ABSTRACT
The brown algal genus Cladostephus (Sphacelariales) is found worldwide in temperate regions. Two species are considered endemic to South America (C. antarcticus Kützing and C. hariotii Sauvageau), whilst specimens from the rest of the world are usually attributed to a third, cosmopolitan species, C. spongiosus (Hudson) C.Agardh. However, comparisons of organellar (plastid rbcL and psbC, mitochondrial COI-5P) and nuclear (ITS nrDNA) markers for samples collected throughout the geographic range of C. spongiosus suggest that two genetic entities are treated under this name, which correspond to previously recognized morphological entities. Specimens with ‘spongiose’ morphology (C. spongiosus s.s.) were found to be limited to the Atlantic coast of Western Europe. The entity showing ‘verticillate’ morphology (‘C. verticillatus’) emerged as the cosmopolitan species: specimens were found on the Atlantic coasts of western Europe and the USA, in the Mediterranean, in New Zealand, Australia and on the Pacific coast of Mexico. Since the name C. verticillatus (Lightfoot) Lyngbye is illegitimate, this entity is herein referred to as C. hirsutus (L.) Boudouresque & M.Perret. A third genetic entity was only encountered in the southern hemisphere, i.e. in New Zealand, Australia, southern Chile and Falkland Islands. Comparisons with descriptions and images of type specimens suggest that this southern entity conforms most closely to the description of Cladostephus australis Kützing, nom. illeg., and it is herein renamed as Cladostephus kuetzingii Heesch, Rindi & W.A.Nelson.
Introduction
The genus Cladostephus C.Agardh (Agardh Citation1817: xxv) is referred to the Sphacelariales, an early-diverging order in the class Phaeophyceae (Silberfeld et al., Citation2010, Citation2014). Apart from the morphological characters typical for the order (summarized by Reviers & Rousseau, Citation1999), members of this genus are characterized by pseudoparenchymatous erect axes that arise from a basal crust, with auxocaulous growth and subdichotomous branching, which bear whorls of determinate branches (Womersley, Citation1987; Prud’homme van Reine, Citation1993). The life history is diplohaplontic, consisting of alternating isomorphic sporophytes, which produce meiospores in unilocular sporangia, and gametophytes, which produce isogametes in plurilocular gametangia (Cormaci et al., Citation2012).
The placement of Cladostephus at family level has varied (Draisma et al., Citation2002, Citation2010). At present, the genus is placed in its own, monotypic family Cladostephaceae Oltmanns (Draisma et al., Citation2010), and includes three species distributed mainly in temperate seas (Guiry & Guiry, Citation2019). The current infrageneric taxonomy, however, has been established on morphological grounds and is still unsettled.
The lectotype species of the genus, Cladostephus spongiosus (Hudson) C.Agardh (Citation1817), designated by Bonnemaison (Citation1822: 182), is based on Conferva spongiosa Hudson, described from an unknown locality in England (Hudson Citation1762: 480; ‘Anglis’). Another entity widely distributed around European coasts was originally described as a separate species, C. verticillatus (Lightfoot) Lyngbye, based on Conferva verticillata described by Lightfoot from Scotland (Lightfoot Citation1777: 984; ‘Frith [sic] of Forth and many other places’). The two species are distinguished mainly by the arrangement of the whorled branches: in C. verticillatus, the whorls are separated by long internodes resulting in an obvious verticillate arrangement; conversely, in C. spongiosus, the whorls are not clearly separated and the branches form a dense, continuous cover (e.g. Harvey, Citation1850: pl. XXXIII; Hauck, Citation1884; Sauvageau, Citation1914; Hamel, Citation1931; Newton, Citation1931; Womersley, Citation1987). Not infrequently, however, the distinction is uncertain, and intermediate morphologies occur (e.g. Sauvageau, Citation1914). Prud’homme van Reine (Citation1972) therefore reduced C. verticillatus to a forma of C. spongiosus, C. spongiosus f. verticillatus (Lightfoot) Prud’homme van Reine.
In Atlantic Europe, the two forms are found intertidally in adjacent habitats, with the verticillate form usually lower than the spongiose form (Prud’homme van Reine, Citation1972; Kornmann & Sahling, Citation1977), possibly due to differences in environmental conditions, for example, salinity and tidal movement. Prud’homme van Reine (Citation1972) suggested that this is why only verticillate Cladostephus is found in the Mediterranean. Womersley (Citation1987: 187), concurred that ‘the two forms recognised by Prud’homme van Reine … are ecologically based and doubtfully worth separating taxonomically’.
Prud’homme von Reine (Citation1972) also examined the type of Fucus hirsutus Linnaeus (Citation1767), which has generally been included as a synonym of C. spongiosus sensu stricto, and identified it as reproductive material of C. spongiosus f. verticillatus. Since the name C. verticillatus (Lightfoot) Lyngbye (1819) is illegitimate (Silva et al., Citation1996: 574), and F. hirsutus is the oldest valid name, he concluded that, if the spongiose and the verticillate Cladostephus ‘ … are retained as separate species, [the latter] should bear the epithet hirsutus’ (Prud’homme van Reine, Citation1972: 141). This was formalized by Boudouresque et al. (Citation1984) as C. hirsutus (L.) Boudouresque & M.Perret. However, due to the current synonymization of C. verticillatus with C. spongiosus, the latter name currently also includes C. hirsutus (Guiry & Guiry, Citation2019).
Prud’homme van Reine (Citation1972) included two more Cladostephus species as synonyms of C. spongiosus, retaining them as forms: C. spongiosus f. laxus (C.Agardh) iAreschoug, a loose-lying form from seagrass beds in Scandinavia; and C. spongiosus f. hedwigioides (Bory) Prud’homme van Reine from Greece (Bory de Saint-Vincent, Citation1832). Skottsberg (Citation1921: 44) suggested that C. setaceus Suhr (1836) may be the same species as C. hariotii Sauvageau (Citation1914; see below), since he considered the former also to originate from South America. However, Prud’homme van Reine (Citation1972) pointed out that the type of C. setaceus is probably from Greece, not Chile, and he regarded it as conspecific with C. hedwigioides, thus including it in his C. spongiosus f. hedwigioides (1972). As ‘all […] European material of Cladostephus consists […] of forms of C. spongiosus’ (Prud’homme van Reine, Citation1972: 139), Mediterranean specimens are referred to either C. spongiosus f. verticillatus or f. hedwigioides (Cormaci et al., Citation2000, Citation2012; Rindi et al., Citation2002).
Other Cladostephus species names are not available for various reasons such as being based on red algae, e.g. Cladostephus wiggii (Turner) Sprengel, C. dubius Bory and C. lycopodium (C.Agardh) C.Agardh (see Guiry & Guiry Citation2019, for synonymies). Cladostephus australis Kützing and Cladostephus myriophyllum Bory are illegitimate, and Cladostephus plumosus (Lyngbye) Fries is currently referred to Sphacelaria plumosa Lyngbye. C. ceratophyllum (Roth) C.Agardh was placed in synonymy with C. verticillatus by C.Agardh, according to Sauvageau (Citation1914). The type of Cladostephus densus Kützing (Citation1856: 4, pl. 7: ) from Helgoland includes two specimens, one of which has been identified as C. spongiosus f. spongiosus by Goudswaard (De Mesquita Rodrigues, Citation1963).
Fig. 1. Map showing geographic coverage of the examined samples. Insert: European Atlantic and Mediterranean Coasts. Light grey circle: Cladostephus spongiosus; grey quadrat: C. hirsutus; black triangle: C. kuetzingii. (Map courtesy of VectorWorldMap.com, vs. 2.1, Copyright 2009, Graphics Factory CC.)
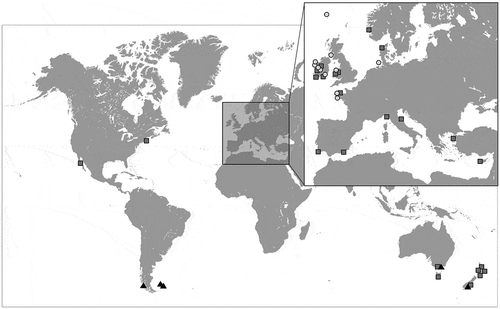
Only two South American species were not synonymized by Prud’homme van Reine (Guiry & Guiry, Citation2019): C. antarcticus Kützing, described from Cape Horn (Kützing Citation1856: 4, pl. 8: fig. II), and C. hariotii Sauvageau, collected on Tierra del Fuego, Chile (Sauvageau Citation1914: 604, ‘harioti’). Consequently, the name C. spongiosus is currently applied to all specimens found outside South America, making this a truly cosmopolitan species (Guiry & Guiry, Citation2019).
Within the brown algal order Sphacelariales, the use of molecular data has helped to clarify relationships at family level (Draisma et al., Citation2002, Citation2010). The DNA dataset for Cladostephus currently available consists of 15 sequences of various ribosomal and organellar markers produced in two phylogenetic studies (Draisma et al., Citation2002, Citation2010) and several other studies not specifically focusing on this order (Rousseau et al., Citation1997; Rousseau & de Reviers, Citation1999; Draisma et al., Citation2001; Bittner et al., Citation2008; Phillips et al., Citation2008; Silberfeld et al., Citation2010; Mystikou et al., Citation2016). In Draisma et al.’s (Citation2010) analysis of psbC and rbcL sequences, Cladostephus formed a well-supported lineage recognized as the Cladostephaceae and sister to the Sphacelariaceae. However, to date, there are insufficient molecular data to clarify the infrageneric taxonomy and biogeography of Cladostephus. We present here the first large-scale molecular study on this genus, with special focus on the complex C. spongiosus sensu lato. Using sequence data for specimens from a wide geographic area, we reassess the taxonomic identity and distribution of C. spongiosus, concluding that spongiose and verticillate forms are best recognized as separate species.
Materials and methods
Sampling and voucher preparation
Specimens of Cladostephus were collected at low tide, or by snorkelling or SCUBA diving, either by the authors or by colleagues (, ). Apical fragments of thalli, placed in silica gel for quick desiccation, were used for DNA extractions. Care was taken to remove sediment, epiphytes and other contaminants from the material prior to drying. The remainder of each specimen was preserved as herbarium voucher. Vouchers were deposited in the herbarium of the National University of Ireland Galway, Galway, Ireland (GALW; Thiers, continuously updated), and the Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand (WELT) (). Microphotographs were taken with a Canon EOS 6D camera (Canon Corporation, Tokyo, Japan) mounted on a Zeiss Axioskop microscope (Carl Zeiss, Oberkochen, Germany).
DNA extraction and amplification
Total DNA was extracted from c. 10–20 mg of silica-dried material using the Macherey-Nagel Nucleospin II Plant kit for DNA extraction (Macherey-Nagel, Düren, Germany), according to the manufacturers’ instructions. The DNA extract was diluted (1:10 or 1:100) with distilled water for PCR amplifications. Four gene regions of the three cell compartments were targeted for phylogenetic inference: the plastid genes coding for the large subunit of the RuBisCO (rbcL) and the photosystem II CP43 chlorophyll Apo protein (psbC), as well as the mitochondrial cytochrome oxidase 1 (cox1/COI-5P) gene and the internal transcribed spacers (ITS1 and ITS2) of the nuclear ribosomal DNA region (including partial 18S, 5.8S and partial 28S genes). PCR amplifications used published and newly developed primers at the annealing temperatures reported in . In all other aspects, amplifications and purification of PCR products followed details given in Heesch et al. (Citation2016). PCR products were commercially Sanger-sequenced by GATC Biotech (Konstanz, Germany).
Table 1. Details of specimens examined in this study
Table 2. Primers used for amplification and sequencing
Alignment features
Sequences were checked by eye in 4Peaks (Griekspoor & Groothuis, Citation2015) and manually aligned using Se-Al v2.0a11 (Rambaut, Citation2007). Outgroup taxa were chosen from the most closely related genera within the Sphacelariales, i.e. Battersia, Chaetopteris and Sphacelorbus (Draisma et al., Citation2002, Citation2010; see Supplementary table S1 for details).
Partial sequences of one or more of the four markers considered (rbcL, psbC, COI-5P and nrDNA) were obtained for a total of 49 samples of Cladostephus collected from its whole geographic range; for 20 of these samples, sequences of all four markers were generated (Table 1). Phylogenetic analyses were performed on six different datasets, based either on individual markers or concatenation of multiple markers: (1) rbcL; (2) psbC; (3) plastid genes (rbcL + psbC); (4) COI-5P; (5) organellar genes (rbcL + psbC + COI-5P); (6) ITS region. To avoid ambiguities, only sequences of specimens for which all respective markers were available were included in concatenated alignments.
The rbcL alignment contained 45 sequences, including eight outgroup sequences (Sphacelorbus nanus (Nägeli ex Kützing) Draisma et al., Battersia arctica (Harvey) Draisma et al., and two sequences each of Battersia plumigera (Holmes) Draisma et al., Battersia racemosa (Greville) Draisma et al. and Chaetopteris plumosa (Lyngbye) Kützing). It was 943 bp long, 64% of the gene (1467 bp, Siemer et al., Citation1998; Draisma et al., Citation2002). The psbC alignment was 1138 bp long, comprising 41 sequences and the same outgroup taxa including one sequence of C. plumosa. The concatenated plastid data set of rbcL-psbC contained 37 sequences, including the same outgroup taxa as the psbC data set, and was 2081 bp long. The COI-5P dataset included 48 samples and was 543 bp long, with B. racemosa used as outgroup. The outgroup for the concatenated organellar data set (rbcL-psbC-COI-5P) likewise comprised one sample of B. racemosa, as this was the only sample outside the Cladostephus clade for which all three markers were available. This dataset included a total of 29 sequences and was 2437 bp long.
The length of the ITS region varied considerably due to the presence of several indels, leading to some ambiguously aligned sections. Treatment with the online tool Gblocks vs 0.91b (Castresana, Citation2000), removing ambiguous or unalignable regions, reduced the alignment of 26 sequences to 805 bp.
Alignments were transformed to formats suitable for phylogenetic programs with the online tool ALTER (Glez-Peña et al., Citation2010). Alignments are available on request from the corresponding author.
Phylogenetic analyses
Alignments were partitioned for codon positions and, where appropriate, for gene regions prior to phylogenetic analyses. Phylogenetic analyses were conducted under the Maximum likelihood (ML) criterion using RAxML v.7.2.2 (Stamatakis, Citation2006), implementing the default GTRgamma model of rate heterogeneity. Support was estimated using 1000 thorough bootstrap replicates. Bayesian posterior probabilities were calculated using MrBayes v. 3.2.6 x64 (Huelsenbeck & Ronquist, Citation2001; Ronquist et al., Citation2012), following the methods of Heesch et al. (Citation2016).
Haplotype networks
Prior to haplotype network analyses, outgroup taxa were removed from the COI-5P and ITS alignments, which contained 47 and 25 sequences, respectively. TCS v. 1.21 (Clement et al., Citation2000) was used to construct parsimony networks based on all available COI-5P and ITS sequences. Alignments were analysed under standard settings, with gaps treated as missing and the connection limit fixed at 50. Networks were visualized with tcsBU (Santos et al., Citation2016) and edited in PowerPoint.
Distance matrices
Estimates of evolutionary divergence between sequences were obtained by calculating uncorrected basepair differences between sequences in MEGA5 (Tamura et al., Citation2011). Analyses involved all codon positions, with ambiguous positions being removed with Gblocks vs 0.91b (Castresana, Citation2000) prior to analysis for each sequence pair.
Nomenclature
Nomenclature follows the Shenzhen Code (Turland et al., Citation2018). The genus name Cladostephus is formed from clados (κλάδος), a branch, and stephos (στέφος), a wreath or crown (Newton, Citation1931). Even though stephos is neuter in Greek (Stearn Citation1973: 279), in botanical nomenclature all genera ending in -stephus are treated as masculine; following Art 62.1 of the Shenzhen Code (Turland et al., Citation2018) we therefore recommend that the masculine gender be retained. Nomenclatural authorities follow Brummitt & Powell (Citation1992) and the conventions therein (including ‘Prud’homme’ for W.F. Prud’homme van Reine and ‘Bory’ for Bory de Saint-Vincent).
Results
Molecular phylogeny
Overall, the phylogenies obtained from plastid (rbcL, psbC), mitochondrial (COI-5P) and nuclear (ITS; data not shown) markers were congruent among single-marker and concatenated data sets (; Supplementary figs S1, S2, S3, S4). In all analyses Cladostephus formed a well-supported monophyletic group, in which three clades, hereby indicated as Clades 1, 2 and 3 (, ) were consistently recovered. The only exception was the rbcL tree (Supplementary fig. S1), where the Mediterranean samples (which in all other analyses formed a subclade within Clade 2) were scattered at the base of Cladostephus without any bootstrap support, presumably due to the low variation of this gene within the genus.
Fig. 2. Maximum likelihood phylogram based on concatenated partial rbcL, psbC and COI-5P sequences (‘organellar data set’) of the genus Cladostephus. Values to the left indicate ML bootstrap support (BP), values to the right Bayesian posterior probabilities (PP). BP lower than 60% and PP lower than 0.9 are not reported. The scale indicates substitutions/site
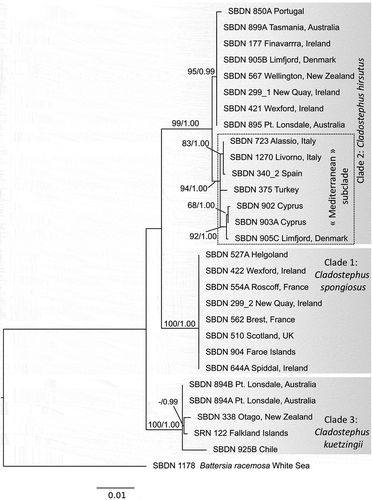
Fig. 3. Haplotype networks based on (A) COI-5P and (B) ITS sequences of Cladostephus (unalignable regions removed by Gblocks; Castresana, Citation2000). Lengths of lines are not to scale, while numbers and open dots refer to base changes between encountered haplotypes (full circles); sizes of circles are proportional to numbers of sequences included
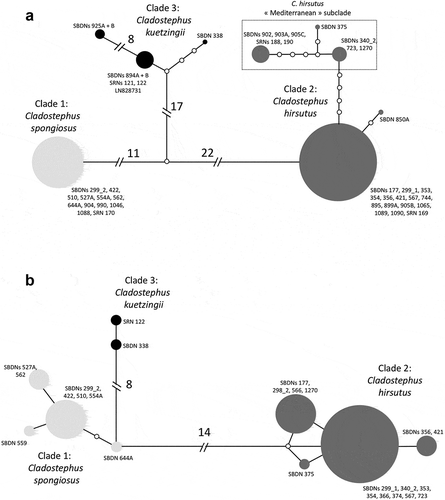
Clade 1 samples were all from the Atlantic coast of Western Europe (). Specimens of Clade 2 occurred on the Atlantic coast of Western Europe and North America, in New Zealand, Australia and in Mexico and included all Mediterranean samples (). Clade 3 was collected only in the southern hemisphere, New Zealand, Tasmania and South America ().
Within clades, in general, coding regions showed limited or no pairwise divergences (Supplementary table S2) except that in Clade 2, collections from the Mediterranean and some Scandinavian samples formed a ‘Mediterranean subclade’ distinct from extra-Mediterranean samples (). A sample from Denmark (SBDN 905c) as well as two samples from Norway (SRN 188 and SRN 190) had the same COI-5P haplotype as the two samples from Cyprus (, Supplementary fig. S4), while the plastid sequences of the Danish sample diverged from Cyprus by 1 bp. For ITS, there was no clear separation between the Mediterranean subclade and extra-Mediterranean samples, possibly due to not all sequences covering the whole length of the alignment (, Supplementary fig. S4).
Among the three clades, the plastid genes showed slight pairwise divergence (rbcL: 0.2–1.4%; psbC: 0.7–1.8%) (Supplementary table S2); ITS divergences ranged from 1.5% (Clades 2 and 3) to 5.8% (Clades 1 and 2). For COI-5P, over the length of the 543 bp sequenced, individual sequences of Clades 1 and 2 differed by 6.3–7.7%, Clades 1 and 3 by 5.3–7.2% and Clades 2 and 3 by 6.8–8.8% (Supplementary table S2; see COI-5P haplotype network in ).
Morphology
Morphological characters for the currently recognized species of Cladostephus are summarized in , and illustrated in – (Clade 1), – (Clade 2) and – (Clade 3). Specimens were generally smaller in Clade 1 than in Clade 2 and had a bushy appearance, with numerous overlapping branches that obscured the whorled habit (–). Clade 2 had characteristic, distinct whorls of branches (–). While tips of Clade 1 specimens were dense, round and resembled a shaving brush (), those of Clade 2 were more slender to sharply pointed, resembling a calligraphy brush (–). However, the verticillate habit was not always obvious in Clade 2. Specimens SRN 188 and SRN 190, collected loose-lying in Norway, consisted of irregularly branched and partly bare axes, with only a few whorled branches near some tips; they corresponded to Cladostephus laxus (Fig. S5).
Table 3. Morphology and habitat of the currently recognized Cladostephus species: Observations on C. spongiosus (Clade 1), C. hirsutus (Clade 2) and C. kuetzingii (Clade 3) from this studyA, complemented by information from Sauvageau (Citation1914)B, Lindauer et al. (Citation1961)C, Prud’homme van Reine (Citation1972)D, Womersley (Citation1987)E, Kornmann & Sahling (Citation1977)F; information on C. antarcticus and C. hariotii taken from Kützing (Citation1856)G and Sauvageau (Citation1914)B
Figs 4–11. Cladostephus spongiosus. Fig. 4. Habit of a specimen in the field. Fig. 5. Habit of erect axes. Fig. 6. Detail of apex of an erect axis. Fig. 7. Cross section of an erect axis. Fig. 8. Detail of an erect axis with whorled branches. Fig. 9. An unbranched whorled branch. Fig. 10. A tuft of hairs formed in the axil of a branchlet. Fig. 11. Detail of unilocular sporangia. Scale bars: Fig. 5 = 4.3 mm; Fig. 6 = 500 μm; Fig. 7 = 100 μm; Figs 8–9 = 300 μm; Fig. 10 = 50 μm; Fig. 11 = 30 μm
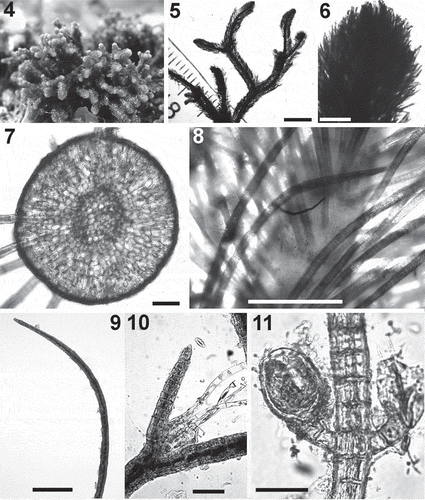
Figs 12–20. Cladostephus hirsutus. Fig. 12. Apical parts of two erect axes. Fig. 13. Detail of apex of an erect axis. Fig. 14. Detail of verticillate habit. Fig. 15. Cross section of an erect axis. Fig. 16. A sickle-shaped whorled branch. Fig. 17. An adventitious branch bearing unilocular sporangia. Fig. 18. Details of unilocular sporangia. Fig. 19. An adventitious branch bearing plurilocular gametangia. Fig. 20. Details of plurilocular gametangia. Scale bars: Fig. 12 = 3.9 mm; Figs 13–14 = 500 μm; Figs 15,17, 19: 100 μm; Figs 16, 18, 20: 50 μm
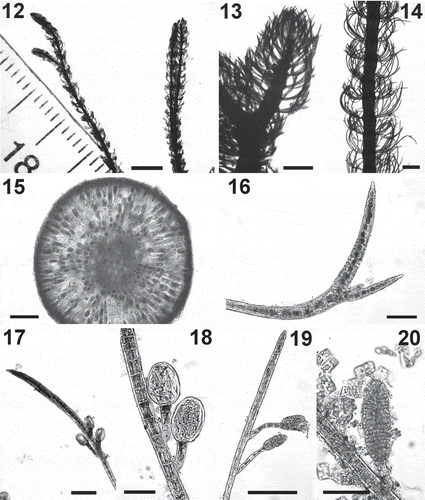
Figs 21–28. Cladostephus kuetzingii. Fig. 21. Habit of herbarium specimens. Fig. 22. Detail of erect axes. Fig. 23. Detail of apex of erect axis. Fig. 24. Detail of an erect axis in cross section. Fig. 25. Whorled branches bearing tufts of hairs. Fig. 26. An unbranched whorled branch. Fig. 27. An adventitious branch bearing unilocular sporangia. Fig. 28. Detail of unilocular sporangia . Scale bars: Fig. 21 = 1 cm; Fig. 22 = 1 mm; Fig. 23 = 200 μm; Figs 24–28 = 50 μm; Figs 25–27 = 100 μm
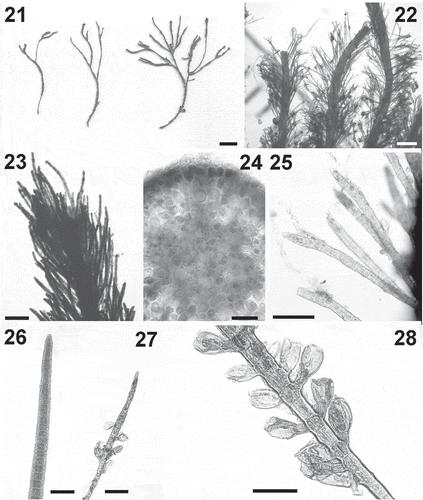
Clade 1 samples agreed well with observations of Cladostephus spongiosus s.s. by Sauvageau (Citation1914) and Prud’homme van Reine (Citation1972); this is therefore the binomial that we assign to this group, while the verticillate entity of Clade 2, following the suggestions of Prud’homme van Reine (Citation1972) and Boudouresque et al. (Citation1984), is henceforth referred to as Cladostephus hirsutus.
The entity represented by Clade 3 was of slightly smaller size than C. hirsutus (, ) and had whorled branches that, in comparison to those of C. hirsutus and C. spongiosus, appeared straighter (–, –) and overlapping, with a bushier appearance than C. hirsutus (). Unilocular sporangia were borne at the top of a pedicel formed by a single cell (–), in contrast to those of C. spongiosus and C. hirsutus, which consisted of 2–5 cells (, –). Plurilocular gametangia in Clade 2 had pedicels formed by 2–8 cells (–). We did not observe plurilocular gametangia in our material of Clades 1 and 3.
Comparisons with the descriptions of southern hemisphere species and images of type specimens (Kützing, Citation1856; Sauvageau, Citation1914; Lindauer et al., Citation1961; Supplementay figs S6–S8) suggest that this southern entity conforms most closely to the description of C. australis Kützing nom. illeg., here replaced by Cladostephus kuetzingii Heesch, Rindi & W.A.Nelson (see Discussion).
Discussion
The present study represents the first comprehensive molecular phylogenetic investigation of the genus Cladostephus. Phylogenetic analyses of molecular markers from all three organismal genomes resolved three well-supported clades within the genus. We found that psbC and rbcL genes, while useful at the ordinal level (Draisma et al., Citation2010) have a very low substitution rate in Cladostephus, which complicates assessments at species level. In contrast, divergences in COI-5P between clades ranged from 5 to 8%, well above the values considered indicative of species-level separation in many studies on brown algae (e.g. 3% in Fucus, Kucera & Saunders (Citation2008); 3% in many brown algal genera, McDevit & Saunders (Citation2009); 2.2–4.7% in Alaria, Lane et al. (Citation2007); 1–7% in Sargassum, Mattio & Payri (Citation2010;) 4–4.8% in the Laminariaceae, McDevit & Saunders (Citation2010); 1.8% in microscopic Ectocarpales, Peters et al. (Citation2015); 4.3–4.7% in Durvillaea, Weber et al. (Citation2017).
Based on combined molecular and morphological data, we conclude that our three phylogenetic clades represent three distinct species, to which we assign the binomials Cladostephus spongiosus (Clade 1), Cladostephus hirsutus (Clade 2) and Cladostephus kuetzingii (Clade 3). Macroscopically, these three species are distinct: in general, the verticillate/spongy habit was confirmed as a taxonomically valid feature, separating C. hirsutus from C. spongiosus and C. kuetzingii. C. hirsutus growing in favourable environmental conditions shows no morphological overlap with the other species. However, when in unfavourable conditions, e.g. detached from the substratum or during the cold season, C. hirsutus morphology can diverge from the typical verticillate form (e.g. upper parts of sample SBDN 902, collected in Cyprus in autumn, consisted of completely bare erect axes). The whorled branches of C. hirsutus are generally more strongly curved than in the other two species, often sickle-shaped. Whorled branches in C. kuetzingii are clearly less bent. We do not know whether the number of cells that form the pedicel supporting the unilocular sporangia is a consistent morphological character distinguishing C. kuetzingii (single cell) from C. hirsutus and C. spongiosus (2–5 in both); this will have to be studied in more specimens, as Lindauer et al. (Citation1961) report 1–9 cells for presumptive C. kuetzingii (as ‘C. australis’).
Our results have important nomenclatural implications. For Clade 1, the use of Cladostephus spongiosus sensu stricto is justified by the excellent morphological correspondence. C. hirsutus is the correct name to use for the species with verticillate habit (Clade 2). For the southern hemisphere entity represented by Clade 3, after scrutiny of the names available in the literature, we conclude that there are three potential candidate names, C. antarcticus, C. australis Kützing nom. illeg. (Kützing, Citation1856) and C. hariotii (Sauvageau, Citation1914), all described from the southern hemisphere, including regions we sampled (e.g. Cape Horn and Orange Harbour are near Isla Riesco, i.e. the southernmost part of Chile).
Cladostephus antarcticus, originally collected at Cape Horn, was described by Kützing (Citation1856) as having a silky appearance, but had a verticillate habit resembling C. hirsutus, as clearly shown in the original illustration (Kützing, Citation1856: 4; Supplementary fig. S6). While no original specimens of C. antarcticus were available to us, the Clade 3 specimens we examined consistently lacked the distinct verticillation of C. antarcticus; therefore, we consider it very unlikely that C. antarcticus and our southern hemisphere species represent the same taxonomic entity.
Cladostephus hariotii has long straight secondary branches obscuring the whorls, like Clade 3. Indeed, the gross morphology observed in specimens of our southern hemisphere entity agrees with the type specimen of C. hariotii, one of two specimens on the sheet labelled as ‘typus!’ at the Muséum National d’Histoire Naturelle in Paris (PC 0488012; Supplementary fig. S8). C. hariotii is described as typically having whorls of 6–10 branches, but sometimes 3–4 or, in the case of young whorls, only two opposite branches arranged distichously (Hariot, Citation1889; Sauvageau, Citation1914), simple and devoid of hairs. In contrast, our Clade 3 specimens have more whorled branches, often di- to trichotomously branched, with abundant hairs, and we never observed the distichous arrangement. We therefore do not consider C. hariotii a suitable attribution for our southern hemisphere entity.
The third candidate, C. australis Kützing nom. illeg., was originally described from Australia (‘Neu Holland’; Kützing, Citation1856: 5; Supplementary fig. S7). Like C. antarcticus, it has up to 20 branches per whorl, openly spaced in the lower parts of the axes and densely arranged in the upper parts, dichotomously branched with repeated bifurcations, and often bearing tufts of hairs. Kützing (Citation1856: 5) noted that the medullary cells are unordered and their cell walls are thickened; the unordered arrangement of medullary cells was a consistent feature of Clade 3 but not thickened walls. However, the taxonomic significance of this character is questionable, as it varies amongst specimens. We conclude that overall Kützing’s C. australis falls within the morphological variation of Clade 3 and this is therefore the most appropriate binomial for it; we replace it here with the new name Cladostephus kuetzingii. Inevitably this is a subjective decision. The only unequivocal way to link taxonomic names with molecular phylogenetic clades is to obtain sequences from type specimens, as achieved in green and red algae (e.g. Hernandez-Kantun et al., Citation2015; Hughey et al., Citation2019); the increasing use of high throughput sequencing will make it more widespread in the future (Oliveira et al., Citation2018). However, to date, very few authors have succeeded in genotyping older type material of brown algae (e.g. Kawai et al., Citation2019). Apart from technical difficulties, for the three candidate species for our Clade 3, this approach is impossible: Kützing’s herbarium is in the Leiden section of the Naturalis Biodiversity Center (L), whose policy is not to allow destructive practices (such as DNA extraction) on their types.
We also propose a new combination for Cladostephus laxus C. Agardh. In our phylogenies, unattached Cladostephus specimens from southern Norway (SBDN 188, SBDN 190), which corresponded morphologically to C. spongiosus f. laxus (K. Sjøtun, pers. comm.), as well as a specimen of C. hirsutus from the type site of C. laxus, Limfjorden in Denmark (SBDN 905C), clustered with Mediterranean samples including the attached C. hirsutus population from Cyprus. We do not know whether the Danish specimen was attached but given the morphological and geographic correspondence, we consider our samples to be representative of genuine Cladostephus laxus, and therefore we propose the new combination: Cladostephus hirsutus f. laxus (C.Agardh) Heesch, Rindi & W.A.Nelson, comb. nov.
Biogeography
The recognition of C. hirsutus, C. spongiosus and C. kuetzingii as distinct species is also supported by their different geographic distributions. C. spongiosus appears to be limited to the western European Atlantic, while C. hirsutus emerges as the cosmopolitan Cladostephus species, occurring along all European coasts including the Mediterranean, and also in the western Atlantic, in Mexico and in Australia and New Zealand. A Mexican population of C. hirsutus is considered a recent introduction to Pacific Mexico (Mazariegos-Villareal et al., Citation2010).
C. spongiosus and C. hirsutus occupy different ecological niches, confirming the opinions of Sauvageau (Citation1914) and Kornmann & Sahling (Citation1977). This difference is especially noticeable in Ireland, where C. spongiosus occupies a higher position on the shore (it was often found in the mid-intertidal zone, growing amongst Fucus holdfasts), while C. hirsutus occurs below the Fucus belt, e.g. on smaller rocks in sandy stretches and in tidepools from the mid-intertidal down to the shallow subtidal (SH, pers. obs.). The view of some authors that this seemingly different zonation is attributable to different ecological forms of the same species (e.g. Prud’homme van Reine, Citation1972; Womersley, Citation1987) is clearly refuted by our molecular results, which document unambiguously the genetic nature of the distinction between spongiose and verticillate forms.
The current conspecificity of C. spongiosus and C. hirsutus could also be the reason for the apparent absence of C. spongiosus outside Europe, due to a potential collection bias resulting from the size difference between C. hirsutus and the smaller C. spongiosus. Moreover, along the European Atlantic coasts, specimens of C. spongiosus are often covered by Fucus thalli (Sauvageau, Citation1914; SH, pers. obs.) and may therefore have simply been overlooked. Since we included only a single collection from the north-western Atlantic (C. hirsutus from Massachusetts, SBDN 744), the distribution of Cladostephus species in this geographic area requires further investigation.
The southern distribution limit of C. spongiosus along eastern Atlantic coasts is not clear. Unfortunately, we could not obtain any specimens from the Azores, Madeira or the Canary and Salvage Islands, where C. spongiosus has previously been recorded (e.g. Haroun et al., Citation2002; John et al., Citation2004; Parente, Citation2010). Since these records refer to the verticillate form (C. spongiosus f. verticillatus, i.e. C. hirsutus), it is currently unknown whether the real C. spongiosus occurs there.
While all extra-Mediterranean samples of C. hirsutus appeared genetically identical, the Mediterranean Sea boasted a variety of COI 5-P haplotypes, including almost all the genetic variability detected in C. hirsutus. This conclusion is in agreement with several recent investigations on other Mediterranean seaweeds, which have shown great genetic diversity and some striking cases of cryptic speciation (e.g. De Jode et al., Citation2019; Pezzolesi et al., Citation2019; Vitales et al., Citation2019). Taken together, these studies support the role of the Mediterranean as a hotspot of genetic diversity for marine organisms, a scenario supported by numerous phylogeographic investigations (Patarnello et al., Citation2007, and references therein; Pascual et al., Citation2017). Cladostephus hedwigioides and C. setaceus were described from the Mediterranean. In our study, only C. hirsutus was collected in the Mediterranean, but it showed high haplotype diversity. Whether this region may constitute a potential ice-age refugium for C. hirsutus (Lüning, Citation1990) should be tested with an extended sampling strategy in Mediterranean and extra-Mediterranean populations. By contrast, the apparent lack of genetic variability outside the Mediterranean could be a relatively recent spread of this species, possibly a relatively recent, human-mediated expansion, like the Mexican non-native population, which was first observed in 2010 (Mazariegos-Villareal et al., Citation2010; Aguilar-Rosas et al., Citation2012). There are reports that populations of C. hirsutus in New Zealand are increasing in size and distributional range (N. Shears, pers. comm.), with dynamics typical of introduced species.
The known geographic distribution of C. kuetzingii comprises the southern hemisphere, i.e. New Zealand, Australia (Tasmania), southern Chile and Falkland Islands, with different haplotypes present in Chile and New Zealand compared with Australia and the Falklands. Only one or few samples from each location were included in this study.
Lindauer et al. (Citation1961) listed two species for the New Zealand marine flora, C. verticillatus and C. australis, stating that the second species is very variable, and it is ‘difficult to segregate [it] from certain closely related plants’ (Lindauer et al., Citation1961: 178). While only C. spongiosus is currently recognized in the New Zealand marine flora, our study clearly shows the presence of both C. hirsutus and C. kuetzingii. Interestingly, Lindauer et al. (Citation1961) noticed that unilocular sporangia were not observed for C. hirsutus (as ‘C. verticillatus’) in New Zealand, but only for C. kuetzingii (as ‘C. australis’). Moreover, Gibson (Citation1994), during her studies on Cladostephus populations in southern Australia, found unilocular sporangia only at one site (Pt. Lonsdale, Victoria) and only in one, but not in all populations there. She did not distinguish between entities, but only between populations of what was then known as C. spongiosus. However, our study revealed the presence of both species at the Pt. Lonsdale site.
Taxonomy and nomenclature
Cladostephus hirsutus (Linnaeus) Boudouresque & M.Perret, 1984: 51
Basionym: Fucus hirsutus Linnaeus Citation1767b: 134 (‘Hab. In Pelago, D. Gunnerus.’).
Notes: Linnaeus’s Systema naturae, ed. 12, Vol. II (Linnaeus Citation1767a) and his Mantissa plantarum Gen. ed. VI sp. ed. II (Linnaeus Citation1767b) both appeared in 1767, in the second half of October, according to Stafleu & Cowan (Citation1981: 107). Fucus hirsutus L. appears in both. However, Linnaeus (Citation1767a: 717) cited his Mantissa and the Mantissa page number for Fucus hirsutus, so it is possible – but by no means certain – that the first part of the Mantissa appeared before Vol. II of ed. 12 of Systema naturae. Spencer et al. (Citation2009: 249) give Systema naturae as the place of publication, but Silva’s authoritative Index Nominum Algarum (http://ucjeps.berkeley.edu/cgi-bin/porp_cgi.pl?566824) gives the Mantissa as the place of publication. This confusion has led Boudouresque et al. (Citation1984) inadvertently to cite Linnaeus (Citation1767a) instead of Linnaeus (Citation1767b) in their nomenclatural treatment of C. hirsutus; this error is hereby corrected but does not negate their nomenclatural act.
A lectotype of Fucus hirsutus Linnaeus was designated in Spencer et al. (Citation2009: 249) as herb. LINN 1274-18, sine loco (LINN, http://linnean-online.org/13548/), even though the sheet has no information other than a number given by the Linnean herbarium; however, the deleted annotation ‘ericoides’ suggests that this is indeed the material Linnaeus had to hand, as Linnaeus (Citation1767a: 717) wrote ‘Proxime accedit ad Fucus ericoidem.’
Linnaeus (Citation1767b: 134) gave ‘Hab[itat]. in Pelago, D[omine]. Gunnerus’ [It grows on the shore, Lord [Bishop] Gunnerus] but no locality information. Johan Ernst Gunnerus (1718–1773) was Bishop of the Diocese of Nidaros in Trondheim, Norway from 1758, and it thus seems likely that the specimen was collected in Norway. The supposed lectotype specimen is heavily epiphytized by Jania rubens L., a species that also occurs in Norway. The question arises as to whether the lectotype designation by Spencer et al. (Citation2009: 249) was necessary as Linnaeus seemed to possess only an ‘entire gathering’ (ICN Art. 40.2; Turland et al., Citation2018) from one location and as there appears to be only one specimen in LINN under this name, this should probably be considered the holotype.
Heterotypic synonyms: Conferva verticillata Lightfoot, Citation1777: 984
Cladostephus verticillatus (Lightfoot) Lyngbye, 1819: 102, nom. illeg., non Cladostephus verticillatus C.Agardh, Citation1817: xxvi (as ‘Verticillatis’)
Cladostephus spongiosus f. verticillatus (Lightfoot) Prud’homme van Reine, Citation1972: 142
Conferva myriophyllum Roth, 1801: 335, nom. illeg.
Cladostephus myriophyllum Bory, Citation1823: 182, nom. illeg.
Notes: Since the name Cladostephus verticillatus is illegitimate, and the name Fucus hirsutus Linnaeus pre-dates its basionym Conferva verticillata Lightfoot, following Prud’homme van Reine (Citation1972) and Boudouresque et al. (Citation1984), this taxon is herein reinstated as Cladostephus hirsutus. Conferva myriophyllum Roth and Cladostephus myriophyllum Bory are both illegitimate names, as they represent an unwarranted change of epithet for Conferva verticillata Lightfoot, Citation1777. Further details on the tortuous nomenclatural history of the names associated with this taxon can be found in Silva et al. (Citation1996: 574).
Consequent on the separation of C. hirsutus from C. spongiosus, the following new infraspecific combination is required:
Cladostephus hirsutus f. laxus (C.Agardh) Heesch, Rindi & W.A.Nelson, comb. nov
Basionym: Cladostephus laxus C.Agardh, Systema algarum, p. 169, 1824
Homotypic synonym: Cladostephus spongiosus f. laxus (C.Agardh) Areschoug p. 388, Citation1850.
Notes: Since we did not examine specimens of C. spongiosus f. hedwigioides (Bory) Prud’homme and C. verticillatus f. ponticus (Sperk) Woronichin, we could not verify whether their morphologies and genetic markers match that of C. hirsutus. We therefore refrain from proposing new combinations to incorporate these two forms into C. hirsutus. However, it is worth mentioning, that this leads to the species C. spongiosus officially being recognized as part of the Mediterranean flora (as C. spongiosus f. hedwigioides), thus contradicting the observation of Prud’homme van Reine (Citation1972) that only verticillate Cladostephus, i.e. C. hirsutus, occurs in the Mediterranean Sea.
Cladostephus kuetzingii Heesch, Rindi & W.A.Nelson, nom. nov
Replaced Synonym: Cladostephus australis Tab. Phyc. Vol. 4: 5, pl. 9: fig. II, 1856, nom. illeg., non Cladostephus australis C.Agardh, Citation1824: 169.
Original description and illustration: Kützing Tab. Phyc. Vol. 4: 5, pl. 9: fig. II, 1856.
Holotype: Illustration in Kützing Tab. Phyc. Vol. 4: 5, pl. 9: fig. II, 1856.
Type locality: ‘Neu-Holland’ [Australia].
Epitype (here designated for above holotype): SBDN 338, Weller’s Rock, Otago Harbour, New Zealand (Fig. S9); coll. W.A. Nelson, 16 Aug 2012 (ASM 303); WELT A033592; sequence information: GenBank/ENA accession numbers LR736118 (COI-5P), LR736119 (psbC), LR736120 (rbcL), LR736190 (ITS nrDNA).
Description (–): Dark brown, wiry alga with erect axes up to 15 cm tall, 200–500 μm thick, irregularly branched; densely covered with whorled determinate branches, straight or slightly curved, 40–55 μm wide and 600–3130 μm long, unbranched, or branched dichotomously or trichotomously, with frequent tufts of hairs in their axils. In cross section, medulla formed by rounded, polygonal or irregularly shaped cells (20–40 μm in diameter); cells in the cortex round, radially elongated or irregularly shaped, 20–50 μm long and wide; surface covered with small, pigmented cells, 10–25 μm long and wide. Adventitious secondary branches 20–45 μm wide and 260–1100 μm long, occurring at some distance from the apex, sometimes densely aggregated in patches resembling a thin turf; unilocular sporangia issued singly or in short series (either unilateral or opposite) along the adventitious branches, ovoid, 25–40 μm wide and 25–55 μm long, with pedicel formed by a single cell (or more); plurilocular gametangia not observed.
Specimens examined: See .
Etymology: The species epithet honours Friedrich Traugott Kützing (1807–1893), who first recognized this entity from Australasia as a distinct species.
Note: Cladostephus spongiosus var. australis J.Agardh (Citation1848: 43), briefly but validly described also from ‘Novae Hollandiae’, is represented in herb. Agardh (LD) by three specimens all collected from Australia: 45671 (collected by Binder), 45672 (Harvey’s Australian Algae) and 45673 with no collector name. All are probably representative of Cladostephus kuetzingii nom. nov., but if they are, the name does not affect the legitimacy of the latter, as taxa only have priority in their own rank (ICN Art. 11.2, Turland et al. Citation2018).
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at 10.1080/09670262.2020.1740947
Supplementary table S1: GenBank/ENA accession numbers of published sequences used in the present study.
Supplementary table S2: Uncorrected base pair differences (absolute and percentage) within and between species of Cladostephus.
Supplementary fig. S1. Maximum likelihood phylogram of the genus Cladostephus based on partial rbcL sequences. Values to the left indicate ML bootstrap support (BP), values to the right Bayesian posterior probabilities (PP). BP less than 60% and PP less than 90% are not reported. The scale indicates substitutions/site.
Supplementary fig. S2. Maximum likelihood phylogram of the genus Cladostephus based on partial psbC sequences. Values to the left indicate ML bootstrap support (BP), values to the right Bayesian posterior probabilities (PP). BP less than 60% and PP less than 90% are not reported. The scale indicates substitutions/site.
Supplementary fig. S3. Maximum likelihood phylogram based on concatenated partial rbcL and psbC sequences (‘plastid data set’) of the genus Cladostephus. Values to the left indicate ML bootstrap support (BP), values to the right Bayesian posterior probabilities (PP). BP lower than 60% and PP lower than 0.9 are not reported. The scale indicates substitutions/site.
Supplementary fig. S4. Maximum likelihood phylogram based on partial COI-5P sequences of the genus Cladostephus. Values to the left indicate ML bootstrap support (BP), values to the right Bayesian posterior probabilities (PP). BP lower than 60% and PP lower than 0.9 are not reported. The scale indicates substitutions/site.
Supplementary fig. S5. Cladostephus hirsutus f. laxus. Detail of samples SRN 188 and SRN 120 (Eggholme, Nordaland, Norway; loose-lying forms).
Supplementary figs S6–S8. Original illustration and specimens of species considered candidates to represent the Cladostephus species of Clade 3. Fig. S6. C. antarcticus (plate 8 in Kützing Citation1856). Fig. S7. C. australis (herein replaced by C. kuetzingii) (plate 9 in Kützing Citation1856). Fig. S8. C. hariotii; specimen MNHM-PC-PC0488012.
Supplementary fig. S9. Cladostephus kuetzingii. Sample SBDN 338 (ASM 303) from Weller’s Rock, Otago Harbour, New Zealand. The specimen marked with an arrow is designated as epitype specimen.
Author contributions
S. Heesch: original concept, sample collections, molecular genetic work, phylogenetic analyses, microphotography, drafting manuscript; manuscript editing; F. Rindi: sample collections, morphological observations, microphotography, manuscript editing; W.A. Nelson: sample collections, microphotography, manuscript editing. M.D. Guiry: nomenclature and manuscript editing. All authors read and approved the manuscript.
TEJP-2019-0110-File008.docx
Download MS Word (27.3 KB)Acknowledgements
We should like to thank the following colleagues for providing specimens: Gerald Kraft, Luis Aguilar-Rosas, Jazmin Hernandez-Kantun, Akira Peters, Francis Bunker, Paul Brazier, Ergün Taskin, Dimitri Kletou, Craig Schneider, Kjersti Sjøtun, Kate Neill, Tracy Farr, Roberta D’Archino, Agnes Mols-Mortensen, Gareth Pearson, Fabio Bulleri, Erasmo Macaya Horta, Tania Mikhaylova, Jens Deding, Svend Åge Bendtsen and J. Hiscock. Also, many thanks for their help during various field trips go to Claire Gachon, Louise Firth, Helka Folch, Roland Gautier, Nestor Robinson, Jyotsna Mishra, Simon Gerard, Lisa Grant, Martina Strittmatter, Henrike Wilken, Rochelle Dewdney, Nick Chapman, and Christian Bruckner. Judy Sutherland provided helpful comments and a lot of encouragement. We thank two anonymous reviewers for their valuable comments, as well as Richard L. Moe for nomenclatural assistance. We are also grateful to Christine Maggs for editorial assistance that contributed to improving the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental Material
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2020.1740947.
References
- Agardh, C.A. (1817). Synopsis algarum Scandinaviae, adjecta dispositione universali algarum. pp. [i]–xl, [1]–135. ex officina Berlingiana, Lundae [Lund].
- Agardh, C.A (1824). Systema algarum. pp. [i]–xxxvii, [1]–312. Literis Berlingianis [Berling], Lundae [Lund].
- Agardh, J.G. (1842). Algae maris Mediterranei et Adriatici, observationes in diagnosin specierum et dispositionem generum. pp. [i]–x, 1–164. Apud Fortin, Masson et Cie, Parisiis [Paris].
- Agardh, J.G. (1848). Species genera et ordines algarum, seu descriptiones succinctae specierum, generum et ordinum, quibus algarum regnum constituitur. Volumen Primum. Algas fucoideas complectens. pp. [i–vi], [i]–viii, [1]–363. C.W.K. Gleerup, Lundae [Lund].
- Aguilar-Rosas, L.E., Núñez-Cebrero, F. & Aguilar-Rosas, C.V. (2012). La presencia del alga europea Cladostephus spongiosus(Hudson) C. Agardh (Sphacelariales, Ochrophyta) en la Península de Baja California, México: especie introducida. Polibotánica, 34: 127–136.
- Areschoug, J.E. (1850). Phycearum, quae in maribus scandinaviae crescunt, enumeratio. Sectio posterio Ulvaceas continen. Nova Acta Regiae Societatis Scientiarum Upsaliensis, 14: 385–454, 3 pl.
- Bittner, L., Payri, C.E., Couloux, A., Cruaud, C., De Reviers, B. & Rousseau, F. (2008). Molecular phylogeny of the Dictyotales and their position within the Phaeophyceae, based on nuclear, plastid and mitochondrial DNA sequence data. Molecular Phylogenetics and Evolution, 49: 211–226.
- Bonnemaison, T. (1822). Essai d’une classification des hydrophites loculées, ou plantes marines articulées qui croissent en France. Journal de Physique, de Chimie, d’Histoire Naturelle et des Arts, 94: 138–148; 174–203.
- Bory de Saint-Vincent, J.B.G.M. (1823). Cladostephe Cladostephus. In Dictionnaire Classique d’Histoire Naturelle. (Audouin, I. et al., editors), 4: 182–183. Rey et Gravier; Baudouin Frères, Paris.
- Bory de Saint-Vincent, J.B.G.M. (1832). Cryptogamie. Expedition scientifique de Moree. Section des Sciences physiques, 3: 281–337.
- Boudouresque, C.-F., Perret-Boudouresque, M. & Knoepffler-Peguy, M. (1984). Inventaire des algues marine benthiques dans le Pyrénées-Orientales (Méditerranée, France). Vie Milieu, 34: 41–59.
- Brummitt, R.K. & Powell, C.E., Eds (1992). Authors of Plant Names. A list of authors of scientific names of plants, with recommended standard forms of these names including abbreviations. pp. [i–iv], 1–732. Royal Botanic Gardens, Kew.
- Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution, 17: 540–552.
- Clement, M., Posada, D. & Crandall, K.A. (2000). TCS: a computer program to estimate gene genealogies. Molecular Ecology, 9: 1657–1660.
- Coleman, A.W. & Vacquier, V.D. (2002). Exploring the phylogenetic utility of ITS sequences for animals: a test case for abalone (Haliotis). Journal of Molecular Evolution, 54: 246–257.
- Cormaci, M., Furnari, G., Alongi, G., Catra, M. & Serio, D. (2000). The benthic algal flora on rocky substrata of the Tremiti Islands (Adriatic Sea). Plant Biosystems, 134: 133–152.
- Cormaci, M., Furnari, G., Catra, M., Alongi, G. & Giaccone G. (2012). Flora marina bentonica del Mediterraneo: Phaeophyceae. Bollettino Accademia Gioenia di Scienze Naturali, 45: 1–508.
- De Jode, A., David, R., Haguenauera, A., Cahill, A.E., Ergaa, Z., Guillemain, D., Sartoretto, S., Rochera, C., Selvaa, M., Le Gall, L., Féral, J.P. & Chenuil A. (2019). From seascape ecology to population genomics and back. Spatial and ecological differentiation among cryptic species of the red algae Lithophyllum stictiforme/L. cabiochiae, main bioconstructors of coralligenous habitats. Molecular Phylogenetics and Evolution, 137: 104–113.
- De Mesquita Rodrigues, J.E. (1963). Contribuiçâo para o conhecimento das Phaeophyceae da Costa Portuguesa. Memórias da Sociedade Broteriana, 16: 5–124.
- Draisma, S.G.A., Prud’homme van Reine, W.F., Stam, W.T. & Olsen, J.L. (2001). A reassessment of phylogenetic relationships within the Phaeophyceae based on Rubisco large subunit and ribosomal DNA sequences. Journal of Phycology, 37: 586–605.
- Draisma, S.G.A., Prud’homme van Reine, W.F. & Kawai, H. (2010). A revised classification of the Sphacelariales (Phaeophyceae) inferred from a psbC and rbcL based phylogeny. European Journal of Phycology, 45: 308–326.
- Draisma, S.G.A., Olsen, J.L., Stam, W.T. & Prud’homme van Reine, W.F. (2002). Phylogenetic relationships within the Sphacelariales (Phaeophyceae): rbcL, Rubisco spacer and morphology. European Journal of Phycology, 37: 385–401.
- Gibson, M. (1994). Reproduction in Cladostephus spongiosus in southern Australia (Sphacelariales, Phaeophyceae). Phycologia, 33: 378–383.
- Glez-Peña, D., Gómez-Blanco, D., Reboiro-Jato, M., Fdez-Riverola, F. & Posada, D. (2010). ALTER: program-oriented format conversion of DNA and protein alignments. Nucleic Acids Research. Web Server issue. ISSN: 0305-1048. http://dx.doi.org/10.1093/nar/gkq321
- Griekspoor, A., & Tom Groothuis, T. (2015). 4peaks. http://www.nucleobytes.com
- Guiry, M.D. & Guiry, G.M. (2019). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org searched August 2019.
- Hamel, G. (1931). Sur le Cladostephus dubius Bory. In Travaux Cryptogamiques, dédiés à Louis Mangin. (Anon. Eds), pp. 309–312. Laboratoire de Cryptogamie, Muséum National d’Historie Naturelle, Paris.
- Hariot, M.P. (1889). Mission Scientifique du Cap Horn 1882–1883. Tome V: Botanique. Gauthier-Villars et Fils, Paris, 400 pp, 12 plates.
- Haroun, R.J., Gil-Rodríguez, M.C., Díaz de Castro, J. & Prud’homme van Reine, W.F. (2002). A checklist of the marine plants from the Canary Islands (central eastern Atlantic Ocean). Botanica Marina, 45: 139–169.
- Harvey, W.H. (1850). Phycologia britannica, or, a history of British sea-weeds: containing coloured figures, generic and specific characters, synonymes, and descriptions of all the species of algae inhabiting the shores of the British Islands. pp. text with plates, pls CCLXXI–CCCXVIII [271–318]. Reeve & Benham, London.
- Hauck, F. (1884). Die Meeresalgen Deutschlands und Österreichs. In Kryptogamen-Flora von Deutschland, Österreich und der Schweiz, Zweite Auflage. (Rabenhorst, L. Eds) 2: 321–512. Euard Kummer, Leipzig.
- Heesch, S., Pažoutová, M., Moniz, M.B.J. & Rindi, F. (2016). Prasiolales (Trebouxiophyceae, Chlorophyta) of the Svalbard Archipelago: diversity, biogeography, and description of the new genera Prasionella and Prasionema. European Journal of Phycology, 51: 171–187.
- Hernandez-Kantun, J.J., Rindi, F., Adey, W., Heesch, S., Peña, V. & Gabrielson, P.W. (2015). Sequencing type material resolves the identity and distribution of the generitype Lithophyllum incrustans, and related European species L. hibernicum and L. bathyporum (Corallinales, Rhodophyta). Journal of Phycology, 51: 791–807.
- Hudson, W. (1762). Flora anglica; exhibens plantas per regnum angliae sponte crescentes, distributas secundum systema sexuale: cum differentiis specierum, synonymis auctorum, nominibus incolarum, solo locorum, tempore florendi, ofììcinalibus pharmacopoeorum. pp. [i]–viii, [1– 8], 1–506, [1–22, ind.]. Impensis auctoris: Prostant venales apud J. Nourse in the Strand, et C. Moran in Covent-Garden, Londini [London].
- Huelsenbeck, J.P. & Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogeny. Bioinformatics, 17: 754–755.
- Hughey, J.R., Maggs, C.A., Mineur, F., Jarvis, C., Miller, K.A., Shabaka, S.H. & Gabrielson, P.W. (2019). Genetic analysis of the Linnaean Ulva lactuca (Ulvales, Chlorophyta) holotype and related type specimens reveals name misapplications, unexpected origins, and new synonymies. Journal of Phycology, 55: 503–508.
- John, D.M., Prud’homme van Reine, W.F., Lawson, G.W., Kostermans, T.B. & Price, J.H. (2004). A taxonomic and geographical catalogue of the seaweeds of the western coast of Africa and adjacent islands. Beihefte zur Nova Hedwigia, 127: 1–339.
- Kawai, H., Hanyuda, T., Sun, Z., Bárbara, I. & Peters, A.F. (2019). Taxonomic revision of Eudesme (Ectocarpales s.l., Phaeophyceae) proposing a new species E. borealis sp. nov. Phycologia, 58: 351–358.
- Kornmann, P. & Sahling, P.-H. (1977). Meeresalgen von Helgoland. Benthische Grün-, Braun und Rotalgen. Helgoländer Wissenschaftliche Meeresuntersuchungen, 29: 1–289.
- Kucera, H. & Saunders, G.W. (2008). Assigning morphological variants of Fucus (Fucales, Phaeophyceae) in Canadian waters to recognized species using DNA barcoding. Botany, 86: 1065–1079.
- Kützing, F.T. (1856). Tabulae phycologicae; oder Abbildungen der Tange. Vol. 6, pp. i–iv, 1–35, 100 pls. Gedruckt auf Kosten des Verfassers (in Commission bei W. Köhne), Nordhausen.
- Lane, C.E., Lindstrom, S.C. & Saunders, G.W. (2007). A molecular assessment of northern Pacific Alaria species (Laminariales, Phaeophyceae) with reference to the utility of DNA barcoding. Molecular Phylogenetics and Evolution, 44: 634–648.
- Lightfoot, J. (1777). Flora scotica: or, a systematic arrangement, in the Linnaean method, of the native plants of Scotland and the Hebrides. Vol. II. pp. 545–1151 [1–24], pls 1–35. Printed for B. White at Horace’s Head, in Fleet Street, London.
- Lindauer, V.W., Chapman, V.J. & Aiken, M. (1961). The marine algae of New Zealand II: Phaeophyceae. Nova Hedwigia, 3: 129–350.
- Linnaeus, C. (1767a). Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus & differentiis. Tomus II. Editio duodecima, reformata. pp. [1]–736, [1–16]]. Impensis direct. Laurentii Salvii, Homiae [Stockholm]. Published Oct. 1767.
- Linnaeus, C. (1767b). Mantissa plantarum Generum editionis VI. et specierum editionis II. pp. [1]–142 [as “742”] + index. [Impensis direct. Laurentiis Salvii], Homiae [Stockholm].
- Lüning, K. (1990). Seaweeds: Their Environment, Biogeography and Ecophysiology. 2nd ed. Wiley-Interscience, New York.
- Mattio, L. & Payri, C. (2010). Assessment of five markers as potential barcodes for identifying Sargassum subgenus Sargassum species (Phaeophyceae, Fucales). Cryptogamie, Algologie, 31: 467–485.
- Mazariegos-Villareal, A., Riosmena-Rodriguez, R., Rivera-Camacho, A.R. & Serviere-Zaragoza, E. (2010). First report of Cladostephus spongiosus (Sphacelariales: Phaeophyta) from the Pacific coast of Mexico. Botanica Marina, 53: 153–157.
- McDevit, D.C. & Saunders, G.W. (2009). On the utility of DNA barcoding for species differentiation among brown macroalgae (Phaeophyceae) including a novel extraction protocol. Phycological Research, 57: 131–141.
- McDevit, D.C. & Saunders, G.W. (2010). A DNA barcode examination of the Laminariaceae (Phaeophyceae) in Canada reveals novel biogeographical and evolutionary insights. Phycologia, 49: 235–248.
- Mystikou, A., Asensi, A.O., De Clerck, O., Müller, D.G., Peters, A.F., Tsiamis, K., Fletcher, K.I., Westermeier, R., Brickle, P., van West, P. & Küpper, F. (2016). New records and observations of macroalgae and associated pathogens from the Falkland Islands, Patagonia and Tierra del Fuego. Botanica Marina, 59: 105–121.
- Newton, L. (1931). A Handbook of the British Seaweeds. The Trustees of the British Museum, British Museum (Natural History), London.
- Oliveira, M.C., Repetti, S.I., Iha, C., Jackson, C.J., Díaz-Tapia, P., Lubiana, K.M.F., Cassano, V., Costa, J.F., Cremen, M.C.M., Marcelino, V.R. & Verbruggen, H. (2018). High-throughput sequencing for algal systematics. European Journal of Phycology, 53: 256–272.
- Parente, M.I. (2010). Lista das macroalgas marinhas (Rhodophyta, Chlorophyta e Phaeophyceae) – List of marine macroalgae (Rhodophyta, Chlorophyta and Phaeophyceae). In Listagem dos organismos terrestres e marinhos dos Açores (A list of the terrestrial and marine biota from the Azores) (Borges, P.A.V., et al., editors), 275–286. Princípia Editora, Cascais.
- Pascual, M., Rives, B., Schunter, C. & Macpherson, E. (2017). Impact of life history traits on gene flow: a multispecies systematic review across oceanographic barriers in the Mediterranean Sea. PLoS ONE, 12(5): e0176419.
- Patarnello, T., Volckaert, F.A.M.J. & Castilho R. (2007). Pillars of Hercules: is the Atlantic-Mediterranean transition a phylogeographical break? Molecular Ecology, 16: 4426–4444.
- Peters, A.F., Couceiro, L. Tsiamis, K., Küpper, F. & Valero, M. (2015). Barcoding of cryptic stages of marine brown algae isolated from incubated substratum reveals high diversity in Acinetosporaceae (Ectocarpales, Phaeophyceae). Cryptogamie, Algologie, 36: 3–29.
- Peters, A.F. & Ramirez, M.E. (2001). Molecular phylogeny of small brown algae, with special reference to the systematic position of Caepidium antarcticum (Adenocystaceae, Ectocarpales). Cryptogamie, Algologie, 22: 187–200.
- Pezzolesi, L., Peña, V., Le Gall, L., Gabrielson, P.W., Kaleb, S., Hughey, J.R., Rodondi, G., Hernandez-Kantun, J.J., Falace, A., Basso, D., Cerrano, C. & Rindi, F. (2019). Mediterranean Lithophyllum stictiforme is a genetically diverse species complex: implications for species circumscription, biogeography and conservation of coralligenous habitats. Journal of Phycology, 55: 473–492.
- Phillips, N., Burrowes, R., Rousseau, F., De Reviers, B. & Saunders, G.W. (2008). Resolving evolutionary relationships among the brown algae using chloroplast and nuclear genes. Journal of Phycology, 44: 394–405.
- Prud’homme van Reine, W.F. (1972). Notes on Sphacelariales II: On the identity of Cladostephus setaceus Suhr and remarks on European Cladostephus. Blumea, 20: 138–144.
- Prud’homme van Reine, W.F. (1993). Sphacelariales of the World, a new synthesis. Korean Journal of Phycology, 8: 145–160.
- Rambaut, A. (2007). Se-al: Sequence alignment editor; available at http://tree.Bio.Ed.Ac.Uk/software/seal/.
- Reviers, B. de & Rousseau, F. (1999). Towards a new classification of the brown algae. Progress in Phycological Research, 13: 107–201.
- Rindi, F., Sartoni, G. & Cinelli, F. (2002). A floristic account of the benthic marine algae of Tuscany (Western Mediterranean Sea). Nova Hedwigia, 74: 201–250.
- Ronquist, F., Teslenk, M., Van der Mark, P., Ayres, D., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Rousseau, F. & De Reviers, B. (1999). Circumscription of the order Ectocarpales (Phaeophyceae): bibliographical synthesis and molecular evidence. Cryptogamie, Algologie, 20: 5–18.
- Rousseau, F., Leclerc, M.-C. & De Reviers, B. (1997). Molecular phylogeny of European Fucales (Phaeophyceae) based on partial large-subunit rDNA sequence comparisons. Phycologia, 36: 438–446.
- Santos, A.M., Cabezas, M.P., Tavares, A.I., Xavier, R. & Branco, M. (2016). tcsBU: a tool to extend TCS network layout and visualization. Bioinformatics, btv636.
- Saunders, G.W. (2005). Applying dna barcoding to red macroalgae: A preliminary appraisal holds promise for future applications. Philosophical Transactions of the Royal Society B: Biological Sciences, 360: 1879–1888.
- Sauvageau, C. (1914). Remarques sur les Sphacélariacées. Vol. 3, pp. iii–xii, 481–634, 128 figs. Bordeaux.
- Siemer, B.L., Stam, W.T., Olsen, J.L. & Pedersen, P.M. (1998). Phylogenetic relationships of the brown algal orders Ectocarpales, Chordariales, Dictyosiphonales, and Tilopteridales (Phaeophyceae) based on RUBISCO large subunit and spacer sequences. Journal of Phycology, 34: 1038–1048.
- Silberfeld, T., Leigh, J.W., Verbruggen, H., Cruaud, C., de Reviers, B. & Rousseau, F. (2010). A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): investigating the evolutionary nature of the brown algal crown radiation. Molecular Phylogenetics and Evolution, 56: 659–674.
- Silberfeld, T., Rousseau, F. & de Reviers, B. (2014). An updated classification of brown algae (Ochrophyta, Phaeophyceae). Cryptogamie, Algologie, 35: 117–156.
- Silva, P.C., Basson, P.W. & Moe, R.L. (1996). Catalogue of the Benthic Marine Algae of the Indian Ocean. University of California Press, 1259 pp.
- Skottsberg, C. (1921). Botanische Ergebnisse der schwedischen Expedition nach Patagonien und dem Feuerlande 1907–1909. VIII. Marine algae. I. Phaeophyceae. Kungliga Svenska Vetenskapsakademiens Handlingar, 61 (11):[1]– 56, 20 figs.
- Spencer, M.A., Irvine, L.M. & Jarvis, C.E. (2009). Typification of Linnaean names relevant to algal nomenclature. Taxon, 58: 237–260.
- Stafleu, F.A. & Cowan, R.S. (1981). Taxonomic literature … Volume III: Lh-O. Vol. 105 pp. XII + 980. Bohn, Scheltema & Holkema, Utrecht.
- Stamatakis, A. (2006). Raxml-vi-hpc: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22: 2688–2690.
- Stearn, W.T. (1973). Botanical Latin. History, grammar, syntax, terminology and vocabulary. 2nd ed. pp. i–xiv, [1]–566, 41 figs. David & Charles, Newton Abbott.
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011). MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28: 2731–2739.
- Thiers, B. [continuously updated]. Index herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih/.
- Turland, N.J., Wiersema, J.H., Barrie, F.R., Greuter, W., Hawksworth, D.L., Herendeen, P.S., Knapp, S., Kusber, W.-H., Li, D.-Z., Marhold, K., May, T.W., McNeill, J., Monro, A.M., Prado, J., Price, M.J. & Smith, G.F., editors (2018). International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile, Vol. 159. pp. [i]–xxxviii, 1–253. Koeltz Botanical Books, Glashütten.
- Vitales, D., Aragay, J., Garnatje, J., Gómez-Garreta, A. & Rull Luch, J. (2019). Phylogeography of Dictyota fasciola and Dictyota mediterranea (Dictyotales, Phaeophyceae): unexpected patterns on the Atlantic-Mediterranean marine transition and taxonomic implications. Peer J, doi: 10.7717/peerj.6916.
- Weber, X.A., Edgar, G.J., Banks, S.C., Waters, J.M. & Fraser, C.I. (2017). A morphological and phylogenetic investigation into divergence among sympatric Australian southern bull kelps (Durvillaea potatorum and D. amatheiae sp. nov.). Molecular Phylogenetics and Evolution, 107: 630–643.
- Womersley, H.B.S. (1987). The Marine Benthic Flora of Australia. Part II: Phaeophyceae. South Australian Government Printing Division, Adelaide.
