ABSTRACT
In the past, large, foliose and unbranched Halymenia-like plants from Europe were often identified as ‘Halymenia latifolia’, however recent taxonomic studies have shown that ‘H. latifolia’ was a catch-all name for at least three cryptic species: Neofolia rosea, Nesoia mediterranea, and the genuine H. latifolia, which has been transferred to the recently established genus Nesoia. In this study, we further refine the taxonomy of European Halymeniaceae by examining new collections of foliose taxa from the Mediterranean Sea and Macaronesia. Analyses of rbcL gene sequences showed that unbranched foliose Halymeniaceae from Europe can be separated into five species, including three previously described species in Neofolia (N. rosea) and Nesoia (N. latifolia and N. mediterranea) and two new species belonging to Halymenia (H. ballesterosii sp. nov.) and Nesoia (N. hommersandii sp. nov.). Halymenia ballesterosii is the only known unbranched foliose species in the genus from Europe and can be separated from morphologically similar species in Neofolia and Nesoia by protruding surface cells, two-celled carpogonial branches, a basal nutritive cell cluster in the carpogonial branch ampullae, and secondary connecting filaments issued from basal cells of ampullar filaments. Nesoia hommersandii differs from N. latifolia by the lack of swollen arms in medullary stellate cells and cystocarps that slightly protrude above the thallus surface. We expect that the species diversity of foliose Halymeniaceae may increase when more foliose plants are collected and examined from different locations in Europe.
Introduction
The Halymeniales (Rhodophyta) was one of the first orders segregated from the Gigartinales (Saunders & Kraft, Citation1996) following the merger of that order with the Cryptonemiales by Kraft & Robins (Citation1985), characterized by its non-procarpic female reproductive structures, in which the carpogonial branches and auxiliary cells are protected by ampullar filaments (known as ampullae). Currently, the Halymeniales includes ~37 genera: one in the Tsengiaceae and 36 in the Halymeniaceae. Among them, the genera Grateloupia C.Agardh and Halymenia C.Agardh are by far the most species-rich, including, respectively, 97 and 80 described species (Guiry & Guiry, Citation2019). Previous studies have highlighted the polyphyletic nature of Halymenia (Hernández-Kantun et al., Citation2009; D’Archino et al., Citation2014; Lee & Kim, Citation2019). Recently, our research group undertook a series of systematic studies on European Halymeniales, resulting in the description of a new genus, Neofolia S.-M.Lin, Rodríguez-Prieto, De Clerck & Huisman, and recognition of several cryptic species (Rodríguez-Prieto et al., Citation2018, Citation2019) by characterizing the pre- and post-fertilization development of female reproductive structures in the generitype of Halymenia, H. floresii (Clemente) C.Agardh. A year later, Lee & Kim (Citation2019) erected a new genus, Nesoia H.W.Lee & M.S.Kim, based on a foliose species, N. delicatula H.W.Lee & M.S.Kim from Jeju Island, South Korea, and transferred Halymenia latifolia to Nesoia, as N. latifolia (P.Crouan & H.Crouan in Lloyd ex Kützing) H.W.Lee & M.S.Kim. In the same year, we characterized the Halymenia-like species, Nesoia latifolia, and described a new species, N. mediterranea Rodríguez-Prieto, S.-M.Lin, De Clerck & Huisman, a species morphologically similar to N. latifolia. Our studies detailing the ontogeny of female reproductive structures and post-fertilization development in the Halymeniaceae provide some useful clues for resolving the polyphyletic genus Halymenia.
In this study, we continue our systematic studies on European Halymeniaceae based on our recent new collections of specimens, which were difficult to assign to any named species based on vegetative structures alone. Therefore, we focused on these Nesoia latifolia-like specimens, examined their vegetative and reproductive structures and sequenced related species from the North-eastern Atlantic Ocean, Macaronesia and the Mediterranean Sea in order to infer their phylogenetic relationships. These investigations led to the recognition of two new species, one in each of the genera Halymenia and Nesoia.
Materials and methods
Collections were made by SCUBA or trawling on circalittoral detritic bottoms. Algal samples were preserved in 5% formalin-seawater for the morphological study or pressed as herbarium vouchers. Materials used in the molecular studies were desiccated in silica gel or preserved in 95% ethanol. Specimens were sectioned by hand or with a freezing microtome and were stained with 1% aniline blue acidified with 1% HCl or were treated with Wittmann’s aceto-iron-haematoxylin-chloral hydrate (Wittmann, Citation1965) modified by Rodríguez-Prieto & Hommersand (Citation2009), and mounted in 50% Hoyer’s mounting medium. Habit images were taken with a Canon EOS 350D (Canon, Tokyo, Japan) and photomicrographs were made with an AxioCam MRc attached to an Axioskop 2 plus microscope (Carl Zeiss, Oberchohen, Germany). Voucher specimens were deposited in the Herbarium of the University of Girona, Spain (HGI) and in the herbarium of the University of La Laguna (TFC). Herbarium abbreviations follow Thiers (Citation2019).
DNA samples were prepared using a DNeasy Plant Mini Kit (Qiagen, Valencia, California, USA) following the manufacturer’s instructions. DNA sequencing procedures of the rbcL gene are as described in De Clerck et al. (Citation2005a, Citation2005b) and Lin et al. (Citation2008). Newly generated sequence data were combined with a selection of sequences available from GenBank and aligned with Sequencher (Gene Codes Corp., Ann Arbor, Michigan, USA). The taxon sampling aimed to present the diversity of Halymeniales at the genus-level as comprehensively as possible, while expanding sequence data on Halymenia, Neofolia and Nesoia (see Supplementary table 1). A Maximum likelihood (ML) species tree was generated using MEGA6 under the Tamura 3-parameter model (Tamura et al., Citation2013). The robustness of the resulting phylogeny was tested using 500 replicates of a rapid bootstrap heuristic (Tamura et al., Citation2013). In addition, a Bayesian tree was estimated using MrBayes 3.2. (Ronquist et al., Citation2012), applying a GTR model. Two runs consisting of 4 chains each were run for 5 million generations. Stationarity and convergence of the runs were assessed visually using Tracer v.1.6 (Rambaut et al., Citation2014) and a majority rule consensus tree was calculated after removal of a burn-in fraction of 10%.
Results
Molecular analyses
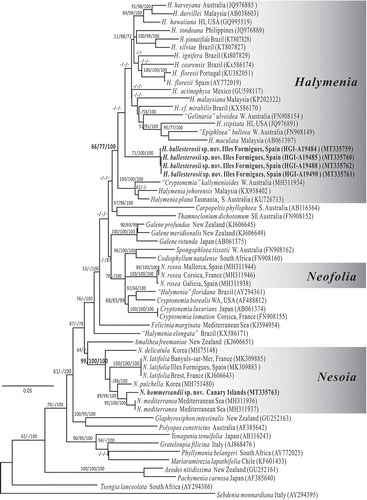
A total of 57 rbcL sequences from representative taxa belonging to the genera Halymenia, Nesoia and Neofolia along with related genera in the family Halymeniaceae were selected for analysis, with Sebdenia monnardiana (Montagne) Berthold and Tsengia lanceolata (J.Agardh) Saunders & Kraft serving as outgroup (). The alignment included 1257 sites, omitting 106 and 103 base pairs from the 5’ and 3’ end of rbcL (1467 bp) gene due to missing information. The ML, Maximum parsimony and Bayesian trees were overall congruent, differing only in the relative placement of clades that received only low support. Foliose Halymeniacean taxa from Europe were clustered into three natural lineages: Halymenia, Neofolia and Nesoia. Our proposed new foliose species, Halymenia ballesterosii sp. nov. was resolved in a moderately well-supported clade containing the generitype of Halymenia (H. floresii) and most named Halymenia species worldwide, along with Epiphloea bullosa (Harvey) De Toni and Gelinaria ulvoidea Sonder, two monospecific genera endemic to Australia. The proposed species Nesoia hommersandii sp. nov. was resolved in a well-supported clade containing the generitype of Nesoia (N. delicatula) and all other currently known species of Nesoia. Our analyses showed that the foliose Halymenia latifolia from Europe contained five species belonging to three genera: Halymenia (H. ballesterosii sp. nov. herein), Neofolia (N. rosea) and Nesoia (N. latifolia, N. mediterranea, N. hommersandii sp. nov. herein).
Fig. 1. Maximum likelihood phylogeny of the Halymeniales based on the rbcL gene using the Tamura 3-parameter + GAMMA model in MEGA6 (–Ln = 11385.4511). Support values resulting from the Maximum likelihood, Maximum parsimony and Bayesian analyses are indicated as branch labels (ML/MP/BI). Scale indicates substitutions per site
Morphological observations
Halymenia ballesterosii Rodríguez-Prieto, S.-M. Lin, De Clerck & Huisman sp. nov.()
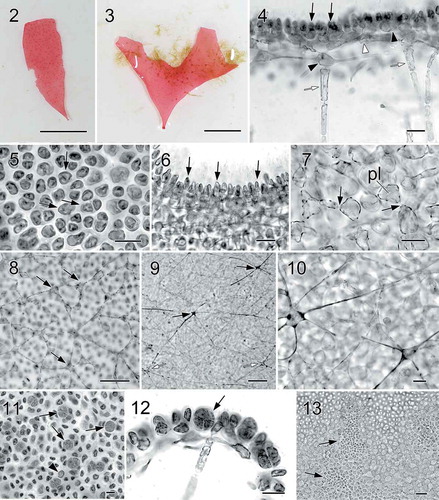
Figs 2–13. Halymenia ballesterosii Rodríguez-Prieto, S.-M. Lin, De Clerck & Huisman sp. nov. Thallus morphology, vegetative structure, sporophyte and male reproductive structure. Fig. 2. Holotype, a monoecious specimen with cystocarps scattered over fertile thallus surface (HGI-A 19484). Fig. 3. Isotype, a female gametophyte consisting of a bifurcating and apically eroded blade (HGI-A 19490). Fig. 4. Cross section of a young blade showing ovoid surface cells (black arrows), ovoid subcortical pit-connected cells (black arrowhead at right), third layer of stellate cells with short arms (white arrowhead), inner cortical cell with long arms (black arrowhead at left) and anticlinally arranged medullary filament (white arrows) (HGI-A 19490). Fig. 5. Surface view of a blade showing rounded to irregularly polygonal cortical cells with reticulate plastid (arrows) (HGI-A 19484). Fig. 6. Close up of a cross section of the blade showing ovoid and protruding surface cells (arrows) (HGI-A 19499). Fig. 7. Subsurface view of thallus blade showing subcortical ovoid to slightly stellate cells with very short arms. Note that cells are secondarily pit connected (arrows) and bear a reticulate plastid (pl) (HGI-A 19490). Fig. 8. Subsurface view of thallus blade showing the inner subcortical cells layer, composed of stellate cells with quite long arms (arrows) (HGI-A 11077). Fig. 9. Subsurface view of thallus blade showing the network of medullary stellate cells with long arms (arrows). Note that the network is composed both by darkly staining (black arrows) and non-darkly staining medullary cells (white arrows) (HGI-A 6552). Fig. 10. Detail of the medulla and medullary stellate cells of Fig. 9 (HGI-A 6552). Fig. 11. Subsurface view showing a tetrasporangial initial (arrowhead) and many scattered tetrasporangial initials that are divided once (arrows) (HGI-A 19488). Fig. 12. Cross section through a tetrasporangial sorus showing cruciately divided tetrasporangium (arrow) (HGI-A 19498). Fig. 13. Surface view of spermatangial sori (arrows) (HGI-A 19477). Haematoxylin (Figs 4, 5); Aniline blue (Figs 6–12); Not stained (Fig. 13). Scale bars: Figs 2, 3 = 1 cm; Figs 4, 5, 7, 12, 13 = 20 µm; Figs 6, 10, 11 = 10 µm; Fig. 8 = 50 µm; Fig. 9 = 100 µm
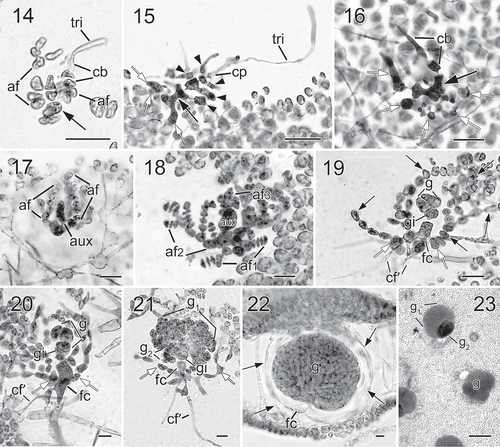
Figs 14−23. Halymenia ballesterosii Rodríguez-Prieto, S.-M. Lin, De Clerck & Huisman sp. nov. Morphology of carpogonial branch and auxiliary cell ampullae and development of cystocarp. Fig. 14. Close up of a carpogonial branch ampulla showing a 2-celled carpogonial branch with a short trichogyne and short branched ampullar filament (af) borne on a subcortical cell (arrow) (HGI-A 19485). Fig. 15. Early post-fertilization of carpogonial branch ampulla showing a 2-celled carpogonial branch with a fertilized carpogonium (cp) and a well-developed distally swelled trichogyne (tri). Note that the cells of carpogonial branch and the cells of the ampullar filaments cut off lateral branches (arrowheads) and the enlarged basal cell (black arrow) fused with neighbouring cells to form a fusion cell and became darkly stained. Few small derived cells (= nutritive cells, white arrows) were cut off from the basal part of the fusion cell (HGI-A 11077). Fig. 16. A later stage of Fig. 15, showing the carpogonial branch (cb) and the fusion cell (black arrow) cutting off more nutritive cells/filaments (white arrows) (HGI-A 11077). Fig. 17. A young auxiliary cell ampulla showing ampullar filaments (af) and the auxiliary cell (aux) (HGI-A 19490). Fig. 18. A fully developed auxiliary cell ampulla showing the three orders of branched ampullar filaments (af1, af2 & af3) and auxiliary cell (aux) (HGI-A 19490) Fig. 19. Early stage after diploidization showing two primary gonimoblast cells (g) just cut off from the gonimoblast initial (gi) borne on the newly formed fusion cell (fc), flanking ampullar filaments (black arrows), and two secondarily produced connecting filaments (cf’). Note that the ampullar cells close to the fusion cell (white arrows) enlarged slightly (HGI-A 19490). Fig. 20. A slightly later stage of Fig. 19, showing primary gonimoblasts (g) cut off from the gonimoblast initial (gi) borne on fusion cell (fc), cells of ampullar filaments (white arrows), and a secondarily produced connecting filament (cf’) (HGI-A 19490). Fig. 21. Immature carposporophyte showing gonimoblast initial (gi), two gonimoblasts (g1 & g2) borne on a small fusion cell (fc), and a secondarily produced connecting filament (cf’). Note that most cells (white arrows) of ampullar filament near the fusion cell are darkly stained but do not branch further (HGI-A 19490). Fig. 22. Cross section of a fully developed cystocarp showing gonimoblasts (g) borne on a conspicuous basal fusion cell (fc) and ampullar filaments forming a filamentous pericarp (arrows) (HGI-A 19484). Fig. 23. Subsurface view of cystocarp-bearing blade showing gonimoblasts (g) and one of the mature cystocarps composed of two gonimolobes (g1 & g2) (HGI-A 19499). Aniline blue (Figs 14–21, 23); Not stained (Fig. 22). Scale bars: Figs 14, 16–22 = 20 µm; Figs 15, 23 = 50 µm
Diagnosis: Thalli erect, foliose, simple or rarely bifurcate, 3–13 cm long by 1.4–4.0 cm wide, rose-pink in colour, soft and gelatinous in texture; blades 90–345 µm thick in upper parts; cortex made up of 2–4 cell layers, outer cortical cells ovoid and protruding above thallus surface, 6.5–11.6 µm long in longitudinal view; medulla mostly hollow, composed of an open network of stellate cells with long arms and sparse filaments, anticlinal to thallus surface, up to 83–340 µm long and 5–17 µm in diameter. Gametophytes and tetrasporophytes isomorphic. Tetrasporangia cruciately divided, 14.8–21.0 µm long by 11.5–19.0 µm wide. Gametophytes monoecious. Spermatangial sori scattered over both thallus surfaces; spermatangia 11.2–17.7 µm long by 2.9–5.4 µm wide. Carpogonial branch ampullae composed of a two-celled carpogonial branch with a small network of nutritive cells; auxiliary cell ampullae abundant, composed of three orders of branched ampullar filaments; mature carposporophyte composed of two gonimolobes up to 60–195 µm in diameter; carposporangia 12.5–15.0 µm in diameter; mature cystocarps ostiolate, slightly swelling the thallus.
Etymology: The species name honours Dr Enric Ballesteros, for his contribution to the knowledge of the biodiversity and ecology of marine macroalgae from the Mediterranean Sea.
Type collection: Holotype, monoecious gametophyte, collected from a detritic bottom off the Formigues Islands, Palamós, Spain, 08 October 2017 by C. Rodríguez-Prieto, at a depth of 37 m (), currently deposited in the algae section of the Herbarium of the University of Girona (HGI-A 19484). Isotypes in HGI-A # 19485 (female), 19488 (tetrasporic), 19490 (, female), 19498 (tetrasporic), and 19499 (male and female).
Type locality: Formigues Islands, Palamós, Spain (North-western Mediterranean).
Distribution: This species is so far known only from the Western Mediterranean (Banyuls-sur-Mer, France and Catalonia and Balearic Islands, Spain).
Habitat and seasonality: This is a rare species. The plant from Banyuls-sur-Mer was found in August at −12 m, those from Catalonia were found between August and October at depths of 30–37 m, and those from the Balearic Islands were found in May, September and October, at depths of 50–87 m. Plants grew on circalittoral detritic bottoms, often mixed with Nesoia latifolia and N. mediterranea.
Specimens examined: Spain: (1) Palamós, Formigues Islands, −37 m, 08.x.2017, coll. C. Rodríguez-Prieto, HGI-A 19484 (male and female), HGI-A 19485 (female), HGI-A 19488 (tetrasporic), HGI-A 19490 (female), HGI-A 19498 (tetrasporic), HGI-A 19499 (male & female); Platja d’Aro, La Roja, −30 m, 17.ix.2017, coll. C. Rodríguez-Prieto, HGI-A 19477 (male and female). (2) Balearic Islands, Menorca Channel, −65 m, 07.ix.2011, coll. S. Joher, HGI-A 11077 (tetrasporic and female); Balearic Islands, Eivissa, −50 m, 16.ix.2004, coll. E. Ballesteros, HGI-A 6552 (tetrasporic, male and female). France: Banyuls-sur-Mer, −12 m, 10.viii.1997, coll. C. Rodríguez-Prieto, HGI-A 4246.
Habit and vegetative structure: Thalli erect, foliose, simple () or seldom bifurcate (), usually minute, up to 3.0 cm long and 1.4 cm wide, occasionally up to 13 cm long and 4 cm wide when growing in nutrient-rich waters. Thalli arise from a small discoid holdfast and a well-defined stipe is absent (, ). Blades are rose-pink, with a thin, slightly darker margin, and are very soft and gelatinous in texture. Upper parts of blades are 90–345 µm thick, usually rounded, and with an entire margin, but upper parts of old blades are often broken. The cortex is composed of 2 cell layers in young parts and 4 layers in older parts (). Outer cortical cells are rounded to irregularly polygonal, not very compacted and up to 5.8–14.3 × 3.8–9.4 µm in surface view (), protruding above thallus surface and 6.5–11.6 µm broad in cross section (). Secondary pit-connections are absent between outer cortical cells ().
The subcortical cells in old thalli form a network comprised of three layers, one of rounded cells (), one of ovoid cells with short arms (), and a third layer of inner cortical stellate cells with relatively long arms (), all of them interconnected via secondary pit-connections. The medulla is either hollow or loosely filled with a network (–) of small stellate cells with long, thin and straight arms parallel to the thallus surface and situated just under the inner cortical cells, and elongated filaments (, white arrows), more-or-less anticlinal to the thallus surface, 83–340 µm long and 5–17 µm thick. Outermost cortical cells are more pigmented than inner cortical cells or medullary cells, but all of them have a reticulated plastid.
Reproductive structures. Tetrasporangia are initially produced from subcortical cells and scattered over the thallus surface (). Fully developed tetrasporangia are rounded or ovoid, cruciately divided, and 14.8–21.0 µm long by 11.5–19.0 µm wide ().
The species is monoecious. Spermatangial parent cells are initially produced from surface cells and formed in sori scattered over the fertile blade (). Mature spermatangia are 11.2–17.7 µm long by 2.9–5.4 µm wide. The fully developed carpogonial branch ampulla () consists of at least one ampullar filament and a two-celled carpogonial branch, often bearing a long trichogyne with a distal, swollen end (). After fertilization, the basal cell of the carpogonial branch enlarges and fuses with neighbouring ampullar cells to form a darkly stained ‘carpogonial’ fusion cell ( black arrows). Notably, a cluster of small cells, presumably functioning as nutritive cells, is produced at the basal part of the fertilized carpogonial branch ampulla (, white arrows). This cluster is similar to the one observed in the generitype, H. floresii (Rodríguez-Prieto et al., Citation2018). Auxiliary cell ampullae are abundant, developing outwardly from inner stellate subcortical cells. The auxiliary cell is the basal cell of the third-order ampullar filament and enlarges when fully developed ().
Very early diploidization stages were not found. After presumed diploidization, the diploidized auxiliary cell soon produces a gonimoblast initial (). In turn, several primary gonimoblast cells are cut off from the gonimoblast initial acropetally (). At the same time, the auxiliary cell remnant fuses with the ampullar cells nearby to form a fusion cell and several secondary connecting filaments are cut off from basal ampullar cells near the fusion cell (). As gonimoblast development continues, the fusion cell fuses with more basal ampullar cells and most ampullar cells elongate slightly but do not branch further (). Fully developed, mature carposporangia-bearing gonimoblasts are composed of at least two different-sized gonimolobes and are 60–195 µm in diameter (). Most cells of the gonimolobes are transformed into pyriform or rounded carposporangia, 12.5–15.0 µm in diameter. At this stage, the fusion cell remains distinguishable at the base of mature gonimoblasts (). Fully developed cystocarps are ostiolate, scattered over the thallus surface, and slightly protruding ().
Nesoia hommersandii Rodríguez-Prieto, S.-M. Lin, Afonso-Carrillo, De Clerck & Huisman sp. nov. (–)
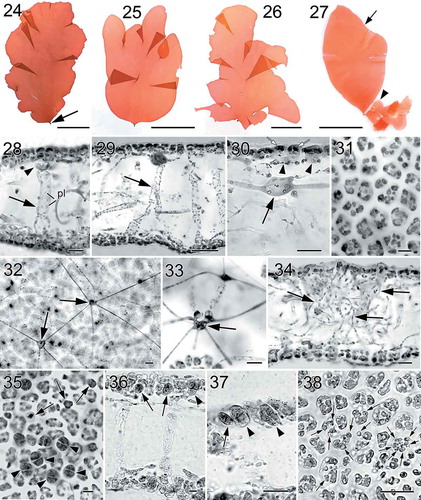
Figs 24–38. Nesoia hommersandii Rodríguez-Prieto, S.-M.Lin, Afonso-Carrillo, De Clerck & Huisman sp. nov. Thallus morphology, vegetative, and reproductive structure. Fig. 24. Holotype, a sporophyte with a single, simple blade and a short stipe (arrow) (TFC Phyc 15296). Figs 25–26. Isotypes, sporophytes bearing lobed margins or bladeletes (TFC Phyc 15297 and 15294). Fig. 27. Isotypes, a small monoecious gametophyte (arrowhead) attached to the basal part of a big sporophyte (arrow). Note that the gametophyte bears many cystocarps that can be distinguished at naked eye (= red dots) (TFC Phyc 15300). Fig. 28. Transverse section of a young blade showing a thin cortex, subcortical cell (arrowhead) and a large medulla with anticlinal medullary filaments (arrow). Note that plastids (pl) are network-like (TFC Phyc 15300). Fig. 29. Transverse section of another young blade showing a thin cortex and broadened medullary filaments (arrow) (TFC Phyc 15300). Fig. 30. Cross section of a young blade showing flattened surface cells (white arrows), ovoid subcortical pit-connected cells with short arms (arrowheads), and inner cortical cell with long arms (black arrow) (TFC Phyc 15300). Fig. 31. Polygonal outer cortical cells in surface view (TFC Phyc 15300). Fig. 32. Subsurface view of thallus blade showing stellate cells of medullary network (arrows) interconnected by long and thin arms (TFC Phyc 15301). Fig. 33. Transverse section showing cells of the anticlinal medullary filaments (arrow) (TFC Phyc 15300). Fig. 34. Transverse section of an old blade showing many rhizoidal filaments (arrows) within the medulla (TFC Phyc 15301). Fig. 35. Subsurface view of a tetrasporangial sorus showing immature tetrasporangia (arrows) and fully divided tetrasporangia (arrowheads) (TFC Phyc 15301). Fig. 36. Cross section through a tetrasporangial sorus showing immature tetrasporangia (arrows) and nearly mature tetrasporangia (arrowhead) (TFC Phyc 15301). Fig. 37. Close up of immature tetrasporangia (arrows) and mature tetrasporangia (arrowhead) (TFC Phyc 15300). Fig. 38. Subsurface view of a spermatangial sorus showing mature spermatangia (arrows) (TFC Phyc 15300). Haematoxylin (Figs 28, 36, 37). Aniline blue (Figs 29–35, 38). Scale bars: Figs 24–27 = 5 cm; Figs 28, 30, 33, 34, 35, 37, 38 = 20 µm; Figs 29 = 50 µm; Figs 31, 32, 36 = 10 µm
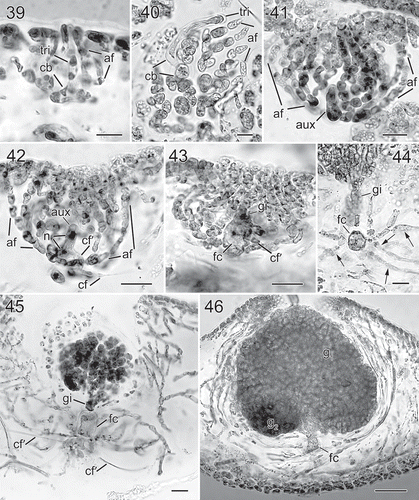
Figs 39–46. Nesoia hommersandii Rodríguez-Prieto, S.-M.Lin, Afonso-Carrillo, De Clerck & Huisman sp. nov. Morphology of carpogonial branch and auxiliary cell ampullae and cystocarp development. Fig. 39. Close up of a carpogonial branch ampulla showing a young carpogonial branch (cb) prolonged by a short trichogyne (tri) surrounded by short branched ampullar filaments (af) (TFC Phyc 15300). Fig. 40. Subsurface view of a fully developed carpogonial branch (cb) ampulla showing an enlarged trichogyne (tri) and highly branched ampullar filaments (af) (TFC Phyc 15300). Fig. 41. Close up of a developing auxiliary cell ampulla showing ampullar filaments (af) and the auxiliary cell (aux) (TFC Phyc 15300). Fig. 42. A fully developed auxiliary cell ampulla showing connecting filament (cf), ampullar filaments (af) and an enlarged auxiliary cell (aux) with stained nuclei (n). Note that a secondary connecting filament (cf’) is cut off from the basal part of the auxiliary cell (aux) (TFC Phyc 15300). Fig. 43. Diploidized auxiliary cell ampulla showing a gonimoblast initial (gi), a secondary connecting filament (cf’) cut off from basal part of newly formed fusion cell (fc) (TFC Phyc 15300). Fig. 44. Close up of a young carposporophyte showing an elongated gonimoblast initial (gi) and a fusion cell (fc) that cut off several secondarily connecting filaments (cf’) (TFC Phyc 15300). Fig. 45. An immature carposporophyte showing a gonimoblast initial (gi) and a fusion cell (fc) which is sending out two secondarily connecting filaments (cf’) (TFC Phyc 15300). Fig. 46. A fully developed carposporophyte showing fusion cell (fc) and two gonimolobes (g1 & g2) (TFC Phyc 15300). Aniline blue (Figs 39, 40, 44, 46); Haematoxylin (Figs 41–43, 45). Scale bars: Figs 39, 40 = 10 µm; Figs 41–45 = 20 µm; Fig. 46 = 50 µm
Diagnosis: Thalli erect, rose to dark red in colour, foliose, consisting of a simple, oval or lobate blade, 16–20 cm long and 10–14 cm wide, arising from short stipes, soft and gelatinous in texture. Blades 100–260 µm thick in upper parts, cortex made up of 2–3 cell layers, outer cortical cells not secondarily pit-connected, flattened or irregularly polygonal, 8.0–15.9 µm wide by 6.0–12.1 µm long in surface view, 5.5–16.4 in height by 6.49–13.6 µm in width in longitudinal view, subcortex composed of a network of rounded or ovoid cells with short arms, subtended by a network of stellate cells with long arms. Medulla composed of a network of stellate cells with long arms, and sparse filaments, anticlinal to thallus surface, 57–126 µm long and 8–20 µm thick. Tetrasporangia cruciately, cruciately-decussately, or (rarely) irregularly divided, 14.7–17.7 µm long by 10.9–14.5 µm wide. Gametophytes monoecious. Carpogonial branches two-celled; auxiliary cell ampullae abundant and composed of three orders of ampullar filaments, up to 11–15 cells long when fully developed; carposporophyte composed of two gonimolobes, up to 126–209 µm in diameter; carposporangia 7.6–13.4 µm in diameter; mature cystocarps ostiolate, protruding above the thallus surface.
Etymology: The species name is dedicated to Professor Max H. Hommersand, for his immense contribution to the systematics of red algae, and especially to the knowledge of female reproduction in red algae.
Type collection: Holotype, a tetrasporic plant () growing in a mixed sand-rock bottom at La Rapadura, Santa Úrsula, Tenerife, Canary Islands, Spain, 01 October 2016, collected by R. Martel, at a depth of 40 m. The specimen is deposited at the algae section of the Herbarium of the University of La Laguna (TFC Phyc 15296). Isotypes: TFC Phyc 15297 (, tetrasporic), TFC Phyc 15294 (, tetrasporic), and TFC Phyc 15300 ( containing tetrasporic, male and female).
Type locality: La Rapadura, Santa Úrsula, Tenerife, Canary Islands, Spain (Macaronesia). Distribution: This species has only been found in Santa Úrsula (Tenerife, Canary Islands, Spain).
Habitat and seasonality: The species was found in October at a depth of 40 m, on a mixed sand-rock bottom.
Specimens examined: La Rapadura, Santa Úrsula, Tenerife, Canary Islands, Spain, −40 m, 01.x.2016, coll. R. Martel, TFC Phyc 15296 (tetrasporic); TFC Phyc 15297 (tetrasporic), TFC Phyc 15294 (tetrasporic), TFC Phyc 15300 (tetrasporic, male and female), TFC Phyc 15301 (tetrasporic) and TFC Phyc 15299 (tetrasporic).
Habit and vegetative structure: Thalli are foliose, 16–20 cm long and 10–14 cm wide, composed of simple or lobate blades, arising from small discoid holdfasts with short stipes (–). Blades are soft and gelatinous in texture, rose to dark red with a thin darker margin. Blades are 100–260 µm thick, composed of two thin layers of cortex and a large lax medulla (–). The cortex is made up of 2 cell layers in young thalli and becomes 3 cell layers in older parts (). Outer cortical cells are 8.0–15.9 µm wide by 6.0–12.1 µm long in surface view and 5.5–16.4 µm high by 6.49–13.6 µm wide in longitudinal view, not protruding at thallus surface (), and are irregularly polygonal and compactly arranged in surface view (). Surface cells lack secondary pit-connections (). The subcortex in fully developed thalli is composed of two networks of secondarily connected stellate cells: an outer layer of rounded or ovoid cells with short arms (, arrowheads) and the innermost layer with sometimes darkly stained stellate cells possessing relatively long arms (, arrow). However, the network of stellate cells with short arms is lacking in the young subcortex. The medulla is composed of a network of stellate cells parallel to the thallus surface and situated mostly just under inner cortical cells (, arrows), and some sparsely arranged anticlinal filaments of elongated cells (–, arrows) that are produced from innermost cortical cells, and that can be up to 57–126 µm long and 8–20 µm thick at the apex of the thallus. Medullary cells bearing long arms cannot be easily observed in transverse section but can be seen in subsurface view after treated with haematoxylin or aniline blue stain. Occasionally, some clusters of rhizoidal filaments are found in the old medulla (). Outer cortical cells have more pigmented plastids than cells in the subcortex and medulla but all of them have reticulated plastids (, pl).
Reproductive structures. Tetrasporangial sori are scattered over the thallus surface (). Tetrasporangial initials are cut off from subcortical cells and different ages of developing tetrasporangia can be found simultaneously (–). Fully developed tetrasporangia are rounded or ovoid, cruciately, cruciately-decussate or irregularly divided, 14.7–17.7 µm long by 10.9–14.5 µm wide (–).
Gametophytes are monoecious. Spermatangial sori are scattered over the thallus surface (). The carpogonial branch ampulla is composed of a two-celled carpogonial branch () with a distally swollen trichogyne and two ampullar filaments when fully developed (). Auxiliary cell ampullae are abundant, developing outwardly from inner stellate, subcortical cells with short arms. The auxiliary cells are always the basal cell of the third-order ampullar filament ().
Direct diploidization was not observed. After presumed diploidization via a connecting filament, the auxiliary cell elongates, its nucleus divides once (), cutting off a gonimoblast initial at its distal end (). Soon after, the remnant of the auxiliary cell fuses with several basal ampullar cells to form a primary fusion cell () while the distal cells of first- and second-order ampullar filaments produce lateral branches. At the same time, the fusion cell cuts off one or several secondary connecting filaments (). As gonimoblast development continues, several secondary connecting filaments are issued from enlarged, multinucleate fusion cells (–). Fully developed, mature carposporangia-bearing gonimoblasts are composed of at least two different sized gonimolobes and are 126–209 µm in diameter (). Most cells of the gonimolobes are transformed into pyriform or rounded carposporangia, 7.6–13.4 µm in diameter (). At this stage, the fusion cell remains distinguishable at the base of mature gonimoblasts and fully developed cystocarps are ostiolate and slightly protruding and scattered over the thallus surface ().
Discussion
Based on rbcL sequence analyses (), most described species of Halymenia from the Mediterranean Sea, Atlantic and Pacific oceans, together with two monotypic genera, Gelinaria ulvoidea and Epiphloea bullosa, from Western Australia, are clustered into a large clade (Halymenia sensu stricto herein). Moreover, foliose Halymeniaceae from Europe and the Mediterranean Sea are separated into five distinct species: Halymenia ballesterosii (a new species described in this study), Neofolia rosea (Rodríguez-Prieto et al., Citation2018), Nesoia hommersandii (a new species described in this study), Nesoia mediterranea (Rodríguez-Prieto et al., Citation2019) and N. latifolia Lee & Kim (Citation2019). The former four species were previously confused with Nesoia latifolia, which was previously known as ‘Halymenia’ latifolia and was once regarded as having a wide distribution in both the Atlantic and Pacific oceans and the Mediterranean Sea (D’Archino et al., Citation2014; Rodríguez-Prieto et al., Citation2019).
In the field, H. ballesterosii is difficult to separate from Nesoia latifolia and N. mediterranea as the three species share a similar thallus morphology (i.e. blade colour and texture). Halymenia ballesterosii is also very similar to Neofolia rosea, although the latter is a little darker and tougher than the other three taxa (Rodríguez-Prieto et al., Citation2018, Citation2019). However, the vegetative and female reproductive structure of H. ballesterosii is similar to the generitype, H. floresii, in terms of having protruding surface cells, two-celled carpogonial branches, and possessing basal nutritive cell cluster in the carpogonial branch ampullae, secondary connecting filaments issued from basal cells of ampullar filaments as well as mature cystocarps immersed in the medulla (Rodríguez-Prieto et al., Citation2018, Citation2019, this study). Moreover, the surface cells in Nesoia and Neofolia are level with the thallus surface and both genera lack a nutritive cellular cluster at the base of the carpogonial branch ampullae (Rodríguez-Prieto et al., Citation2018, Citation2019). A morphological comparison of the five foliose species of Halymenia, Neofolia and Nesoia from Europe is given in .
Table 1. Morphological comparison among foliose species of Halymenia, Neofolia and Nesoia from Europe
Nesoia hommersandii can be confused with Nesoia latifolia in having a similar thallus morphology. However, N. hommersandii (lacking swollen arms) differs from N. latifolia (having swollen arms) in the morphology of medullary stellate cells. On the other hand, young or smaller plants of N. hommersandii (2-celled carpogonial branches) are sometimes indistinguishable from Neofolia rosea (having swollen arms in medullary cells, 3-celled carpogonial branches) in habit, but they differ in vegetative and female reproductive morphology (see ).
The observations on female reproductive structures in the two newly described species corroborate the diagnostic characters proposed to describe or redefine Halymenia, Neofolia and Nesoia (Rodríguez-Prieto et al., Citation2018). Traditionally, all species in these genera were considered to possess fairly uniform female reproductive and post-fertilization structures. Careful observations however revealed a subtle but distinct set of characters that allow separation at the genus level. The structures reported for the new Halymenia and Nesoia species agree with the defining characteristics of both genera. From a more general point of view, the combined molecular-morphological approach taken in our studies is promising to redefine the classification and biogeography of many of the remaining Halymenia species worldwide. As seen in the literature, the name ‘Halymenia latifolia’ was widely used for large, unbranched foliose plants occurring in Europe (Maggs & Guiry, Citation1982; Loiseaux-de Goër & Noailles, Citation2008; Rodríguez-Prieto et al., Citation2013; Athanasiadis, Citation2016). The exact biogeographic ranges of H. ballesterosii and Nesoia hommersandii are difficult to delineate at present as many records of ‘Halymenia latifolia’ need to be reexamined. Nevertheless, H. ballesterosii has been currently confirmed only for Banyuls-sur-Mer, Catalonia and the Balearic Islands (Western Mediterranean) while N. hommersandii is present only in the Canary Islands (Macaronesia).
Supplementary Information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2020.1749888.
Supplementary table 1. List of species used in rbcL analysis and accession numbers in GenBank. Accession numbers in bold were determined for this study.
Author contributions
C. Rodrí guez-Prieto & S.-M. Lin: original concept and drafting manuscript; C. Rodrí guez-Prieto: light microscopy; C. Rodrí guez-Prieto & J. Afonso-Carrillo: field investigations; S.-M. Lin & O. De Clerck: analysis of molecular data; C. Rodrí guez-Prieto, Julio Afonso-Carrillo, Olivier De Clerck, John M. Huisman & S.-M. Lin all review & approved the final manuscript.
Supplemental Material
Download MS Word (32.7 KB)Acknowledgements
We thank Miss Y.-S. Chiou and Sofie D’Hondt for helping with DNA sequencing. We thank Sergi Joher, Enric Ballesteros and Ramiro Martel for collecting some of the samples used in this work. We are thankful to the crews of the research vessels Cornide de Saavedra, Miguel Oliver and Ramón Margalef.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental Material
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2020.1749888.
Additional information
Funding
References
- Athanasiadis, A. (2016). Phycologia Europaea Rhodophyta Vol. I. pp. [i] –xxxxviii, 1–762. Published and distributed by the author, Thessaloniki.
- D’Archino, R., Nelson, W.A. & Zuccarello, G.C. (2014). Amalthea and Galene, two new genera of Halymeniaceae (Rhodophyta) from New Zealand. Botanica Marina, 57: 185–201.
- De Clerck, O., Gavio, B., Fredericq, S., Bárbara, I. & Coppejans, E. (2005a). Systematics of Grateloupia filicina (Halymeniaceae, Rhodophyta) based on rbcL sequence analyses and morphological evidence, including the reinstatement of G. minima and the description of G. capensis sp. nov. Journal of Phycology, 41: 391–410.
- De Clerck, O., Gavio, B., Fredericq, S., Cocquyt, E. & Coppejans, E. (2005b). Systematic reassessment of the red algal genus Phyllymenia (Halymeniaceae, Rhodophyta). European Journal of Phycology, 40: 169–178.
- Guiry, M.D. & Guiry, G.M. (2019). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; last searched on 2 September 2019.
- Hernández-Kantun, J.J., Riosmena-Rodríguez, R. & León-Cisneros, K. (2009). Morphology and anatomy of Halymenia actinophysa (Halymeniales, Rhodophyta) from the southwestern Gulf of California, Mexico. Botanica Marina, 52: 248–255.
- Kraft, G.T. & Robins, P.A. (1985). Is the Order Cryptonemiales (Rhodophyta) defensible? Phycologia, 24: 67–77.
- Lee, H.W. & Kim, M.S. (2019). Female reproductive structures define the novel genus, Nesoia (Halymeniaceae, Rhodophyta). European Journal of Phycology, 54: 66–77.
- Lin, S.-M., Liang, H.-Y. & Hommersand, M.H. (2008). Two types of auxiliary cell ampullae in Grateloupia (Halymeniaceae) including G. taiwanensis sp. nov. and G. orientalis sp. nov. from Taiwan based on rbcL gene sequence analysis and cystocarp development. Journal of Phycology, 44: 196–214.
- Loiseaux-de Goër, S. & Noailles, M.-C. (2008). Algues de Roscoff. Editions de la Station Biologique de Roscoff, Roscoff.
- Maggs, C. & Guiry, M. (1982). Morphology, phenology and photoperiodism in Halymenia latifolia Kütz. (Rhodophyta) from Ireland. Botanica Marina, 25: 589–600.
- Rambaut, A., Suchard, M.A., Xie, D. & Drummond, A.J. (2014). Tracer v1.6. Available from http://tree.bio.ed.ac.uk/software/tracer.
- Rodríguez-Prieto, C., Ballesteros, E., Boisset, F. & Afonso-Carrillo, J. (2013). Guía de las macroalgas y fanerógamas marinas del Mediterráneo occidental. Ediciones Omega, S.A., Barcelona.
- Rodríguez-Prieto, C., De Clerck, O., Huisman, J.M. & Lin, S.M. (2018). Systematics of the red algal genus Halymenia (Halymeniaceae, Rhodophyta). 1. Characterization of the generitype H. floresii and description of Neofolia rosea gen. & sp. nov. European Journal of Phycology, 53: 520–536.
- Rodríguez-Prieto, C., De Clerck, O., Huisman, J.M. & Lin, S.M. (2019). Characterisation of Nesoia latifolia (Halymeniaceae, Rhodophyta) from Europe with emphasis on cystocarp development and description of Nesoia mediterranea sp. nov. Phycologia, 58: 393–404.
- Rodríguez-Prieto, C. & Hommersand, M.H. (2009). Behaviour of the nuclei in pre- and post-fertilization stages in Kallymenia (Kallymeniaceae, Rhodophyta). Phycologia, 48: 138–155.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L, Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M. A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Saunders, G.W. & Kraft, G.T. (1996). Small-subunit rRNA gene sequences from representatives of selected families of the Gigartinales and Rhodymeniales (Rhodophyta). II. Recognition of the Halymeniales ord. nov. Canadian Journal of Botany, 74: 694–707.
- Tamura, K., Stecher, G., Peterson, P., Filipski, A. & Kumar, S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biololgy and Evolution, 30: 2725–2729.
- Thiers, B. (2019). (continuously updated) Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/.
- Wittmann, W. (1965). Aceto-iron-haematoxylin-chloral hydrate for chromosome staining. Stain Technology, 40: 161–164.
