ABSTRACT
We documented male and female gametogenesis, auxospore structure and development of the biddulphioid diatom species Biddulphia tridens (Ehrenberg) Ehrenberg, a close relative of the generitype. Our mating experiments showed that the B. tridens clones were homothallic, self-fertile and produced two eggs per oogonium. During spermatogenesis, spermatogonia developed by depauperating mitosis from spermatogonangium cells and four hologenous uniflagellate male gametes were produced per spermatocyte. In the early stage of auxospore development, cells were globular to sub-globular in shape and covered with a siliceous envelope. The incunabular scales on these auxospores showed a great diversity in size and shape. In the later stages of development, auxospores grew anisodiametrically, facilitated by transverse perizonial bands. Initial epivalve formation was preceded by the retraction of the protoplast from the auxospore wall. The structural features of sex cells such as incunabular scales found in B. tridens are distinct from other broadly defined biddulphioid diatoms thus far examined. This is consistent with the distant relationship between true Biddulphia species and the Biddulphia-like diatoms indicated by molecular phylogenies. Morphological and molecular examination of B. tridens clones were performed here only to confirm species identity. This complete reproductive dataset from a true Biddulphia species will provide valuable insights for future studies of the relationships between Biddulphia-like diatoms and true Biddulphia species.
Introduction
A unique and intriguing feature of the diatom life cycle is the gradual mean population cell size reduction over the course of mitotic propagation of diploid vegetative individuals (Round et al., Citation1990), which cannot go on indefinitely. The auxospore is a specialized zygote, known only in diatoms, which can generate a species-specific maximal cell size via production of large, postsexual progeny individuals. These individuals are generally much larger than both parents combined (Davidovich, Citation2001, and references therein), and so are in the position to commence a new round of mitoses and cell size diminution. The unique feature of diminution in the life cycle makes sexuality necessary in the majority of diatom species. In the course of diminution over the long lifetime of a diatom genotype, the species-specific valve morphology may also change in response to varying environment. In contrast, auxospore morphology is similar for members of a genus and related genera (Hasle et al., Citation1983; Kaczmarska et al., Citation2019).
The broadly defined biddulphioid diatoms are a relatively large group of bipolar centric diatoms, commonly reported worldwide. The existing genera considered to be biddulphioid diatoms have readily recognizable valves that are porose, loculate and carry a pseudocellus or ocellus (Ross & Sims, Citation1971; Round et al., Citation1990). However, their taxonomy is in need of reappraisal (Ashworth et al., Citation2013). Several taxa formerly attributed to the genus Biddulphia Gray have already been transferred to other existing genera or elevated to new genera based on their frustule, valve morphology and/or molecular phylogenies (Hoban, Citation1983; Ashworth et al., Citation2013), a reflection of the absence of generic valvar morphological synapomorphies in Biddulphia (sensu lato, or s.l.). In some but not all current molecular phylogenetic trees, Biddulphia species form a clade which is sister to the morphologically distinct genus Attheya (Ashworth et al., Citation2013; Parks et al., Citation2018). Presently, molecular data are available only for the Biddulphia generitype and three species that cluster around it: the generitype B. biddulphiana (Smith) Boyer, B. tridens (Ehrenberg) Ehrenberg, B. alternans (Bailey) Van Heurck, and B. reticulum (Ehrenberg) Boyer. Therefore, we consider these diatoms as true Biddulphia species (Biddulphia sensu stricto, or s.s.) in this paper. All these taxa have pseudocelli and similar occluded simple pores across the valve (Ashworth et al., Citation2013).
The life cycles of diatoms in general and polar centrics in particular are poorly understood. Only a small number of known species (relative to all known polar centric species) have been investigated in detail, and few species have been added since the seminal work of von Stosch and his co-workers (reviewed in von Stosch, Citation1982). Some notable exceptions are Ardissonea (Davidovich et al., Citation2017; Kaczmarska et al., Citation2018), Hydrosera (Idei et al., Citation2015), Chaetoceros (Kaczmarska et al., Citation2019) and cymatosiroids (Samanta et al., Citation2017, Citation2018). Even less is known about the life histories of true biddulphioid diatoms. Among the species belonging to the genus Biddulphia s.s., only B. biddulphiana (as B. pulchella in von Stosch, Citation1965, Citation1982) has been investigated thus far. The structural details of the B. biddulphiana auxospore wall were only generally outlined because the heavily silicified wall components in this species were not suitable for TEM examination available then. Von Stosch (Citation1956) investigated sexual reproduction in Biddulphia-like diatoms such as B. rhombus (Ehrenberg) Smith and B. granulata Roper in detail, but later these two species were found to belong to the eupodiscacean genus Odontella. More recently, Idei et al. (Citation2015) investigated details of the auxospore structure of another biddulphioid diatom, Hydrosera triquetra Wallich. However, this species is only distantly related to the genus Biddulphia s.s. (Ashworth et al., Citation2013). A detailed study of sexual reproductive stages and auxospore structure of the biddulphioid diatoms from these different lineages may provide significant and independent lines of evidence to better our understanding of their evolutionary history.
The objective of this study was to examine the structure, development and behaviour of sex-cells in B. tridens, a close relative of the generitype, B. biddulphiana. The phylogenetic relationship between B. tridens and B. biddulphiana has been demonstrated by Ashworth et al. (Citation2013). We documented the details of gametogenesis for both sexes, auxospore structure and development of this species, and compared our findings to other Biddulphia, Biddulphia-like species and other polar diatoms, both centric and pennate. Morphological and molecular examination of the B. tridens clones used was performed, but only to confirm species identity.
Materials and methods
Sampling and clonal culture establishment
Four monoclonal cultures of B. tridens were established from coastal seawater. Clones Mex-A and Mex-A(?) were collected from Baja California, Mexico and clone PLB-B3 was collected from Puerto Lima Bay, Costa Rica. Clone ECT3902 was collected by Dr M. Ashworth from Florida, USA. Details of the collection and establishment of clones can be found on BOLD for our clones (http://www.boldsystems.org/index.php/MAS_Management_DataConsole?codes=BIDDU; process ID: BIDDU001-18, BIDDU002-18, BIDDU003-18) and on Protist Central (http://www.protistcentral.org/Photo/get/photo_id/3527; voucher ID: HK327, ECT3902) for the Floridian clone. Our B. tridens clones were isolated by micro-pipetting (Andersen, Citation2005) individual clonal chains using a Zeiss Axiovert 200 inverted light microscope (Carl Zeiss, Oberkochen, Germany). Each chain was washed several times with sterile seawater until free from other biota, placed in a 12-well culture plate (Corning Incorporated, Corning, New York, USA) with f/2 medium, salinity 22 or 27 psu (the latter being used only prior to some induction trials, see below), and kept at 20°C with a photoperiod of 12:12 h light:dark (L:D) and an irradiance of ~26 µmol photons m–2 s–1 for growth. The monoclonal cultures were then scaled up to 50 ml volume flasks under the same growth conditions as above.
Molecular identification
Our clonal cultures were harvested from exponential growth phase and concentrated by centrifugation. DNA was extracted using an UltraClean® Soil DNA Isolation Kit (Qiagen Sciences, Germantown, Maryland, USA (formerly Mo Bio Laboratories)) as per the manufacturer’s instructions. Three DNA fragments were amplified with specific primers for the 321 bp conservative region of the nuclear-encoded 18S rRNA including partial V2 and V3 variable regions (Iwatani et al., Citation2005), a 333 bp fragment of the V4 region of 18S rRNA (Zimmermann et al., Citation2011), and a 540 bp fragment of the plastid encoded rbcL gene (MacGillivary & Kaczmarska, Citation2011). The targeted DNA fragments were amplified and sequenced following Samanta et al. (Citation2017). The efficacy of these fragments in species identification has been demonstrated by Zimmerman et al. (Citation2011) and MacGillivary & Kaczmarska (Citation2011). GenBank accession numbers for the sequences are MK542653-55 and MK558229-34. Sequences of 18 related species obtained from GenBank were added to the analyses.
For the construction of multigene datasets, sequences were concatenated head to tail and aligned using MUSCLE (http://www.ebi.ac.uk/Tools/msa/muscle/; Edgar, Citation2004). Bayesian inference (BI) was performed using MrBayes v3.2 (Ronquist et al., Citation2012). The multi-gene dataset was partitioned by genes and the substitution model was estimated from the data. A four-chain run for 20 000 000 generations was used and trees were sampled every 5000 generations. Posterior probabilities (PP) were estimated with 50% burn-in, and a majority rule consensus tree was constructed. An online version of PHYML (Phylogenetic inferences using Maximum likelihood; http://www.atgc-montpellier.fr/phyml/; Guindon et al., Citation2010) was used to construct the ML tree by selecting AIC (Akaike Information Criterion; Posada & Buckley, Citation2004) for the appropriate substitution model (Lefort et al., Citation2017) and NNI (Nearest-neighbour interchanges; Felsenstein, Citation2004) for branch swapping. Bootstrap supports (Felsenstein, Citation1985) were obtained based on 1000 replicates for ML analysis. Sequences of Brockmanniella brockmannii (Hustedt) Hasle, Stosch & Syvertsen were used as outgroups for phylogenetic analyses.
Sexual induction protocol
Before sexual induction, stock cultures were maintained in exponential growth-stage for weeks by their bi-weekly transfer to fresh f/2 growth medium and then grown at room temperature (~22°C) with a photoperiod of 8:16 h L:D and an irradiance of ~26 µmol photons m–2 s–1. Under these growth conditions none of the clones ever sexualized. Each clone individually (control) and in pairs was subjected to the following sexualizing protocols. After 3 hours of light exposure, 15-day-old flask cultures were inoculated into a mixture of f/2 and L1 growth medium (1:1 ratio) in 6-well culture plates. The induced plates were kept at the same growth conditions used for exponential growth (22 psu salinity (protocol 1), with several trials at 27 psu (protocol 2)) using the local coastal waters.
In a third protocol, 19 inductions were conducted using high salinity f/2 growth medium consisting of a 1:3 v/v mixture of local seawater (27 psu) and seawater from the Gulf of Mexico (34 psu). In these inductions, the 4 to 7-week-old culture material grown as indicated above was placed in 6-well plates, individually (control) and pair-wise mixed. They were then moved to a growth cabinet at ~17°C, 12:12 h L:D cycle and an irradiance of ~26 µmol photons m–2 s–1 for 24 h. Following this, the induction trays were moved back to the laboratory. In the lab, growth conditions were 22–24°C, continuous light for 2 days at light intensity of 24 µmol photons m−2 s−1. This was followed by 8:16 h L:D for as long as sexualized cells grew.
In all protocols, individual and pairwise induction trials were performed at least in triplicate. Sexual activities were monitored using the inverted microscope every few days. When required, cultures were fixed in 2.5% (v/v) glutaraldehyde in the growth media with a final dilution of 1:10 v/v culture:fixative and refrigerated until further analysis.
Light microscopy of sexual stages
To examine the nuclear behaviour during various stages of sexual reproduction, cells were stained with Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, California, USA) as per the manufacturer’s instructions. Silicification was visualized by tracing incorporation of PDMPO ((2-(4-pyridyl)-5-((4-(2dimethylaminoethylaminocarbamoyl)methoxy)phenyl)oxazole); Thermo Fisher Scientific, Waltham, Massachusetts, USA) into developing cell walls. A final concentration of 0.125 µM PDMPO was added to wells of the culture plates at the beginning of the induction experiment (Leblanc & Hutchins, Citation2005). Brightfield, DAPI and PDMPO epifluorescence light microscopy were performed using a Zeiss AxioSkop 2 plus upright microscope equipped with an AxioCam HR colour camera and HBO 100 mercury vapour illumination source.
Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) of sexual stages
Vegetative frustules from each of our clonal cultures were prepared for SEM/EDS examination following Kaczmarska et al. (Citation2005). Lightly- and non-silicified cells were fixed for at least 1 h with 2.5% glutaraldehyde in f/2 medium, then rinsed four times with distilled water (~100 ml) every 10 min, with gentle vacuum, in a filtration tower onto a 25 mm diameter, 1 µm pore size polycarbonate filter (Sterlitech Corporation, Kent, Washington, USA). Filters were removed from the tower while still moist and stacked with stainless steel washers (OD 25 mm, ID 15.5 mm) and an additional filter in the order: washer/specimen filter/washer/additional filter/washer and clamped together with a pair of 0.75 in (19 mm) stainless steel foldover binder clips (Staples® Model 24169-CA) to contain the specimen side of the filter in a flow-through chamber (Supplementary fig. 1). Binder clip handles were removed to allow the assembly to fit into the critical point dryer chamber (see below). Specimens were then post-fixed in 1% osmium tetroxide for 1 h, and dehydrated using 10 min changes of 20%, 50%, 70%, 85%, 95% ethanol:distilled water, followed by four 10 min changes of 100% anhydrous ethanol. Filter assemblies were dried with liquid CO2 in a Denton DCP-1 critical point dryer (Denton Vacuum, Moorestown, New Jersey, USA). For examination of auxospore wall components, specimens were prepared in two ways. In the first method, to remove fixative samples were rinsed onto filters as above, resuspended in ~1 ml distilled water and subjected to a brief (5 min) acid wash (15 ml 1:1 sulphuric:nitric acid) with no heating other than that produced by mixing the two acids. The acid solution was then diluted in 100 ml distilled water, transferred to the filtration tower containing a fresh filter, rinsed with 250 ml distilled water and air-dried. The second preparation method omitted the acid treatment and the sample was simply washed with 250 ml distilled water onto the filter and air-dried. Filters bearing specimens prepared by all methods were mounted on aluminium stubs with double-sided tape, rimmed with colloidal carbon, and coated with ~15 nm of gold using a Hummer 6.2 sputtering unit (Anatech Ltd, Union City, California, USA). Images were acquired using a Hitachi SU3500 SEM (Hitachi High Technologies, Toronto, Canada) at a working distance of 5 mm and 10 kV accelerating voltage. EDS was performed with the same instrument equipped with an Oxford AZtec/X-Max 20 EDS system (Oxford Instruments, High Wycombe, UK) at 10 mm working distance. Since the only element of interest in this study was silicon (Si-Kα, X-ray energy 1.74 keV), an accelerating voltage of 10 kV provided sufficient overvoltage for efficient X-ray excitation. Spectra were acquired for 100 s (dead time corrected) at 0.1 nA beam current, energy range 0–10 keV into 1024 channels. The EDS spectra were collected from intact and unobstructed structures and/or auxospores. Spectra from the polycarbonate support filter adjacent to the auxospores were also routinely taken and showed no remote excitation from neighbouring siliceous components (if present) at distances as close as 3 µm.
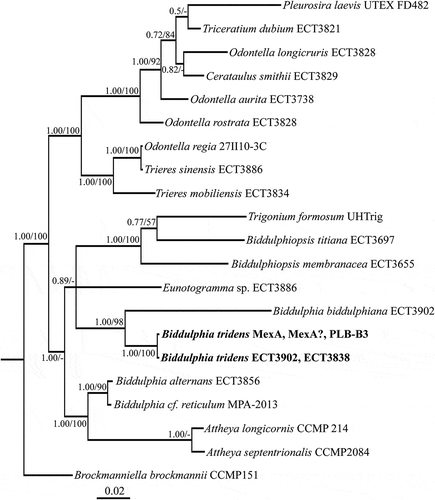
Fig. 1. Phylogenetic tree from Bayesian inference (BI) based on concatenated conservative and V4 regions of 18S rRNA gene, and a partial rbcL gene sequence. Support values at nodes from left to right are posterior probabilities (BI) and bootstrap percentages (ML). Scale bar indicates substitutions per site.
The terminology used here for auxospores and sexual stages conforms to Kaczmarska et al. (Citation2013) while for frustule structure it follows Anonymous (Citation1975) and Ross et al. (Citation1979).
Results
Molecular and morphological identity of the clones
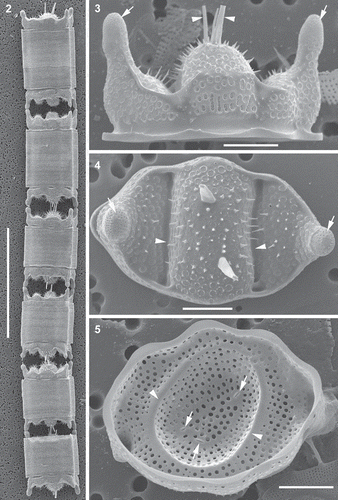
All four B. tridens clones were 100% identical to each other at the nucleotide level for all three genetic loci (i.e. conservative and V4 regions of 18S rRNA and partial rbcL) regardless of collection site. In our multi-gene phylogenetic tree, B. tridens clones were recovered as a sister taxon to the generitype B. biddulphiana () and available curated sequences of B. tridens from other locations. The valves of our clones also conformed well to the delineation of B. tridens (Ashworth et al., Citation2013). Cells were chain-forming (). All the B. tridens clones had pseudocelli on extended elevations of apices (, ). The apical length of vegetative cells in culture was 14–134 μm. Areolae were large with perforated vela (, ). The stria density was 4.5–7.2 in 10 μm. Spines were scattered across the valve face (, ). One to several rimoportulae were found in the central elevation of the valve with long external tubes (–). The internal ridges or ribs (pseudosepta) were present and corresponded to the external indentation of the valve face.
Figs 2–5. Morphology of representative vegetative frustules and valves, scanning electron microscopy (SEM). Fig. 2. Clone PLB-B3; shows six frustules in a chain. Fig. 3. Clone PLB-B3; shows girdle view of valve, pseudocelli (arrows) and external tubes of the rimoportulae (arrowheads). Fig. 4. Clone Mex-A; shows external valve surface, valve outline, pseudocelli (arrows), spines scattered on valve face, hyaline area of the valve where the valve face is indented (arrowheads), and pores. Fig. 5. Clone PLB-B3; internal view of valve, openings of the rimoportulae (arrows) and a hyaline ring corresponding to a circular indentation present in small valves with nearly circular outline (arrowheads). Scale bars: Fig. 2, 100 µm; Figs 3–5, 10 µm.
Sexual induction and reproductive stages
Three different protocols were used to sexualize the clones and all were successful in inducing male and female gametogenesis within 2–3 days, at times profusely albeit inconsistently. Similarly, three out of four clones (Mex-A, Mex-A(?) and PLB-B3) produced spherical and anisodiametric auxospores, regardless of protocol used. Spherical auxospores were present in both control wells and when paired with others in low (22 psu) and higher (27 psu) salinity Atlantic seawater-based f/2. Complete successful sexualization, including spherical and anisodiametric auxospores that produced initial cells, was found only in wells of clone Mex-A(?) and only under the third induction protocol where the growth media consisted of a mixture of Atlantic and Gulf of Mexico seawater. Here too, male and female gametogenesis was in evidence 2 days following cold treatment; spherical auxospores developed very soon thereafter while anisodiametric auxospores and initial cells developed 5 days later.
Spermatogenesis
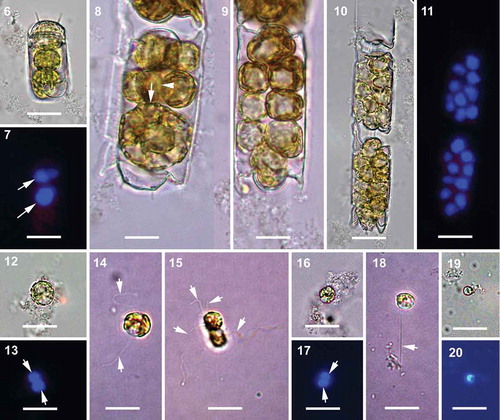
The entire protoplast of the spermatogonangium underwent multiple rounds of depauperating mitotic division to produce naked spermatogonia, and eventually primary spermatocytes (–). No vestigial valves were detected by PDMPO staining. The apical length of the spermatogonangium valves was 16–38 µm. Up to 16 primary spermatocytes per spermatogonangium could be counted. The diploid nucleus of each primary spermatocyte stained strongly with DAPI (). The diameter of the primary spermatocytes was 8–14 µm. The diploid nucleus of the primary spermatocyte underwent nuclear division of meiosis I to produce two haploid nuclei (, ) and subsequently the cytokinetic division of meiosis I produced two secondary spermatocytes (–). Tetraflagellated primary spermatocytes were found at cytokinetic meiosis I (). We also observed biflagellate primary spermatocytes in the same samples (). Secondary spermatocytes were 6–8 µm in diameter. The secondary spermatocytes then underwent meiosis II to produce haploid uniflagellate male gametes (–). The final stage of male gametes observed suggests they were hologenous, 3–6 µm in diameter.
Figs 6–20. Spermatogenesis, brightfield and epifluorescence microscopy. All the cells with flagella are from live samples. Fig. 6. Initiation of depauperating mitoses within the spermatogonangium. Fig. 7. Corresponding epifluorescence image of Fig. 6, arrows indicating two DAPI stained diploid nuclei. Figs 8, 9. Intermediate stages further along in a series of depauperating mitoses within the spermatogonangium. In Fig. 8, arrow indicates the plane of first mitotic division (parallel to apical axis) and arrowhead indicates the subsequent second plane of mitotic division in a more advanced sibling cell (parallel to pervalvar axis). Fig. 10. Primary spermatocytes within the spermatogonangial frustule resulting from several rounds of depauperating mitoses. Fig. 11. Corresponding epifluorescence image of Fig. 10 showing strongly DAPI stained diploid nuclei. Fig. 12. Primary spermatocyte. Fig. 13. Corresponding epifluorescence image of Fig. 12. Arrows indicate the DAPI stained nuclei after meiosis I. Fig. 14. Primary spermatocyte with two flagella (arrows). Fig. 15. Quadriflagellated primary spermatocyte during cytokinesis following meiosis I. Arrows indicate the flagella. Fig. 16. Secondary spermatocytes with flagella lost due to fixation. Fig. 17. Corresponding epifluorescence image of Fig. 16. Arrows indicate the DAPI stained nuclei following meiosis II. Fig. 18. A uniflagellated (arrow) secondary spermatocyte, another flagellum might have been lost. Fig. 19. Male gamete; flagellum lost during sample preparation. Fig. 20. Corresponding epifluorescence image of Fig. 19. Scale bars: 20 µm.
Oogenesis and auxospore development
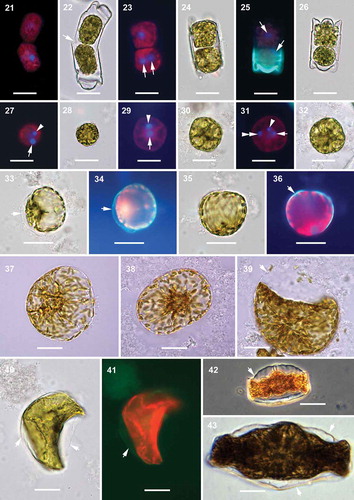
The apical length of the oogonia varied from 21 to 33 µm. Prior to meiosis, the oogonium frustule elongated and eventually bent (, ). During oogenesis, the first nuclear division (meiosis I) was followed by cytokinesis and produced two secondary oocytes per oogonium (–), each containing a haploid nucleus and no PDMPO-traced silica in their walls. Sometimes, the two secondary oocytes produced were of somewhat different size. After that, the oogonial frustules dehisced and the secondary oocytes were released into the growth medium. The second nuclear division (meiosis II) in each secondary oocyte produced two sister nuclei, but no cytokinesis followed (, , –). In the secondary oocyte, one of the two sister nuclei produced by meiosis II became pyknotic (–) although sometimes meiosis II and pyknosis of one of its two sister nuclei occurred in secondary oocytes within the oogonial frustule () before they were liberated into the growth media. It is unknown how soon pyknosis started or how long it lasted. As a result, two eggs were produced per oogonium and each egg contained one functional and one pyknotic nucleus. EDS analysis did not detect silicon in the egg cell wall (Supplementary fig. 2). The diameter of the secondary oocytes and eggs varied from 17 to 28 µm. We also found trinucleated cells in culture wells subjected to our induction protocols, which suggests that fertilization took place although syngamy was not observed (, ).
Figs 21–43. Oogenesis and auxospore development, epifluorescence and brightfield microscopy. Fig. 21. Two secondary oocytes produced by oogonium. Fig. 22. Corresponding brightfield image of Fig. 21. Arrow indicates the opening of the oogonial theca to release the secondary oocytes, or possibly allow sperm penetration. Fig. 23. Two secondary oocytes produced by the oogonium, one of them with two DAPI stained nuclei of equal size (arrows) after meiosis II. Fig. 24. Corresponding brightfield image of Fig. 23. Fig. 25. Two secondary oocytes with DAPI stained nuclei (arrows) after meiosis I. Green fluorescence represents PDMPO incorporation into the new hypovalve of the oogonial frustule. Fig. 26. Corresponding brightfield image of Fig. 25. Fig. 27. Free egg cell with one functional (arrow) and one pyknotic (arrowhead) DAPI stained nucleus. Fig. 28. Corresponding brightfield image of Fig. 27. Fig. 29. Another free egg cell with one functional (arrow) and one pyknotic (arrowhead) DAPI stained nucleus. Fig. 30. Corresponding brightfield image of Fig. 29. Fig. 31. Tri-nucleated egg cell; with one functional (arrow) and one pyknotic (arrowhead) nuclei of the egg and another functional nucleus (double arrowhead) possibly from a sperm cell. Fig. 32. Corresponding brightfield image of Fig. 31. Fig. 33. Initiation of plasmolysis of the protoplast in the young sub-globular auxospore. Arrow indicates auxospore envelope. Fig. 34. Corresponding epifluorescence image of Fig. 33. Arrow indicates PDMPO stained young auxospore envelope (green). Fig. 35. Sub-globular auxospore. Fig. 36. Corresponding epifluorescence image of Fig. 35. Arrow indicates PDMPO stained young auxospore envelope (green). Figs 37, 38. Further anisodiametric expansion of the globular auxospore. Fig. 39. Constriction of the protoplast and separation of plasma membrane from the auxospore envelope. Arrow indicates eroding auxospore wall. Fig. 40. Initiation of initial cell formation within the auxospore envelope. Arrows indicate the auxospore envelope. Fig. 41. Corresponding epifluorescence image of Fig. 40. Arrow indicates PDMPO incorporation in auxospore envelope (green). Chloroplast auto-fluorescence appears in red. Fig. 42. Phase contrast image of the initial cell within the auxospore envelope (arrow). Fig. 43. The complete initial cell. Arrows indicate the auxospore envelope still attached. Scale bars: Figs 21–32, 20 µm; Figs 33–43, 25 µm.
The youngest auxospores were found free in the media with no contact with oogonial thecae, were globular to sub-globular in shape and covered with a siliceous envelope (–). In the second growth stage, auxospores grew more anisodiametric (–). Eventually, the protoplast retracted from the auxospore wall before the initial epivalve formed. As development continued, the heterovalvate initial frustule was formed within the auxospore envelope thereby completing the final, third stage of development, the deposition of the initial frustule (, ).
Observed in SEM, the dehisced oogonial theca cingulum consisted of at least 12 copulae (), compared with only about half a dozen in normal vegetative thecae ( vs –). The last-deposited oogonial copulae demonstrated imperfect structure (compare the last three copulae of the same theca to the first three; vs ). Spherical to ellipsoidal cells up to 37 µm in diameter and with no silicon in their cell walls (undetectable by PDMPO or EDS; Supplementary fig. 2) were considered either eggs or spermatocytes (, , ). The auxospore wall structure varied depending on its stage of development. In the youngest spherical auxospores (30–85 µm in diameter), walls contained only siliceous incunabular scales. Incunabular scaly siliceous elements of auxospore walls were organized into several layers; generally larger scales were located in deeper layers (). The incunabular scales demonstrated great diversity in size and shape, some of which have not been reported in published literature (–). Scale structure and ornamentation varied from small thick rings to those that were roughly elliptical with branching lateral ribs, with and without an annulus. Some scales were circular with ribbing structure mimicking the ornamentation pattern of vegetative valves in some thalassiosiroid diatom species (). Some of the elongated scales had a median rib that was somewhat similar to pinnate transverse perizonia of pennate auxospores.
Figs 44–48. Mitotically dividing cells, oocytes, eggs and spherical cells, scanning electron microscopy. Fig. 44. A fully extended frustule, possibly following mitosis, demonstrating the structure of cingula in a vegetative frustule. Fig. 45. An oogonial theca with a secondary oocyte (or an egg) showing copulae similar to those in a vegetative frustule (arrowheads). Fig. 46. A close-up of the distal part of the oogonial cingulum in Fig. 45 showing the structure of copulae (arrowheads) different from those located closer to the valvocopula indicated in Fig. 45. Fig. 47. A close up of specimen from Fig. 45 showing no incunabular scales. Fig. 48. A spherical cell, possibly a small egg, showing the very delicate cell membrane (preserved on smooth surface of the bottom part of the cell) with no siliceous structure in evidence. Scale bars: Fig. 44, 50 µm; Fig. 45, 25 µm; Fig. 46, 5 µm; Figs 47, 48, 10 µm.
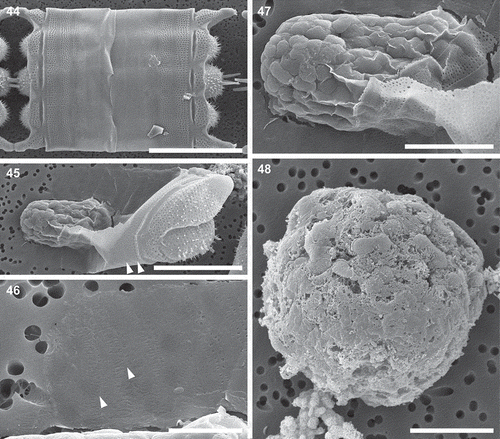
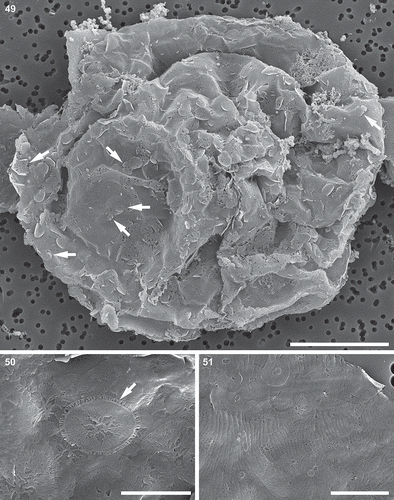
Figs 49–51. Auxospore, scanning electron microscopy. Fig. 49. A slightly collapsed, subglobular auxospore showing siliceous components of the cell wall. Arrows indicate scales with a variety of shapes. This figure is a digitally constructed montage of four individual SEM images. Fig. 50. Various incunabular scales in the outermost layer of the auxospore wall. Arrow indicates scale with pattern mimicking the ornamentation pattern of vegetative valves in some thalassiosiroid diatom species. Fig. 51. Perizonial bands overlaid by a layer of incunabular scales. Scale bars: Fig. 49, 20 µm; Fig. 50, 3 µm; Fig. 51, 5 µm.
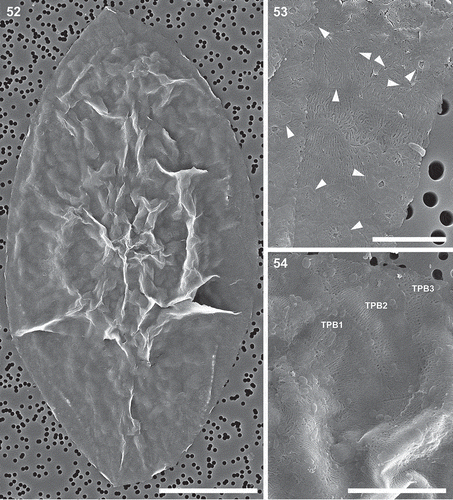
In the second, anisodiametric stage of growth, transverse perizonial bands were deposited over the expanding auxospore cell surface (). The bands were concentrated at the flatter, dorsal side of the cell; some of the bands were overlaid by a single scale or a cluster of scales (, ). The bands were wide and overlapped even in mature auxospores (). Open ends of the bands () and their complete widths () were rarely observed because the organic matrix, scales and other bands often obscured them. Individual band structure was complex, with an ill-defined, wide midregion which ran between lateral rows of pores (, ). Pores diminished in size along the costae extending perpendicularly from the midregion. The band structure was asymmetric; the underlying part of the band was narrower than the overlapping part (). In auxospores containing initial cells, the bands straddled the initial epivalve surface (, ). We did not find longitudinal bands. The transverse perizonium thinned and/or otherwise deteriorated as the auxospore matured; only a delicate veil covered the completed initial valve and valve face spines punctured the transverse perizonium (, ). Anisodiametric auxospore apical axes were 76–121 µm; transapical axes were 60–87 µm.
Figs 52–54. Auxospore cell wall, scanning electron microscopy. Fig. 52. A nearly mature, anisodiametric auxospore showing siliceous components of the cell wall. This figure is a digitally constructed montage of six individual SEM images. Fig. 53. Incunabular scales of various sizes, boundaries and patterning centres indicated with arrowheads. Fig. 54. Structure of three overlapping transverse perizonial bands (TPB1–3). Scale bars: Fig. 52, 25 µm; Fig. 53, 5 µm; Fig. 54, 10 µm.
Figs 55–58. Auxospore cell wall and initial cell, scanning electron microscopy. Fig. 55. The ends of the open transverse perizonial bands (arrows). Fig. 56. Segment of transverse perizonial band showing asymmetry across its width (arrows) relative to the loosely defined central rib (arrowheads). Fig. 57. Enlarged detail of initial valve in Fig. 58, showing its spines puncturing transverse perizonial bands (arrows). Fig. 58. An initial frustule shrouded in remnants of the dorsal part of the auxospore wall. Note transverse perizonial bands straddling the initial epivalve, low-topography valve face profile and imperfectly formed apical elevations. This figure is a digitally constructed montage of four individual SEM images. Scale bars: Fig. 55, 10 µm; Fig. 56, 1 µm; Fig. 57, 5 µm; Fig. 58, 20 µm.
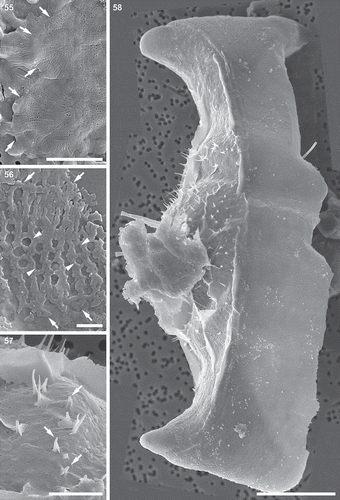
Recognizable initial valves were deposited underneath the transverse perizonium. The initial valve was imperfect; apical elevations and their pseudocelli were underdeveloped and shallow internal indentations produced uncharacteristically flat valve face topography (, , ). The initial hypovalve morphology was more similar to typical vegetative valves than the initial epivalve.
Discussion
Taxon identity and sexual induction
The morphological and molecular characters of our clones meet all diagnostic criteria for B. tridens (Ashworth et al., Citation2013). According to the most recent taxonomic reappraisal of the biddulphioid taxa, only four species (B. tridens, B. biddulphiana, B. alternans and B. reticulum) are genetically sufficiently known and similar to the generitype to warrant their position in the genus Biddulphia. However, variability in valve morphological characters still exists among the members of the genus. Internal ridges or ribs (pseudosepta) are found in B. tridens, B. biddulphiana and B. alternans, but are absent in B. reticulum (Ashworth et al., Citation2013), although all the species have pseudocelli. It is important to note that other pseudocellate taxa are scattered across the Mediophyceae, such as Isthmia minima C. Agardh, Trigonium formosum Cleve, Terpsinoë musica Ehrenberg, and Hydrosera triquetra and Biddulphia-like species now attributed to the genus Odontella. This suggests that further family-level revisions may be required for the broadly defined biddulphioid diatoms.
Our mating experiments show that the B. tridens clones are homothallic and some are self-fertile, although initial cell production was low despite the production of a considerable number of gametes and spherical auxospores. Spherical auxospore production starts nearly concurrently with male and female gametogenesis in the population of cells in the same induction well. Sexualized individuals responded to the induction cues promptly and concurrently, and so within a few hours both gametes and auxospores were present. But anisodiametric development of auxospores and formation of initial cells took longer. Furthermore, initial cells were only found in wells containing one out of four clones sexualized. Our results indicate that higher salinity and/or yet undetermined constituents of the regional seawater could be an important necessary factor for completion of sexual reproduction in clone Mex-A(?). The specific salinity might be necessary to maintain osmotic balance in the non-spherical auxospore during plasmolytic retraction of the plasma membrane before deposition of the initial valve. Similarly, the requirement for specific micronutrients (addition of sodium selenite to the growth medium, for example) was found essential for the growth of Minutocellus polymorphus (Hargraves & Guillard) Hasle, Stosch & Syvertsen and other cymatosiroid diatoms isolated from certain locations (Hasle et al., Citation1983). The completion of the anisodiametric development in the presence of Gulf of Mexico seawater suggests that some micronutrients, or other physico-chemical characteristics of the Mexican seawater absent in Canadian Atlantic waters, might be necessary to complete auxospore development in these Central American species. Nonetheless, it remains uncertain why auxospores developing in the high salinity f/2 media containing Gulf of Mexico water aborted in the other three clones.
Gametogenesis
In the majority of oogamous diatoms, the final stage of spermatogenesis is meiosis, by which a diploid primary spermatocyte produces sperm. In several polar and non-polar centrics, before meiosis takes place in the primary spermatocyte, the male-determined vegetative cells (i.e. spermatogonangia) undergo a successive differentiating step (mitoses and cytokineses without cell growth), called depauperating cell divisions, to produce primary spermatocytes (von Stosch et al., Citation1973; Drebes, Citation1977b). Deposition of a vestigial valve may accompany some of these mitoses (Drebes, Citation1977b). B. tridens produces a variable number of primary spermatocytes by depauperating cell divisions, but without the formation of vestigial valves within the spermatogonangium. On the other hand, some polar centric diatoms such as the cymatosiroid Brockmanniella brockmannii male-determined vegetative cells directly undergo meiosis to produce sperm, without any prior depauperating cell divisions, at least in some size-ranges (Samanta et al., Citation2017). Similar to B. tridens, some polar centric species, such as Lithodesmium undulatum Ehrenberg (Drebes, Citation1977b), Streptotheca tamesis Shrubsole (Drebes, Citation1977b), and Bellerochea malleus (Brightwell) Van Heurck (von Stosch, Citation1977) and non-polar centric diatom species such as Coscinodiscus granii Gough (Schmid, Citation1995) and Rhizosolenia setigera Brightwell (Drebes, Citation1977b), also produce entirely valve-less spermatogonia. The spermatogonia of Odontella (=Biddulphia) rhombus (Ehrenberg) Kützing, O. granulata (Roper) Ross, Cerataulus smithii Ralfs, and Plagiogrammopsis vanheurckii (Grunow) Hasle, Stosch & Syvertsen are furnished with vestigial valves (von Stosch, Citation1956; Samanta et al., Citation2018). It is important to note that in Trieres (=Odontella) sinensis (Greville) Ashworth & Theriot, T. regia (Schultze) Ashworth & Theriot and T. mobiliensis (Bailey) Ashworth & Theriot, the vestigial valves are produced only after the first depauperating mitosis which divides the spermatogonangium into two halves (Drebes, Citation1977b; Hoppenrath et al., Citation2009). A reduced form of depauperating mitosis is found in Guinardia flaccida (Castracane) Peragallo (Drebes, Citation1977b), Pleurosira laevis (Ehrenberg) Compère (Heath & Darley, Citation1972) and Hydrosera triquetra (Idei et al., Citation2015), where spermatogonangia become multinucleate plasmodia because several rounds of nuclear divisions proceed without cytokinesis. Therefore, spermatogenesis may be significantly different among the oogamous diatoms at the initial stages.
Except for the plasmodial type above, nuclear division in a primary spermatocyte results in four haploid sperm. There are two major types of sperm: hologenous (chloroplasts retained) and merogenous (chloroplasts expelled). In B. tridens, sperm are hologenous throughout meiosis, which is in contrast to the congener B. biddulphiana (the sister taxon of B. tridens), the only other species examined (von Stosch, Citation1982). The merogenous type spermatogenesis found in B. biddulphiana seems to be unique. In this species, the plasmatic residual body which retains all chloroplasts first buds off from the primary spermatocyte, then the colourless cell’s nucleus undergoes meiosis (von Stosch Citation1965, Citation1982). The other polar centric species shown to possess merogenous spermatogenesis are O. granulata, O. rhombus, Cerataulus smithii, Pleurosira lavis and Plagiogrammopsis vanheurckii (Samanta et al., Citation2018). Hologenous spermatogenesis is also found in other ocellus-bearing taxa such as O. aurita (Lyngbye) Agardh, T. regia, T. sinensis, T. mobiliensis (Drebes, Citation1974; von Stosch, Citation1954, Citation1982). Thus, the type of spermatogenesis varies from species to species.
In contrast to spermatogenesis which undergoes full-course meiosis resulting in four viable gametes, diatom oogenesis exhibits reduced forms of meiosis resulting in only one or two cells functioning as female gametes. There are three known types of oogenesis, depending on the number of eggs and residual cells produced per oogonium (see Mizuno, Citation2006 for details). In B. tridens, two eggs are produced per oogonium, whereas in B. biddulphiana only one egg is produced (von Stosch, Citation1982). The Biddulphia-like taxa such as O. aurita, O. granulata, T. mobiliensis, T. sinensis and T. regia also produce two eggs per oogonium (von Stosch, Citation1954, Citation1982; Drebes, Citation1974). Among other polar centric diatoms, some members of Chaetocerotales (Attheya armatus (West) Crawford, A. decora West, A. septentrionalis (Østrup) Crawford), Lithodesmiales (Ditylum brightwellii (West) Grunow, Lithodesmium intricatum Ehrenberg, L. undulatum), and Cymatosirales (Brockmanniella brockmannii) also produce two eggs (von Stosch, Citation1954; Drebes, Citation1977a; Koester et al., Citation2007; Samanta et al., Citation2017). One egg per oogonium is produced by many of the non-polar centric diatoms studied so far (reviewed by Mizuno, Citation2008) and some polar centric members of Chaetocerotales (Bacteriastrum hyalinum Lauder, Chaetoceros crucifer Gran), Lithodesmiales (Bellerochea malleus, Streptotheca thamensis), Eupodiscales (Amphitetras antediluviana Ehrenb., Cerataulus laevis (Ehrenberg) Ralfs) and Biddulphiales (Lampriscus sp.) (von Stosch, Citation1954, Citation1982; Drebes, Citation1974; Ehrlich et al., Citation1982; French & Hargraves, Citation1985; Idei & Nagumo, Citation2002). All the thalassiosiroid diatoms studied to date produce only one egg (e.g. Migita, Citation1967; Drebes, Citation1974; Chepurnov et al., Citation2004, Citation2006; von Dassow et al., Citation2006). Only two species of polar centric diatoms, Odontella rhombus and Cerataulus smithii have been shown to produce one egg and one residual cell per oogonium (von Stosch, Citation1956). Like the cymatosiroid diatom B. brockmannii, the eggs in B. tridens are found free in the culture media. It should be kept in mind that the eggs in diatoms are in fact multinucleate cells (although some of the nuclei may be pyknotic), unlike uninucleated eggs in the majority of algae and metazoan animals from which the term ‘egg’ was adopted for diatoms.
Auxospore development
Incunabular scales are present within the auxospore wall in most centric diatoms and their morphology is diverse in terms of size, shape and structure (Kaczmarska et al., Citation2013). We found scales even more unusually ornamented than reported to date. Spinescent scales are present in diatoms formerly attributed to the family Biddulphiaceae (von Stosch, Citation1982), but now these taxa have been placed in the Eupodiscaceae (Ashworth et al., Citation2013). Members of another polar centric family, Cymatosiraceae, also have spinescent scales on their auxospore envelope, but their structure is different from the former group (von Stosch, Citation1982; Samanta et al., Citation2017). The spinescent scales are long, hair-like and mostly unbranched in Cymatosiraceae (Samanta et al., Citation2017), but in Eupodiscaceae, scales consist of a small ‘prostrate system’ of basal branched threads, from which highly branched spines arise (von Stosch, Citation1982). Neither B. tridens nor B. biddulphiana (Biddulphia s.s.) have spinescent scales. An interesting feature of B. tridens is circular to elongated incunabular scales of various sizes, whereas in B. biddulphiana they are large and roughly circular (von Stosch, Citation1982). Like our species, another biddulphioid diatom, Hydrosera triquetra, also has circular to elongated scales ( in Idei et al., Citation2015), but the structural details are different in the two species. In H. triquetra, scales are porous and bear branching ribs, whereas in B. tridens, scales mostly have branching ribs but no pores. This apparent dissimilarity between these phylogenetically distantly related biddulphioid diatoms (B. tridens and H. triquetra; Ashworth et al., Citation2013) indicates that their ornamentation may have evolved independently and also suggest the flexible nature of scale morphogenesis during the course of auxospore development.
Among the species currently comprising the genus Biddulphia (Ashworth et al., Citation2013), only in B. biddulphiana has the auxospore been studied in any detail thus far (von Stosch, Citation1982: ). As in our species, in B. biddulphiana the anisodiametric expansion of the auxospore is free of the maternal theca. The contraction of the protoplast away from the auxospore wall in our species is very similar to the process observed in the Biddulphia-like species T. regia, but the anisodiametric auxospore in this case is still attached to the maternal theca (Hoppenrath et al., Citation2009).
Similar to the majority of polar centric diatoms, anisodiametric growth of the auxospore in our species is facilitated by transverse perizonial bands. Von Stosch (Citation1982) postulated that longitudinal perizonial bands were also involved during the anisodiametric expansion of the auxospore in B. biddulphiana, but we did not find such bands in our species. It is interesting to note that anisodiametric auxospore development in H. triquetra is not controlled by perizonial bands like other polar centrics. Instead, entangled incunabular strips of unperforated silica restrict the polarized growth of the initial cells (Idei et al., Citation2015) in the way and in the positions that the perizonial bands function in other polar centric auxospores. A similar type of incunabular strip is known in some raphid pennate diatom auxospores, such as Nitzschia (Trobajo et al., Citation2006) and Pinnularia (Poulíčková et al., Citation2007), but in addition to the transverse perizonial bands. In contrast to H. triquetra, anisodiametric growth in Terpsinoë musica Ehrenberg, which is a phylogenetic sister to Hydrosera (Ashworth et al., Citation2013), is controlled by perizonial bands rather than incunabular strips (Müller, Citation1889). It is important to note that both of these genera are phylogenetically distantly related to the genetically characterized genus Biddulphia (Ashworth et al., Citation2013), but they are formally still considered to belong to the Biddulphiaceae family (Round et al., Citation1990). Molecular phylogeny suggests that the Biddulphia clade is sister to the morphologically distant genus Attheya. However, the transverse perizonial band structure differs somewhat between these two genera. Biddulphia has open transverse perizonial bands, whereas bands in Attheya are closed as they are in Chaetocerotales. The auxospore envelope structure of Attheya is more similar to Chaetoceros species than to Biddulphia s.s. (von Stosch, Citation1982).
In summary, after several taxonomic reappraisals of the morphologically defined biddulphioid taxa, only four species can be currently confirmed to hold their position in the genus Biddulphia based on genetic data. We documented the complete life cycle of one of these species, B. tridens, and highlighted differences between this species, the generitype and other morphologically but not genetically similar diatoms. Our results show that structural features of sex-cells in B. tridens are distinct from the other biddulphioid diatoms, which is consistent with the contention that they are phylogenetically distant from each other. The complete reproductive dataset for other true Biddulphia species may add valuable information for future phylogenetic studies of all these diatoms. Finally, our sexual induction experiments suggest that diatom sexualization is a highly species-specific process, and the physico-chemical properties of the native seawater may be an important factor for auxospore maturation. All this makes studies of diatom sexual reproduction so much more challenging and interesting.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2020.1716077
Supplementary fig. 1. Flow-through chamber assembly for processing fixed and critical point dried specimens. A. Disassembled components. B. Sample filter (specimen side up) placed on first stainless steel washer. C. Second washer placed on sample filter. D. Additional filter placed on second washer. E. Third washer placed on additional filter. F. Washer/filter stack clamped together with binder clips. G. Binder clip handles removed.
Supplementary fig. 2. SEM/EDS of putative egg cell. Areas analysed indicated by boxes 1 and 2 in SEM image (top) correspond to overlaid EDS spectra (bottom), showing absence of detectable silicon in both egg cell and specimen filter substrate.
Author contributions
B. Samanta: original concept, light microscopy, culture work, molecular analysis, drafting and editing manuscript; I. Kaczmarska: original concept, light and electron microscopy, culture work, drafting and editing manuscript; J.M. Ehrman: electron microscopy, EDS analysis, drafting and editing manuscript.
TEJP-2019-0036-File011.tif
Download TIFF Image (6 MB)TEJP-2019-0036-File010.tif
Download TIFF Image (7 MB)Acknowledgements
We thank Mike MacGillivary for establishing clones of B. tridens and M. Ashworth for the Florida clone and Texan f/2 growth media. Anonymous reviewers contributed greatly to the clarity of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andersen, R.A. (2005). Algal Culturing Techniques. Elsevier Academic Press, San Diego.
- Anonymous (1975). Proposals for a standardization of diatom terminology and diagnoses. Nova Hedwigia, Beiheft, 53: 323–354.
- Ashworth, M.P., Nakov, T. & Theriot, E.C. (2013). Revisiting Ross and Sims (1971): toward a molecular phylogeny of the Biddulphiaceae and Eupodiscaceae (Bacillariophyceae). Journal of Phycology, 49: 1207–1222.
- Chepurnov, V.A., Mann, D.G., Sabbe, K. & Vyverman, W. (2004). Experimental studies on sexual reproduction in diatoms. International Review of Cytology, 237: 91–154.
- Chepurnov, V.A., Mann, D.G., von Dassow, P., Armbrust, E.V., Sabbe, K., Dasseville, R. & Vyverman, W. (2006). Oogamous reproduction, with two-step auxosporulation, in the centric diatom Thalassiosira punctigera (Bacillariophyta). Journal of Phycology, 42: 845–858.
- Davidovich, N.A. (2001). Species-specific sizes and size range of sexual reproduction in diatoms. In Proceedings of the 16th International Diatom Symposium (Economou-Amilli, A., editor), 191–196. Amvrosiou Press, Athens.
- Davidovich, N.A., Davidovich, O.I., Podunay, Y.A., Gastineau, R., Kaczmarska, I., Poulíčková, A. & Witkowski, A. (2017). Ardissonea crystallina has a type of sexual reproduction that is unusual for centric diatoms. Scientific Reports, 7: 14670.
- Drebes, G. (1974). Marines Phytoplankton – Eine Auswahl der Helgoländer Planktonalgen (Diatomeen, Peridineen). Thieme, Stuttgart.
- Drebes, G. (1977a). Cell structure, cell division, and sexual reproduction of Attheya decora West (Bacillariophyceae, Biddulphiineae). Nova Hedwigia, 54: 167–178.
- Drebes, G. (1977b). Sexuality. In The Biology of Diatoms (Werner, D., editor), 250–283. Blackwell Science Publishers, Oxford.
- Edgar, R.C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32: 1792–1797.
- Ehrlich, A., Crawford, R.M. & Round, F.E. (1982). A study of the diatom Cerataulus laevis – the structure of the auxospore and the initial cell. British Phycological Journal, 17: 205–214.
- French III, F.W. & Hargraves, P.E. (1985). Spore formation in the life cycle of diatoms Chaetoceros diadema and Leptocylindrus danicus. Journal of Phycology, 21: 477–483.
- Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39: 1993–2002.
- Felsenstein, J. (2004). Inferring Phylogenies. Sinauer Associates, Sunderland, MA.
- Guindon, S., Dufayard, J.F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59: 307–321.
- Hasle, G.R., von Stosch, H.A. & Syvertsen, E.E. (1983). Cymatosiraceae, a new diatom family. Bacillaria, 6: 9–156.
- Heath, I.B. & Darley, W.M. (1972). Observations on the ultrastructure of the male gametes of Biddulphia laevis Ehr. Journal of Phycology, 8: 51–59.
- Hoban, M.A. (1983). Biddulphioid diatoms. II. The morphology and systematics of the Pseudocellate species, Biddulphia biddulphiana (Smith) Boyer, B. alternans (Bailey) Van Heurck, and Trigonium arcticum (Brightwell) Cleve. Botanica Marina, 26: 271–284.
- Hoppenrath, M., Elbrächter, M. & Drebes, G. (2009). Marine Phytoplankton: Selected Microphytoplankton Species from the North Sea around Helgoland and Sylt. Schweizerbart Science Publishers, Stuttgart.
- Idei, M. & Nagumo, T. (2002). Auxospore structure of the marine diatom genus Lampriscus with triangular/quadrangular forms. In Abstracts of the 17th International Diatom Symposium ( Poulin, M., editor). International Society for Diatom Research, Ottawa.
- Idei, M., Sato, S., Nagasato, C., Motomura, T., Toyoda, K., Nagumo, T. & Mann, D.G. (2015). Spermatogenesis and auxospore structure in the multipolar centric diatom Hydrosera. Journal of Phycology, 51: 144–158.
- Iwatani, N., Murakami, S. & Suzuki, Y. (2005). A sequencing protocol of some DNA regions in nuclear, chloroplastic and mitochondrial genomes with an individual colony of Thalassiosira nordenskioeldii Cleve (Bacillariophyceae). Polar Bioscience, 18: 35–45.
- Kaczmarska, I., LeGresley, M.M., Martin, J.L. & Ehrman, J. (2005). Diversity of the diatom genus Pseudo-nitzschia Peragallo in the Quoddy region of the Bay of Fundy, Canada. Harmful Algae, 4: 1–19.
- Kaczmarska, I., Poulíčková, A., Sato, S., Edlund, M.B., Idei, M., Watanabe, T. & Mann, D.G. (2013). Proposals for a terminology for diatom sexual reproduction, auxospores and resting stages. Diatom Research, 28: 263–294.
- Kaczmarska, I., Ehrman, J.M., Davidovich, N.A., Davidovich, O.I. & Podunay, Y.A. (2018). Structure and development of auxospore in Ardissonea crystallina (C. Agardh) Grunow demonstrates another way for a centric to look like a pennate. Protist, 169: 466–483.
- Kaczmarska I., Samanta B., Ehrman J.M. & Porcher E.M.A. (2019). Auxosporulation in Chaetoceros acadianus sp. nov. (Bacillariophyceae), a new member of the Section Compressa. European Journal of Phycology, 54: 206–221.
- Koester, J.A., Brawley, S.A., Karp-Boss, L. & Mann, D.G. (2007). Sexual reproduction in the marine centric diatom Ditylum brightwellii (Bacillariophyta). European Journal of Phycology, 42: 351–366.
- Leblanc, K. & Hutchins, D.A. (2005). New applications of a biogenic silica deposition fluorophore in the study of oceanic diatoms. Limnology and Oceanography Methods, 3: 462–476.
- Lefort, V., Longueville, J.E. & Gascuel, O. (2017). SMS: Smart Model Selection in PhyML. Molecular Biology and Evolution, 34: 2422–2424.
- MacGillivary, M.L. & Kaczmarska, I. (2011). Survey of the efficiency of a short fragment of the rbcL gene as a supplemental DNA barcode for diatoms. Journal of Eukaryotic Microbiology, 58: 529–536.
- Migita, S. (1967). Sexual reproduction of centric diatom Skeletonema costatum. Bulletin of the Japanese Society of Scientific Fisheries, 33: 392–398.
- Mizuno, M. (2006). Evolution of meiotic patterns of oogenesis and spermatogenesis in centric diatoms. Phycological Research, 54: 57–64
- Mizuno, M. (2008). Evolution of centric diatoms inferred from patterns of oogenesis and spermatogenesis. Phycological Research, 56: 156–165.
- Müller, O. (1889). Auxosporen von Terpsinoë musica Ehr. Berichte der Deutschen Botanischen Gesellschaft, 7: 181–183.
- Parks, M.B., Wickett, N.J. & Alverson, A.J. (2018). Signal, uncertainty, and conflict in phylogenetic data for a diverse lineage of microbial eukaryotes (Diatoms, Bacillariophyta). Molecular Biology and Evolution, 35: 80–93.
- Posada, D. & Buckley, T.R. (2004). Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology, 53: 793–808.
- Poulíčková, A., Mayama, S., Chepurnov, V.A. & Mann D.G. (2007). Heterothallic auxosporulation, incunabula and perizonium in Pinnularia (Bacillariophyceae). European Journal of Phycology, 42: 367–390.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Ross, R. & Sims, P.A. (1971). Generic limits in the Biddulphiaceae as indicated in the scanning electron microscope. In Scanning Electron Microscopy, Systematic and Evolutionary Application ( Heywood, V.H., editor), 155–177. Academic Press, London.
- Ross, R., Cox, E.J., Karayeva, N.I., Mann, D.G., Paddock, T.B.B., Simonsen, R. & Sims, P.A. (1979). An amended terminology for the siliceous components of the diatom cell. Nova Hedwigia, Beiheft, 64: 513–533.
- Round, F.E., Crawford, R.M. & Mann, D.G. (1990). The Diatoms. Biology & Morphology of the Genera. Cambridge University Press, Cambridge.
- Samanta, B., Kinney, M.E., Heffell, Q., Ehrman, J.M. & Kaczmarska, I. (2017). Gametogenesis and auxospore development in the bipolar centric diatom Brockmanniella brockmannii (Family Cymatosiraceae). Protist, 168: 527–545.
- Samanta, B., Heffell, Q., Ehrman, J.M. & Kaczmarska, I. (2018). Spermatogenesis in the bipolar centric diatom Plagiogrammopsis vanheurckii (Mediophyceae). Phycologia, 57: 354–359.
- Schmid, A.M.M. (1995). Sexual reproduction in Coscinodiscus granii Gough in culture: a preliminary report. In Proceedings of the 13th International Diatom Symposium (Marino, D. & Montresor, M., editors), 139–159. Biopress, Bristol.
- Trobajo, R., Mann, D.G., Chepurnov, V.A., Clavero, E. & Cox, E.J. (2006). Auxosporulation and size reduction pattern in Nitzschia fonticola (Bacillariophyta). Journal of Phycology, 42: 1353–1372.
- von Dassow, P., Chepurnov, V.A. & Armbrust, E.V. (2006). Relationships between growth rate, cell size, and induction of spermatogenesis in the centric diatom Thalassiosira weissflogii (Bacillariophyta). Journal of Phycology, 42: 887–899.
- von Stosch, H.A. (1954). Die Oogamie von Biddulphia mobiliensis und die bisher bekannten auxosporenbildungen bei den centrales. Huitième Congrès International de Botanique Paris 1953, Rapports et Communicatons parvenus avant le Congrès a la Section, 17: 58–68.
- von Stosch, H.A. (1956). Entwicklungsgeschichtliche Untersuchungen an zentrischen Diatomeen. II. Geschlechtszellenreifung, Befruchtung und Auxosporenbildung einiger grundbewohnender Biddulphiaceen der Nordsee. Archiv für Mikrobiologie, 23: 327–365.
- von Stosch, H.A. (1965). Manipulierung der Zellgrösse von Diatomeen im Experiment. Phycologia, 5: 21–44.
- von Stosch, H.A. (1977). Observations on Bellerochea and Streptotheca, including descriptions of three new planktonic diatom species. Nova Hedwigia, Beiheft, 54: 113–166.
- von Stosch, H.A. (1982). On auxospore envelopes in diatoms. Bacillaria, 5: 127–156.
- von Stosch, H.A., Theil, G. & Kowallik, K.V. (1973). Entwicklungsgeschichtliche Untersuchungen an zentrischen Diatomeen. V. Bau und Lebenszyklus von Chaetoceros didymum, mit Beobachtungen über einige andere Arten der Gattung. Helgoländer Wissenschaflicher Meeresuntersuchungen, 25: 384–445.
- Zimmermann, J., Jahn, R. & Gemeinholzer, B. (2011). Barcoding diatoms: evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Organisms Diversity & Evolution, 11: 173–192.
