erosion/dissolution) and represent potential avenues of research (e.g. see Granier, Citation2012 for a review on the contribution of green algae to limestone production).
Vegetative reproduction by below-ground rhizomes (or stolons) is well recognized as an important attribute for growth and persistence of both macroalgae and seagrasses on a range of scales from ramets to meadows (Stockman et al., Citation1967; Tomlinson, Citation1974; Friedmann & Roth, Citation1977; Hillis-Colinvaux, Citation1988; Littler & Littler, Citation1990, Citation1992; Williams, Citation1990; Fourqurean & Rutten, Citation2004; Littler et al., Citation2005). Littler & Littler (Citation1990) reported on the existence of a translucent and delicate but macroscopic subterranean rhizomatous system used for lateral vegetative reproduction in Udotea spp.; however, their observations seem to be restricted to ‘false’ Udotea spp. (e.g. U. cyathiformis, U. fibrosa Littler & Littler, and U. unistratea Littler & Littler). Here, U. geppiorum specimens maintained in an aquarium for > 36 months (C. Smith Laboratory) started to produce shoots remotely, but we could not identify macroscopic rhizoids linking thalli, a behaviour we also previously witnessed for Halimeda species (T. Sauvage and K. Peyton, unpublished data). Recent metabarcoding studies of endolithic communities have shown that vegetative reproduction of members of the Bryopsidales may also occur by microscopic rhizoids as deduced by the molecular detection of otherwise macroscopic taxa such Halimeda species in this microhabitat (Sauvage et al., Citation2016). Such microscopic siphons could thus mediate the lateral spread of U. geppiorum, although the existence of a macroscopic rhizomatous system (or lack thereof) also needs to be further investigated in well-developed mesophotic meadows. Overall, we hypothesize that in the field, the ramets of U. geppiorum can radiate into patches by rhizoids spreading laterally in the substratum (via macroscopic or microscopic rhizoids; to be determined), leading to the establishment of patches, which then further coalesce into meadows, similar to the vegetative propagation described for seagrass meadows via rhizomes/stolons (Williams, Citation1990; Rasheed, Citation2004).
Udotea geppiorum meadows are more reminiscent of seagrass canopies than those of Halimeda spp. Indeed, like seagrass species, the blades of U. geppiorum form a nearly closed canopy. In contrast, H. kanaloana Vroom, which also forms extensive meadows in the Hawaiian archipelago, off Maui, Moloka‘i, Kaho‘olawe and Lāna‘i (Abbott & Huisman, Citation2004; Kahng & Kelley, Citation2007) exhibit a somewhat uniform dispersion pattern in which individuals are spaced > 7.0 cm apart (K. Peyton, unpublished data) and may thus not provide as much habitat or potential shelter for fauna as the dense blades of U. geppiorum. The ecological services of the meadows formed by U. geppiorum in Hawai‘i, including the support of associated biodiversity and trophic interactions, from detritivores to apex predators (e.g. Carangoides ferdau), are poorly known and thus offer opportunities for further research in mesophotic habitats of the archipelago.
Status in Hawai‘i
Bailey-Brock & Magalhães (Citation2010) reported ‘U. argentea’ from O‘ahu (revised here as U. geppiorum) as a possible introduced species because of the proximity of their collections to the Honolulu’s south shore harbours which are subjected to intense shipping, and the absence of an existing Udotea record in Hawai‘i prior to 2007. Our collections of U. geppiorum, dating from as early as 2000, span two regions of the Main Hawaiian Islands (O‘ahu and Maui), including pristine mesophotic habitats distant from harbours, anchoring or recently dredged areas, which would warrant recognition of the species as an indigenous member of the Hawaiian marine flora. In contrast, the recent record of the Bryopsidalean taxa Avrainvillea cf. erecta (Wade et al., Citation2018) is indeed of ambiguous indigeneity because it has so far only been documented in the vicinity of harbours in disturbed dredged habitats. Future genomics efforts focused on deciphering population-level differences across Indo-Pacific localities will represent key approaches in determining the natural history of such taxa and their biogeographic origin.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2019.1668061
Supplementary fig. S1. Photographic illustration of a shallow U. geppiorum specimen collected off Kalaeloa south-west O‘ahu from ~6 m depth by Dr R. Okano (BISH720045). Image downloaded from the Macroalgal Herbarium Portal.
Supplementary fig. S2. Photographic illustration of Yamada’s syntype from Palau (SAP8601). The specimen is identical to fig. 1 in Yamada (Citation1930).
Supplementary fig. S3. Photographic illustration of Udotea flabellata J.V.Lamouroux nom. illeg. syntype held at NY (Harvey’s ‘Friendly Isl. Alg. no. 94’, NY02112272). Image downloaded from the Macroalgal Herbarium Portal.
Supplementary fig. S4. Photographic Illustration of U. flabellata J.V.Lamouroux nom. illeg. at MICH (Harvey’s ‘Friendly Isl. Alg no.94’, MICH1306130). Note M.J. Wynne’s annotation ‘Udotea geppiorum Yamada Citation1930 syntypes!’. Image downloaded from the Macroalgal Herbarium Portal.
Supplementary fig. S5. Photographic illustration of Hawaiian specimen of U. geppiorum (ARS08534) sequenced for the present study and deposited in BISH under accession BISH775782.
Supplementary table S1. Newly and previously published tufA accessions included in the phylogenetic analyses.
Author contributions
T. Sauvage, K.A. Peyton and D.L. Ballantine wrote the manuscript. K.A. Peyton conducted submersible dives and field observations. D.L. Ballantine conducted morphological observations. T. Sauvage and R.M. Wade conducted molecular work and phylogenetic analyses. A.R. Sherwood, S. Keeley and C. Smith contributed to data interpretation and revision of the manuscript. All authors read and approved the final manuscript.
TEJP-2019-0025-File012.pdf
Download PDF (84.9 KB)TEJP-2019-0025-File011.tif
Download TIFF Image (155.7 MB)TEJP-2019-0025-File010.tif
Download TIFF Image (10.6 MB)TEJP-2019-0025-File009.tif
Download TIFF Image (36.7 MB)TEJP-2019-0025-File008.tif
Download TIFF Image (49.8 MB)TEJP-2019-0025-File007.tif
Download TIFF Image (11 MB)Acknowledgements
Collaborative fieldwork in mesophotic habitats was funded by several awards to the University of Hawai‘i by the National Oceanic and Atmospheric Administration (NOAA) Coastal Ocean Program, NOAA Undersea Research Program’s Hawai‘i Undersea Research Laboratory, NOAA Coral Reef Conservation Program and NOAA’s Office of Ocean Exploration. TS is thankful for an invitation to participate in the Maui HURL mesophotic algae campaign led by Dr Heather Spalding (November 2006). ARS thanks support from the National Science Foundation (DEB-1754117). TS acknowledges support from a G.E. Burch Fellowship for funding residency and research at SMS. We are also thankful to Prof. Kazuhiro Kogame (SAP Herbarium, Hokkaido University, Sapporo) for providing a high-quality picture of Yamada’s syntype and Mike Guiry for discussion on the taxonomic history of U. flabellata and U. flabellum. We also thank Dr Monica Paiano (UHM) and Larissa A. Santos (SMS) for assistance with molecular work. This is SMS contribution No. 1115.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abbott, I. & Huisman, J. (2004). Marine Green and Brown Algae of the Hawaiian Islands. Bishop Museum Press, Honolulu, Hawai‘i.
- Acosta-Calderón, J.A., Hernández-Rodríguez, C., Mendoza-González, A.C. & Mateo-Cid, L.E. (2018). Diversity and distribution of Udotea genus J.V. Lamouroux (Chlorophyta, Udoteaceae) in the Yucatan peninsula littoral, Mexico. Phytotaxa, 345: 179–218.
- Agegian, C. & Abbott, I. (1985). Deep water macroalgal communities: a comparison between Penguin Bank (Hawaii) and Johnson Atoll. Proceedings of the Fifth International Coral Reef Congress. Antenne Museum-EPHE, Moorea, French Polynesia, pp. 47–50.
- Aponte, N. & Ballantine, D. (2001). Depth distribution of algal species on the deep insular fore reef at Lee Stocking Island, Bahamas. Deep-Sea Research, 48: 2185–2194.
- Atmadja, W.S. & Prud’homme van Reine, W.F. (2014). Checklist of the Seaweed Species Biodiversity of Indonesia with their Distribution and Classification: Green Algae (Chlorophyta) and Brown Algae (Phaeophyceae, Ochrophyta). Naturalis Biodiversity Centre and Indonesian Institute of Sciences (LIPI), Leiden & Indonesia.
- Bailey-Brock, J.H. & Magalhães, W.F. (2010). Udotea argentea (Bryopsidales: Udoteaceae), a new record for the Hawaiian Islands. Marine Biodiversity Records, 3: E63.
- Ballantine, D. (1990). Ceramium bisporum sp. nov. (Rhodophyta, Ceramiales), an unusual new species from deep-water habitats in the Caribbean. Phycologia, 29: 146–149.
- Ballantine, D. & Aponte, N. (2002). Botryocladia bahamense sp. nov. (Rhodymeniaceae, Rhodophyta) from the Bahamas, West Atlantic. Cryptogamie, Algologie, 23: 123–130.
- Ballantine, D. & Aponte, N. (2005). An annotated checklist of deep-reef benthic marine algae from Lee Stocking Island, Bahamas II. Rhodophyta. Nova Hedwigia, 80: 147–171.
- Ballantine, D. & Norris, J. (1994). Verdigellas, a new palmelloid genus (Tetrasporales, Chlorophyta) from the tropical West Atlantic. Cryptogamie, Botany, 4: 368–372.
- Brostoff, W.N. (1989). Avrainvillea amadelpha (Codiales, Chlorophyta) from Oahu, Hawaii. Pacific Science, 43: 166–169.
- Cremen, M.C.M., Leliaert, F., West, J., Lam, D.W., Shimada S., Lopez-Bautista, J.M. & Verbruggen, H. (2018). Reassessment of the classification of Bryopsidales (Chlorophyta) based on chloroplast phylogenomic analyses. Molecular Phylogenetics and Evolution, 130: 397–405.
- Doty, M., Gilbert, W. & Abbott, I. (1974). Hawaiian marine algae from seaward of the algal ridge. Phycologia, 13: 345–357.
- Drew, E. (1983). Halimeda biomass, growth rates and sediment generation on reefs in the Central Barrier Reef province. Coral Reefs, 2: 101–110.
- Drew, E. & Abel, K. (1988). Studies on Halimeda I. The distribution and species composition of Halimeda meadows throughout the Great Barrier Reef Province. Coral Reefs, 6: 195–205.
- Ellis, J. & Solander, D. (1786). The natural history of many curious and uncommon zoophytes, collected from various parts of the globe by the late John Ellis … Systematically arranged and described by the late Daniel Solander. pp. xii + 208, 63 Plates. London: B. White & Son.
- Fourqurean, J. & Rutten, L. (2004). The impact of Hurricane Georges on soft-bottom, back reef communities: site- and species-specific effects in South Florida seagrass beds. Bulletin of Marine Science, 75: 239–257.
- Freile, D., Milliman, J. & Hillis, L. (1995). Leeward bank margin Halimeda meadows and draperies and their sedimentary importance on the western Great Bahama Bank slope. Coral Reefs, 14: 27–33.
- Friedmann, E. & Roth, W. (1977). Development of the siphonous green alga Penicillus and the Espera state. Botanical Journal of the Linnean Society, 74: 189–214.
- Gepp, A. & Gepp, E. (1911). The Codiaceae of the Siboga Expedition including a monograph of Flabellarieae and Udoteae. In Siboga-Expeditie Monographie (Weber, M., editor), pp. 1–150, 22 pls. E.J. Brill, Leiden.
- Gepp, A. & Gepp, E. (1908). Marine algae (Chlorophyceae and Phaeophyceae) and marine phanerogams of the “Sealark” Expedition, collected by J. Stanley Gardiner, M.A., F.R.S., F.L.S. Transactions of the Linnean Society of London, Second Series, Botany, 7: 163–88, pls. 22–24.
- Graham, M., Kinlan, B., Druehl, L., Garske, L. & Banks, S. (2007). Deep-water kelp refugia as potential hotspots of tropical marine diversity and productivity. Proceedings of the National Academy of Sciences USA, 104: 16576–16580.
- Granier, B. (2012). The contribution of calcareous green algae to the production of limestones: A review. Geodiversitas, 34: 35-60
- Guiry, M.D.& Guiry, G.M. (2019). AlgaeBase. Worldwide electronic publication, National University of Ireland, Galway. Available at: http://www.algaebase.org, searched on 15 Jan 2018.
- Händeler, K., Grzymbowski, Y.P., Krug, P.J. & Wägele, H. (2009). Functional chloroplasts in metazoan cells – a unique evolutionary strategy in animal life. Frontiers in Zoology, 6: 28.
- Hanisak, M. & Blair, S. (1988). The deep-water macroalgal community of the East Florida continental shelf (USA). Helgolander Meeresuntersuchungen, 42: 133–163.
- Hillis-Colinvaux, L. (1980). Ecology and taxonomy of Halimeda: primary producer of coral reefs. Advances in Marine Biology, 17: 1–327.
- Hillis-Colinvaux, L. (1986). Halimeda growth and diversity on the deep fore-reef of Enewetak Atoll. Coral Reefs, 5: 19–21.
- Hillis-Colinvaux, L. (1988). Characteristics of Halimeda meadows, with emphasis on a meadow near Eniwetok Inlet, Eniwetok Atoll, (Marshall Islands). In Proceedings of the 6th International Coral Reef Symposium, Australia, pp. 119–25.
- Hillis-Colinvaux, L. (1991). Recent calcified Halimedaceae. In Calcareous Algae and Stromatolites (Riding, R., editor), 167–88. Springer, Berlin.
- Hinderstein, L.M., Marr, J.C.A., Martinez, F.A., Dowgiallo, M.J., Puglise, K.A., Pyle, R.L., Zawada, D.G. & Appeldoorn, R. (2010). Mesophotic coral ecosystems: characterization, ecology, and management. Coral Reefs, 29: 247–251.
- Howe, M.A. (1904). Notes on Bahaman algae. Bulletin of the Torrey Botanical Club, 31: 91–100, Plate 6.
- Kahng, S. & Kelley, C. (2007). Vertical zonation of megabenthic taxa on a deep photosynthetic reef (50–140 m) in the Au‘au Channel, Hawai‘i. Coral Reefs, 26: 679–687.
- Kooistra, W. (2002). Molecular phylogenies of Udoteaceae (Bryopsidales, Chlorophyta) reveal nonmonophyly for Udotea, Penicillus and Chlorodesmis. Phycologia, 41: 453–462.
- Lagourgue, L., Puillandre, N. & Payri, C.E. (2018). Exploring the Udoteaceae diversity (Bryopsidales, Chlorophyta) in the Caribbean region based on molecular and morphological data. Molecular Phylogenetics and Evolution, 127: 758–769.
- Lamouroux, J.V.F. (1816). Histoire des polypiers coralligènes flexibles, vulgairement nommés zoophytes. pp. [i]-lxxxiv, chart, [1]-560, [560, err], pls I-XIX, uncol. by author. Caen: De l’imprimerie de F. Poisson.
- Lanfear, R., Calcott, B., Ho, S.Y.W. & Guindon, S. (2012). PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution, 29: 1695–1701.
- Leichter, J., Stokes, M. & Genovese, S. (2008). Deep water macroalgal communities adjacent to the Florida Keys reef tract. Marine Ecology Progress Series, 356: 132–138.
- Littler, D. & Littler, M. (1990). Systematics of Udotea species (Bryopsidales, Chlorophyta) in the tropical western Atlantic. Phycologia, 29: 206–252.
- Littler, D. & Littler, M. (1992). Systematics of Avrainvillea (Bryopsidales, Chlorophyta) in the tropical western Atlantic. Phycologia, 31: 375–418.
- Littler, D.S. & Littler, M.M. (2003). South Pacific Reef Plants. A Diver’s Guide to the Plant Life of the South Pacific Coral Reefs. Washington, DC: OffShore Graphics.
- Littler, M., Littler, D., Blair, S. & Norris, J. (1985). Deepest known plant life discovered on an uncharted seamount. Science, 227: 57–59.
- Littler, M., Littler, D., Blair, S. & Norris, J. (1986). Deep-water plant communities from an uncharted seamount off San Salvador Island, Bahamas: distribution, abundance, and primary productivity. Deep-Sea Research, 33: 881–892.
- Littler, M., Littler, D. & Brooks, B. (2005). Extraordinary mound building Avrainvillea (Chlorophyta): the largest tropical marine plants. Coral Reefs, 24: 555.
- Lobban, C.S. & Tsuda, R.T. (2003). Revised checklist of benthic marine macroalgae and seagrasses of Guam and Micronesia. Micronesica, 35/36: 54–99.
- Norris, J. & Olsen, J. (1991). Deep-water green algae from the Bahamas, including Cladophora vandenhoekii sp. nov. (Cladophorales). Phycologia, 30: 315–328.
- Ohba, H., Victor, S., Golbuu Y. & Yukihira, H. (2007). Tropical Marine Plants of Palau. Palau International Coral Reef Center, Palau.
- Payri, C. (1988). Halimeda contribution to organic and inorganic production in a Tahitian reef system. Coral Reefs, 6: 251–262.
- Rasheed, M. (2004). Recovery and succession in a multi-species tropical seagrass meadow following experimental disturbance: the role of sexual and asexual reproduction. Journal of Experimental Marine Biology and Ecology, 310: 13–45.
- Ronquist, F. & Huelsenbeck, J. (2003). MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572–1574.
- Sangil, C., Martin-Garcia, L., Afonso-Carrillo, J., Barquin, J. & Sansón, M. (2018). Halimeda incrassata (Bryopsidales, Chlorophyta) reaches the Canary Islands: mid-and deep-water meadows in the eastern subtropical Ocean. Botanica Marina, 61: 103–110.
- Sangil, C., Sansón, M., Afonso-Carrillo, J. & Martín-García, L. (2010). Extensive off-shore meadows of the Penicillus capitatus (Udoteaceae, Chlorophyta) in the Canary Islands (eastern Atlantic Ocean). Botanica Marina, 53: 183–187.
- Sauvage, T., Schmidt, W.E., Suda, S. & Fredericq, S. (2016). A metabarcoding framework for facilitated survey of endolithic phototrophs with tufA. BMC Ecology, 16: 8.
- Silva, P.C., Meñez, E.G. & Moe, R.L. (1987). Catalog of the benthic marine algae of the Philippines. Smithsonian Contributions to Marine Sciences, 27: [i–ii] iii–iv, 1–179.
- Smith, J., Runcie, J. & Smith, C. (2005). Characterization of a large-scale ephemeral bloom of the green alga Cladophora sericea on the coral reefs of Maui, Hawai‘i. Marine Ecology Progress Series, 302: 77–91.
- Spalding, H.L. (2012). Ecology of mesophotic macroalgae and Halimeda kanaloana meadows in the Main Hawaiian Islands. PhD Dissertation, University of Hawai‘i at Mānoa. Proquest UMI 3534581.
- Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312–1313.
- Stockman, K., Ginsburg, R. & Shinn, E. (1967). The production of lime mud by algae in South Florida. Journal of Sedimentary Petrology, 37: 633–648.
- Thiers, B. (2019). Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium, Bronx, NY. (continuously updated, http://sweetgum.nybg.org/ih/).
- Tomlinson, P. (1974). Vegetative morphology and meristem dependence: the foundation of productivity in seagrasses. Aquaculture, 4: 107–130.
- Tsuda, R.T. (2003). Checklist and bibliography of the marine benthic algae from the Mariana Islands (Guam and CNMI). Technical Report. University of Guam Marine Laboratory, 107: i–v, 1–49.
- Wade, R.M. & Sherwood, A.R. (2017). Molecular determination of kleptoplast origins from the sea slug Plakobranchus ocellatus (Sacoglossa, Gastropoda) reveals cryptic bryopsidalean (Chlorophyta) diversity in the Hawaiian Islands. Journal of Phycology, 53: 467–475.
- Wade, R.M, Spalding, H.L., Peyton, K.A., Foster, K., Sauvage, T., Ross, M. & Sherwood, A.R. (2018). A new record of Avrainvillea cf. erecta (Berkeley) A. Gepp & E. S. Gepp (Bryopsidales, Chlorophyta) from urbanized estuaries in the Hawaiian Islands. Biodiversity Data Journal, 6: e21617.
- Williams, S. (1990). Experimental studies of Caribbean seagrass development. Ecological Monographs, 60: 449–469.
- Yamada, Y. (1930). Une nouvelle espèce d’ Udotea du Pacifique: Udotea geppii sp. nov. Revue Algologique, 5: 139–142.
- Zanardini, G. (1858 ‘1857’). Plantarum in mari Rubro hucusque collectarum enumerato (juvante A. Figari). Memoirie del Reale Istituto Veneto di Scienze, Lettere ed Arti, 7: 209–309, pls III–XIV.
