ABSTRACT
Fifty-six specimens of the red algal genus Martensia from the Main and Northwestern Hawaiian Islands, representing both shallow (36 specimens from 0–20 m depth) and mesophotic habitats (20 specimens from 62–93 m depth), were collected and characterized using morphological and molecular analyses. Phylogenetic analyses of the rbcL gene resolved five distinct clades of Martensia from the Hawaiian Islands, of which none could be attributed to the two previously used taxonomic names for this flora (M. fragilis Harvey and M. flabelliformis Harvey ex J.Agardh). Analyses of the COI barcoding region were consistent with rbcL trends, although fewer specimens amplified and sequenced for this marker. Four rbcL clades (lineages 1–4) were distinct from each other and from other described species of Martensia based on both phylogenetic position and morphological characters, and are here described as new species: Martensia abbottiae A.R.Sherwood & S.-M. Lin sp. nov., Martensia hawaiiensis A.R.Sherwood & S.-M. Lin sp. nov., Martensia tsudae A.R.Sherwood & S.-M. Lin sp. nov., and Martensia lauhiekoeloa A.R.Sherwood & S.-M. Lin sp. nov. Lineage 5 is attributable to M. albida Y.Lee. All five species in the Hawaiian flora occur in mesophotic habitats, while M. hawaiiensis, M. tsudae, and M. albida also inhabit shallow waters of the Main Hawaiian Islands. This study provides additional support for the degree of uncharacterized biodiversity from mesophotic depths in the Hawaiian Archipelago.
Introduction
The red algal genus Martensia is characterized by its distinctive vegetative morphology of one or more bands of net-like tissue disrupting a membranous blade. Eighteen species of Martensia are currently recognized worldwide (Guiry & Guiry, Citation2019). Abbott (Citation1999) recognized two species of Martensia in the Hawaiian flora: M. fragilis Harvey and M. flabelliformis Harvey ex J.Agardh (as Neomartensia flabelliformis (Harvey ex J.Agardh) Yoshida & Mikami). Martensia is common in the shallow, nearshore coral reef habitats of the Hawaiian Islands and is easily recognized in the field by its distinctive thallus morphology and bluish-purple iridescence.
Martensia fragilis has been reported widely from warm-temperate to tropical locations. Lin et al. (Citation2013) compared sequence data for specimens morphologically identified as M. fragilis from numerous geographic locations, as well as from the type locality of Sri Lanka, and demonstrated a remarkable degree of sequence variation in the rbcL marker for these collections. Moreover, the specimen from Sri Lanka was very distinctive in DNA sequence from others, suggesting the presence of a number of cryptic species within a complex more broadly known as ‘M. fragilis’. Several taxa have already been described as distinct entities from this complex (M. jejuensis Y. Lee, M. lewisiae S.-M. Lin, Hommersand & Fredericq) in recent decades (Lee, Citation2004; Lin et al., Citation2004). A specimen from the Hawaiian Islands was included in Lin et al. (Citation2013), and its phylogenetic placement, which was distinct from Sri Lankan material, clearly demonstrated that ‘M. fragilis’ from Hawai‘i needed to be re-evaluated.
The second species reported from Hawai‘i, Martensia flabelliformis, was described from material from Tonga (Agardh, Citation1863), and the species is reported as widely distributed throughout the western and south-eastern Pacific Ocean, the central Pacific (Hawaiian Islands) and South Africa (Guiry & Guiry, Citation2019). However, less is known about the DNA sequence diversity of specimens from these localities, and material from the type locality has not yet been analysed. While Martensia is well documented and sampled in the shallow environments of the Hawaiian Islands, it is possible that it may also be a member of the Mesophotic Coral Ecosystem (MCE), as has been demonstrated for other genera (e.g. Spalding et al., Citation2016).
MCEs are characterized by the presence of light-dependent organisms and are typically found starting at depths of 30–40 m, but can extend to 150 m or more in the tropics and subtropics (Hinderstein et al., Citation2010). Submersible surveys of Hawaiian MCEs have demonstrated a high coverage by macroalgae (Kahng & Kelley, Citation2007; Rooney et al., Citation2010), suggesting an important ecological role for these organisms, but these surveys have been lacking in detail regarding the species composition and spatial heterogeneity of these algal assemblages. Recent access to submersibles and advances in technical diving technology have allowed extensive in situ collections at mesophotic depths throughout the Hawaiian Archipelago, and results thus far illustrate that much work remains to be done to adequately describe the unique treasure-trove of algal species in this unique flora. For example, over 45% of the mesophotic flora based on morphological identifications of algae from MCEs in the Main Hawaiian Islands (MHI) were new records for Hawai‘i, or species new to science (Spalding, Citation2012), and 100% of species of Ulvaceae characterized using molecular tools were undescribed (Spalding et al., Citation2016). Thus, detailed analyses using both morphological and molecular techniques are necessary to properly describe this mesophotic flora, and to test species-level hypotheses regarding biogeography, endemism and deep reef refugia (e.g. Bongaerts et al., Citation2010).
In this study we present a molecular phylogenetic and morphological analysis of specimens collected from both shallow and mesophotic sites in the Main Hawaiian Islands within the context of previous molecular studies of the genus, and demonstrate that the Hawaiian flora contains five species attributable to this genus, of which four are undescribed, and one is a new record of a species described from Korea.
Materials and methods
Specimens of Martensia were collected during the Hawaiian Rhodophyta Biodiversity Survey of the Main Hawaiian Islands (MHI) (Sherwood et al., Citation2010a, Citation2010b), and during mesophotic submersible and technical diving surveys of the MHI (2006, 2007, 2008, 2009, 2016) and technical diving cruises to the NWHI (2012, 2013, 2016, and 2018) (Supplementary table S1). Specimens were preserved as herbarium presses, in silica gel for DNA extraction and as formalin vouchers (in some cases). Silica gel vouchers were extracted for genomic DNA using either a Qiagen DNeasy Plant Mini Kit (Qiagen, Valencia, California, USA) or an OMEGA E.Z.N.A.® Plant DNA DS Kit (OMEGA Biotek, Norcross, Georgia, USA). Portions of the COI (cytochrome oxidase subunit I, 658 bp) barcoding region were amplified using the GazF2 and GazR2 primers (Saunders, Citation2005; Lane et al., Citation2007). Similarly, portions of the rbcL gene (ribulose 1,5-bisphosphate carboxylase/oxygenase large subunit; 1442 bp) were sequenced and assembled by amplifying two overlapping fragments using the primers rbcLF7 (Gavio & Fredericq, Citation2002) and rbcLJNR1 (Kang & Kim, Citation2013) for the first fragment, and rbcLF762 and rbcLR1442 (Kim et al., Citation2010) for the second fragment, as suggested by Kang et al. (Citation2015). For most Martensia DNA extracts, a 1:100 dilution was required for successful amplification and sequencing. Successful PCR products were submitted for sequencing to the University of Hawai‘i at Mānoa Advanced Studies in Genomics, Proteomics, and Bioinformatics core facility. Raw sequence reads for each gene were edited and assembled in Geneious v. 11.1.5 (Biomatters, Auckland, NZ), and aligned using the MUSCLE plug-in (Edgar Citation2004) in Geneious. Consensus sequences were then aligned with reference sequences and analysed with PartitionFinder v. 1.1.1 (Lanfear et al., Citation2012). Phylogenetic reconstruction was performed using RAxML v. 8.2.10 (Stamatakis, Citation2014) with 1000 bootstrap replicates and MrBayes v. 3.2.6 (Ronquist et al., Citation2012) via the CIPRES gateway (Miller et al, Citation2010). PartitionFinder suggested no partitioning for both Maximum likelihood (ML) and Bayesian inference analyses, and the models GTR+I+G (AICc) and TrN+G (BIC) were selected for these analyses, respectively. Identical sequences were removed from alignments prior to running phylogenetic analyses, but all samples are indicated on the final phylogenetic tree figure. Sequence alignments are available upon request and newly generated sequences were submitted to GenBank (MN164719–MN164752 and MN170745–MN170773).
Hand sections were stained with 1% aniline blue acidified with 1% HCl and mounted in 30–50% corn syrup solution. Photomicrographs were taken on a Zeiss AxioImager A1 compound light microscope (Carl Zeiss) with an Infinity2-1RC digital camera (Lumenera Corporation, Ottawa, Ontario, Canada). Herbarium sheets were digitized in the Joseph F. Rock Herbarium (HAW) using a Canon EOS 5D Mark II Digital Camera, a copy stand, and a MK Direct Photo-eBox PLUS 1419.
Results
Martensia rbcL phylogeny
Maximum likelihood and Bayesian analysis of the rbcL alignment for Hawaiian Martensia and related sequences from GenBank yielded phylogenetic trees with very similar topology, and so only the ML tree is shown, with support values from ML bootstrap (first value) and Bayesian posterior probabilities (second value) superimposed on the ML tree (). The genus Martensia was clearly resolved as distinct from the outgroups in the analyses (Nitophyllum and Augophyllum). Five main lineages of Martensia from the Hawaiian Islands were resolved in the analyses (labelled as lineages 1–5 in ), all of which had full or near-full support, except for lineage 3, which had 69% bootstrap support and 0.90 posterior probability support. One clade (lineage 5) is assignable to Martensia albida, which was described from Korea in 2006 (Lee, Citation2006) and represents a new record for the Hawaiian Islands, while the remaining four species cannot be reliably assigned to any previously described species of Martensia.
Fig. 1. Maximum likelihood phylogeny (rbcL gene) of Hawaiian Martensia specimens in the context of available GenBank sequences for Martensia and close relatives. Scale bar = substitutions per site. Numbers at nodes indicate bootstrap support (first value) and Bayesian posterior probabilities (second value). Full support is indicated by *. The five lineages of Hawaiian Martensia are numbered at their respective clades.
COI DNA barcoding comparisons
The neighbour-joining framework of Martensia COI DNA barcode sequences revealed groupings of Hawaiian Martensia corresponding to lineages 2, 3 and 5 (Supplementary fig. S1). No ‘clean’ COI sequences were generated for either lineages 1 or 4. Hawaiian Martensia sequences in lineages 2 and 3 were very distinct from other sequences, while those in lineage 5 were in a separate, but similar group of Korean sequences of M. albida (Supplementary fig. S1).
Geographic and depth distribution of Hawaiian Martensia specimens
Although the number of specimens analysed from the NWHI was modest (13), all five lineages of Hawaiian Martensia had a geographic distribution that included this protected part of the archipelago. Conversely, four of the five lineages had geographic distributions that included the MHI. Lineage 4 was the most restricted in geographic distribution, being known thus far only from French Frigate Shoals, NWHI (Supplementary fig. S2).
Depth distribution also varied by lineage. Lineages 1 and 4 are thus far only known from mesophotic depths, with lineage 1 collected from 65–93 m and lineage 4 from 61–67 m depth. Lineage 2 was primarily from shallow water habitats, with a single collection (BISH 776255, ARS 09463, Midway Atoll) from mesophotic depths (65 m). Lineage 3 was also primarily from shallow-water habitats around the MHI but included two collections from the mesophotic (ARS 09462 – Penguin Bank, MHI (no voucher); BISH 776267, ARS 09454 – Nihoa, NWHI). Lineage 5 was represented by numerous specimens from both shallow and mesophotic depths.
Given that five distinct and well-supported lineages of Martensia from the Hawaiian Islands have been resolved based on molecular phylogenetic analyses, below we describe four new species (corresponding to lineages 1, 2, 3 and 4) that, as far as is known, occur only in the Hawaiian Islands.
Morphological observations
The rbcL sequence analyses revealed that the collections identified as Martensia ‘fragilis’ and similar species from the Hawaiian Islands were split into at least five evolutionary lineages. We could not unequivocally demonstrate the occurrence of genuine M. fragilis outside of its type locality. Accordingly, we recognize four new species, Martensia abbottiae A.R. Sherwood & S.-M. Lin, sp. nov., Martensia hawaiiensis A.R. Sherwood & S.-M. Lin, sp. nov. Martensia tsudae A.R. Sherwood & S.-M. Lin, sp. nov. and Martensia lauhiekoeloa A.R. Sherwood & S.-M. Lin, sp. nov., for the four unnamed clades detected by molecular analyses from the Hawaiian Islands.
A key to the four new species and the closely related species as detected from rbcL sequence analyses can be constructed using morphological characteristics, as follows:
Key to the species of Martensia with variable sizes of networks from the northern Pacific
1a. Thalli large, up to 10 (–30) cm in height or in width at maturity 2
1b. Thalli small, less than 10 cm in height or in width at maturity 5
2a. Thalli consisting of linear axes, bearing marginal flabellate bladelets 3
2b. Thalli flabellate and subdichotomously divided, but not bearing marginal lobes or bladelets M. albida
3a Thalli bushy, up to 30 cm long, old networks fragmented or becoming fimbriate, margins of networks covered with few teeth-like protrusionsM. jejuensis
3b Thalli not bushy, networks not fragmented or becoming fimbriate 4
4a. Thalli composed of 2–7 blades with a single band of network, up to 23 cm in height and 29 cm in width, subdichotomously divided M. abbottiae
4b. Thalli composed of a single blade with multiple bands of networks, 6–10 cm tall and 6–15 cm wide M. lauhiekoeloa
5a. Thallus blades with a single band of networks 6
5a. Thallus blades with multiple bands of networks 7
6a. Thallus upright, attached by a short stipe, basal thallus 4–5 cell layers M. hawaiiensis
6b. Thallus upright or prostrate, lacking a stipe, basal thallus 1–2 cell layers thickM. tsudae
7a. Thalli bushy, 5–9 cm long, each main blade bearing 5–9 bladelets, which also bear several new marginal lobes, up to 5 orders of alternation of producing marginal bladelets or lobes M. fragilis
7b. Thalli prostrate, 2–4 cm long, blades bearing conspicuous bands of networks 8
8a. Thalli with thin membranous blades of 2–3 cell layers (less than 80 μm thick), network not fully covering the main blades 9
8b. Thalli with thick membranous blades, 4–5 cell layers (120–170 μm thick), network nearly fully covering the main blades M. kentingii
9a. Main blades slightly to deeply cleft, marginal bladelets mostly arising from the primary longitudinal lamellaeM. taiwanifretensis
9b. Main blades mainly flabellate, marginal bladelets mostly arising from the membranous margins of blades M. leei
Martensia abbottiae A.R. Sherwood & S.-M. Lin, sp. nov. (–)
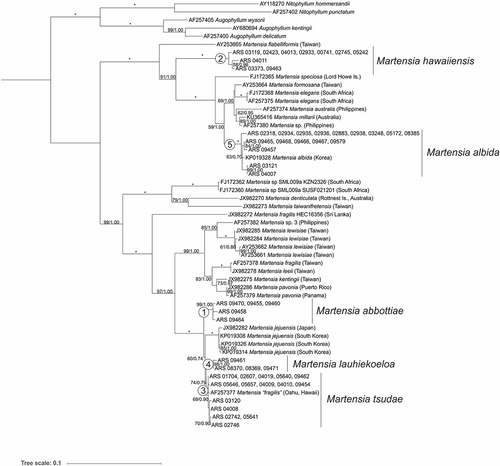
Figs 2–10. Habit and morphology of Martensia abbottiae sp. nov. Fig. 2. Holotype specimen BISH 776243 (arrow) (from collection ARS 09458). Fig. 3. Voucher specimen BISH 776245 (ARS 09464), a female gametophyte. Fig. 4. Dentations and finger-like projections along leading margin of networks (BISH 776246; ARS 09470). Fig. 5. One cell-layered primary longitudinal (arrows) and cross-connecting lamellae (arrowheads) of network (BISH 776243; from collection ARS 09458). Fig. 6. Older network (BISH 776246; ARS 09470). Fig. 7. Basal part of developing network showing elongated longitudinal lamellae (arrows) (BISH 776246; ARS 09470). Fig. 8. Cross section through upper part of membranous blade (BISH 776246; ARS 09470). Fig. 9. Cross section through basal part of membranous blade (BISH 776243; from collection ARS 09458). Fig. 10. Squashed mature cystocarp showing extruded carpospores (BISH 776245; ARS 09464). Scale bars = 2 cm (Fig. 2), 3 cm (Fig. 3), 500 µm (Figs 4, 10), 100 µm (Figs 6–7), 50 µm (Figs 5, 8–9).
Description: Thallus upright, 4–23 cm in height and 8–29 cm in width, attached by a stipe (5–8 mm long), rose-red to dark-rose. Thallus constructed of 2–7 blades, spatulate to rounded in shape, subdichotomously divided one- to several times. A single network per blade, network margin with finger-like projections. Network having secondary needle-like longitudinal but cross-connecting strands rare. Basal thallus 1–2 (–4) cell layers (45–122 µm thick). Cystocarps 800–1350 µm in diameter and 1050–1360 µm in height, fully developed carposporangia 26–30 µm wide by 49–68 µm long.
Holotype: BISH 776243 (ARS 09458; Penguin Bank, west Molokaʻi, Hawaiʻi (21.04582, −157.35472), 76 m, 17.XI.2006, T. Kerby (P4-184 #113)). GenBank accession MN170770.
Isotype: BISH 776244 (ARS 09460; Penguin Bank, west Molokaʻi, Hawaiʻi (21.04582, −157.35472), 76 m, 17.XI.2006, T. Kerby (P4-184 #127)). GenBank accession MN170772.
Paratype: HAW-A-02849 (ARS 09470; Penguin Bank (W. Molokaʻi), Hawaiʻi (21.04545, −157.35240), 68 m, 28.XI.2006, M. Cremer (P4-189 #334). GenBank accession MN170769.
Etymology: This species is named in honour of Dr Isabella Aiona Abbott, who made enormous contributions to the field of Hawaiian phycology over her illustrious career, and who was the first native Hawaiian to earn a PhD. She was born in Hāna, Maui; this species is named to celebrate her 100th birthday (20 June 2019).
Distribution: Mesophotic depths (65–93 m) off Maui, Penguin Bank, and Nihoa of the Hawaiian Islands.
Specimens examined: BISH 776242 (ARS 09455), BISH 776243 (ARS 09458), BISH 776244 (ARS 09460), BISH 776245 (ARS 09464), BISH 776246 (ARS 09470).
DNA Sequence data: GenBank accessions MN164731, MN170769–MN170772.
Note: This species represents lineage 1 in our rbcL phylogenetic analyses ().
Habit and vegetative morphology
Thalli are upright, 4–23 cm in height and 8–29 cm in width, attached by a stipe, 5–8 mm long, rose-red to dark-rose in colour (–). Thalli are composed of 2–7 membranous blades, which are spatulate to rounded in shape, subdichotomously divided one- to several times. Blade margins are entire or with occasional dentation or rounded lobes. Each blade possesses a single band of network with finger-like projections of margin material (). Networks are mostly composed of primary longitudinal and cross-connecting lamellae (–); secondary needle-like longitudinal and cross-connecting strands are rare, developing unidirectionally. Upper part of membranous blade is monostromatic (), whereas the basal part of old blades consists of 2–4 cell layers, 48–132 µm thick (). The membranous cells are 25–57 µm in width.
Reproductive morphology
Tetrasporophytes and male gametophytes were not found. Cystocarps are scattered over the entire network, 800–1350 µm in diameter and 1050–1360 µm in height. Mature carposporangia are 26–30 µm wide by 49–68 µm long, pyriform in shape ().
Martensia hawaiiensis A.R. Sherwood & S.-M. Lin, sp. nov. (–)
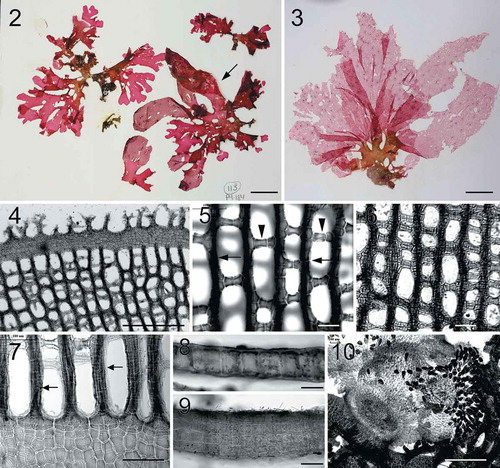
Figs 11–20. Habit and morphology of Martensia hawaiiensis sp. nov. Fig. 11. Holotype specimen BISH 776253 (from collection ARS 04013). Fig. 12. Isotype specimen BISH 775969 (from collection ARS 04013), a female gametophyte. Fig. 13. Voucher specimen BISH 776252 (from collection ARS 04011), a tetrasporic plant. Fig. 14. Basal part of a developing network (BISH 776254; ARS 05242). Fig. 15. Network of thickened longitudinal lamellae (arrows) and cross-connecting strands (arrowheads) (holotype BISH 776253; ARS 04013). Fig. 16. Discoidal plastids (arrows) in a surface view of cells of membranous region of thallus (BISH 776254; ARS 05242). Fig. 17. Cross section through basal part of membranous blade (BISH 776254; ARS 05242). Fig. 18. Mature cystocarp containing carposporangia (BISH 776251; ARS 03373). Fig. 19. Close up view of tetrasporangial sori embedded in thickened longitudinal lamellae (BISH 776254; ARS 05242). Fig. 20. Subsurface view of a tetrasporangial sorus containing tetrasporangia (arrows) (BISH 776254; ARS 05242). Scale bars = 1 cm (Figs 11–13), 100 µm (Figs 14–15, 17–20), 50 µm (Fig. 16).
Description: Thallus upright, rose- to blood-red in colour, 1.5–5.5 cm in height and 0.6–6 cm in width, attached by a short stipe. Thallus composed of 1–3 flabellate to rounded blades, with a single distal band of network per blade. Basal thallus 4–6 cell layers (265–310 µm in thickness). Network thick, having needle-like secondary cross-connecting and longitudinal lamellae. Cystocarps 900–1250 µm in diameter and 1070–1300 µm in height; carposporangia 25–30 µm by 62–70 µm. Tetrasporangial sori formed on network and irregularly shaped, fully developed tetrasporangia 33–45 µm in diameter.
Holotype: BISH 776253 (from collection ARS 04013; Shipwreck Beach, Lānaʻi, Hawaiʻi (20.912473, −156.895167), shallow subtidal, 26.III.2008, A. Kurihara). GenBank accession MN170757.
Isotype: BISH 775969 (from collection ARS 04013; Shipwreck Beach, Lānaʻi, Hawaiʻi (20.912473, −156.895167), shallow subtidal, 26.III.2008, A. Kurihara).
Paratype: HAW-A-02850 (ARS 03373; On basalt rock, Mahukona, Hawaiʻi (20.164702, −155.900085), shallow subtidal, 21.II.2008, K. Conklin). GenBank accessions MN164749 and MN164728.
Etymology: Named for the Hawaiian Islands, where it is very widespread and common, especially in the shallow waters of the MHI.
Distribution: Shallow coastal waters of Kauaʻi, Oʻahu, Lānaʻi, and Maui in the MHI, and mesophotic depths (65 m) at Midway Atoll of the NWHI.
Specimens examined: BISH 669202 (ARS 00741), BISH 776247 (ARS 02423), BISH 776248 (ARS 02745), BISH 776249 (ARS 02933), BISH 776250 (ARS 03119), BISH 776251 (ARS 03373), BISH 776252 (ARS 04011), BISH 776253 (from collection ARS 04013), BISH 775969 (from collection ARS 04013), BISH 776254 (ARS 05242), BISH 776255 (ARS 09463).
DNA Sequence data: GenBank accessions MN164728–164730, MN164748–MN164751, MN170751–MN170753, MN170756–MN170758.
Note: This species represents lineage 2 in our rbcL phylogenetic analyses ().
Habit and vegetative morphology
Thalli (–) are upright, rose- to blood-red in colour, composed of 1–3 flabellate to rounded blades, 1.5–5.5 cm in height and 0.6–6 cm in width, attached on rocks or coral by a short stipe, 2–12 mm long. Each blade bears a single band of network with a smooth margin () or covered in small interconnected lobes (–). The membranous cells are variable in size, 24–86 µm in width (). Basal parts of thalli are constructed of 4–6 cell layers, 265–310 µm in thickness (). Networks are composed of primary longitudinal and cross-connecting lamellae and primary lamellae are relatively thick, consisting of multiple strands of cells (). In old networks, primary longitudinal lamellae are broadened () and the pit-connections between cells of cross-connecting lamellae break down, giving network a thickened and ragged appearance (–).
Reproductive morphology
Cystocarps are borne on network margins or scattered over network, 900–1250 µm in diameter and 1070–1300 µm in height (). Fully developed carposporangia are 25–30 µm wide by 62–70 µm long, pyriform in shape. Tetrasporangial sori are relatively small and irregularly shaped, borne on broadened longitudinal lamellae (). Mature tetrasporangia are 45–63 µm in diameter.
Martensia tsudae A.R. Sherwood & S.-M. Lin, sp. nov. (–)
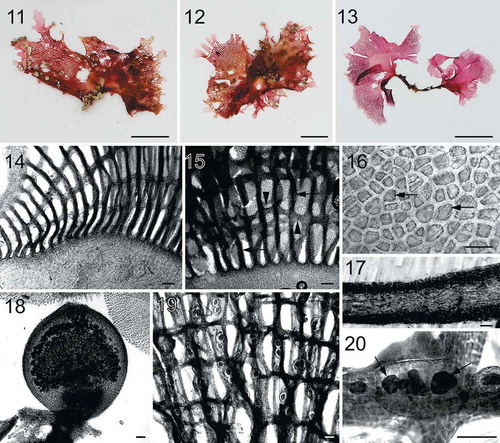
Figs 21–29. Habit and morphology of Martensia tsudae sp. nov. Fig. 21. Holotype specimen BISH 776264 (from collection ARS 05641), a female gametophyte. Fig. 22. Isotype specimen BISH 775970 (from collection ARS 05641), a tetrasporic plant. Fig. 23. Voucher specimen BISH 776266 (ARS 05657), a tetrasporic plant. Fig. 24. Part of a young network showing blade margin with connected dentations or lobes (BISH 776265; ARS 05646). Fig. 25. Part of a developing network showing broadened membranous margin and elongated longitudinal lamellae (BISH 776262; ARS 04010). Fig. 26. A younger network showing primary longitudinal lamellae (arrows) and cross-connecting strands (arrowheads) (BISH 776267; ARS 09454). Fig. 27. Basal part of a young network (BISH 776267; ARS 09454). Fig. 28. Close up view of spermatangial sori embedded in thickened longitudinal lamellae (BISH 776265; ARS 05646). Fig. 29. Close up view of tetrasporangial sori embedded in thickened longitudinal lamellae (BISH 776265; ARS 05646). Scale bars = 1 cm (Figs 21–23), 100 µm (Figs 25–26, 28–29), 50 µm (Figs 24, 27).
Description: Thalli upright or prostrate, dark rose-red to wine-red in colour, 1–6 cm in height and 1.5–6 cm in width, lacking a stipe. Thalli consisting of one to several blades terminating in a single, fan-shaped network. Basal thallus 1–2 cell layers thick (60–95 µm in thickness). Blade margins with connected dentations or lobes. Network composed of thick primary longitudinal lamellae (up to 10 cells wide), with cross-connecting lamellae (2–5 cells in width). Only tetrasporophytes and male gametophytes known; mature tetrasporangia 44–55 µm in diameter.
Holotype: BISH 776264 (from collection ARS 05641; Kanahā Beach Park, Maui, Hawaiʻi (20.900281, −156.440988), shallow subtidal, 06.IV.2008, A. Kurihara). GenBank accession MN170767.
Isotypes: BISH 775970 (from collection ARS 05641; Kanahā Beach Park, Maui, Hawaiʻi (20.900281, −156.440988), shallow subtidal, 06.IV.2008, A. Kurihara); HAW-A-02851 (ARS 02746; North end of Keomoku Rd., south end of Shipwreck Beach, Lānaʻi, Hawaiʻi (20.91450, −156.90041), shallow subtidal, 03.III.2007, A. Kurihara and T. Sauvage), GenBank accessions MN170746 and MN164726.
Etymology: This species is named in honour of our colleague, Dr Roy Tsuda, who has made many contributions to our understanding of the flora of the western Pacific, and who continues to collaborate with us on the taxonomy of the mesophotic flora of the Hawaiian Islands.
Distribution: Mesophotic depths at Nihoa (64 m), NWHI, and Molokaʻi (83-126 m), MHI, and shallow depths at Oʻahu, Molokaʻi, Lānaʻi, Maui, Hawaiʻi, MHI.
Specimens examined: BISH 776256 (ARS 01704), BISH 776257 (ARS 02607), BISH 776258 (ARS 02746), BISH 776259 (ARS 03120), BISH 776260 (ARS 04008), BISH 776261 (ARS 04009), BISH 776262 (ARS 04010), BISH 776263 (ARS 04019), BISH 775967 (ARS 05640), BISH 776264 (from collection ARS 05641), BISH 775970 (from collection ARS 05641), BISH 776265 (ARS 05646), BISH 776266 (ARS 05657), BISH 776267 (ARS 09454), BISH 776268 (ARS 02742).
DNA Sequence data: GenBank accessions MN164757–MN164727, MN164732, MN164735–MN164736, MN170745–MN170747, MN170755, MN170761–MN170765, MN170767–MN170768.
Note: This species represents lineage 3 in our rbcL phylogenetic analyses ().
Habit and vegetative morphology
Thalli (–) are upright or prostrate, dark rose-red to wine-red in colour, 1–6 cm in height and 1.5–6 cm in width, lacking a stipe. Thalli consisting of one to several blades terminating in a single, fan-shaped network. Networks occasionally bear numerous bladelets at the margin (). Basal portion of thallus blades ranges from 1–2 cell layers thick, 60–95 µm in thickness. Blade margins bear connected dentations () or lobes (). Old networks are composed of broadened primary longitudinal lamellae (up to 10 cells wide), with cross-connecting lamellae (2–5 cells in width). Needle-like secondary cross-connecting and longitudinal lamellae develop unidirectionally or bidirectionally (–).
Reproductive morphology
Female gametophytes not observed. Spermatangial sori are scattered over broadened longitudinal lamellae (). Tetrasporangial sori are borne on either membranous blades () or scattered over network (). Tetrasporangia tetrahedrally divided and globose when mature, 44–55 µm in diameter.
Martensia lauhiekoeloa A.R. Sherwood & S.-M. Lin, sp. nov. (–)
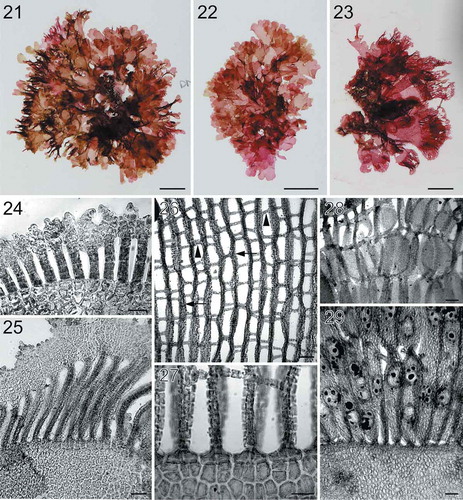
Figs 30–41. Habit and morphology of Martensia lauhiekoeloa sp. nov. Fig. 30. Isotype specimen BISH 775971 (from collection ARS 08369). Fig. 31. Holotype specimen BISH 776270 (from collection ARS 08369), a mature tetrasporic plant. Fig. 32. Voucher specimen BISH 776272 (ARS 08370), a tetrasporic plant photographed in situ. Fig. 33. Upper part of a young network showing dentate margin (BISH 776269; ARS 09461). Fig. 34. Middle portion of young network composed of primary (arrows) and secondary (arrowheads) longitudinal lamellae and cross-connecting strands (double arrowheads) making a box-like network (BISH 776269; ARS 09461). Fig. 35. Basal part of a young network showing elongated longitudinal lamellae (BISH 776272; ARS 08370). Fig. 36. Upper part of an older network showing thickened longitudinal lamellae and membranous margin with small dentations (BISH 776272; ARS 08370). Fig. 37. Older network with numerous secondarily produced short longitudinal lamellae (arrows) (BISH 776271; ARS 09471). Fig. 38. Close up of an immature cystocarp borne on network (BISH 776269; ARS 09461). Fig. 39. Interior of the immature cystocarp bearing terminal carposporangial initials (arrows) (BISH 776269; ARS 09461). Fig. 40. Close up of a tetrasporangium borne on network (BISH 776270; from collection ARS 08369). Fig. 41. Solitary, small tetrasporangia (arrows) borne on network (BISH 776270; from collection ARS 08369). Scale bars = 2 cm (Fig. 30), cm (Figs 31–32), 100 µm (Figs 33–34, 36–39, 41), 50 µm (Figs 35, 40).
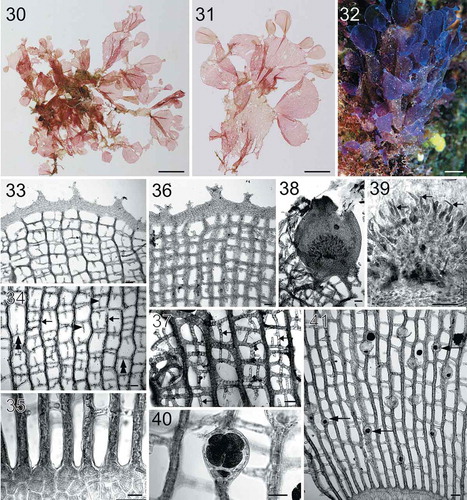
Description: Thallus upright, 6–10 cm tall and 6–15 cm wide. Stipe present, 4–6 mm in length. Thallus composed of a single blade with multiple bands of networks (up to 6 per blade). Blades mostly dichotomously divided in older parts of thallus, rounded to spatulate in shape, with more network lobes (3–6) in youngest parts of the blade. Blades greenish-coloured in older sections when dried, and progressing to rose-pink at the apices (when dried). Basal thallus 1–2 cell layers thick (51–69 µm in thickness). Needle-like secondary longitudinal and cross-connecting strands variable in abundance. Cystocarps 880–910 µm in diameter and 1040–1065 µm in height; carposporangia 62–88 µm in length and 10–15 µm in width. Tetrasporangia formed on networks, 50–66 µm in diameter at maturity.
Holotype: BISH 776270 (from collection ARS 08369; French Frigate Shoals, Hawaiʻi (23.6384667, −166.2513833), 61 m, 08.IX.2012, D. Wagner (NWHI 69)). GenBank accession MN164733.
Isotypes: BISH 775971 and HAW-A-02852 (from collection ARS 08369; French Frigate Shoals, Hawaiʻi (23.6384667, −166.2513833), 61 m, 08.IX.2012, D. Wagner (NWHI 69)).
Etymology: Traditional Hawaiian approaches to naming species take inspiration from, and relate meaning to, the world around us. Names may be tied to events around the time of a child’s birth, recognize the importance of a deity, or derive from detailed observations of a species’ habitat and morphology. Thus, names become precious possessions for Hawaiians (Abbott, Citation1992). The specific nomenclature for Martensia lauhiekoeloa is in the Hawaiian language and was developed by some members of the Cultural Working Group of the Papahānaumokuākea Marine National Monument, based on a collaborative exchange of information, and discussion of appropriate Hawaiian terms. This group, dedicated to the study of limu (marine algae), took into consideration first impressions, unique characteristics of this species, comparisons to other species, descriptions of its habitat, and other imagery brought to mind by the appearance of the holotype and isotype specimens. Through subsequent discussion and deliberation, several potential names were discussed, and M. lauhiekoeloa was arrived at by consensus.
The species epithet, lauhiekoeloa, represents a Latinized combination of three descriptive terms. Lau (noun; literally, leaf) represents the leafy character of the blades, the many extensions from one holdfast, and patterns visible throughout the entire thallus. Hie (adjective; attractive, dignified, noble) refers to the distinguished appearance of this alga that is attractive and mesmerizing to the viewer. Koelo (intransitive verb; literally, to stream, flutter wave; to trail behind, as the train of a gown) represents the imagery of the thallus being pushed by the current and resembling the elegant train of a holoku dress. Translations from Pukui & Elbert (Citation1986).
Distribution: Mesophotic depths (61–67 m) at French Frigate Shoals, NWHI.
Specimens examined: BISH 776269 (ARS 09461), BISH 776270 (from collection ARS 08369), BISH 775971 (from collection ARS 08369), BISH 776271 (ARS 09471), BISH 776272 (ARS 08370).
DNA Sequence data: GenBank accessions MN164733–MN164734, MN164752, MN170773.
Note: This species represents lineage 4 in our rbcL phylogenetic analyses ().
Habit and vegetative morphology
Thalli (–) are upright, 6–10 cm tall and 6–15 cm wide, attached to coral by a short stipe, 4–6 mm in length. Thalli are mostly composed of solitary blades, which bear multiple bands of networks (up to 6 per blade). Blades are mostly dichotomously divided in older thalli, rounded to spatulate in shape () and produce several network lobes (3–6) in youngest parts of the blades (). Blades are purplish when alive (), but turning greenish in basal parts and progressing to rose-pink at the apices when dried. Blade margins are covered with small dentations (, ). Basal parts of thalli are 1–2 cell layers thick (), 51–69 µm in thickness. Networks are composed of primary longitudinal lamellae, up to 5 cells thick, linked by cross-connecting lamellae 1–2 cells thick, resulting in a box-like structure (–, ). Needle-like secondary longitudinal and cross-connecting strands are variable in abundance, mostly unidirectionally formed (, ).
Reproductive morphology
Cystocarps () are scattered on networks, 880–910 µm in diameter and 1040–1065 µm in height. Fully developed carposporangia are 62–88 µm in length and 10–15 µm in width and elongated and vermiform in shape (). Tetrasporangia occur singly, not in groups, and are relatively small and embedded in primary longitudinal lamellae (–) on networks. Tetrasporangia ranging from 50–66 µm in diameter, globose in shape and tetrahedrally divided to produce four tetraspores ().
Martensia albida Y.Lee Citation2006: 22, fig. 2 (–)
Figs 42–48. Habit of Hawaiian specimens of Martensia albida. Fig. 42. Thallus with a thickened band of network; voucher specimen BISH 776275 (ARS 02934). Fig. 43. A tetrasporic thallus with a thin and broadened network; voucher specimen BISH 776285 (ARS 09466). Fig. 44. A female gametophyte with a thin and extensive network; voucher specimen BISH 776284 (ARS 09465). Fig. 45. A young tetrasporic thallus; voucher specimen BISH 776287 (ARS 09468). Fig. 46. Old thallus with a thickened and broadened network; voucher specimen BISH 776280 (ARS 03248). Fig. 47. Old thallus with broadened network and broken margin; voucher specimen BISH 775972 (ARS 09465). Fig. 48. Old thallus with broadened network and entire margin; voucher specimen BISH 775973 (ARS 09465). Scale bars = 1 cm (Figs 42, 46, 48), scale bar = 2 cm (Figs 43–45, 47).

Distribution: Shallow depths in the MHI (Kaua‘i, O‘ahu, Maui, Hawai‘i, Ni‘ihau) and mesophotic depths (82–91 m) in the NWHI (French Frigate Shoals) and MHI (O‘ahu).
Specimens examined: BISH 776273 (ARS 02318), BISH 776274 (ARS 02883), BISH 776275 (ARS 02934), BISH 776276 (ARS 02935), BISH 776277 (ARS 02936), BISH 776278 (ARS 02938), BISH 776279 (ARS 03121), BISH 776280 (ARS 03248), BISH 776281 (ARS 04007), BISH 776282 (ARS 05172), BISH 776283 (ARS 09457), BISH 776284 (from collection ARS 09465), BISH 776285 (ARS 09466), BISH 776286 (ARS 09467), BISH 776287 (ARS 09468), BISH 775972 (from collection ARS 09465), BISH 775973 (from collection ARS 09465), BISH 775968 (ARS 09579).
DNA Sequence data: GenBank accessions MN164737–MN164747, MN164719–MN164724, MN170748–MN170750, MN170754, MN170759–MN170760.
Note: This species represents lineage 5 in our phylogenetic analyses ().
Habit and vegetative morphology
Thalli (–) are upright, rose-coloured, except greenish in older parts of networks, composed of one to several blades, 3–13 cm in height and 4–27 cm in width, attached on rocks or coral by a small stipe, 1–10 mm in length. Thallus blades further divided into two to several bladelets; a single, terminal, broadly rounded network present on each bladelet; network margins entire. Networks are composed of primary longitudinal and cross-connecting lamellae, with thick primary lamellae so that network appears ‘closed’ in places; needle-like secondary cross-connecting strands and longitudinal lamellae present, developing unidirectionally or bidirectionally.
Reproductive morphology
Cystocarps are scattered over the networks, sometimes on both surfaces, 1150–1420 µm in diameter and 1500–1870 µm in height. Fully developed carposporangia are 25–35 µm wide by 71–100 µm long, tear-drop shaped. Tetrasporangial sori globose-to-ovoid, borne on longitudinal lamellae. Mature tetrasporangia are 65–80 µm in diameter.
Discussion
Until now, two widely applied species names of Martensia were used to describe the diversity of this red algal genus in the Hawaiian Islands (Abbott, Citation1999). Martensia fragilis was used for those species capable of forming multiple networks, while M. flabelliformis (as Neomartensia flabelliformis in Abbott, Citation1999) was used for those specimens forming single, thick networks and with a prominent stipe. In the current study we demonstrate that the species diversity of Martensia in the Hawaiian Islands comprises more than double that number of species, and that none of the five lineages represented in our phylogenetic analyses likely correspond to either M. fragilis or M. flabelliformis.
Previous reports of Martensia from Hawaiian MCEs are all as M. fragilis, and include records by Spalding (Citation2012) from 50–102 m depth in the MHI, and by Doty et al. (Citation1974) from 47 m depth from dredge haul specimens in the MHI. Abbott (Citation1989) recorded M. fragilis from Laysan, NWHI (the first record of Martensia in the NWHI) in a study that included numerous specimens collected from hauled lobster traps, but it is not known for certain whether the specimens originated from mesophotic depths. In contrast, Agegian & Abbott (Citation1985) did not report any specimens of Martensia from their submersible surveys of Penguin Bank (MHI) and Johnston Atoll. The specimens included in the current study confirm the presence of Martensia in the mesophotic zone of the NWHI (Nihoa, French Frigate Shoals and Midway Atoll), and in addition, increase the known diversity of the genus in the NWHI to four species. Moreover, the mesophotic specimens analysed in the present study correspond to those with multiple networks (i.e. M. ‘fragilis’) as well as those with single networks (i.e. M. ‘flabelliformis’) (–).
Our phylogenetic analyses demonstrate that Hawaiian specimens corresponding to what was previously called M. fragilis (i.e. lineage 3, and some specimens in lineage 5; ) are not closely allied with either material from Sri Lanka (the type locality for the species), or material known from any other location (; Kang et al., Citation2015), and thus represent an undescribed species (most of the specimens are represented by the species described here as M. tsudae). The taxonomic situation for specimens previously referred to as M. flabelliformis (lineage 2, and some specimens in lineage 5, ) is not as clear. Type or topotype material has not yet been sequenced for this species, and the two available sequences on GenBank labelled M. flabelliformis are identical (except for a series of ambiguous nucleotides in one). Both rbcL sequences are derived from material from Taiwan (Lin et al., Citation2004, Citation2009), rather than the type locality of Tonga, and thus may or may not represent M. flabelliformis. However, we advocate here for naming the Hawaiian Martensia specimens in lineage 2 as a new species as the best path forward, for several reasons. First, type material may not become available for DNA sequencing in the future, or may not be in adequate condition for successful sequencing (M. flabelliformis was described in 1863 (Agardh, Citation1863), and the overall success rate of DNA sequencing for Martensia is low, in our experience, compared with many other red algal taxa). Second, if the species is not described at this point, the opportunity may be largely missed, given the sheer number of algal taxonomic lineages from the Hawaiian Islands that require substantial systematic and taxonomic attention. Third, it is unlikely that M. flabelliformis, as described from Tonga, is identical in genotype with the Hawaiian specimens. At least in the Hawaiian mesophotic algae lineages examined in detail to date, genera with multiple species tend to display high endemicity (Spalding et al., Citation2016, Spalding et al., in prep. work on the genus Distromium). Finally, should our position be proven wrong in the future, the specimens can be taxonomically re-assigned, and yet still benefit in the meantime from the recognition provided by a taxonomic name.
Surprisingly, the Hawaiian Martensia specimens in lineage 5 corresponded to M. albida in the rbcL phylogeny (), with M. albida sequences from GenBank (derived from Korean material) nested within the more diverse Hawaiian clade. Lineage 5 sequences were separate, but similar to M. albida sequences in the COI analysis (Supplementary fig. S1). Martensia albida has not been previously reported outside of Korea (Guiry & Guiry, Citation2019), from where it was described in 2006 (Lee, Citation2006). Our specimens are similar in morphology to M. albida in many features (e.g. thallus size, blades with single network, entire margins, tetrasporangial sori on network), but the Hawaiian specimens include cystocarpic plants, which were not reported in Lee’s (Citation2006) description of Korean material, and the ‘white’ or ‘milky white’ colour of the blades when underwater reported for M. albida has not yet been confirmed for the Hawaiian specimens. Nevertheless, given the correspondence of other morphological characters and the phylogenetic relationship indicated for the specimens from Hawaii and Korea based on the rbcL and COI markers, the Hawaiian specimens are assigned to M. albida at this time.
Martensia has been reported from mesophotic depths in several other locations around the world. For example, Martensia pavonia Hering was recorded by Ballantine et al. (Citation2016) as a common member of the deep-water algal turf communities of Puerto Rico, from 36–82 m. Hanisak & Blair (Citation1988) also reported M. pavonia off the east coast of Florida, from 25–42 m depth. Littler & Littler (Citation2003) reported six species of Martensia (including Neomartensia) from the islands of the South Pacific, many of which extended to the limits of the scuba methods employed for the collections (30 m), and thus also likely occur at mesophotic depths. As more scientists are able to access specimens from mesophotic depths, this list will probably increase.
The Deep Reef Refugia Hypothesis (DRRH; Bongaerts et al., Citation2010) postulates that mesophotic reefs may serve as refugia for shallow-water organisms; however, this hypothesis has been tested only on a limited basis, thus far. Rocha et al. (Citation2018) provided evidence that most mesophotic reefs differ from their shallow counterparts, which casts doubt on the ability of mesophotic reefs to support populations of shallow-water species. Similarly, Hurley et al. (Citation2016) demonstrated significant differences between the brachyuran crab communities of shallow and deep environments in the Hawaiian Islands. In contrast, population genetic analysis of an endemic Hawaiian damselfish illustrated a lack of significant genetic differentiation by depth, highlighting the potential of mesophotic reefs to act as refugia for at least some, more motile reef species (Tenggardjaja et al., Citation2014). Our work on the red algal genus Martensia is inconclusive with respect to the DRRH: while three of the species of Martensia in Hawai‘i are known from both shallow and mesophotic habitats (M. hawaiiensis, M. tsudae and M. albida), two were collected only from deep locations (M. abbottiae, M. lauhiekoeloa).
Ongoing systematic research on mesophotic algae from several locations around the world in recent years has demonstrated the presence of undescribed species (e.g. Cianciola et al., Citation2010; Ballantine & Ruiz, Citation2010). The current effort to characterize the mesophotic algae from the length of the Hawaiian Archipelago is, thus far, supporting this trend. In the present study we demonstrate that the Hawaiian flora contains five species of the red algal genus Martensia, of which four are new species, and one a new record for the Islands. Similar efforts will be ongoing for the next several years in an attempt to develop an accurate understanding of the species-level biodiversity and their evolutionary relationships for Hawaiian mesophotic algae. Ultimately, these data will be critical for addressing the broader questions of whether MCEs may serve as refugia for species experiencing stress on shallow reefs (Bongaerts et al., Citation2010), and whether Hawaiian mesophotic algae display levels of endemicity that differ from the shallow-water flora and will allow biogeographic comparisons to mesophotic floras from other regions of the world.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2019.1668062.
Supplementary table S1: Collection details for Martensia specimens analysed in the current study.
Supplementary fig. S1: Neighbour-joining phylogram of COI sequences of Martensia from Hawai‘i and elsewhere, indicating the presence of three (out of five total) groupings of the genus for Hawaiian specimens.
Supplementary fig. S2: Map of collection sites (yellow dots) for the Martensia specimens analysed in the current study.
Author contributions
A.R. Sherwood: original concept, sample collection, generation of morphological data, data analysis, writing of manuscript. S.-M. Lin: analysis of morphological data, writing/editing of manuscript. R. M. Wade: generation and analysis of molecular data, editing of manuscript. H. L. Spalding: sample collection, editing of manuscript. C. M. Smith: sample collection, editing of manuscript. R. K. Kosaki: sample collection, editing of manuscript, coordination of PMNM CWG.
TEJP-2019-0083-File010.pptx
Download MS Power Point (296.8 KB)TEJP-2019-0083-File009.pdf
Download PDF (485.9 KB)TEJP-2019-0083-File008.docx
Download MS Word (25.2 KB)Acknowledgements
We thank the many collectors of specimens for this project (listed in Supplementary table S1). A special thank you to D. Wagner and the other accomplished divers associated with the Papahānaumokuākea Marine National Monument, the HURL Pisces IV and Pisces V submersible and RCV-150 pilots, crew, and support staff, the officers and crew of the NOAA ship Hi‘ialakai, as well as the crew of the R/V Ka‘imikai-o-Kanaloa, for access to the Hawaiian MCEs. Thank you to the Papahānaumokuākea Cultural Working Group’s nomenclature committee (Pelikaʻoʻiʻo Andrade, Pam Lota Fujii, Wally Ito, Solomon Kahoʻohalahala, Kim Kanoeʻulalani Morishige, Kalani Quiocho and Lei Wann) for developing the specific epithet for M. lauhiekoeloa.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abbott, I.A. (1989). Marine algae of the Northwest Hawaiian Islands. Pacific Science, 43: 223–233.
- Abbott, I.A. (1992). Lā‘au: Traditional Hawaiian Uses of Plants. Bishop Museum Press, Honolulu. 176 pp.
- Abbott, I.A. (1999). Marine Red Algae of the Hawaiian Islands. Bishop Museum Press, Honolulu.
- Agardh, J.G. (1863). Species genera et ordines algarum, seu descriptiones succinctae specierum, generum et ordinum, quibus algarum regnum constituitur. Volumen secundum: algas florideas complectens. Part 2, fasc. 3. Lundae [Lund]: C.W.K. Gleerup. pp. 787–1138, 1158–1291.
- Agegian, C.R. & Abbott, I.A. (1985). Deep water macroalgal communities: a comparison between Penguin Bank (Hawaii) and Johnston Atoll. Proceedings of the 5th International Coral Reef Congress, 5: 47–51.
- Ballantine, D.L. & Ruiz, H. (2010). Two new deepwater Peyssonnelia species, Peyssonnelia iridescens and Peyssonnelia gigaspora (Peyssonneliaceae, Rhodophyta) from Puerto Rico, Caribbean Sea. Phycologia, 49: 537–544.
- Ballantine, D.L., Ruiz Torres, H.R. & Aponte, N.E. (2016). The Mesophotic, Coral Reef-Associated, Marine Algal Flora of Puerto Rico, Caribbean Sea. Smithsonian Contributions to Botany Number 105. viii + 41 pp.
- Bongaerts, P., Ridgway, T., Sampayo, E.M. & Hoegh-Guldberg, O. (2010). Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs, 29: 309–327.
- Cianciola, E.N., Popolizio, T.R., Schneider, C.W. & Lane C.E. (2010). Using molecular-assisted alpha taxonomy to better understand red algal biodiversity in Bermuda. Diversity, 2: 946–958.
- Doty, M.S., Gilbert, W.J. & Abbott, I.A. (1974). Hawaiian marine algae from seaward of the algal ridge. Phycologia, 13: 345–357.
- Edgar, R.C. (2004). MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics, 5: 113.
- Gavio, B. & Fredericq, S. (2002). Grateloupia turuturu (Halymeniaceae, Rhodophyta) is the correct name of the non-native species in the Atlantic known as Grateloupia doryphora. European Journal of Phycology, 37: 349–359.
- Guiry, M.D. & Guiry, G.M. (2019). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org.
- Hanisak, M.D. & Blair, S.M. (1988). The deep-water macroalgal community of the east Florida continental shelf (USA). Helgolander Meeresuntersuchungen, 42: 133–163.
- Hinderstein, L.M., Marr, J.C.A., Martinez, F.A., Dowgiallo, M.J., Puglise, K.A., Pyle, R.L., Zawada, D.G. & Appeldoorn, R. (2010). Theme section on ‘Mesophotic Coral Ecosystems: Characterization, Ecology, and Management’. Coral Reefs, 29: 247–251.
- Hurley, K.K.C., Timmers, M.A., Godwin, L.S., Copus, J.M., Skillings, D.J. & Toonen, R.J. (2016). An assessment of shallow and mesophotic reef brachyuran crab assemblages on the south shore of O‘ahu, Hawai‘i. Coral Reefs, 35: 103–112.
- Kahng, S.E. & Kelley, C. (2007). Vertical zonation of megabenthic taxa on a deep photosynthetic reef (50–140 m) in the Au’au Channel, Hawai‘i. Coral Reefs, 2: 679–687.
- Kang, J.C. & Kim, M.S. (2013). A novel species Symphyocladia glabra sp. nov. (Rhodomelaceae, Rhodophyta) from Korea based on morphological and molecular analyses. Algae, 28: 149–160.
- Kang, J.C., Yang, M.Y., Lin, S.-M. & Kim, M.S. (2015). Reappraisal of nine species of Martensia (Delesseriaceae, Rhodophyta) reported from Korea based on morphology and molecular analyses. Botanica Marina, 58: 151–166.
- Kim, M.S., Kim, S.Y. & Nelson, W. (2010). Symphyocladia lithophila sp. nov. (Rhodomelaceae, Ceramiales), a new Korean red algal species based on morphology and rbcL sequences. Botanica Marina, 53: 233–241.
- Lane, C.E., Lindstrom, S.C. & Saunders, G.W. (2007). A molecular assessment of northeast Pacific Alaria species (Laminariales, Phaeophyceae) with reference to the utility of DNA barcoding. Molecular Phylogenetics and Evolution, 44: 634–648.
- Lanfear, R., Calcott, B., Ho, S.Y.W. & Guindon, S. (2012). PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution, 29: 1695–1701.
- Lee, Y. (2004). Two new species of Martensia (Delesseriaceae, Rhodophyta) from Jeju Island, Korea. Phycological Research, 52: 255–265.
- Lee, Y. (2006). The genus Martensia Hering (Delesseriaceae, Rhodophyta) with M. albida sp. nov. and M. flammifolia sp. nov on Jeju Island, Korea. Algae, 21: 15–48.
- Lin, S.-M., Hommersand, M.H. & Fredericq, S. (2004). Two new species of Martensia (Delesseriaceae, Rhodophyta) from Kenting National Park, southern Taiwan. Phycologia, 43: 13–25.
- Lin, S.-M., Hommersand, M.H., Fredericq, S. & De Clerck, O. (2009). Characterization of Martensia (Delesseriaceae, Rhodophyta) based on morphological and molecular study of the type species, M. elegans, and M. natalensis sp. nov. from South Africa. Journal of Phycology, 45: 678–691.
- Lin, S.-M., Yang, W.-C., Huisman, J.M., De Clerck, O. & Lee, W.J. (2013). Molecular phylogeny of the widespread Martensia fragilis complex (Delesseriaceae, Rhodophyta) from the Indo-Pacific region reveals three new species of Martensia from Taiwan. European Journal of Phycology, 48: 173–187.
- Littler, D.S. & Littler, M.M. (2003). South Pacific Reef Plants: A Diver’s Guide to the Plant Life of South Pacific Coral Reefs. OffShore Graphics, Washington, DC.
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), 1–8. IEEE, New Orleans, Louisiana.
- Pukui, M.K. & Elbert, S.H. (1986). Hawaiian Dictionary. University of Hawai‘i Press, Honolulu, Hawai‘i.
- Rocha, L.A., Pinheiro, H.T., Shepherd, B., Papastamatiou, Y.P., Luiz, O.J., Pyle, R.L. & Bongaerts, P. (2018). Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science, 361: 281–284.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Rooney, J., Donham, E., Montgomery, A., Spalding, H., Parrish, F., Boland, R., Fenner, D., Gove, J. & Vetter, O. (2010). Mesophotic coral ecosystems in the Hawaiian Archipelago. Coral Reefs, 29: 361–367.
- Saunders, G.W. (2005). Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philosophical Transactions of the Royal Society B: Biological Sciences, 360: 1879–1888.
- Sherwood, A.R., Kurihara, A., Conklin, K.Y., Sauvage, T. & Presting, G.G. (2010a). The Hawaiian Rhodophyta Biodiversity Survey (2006–2010): a summary of principal findings. BMC Plant Biology, 10: 258.
- Sherwood, A.R., Sauvage, T., Kurihara, A., Conklin, K.Y. & Presting, G.G. (2010b). A comparative analysis of COI, LSU and UPA marker data for the Hawaiian florideophyte Rhodophyta: implications for DNA barcoding of red algae. Cryptogamie, Algologie, 31: 451–465.
- Spalding, H.L. (2012). Ecology of mesophotic macroalgae and Halimeda kanaloana meadows in the main Hawaiian Islands. PhD Thesis, University of Hawai’i, Honolulu.
- Spalding, H.L., Conklin, K.Y., Smith, C.M., O’Kelly, C.J. & Sherwood, A.R. (2016). New Ulvaceae (Ulvophyceae, Chlorophyta) from mesophotic ecosystems across the Hawaiian Archipelago. Journal of Phycology, 52: 40–53.
- Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312–1313.
- Tenggardjaja, K.A., Bowen, B.W. & Bernardi, G. (2014). Vertical and horizontal genetic connectivity in Chromis verater, an endemic damselfish found on shallow and mesophotic reefs in the Hawaiian Archipelago and adjacent Johnston Atoll. PLoS ONE, 9: e115493.
