ABSTRACT
The phylogroup Chloromonadinia (Volvocales, Chlorophyceae) encompasses ecologically versatile green microalgae with a predominantly freshwater and snow lifestyle. Although a few species have been described from soils, the evolutionary history of terrestrial volvocalean flagellates is poorly known due to undersampling. Here we provide new insights into the phylogenetic position of terrestrial flagellates within the Chloromonadinia and investigate six unresolved strains from terrestrial and freshwater habitats in both hemispheres. The terrestrial strains differed in cell morphology, ultrastructure and lifestyle from the freshwater ones, therefore, we expected them to represent distinct evolutionary lineages (species or genera). While the terrestrial strain SAG 25.87 (Chloromonas gracillima comb. & stat. nov.) from Czech mountains clustered within the psychrotolerant core Chloromonas clade, the other terrestrial strains formed a phylogenetically separate lineage which we proposed as Ostravamonas gen. nov. The new genus differs from other closely related Chloromonadinia genera by chloroplast, eyespot, papilla and cell-wall morphology. Two terrestrial strains, CAUP G 1401 (coal spoil heap; Czechia) and SAG 12.72 (acidic soil; New Zealand), were designated as Ostravamonas chlorostellata comb. nov. and represent the first Chloromonadinia microalgae discovered in acidic terrestrial environments. Closely related ‘Chlamydomonas’ strains SAG 75.81 (ditch; France) and CCAP 11/108 (origin unknown) were reassigned to Ostravamonas meslinii comb. nov. and Ostravamonas tenuiincisa sp. nov., respectively. Our study shows that the traditional ‘Chlamydomonas’ still hides an overlooked diversity of species with a terrestrial lifestyle that are nested phylogenetically within the Chloromonadinia phylogroup.
Introduction
The phylogroup Chloromonadinia (Volvocales, Chlorophyceae) encompasses four genera of unicellular flagellates (Chloromonas Gobi, Gloeomonas G.A. Klebs, Chlainomonas Christen and Ixipapillifera Nakada) isolated mainly from fresh water and snow. The most widespread and species-rich genus is Chloromonas, accommodating morphologically, ecologically and physiologically diverse organisms. Barcytė et al. (Citation2018a) demonstrated that the current concept of Chloromonas is paraphyletic, and suggested that the genus should be split into a number of smaller taxonomic entities for better systematic organization of Chloromonadinia. The genus Chloromonas was identified as the monophyletic clade containing the type species Chloromonas reticulata, and corresponding to the Chloromonas reticulata clade sensu Pröschold et al. (Citation2001) (Barcytė et al., Citation2018a). Both pyrenoid-lacking and -bearing species fall into this circumscription, as previously demonstrated, including species formerly classified as Chlamydomonas (Pröschold et al. Citation2001; Matsuzaki et al., Citation2012). Having been subdivided into three main clades (psychrophilic, psychrotolerant and mesophilic clades; Barcytė et al. Citation2018a), Chloromonas now awaits an integrative taxonomic revision with the establishment of new genera. Since both morphology and phylogeny have certain limitations, polyphasic taxonomic approaches are currently the most popular way to classify and reclassify microorganisms. For example, by combining morphological and molecular analyses, Matsuzaki et al. (Citation2012, Citation2014, Citation2018, Citation2019) identified and re-identified multiple Chloromonas strains and proposed new species combinations in some cases, and new descriptions in others. However, there have as yet been no attempts to reclassify Chloromonadinia microalgae at the genus level based on current systematic methods. The taxonomic history of Chloromonas-like flagellates is not simple, especially for snow-dwelling species (many of which have resting stages that were placed in chlorococcalean genera such as Scotiella F.E. Fritsch (Hoham & Mullet, Citation1978; Hoham et al., Citation1983) and Cryocystis Kol (Hoham et al., Citation1979)).
In contrast to freshwater and snow Chloromonadinia taxa, the majority of which are psychrotolerant and psychrophilic Chloromonas species (Hoham et al., Citation2002; Matsuzaki et al., Citation2014, Citation2018, Citation2019; Barcytė et al., Citation2018a, Citationb), much less attention has been paid to the terrestrial species of Chloromonadinia. Consequently, the uncharacterized strains limit our understanding of Chloromonas-like morphotypes and phylotypes thriving in (aero-)terrestrial environments. For example, our current knowledge is limited to several sequenced strains isolated from soils in different parts of the world, and taxa with terrestrial lifestyles are under-studied.
Other genera of the Chloromonadinia phylogroup include the aforementioned freshwater Gloeomonas, which is a sister lineage to the psychrotolerant Chloromonas; the snow alga Chlainomonas, a close relative of the psychrophilic Chloromonas; and terrestrial and freshwater Ixipapillifera whose phylogenetic placement within Chloromonadinia continues to be debated. The phylogenetic position of freshwater Chloromonas pseudoplatyrhyncha (Pascher) P.C. Silva is also unclear, since it does not cluster within any of the previously defined Chloromonas clades (Barcytė et al., Citation2018a).
In this paper, we have focused on terrestrial members of the Chloromonadinia phylogroup. We studied the new volvocalean flagellate strain CAUP G 1401, isolated from a highly unusual terrestrial habitat – a burning coal spoil heap. We compared it with morphologically similar authentic terrestrial strains of the species Chlamydomonas chlorostellata E.A. Flint & H. Ettl SAG 12.72 and C. chlorostellata var. gracillima H. Ettl SAG 25.87, both assigned to the freshwater species Chlamydomonas meslinii Bourrelly (authentic strain SAG 75.81). For comparison and taxonomic implications, we have also studied a new isolate of C. reticulata CAUP G 1302 and Chlamydomonas moewusii var. microstigmata CCAP 11/108. We aimed to evaluate or re-evaluate the taxonomic status of the new and authentic strains. Based on the results, we discuss relationships between species circumscribed within the Chloromonadinia phylogroup. For the purpose of accommodating the investigated strains within Chloromonadinia, we propose here a new genus-level taxonomy.
Materials and methods
Strain origins, cultivation and microscopy
Two strains used in this study were newly isolated () using multiple serial dilutions and an inverted phase contrast Nikon Diaphot 200 microscope (Nikon Corp., Tokyo, Japan). These strains have been deposited in the Culture Collection of Algae of Charles University in Prague, Czechia (CAUP; https://botany.natur.cuni.cz/algo/caup.html). Three strains were obtained from the Culture Collection of Algae at the University of Göttingen, Germany (SAG; http://sagdb.uni-goettingen.de/). An additional strain was purchased from the Culture Collection of Algae and Protozoa (CCAP; https://www.ccap.ac.uk/; ).
Table 1. List of studied strains.
Cultures of each strain were maintained in Bold’s Basal Medium (BBM; Bischoff & Bold, Citation1963) in a Q-Cell 200 incubator (PolLab, Wilkowice, Poland) at 20°C. Continuous light was provided by cool white 8W fluorescent tubes (Osram, Munich, Germany) at ~30 µmol photons m–2 s–1.
Morphological investigations of cultures were conducted using an Olympus BX43 light microscope (Tokyo, Japan). Microphotographs were taken with an Olympus DP27 digital camera (Tokyo, Japan). The Olympus micro imaging software cellSens v1.15 (Tokyo, Japan) was used for morphometric measurements of the algae. Vegetative cells were observed and documented using 1-month-old cultures. One hundred cells of each strain were measured for size comparisons. The ultrastructure of cells was studied using transmission electron microscopy (TEM) as previously described in Barcytė et al. (Citation2017).
DNA extraction, fragment amplification and sequencing
Genomic DNA from each investigated strain was extracted using a Geneaid Plant Genomic DNA Mini Kit (New Taipei City, Taiwan). Nucleotide sequences of 18S-ITS1-5.8-ITS2 rDNA regions were amplified using the following primer pairs: 18SF/18SR (Katana et al., Citation2001), AL1500af (Helms et al., Citation2001)/ITS2 and ITS1/ITS4 (White et al., Citation1990). A partial fragment of a ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit gene (rbcL) was amplified with primers rbcL1F and rbcL23R (Hoham et al., Citation2002). Polymerase chain reactions (PCRs) were performed using PPP Master Mix (Top-Bio, Prague, Czechia) in a total volume of 25 µl, or a Qiagen Multiplex PCR Plus Kit (Hilden, Germany) in a total volume of 10 µl. Cycling conditions have been previously described in Barcytė et al. (Citation2018a) and Hoham et al. (Citation2002). The PCR products were cleaned using ethanol or ExoSAP-IT (Affymetrix, Cleveland, USA). Sequencing was performed by Macrogen (Amsterdam, the Netherlands) in both directions using the PCR primers described above, and the internal primers 985R, 1422F (Remias et al., Citation2012) and 1422R (5ʹ-CTA AGG GCA TCA CAG ACC TG-3ʹ; T. Friedl, unpubl.). Sequences were assembled using SeqAssem ver. 07/2008 (Hepperle, Citation2004). These have been deposited in GenBank under accession numbers MK908411–MK908414, MK912124–MK912127, MK912141–MK912145 and MN380030.
Phylogenetic analyses
Employing the BLAST search algorithm (Altschul et al., Citation1997), we obtained sequences from GenBank that are closely related to our newly sequenced strains. Phylogenetic analyses relied upon 63 nuclear 18S rDNA sequences, and 55 plastid rbcL sequences. Multiple sequence alignments were computed using the MAFFT algorithm (Katoh & Toh, Citation2008) employing Q-INS-i (18S rDNA) and FFT-NS-1 (rbcL) strategies. We checked the alignments for misaligned positions using the program BioEdit 7.0.9.0 (Hall, Citation1999). The 18S rDNA alignment covered 1687 positions (336 variable, 230 parsimony-informative), while the rbcL alignment covered 936 positions (353 variable, 297 parsimony-informative). The Akaike information criterion (AIC) implemented in the software jModelTest 0.1.1 (Posada, Citation2008) selected the best fitting nucleotide substitution model for the 18S rDNA dataset (GTR+Γ+I) and the rbcL dataset (GTR+Γ+I for the rbcL 1st codon position, JC+I for the rbcL 2nd codon position and GTR+Γ+I for the rbcL 3rd codon position). The 18S-based maximum-likelihood phylogeny was computed in RAxML 7.0.4 (Stamatakis et al., Citation2008) under the proposed model. Statistical support values were acquired by rapid bootstrapping (1000 replicates) in the same program. The rbcL-based maximum-likelihood phylogeny was computed with the program Garli 2.01 (Zwickl, Citation2006) in accord with the above-mentioned nucleotide substitution models, with 1000 bootstrap replicates. Bayesian posterior probabilities were computed in MrBayes 3.2.1 x86 (Ronquist et al., Citation2012) using the non-partitioned 18S rDNA alignment, and the rbcL alignment partitioned into each of the three codon positions. In both cases, we performed two Markov chain Monte Carlo (MCMC) runs for 106 generations with one cold and three heated chains under the proposed evolutionary models, with trees sampled every 100 generations and a burn-in of the first 25%. After 106 generations, the average standard deviation of split frequencies dropped below 0.008, and the potential scale reduction factor (PSRF) approached 1.000–1.003 for convergence diagnostic parameters. For visualization of the phylogenetic trees, we used the program FigTree v1.4.2 (Rambaut, Citation2007). Sequence comparisons based on numbers of different nucleotides were conducted using the software MEGA6 (Tamura et al., Citation2013).
ITS2 rDNA secondary structure analysis
The ITS2 region between 5.8S and 28S rDNA flanking regions was annotated using the ITS2 database (Koetschan et al., Citation2012). Secondary structure models of the annotated ITS2 regions were folded based on the minimum energy criterion in the program RNAstructure 5.3 (Reuter & Mathews, Citation2010) and visualized by Varna 3.8 (Darty et al., Citation2009). The primary ITS2 sequences along with their secondary structures were aligned employing the ClustalW algorithm implemented in the program 4SALE 1.7. (Seibel et al., Citation2008). The sequence+structure alignment had 320 positions and was analysed for the presence of compensatory base changes (CBCs; Wolf et al., Citation2013) among sequences in 4SALE 1.7.
Results
Morphology/cytology and ultrastructure of the studied strains have been investigated and captured using light microscopy (–) and transmission electron microscopy (–), and summarized in the form of line drawings (–).
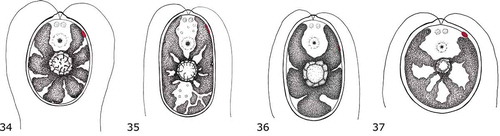
Figs 1–18. Morphology and reproduction of studied strains. Figs 1–3. Ostravamonas chlorostellata CAUP G 1401. Figs 4–6. O. chlorostellata SAG 12.72. Figs 7–9. Chloromonas gracillima SAG 25.87. Figs 10–12. O. meslinii SAG 75.81. Figs 13–15. O. tenuiincisa CCAP 11/108. Figs 16–18. C. reticulata CAUP G 1302. Yellow arrowheads indicate papilla, red arrowheads indicate eyespot. Scale bars = 10 µm.
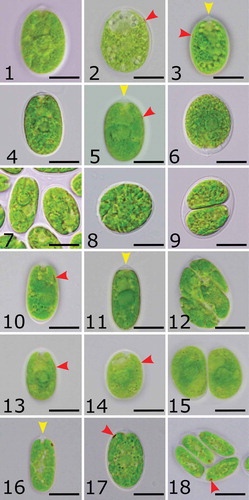
Figs 19–29. Transmission electron micrographs of studied strains. Fig. 19. Chloromonas reticulata CAUP G 1302. Fig. 20. Ostravamonas chlorostellata CAUP G 1401. Fig. 21. O. chlorostellata SAG 12.72. Fig. 22. O. meslinii SAG 75.81. Fig. 23. C. gracillima SAG 25.87. Papillae of strains: Figs 24–25. CAUP G 1401. Fig. 26. SAG 12.72. Fig. 27 SAG 75.81. Fig. 28. SAG 25.87. Fig. 29. CAUP G 1302. White arrowheads indicate cell wall thickenings, black arrowheads indicate papillae. Abbreviations: Ch, chloroplast; L, lipid droplet; N, nucleus, Nu, nucleolus; P, pyrenoid; S, starch; V, vacuole. Scale bars: Figs 24, 28 = 1 µm. Figs 19–23, 25–27, 29 = 2 µm.
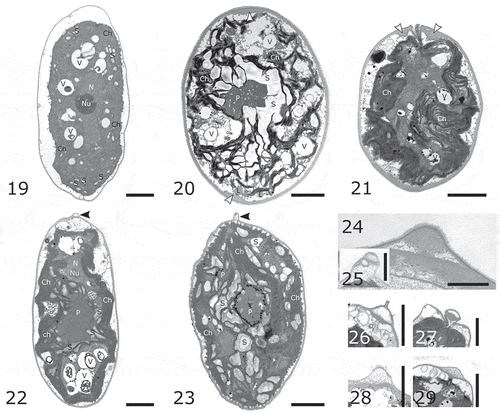
Figs 30–33. Pyrenoid ultrastructure. Fig. 27. Ostravamonas chlorostellata CAUP G 1401. Fig. 28. O. chlorostellata SAG 12.72. Fig. 29. O. meslinii SAG 75.81. Fig. 30. Chloromonas gracillima SAG 25.87. Scale bars = 1 µm.
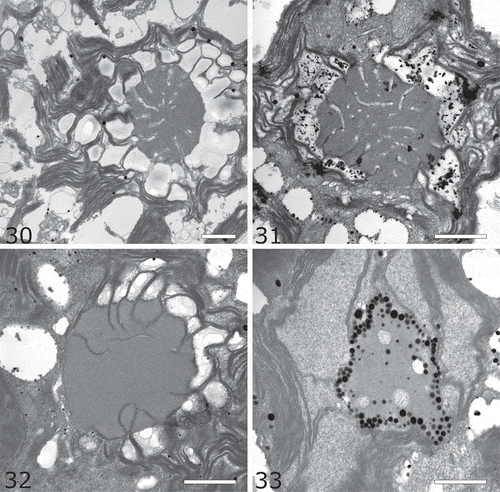
Figs 34–37. Line drawings of re-examined species. Fig. 34. Ostravamonas chlorostellata. Fig. 35. O. meslinii. Fig. 36. O. tenuiincisa. Fig. 37. Chloromonas gracillima.
Morphology/cytology
Cells of the new terrestrial Czech strain CAUP G 1401, herein classified as Ostravamonas chlorostellata, had broad ellipsoidal or broad cylindrical, sometimes almost spherical cells, with a radially lobed chloroplast containing a central pyrenoid (–). Two equal flagella emerged from a low but prominent papilla, which was hemispherical, cone-shaped with a round face, or keel-shaped (). The flagella were about 1.5 times as long as the cell and two prominent contractile vacuoles were present below the papilla (). The eyespot was elliptical and lateral, located in the second third of the anterior end of the cell (, , ). The eyespot was often camouflaged by the massive chloroplast (). Cell walls were smooth and robust. They were often somewhat remote from the protoplast, especially towards the posterior end of cells. Cells of the strain CAUP G 1401 ranged from 16–27 µm in length and 11–22 µm in width. The alga reproduced asexually, forming two or four zoospores.
The curated strain of the terrestrial Chlamydomonas chlorostellata SAG 12.72 (=genus Ostravamonas) had broadly ellipsoidal or broadly cylindrical cells with a broadly rounded posterior end (–). Sometimes the cells were almost spherical. The cells had a rather thick outline and a low hemispherical, cone-shaped with a round face, or keel-shaped papilla (, ). An extra layer of mother cell walls often coated the cells. Its chloroplast was radially lobed, and had a prominent pyrenoid in the middle (–, ). The eyespot was elliptical, located in the lateral position of the chloroplast, in the second or the last third of the cell’s anterior end (, ). In mature cells it was often hidden (, ). The posterior end of the cell was sometimes not fully filled by the chloroplast and appeared empty (). Two equal-length flagella, longer than the cell, were present. Cells of O. chlorostellata SAG 12.72 were 15–27 µm long and 9–22 µm wide. The strain reproduced asexually, forming two to four zoospores.
The second terrestrial Czech isolate, the authentic strain of the species C. chlorostellata var. gracillima (hereafter Chloromonas gracillima) SAG 25.87 had broadly ellipsoidal or cylindrical (), sometimes almost spherical cells (). Its cell walls were thick, with a distinct hemispherical papilla in the front (). Cells had two flagella, which were approximately as long as the cell. The chloroplast was composed of large parietal lobes, some of which extended towards the cell’s centre, and surrounded the pyrenoid (, ). In mature cells, the chloroplast was highly divided and asteroid-shaped (). Occasionally, cells were observed to contain two pyrenoids (). An ellipsoidal or slightly roundish eyespot was present in the lateral anterior end of the chloroplast, in the first third of the anterior end of the cell (). The eyespot was often hidden. Cells were 12–22 µm in length, and 7–18 µm in width. The alga reproduced by forming two, four or eight zoospores ().
The authentic strain of freshwater species Chlamydomonas meslinii SAG 75.81 (=genus Ostravamonas) had ellipsoidal or cylindrical cells with rounded ends (, ). Sometimes the posterior end of the cell was pointed. A small distinct hemispherical papilla was present in the front (, ). Cells possessed two equal flagella of variable length, shorter than, equal to or longer than the cell. A contractile vacuole was located close to each flagellar base. The cell walls were approximately as thick as the membranes. The chloroplast filled most of the cell’s volume and was composed of radially emerging lobes, along with additional parietal or central ellipsoidal discs (, , ). A large pyrenoid was located approximately in the middle of the cell (, ). Occasionally, two pyrenoids appeared. A long, thin, string-like eyespot was present at the lateral margins of the chloroplast, in the second third of the cell’s anterior (, ). Cells were 16–27 µm long and 7–20 µm wide. Asexual reproduction forming two or four zoospores was observed ().
The curated strain of unknown origin but assigned to the species Chlamydomonas moewusii var. microstigmata CCAP 11/108 (= Ostravamonas tenuiincisa) had broadly ellipsoidal to ovoid cells with broadly rounded ends (–). The cell wall was thicker than the membrane and had a low keel-shaped papilla in the front. Two equal length flagella were about as long as the cell. Two contractile vacuoles were present in the apical opening of the chloroplast. The plastid filled most of the cell’s volume and was essentially cup-shaped but had radial slits. The central pyrenoid was spherical in shape and covered by starch plates (). A small, thin, string-like or narrowly ellipsoidal eyespot was present at the lateral margins of the chloroplast, in the second or last third of the anterior end of the cell (, , ). However, it was often hidden. Cells were 14–25 µm long and 7–21 µm wide. Asexual reproduction by formation of two or four, rarely eight zoospores was present ().
By contrast, cells of the newly isolated freshwater Arctic strain CAUP G 1302, belonging to Chloromonas reticulata, were ellipsoidal or ellipsoidal-cylindrical, occasionally ovoid (, ). They had two equal flagella about as long as the cell, and a thin cell wall with a hemispherical papilla (). Sometimes the top of the papilla was flat. The chloroplast was parietal and cup-shaped, composed of multiple parietal lobes, which were always connected to adjacent lobes (, ). The eyespot was located in the lateral anterior end of the chloroplast, in the first third of the anterior end of the cell, and was ellipsoidal or D-shaped (–). The pyrenoid was absent. Cells were 10–20 µm long and 5–13 µm wide. Asexual reproduction yielded four or eight zoospores ().
Ultrastructure
TEM confirmed observed differences in chloroplast exteriors in the studied strains (–). For example, only C. reticulata CAUP G 1302 had a cup-shaped chloroplast with parietal lobes that did not enter the cell centre, and lacked a pyrenoid (). The other examined strains (O. microstigmata CCAP 11/108 was not included in the TEM study) possessed a chloroplast radiating from the cell periphery towards its centre, where a pyrenoid was located (–; ). In the two O. chlorostellata strains CAUP G 1401 and SAG 12.72, chloroplast thylakoids undulated along the periphery of the chloroplast envelope, and extended radially where they surrounded the pyrenoid, which was spherical or subspherical in shape (). The chloroplast of O. meslinii SAG 75.81 had rather distinct peripheral lobes, some of which extended radially and encircled the pyrenoid (). By contast, Chloromonas gracillima SAG 25.87 had both radially and upright stretching chloroplast thylakoids, forming a net-like structure in which the pyrenoid was entangled (). For a detailed pyrenoid description, see below.
The cell walls of the investigated strains were smooth and without ornamentation (–). Presence of cells with thick walls (≥ 200 nm) was confirmed in the O. chlorostellata strains CAUP G 1401 and SAG 12.72, and C. gracillima SAG 25.87. The cell walls of O. chlorostellata sometimes had prominent anterior and posterior thickenings (, ). TEM confirmed the presence of hemispherical papillae in all examined strains (, , –), including the presence of a keel-shaped papilla in O. chlorostellata (, ). A nucleus with a prominent nucleolus was located in the cell centre in C. reticulata CAUP G 1302 (), while in the other strains it was placed anterior to the pyrenoid (, –). All investigated strains had numerous cytoplasmic vacuoles, some of which contained electron-dense structures (–). Lipid globules were detected in the cytosol only in O. chlorostellata strains CAUP G 1401 and SAG 12.72 ().
The pyrenoids of O. chlorostellata CAUP G 1401 and SAG 12.72 were surrounded by numerous starch plates and granules, and penetrated by both thylakoids and fine starch grains (, , ). The pyrenoid of O. meslinii SAG 75.81 was also surrounded by starch, and thylakoids penetrated the pyrenoid’s matrix, but starch grains usually did not (, ). The pyrenoid of strain C. gracillima SAG 25.87 was either surrounded by starch plates and grains () or naked (). In addition, numerous plastoglobuli surrounded the pyrenoid, which was not observed in any other strain investigated in this study (, ). Several starch grains were present in the pyrenoid matrix in C. gracillima SAG 25.87 as well (). Of note, the pyrenoid-less C. reticulata CAUP G 1302 never contained starch granules as large as those in other strains, just fine grains scattered within the interthylakoidal spaces ().
Molecular phylogeny based on 18S rRNA and rbcL genes
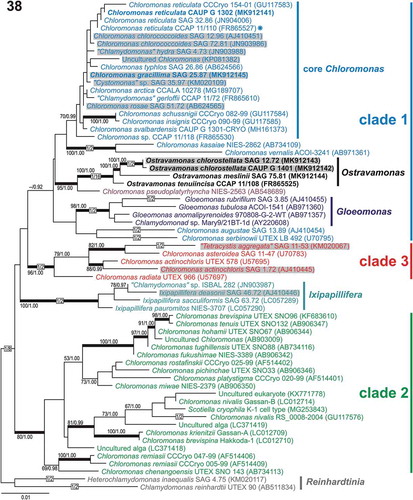
Phylogenetic analysis of 18S rDNA revealed that the six strains investigated in this study clustered into two main phylogenetic lineages within the Chloromonadinia phylogroup (). In the 18S rDNA phylogenetic tree, all strains were nested within a highly diversified clade 1 (sensu Hoham et al., Citation2002), which was unlikely to be monophyletic. The Arctic C. reticulata CAUP G 1302 and Czech C. gracillima SAG 25.87 clustered within the core Chloromonas clade, with moderate support for being monophyletic. The strains of the newly proposed genus Ostravamonas (CAUP G 1401 and SAG 12.72 as O. chlorostellata, SAG 75.81 as O. meslinii and CCAP 11/108 as O. tenuiincisa) were placed as sister lineages to C. pseudoplatyrhyncha NIES-2563 (). Comparison of 1687 nucleotide positions in the 18S rDNA between the newly isolated C. reticulata strain CAUP G 1302 and the authentic strain CCAP 11/110 revealed just one nucleotide difference. Chloromonas gracillima SAG 25.87 also differed from its closest revealed relative ‘Cystomonas’ sp. SAG 35.97 by one nucleotide substitution. The two strains of O. chlorostellata SAG 12.72 and CAUP G 1401 differed from O. tenuiincisa CCAP 11/108 by 16 or 17 nucleotide substitutions, respectively, and by one nucleotide from each other. The closest known relative of O. chlorostellata strains SAG 12.72 and CAUP G 1401 was the newly sequenced O. meslinii SAG 75.81, which differed from them by 24 or 25 nucleotide substitutions, respectively. Ostravamonas meslinii SAG 75.81 differed by a considerable number of nucleotide substitutions (18 nucleotides) from O. tenuiincisa CCAP 11/108. Assignment of the whole Ostravamonas clade was strongly statistically supported. Assignment of C. pseudoplatyrhyncha NIES-2563 as its sister lineage has moderate statistical support ().
Fig. 38. Maximum-likelihood tree of the Chloromonadinia phylogroup based on 18S rDNA sequences. Numbers next to branches indicate statistical support values: ML bootstraps and BI posterior probabilities. Thick lines indicate branches with statistical support: ML ≥ 80/BI ≥ 0.95. Chloromonas clades 1, 2 and 3 were delimited according to Hoham et al. (Citation2002) and adopted in the previous studies of Barcytė et al. (Citation2018a, Citationb). New sequences are in bold. Grey shaded strains were isolated from terrestrial habitats. The asterisk indicates the authentic strain of C. reticulata, which is the type species of the genus Chloromonas.
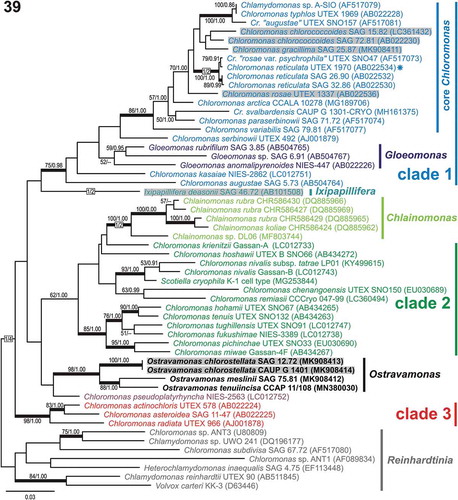
In contrast to the 18S rDNA phylogenetic tree topology, the rbcL-based phylogeny statistically supported monophyly of both clade 1 and the core Chloromonas clade (). In addition, the four Ostravamonas strains (i.e. CAUP G 1401, SAG 12.72, SAG 75.81, CCAP 11/108) clustered out of clade 1 (). Similar to the 18S rDNA inference, C. pseudoplatyrhyncha NIES-2563 appeared to be closely related to the four strains mentioned above, but failed to achieve statistical support. Ostravamonas chlorostellata CAUP G 1401 and SAG 12.72 were identical in their rbcL sequence (936 aligned nucleotides) and differed from O. meslinii SAG 75.81 by 52 nucleotide substitutions. Meanwhile, C. gracillima SAG 25.87 differed from the most similar sequence of C. chlorococcoides SAG 72.81 by 19 nucleotide substitutions, and there is no support for the position of C. gracillima (). Despite multiple efforts to amplify the rbcL sequence of C. reticulata CAUP G 1302, we failed to obtain an rbcL fragment for analysis. However, as we will show in the following ITS2 comparisons, the strain is phylogenetically closely related to C. reticulata CCAP 11/110 (), as was also demonstrated by 18S rDNA phylogeny ().
Fig. 39. Maximum-likelihood tree of the Chloromonadinia phylogroup based on rbcL sequences. Numbers next to branches indicate statistical support values: ML bootstraps and BI posterior probabilities. Thick lines indicate branches with statistical support: ML ≥ 80/BI ≥ 0.95. Chloromonas clades 1, 2 and 3 were delimited according to Hoham et al. (Citation2002) and adopted in the previous studies of Barcytė et al. (Citation2018a, Citationb). New sequences are in bold. Grey shaded strains were isolated from terrestrial habitats. The asterisk shows the authentic strain of C. reticulata, which is the type species of the genus Chloromonas.
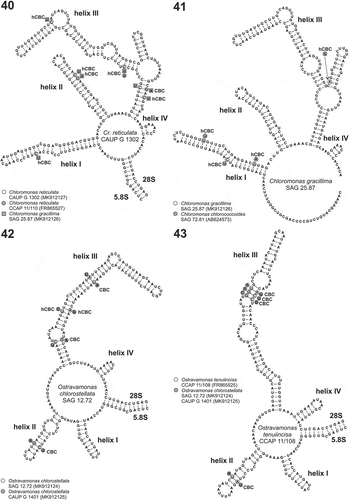
Figs 40–43. Secondary structure models of the nuclear ITS2. Fig. 40. ITS2 secondary structure model of the strain Chloromonas reticulata CAUP G 1302 isolated in the Arctic compared with two closely related strains of the core Chloromonas lineage. The strain CCAP 11/110 represents the authentic strain of C. reticulata, and the strain SAG 25.87 represents C. gracillima. Differences in double-strand compensatory base changes (CBC) and single strand compensatory base changes (hCBC) are marked as grey circles (CCAP 11/110) or squares (SAG 25.87) Fig. 41. ITS2 secondary structure model of C. gracillima SAG 25.87 compared with the closely related C. chlorococcoides strain SAG 72.81. Differences in double-strand compensatory base changes (CBC) and single strand compensatory base changes (hCBC) are marked as grey circles. Fig. 42. ITS2 secondary structure model of O. chlorostellata SAG 12.72 compared with the conspecific strain CAUP G 1401. Differences in double-strand compensatory base changes (CBC) and single strand compensatory base changes (hCBC) are marked as grey circles. Fig. 43. ITS2 secondary structure model of O. tenuiincisa CCAP 11/108 compared with O. chlorostellata SAG 12.72 and CAUP G 1401. Differences in double-strand compensatory base changes (CBC) are marked as grey circles.
Comparison of ITS2 rDNA secondary structures
Comparing 275 aligned nucleotides of the core Chloromonas strains, C. reticulata CAUP G 1302 and C. gracillima SAG 25.87, identified 38 nucleotide substitutions in the primary sequences of the ITS2 region. The two sequences exhibited one compensatory base change (CBC; helix III; ) and six one-sided mutations (hCBC) when their secondary structure models were compared. The strain CAUP G 1302 differed by three nucleotide substitutions (including one hCBC) from the authentic strain of C. reticulata CCAP 11/110 and also from C. rosae var. psychrophila UTEX SNO47. Meanwhile, C. gracillima SAG 25.87 was most similar (with one nucleotide difference) to an uncultured Chlamydomonadales clone 750 (GenBank accession number MF481961) isolated from soil.
Chloromonas gracillima SAG 25.87, which had the rbcL sequence most similar to C. chlorococcoides SAG 72.81 (), differed from this strain by 21 nucleotides within the ITS2 region (251 alignable positions), comprising four hCBCs ().
Ostravamonas chlorostellata SAG 12.72 and CAUP G 1401, which had identical rbcL sequences and almost identical 18S rDNA sequences, differed by 13 nucleotides within the ITS2 regions (231 alignable positions). The nucleotide substitutions between SAG 12.72 and CAUP G 1401 comprised also three CBCs (1× helix II; 2× helix III; ) and two hCBC (helix III). In the case of O. meslinii SAG 75.81, repeated amplifications of the ITS region failed to provide the full-length ITS2 sequence. We succeeded in sequencing a 63 base pair fragment, which could be annotated as 27 bp of the 5.8S, and 36 bp of the very beginning of the ITS2 region. This short sequence was most similar to ‘Chlamydomonas’ sp. CCAP 11/122 (six nucleotide differences) and O. tenuiincisa CCAP 11/108 (eight nucleotide differences), in agreement with the above described phylogenetic placement based on the 18S rDNA molecular marker. Meanwhile, O. tenuiincisa CCAP 11/108 and O. chlorostellata SAG 12.72 and CAUP G 1401 displayed four CBCs (1× helix II; 3× helix III; ).
Taxonomic proposals
Ostravamonas Barcytė & Hodač, gen. nov.
Description: Vegetative cells ellipsoidal, broadly ellipsoidal, or broadly cylindrical, and occasionally spherical. Two equivalent flagella of variable length. Cell wall robust with a smooth surface. Low but prominent hemispherical, cone-shaped with a round face, or keel-shaped papilla. Two apical contractile vacuoles. Chloroplasts single and radially lobed, or cup-shaped with radial slits and with additional discs. Pyrenoid central, with thylakoids entering its matrix, and surrounded by starch plates and granules. Eyespot elliptical or string-like, and located in the lateral anterior end of the chloroplast, in the second or last third of the anterior end of the cell. Nucleus is anterior to the pyrenoid. The posterior end of the cell often stands away from the protoplast. Cells reproduce asexually by forming two, four or eight biflagellate zoospores. Sexual reproduction unknown.
Identification: The genus is supported by molecular phylogenetic analyses using 18S-ITS1-5.8S-ITS2 rDNA, and rbcL sequence comparisons (including ITS2 secondary structures). The genus can be recognized by the presence of radially lobed chloroplast, additional disconnected chloroplast discs, string-like eyespot or cell wall standing away from the protoplast.
Etymology: The name refers to the City of Ostrava where the genus was rediscovered.
Type species: Ostravamonas chlorostellata (E.A. Flint & H. Ettl) Barcytė & Hodač.
Ostravamonas chlorostellata (E.A. Flint & H. Ettl) Barcytė & Hodač, comb. nov. (–, , )
Basionym: Chlamydomonas chlorostellata E.A. Flint & H. Ettl, 1966, New Zealand Journal of Botany 4: 420.
Holotype: Flint & Ettl (Citation1966), fig. 2.
Epitype (designated here): The authentic SAG 12.72 strain is permanently cryopreserved in a metabolically inactive state in the Culture Collection of Algae at Göttingen University (SAG) in Germany.
Type locality: Tekoa, Southern Alps at Bealey, New Zealand.
Note: Additional strain CAUP G 1401 is housed in the Culture Collection of Algae of Charles University in Prague (CAUP), Czechia.
Ostravamonas meslinii (Bourelly) Barcytė & Hodač, comb. nov. (–, , )
Basionym: Chlamydomonas meslinii Bourrelly, Citation1951, Hydrobiologia 3: 258.
Holotype: Bourelly (Citation1951), pl. 3 fig. 52.
Epitype (designated here): The authentic strain SAG 75.81 is permanently cryopreserved in a metabolically inactive state in the Culture Collection of Algae at Göttingen University (SAG) in Germany.
Type locality: Alpine garden of the National Museum in Paris, France.
Ostravamonas tenuiincisa Barcytė & Hodač, sp. nov.
Description: Vegetative cells unicellular, biflagellate, broadly ellipsoidal to ovoid, with both ends rounded. Two equal flagella approximately the same size as the cell. Anterior papilla low and keel-shaped. Eyespot small, string-like or narrowly ellipsoidal in the lateral anterior end of the chloroplast, in the anterior second or last third of the cell. Chloroplast cup-shaped with radial slits. Central pyrenoid spherical, covered with starch plates. Nucleus anterior to the pyrenoid and two apical contractile vacuoles. Asexual reproduction by forming two to four, or eight zoospores. Sexual reproduction unknown.
Holotype (designated here): The authentic strain CCAP 11/108 is permanently cryopreserved in a metabolically inactive state at the Culture Collection of Algae and Protozoa (CCAP) in Scotland. – show the morphology of the holotype.
Type locality: Unknown.
Etymology: The specific epithet ‘tenuiincisa’ refers to the presence of short radial slits in the cup-shaped chloroplast.
Chloromonas gracillima (H. Ettl) Barcytė & Hodač, comb. & stat. nov. (–, , )
Basionym: Chlamydomonas chlorostellata var. gracillima H. Ett, 1976, Nowa Hedwigia 49: 401.
Holotype: Ettl (Citation1976), pl. 61, fig. 1.
Epitype (designated here): The authentic SAG 25.87 strain is permanently cryopreserved in a metabolically inactive state in the Culture Collection of Algae at Göttingen University (SAG) in Germany.
Type locality: Hrubý Jeseník mountains, Czechia.
Discussion
Morphological comparison and search for distinctive features
The newly isolated O. chlorostellata CAUP G 1401, falling within the Chloromonadinia phylogroup and constituting the novel genus Ostravamonas, exhibited several morphological differences from the monophyletic genus Chloromonas (=core Chloromonas clade; , ), whose members usually possess a cup- or urn-shaped chloroplast with parietal lobes, and generally lack pyrenoids (e.g. Barcytė et al., Citation2018a, Citationb). For example, the morphology of the new Arctic isolate CAUP G 1302 fits well within this circumscription. In addition, the shape of the papilla along with the position and shape of the eyespot hinted at its affiliation with the Chloromonas type species, C. reticulata (Matsuzaki et al., Citation2012). Their conspecificity was also supported by phylogenetic analysis of 18S rRNA gene sequences (), and confirmed by ITS2 secondary structure analysis (). A few exceptions do exist (Matsuzaki et al., Citation2012). For example, the re-examined C. gracillima SAG 25.87 had a parietal and radially lobed chloroplast, and one to two pyrenoids ().
When compared with other taxa, O. chlorostellata CAUP G 1401 morphologically resembled numerous species of Chlamydomonas Ehrenberg sensu lato (Ettl Citation1976, Citation1983). For example, it was similar to C. radiata Deason & Bold, C. actinochloris Deason & Bold, C. augustae var. eupapillata H. Ettl, C. macrostellata J.W.G. Lund, C. gerloffii H. Ettl or C. spinosa H. Ettl in bearing a massive, radially lobed chloroplast with a centrally located pyrenoid. However, C. radiata and C. actinochloris do not bear a papilla, and the nucleus is located posterior to the pyrenoid in the former. C. augustae var. eupapillata and C. spinosa have clearly expressed papillae as large as that of the newly investigated strain CAUP G 1401 of the species O. chlorostellata. However, the papilla of C. augustae var. eupapillata has an upraised keel-shaped face, while C. spinosa has a cone-shaped papilla with an acute face. Chlamydomonas macrostellata and C. gerloffii have much smaller hemispherical papillae. Other morphological comparisons, including shape of the chloroplast, shape and position of the eyespot, position of the pyrenoid and nucleus, and cell size, are summarized in .
Table 2. Morphological comparison of similar Chloromonadinia species.
The most morphologically similar species to the studied O. chlorostellata CAUP G 1401 was Chlamydomonas chlorostellata. The authentic strain (SAG 12.72) of this species was available for comparison (see Results section). Apart from the similar chloroplast outline (–), the two isolates had the same shape of papilla and eyespot and the same type of pyrenoid (, ), and they both shared an empty posterior end of their cells, along with anterior and posterior cell wall thickenings, suggesting conspecificity of the two strains. The molecular phylogenetic results confirmed that the strain SAG 12.72 belongs among the Chloromonadinia, and also is a close relative of the strain CAUP G 1401 (, ). Since Chlamydomonas is polyphyletic, and species in clades other than the clade containing the type species C. reinhardtii should be transfered to other genera (Pröschold et al., Citation2001), we have classified both strains SAG 12.72 and CAUP G 1401 as O. chlorostellata. Moreover, our investigation of the morphologically similar species C. meslinii SAG 75.81 and C. moewusii var. microstigmata CCAP 11/108 confirmed their close phylogenetic relationships to the discussed species, resulting in their accommodation within the genus Ostravamonas as well (, ). Additionally, we have classified the latter strain as the new species, O. tenuiincisa, because our morphological investigation did not match the taxon description to which strain CCAP 11/108 was formerly assigned. For example, C. moewusii var. microstigmata has a cup-shaped chloroplast with a lateral thickening in the (approximately) half of the cell’s height where a pyrenoid is usually nested, and the nucleus is posterior to the pyrenoid (Lund, Citation1947). Furthermore, C. moewusii was shown to be a part of the Moewusinia phylogroup (Nakada et al., Citation2008) and it is reasonable to assume that morphologically similar C. moewusii var. microstigmata could be a part of Moewusinia rather than Chloromonadinia.
Of note, numerous species within the genus Chlamydomonas sensu lato, such as the aforementioned C. radiata, C. actinochloris and C. augustae var. eupapillata have been transferred to the genus Chloromonas (Pröschold et al., Citation2001). They are divided among Chloromonas clade 1 and clade 3 sensu Hoham et al., Citation2002 (, ). Furthermore, the previous (Barcytė et al., Citation2018a, Citationb) and the current 18S rDNA-based phylogenetic analyses demonstrated that the authentic strains of Chlamydomonas gerloffii CCAP 11/72 and C. hydra var. micropapillata SAG 4.73 are part of the core Chloromonas clade, as is the currently re-examined Chloromonas gracillima SAG 25.87. This shows that morphological identification of the genus Chloromonas is not that simple, and that the core Chloromonas clade contains great morphological diversity (). The electron-dense globules surrounding the pyrenoid which we detected only in C. gracillima SAG 25.87 () were also reported for C. chlorococcoides (Matsuzaki et al., Citation2012), supporting the close affiliation of the two taxa (, ).
Several paraphyletic lineages share very similar morphological and ultrastructual characteristics (plesiomorphic features) within the Chloromonadinia. As a consequence, light microscope based identification of the related taxa is not always straightforward. However, the search for morphological and ecological differences is an important complement to phylogenetic species delimitations. For example, O. chlorostellata differs from the species of the Chloromonas clade 3 and Chloromonas augustae by a set of features () and by often having a posterior cell end that stands away from the protoplast (). Meanwhile, O. meslinii has a radial chloroplast with additional discs, which has not been reported for any other species within the Chloromonadinia (). The newly proposed species O. tenuiincisa has a cup-shaped chloroplast with radial slits, resulting in large blunt lobes of the plastid (). In addition, the sister lineage of Ostravamonas, C. pseudoplatyrhyncha NIES-2563, has ovoid to spherical cells with a cup-shaped chloroplast, and multiple atypical pyrenoids, including a large D- or rod-shaped eyespot positioned in the lateral central part of the cell (Matsuzaki et al., Citation2010). Moreover, Chlainomonas, Gloeomonas and Ixipapillifera have a set of unique morphological features, effectively discriminating them from other Chloromonadinia (, ). For example, Gloeomonas has two flagellar bases that are remote from each other (Klebs, Citation1886), Chlainomonas has four flagella and a swollen or gelatinous cell wall separated from the protoplast (Christen, Citation1959), and Ixipapillifera has the X-shaped papilla (Nakada et al., Citation2016).
Since morphology of the volvocalean green algae can be confusing, attempts to use DNA to characterize and match new isolates with historical strains, and re-examination of authentic strains is of particular importance in revising algal taxonomy.
Interpreting Chloromonadinia diversity using conserved and variable molecular markers
The phylogenetic species concept dominates algal taxonomy, with 18S rDNA and rbcL being the most used molecular markers to date. Phylogenetic analyses employing the two markers support the existence of three main Chloromonadinia superclades (, ), which although topologically consistent between markers, sometimes lack statistical support. Smaller monophyletic clades, such as, for example, the core Chloromonas, Gloeomonas or Ixipapillifera are also supported (, ). On the other hand, the sister relationships of the clades are not robust, and might vary using additional sequences or even concatenated data (see discussion in Barcytė et al., Citation2018a).
Delineation of closely related species, especially sister species, using 18S rDNA and rbcL is not straightforward (, ). The ITS2 rDNA, especially its secondary structure analysis, is the most popular tool to help resolve ambiguous sister relationships at the molecular level (e.g. Matsuzaki et al., Citation2012; Wolf et al., Citation2013; Barcytė et al., Citation2018a, Citationb). For instance, ITS2 rDNA secondary structure analysis strongly supports classification of morphologically different Chloromonas reticulata CAUP G 1302 and C. gracillima SAG 25.87 as separate species ().
According to the ITS2/CBC approach, the two Ostravamonas strains CAUP G 1401 and SAG 12.72 should be considered distinct species as well (). However, one nucleotide difference in the 18S rDNA sequences () and identical rbcL sequences (), together with similar morphology, ultrastructure and ecology (acidic habitats; see discussion below) do not support such separation. While ITS2 is useful for investigating closely related species, as discussed above, use of this marker to delineate species without additional supporting data might be tricky. For example, morphologically different C. gracillima SAG 25.87 and C. chlorococcoides SAG 72.81 are well separated by 18S rDNA phylogeny (), closely related by rbcL phylogeny () and the CBC species concept does not support their representation as different species (). Meanwhile, morphologically different O. tenuiincisa and O. chlorostellata are supported as separate species by all molecular markers (, , ).
Direct sequencing of the amplified ITS2 region of the strain CAUP G 1401 identified several ambiguous positions (i.e. single nucleotide polymorphisms) in independent reads, suggesting the presence of several ITS2 paralogues. For example, Stat et al. (Citation2011) showed that the use of ITS2 to delineate different Symbiodinium (Dinophyceae) is problematic due to Symbiodinium carrying multiple copies of ITS2, and displaying intragenomic variability. Divergent intragenomic ITS2 paralogues were also revealed in Heterococcus (Xantophyceae; Rybalka et al., Citation2013) and several Eustigmatophyceae strains (Kryvenda et al., Citation2018). On the other hand, three CBCs have never been reported for the same species as has been found in the O. chlorostellata strains CAUP G 1401 and SAG 12.72. At least one CBC was supported among Chloromonas tughillensis strains (Matsuzaki et al., Citation2014). The fact that O. chlorostellata represents a species covering a large distribution area that is comprised of genetically diverse local populations may offer an explanation for this puzzle. On the other hand, the same is not true for C. reticulata, which occurs in cold environments including the Arctic and Antarctic. Discovery and isolation of other related strains could better characterize the ongoing diversification of the O. chlorostellata lineage.
Identification of the sister lineages O. chlorostellata and O. meslinii SAG 75.81 as a separate taxon is well supported by 18S rDNA and rbcL gene analyses (, ). We repeatedly sequenced both flanking regions of its annotatable 5.8S-ITS2 rDNA fragment, and the resulting high-quality sequences did not match any algae or related organism. Based on that, we conclude that the strain SAG 75.81 contains large intronic or pseudogenous indels within its ITS regions. This may constitute additional evidence supporting the unique phylogenetic placement of this microalga.
In conclusion, the value of conserved molecular markers should not be underestimated. They still provide the first crucial insights into algal taxonomy. The ITS2/CBC concept provides complementary information for species delimitation, but it should not be taken for granted because different algal groups and taxa have different evolutionary histories. More detailed exploration of both ITS1 and ITS2 multi-copy species could improve our understanding of the evolution of Chloromonadinia, and all Volvocales. Moreover, morphology, and especially ecology, are important criteria for circumscribing species and should get more attention in current molecular data based taxonomy papers.
Terrestrial lifestyle within the Chloromonadinia
Members of the Chloromonadinia phylogroup with terrestrial lifestyles are still very little understood. Apart from the studied Chloromonas gracillima SAG 25.87, terrestrial members of the core Chloromonas clade may include C. rosae SAG 51.72 isolated from soil in the High Tatra Mountains (Slovakia). It is also possible that the organism originated in the snowfields on the mountain, considering its close relative, C. reticulata. Meanwhile, C. chlorococcoides strains SAG 12.96 and SAG 72.81 were obtained from soils in Germany and Australia, respectively. ‘Chlamydomonas’ hydra SAG 4.73 was isolated from soil in a beech forest in Czechia, while ‘Cystomonas’ sp. SAG 35.97 was isolated from a branch of a spruce tree in Germany. Chloromonas actinochloris SAG 1.72 and ‘Tetracystis aggregata’ SAG 11-53 were found in dry soils in the USA and Mexico, respectively. Both organisms belong to the mesophilic Chloromonas clade (clade 3). In addition, Ixipapillifera deasonii SAG 46.72 was isolated from soil in the USA ().
Ostravamonas chlorostellata strains CAUP G 1401 and SAG 12.72 were isolated from acidic terrestrial habitats. The pH of the tree bark where the strain CAUP G 1401 was discovered is unknown. However, residues of trees on the Heřmanice spoil heap are acidic enough to accomodate acidophilic algal species (Barcytė et al., Citation2018Citationc). The strain SAG 12.72 was obtained from acidic soil (pH = 5.3) (Flint & Ettl, Citation1966). Since these two strains were isolated from different hemispheres, the genus might have a worldwide distribution, and new isolates of Ostravamonas might be discovered in other acidic habitats. Ostravamonas meslinii is a freshwater species, but since it was isolated from a ditch, we cannot reject the possibility that the alga occurrs in terrestrial habitats as well. Ostravamonas tenuiincisa may also be a terrestrial alga considering its former classification as Chlamydomonas moewusii var. microstigmata, a taxon known from soil samples (Lund, Citation1947).
The low number of terrestrial species and strains is probably a result of limited sampling efforts rather than rarity of such lifestyles among the Chloromonadinia. More detailed studies of Chloromonas-related microalgae could improve understanding of evolution driven by ecological speciation, and colonization of terrestrial environments by Chloromonadinia algae.
Author contributions
D. Barcytė: concept of the paper, isolation and cultivation of strains, light and electron microscopy, molecular lab work, drafting and editing of manuscript; L. Hodač: analyses of molecular data, drafting and editing of manuscript; L. Nedbalová: reading of the draft and financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Altschul, S.F., Madden, T.L., Schäffer, A.A., Zhang, J., Zhang, Z., Miller, W. & Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research, 25: 3389–3402.
- Barcytė, D., Hodač, L. & Nedbalová, L. (2017). Lunachloris lukesovae gen. et sp. nov. (Trebouxiophyceae, Chlorophyta), a novel coccoid green alga isolated from soil in South Bohemia, Czech Republic. European Journal of Phycology, 52: 281–291.
- Barcytė, D., Hodač, L., Nedbalová, L. & Elster, J. (2018a). Chloromonas svalbardensis n. sp. with insights into the phylogroup Chloromonadinia (Chlorophyceae). Journal of Eukaryotic Microbiology, 65: 882–892.
- Barcytė, D., Hodač, L., Nedbalová, L. & Elster, J. (2018b). Chloromonas arctica sp. nov., a psychrotolerant alga from snow in the High Arctic (Chlamydomonadales, Chlorophyta). International Journal of Systematic and Evolutionary Microbiology, 68: 851–859.
- Barcytė, D., Nedbalová, L., Culka, A., Košek, F. & Jehlička, J. (2018c). Burning coal spoil heaps as a new habitat for the extremophilic red alga Galdieria sulphuraria. Fottea, 18: 19–29.
- Bischoff, H.W. & Bold, H.C. (1963). Phycological studies IV. Some soil algae from Enchanted Rock and related algal species. University of Texas Publication, 6318: 1–95.
- Bourrelly, P. (1951). Volvocales rares ou nouvelles. Hydrobiologia, 3: 251–281.
- Christen, H.R. (1959). Flagellaten aus dem Schützenweiher bei Veltheim. Mitteilungen der naturwissenschaftlichen gesellschaft in Winterthur, 29: 167–189.
- Darty, K., Denise, A. & Ponty, Y. (2009). VARNA: interactive drawing and editing of the RNA secondary structure. Bioinformatics, 25: 1974–1975.
- Ettl, H. (1976). Die Gattung Chlamydomonas Ehrenberg. Beihefte zur Nova Hedwigia, 49: 1–1122.
- Ettl, H. (1983). Chlorophyta I. Phytomonadina. In Süßwasserflora von Mitteleuropa (Ettl, H., Gerloff, J., Heynig, H. & Mollenhauer, D., editors). Gustav Fischer Verlag, Jena.
- Flint, E.A. & Ettl, H. (1966). Some new and uncommon Chlamydomonas species from New Zealand. New Zealand Journal of Botany, 4: 418–433.
- Hall, T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41: 95–98.
- Helms, G., Friedl, T., Rambold, G. & Mayrhofer, H. (2001). Identification of photobionts from the lichen family Physciaceae using algal-specific ITS rDNA sequences. Lichenologist, 33: 73–86.
- Hepperle, D. (2004). SeqAssem©. A sequence analysis tool, contig assembler and trace data visualization tool for molecular sequences. Win32-Version. Distributed by the author via: http://www.sequentix.de.
- Hoham, R.W., Bonome, T.A., Martin, C.W. & Leebens-Mack, J.H. (2002). A combined 18S rDNA and rbcL phylogenetic analyses of Chloromonas and Chlamydomonas (Chlorophyceae, Volvocales) emphasizing snow and other cold-temperature habitats. Journal of Phycology, 38: 1051–1064.
- Hoham, R.W. & Mullet, J.E. (1978). Chloromonas nivalis (Chod.) Hoh. & Mull. comb. nov., and additional comments on the snow alga, Scotiella. Phycologia, 17: 106–107.
- Hoham, R.W., Mullet, J.E. & Roemer, S.C. (1983). The life history and ecology of the snow alga Chloromonas polyptera comb. nov. (Chlorophyta, Volvocales). Canadian Journal of Botany, 61: 2416–2429.
- Hoham, R.W., Roemer, S.C. & Mullet, J.E. (1979). The life history and ecology of the snow alga Chloromonas brevispina comb. nov. (Chlorophyta, Volvocales). Phycologia, 18: 55–70.
- Katana, A., Kwiatowski, J., Spalik, K., Zakryś, B., Szalacha, E. & Szymańska, H. (2001). Phylogenetic position of Koliella (Chlorophyta) as inferred from nuclear and chloroplast small subunit rDNA. Journal of Phycology, 37: 443–451.
- Katoh, K. & Toh, H. (2008). Recent developments in the MAFFT multiple sequence alignment program. Briefing in Bioinformatics, 9: 286–298.
- Klebs, G.A. (1886). Über die Organisation der Gallerte bei einigen Algen und Flagellaten. Untersuchungen Botanische Institut Tübingen, 2: 333–417, pls III, IV.
- Koetschan, C., Hackl, T., Müller, T., Wolf, M., Förster, F. & Schultz, J. (2012). ITS2 database IV: interactive taxon sampling for internal transcribed spacer 2 based phylogenies. Molecular Phylogeny and Evolution, 63: 585–588.
- Kryvenda, A., Rybalka, N., Wolf, M. & Friedl, T. (2018). Species distinctions among closely related strains of Eustigmatophyceae (Stramenopiles) emphasizing ITS2 sequence-structure data: Eustigmatos and Vischeria. European Journal of Phycology, 53: 471–491.
- Lund, J.W.G. (1947). Observations on soil algae. III. Species of Chlamydomonas Ehr. in relation to variability within the genus. New Phytologist, 46: 185–194.
- Matsuzaki, R., Hara, Y. & Nozaki, H. (2012). A taxonomic revision of Chloromonas reticulata (Volvocales, Chlorophyceae), the type species of the genus Chloromonas, based on multigene phylogeny and comparative light and electron microscopy. Phycologia, 51: 74–85.
- Matsuzaki, R., Hara, Y. & Nozaki, H. (2014). A taxonomic study of snow Chloromonas species (Volvocales, Chlorophyceae) based on light and electron microscopy and molecular analysis of cultured material. Phycologia, 53: 293–304.
- Matsuzaki, R., Nakada, T., Hara, Y. & Nozaki, H. (2010). Light and electron microscopy and molecular phylogenetic analyses of Chloromonas pseudoplatyrhyncha (Volvocales, Chlorophyceae). Phycological Research, 58: 202–209.
- Matsuzaki, R., Nozaki, H. & Kawachi, M. (2018). Taxonomic revision of Chloromonas nivalis (Volvocales, Chlorophyceae) strains, with the new description of two snow-inhabiting Chloromonas species. PLoS ONE, 13: e0193603.
- Matsuzaki, R., Nozaki, H., Takeuchi, N., Hara, Y. & Kawachi, M. (2019). Taxonomic re-examination of “Chloromonas nivalis (Volvocales, Chlorophyceae) zygotes” from Japan and description of C. muramotoi sp. nov. PLoS ONE, 14: e0210986.
- Nakada, T., Misawa, K. & Nozaki, H. (2008). Molecular systematics of Volvocales (Chlorophyceae, Chlorophyta) based on exhaustive 18S rRNA phylogenetic analyses. Molecular Phylogenetics and Evolution, 48: 281–291.
- Nakada, T., Tomita, M., Wu, J.T. & Nozaki, H. (2016). Taxonomic revision of Chlamydomonas subg. Amphichloris (Volvocales, Chlorophyceae), with resurrection of the genus Dangeardinia and descriptions of Ixipapillifera gen. nov. and Rhysamphichloris gen. nov. Journal of Phycology, 52: 283–304.
- Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25: 1253–1256.
- Pröschold, T., Marin, B., Schlösser, U.G. & Melkonian, M. (2001). Molecular phylogeny and taxonomic revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas Ehrenberg and Chloromonas Gobi, and description of Oogamochlamys gen. nov. and Lobochlamys gen. nov. Protist, 152: 265–300.
- Rambaut, A. (2007). Figtree, a graphical viewer of phylogenetic trees. In http://tree.bio.ed.ac.uk/software/figtree/.
- Remias, D., Schwaiger, S., Aigner, S., Leya, T., Stuppner, H. & Lütz, C. (2012). Characterization of an UV- and VIS-absorbing, purpurogallin-derived secondary pigment new to algae and highly abundant in Mesotaenium berggrenii (Zygnematophyceae, Chlorophyta), an extremophyte living on glaciers. FEMS Microbiology Ecology, 79: 638–648.
- Reuter, J.S. & Mathews, D.H. (2010). RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinformatics, 11: 129.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Rybalka, N., Wolf, M., Andersen, R.A. & Friedl, T. (2013). Congruence of chloroplast- and nuclear-encoded DNA sequence variations used to assess species boundaries in the soil microalga Heterococcus (Stramenopiles, Xanthophyceae). BMC Evolutionary Biology, 13: 39.
- Seibel, P.N., Müller, T., Dandekar, T. & Wolf, M. (2008). Synchronous visual analysis and editing of RNA sequence and secondary structure alignments using 4SALE. BMC Research Notes, 1: 91.
- Stamatakis, A., Hoover, P. & Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology, 57: 758–771.
- Stat, M., Bird, C.E., Pochon, X., Chasqui, L., Chauka, L.J., Concepcion, G.T., Logan, D., Takabayashi, M., Toonen, R.J. & Gates, R.D. (2011). Variation in Symbiodinium ITS2 sequence assemblages among coral colonies. PLoS ONE, 6: e15854.
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30: 2725–2729.
- White, T.J., Bruns, T., Lee, S. & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols: A Guide to Methods and Applications (Innis, N., Gelfand, D., Sninsky, J. & White, T., editors), 315–322. Academic Press, New York.
- Wolf, M., Chen, S., Song, J., Ankenbrand, M. & Müller, T. (2013). Compensatory base changes in ITS2 secondary structures correlate with the biological species concept despite intragenomic variability in ITS2 sequences – a proof of concept. PLoS ONE, 8: e66726.
- Zwickl, D.J. (2006). Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD dissertation, The University of Texas at Austin.
