?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Floating algae can be found in high abundances at mid and high latitudes, their prolonged positive buoyancy allowing long-distance dispersal. However, despite their importance to dispersal and ecological and evolutionary meta-population dynamics, little is known about the buoyancy responses of high latitude algae to the conditions at the sea surface. Indeed, even at 60°N environmental conditions during spring/summer can be challenging, and may cause the demise and sinking of floating algae. The bladderwrack Fucus vesiculosus from the Northern Baltic Sea floats on the sea surface when detached from the benthic substratum. We conducted a field experiment with tethered individuals during their reproductive period to measure variation in floating time and how that is related to morphological traits such as occurrence of vesicles and/or receptacles, and to measure growth and photosynthesis while afloat. Algal individuals with receptacles tended to sink quickly, whereas the longest floating time was evident for individuals carrying vesicles but lacking receptacles. While afloat, all individuals grew in size, showed photosynthetic acclimation to sea surface conditions and had a few invertebrates associated with them. Our results showed that rafts of F. vesiculosus were physiologically viable until their day of sinking and that morphological traits such as the occurrence of vesicles and receptacles modified their floating time. Accordingly, floating algae with a similar morphological set-up, and thus also reproductive phenology, to F. vesiculosus can have a high floating persistence, but, depending on their reproductive structures, they may mostly serve as long-range dispersal vehicles for associated organisms.
Introduction
Macroalgae with positively buoyant features such as gas-filled vesicles or honeycomb structures (Rothäusler et al., Citation2015 for Fucus vesiculosus L.; Tala et al., Citation2013 for Durvillaea antarctica) can float on the sea surface when they become detached from the benthic substratum and remain buoyant for weeks or even months (Hobday, Citation2000a; Vandendriessche et al., Citation2007; Yatsuya, Citation2008; Graiff et al., Citation2013, Citation2016; Tala et al., Citation2019). Prolonged buoyancy provides the potential to float for long distances (e.g. Fraser et al., Citation2009; Rothäusler et al., Citation2015; van Hees et al., Citation2018) and these long-distance dispersers can contribute to population connectivity and to the (re-) colonization of new habitats (Muhlin et al., Citation2008; Coyer et al., Citation2011). Therefore, dispersal ability and range primarily depend on the abundance of floating individuals and their persistence time on the sea surface. There is also evidence that these algae can travel considerable distances at the mercy of currents and winds, and have the potential to release gametes or spores at one or more new and suitable sites, such as has been shown for the subantarctic regions where populations of D. antarctica and Macrocystis pyrifera are connected between distant islands or land masses, driven by the West Wind Drift (Fraser et al., Citation2009, Citation2010; Macaya & Zuccarello, Citation2010).
At high latitudes in the Baltic Sea, F. vesiculosus is the principal foundation species covering large areas of the shallow rocky subtidal. Consequently, floating thalli of F. vesiculosus are highly abundant between the Finnish and the Swedish Archipelagoes (Rothäusler et al., Citation2015) and most probably around the whole Baltic Sea. Rafts of F. vesiculosus have also been described from the North Atlantic and adjacent seas (Tully & Ó Céidigh, Citation1986; Ingólfsson, Citation1998; Ólafsson et al., Citation2001; Vandendriessche et al., Citation2006; Khalaman & Berger, Citation2006; Muhlin et al., Citation2008; Thiel et al., Citation2011).
Fucus vesiculosus is known for its gas-filled vesicles, which are found in pairs on the algae. When individuals get older they break and leave the vesicles without gas, therefore, after a couple of years, adult specimens have vesicles only towards their younger end (Rothäusler, personal observation). These ends, also known as the vegetative apical tips, are where the meristem is located and they either transform into reproductive structures or they remain vegetative. Only the vegetative tips keep forming vesicles.
In the Baltic Sea, in addition to gas-filled vesicles, F. vesiculosus possess enlarged air pockets in their reproductive apices (hereafter receptacles). These may provide additional buoyancy during the reproductive season in spring/summer in comparison with Atlantic F. vesiculosus which lack such air-filled receptacles (Bäck et al., Citation1993). After releasing gametes, receptacles disintegrate (Rothäusler, personal observation) and thus the algal rafts may lose their buoyancy if that is based solely on receptacles. Therefore, it seems likely that different morphological traits, such as the occurrence of receptacles and vesicles, can influence the floating time of detached F. vesiculosus.
Once dislodged, algae begin their journey via surface winds and currents and have to withstand the prevailing higher solar radiation and temperature at the sea surface, which requires an efficient physiological acclimation (Rothäusler et al., Citation2009 for Macrocystis pyrifera; Graiff et al., Citation2016 for Durvillaea antarctica; van Hees et al., Citation2018 for Sargassum spinuligerum). Acclimatization processes, such as pigment adjustment, dynamic photoinhibition and/or repair mechanisms, enable floating individuals to retain growth and reproductive ability, as well as to persist and disperse before sinking (Rothäusler et al., Citation2011a, Citation2018a; Graiff et al., Citation2013, Citation2016; Tala et al., Citation2013; van Hees et al., Citation2018). Photosynthetic performance, which largely determines the ability for these acclimation processes, has been found to be highly variable among individuals in benthic fucoid species (Johannesson et al., Citation2012; Rothäusler et al., Citation2016). This implies that floating duration may vary because some individuals are better adapted to the conditions on the sea-surface than others and thus persist afloat for longer.
While the persistence of floating algae in terms of physiology and growth has been relatively well studied at mid latitudes in both hemispheres (Hobday, Citation2000a; Yatsuya, Citation2008; Rothäusler et al., Citation2009, Citation2011a, Citationb, Citationc, Citation2018a; Graiff et al., Citation2013, Citation2016; Tala et al., Citation2013), corresponding data at high latitudes are scarce (Tala et al., Citation2016, Citation2019). At high latitudes the overall modest surface conditions in spring/summer (low irradiance, temperature and epibiosis), in comparison with mid and low latitudes, may favour the survival of floating algae (Ingólfsson, Citation1998; Rothäusler et al., Citation2009, Citation2011b, Citation2018a; Graiff et al., Citation2013, Citation2016; Tala et al., Citation2016). Several studies document successful dispersal of macroalgae (e.g. Fraser et al., Citation2009; Macaya & Zuccarello, Citation2010; Olsen et al., Citation2010), thus, attesting to facilitation of long-distance dispersal under benign climate conditions. However, these studies provide little information about the functional traits of the algae, such as morphology and photosynthetic acclimation potential, that allow persistent buoyancy.
Rafting, whereby other organisms hitchhike on buoyant objects such as algae, can affect their floating persistence (Vandendriessche et al., Citation2007; Rothäusler et al., Citation2009, Citation2011b) and hence reduce their dispersal distances. Herbivorous hitchhikers (e.g. amphipods and isopods) actively feed on their rafts, which at increasing temperatures and densities can reduce photosynthetic thalli (Vandendriessche et al., Citation2007; Rothäusler et al., Citation2009). At the same time epibionts (e.g. non-buoyant algae, bryozoans and barnacles) can successively cover their floating host, which may suppress algal photosynthesis (Oswald et al., Citation1984; Rothäusler et al., Citation2011c), decrease buoyancy and ultimately cause sinking (Graiff et al., Citation2016).
Although the potential to persist at the sea surface is a key factor for the dispersal of macroalgae, our understanding of the factors behind variation in the floating duration of algal individuals is in its infancy. Furthermore, climate change, with increasing sea surface temperatures (Lehmann et al., Citation2011) and intensifying solar radiation (Wild Citation2005), may further challenge the floating dispersal ability of high-latitude macroalgae, increasing the need for understanding of their functional traits such as morphology (vesicles and receptacles) and photosynthetic acclimation potential. Therefore, we conducted a field experiment with detached F. vesiculosus during its reproductive season. We hypothesized that (1) floating time differs among individuals, depending on morphological traits such as the presence of air-filled vesicles and receptacles and (2) the individuals able to maintain their photosynthetic performance and growth while floating will persist afloat for longer than those less able to acclimate to sea surface conditions. Further, we quantified the accumulation of invertebrates on macroalgae with floating duration.
Materials and methods
Collection of algal material
We collected adult individuals of F. vesiculosus on 2 June 2014 by snorkelling within a continuous algal belt in the south-western Archipelago Sea (60°08′N, 22°17′E), Finland. A total of 14 mature individuals with their disc-shaped holdfast were detached at 5 m intervals at ~1.5 m depth, stored in buckets filled with seawater and brought to the Archipelago Research Institute of the University of Turku at the island of Seili (60°14′N, 21°57′E). They were kept overnight in flow-through seawater tanks before being measured and tethered in the field. Algae were prepared for experimentation by rinsing them carefully with seawater in order to remove associated grazers and epiphytes.
The 14 adult individuals varied in shape and biomass (mean ± SD, 175 ± 76 g) thus representing the natural morphological variation of the sampled population. However, all individuals were reproductive (mean ± SD, 166 ± 14) and presented vegetative meristems (109 ± 18), but four individuals did not carry vesicles (n = 10, 32 ± 6). We split each individual into three approximately equal-sized thalli without a holdfast (mean ± SD, largest thallus 97 ± 13 g; smallest thallus 23 ± 5 g). After cutting, each of the replicated thalli consisted of several apical tips with meristems, but not all of them had receptacles and/or vesicles. Hereafter, the mean of the three thalli from the same individual represents one genetic individual. Throughout the manuscript, when referring to one thallus it represents one replicate of the genetic individual. During the course of our experiment, no new receptacles were formed.
Experimental design
The experiment was set up close to the island of Seili, ~300 m from the shore of the nearest island. This region is characterized by skerry landscapes, consisting of many small islands. Benthic F. vesiculosus inhabits the waters around these islands from ~1 to 4 m depth. Via snorkelling we built up, side by side, a total of three buoyant long-lines (25 m in length) in ~5 m depth. At their ends, we anchored these long-lines to the sea bottom.
Each of the 42 thalli received a unique identification tag attached to the base of the stipe and a floating cord of 50 cm length. In order to avoid physical damage to the stipe, we surrounded it with a rubber hose that was fixed with cable ties to the cord. Then we distributed the thalli over the three experimental lines, so that one thallus from each genetic individual (n = 14) was represented on each line and spaced 1.5 m apart, allowing them to float freely on the sea surface.
We checked the 42 thalli every third day to monitor their date of sinking. For logistical reasons, after six weeks of experimentation, floating thalli were checked only once a week. We considered the thalli as sunken when they were completely submerged and no part remained above the sea surface. Four thalli were lost from the experimental lines during our experiment.
Sea surface conditions: solar radiation, UV and temperature
Sea surface light intensity (µmol photons m–2 s–1) and water temperature (°C) were monitored every 20 min during the course of experimentation using HOBO (Pendant temp/light, Onset, USA) light and temperature loggers. A total of six loggers, two on each of the buoyant experimental lines were attached at 30 cm below the sea surface. In addition, UV-index data were measured by the Seili weather station. Light intensity and UV-index were presented as average monthly midday values, ranging from 11:00 to 14:00, while water temperature was calculated as monthly average over the duration of the experiment ().
Table 1. Environmental conditions of surface water temperature, solar radiation and UV-index during the course of experimentation in 2014.
During experimentation (June to September) we found a progression in sea surface temperature, with the lowest value at the start of the experiment in June (mean ± SD, 14.4 ± 1.3°C), reaching a peak in August (21.0 ± 1.9°C) and a decline in September (18.1 ± 0.4°C). Our irradiance data, such as solar radiation and UV-index, showed highest values in June and July ().
Measurements of Fucus vesiculosus
Photosynthetic performance values, such as ETRmax, Ek and alpha (αETR), were determined from all 14 genetic individuals at the beginning of experimentation (day 0, initial) before they were split, and again at their observed day of sinking (final) from each replicated thallus. Morphological features such as the initial number of vesicles, number of receptacles, number of meristems, length of thalli (mm) as well as their wet biomass (g) were measured after their splitting in the beginning, and at their day of sinking. We calculated growth rate while floating, in terms of biomass, meristems and length, as follows: (final size−initial size)/floating time (days).
We measured the ETRmax, Ek and alpha from apical meristematic tips via photosynthetic versus light intensity (P-I) curves. This was done in vivo using a computer-aided portable pulse amplitude-modulated fluorometer (PAM 2000, Walz, Effeltrich, Germany). Three samples of the respective tissue were put separately into test tubes that were wrapped with aluminium foil and irradiated individually with increasing intensities of photosynthetic active radiation (PAR: 0–500 µmol photons m–2 s–1), which was provided by a light-emitting-diode lamp of the PAM device (Schreiber et al., Citation1995). The ETR was estimated by relating the effective quantum yield (PSII) and the intensity of the radiation as described in Rothäusler et al. (Citation2011b). The hyperbolic tangent model of Jassby & Platt (Citation1976) was fitted to each data set as follows:
where ETRmax (µmol e– m–2 s–1) is the maximal electron transport rate; tan h is the hyperbolic tangent function; αETR (µmol e– m–2 s–1 [µmol photons m–2 s–1]) is the initial slope of the P-I curve and stands for the electron transport efficiency, and I is the photon fluence rate of PAR. The saturation irradiance for electron transport Ek (µmol m–2 s–1) was also calculated as the intercept between α, and the ETRmax values (ETRmax/αETR). The mean value of the three replicated measurements represented the photosynthetic parameter of one thallus, while the grand mean of the three thalli represented one genetic individual.
Associated organisms
Before each sunken algal thallus was collected, we enclosed it carefully in a mesh bag (1 mm mesh size) in order to retain the mobile invertebrates that colonized it while afloat. The mesh bags were brought to the laboratory at Seili and the associated invertebrates dislodged from each individual thallus, along with those clinging to the thallus, were counted and identified.
Statistical analysis
To test whether different genetic individuals of F. vesiculosus (random factor) had different growth rates (measured as number of meristems, length and biomass in respect to their days afloat) and varied among experimental lines (random factor), we applied Generalized Linear Mixed Models (GLMM) implemented with the R package ‘lme4’ (Bates et al., Citation2014). The same models were used for the physiological responses of ETRmax, Ek and αETR at the end of the floating time.
Within all GLMM analyses, we calculated the amount of variation due to random factors and tested the significance of them and their interactions with the likelihood-ratio test between the models with and without the random factors with the R package ‘lmtest’ (Zeileis & Hothorn, Citation2002), and then simplified the model by excluding the non-significant effects, with the aid of the Akaike Information Criterion (AIC). The error variance distribution of the response variables was Gaussian, which was checked by visual inspection of the residual plot. We derived the individual estimates for each independent variable from the model as a best linear unbiased predictor (BLUP; Littell et al., Citation2006). In addition, we tested whether being afloat affected the physiological responses of the same genetic individual with repeated-measures ANOVA (Proc GLM). We included in this analysis the initial and the final photosynthetic measurements. This last analysis was done using SAS 9.4 (SAS Institute Inc., Citation2014).
We conducted structural equation modelling (SEM) to construct three plausible models to determine whether morphological (initial wet biomass, initial number of receptacles, initial presence of vesicles) and physiological traits (initial ETRmax, growth rate in terms of biomass) were significant predictors of floating time (dependent variable). In model I all observed variables were included, and initial biomass and ETRmax were found not significant. In model II, initial biomass was excluded and ETRmax was still not significant. By deleting the two non-significant observed variables (initial biomass and ETRmax) we found the best model fit in model III, which was guided by the goodness of fit as suggested by Kline (Citation2015). Further, we fed model I, allowing all possible correlations among all observed variables, and in the final model III we kept only the significant correlations between initial number of receptacles and initial presence of vesicles. For all models, we estimated standardized path coefficients using a maximum likelihood method. Goodness of fit was evaluated through Root Mean Square Error of Approximation (RMSEA), Comparative Fit Index (CFI) and Akaike Information Criterion (AIC). Genetic individuals and experimental lines were not included because they were not significant in the previous analyses. The Structural Equation Modelling, SEM, was analysed by using the R package Lavaan (Rosseel, Citation2012).
Table 2. Variance components (%) due to the random effects of genetic individual and experimental line with their statistical significance in physiological responses (ETRmax, Ek and αETR) of Fucus vesiculosus at the end of the floating period.
In order to visualize the variation of observed floating duration with the predicted values by the factors found important in SEM, we further conducted a multiple regression analysis of floating duration on the number of receptacles, number of vesicles and growth rate. The SEM and multiple regression approaches gave very similar results. We show the result as a SEM path diagram because SEM allows explicit hypotheses about causalities and covariance structures.
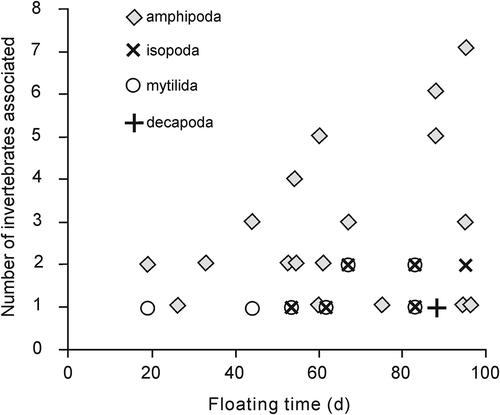
To test whether there was a relationship between the floating time of F. vesiculosus and its associated hitchhikers, we performed Pearson correlations between floating time and the total number of invertebrates, then separately between each group of invertebrates (amphipods, isopods and mytilids) and floating time. These analyses were run with the R-package stats v3.5.1 (R Core Team, Citation2013).
Results
Fucus floating time
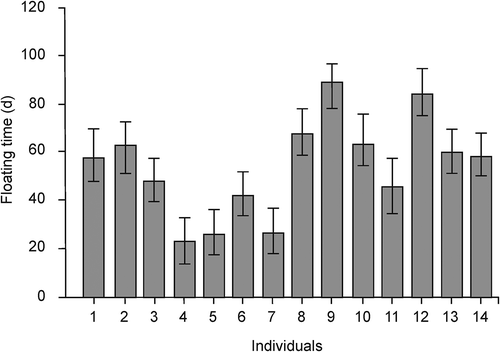
Genetic individuals significantly differed in their floating time at the sea surface during spring/summer (variance component due to individual 61.4%, χ2 = 14.8, p < 0.001), ranging from 23 ± 9 to 88 ± 9 days (mean ± SE) (). The shortest floating time of a single thallus was 12 days while the longest was 96 days. Floating time did not vary among the experimental lines (variance component due to line 0%).
Fig. 1. Floating time (days) of different genetic individuals of Fucus vesiculosus (n = 14) tethered in their native habitat. Data show genetic individual estimates (mean ± SE, based on n = 3 thalli). Due to the loss of four thalli, the mean of the genetic individuals 1, 7, 8 and 10 are based on n = 2 thalli.
Fucus growth and photosynthesis
While afloat, all genetic individuals grew new meristems (mean ± SE, 1.2 ± 0.4), and increased in length (5.4 ± 1.6 mm) and in wet biomass (0.4 ± 0.2 g). None of the three measured growth rates differed among genetic individuals (variance component due to individuals (%); meristems: 17.2%, χ2 = 2.07, p = 0.07; length: 3%, χ2 = 2.32, p = 0.13; biomass: 3%, χ2 = 1.98, p = 0.16) or experimental lines (variance component due to lines (%); meristems: 9.5%, χ2 = 1.88, p = 0.09; length: 0%; biomass: 0%).
Both ETRmax (start: 98.7 ± 3.2; end: 72.0 ± 5.4) and αETR (start: 0.59 ± 0.01; end: 0.38 ± 0.01) of genetic individuals declined (repeated-measures ANOVA, F(1, 36) = 15.8, p < 0.001, F(1, 36) = 83.9, p < 0.001, respectively) by 27% and 36%, respectively, from the start to the end of floating. No such changes were evident for Ek (start: 172.0 ± 7.2; end: 165.7 ± 6.9: F (1, 36) = 0.43; p = 0.51).
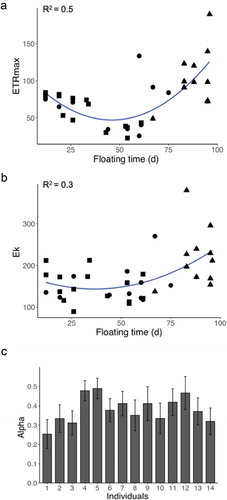
ETRmax and Ek at the end of floating did not vary among genetic individuals (mean ± SE, 73.1 ± 6.0 and 134.2 ± 24.9 µmol photons m–2 s–1, respectively) or experimental lines (, , respectively). Only final αETR significantly varied among genetic individuals with values ranging from 0.3 ± 0.06 to 0.5 ± 0.05 (, ).
Fig. 2. Scatter plots showing the relationship between floating time and final ETRmax (a) as well as floating time and final Ek (b) of all tethered thalli recovered at the end of experimentation (n = 38, four were lost). Different symbols show the n = 38 thalli with different morphologies. Dots = receptacle and vesicle carriers; triangles = only vesicle carriers; and squares = only receptacle carriers. R2 values for the fit of the polynomial regressions are displayed. Bar plot (c) shows the genetic individual estimates (mean of the n = 3 thalli ± SE, n = 14) for the final αETR.
Morphological and physiological traits as predictors of floating time
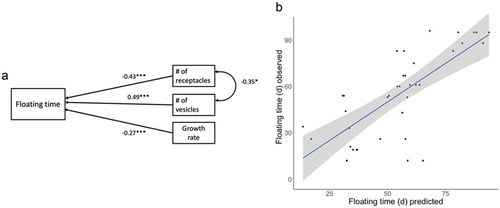
The SEM analysis for model III, which was the best-fitting model, suggested that the floating duration of F. vesiculosus was strongly affected by morphological traits (, ). The strongest negative effect on floating time was caused by the number of receptacles, which was followed by growth rate (biomass); both significantly reduced algal floating times. On the contrary, the presence of gas-filled vesicles had a positive effect on floating time. Finally, genetic individuals with more receptacles carried fewer vesicles (SEM correlation coefficient = −0.35, p < 0.05).
Table 3. Results from the structural equation models.
Fig. 3. Path model III (see ) showing the best fit. (a) Standardized path coefficients are provided for each one-headed arrow, and correlation coefficient for the two-headed one. The asterisk indicates significant regression weights. The curved arrow indicates significant correlation among the observed variables. (b) Multiple regression scatter plot, where the floating time is plotted against the predicted values. The latter ones are generated by a multiple regression model including the three independent variables: # of receptacles (β ± SE: –0.26 ± 0.8, p < 0.01), # of vesicles (1.21 ± 0.32, p < 0.001), and growth rate (−1.74 ± 0.84, p < 0.05), with an R2 of 0.53. The grey area surrounding the regression line represents the confidence interval.
Associated invertebrates
Mobile invertebrates colonized floating F. vesiculosus during experimentation (). In total, 24 thalli carried invertebrates at their date of sinking. There was no relationship between floating time and the total number of invertebrates (r = 0.2, df = 22, p = 0.3) nor for each invertebrate group such as amphipods (r = 0.20, df = 22, p = 0.23), isopods (r = 0.22, df = 22, p = 0.29) and mytilid mussels (r = −0.33, df = 22, p = 0.12). No correlation was run for the decapods, because only one thallus that stayed afloat for 88 days carried a juvenile Rhithropanopeus harrisii. There was a general trend that with floating time the hitchhiking invertebrate community became more variable and diverse, with several thalli having abundant amphipods ().
Discussion
The floating time of F. vesiculosus under spring/summer field conditions ranged on average from 3 weeks to 3 months, and varied among genetic individuals. This variation in buoyancy appears to be largely explained by morphological traits such as the number of receptacles and the presence of vesicles, because all individuals acclimated well to the new surface conditions. Hence our study provides evidence that the composition of buoyancy devices and reproductive structures of an algal individual determine their floating time and thus dispersal potential.
Floating time and morphological traits
Here, we showed evidence that both the number of vesicles (enhance buoyancy) and receptacles (reduce buoyancy when degrading) played a role in determining the floating time of F. vesiculosus. First, the SEM path coefficients and the multiple regression coefficients indicated that when one of them is kept constant the other has a significant effect. Second, when thalli had only vesicles the floating duration was almost always longer (75 to 90 days) than when they carried both vesicles and receptacles (20 to 75 days), and always longer than when they carried only receptacles (20 to 50 days). Similar high floating times have been reported for Chilean Durvillaea antarctica floating at 50°S in summer (Tala et al., Citation2019) and for fragments of North Sea F. vesiculosus (50°N) when kept in an indoor microcosm (Vandendriessche et al., Citation2007). While the buoyancy of D. antarctica decreased as soon as the honeycomb structure inside their thalli started to disintegrate (Graiff et al., Citation2013; Tala et al., Citation2016), we showed that for F. vesiculosus the amounts of receptacles and vesicles jointly determined their floating time.
Genetic individuals with more receptacles carried fewer vesicles and sank earlier. This is because reproductive branches of F. vesiculosus stop growing, and thus also stop forming vesicles. Although all genetic individuals were collected at the same site and at the same time, variation in the receptacle maturation phenology probably influenced the floating time. In fact, some receptacles may already have started the release of gametes when collected in June, while others carried immature receptacles. For several Sargassum species, floating ability decreases with the progressing reproductive stage because the water content of thalli increases due to degradation processes (Yatsuya, Citation2008). Similarly, after F. vesiculosus releases its gametes, receptacles start to disintegrate and become filled with water (Rothäusler, personal observation), causing an increase in biomass. Thus receptacle maturation and subsequent disintegration during the experiment decreased buoyancy and the more receptacles the genetic individuals carried the more prone to sinking they were, despite the presence of vesicles.
In a former study, using a biophysical particle tracking model with flow fields from an ocean circulation model, we showed that Baltic Sea F. vesiculosus with a floating time of 20 days had a mean floating distance of 50 km, whereas a floating time of 100 days increased the mean floating distance to > 200 km (Rothäusler et al., Citation2015). This implies that genetic individuals with receptacles and/or few vesicles (20 to 50 days afloat) disperse over more limited distances than those without receptacles (> 75 days afloat), but on the other hand their chance to release viable gametes when arriving at a new and suitable site might be higher and thus also their fertilization success with a resident bladderwrack. We did not check for the viability of gametes from our floating algae, but rafted fucoid seaweeds from New Zealand and Australia released viable propagules for up to 60 days (Hawes, Citation2008; McKenzie & Bellgrove, Citation2008).
Aggregations of macroalgae as a consequence of entanglement can increase the size of algal patches significantly (e.g. Hinojosa et al., Citation2010) and may disperse low persistence thalli over larger distances, which could be the case here. However, higher frequencies of bigger sized F. vesiculosus items have been only observed in summer, but aggregations were very small (60–150; Rothäusler et al., Citation2015) in comparison with patches of M. pyrifera and D. antarctica (1–4 m in diameter; Hinojosa et al., Citation2010). Hence, these small aggregations with a high load of mature receptacles in summer probably enhance their sinking rather than provide extra buoyancy.
Our findings imply that the floating time of F. vesiculosus, and thus the probability of long-distance dispersal, is probably much longer in seasons when floating individuals are without mature receptacles, which is either in early spring or autumn/winter. During spring, when receptacles are still growing, a long floating time may provide the opportunity for long-range gamete dispersal, but only when receptacle maturation takes place at the new site of arrival e.g. when rafted thalli entangle with benthic conspecifics. In autumn/winter, a higher floating persistence may instead facilitate the dispersal of algal-associated organisms. The strong seasonal variation in weather conditions in the north-eastern Baltic Sea regions, with higher storm probabilities in April/May and again in August/September (Soomere & Räämet, Citation2011), supports the idea of larger travel distances in spring and autumn due to storm-induced winds that can improve floating dispersal.
Physiological performance and associated organisms
On their days of sinking, all genetic individuals responded with lower ETRmax and αETR than at the beginning of their floating (decreased by 27% and 36%, respectively), implying acclimation to new surface conditions while afloat instead of declining physiological functioning. This down-regulation of PSII from the benthic to the floating state is known to protect the photosynthetic apparatus against the prevailing irradiance conditions and has been observed previously for M. pyrifera and D. antarctica (Rothäusler et al., Citation2011a, Citationb, Citationc; Graiff et al., Citation2013; Tala et al., Citation2019). Also the fact that similar low ETRmax values were detected for benthic F. vesiculosus during spring/summer (Ekelund et al., Citation2008; Rothäusler et al., Citation2016), supports our idea of a high acclimation potential and does not indicate deterioration. Moreover, F. vesiculosus continued to grow, as was shown for floating M. pyrifera (Rothäusler et al., Citation2009, Citation2011a, Citationb, Citationc), underscoring the fact that algae stayed physiologically viable.
Rafted genetic individuals responded with varying photosynthetic efficiencies (αETR), implying that they converted the received surface radiation into chemical energy to a different degree. Possibly, these varying responses were generated because some genetic individuals possessed different Chl a content, light harvesting complexes, or their rate of carbon fixation by Rubisco differed.
In the Northern Baltic Sea, benthic F. vesiculosus experience considerable seasonal variations in temperature and light climates (e.g. Lehvo et al., Citation2001), with ice and darkness in autumn/winter and warm water temperatures and high irradiances in spring/summer, accompanied by an extension of daylight hours. Therefore, we suggest that the surface conditions experienced were within the tolerance range of the species, because the algae adjusted their photosynthesis and even continued to grow until sinking. Additionally, we did not observe tissue softness, breakage or fragmentation of thalli, which would indicate deterioration.
Over time, floating macroalgae often become successively inhabited by mesograzers (e.g. isopods and amphipods) (e.g. Ingólfsson, Citation1998; Hobday, Citation2000b; Vandendriessche et al., Citation2006), which at high densities can contribute, via grazing, to the loss of photosynthetic tissues, thereby suppressing algal growth (Rothäusler et al., Citation2011c, Citation2018a). Until sinking, our experimental thalli were colonized by few organisms such as amphipods, isopods, bivalves and decapods. The reason for this could be that at high latitudes in the Baltic Sea, benthic F. vesiculosus individuals are inhabited by relatively low abundances of mobile invertebrates during spring (e.g. Jormalainen et al., Citation2016). Mobile invertebrates can also easily leave the floating thallus (Miranda & Thiel, Citation2008; Gutow et al., Citation2009). Thus, it is unlikely that the mobile invertebrates found herein affected the floating time. Similarly, Ascophyllum nodosum fronds tethered at 64°N were colonized by benthic and pelagic organisms but they did not cause their sinking (Ingólfsson, Citation1998).
During the course of our experiment, we observed natural floating F. vesiculosus rafts entangled in our experimental thalli. If these natural rafts were carrying organisms, they probably contributed to the colonization of our thalli. Overall, at high latitudes, the acclimation to spring/summer surface-water conditions, together with the morphological traits enhancing buoyancy, indicate that floating F. vesiculosus individuals serve to disperse associated organisms.
Implications
Fucus vesiculosus is one of the dominant floating macroalgae in the world’s oceans (Thiel & Gutow, Citation2005; Rothäusler et al., Citation2012) and is commonly found floating in the northern N Atlantic > 45°N (Tully & Ó Céidigh, Citation1986; Ingólfsson, Citation1998; Vandendriessche et al., Citation2006) and also in the Baltic Sea > 57°N (Pereyra et al., Citation2013; Rothäusler et al., Citation2015). Their non-receptacle carrying thalli, with an extended floating time of > 75 days, can travel > 200 km in comparison to reproductive ones (20 to 50 days, travel distance of 50 km) (Rothäusler et al., Citation2015), and therefore have particularly good potential for long-distance dispersal. This suggests that the floating dispersal prior (autumn/winter) to or at the start of (spring) of the reproduction period is likely to be most effective for F. vesiculosus gene flow over long distances.
In the face of global change, understanding the physiology and the reproductive phenology of floating macroalgae is important in order to predict their floating time, long-distance dispersal and thus potential population connectivity over vast distances. Recently it was shown that for benthic F. vesiculosus, ongoing climate change such as the combined effect of warming and hyposalinity (Meier & Eilola, Citation2011) hampered receptacle formation (Rothäusler et al., Citation2018b), and that hyposalinity strongly affected sperm viability and thus also subsequent fertilization success (Rothäusler et al., Citation2019). This negative effect on the reproductive phenology of benthic populations also influences indirectly the supply of floating individuals.
Certainly, global change is already compromising the persistence and reproduction of floating macroalgae, and consequently reducing the distance and frequencies of dispersal, affecting not only the dispersal processes of the macroalgae themselves but also of their associated hitchhikers (Macreadie et al., Citation2011). Future studies are needed in order to determine if different species and individuals of rafted macroalgae vary in their floating persistence and if they can release viable propagules under climate change. This will help to evaluate their rafting implications in a changing world.
Author contributions
E. Rothäusler, V. Jormalainen: original concept, drafting and editing manuscript, set-up of experiment and sampling; L. Rugiu: statistical analyses, set-up of experiment and sampling; T. Tiihonen: set-up of experiment and sampling.
Acknowledgements
We are grateful to Joakim Sjöroos and Juho Yli-Rosti in helping with the set-up of the experiment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bäck, S., Collins, J.C. & Russell, G. (1993). Comparative reproductive biology of the Gulf of Finland and the Irish Sea Fucus vesiculosus L. Sarsia, 78: 265–272.
- Bates, D., Mächler, M., Bolker, B. & Walker, S. (2014). Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823.
- Coyer, J.A., Hoarau, G., Van Schaik, J., Luijckx, P. & Olsen, J.L. (2011). Trans-Pacific and trans-Arctic pathways of the intertidal macroalga Fucus distichus L. reveal multiple glacial refugia and colonizations from the North Pacific to the North Atlantic. Journal of Biogeography, 38: 756–771.
- Ekelund, N.G.A., Nygård, C.A., Nordström R. & Gylle, M.A. (2008). In situ study of relative electron transport rates in the marine macroalga Fucus vesiculosus in the Baltic Sea at different depths and times of year. Journal of Applied Phycology, 20: 751–756.
- Fraser, C.I., Nikula, R., Spencer, H.G. & Waters, J.M. (2009). Kelp genes reveal effects of subantarctic sea ice during the Last Glacial Maximum. Proceedings of the National Academy of Sciences USA, 106: 3249–3253.
- Fraser, C.I., Thiel, M., Spencer, H.G. & Waters, J.M. (2010). Contemporary habitat discontinuity and historic glacial ice drive genetic divergence in Chilean kelp. BMC Evolutionary Biology, 10: 203–214.
- Graiff, A., Karsten, U., Meyer, S., Pfender, D., Tala, F. & Thiel, M. (2013). Seasonal variation in floating persistence of detached Durvillaea antarctica (Chamisso) Hariot thalli. Botanica Marina, 56: 3–14.
- Graiff, A., Pantoja, J.F., Tala, F. & Thiel, M. (2016). Epibiont load causes sinking of viable kelp rafts: seasonal variation in floating persistence of giant kelp Macrocystis pyrifera. Marine Biology, 163: 191.
- Gutow, L., Giménez, L., Boos, K. & Saborowski, R. (2009). Rapid changes in the epifaunal community after detachment of buoyant benthic macroalgae. Journal of the Marine Biological Association of the United Kingdom, 89: 323.
- Hawes, N.A. (2008). Nearshore dispersal and reproductive viability of intertidal fucoid algae: how effective is drift in local to regional dispersal? University of Canterbury, Master’s thesis.
- Hinojosa, I. A., Pizarro, M., Ramos, M. & Thiel, M. (2010). Spatial and temporal distribution of floating kelp in the channels and fjords of southern Chile. Estuarine and Coastal Shelf Sciences, 87: 367–377.
- Hobday, A.J. (2000a). Age of drifting Macrocystis pyrifera (L.) C. Agardh rafts in the Southern California Bight. Journal of Experimental Marine Biology and Ecology, 253: 97–114.
- Hobday, A.J. (2000b). Persistence and transport of fauna on drifting kelp (Macrocystis pyrifera (L.) C. Agardh) rafts in the Southern California Bight. Journal of Experimental Marine Biology and Ecology, 253: 75–96.
- Ingólfsson, A. (1998). Dynamics of macrofaunal communities of floating seaweed clumps off western Iceland: a study of patches on the surface of the sea. Journal of Experimental Marine Biology and Ecology, 231: 119–137.
- Jassby, A.D. & Platt, T. (1976). Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnology and Oceanography, 21: 540–547.
- Johannesson, K., Forslund, H., Capetillo, N., Kautsky, L., Johansson, D., Pereyra, R.T. & Råberg, S. (2012). Phenotypic variation in sexually and asexually recruited individuals of the Baltic Sea endemic macroalga Fucus radicans: in the field and after growth in a common-garden. BMC Ecology, 12: 2.
- Jormalainen, V., Gagnon, K., Sjöroos, J. & Rothäusler, E. (2016). The invasive mud grab enforces a major shift in a rocky littoral invertebrate community of the Baltic Sea. Biological Invasions, 18: 1409–1419.
- Khalaman, V.V. & Berger, V.Y. (2006). Floating seaweeds and associated fauna in the White Sea. Oceanology, 46: 827–833.
- Kline, R.B. (2015). Principles and Practice of Structural Equation Modeling. 4th ed. Guilford Press, New York.
- Lehmann, A., Getzlaff, K. & Harlaß, J. (2011). Detailed assessment of climate variability in the Baltic Sea area for the period 1958 to 2009. Climate Research, 46: 185–196.
- Lehvo, A., Bäck, S. & Kiirikki, M. (2001). Growth of Fucus vesiculosus L. (Phaeophyta) in the Northern Baltic Proper: energy and nitrogen storage in seasonal environment. Botanica Marina, 44: 345–350.
- Littell, R.C., Milliken, G.A., Stroup, W.W., Wolfinger, R.D. & Schabenberger, O. (2006). SAS for Mixed Models. SAS Institute.
- Macaya, E. & Zuccarello, G. (2010). Genetic structure of the giant kelp Macrocystis pyrifera along the southeastern Pacific. Marine Ecology Progress Series, 420: 103–112.
- Macreadie, P.I., Bishop, M.J. & Booth, D.J. (2011). Implications of climate change for macrophytic rafts and their hitchhikers. Marine Ecology Progess Series, 443: 285–292.
- McKenzie, P.F. & Bellgrove, A. (2008). Dispersal of Hormosira banksii (Phaeophyceae) via detached fragments: reproductive viability and longevity. Journal of Phycology, 44: 1108–1115.
- Meier, M. & Eilola, K. (2011). Future projections of ecological patterns in the Baltic Sea. Oceanografi, 107.
- Miranda, L. & Thiel, M. (2008). Active and passive migration in boring isopods Limnoria spp. (Crustacea, Peracarida) from kelp holdfasts. Journal of Sea Research, 60: 176–183.
- Muhlin, J.F., Engel, C.R., Stessel, R., Weatherbee, R.A. & Brawley, S.H. (2008). The influence of coastal topography, circulation patterns, and rafting in structuring populations of an intertidal alga. Molecular Ecology, 17: 1198–1210.
- Ólafsson, E.A., Ingólfsson, A. & Steinarsdottire, M.B. (2001). Harpacticoid copepod communities of floating seaweed: controlling factors and implications for dispersal. Hydrobiologia, 453: 189–200.
- Olsen, J.L., Zechman, F.W., Hoarau, G., Coyer, J.A., Stam, W.T., Valero, M. & Åberg, P. (2010). The phylogeographic architecture of the fucoid seaweed Ascophyllum nodosum: an intertidal ‘marine tree’ and survivor of more than one glacial-interglacial cycle. Journal of Biogeography, 37: 842–856.
- Oswald, R.C., Telford, N., Seed, R. & Happey-Wood, C.M. (1984). The effect of encrusting bryozoans on the photosynthetic activity of Fucus serratus L. Estuarine, Coastal and Shelf Science, 19: 697–702.
- Pereyra, R.T., Huenchuñir, C., Johansson, D., Forslund, H., Kautsky, L., Jonsson, P.R. & Johannesson, K. (2013). Parallel speciation or long-distance dispersal? Lessons from seaweeds (Fucus) in the Baltic Sea. Journal of Evolutionary Biology, 26: 1727–1737.
- R Core Team (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
- Rosseel, Y. (2012). Lavaan: an R package for structural equation modeling. Journal of Statistical Software, 48: 1–36.
- Rothäusler, E., Gómez, I., Hinojosa, I.A., Karsten, U., Tala, F. & Thiel, M. (2009). Effect of temperature on growth and reproduction of floating Macrocystis spp. (Phaeophyceae) along a latitudinal gradient. Journal of Phycology, 45: 547–559.
- Rothäusler, E., Gómez, I., Karsten, U., Tala, F. & Thiel, M. (2011a). Physiological acclimation of floating Macrocystis pyrifera to temperature and irradiance ensures long-term persistence at the sea surface at mid-latitudes. Journal of Experimental Biology and Ecology, 405: 33–41.
- Rothäusler, E., Gómez, I., Hinojosa, I.A., Karsten, U., Tala, F. & Thiel, M. (2011b). Physiological performance of floating giant kelp Macrocystis pyrifera (Phaeophyceae): latitudinal variability in the effects of temperature and grazing. Journal of Phycology, 47: 269–281.
- Rothäusler, E., Gómez, I., Hinojosa, I.A., Karsten, U., Miranda, L., Tala, F. & Thiel, M. (2011c). Kelp rafts in the Humboldt Current: interplay of abiotic and biotic factors limit their floating persistence and dispersal potential. Limnology and Oceanography, 56: 1751–1763.
- Rothäusler, E., Gutow, L. & Thiel, M. (2012). Floating seaweeds and their communities. In Seaweed Biology (Wiencke, C. & Bischof, K., editors), 359–380. Springer, Berlin.
- Rothäusler, E., Corell, H. & Jormalainen, V. (2015). Abundance and dispersal trajectories of floating Fucus vesiculosus in the Northern Baltic Sea. Limnology and Oceanography, 60: 2173–2184.
- Rothäusler, E., Sjöroos, J., Heye, K. & Jormalainen, V. (2016). Genetic variation in photosynthetic performance and tolerance to osmotic stress (desiccation, freezing, hyposalinity) in the rocky littoral foundation species Fucus vesiculosus (Fucales, Phaeophyceae). Journal of Phycology, 52: 877–887.
- Rothäusler, E., Reinwald, H., López, B.A., Tala, F. & Thiel, M. (2018a). High acclimation potential in floating Macrocystis pyrifera to abiotic conditions even under grazing pressure – a field study. Journal of Phycology, 54: 368–379.
- Rothäusler, E., Rugiu, L. & Jormalainen, V. (2018b) Forecast climate change conditions sustain growth and physiology but hamper reproduction in range-margin populations of a foundation rockweed species. Marine Environmental Research, 141: 205–213.
- Rothäusler, E., Uebermuth, C., Haavisto, F. & Jormalainen, V. (2019). Living on the edge: gamete release and subsequent fertilization in Fucus vesiculosus (Phaeophyceae) are weakened by climate change-forced hyposaline conditions. Phycologia, 58: 111–114.
- SAS Institute Inc. (2014). SAS/STAT® 9.4 User’s Guide. SAS Institute Inc., Cary.
- Schreiber, U., Hormann, H., Neubauer, C. & Klughammer, C. (1995). Assessment of photosystem II photochemical quantum yield by chlorophyll fluorescence quenching analysis. Functional Plant Biology, 22: 209–220.
- Soomere, T. & Räämet A. (2011). Spatial patterns of the wave climate in the Baltic Proper and the Gulf of Finland. Oceanologia, 53: 335–371.
- Tala, F., Gómez, I., Luna-Jorquera, G. & Thiel, M. (2013). Morphological, physiological and reproductive conditions of rafting bull kelp (Durvillaea antarctica) in northern-central Chile (30°S). Marine Biology, 160: 1339–1351.
- Tala, F., Velasquez, M., Mansilla, A., Macaya, E.C. & Thiel, M. (2016). Latitudinal and seasonal effects on short-term acclimation of floating kelp species from the South-East Pacific. Journal of Experimental Marine Biology and Ecology, 483: 31–41.
- Tala, F., López, B.A., Velásquez, M., Jeldres, R., Macaya, E.C., Mansilla, A., Ojeda, J. & Thiel, M. (2019). Long-term persistence of the floating bull kelp Durvillaea antarctica from the South-East Pacific: potential contribution to local and transoceanic connectivity. Marine Environmental Research, 149: 67–79.
- Thiel, M. & Gutow, L. (2005). The ecology of rafting in the marine environment. I. The floating substrata. Oceanography and Marine Biology – An Annual Review, 42: 181–264.
- Thiel, M., Hinojosa, I.A., Joschko, T. & Gutow, L. (2011). Spatio-temporal distribution of floating objects in the German Bight (North Sea). Journal of Sea Research, 65: 368–379.
- Tully, O. & Ó Céidigh, P.O. (1986). The ecology of Idotea species (Isopoda) and Gammarus locusta (Amphipoda) on surface driftweed in Galway Bay (west of Ireland). Journal of the Marine Biological Association of the United Kingdom, 66: 931–942.
- van Hees, D.H., Olsen, Y.S., Mattio, L., Ruiz-Montoya, L., Wernberg, T. & Kendrick, G.A. (2018). Cast adrift: physiology and dispersal of benthic Sargassum spinuligerum in surface rafts. Limnology and Oceanography, 64: 526–540.
- Vandendriessche, S., Vincx, M. & Degraer, S. (2006). Floating seaweed in the neustonic environment: a case study from Belgian coastal waters. Journal of Sea Research, 55: 103–112.
- Vandendriessche, S., Vincx M. & Degraer, S. (2007). Floating seaweed and the influences of temperature, grazing and clump size on raft longevity – a microcosm study. Journal of Experimental Marine Biology and Ecology, 343: 64–73.
- Wild, M. (2005). From dimming to brightening: decadal changes in solar radiation at Earth’s surface. Science, 308: 847–850.
- Yatsuya, K. (2008). Floating period of Sargassacean thalli estimated by the change in density. Journal of Applied Phycology, 20: 797–800.
- Zeileis, A. & Hothorn, T.H. (2002). Diagnostic checking in regression relationships. http://cran.r-project.org/doc/Rnews/. R News, 2: 7–10.