ABSTRACT
The composition and patterns of spatial and season variability of epiphytic diatom assemblages of five intertidal species of Gelidiales (Rhodophyta) from three sampling sites off Gran Canaria (Canary Islands, Atlantic Ocean) were investigated during summer (2007) and winter (2008) seasons using scanning electron microscopy (SEM). Dominant species included Cocconeis scutellum var. scutellum, C. scutellum var. posidoniae, Grammatophora oceanica, Lichmophora eherembergi, Navicula sp. and Tabularia fasciculata and these represented 78% of the total diatom assemblage on Gelidiales. Eight of the diatom taxa, Cocconeis maxima, Amphicocconeis debesii, Cocconeis convexa, C. neothumensis var. marina, C. scutellum var. scutellum, C. scutellum var. posidoniae, Gomphonemopsis obscura and Pseudohimantidium pacificum, found in the present study represent new records for the Canary Archipelago. These species showed two distinct patterns of zonation, one in relation to their position at the intertidal area and the other relative to position on the host thalli. Although the vertical distribution of host thalli at the intertidal zone was important when considering the assemblage structure of epiphytic diatoms, the results showed that the cell abundance distribution changed more significantly when considering season rather than spatial distribution. Moreover, even though morphology of host thalli was not a determining factor, their aggregation mode had a significant influence on the abundance and distribution of microalgae. Finally, considering the growth forms of diatom communities, adnate diatoms were more abundant in turf than in clump species.
Introduction
Microphytobenthos, including algal epiphytes, has long been recognized as a significant component of both the biodiversity and ecological function of marine assemblages, being an important contributor of primary production and a source of food for grazing (Kitting et al., Citation1984; D’Antonio, Citation1985; Booth, Citation1987; Underwood & Kromkamp, Citation1999; Moncreiff & Sullivan, Citation2001; Meadows et al., Citation2012; Piccinetti et al., Citation2016; Pennesi & Danovaro, Citation2017). Algal epiphytes, due to their distribution and composition in intertidal habitats, are increasingly recognized as important assemblages with which to study environmental stress, such as nutrient availability (Ballantine, Citation1979; Tomasko & Lapointe, Citation1991; Neckles et al., Citation1993; Cambridge et al., Citation2007; Costa et al., Citation2009, Citation2014; Tuya et al., Citation2013), and wave and tidal exposure (Schanz et al., Citation2002). The diatoms constitute the major fraction of microepiphytes on marine macrophytes (Kita & Harada, Citation1962; Jacobs & Noten, Citation1980; Round et al., Citation1990; Graham & Wilcox, Citation2000), and they play important ecological roles in aquatic ecosystems as important contributors to primary production, as a source of food for grazers and to help to drive global cycling of environmental variables (Round et al., Citation1990). On the northern coasts of Gran Canaria, which are characterized by high wave action, macroalgae of the Order Gelidiales (Kylin, Citation1923; Rhodophyta) are common and form dense assemblages which appear as mixed belts or as well marked successive monospecific belts. They are mainly represented by five species: Gelidium arbuscula, G. canariense, G. pusillum, Pterocladiella capillacea and P. melanoidea. These macroalgae are distributed along a vertical gradient, where G. pusillum and P. melanoidea occupy the upper intertidal, P. capillacea the middle intertidal, G. arbuscula the lower intertidal and G. canariense the upper subtidal. Along the vertical intertidal gradient, the tide influences the distribution of macroalgae via abiotic stresses related to extreme temperature, desiccation, irradiance and osmotic potential (Raffaelli & Hawkins, Citation1996; Domínguez-Álvarez et al., Citation2011). The same factors could affect the abundance and distribution of the epiphytes that inhabit the macroalgae assemblages, although the effect of water movement and tidal gradient on epiphytic diatom assemblages has rarely been considered until now.
Studies on vertical zonation of benthic diatoms on rocks (Aleem, Citation1950; Castenholtz, Citation1963) and in tidal pools (Metaxas & Lewis, Citation1992) are dated, and no data are available for epiphytic diatom assemblage on marine macroalgae. Navarro et al. (Citation1989) found large differences between sites of high and low exposure to wave action in diatom assemblages on different substrata in Puerto Rico. Costa et al. (Citation2009) observed a lower species richness of epiphytic diatoms on thalli of Galaxaura rugosa exposed to trade winds and high water motion. In comparison to other macroalgal-dominated regions, at Gran Canaria the Gelidiales are almost free of epiphytic macroalgae. The zonation of epiphytic diatom taxa on the macroalgae is related to the different thallus morphologies, and the surface of the host available for diatom adhesion (Thomas & Jiang, Citation1986; Mazzella & Spinoccia, Citation1992; Snoeijs, Citation1994, Citation1995; Busse & Snoeijs, Citation2002; Al-Handal & Wulff, Citation2008; Sutherland, Citation2008; Totti et al., Citation2009; Romagnoli et al., Citation2014). Indeed, epiphyte adhesion and distribution are influenced by the same abiotic conditions that affect the vertical zonation of their host, but also by the conformation of the thalli of the macroalgae themselves which could offer different micro-environmental conditions for the establishment and consolidation of epiphytic assemblages (Costa et al., Citation2009). Along with their different position in the intertidal, macroalgae differ in their structure and aggregation mode. In particular, Pterocladiella capillacea, Gelidium arbuscula and G. canariense are characterized by complex cylindrical thalli organized in upright ramets that arise from a holdfast of entangled stolons (i.e. clumps). Thalli of P. capillacea are mostly between 4 and 10 cm long, G. canariense has thalli over 14 cm long, while G. arbuscula shows clumps made from a few small upright ramets (Polifrone et al., Citation2012). The other two species (i.e. P. melanoidea and G. pusillum) are characterized by upright cylindrical axes (1–3 cm long) which arise from a stoloniferous holdfast but they do not form clumps and extend on the rocks as a turf resembling a carpet. The three species that form clumps have a bushy appearance (branching order up to 5) while the smallest species are scarcely branched (branching order up to 2; Seoane Camba, Citation1979; Fredriksen & Rueness, Citation1990; Santelices & Hommersand, Citation1997; Polifrone et al., Citation2012).
The goal of this study was to assess the density and structure of epiphytic diatom assemblages on the species belonging to the family Gelidiaceae. In addition, we explored the role of, and differences in, the diatom assemblage structure for each macroalga in relation to their ecological aggregations (i.e. turf, clump), morphology and their seasonal variability.
Materials and methods
Sampling and sites
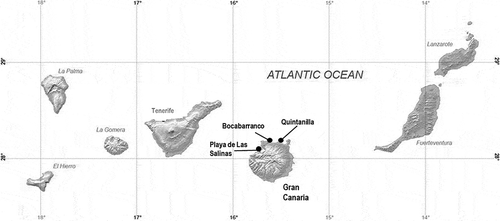
The Canary Islands (Atlantic Ocean, Spain) are an archipelago of volcanic origin located in the northern Atlantic Ocean between 100 and 600 km off the north-west coast of Africa (28°N). They consist of seven main islands (Tenerife, Fuerteventura, Gran Canaria, Lanzarote, La Palma, La Gomera and El Hierro). The sampling area is located on the Gran Canaria Island, where there is a different hydrodynamic regime between the exposed north and north-east facing shores, and the more sheltered south and south-western facing coast of the island (Barton et al., Citation1998; Davenport et al., Citation2002). The climate is temperate. Surface water temperature is between 18°C and 23°C, with constant salinity of 37% and semidiurnal tides with an average high tide of 2 m and an average low tide of 0.8 m. The particular oceanographic conditions of this region (Barton et al., Citation1998; Davenport et al., Citation2002) are associated with high species richness in macroalgae assemblages, with their distribution strictly depending on the hydrodynamic conditions. shows the three sampling sites in Gran Canaria island which showed abundant assemblages of Gelidiales: Quintanilla (Arucas, UTM: 446325.38-3113527.26), Bocabarranco (Gáldar, UTM 434593.26-3114923.25) and Playa de Las Salinas (Agaete, 430126.24-3109173.27). Quantitative measurements of water movement are difficult to apply in wave-exposed habitats; therefore, the wave exposure was described in terms of prevailing currents, wind direction and coastal topography. According to this qualitative estimation, Playa de Las Salinas and Bocabarranco are more exposed sites than Quintanilla. At the three sites, Gelidiales formed a well-defined belt and were characterized by a marked vertical zonation. The sampling of five macroalgal taxa (i.e. G. arbuscula, G. canariense, G. pusillum, P. capillacea, P. melanoidea) was carried out in the rocky intertidal zone in summer 2007 and winter 2008 at low tide. The algae were collected by hand, taking care to collect the whole thallus. Considering the structure of the populations and the aggregation of thalli in clumps or turf through a stoloniferous prostrate system, three upright axes for each species and at each season and locality were randomly collected from aggregations which were at least 20 cm apart, usually on separate rocks or tide pools, in order to avoid the sampling of the same individual. The collected samples (i.e. 18 thalli for each species) were immediately placed in separate plastic bags with 4% formalin/seawater solution and transported inside ice coolers to the laboratory of the Departamento de Biología at the Universidad de Las Palmas de Gran Canaria within 2 hours. The thalli were examined under a Wild M3Z Heerbrugg dissecting microscope and under an Olympus CX41 microscope, and then identified following Santelices & Hommersand (Citation1997) and Afonso-Carrillo & Sansón (Citation1999).
Sample processing and identification of diatoms
Three 1 cm in length pieces (one for each replicate) of upright axes of each seaweed species were cut at the apical, middle and basal parts. In order to preserve the epiphytic diatom assemblages and their mode of attachment to the substratum, the seaweed samples were processed through a critical point drying procedure following the ‘sandwich method’ (Totti et al., Citation2003). After washes with distilled water to remove the excess fixative, samples were dehydrated individually in a serial alcohol gradient (10, 25, 50, 60, 80, 90, 95 and 100%) and finally dried using a Critical Point Dryer (Polaron CPD7501) (Totti et al., Citation2003). Dried samples were attached to aluminium stubs by an adhesive carbon disc, sputter-coated with Au-Pd (Polaron 132 SC7640) and examined in scanning electron microscopy (SEM, Jeol 6060LV) operating at 25 kV. The epiphytic diatoms were counted using SEM microscopy between 80 and 400 visual fields, each having an area of 2976 µm2, at 1500 times magnification, depending on the number of the epiphytic diatom cells on the host thallus. Abundances were expressed as number of cells mm–2 of thallus surface (calculated using predefined spreadsheets in Microsoft Access) and the relative abundance as their proportion to the total number of diatoms. The identification of diatoms to the lowest possible taxonomic rank was made possible through an oxidation of the organic component of their frustules. Epiphytic diatoms were scraped from the seaweed surface and cleaned following von Stosch’s method in Hasle & Syvertsen (Citation1997). A few drops of cleaned diatom material were poured on a 0.2 µm pore size Nucleopore polycarbonate filter fixed to an aluminium stub with double adhesive carbon disc, left to air dry and coated with Au-Pd before examination in SEM. Epiphytic diatoms were subdivided into the following growth forms: adnate for diatoms firmly attached to the substratum through their valve face; erect for diatoms attached to seaweeds through mucus pads or peduncles; and motile for biraphid diatoms freely moving on the substratum. The identification of diatoms was made following Hustedt (Citation1931–1959, Citation1961–1966), Hendey (Citation1964), Peragallo & Peragallo (Citation1897–1908), Poulin et al. (Citation1984a,Citation1984b), De Stefano et al. (Citation2000, Citation2008), Witkowski et al. (Citation2000), De Stefano & Romero (Citation2005) and Kooistra et al. (Citation2008).
Data analysis
The qualitative measure of diatom taxa was classified as follows: very rare (vr), from 2–5 cells mm–2 of host macroalga; rare (r), 6–10 cells mm–2 of host macroalga; sporadic (s), up to 10% of the total cells mm–2 of host macroalga; frequent (f), 10.01–20% of the total cells mm–2 of host macroalga; and common (c), more than 20% of the total cells mm–2 of host macroalga. Statistical analysis on total abundance in summer and winter and ecological forms of diatoms was performed in R (R Development Core Team, Citation2009), and using the routines included in the software package PRIMER® 6 software (Clarke & Gorley, Citation2006). The data were non-normal so we used non-parametric methods to compare groups; the Wilcoxon–Mann–Whitney test to compare central tendencies between two groups and the Kruskal–Wallis test to compare among more than two groups. Pearson’s chi-square test was used to compare different morphologies using data in frequency tables. A cluster canonical correlation analysis was performed in order to relate the structure of diatom forms to the major environmental parameters measured in this study, such as sea surface temperature (SST), global solar radiation, PAR and position of the algae in the intertidal. Results of the latter analysis were not relevant due to the similar environmental conditions present in the three study sites and are not reported.
Results
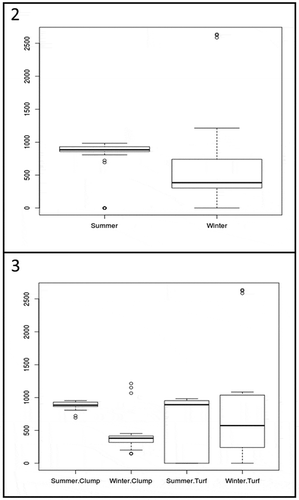
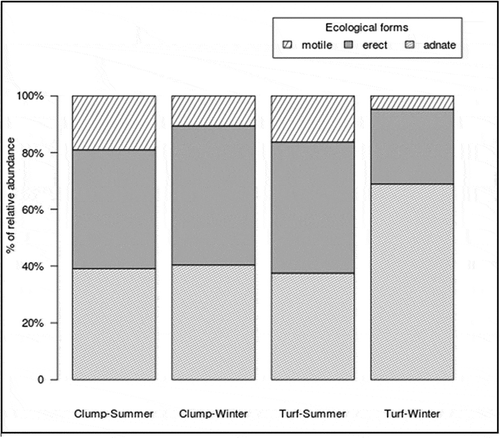
Electron microscope analysis (SEM) showed the presence of a dense layer of filamentous fungi on the thalli of the five Gelidiales analysed (i.e. G. arbuscula, G. canariense, G. pusillum, P. capillacea, P. melanoidea), while diatoms were the most abundant microalgae. shows the diatom taxa observed on the thalli of the five Gelidiales: 27 taxa subdivided in 16 genera of the class Bacillariophyceae, subclasses Bacillariophycidae and Fragilariophycidae were reported. Cocconeis scutellum var. posidonia, Cocconeis scutellum var. scutellum, Grammatophora oceanica, Lichmophora eherembergi, Navicula sp. and Tabularia fasciculata represented 78% of the total diatoms on Gelidiales analysed and were here classified as frequent or common taxa on the different species of macroalgae. Among these taxa, C. scutellum var. posidoniae was the only one that could differentiate the two aggregation modes of macroalgae, being more common on small and less branched species which formed turf. Cocconeis maxima, Amphicocconeis debesi and Cocconeis neothumensis var. marina were found exclusively on G. arbuscula; Amphicocconeis sp. and Cocconeis subtilissima were found only on thalli of G. canariense; and Campyloneis grevillei, Cocconeis pellucida and Gomphonemopsis obscura were exclusive to P. melanoidea. Most of these taxa are here considered rare or very rare due to a density below 10 cells mm–2. Most diatom species were found during both seasons and are probably permanent epiphytes on the Gelidiales in the north coast of Gran Canaria, while only few rare and sporadic species showed a seasonal abundance. In general, higher species richness was observed in winter than in summer for all species except for P. capillacea and G. canariense, where the number of species remained unaltered although the composition varied. A highly significant difference (p < 0.01) was observed between the total diatom abundance in summer and winter (i.e. major in summer) and a Wilcoxon–Mann–Whitney test indicated that the difference was due to the abundance of diatoms on clump algae (p < 0.01) rather than turf (p > 0.05), because the latter presented a more stable structure between the two seasons (). In fact, in the clump species, diatoms were more abundant in summer (294 ± 33 cells mm–2) than in winter (142 ± 107 cells mm–2), while in the turf species the mean number of cells mm–2 was 260 ± 148 in summer and 280 ± 340 in winter. The Wilcoxon–Mann–Whitney test also provided evidence for high differences in the assemblage structure of diatoms on the two host aggregation types and in the two seasons (). The PERMANOVA Main-test showed that the species of the Gelidiales considered (i.e. G. arbuscula, G. canariense, G. pusillum, P. capillacea, P. melanoidea) had a significant effect on the epiphytic species composition in both summer and winter with p = 0.004 and 0.006, respectively. The pairwise comparison of the different species of red algae analysed showed that in winter P. capillacea and G. canariense were similar in diatom species composition (p = 0.021). The adnate diatoms maintained almost the same percentage in summer (39%) and winter (40%) in the clump algae and the erect diatoms increased from 42% in summer to 49% in winter. The proportion of motile diatomsfell from 19% in summer to 11% in winter when the hydrodynamic conditions became harsher. In the turf algae, a higher percentage of adnate diatoms was observed in winter (69%) than in summer (38%) and there was a reduction of both erect (26%) and motile forms (5%) ().
Table 1. Qualitative and quantitative abundance of epiphytic diatoms from five species of Gelidiales collected in summer 2007 and winter 2008 at three localities in Gran Canaria (i.e., Quintanilla, Bocabarranco and Playa de Las Salinas) in relation to their ecological aggregations (turf, clump). vr = very rare; r = rare; s = sporadic; f = frequent; c = common
Fig. 4. Contribution of the different life ecological forms of the benthic diatoms (i.e. motile, erect, adnate) to total diatom abundance at each turf and clump per season (i.e. winter and summer)
Figs 5‒6. Fig. 5. Boxplot showing the difference of the total diatom abundance in the three localities of Playa de Las Salinas (S), Bocabarranco (B) and Quintanilla (Q). Fig. 6. Percentage contribution of abundances for each diatom form (i.e. motile, erect, adnate) in the three localities of Playa de Las Salinas (S), Bocabarranco (B) and Quintanilla (Q)
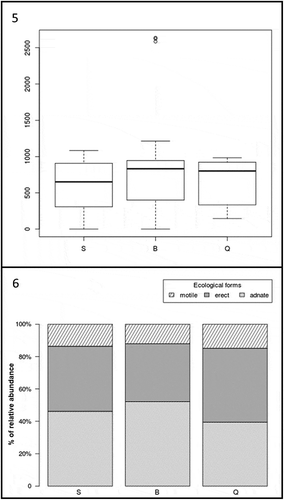
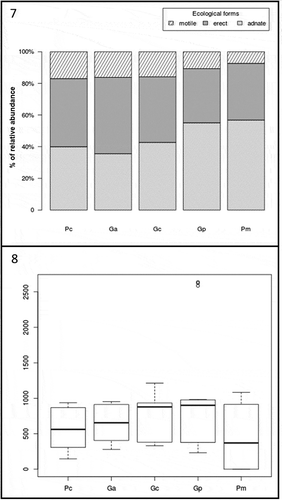
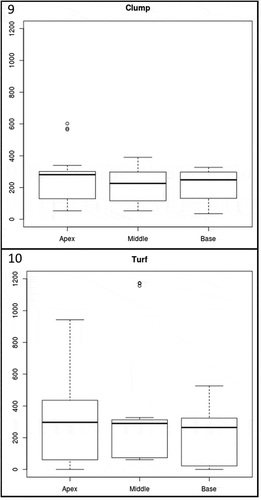
The total abundance of diatoms at each locality did not differ significantly () but there were significant differences in the assemblage structure (), with a higher percentage of adnate (52%) at Bocabarranco (Gáldar) and of erect (46%) diatoms at Quintanilla, while motile diatoms were little represented in all localities (12–15%). Surprisingly, P. melanoidea and G. pusillum were the only host species which lacked epiphytic diatoms on the entire thallus, or at least part of it, at Playa de Las Salinas and Bocabarranco. Nevertheless, Kruskal–Wallis rank sum test indicated no significance (p = 0.05) in the difference in total diatom abundance between host species (). These results suggest that differences between species at different vertical gradients in the intertidal exerted a weak control on epiphytic diatom density. The mean diatom abundance varied from 191 ± 102 cells mm–2 on P. capillacea to 252 ± 120 cells mm–2 on G. canariense, for the clump-forming species; and from 150 ± 205 cells mm–2 on P. melanoidea to 336 ± 284 cells mm–2 on G. pusillum, for the turf-forming species. The percentage of diatom forms on the different host species varied significantly (p < 0.005) within both clump and turf assemblages (, ). Turf algae showed a higher percentage of adnate diatoms than clump, although the percentage of motile diatoms was higher in G. pusillum than in P. melanoidea. Clump species had almost the same pattern of diatom forms shown in turf species, with the exception of G. arbuscula which showed a higher amount of erect forms than the other two clump species (). There were no significant differences in diatom abundance with aggregation type of host (clump or turf) or vertical distribution (p > 0.05). Nevertheless, significant differences were found when performing the Pearson’s chi-squared test to compare the diatom growth forms at each level of thalli for the two-host aggregation mode (, ). In both clump and turf algae, the same pattern was observed, where the portion of adnate diatoms was higher at the base of thalli and reduced at the apex, while the percentage of erect and motile diatoms was higher at the apex and middle part and reduced at the base. However, the turf algae showed a higher percentage of adnate diatoms than the clump species.
Figs 7‒8. Fig. 7. Percentage contribution of abundances for each diatom form (i.e. motile, erect, adnate) in the different host species (i.e. Pc = Pterocladiella capillacea, Ga = Gelidium arbuscula, Gc = G. canariense, Gp = G. pusillum, Pm = P. melanoidea). Fig. 8. Boxplot showing the difference of the total diatom abundance in the different host species (i.e. Pc = Pterocladiella capillacea, Ga = Gelidium arbuscula, Gc = G. canariense, Gp = G. pusillum, Pm = P. melanoidea)
Figs 9‒10. Fig. 9. Boxplot showing the difference of the total diatom abundance in the different levels along the host thalli (i.e. apex, middle, base) per clump. Fig. 10. Boxplot showing the difference of the total diatom abundance in the different levels along the host thalli (i.e. apex, middle, base) per turf
Discussion
Despite the importance diatoms have in contributing to the productivity of coastal areas, only a few studies have been carried out to estimate their abundance and distribution in the Canary Islands, and most of those are related to microphytobenthos associated with soft and rocky bottoms or planktonic diatom assemblages (Gil-Rodríguez et al., Citation2003; Ojeda Rodríguez et al., Citation2005). Marine epiphytic diatoms on macroalgae in the Canary Islands have not been previously studied in detail, and information on their distribution and abundance is scarce (Sansón & Reyes, Citation1995) especially for the order Gelidiales. As has been observed in previous studies of marine benthic organisms from other regions (Round et al., Citation1990; Siqueiros-Beltrones & Hernández-Almeida, Citation2006; Corlett & Jones, Citation2007; Balata et al., Citation2008; Lebreton et al., Citation2009; Gauna et al., Citation2015; Siqueiros-Beltrones et al., Citation2017), diatoms were the predominant epiphytes. Eight of the identified diatom taxa are new records for the Canary Archipelago: Cocconeis maxima, Amphicocconeis debesii, Cocconeis convexa, C. neothumensis var. marina, C. scutellum var. posidoniae, C. scutellum var. scutellum, Gomphonemopsis obscura and Pseudohimantidium pacificum. Although the data show that some species of diatoms prefer specific host thalli, we cannot say for certain that there is a clear specificity for that host due to the scarce abundances measured (e.g. sporadic presence of Achnanthidium maximum only on G. arbuscula). Taxa classified as ‘frequent’ or ‘common’ were present on the five Gelidiales from Gran Canaria. These data agreed with previous studies which analysed epiphytic diatom specificity on a host macrophyte (Siqueiros-Beltrones & Hernández-Almeida, Citation2006; Corlett & Jones, Citation2007; Balata et al., Citation2008; Lebreton et al., Citation2009; Gauna et al., Citation2015; Siqueiros-Beltrones et al., Citation2017). Romagnoli et al. (Citation2007) studied the ecological succession of microalgal assemblages on different substrata and reported that a well-developed community, characterized by the presence of adnate living forms, is established after 3–5 weeks. The species richness of diatom epiphytes on Gelidiales (S = 27) was extremely reduced compared with the epiphytic diatom assemblage on other macroalgae (Siqueiros-Beltrones & Valenzuela-Romero, Citation2004; Argumedo-Hernández & Siqueiros-Beltrones, Citation2008; Hernández-Almeida & Siqueiros-Beltrones, Citation2012; Siqueiros-Beltrones et al., Citation2017), mangroves (Sullivan, Citation1981; Chen et al., Citation2010) or seagrasses (Mazzella et al., Citation1994; Pinckney & Micheli, Citation1998; De Stefano et al., Citation2000; Gambi et al., Citation2000). Previously authors have reported similar results in diatom assemblages of extreme habitats such as hypersaline ponds (Siqueiros-Beltrones, Citation1988, Citation1990) and other coastal habitats with high-intensity hydrodynamic conditions (Costa et al., Citation2009), since species diversity usually declines under a harsh disturbance regime, where only few species are able to survive or settle successfully. As reported in previous studies of macroalgae in other geographic areas (Tanaka & Watanabe, Citation1990; Tuji, Citation2000; Hameed, Citation2003; Costa et al., Citation2009), the presence of a reduced number of motile diatoms and the prevalence of erect and adnate forms indicated a mature assemblage, characterized by species (i.e. Cocconeis scutellum var. scutellum and Grammatophora. oceanica) which adhere firmly to the thalli throughout the year, regardless of the intensity of the water currents. Consequently, we hypothesize that the low richness of diatom species observed on Gelidiaceae in Gran Canaria is due to the microhabitat conditions which result from the hydrodynamic regime; although in the present study we could not compare localities with high or low hydrodynamic conditions because these five species of macroalgae are strictly related to habitats characterized by high water motion at Gran Canaria. Even if the harsh environmental condition could favour the settlement and adhesion of only a few taxa, the assemblage seemed to have reached the final stages of an ecological succession because motile diatoms, scarce on Gelidiales from Gran Canaria, are the most frequent early algal colonizers of natural and artificial substrata through production of the primary biofilm of mucilage which allows the adhesion of other more stable ecological forms (Wetherbee et al., Citation1998; Evans, Citation2003; Higgins et al., Citation2003).
Factors other than abiotic conditions could influence the abundance and structure of epiphytic assemblages, such as grazing, the morphology of the host algae and competition among epiphytes colonizing the host surface (Aumack et al., Citation2011). We observed a homogeneous distribution of diatoms on clump host species, with almost equal relative abundance of several epiphytic diatom species and evidence of competition between them, with a slight prevalence of G. oceanica over other diatoms. However, on turf-forming algal species, the diatom distribution was heterogeneous and patchy, and it was possible to observe portions of thalli or entire thalli with no epiphytes at all. On turf algae thalli C. scutellum var. posidoniae prevailed over the other species. An important factor that could limit the adhesion of diatoms, and possibly influence the microepiphytism as well, was the abundant coverage of fungi observed on most thalli of all species of Gelidiales considered in this study. The proliferation of fungal hyphae which covered a great part of the thalli could make the growth of other epiphytes difficult, reducing the surface available for the adhesion of both macro and microalgae.
Environmental factors such as temperature, and especially light availability, are probably directly responsible for the observed seasonal changes, as was reported by Chen et al. (Citation2010) in the case of epiphytic diatoms on mangroves. Our data show that the density and composition of epiphytic diatom assemblies on the host thalli showed seasonal variation only in the clump-forming algae, while turf-forming species showed stability in diatom abundance. In particular, on the clump-forming algae the percentage of adnate diatoms remained almost unaltered, while erect forms increased, and motile ones decreased as environmental conditions worsened in winter. On the turf-forming algae, the increase in the percentage of adnate and decrease in mobile and erect diatom species was probably due to the higher strength of wave break on the rocks during the winter season, which would detach mobile and erect diatoms allowing the spread of adnate species, which are more likely to be resistant to intense water motion.
The epiphytic diatoms showed low variability in density and species composition between the different sites analysed (i.e. Quintanilla, Bocabarranco and Playa de Las Salinas). This low variation between localities was probably due to the similarity of environmental conditions, such as temperature, water motion and especially irradiance, the intensity of which is known to increase the total biomass and diversity of epibenthonic diatoms (Hudon & Bourget, Citation1983). In contrast, there were differences in the assemblage structure of diatoms (according to their growth form) between sampling sites, which could be indicative of slightly different biotic and abiotic factors at each locality. The presence of a higher density of adnate species (i.e. Cocconeis spp., Achnanthes brevipes Agardh and Navicula spp.), which have higher adhesive strength on the host surface, could be indicative of a higher hydrodynamic exposure at Bocabarranco than at Playa de Las Salinas. In the same way, if we consider the abundance of growth forms of diatoms as a possible indicator of a hydrodynamic gradient, Quintanilla was the less exposed site. Unfortunately, we could not obtain any in situ measurements of environmental parameters (such those based on calcium carbonate) to support our data on diatom assemblages because the intense wave break at the coast made it impossible to site the apparatus normally used.
Aleem (Citation1950) and Castenholtz (Citation1963) observed a zonation pattern in the abundance and distribution of diatoms in the intertidal, mostly owing to tidal height and emersion time. Metaxas & Lewis (Citation1992) observed vertical zonation in diatoms living inside tidal pools, mostly owing to different flushing frequencies. In our study no difference was found in the total abundance and distribution of growth forms of diatoms on species located in the upper intertidal and shallow subtidal zones (i.e. P. capillacea and G. canariense), or between species distributed in different belts in the upper intertidal (i.e. P. melanoidea and G. pusillum). This is probably because the macroalgal thalli mitigate the effect of desiccation, maintaining a wet microenvironment. Instead, the differences observed in the epiphytic diatom assemblage on the five Gelidiales could be mostly due to the different morphology of host macroalgae and their aggregation mode. In fact, the composition of the epiphytic diatom assemblage, in terms of growth forms, was significantly different between the two seaweed aggregation modes. The effect of the host macroalgal morphology on the composition and structure of the epiphytic diatom assemblage has also been considered by other authors (Thomas & Jiang, Citation1986; Al-Handal & Wulff, Citation2008; Sutherland, Citation2008; Totti et al., Citation2009). Our results on the distribution of diatom growth forms on turf-forming algae were different to the observations of Thomas & Jiang (Citation1986), Snoeijs (Citation1994, Citation1995) and Totti et al. (Citation2009), where increasing cell abundances were reported on thalli with a more complex microarchitecture. Despite all the morphological divergences between host species and ecological diatom assemblages, we could find a clear pattern in the distribution of the growth forms of epiphytic diatoms. On the one hand, diatom assemblages within the clump-forming species, G. arbuscula were different to those on G. canariense and P. capillacea, probably due to differences in thallus size of these three host species. Adnate growth forms of diatoms were consequently less represented and substituted by erect forms. On the other hand, the greater anomaly resided in the differences observed between clump- and turf-forming algae. Thomas & Jiang (Citation1986) found a positive relationship between the branching order of the filamentous algae and the number of diatom taxa hosted. Due to the simplified microarchitecture of the turf algae (thalli of about 2 mm long with reduced branching order), it was expected that clump algae would have a higher amount of adnate diatom forms due to a larger surface available for the adhesion of cells. However, we found that adnate forms were more abundant on turf algae than on clump species. We did not find any difference in diatom assemblages between macroalgae with flexible (Pterocladiella) and coarse thalli (Gelidium), in agreement with Round (Citation1981) who studied thalli of Laminaria sp. with smooth and rough surfaces. In regard to the position along the thallus from the base to the apex, Main & McIntire (Citation1974) did not find any difference in diatom distribution along the thalli of several macrophytes. Totti et al. (Citation2009) studied the vertical distribution of epiphytic diatoms on Fucus vesciculosus, F. evanescens and Saccharina latissima (as Laminaria saccharina) and found differences only for F. vesiculosus, with the number of epiphytic diatoms on the apical part significantly higher than on the basal part. In our study, we did not find differences in diatom abundance at three parts of the thalli, but we found a significant difference in their assemblage structure.
Overall, our results imply a role for water motion in limiting the abundance of diatoms on thalli of Gelidiales at the northern shores of Gran Canaria, and no diatom species were specific to any macroalgae. A seasonal variation in diatoms abundance was observed between the sampling sites, mainly due to variation in abundance of diatoms in clump species between summer and winter. Differences in terms of diatom abundance were not significant at any spatial level but they were important in the definition of their assemblage structure. Pattern of the host thalli (i.e. turf or clump) had a significant influence on the abundance and distribution of diatoms. Moreover, the vertical distribution of host thalli in the intertidal was significant when considering the assemblage structure of epiphytic diatoms.
Author contributions
M. Polifrone, original concept, sampling, data analysis and interpretation, drafting, writing and editing manuscript; M.A. Viera-Rodríguez, data analysis and interpretation; C. Pennesi, original concept, data analysis and interpretation, drafting, writing and editing manuscript and Author for correspondence; M.T. Cante, sampling and data analysis; A.S. Del Pino, sampling and data analysis; M. Stroobant, sampling and data analysis; M. De Stefano, original concept, sampling, data analysis and interpretation, drafting and editing manuscript.
Acknowledgements
Prof. De Stefano acknowledges assistance of Sultan Qaboos University.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Afonso-Carrillo, J. & Sansón, M. (1999). Algas hongos y fanerógamas marinas de las Islas Canarias. Clave analítica. Materiales Didácticos Universitarios. Serie Biología/2. Universidad de La Laguna, La Laguna.
- Aleem, A.A. (1950). The diatom community inhabiting the mud-flats at Whitstable. New Phytologist, 49: 174–188.
- Al-Handal, A.Y. & Wulff, A. (2008). Marine epiphytic diatoms from the shallow sublittoral zone in Potter Cove, King George Island, Antarctica. Botanica Maina, 51: 411–435.
- Argumedo-Hernández, U. & Siqueiros-Beltrones, D.A. (2008). Cambios en la estructura de la asociación de diatomeas epífitas de Macrocystis pyrifera (L.) C. Ag. Acta Botanica Mexicana, 82: 43‒66.
- Aumack, C.F., Amsler, C.D., McClintock, J.B. & Baker, B.J. (2011). Impacts of mesograzers on epiphyte and endophyte growth associated with chemically defended macroalge from the western Antarctic Peninsula: a mesocosm experiment. Journal of Phycology, 47: 36–41.
- Balata, D., Bertocci, I., Piazzi, L. & Nesti, U. (2008). Comparison between epiphyte assemblages of leaves and rhizomes of the seagrass Posidonia oceanica subjected to different levels of anthropogenic eutrophication. Estuarine Coastal and Shelf Science, 79: 533–540.
- Ballantine, D.L. (1979). The distribution of algal epiphytes on macrophyte hosts offshore from La Parguera, Puerto Rico. Botanica Marina, 22: 107–110.
- Barton, E.D., Arı́stegui, J., Tett, P., Cantón, M., Garcı́a-Braun, J.A., Hernández-Leon, S., Nykjaer, L., Almeida, C., Almunia, J., Ballasteros, S., Basterretxea, G., Escánez, J., Garcı́a-Weill, J., Hernández-Guerra, A., López-Laatzen, F., Molina, R., Montero, M.F., Navarro-Pérez, E., Rodrı́guez-Pérez, J.M., Van Leening, K., Vélez, H. & Wild, K. (1998). The transition zone of the Canary Current upwelling region. Progress in Oceanography, 41: 457–503.
- Booth, W.E. (1987). Contribution by diatoms to marine algal host-epiphyte photosynthesis. Botanica Marina, 30: 129–140.
- Busse, S. & Snoeijs, P. (2002). Gradient responses of diatom communities in the Bothnian Bay, northern Baltic Sea. Nova Hedwigia, 74: 501–525.
- Cambridge, M.L., How, J.R., Lavery, P.S. & Vanderklift, M.A. (2007). Retrospective analysis of epiphyte assemblages in relation to seagrass loss in a eutrophic coastal embayment. Marine Ecology Progress Series, 346: 97–107.
- Castenholtz, R.W. (1963). An experimental study of the vertical distribution of littoral marine diatoms. Limnology and Oceanography, 8: 450–462.
- Chen, C.P., Ya-Hui, G. & Ling, P. (2010). Geographical and seasonal patterns of epiphytic diatoms on a subtropical mangrove (Kandelia candel) in southern China. Ecological Indicators, 10: 143–147.
- Clarke, K.R. & Gorley, R.N. (2006). PRIMER V6: User Manual/Tutorial. PRIMER-E, Plymouth.
- Corlett, H. & Jones, B. (2007). Epiphyte communities on Thalassia testudinum from Grand Cayman, British West Indies: their composition, structure, and contribution to lagoonal sediments. Sedimentary Geology, 194: 245‒262.
- Costa, M.M.S., Eskinazi-Leҫe, E., Pereira, S.M. & Bandeira-Pedrosa, M.E. (2009). Diatomáceas epífitas em Galaxaura rugosa (J.Ellis & Solander) J. V. Lamouroux no Arquipélago de Fernando de Noronha, PE, Nordeste do Brasil. Acta Botanica Brasilica, 23: 713–719.
- Costa, M.M.S., Pereira S.M.B., De Arruda, P.C. & Leça, E.E. (2014). Quantitative variation of epiphytic diatoms in Galaxaura rugosa (Nemaliales: Rhodophyta). Marine Biodiversity Records, 7: e85.
- D’Antonio, C. (1985). Epiphytes on the rocky intertidal red alga Rhodomela larix (Turner) C. Agardh: negative effects on the host and food for herbivores? Journal of Experimental Marine Biology and Ecology, 86: 197–218.
- Davenport, R., Never, S., Helmke, P., Perez-Moreno, J. & Llinás, O. (2002). Primary productivity in the northern Canary Islands region as inferred from SeaWIFS imagery. Deep-Sea Research Part II, 49: 3481–3496.
- De Stefano, M., Marino, D. & Mazzella, L. (2000). Marine taxa of Cocconeis on leaves of Posidonia oceanica, including a new species and two new varieties. European Journal of Phycology, 35: 225–242.
- De Stefano, M. & Romero, O. (2005). A survey of alveolate species of the diatom genus Cocconeis (Ehr.) with remarks on the new section Alveolatae. Bibliotheca Diatomologica, 52: 1–133.
- De Stefano, M., Romero, O. & Totti, C. (2008). A comparative study of Cocconeis scutellum Ehrenberg and its varieties (Bacillariophyta). Botanica Marina, 51: 506–536.
- Domínguez-Álvarez, S., Rico, J.M. & Gil-Rodríguez, M.C. (2011). Photosynthetic response and zonation of three species of Gelidiales from Tenerife, Canary Islands. Anales Del Jardin Botanico De Madrid, 68: 117‒124.
- Evans, L-V. (2003). Biofilms: Recent Advances in their Study and Control. Harwood Academic, Amsterdam.
- Fredriksen, S. & Rueness, J. (1990). Culture studies on Pterocladiella melanoidea (Schousboe ex Bornet) comb. nov. (Gelidiales, Rhodophyta). Phycologia, 29: 182‒190.
- Gambi, M.C., Zupo, V., Buia, M.C. & Mazzella, L. (2000). Feeding ecology of Platynereis dumerilii (Audouin & Milne-Edwards) in the seagrass Posidonia oceanica system: the role of the epiphytic flora (Polychaeta, Nereididae). Ophelia, 53: 89‒202.
- Gauna, M.C., Cáceres, E.J. & Parodi, E.R. (2015). Spatial and temporal variability in algal epiphytes on Patagonian Dictyota dichotoma (Dictyotales, Phaeophyceae). Aquatic Botany, 120: 338‒345.
- Gil-Rodríguez, M.C., Haroun, R., Ojeda Rodríguez, A., Berecibar Zugasti, E., Domínguez Santana, P. & Herrera Morán, B. (2003). Lista de Especies Marinas de Canarias (algas, hongos, plantas y animales). Consejería de Política Territorial y Medio Ambiente del Gobierno de Canarias.
- Graham, L.E. & Wilcox, L.W. (2000). Algae. Prentice Hall, Upper Saddle River.
- Hameed, H.A. (2003). The colonization of periphytic diatoms species on artificial substrates in the Ashar canal, Basrah, Iraq. Limnologica, 33: 54–61.
- Hasle, G.R. & Syvertsen, E.E. (1997). Marine diatoms. In Identifying Marine Phytoplankton (Tomas, C.R., editor), 5–385. Academic Press, San Diego.
- Hendey, N.I. (1964). An Introductory Account of the Smaller Algae of British Coastal Waters. Part V. Bacillariophyceae (Diatoms). Her Majesty’s Stationery Office, London.
- Hernández-Almeida, O.U. & Siqueiros-Beltrones, D.A. (2012). Substrate-dependent differences between the structures of epiphytic and epilithic diatom assemblages off the southwestern coast of the Gulf of California. Botanica Marina, 55: 149–159.
- Higgins, M.J., Molino, P., Mulvaney, P. & Wetherbee, R. (2003). The structure and nanomechanical properties of the adhesive mucilage that mediates diatom-substratum adhesion and motility. Journal of Phycology, 39: 1181–1193.
- Hudon, C. & Bourget, E. (1983). The effect of light on the vertical structure of epibenthic diatom communities. Botanica Marina, 26: 317–330.
- Hustedt, F. (1931–1959). Die Kieselalgen Deutschlands, Österreichs und der Schweiz. In Kryptogamen-Flora von Deutschland, Österreichs und der Schweiz (Rabenhorst, L., editor), 1–845. Akademische Verlagsgesellschaft, Leipzig.
- Hustedt, F. (1961–1966). Die Kieselalgen Deutschlands, Österreichs und der Schweiz. In Kryptogamen-Flora von Deutschland, Österreich und der Schweiz (Rabenhorst, L., editor), 1–816. Akademische Verlagsgesellschaft, Leipzig.
- Jacobs, R.P.W.M. & Noten, T.M.P.A. (1980). The annual pattern of the diatoms in the epiphyton of eelgrass (Zostera marina L.) at Roscoff, France. Aquatic Botany, 8: 355–370.
- Kita, T. & Harada, E. (1962). Studies on the epiphytic communities. 1. Abundance and distribution of microalgae and small animals on the Zostera marina blades. Publications of the Seto Marine Biological Laboratory, 10: 245–257.
- Kitting, C.L., Fry, B. & Morgan, M.D. (1984). Detection of inconspicuous epiphytic algae supporting food webs in seagrass meadows. Oecologia, 62: 145–149.
- Kooistra, W.H.C.F., Forlani, G. & De Stefano, M. (2008). Adaptations of araphid pennate diatoms to a planktonic existence. Marine Ecology – An Evolutionary Perspective, 30: 1–15.
- Kylin, H. (1923). Studienüber die Entwicklungsgeschichte der Florideen. Kongliga Svenska Vetenskaps-Akademiens Handlingar, Ny Följd 63: 1–139.
- Lebreton, B., Richard, P., Radenac, G., Bordes, M., Bréret, M., Arnaud, C., Mornet, F. & Blanchard, G.F. (2009). Are epiphytes a significant component of intertidal Zostera noltii beds? Aquatic Botany, 91: 82‒90.
- Main, S.P. & McIntire, C.D. (1974). The distribution of epiphytic diatoms in Yaquina Estuary, Oregon (U.S.A.). Botanica Marina, 17: 88–99.
- Mazzella, L., Buia, M.C. & Spinoccia, L. (1994). Biodiversity of epiphytic diatom community on leaves of Posidonia oceanica. In Proceedings of the 13th International Diatom Symposium, Maratea (Italy), 1‒7 September 1994 (Marino, D. & Montresor, M., editors), 241‒251. Biopress, Bristol.
- Mazzella, L. & Spinoccia, L. (1992). Epiphytic diatoms of leaf blades of the Mediterranean seagrass Posidonia oceanica (L.) Delile. Giornale Botanico Italiano, 126: 752‒754.
- Meadows, P.S., Meadows, A. & Murray, J.M.H. (2012). Biological modifiers of marine benthic seascapes: their role as ecosystem engineers. Geomorphology, 157–158: 31–48.
- Metaxas, A. & Lewis A.G. (1992). Diatom communities of tidepools: the effect of intertidal height. Botanica Marina, 35: 1–10.
- Moncreiff, C.A. & Sullivan, M.J. (2001). Trophic importance of epiphytic algae in subtropical seagrass beds: evidence from multiple stable isotope analyses. Marine Ecology Progress Series, 215: 93–106.
- Navarro, J.N., Pérez, C., Arce, N. & Arroyo, B. (1989). Benthic marine diatoms of Caja de Muertos Island, Puerto Rico. Nova Hedwigia, 49: 333–367.
- Neckles, H.A., Wetzel, R.L. & Orth, R.J. (1993). Relative effects of nutrient enrichment and grazing on epiphyte-macrophyte (Zostera marina L.) dynamics. Oecologia, 93: 285–295.
- Ojeda Rodríguez, A., Gil-Rodríguez, M.C. & Moreira-Reyes, A. (2005). Aportaciones al conocimiento de diatomeas bentónicas y ticoplanctónicas del Puerto de Santa Cruz de Tenerife (islas Canarias). Vieraea, 33: 59‒78.
- Pennesi, C. & Danovaro, R. (2017). Assessing marine environmental status through microphytobenthos assemblages colonizing the Autonomous Reef Monitoring Structures (ARMS) and their potential in coastal marine restoration. Maine Pollution Bulletin, 125: 56–65.
- Peragallo, H. & Peragallo, M. (1897–1908). Diatomeès Marines de France et des Districts Maritimes Voisins. Micrographe-Editeur, Grez-sur-Loing, France.
- Piccinetti, C.C., Ricci, R., Pennesi, C., Radaelli, G., Totti, C., Norici, A., Giordano, M. & Olivotto, I. (2016). Herbivory in the soft coral Sinularia flexibilis (Alcyoniidae). Scientific Reports, 6: 22679.
- Pinckney, J.L. & Micheli, F. (1998). Microalgae on seagrass mimics: does epiphyte community structure differ from live seagrasses? Journal of Experimental Marine Biology and Ecology, 221: 59–70.
- Polifrone, M., Gil-Rodríguez, M.C., Domínguez-Álvarez, S., Stroobant, M. & Viera-Rodríguez, M.A. (2012). Reproductive phenology of three species of Gelidiales (Rhodophyta) in two macroalgal communities from Tenerife (Atlantic Ocean, Canary Islands, Spain). An Jardin Botanico Madrid, 69: 247–252.
- Poulin, M., Bérard-Therriault, L. & Cardinal, A. (1984a). Les diatome´es benthiques de substrats durs des eaux marines et saumatres du Quebec. 1. Cocconeioideae (Achnanthales, Achnanthaceae). Naturaliste Canadien, 111: 45–61.
- Poulin, M., Bérard-Therriault, L. & Cardinal, A. (1984b). Les diatome´es benthiques de substrats durs des eaux marines et saumatres du Quebec. 2. Tabellarioideae et Diatomoideae (Fragilariales, Fragilariaceae). Naturaliste Canadien, 111: 275–295.
- R Development Core Team (2009). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
- Raffaelli, D. & Hawkins, S. (1996). Intertidal Ecology. Chapman & Hall, London.
- Romagnoli, T., Bavestrello, G., Cucchiari, E.M., De Stefano, M., Di Camillo, C.G., Pennesi, C., Puce, S. & Totti, C. (2007). Microalgal communities epibiontic on the marine hydroid Eudendrium racemosum in the Ligurian Sea during an annual cycle. Marine Biology, 151: 537‒552.
- Romagnoli, T., Totti, C., Accoroni, S., De Stefano, M. & Pennesi, C. (2014). SEM analysis of the epibenthic diatoms on Eudendrium racemosum (Hydrozoa) from the Mediterranean Sea. Turkish Journal of Botany, 38: 566‒594.
- Round, F.E. (1981). The Ecology of Algae. Cambridge University Press, Cambridge.
- Round, F.E., Crawford, R.M. & Mann, D.G. (1990). The Diatoms. Biology and Morphology of the Genera. Cambridge University Press, Cambridge.
- Sansón, M. & Reyes, J. (1995). Morphological and geographical observations on four species of Ceramiaceae (Rhodophyta) new to Canary Islands. Botanica Marina, 38: 89–95.
- Santelices, B. & Hommersand, M. (1997). Pterocladiella, a new genus in the Gelidiaceae (Gelidiales, Rhodophyta). Phycologia, 36: 114–119.
- Schanz, A., Polte, P. & Asmus, H. (2002). Cascading effects of hydrodynamics on an epiphyte-grazing system in intertidal seagrass beds of the Wadden Sea. Marine Biology, 141: 287–297.
- Seoane-Camba, J. (1979). Sobre algunas gelidiáceas nuevas o poco conocidas de las costas españolas. Acta Botanica Malacitana, 5: 99–112.
- Siqueiros-Beltrones, D.A. (1988). Diatomeas bentónicas de la laguna Figueroa, Baja California. Ciencias Marinas, 14: 85–112.
- Siqueiros-Beltrones, D.A. (1990). Association structure of benthic diatoms in a hypersaline environment. Ciencias Marinas, 16: 101–127.
- Siqueiros-Beltrones, D.A., Argumedo-Hernández, U. & López-Fuerte, F.O. (2017). Diversity of benthic diatoms in the Guerrero Negro Lagoon (El Vizcaíno Biosfere Reserve), Baja California Peninsula, Mexico. Revista Mexicana De Biodiversidad, 88: 21–35.
- Siqueiros-Beltrones, D.A. & Hernández-Almeida, O.U. (2006). Florística de diatomeas epífitas en macroalgas de un manchón subtropical. CICIMAR-Oceánides, 21: 11‒61.
- Siqueiros-Beltrones, D.A. & Valenzuela-Romero, G. (2004). Benthic diatom assemblages in an abalone (Haliotis spp.) habitat from the Baja California peninsula. Pacific Science, 58: 435‒446.
- Snoeijs, P.J.M. (1994). Distribution of epiphytic diatom species composition, diversity and biomass on different macroalgal hosts along seasonal and salinity gradients in the Baltic Sea. Diatom Research, 9: 189–211.
- Snoeijs, P.J.M. (1995). Effects of salinity on epiphytic communities on Pilayella littoralis (Phaeophyceae) in the Baltic Sea. Ecoscience, 2: 382–394.
- Sullivan, M.J. (1981). Community structure of diatoms epiphytic on mangroves and Thalassia in Bimini Harbour, Bahamas. In Proceedings of the Sixth Symposium on Recent and Fossil Diatoms (Ross, R., editor), 385–398. Koeltz Science Publisher Koenigstein, Budapest.
- Sutherland, D.L. (2008). Surface-associated diatoms from marine habitats at Cape Evans, Antarctica, including the first record of living Eunotogramma marginopunctatum. Polar Biology, 31: 879–888.
- Tanaka, S. & Watanabe, T. (1990). The colonization process of a typical epilithic algal community, Homoeothrix janthina–Achnanthes japonica community, in a less polluted river in Japan. Japanese Journal of Phycology, 38: 167–177.
- Thomas, D.P. & Jiang, J. (1986). Epiphytic diatoms of the inshore marine area near Davis Station. Hydrobiology, 140: 193–198.
- Tomasko, D.A. & Lapointe, B.E. (1991). Productivity and biomass of Thalassia testudinum as related to water column nutrient availability and epiphyte levels: field observations and experimental studies. Marine Ecology Progress Series, 75: 9–17.
- Totti, C., De Stefano, M., Facca, C. & Ghirardelli, L.A. (2003). Microfitobenthos. Biologia Marina Mediterranea, 10: 263–284.
- Totti, C., Poulin, M., Romagnoli, T., Perrone, C., Pennesi, C. & De Stefano, M. (2009). Epiphytic diatom communities on intertidal seaweeds from Iceland. Polar Biology, 32: 1681–1691.
- Tuji, A. (2000). Observation of developmental processes in loosely attached diatom (Bacillariophyceae) communities. Phycological Research, 48: 75–84.
- Tuya, F., Viera-Rodríguez, M.A., Guades, R., Espino, F., Haroun, R. & Terrados, J. (2013). Seagrass responses to nutrient enrichment depend on clonal integration, but not flow-on effects on associated biota. Marine Ecology Progress Series, 490: 23–35.
- Underwood, J. & Kromkamp, J. (1999). Primary production by phytoplankton and microphytobenthos in estuaries. Advances in Ecological Research, 29: 93–153.
- Wetherbee, R., Lind, J.L. & Burke, J. (1998). The first kiss: establishment and control of initial adhesion by raphid diatoms. Mini review. Journal of Phycology, 34: 9–15.
- Witkowski, A., Lange-Bertalot, H. & Metzeltin, D. (2000). Diatom flora of marine coasts. I. Iconographia Diatomologica, 7: 1–925.