ABSTRACT
We investigated species referred to as ‘Ahnfeltiopsis flabelliformis’ from the Far Eastern seas of Russia as well as Besa catenata (Yendo) M.S.Calderon & S.M.Boo from Mie Prefecture (Shima Peninsula, Japan, near to its type locality) morphologically and by analysis of sequences of plastid rbcL and mitochondrial COI-5P genes. Morphological and phylogenetic analyses showed that Ahnfeltiopsis flabelliformis does not occur in the Far Eastern seas. All algae previously identified as ‘A. flabelliformis’ from Russian waters belong to two morphologically and genetically distinct species that should be referred to the genus Besa as B. divaricata (Holmes) M.S.Calderon & S.M.Boo and B. japonica (Suringar) A.V. Skriptsova & S.Y.Shibneva, comb. nov. We found an unattached form of B. divaricata and named it as B. divaricata f. ahnfeltioides A.V.Skriptsova & S.Y.Shibneva based on its morphological and ecological features.
Introduction
The family Phyllophoraceae (Gigartinales) is one of the most intensively studied red algal taxa. The systematics of the family was traditionally based on life-cycle features (Silva & DeCew, Citation1992). The Phyllophoraceae includes 16 genera, of which only six are recorded from the North-west Pacific. These include the genera Ahnfeltiopsis P.C.Silva & DeCew and Besa Setchell, which have recently undergone taxonomic revision.
The genus Besa was established in 1912 by W.A. Setchell with a description of the generitype, Besa papillaeformis Setchell, collected off Lands End, San Francisco, California, USA. Besa papillaeformis forms expanded, fleshy, cartilaginous crusts bearing diminutive reproductive papillae up to 0.45 mm tall (Fredericq & Lopez-Bautista, Citation2002). Before 2016, only two species were attributed to the genus Besa: B. papillaeformis and B. stipitata Hollenberg & Abbott. The isomorphic alteration of generations was the main distinguishing feature of Besa. The genus Ahnfeltiopsis was separated from Gymnogongrus C.Martius in 1992 (Silva & DeCew, Citation1992). It was proposed for members of the Phyllophoraceae in which the female gametophyte has an erect compressed thallus, with internal cystocarps developing in the medulla, and a heteromorphic life cycle in which a free-living, crustose tetrasporophyte alternates with the erect gametophyte. Subsequently, based on molecular phylogenetic analyses, it was shown that the genus Ahnfeltiopsis is not monophyletic, and is divided into several (four to six) distantly related groups, which include members of other genera of the Phyllophoraceae (Fredericq & Lopez-Bautista, Citation2002; Fredericq et al., Citation2003; Le Gall & Saunders, Citation2010; Maggs et al., Citation2013; Calderon & Boo, Citation2016; Calderon et al., Citation2016). Therefore, it was suggested that life cycle pattern, the basis of some generic concepts in the Phyllophoraceae, cannot be used as a character to recognize genera (Fredericq & Lopez-Bautista, Citation2002; Le Gall & Saunders, Citation2010).
In a series of recent investigations based on molecular analyses, some new genera have been segregated from Ahnfeltiopsis (Maggs et al., Citation2013; Calderon & Boo, Citation2016) and four species of Ahnfeltiopsis were transferred to Besa as B. divaricata (Holmes) M.S.Calderon & S.M.Boo, B. catenata (Yendo) M.S.Calderon & S.M.Boo, B. paradoxa (Suringar) M.S.Calderon & S.M.Boo and B. leptophylla (J.Agardh) M.S.Calderon & K.A.Miller. This has changed the concept of the genus Besa. At present, this genus includes species with both erect and diminutive crustose thalli characterized by the following features: the formation of small, downwardly directed gonimoblast initials, medullary cells laterally connected to clusters of carposporangia, and the fusion of fusiform gonimoblast conjunctor cells with nearby vegetative cells (Calderon et al., Citation2016). The new concept of Besa includes both heteromorphic and isomorphic life cycles. B. papillaeformis and B. stipitata with crustose thalli are considered as an example of paedomorphosis in which precocious sexual maturation has been evolutionarily fixed in a putative morphological juvenile stage as a possible consequence of progenesis (Fredericq & Lopes-Bautista, Citation2002). As a result, the erect axes of the gametophyte remain strongly reduced, so that the gametophyte thallus is effectively crustose, and the life cycle is considered as isomorphic.
According to the current concept, the genus Ahnfeltiopsis includes species with heteromorphic alternation of generations, characterized by the presence of heterokaryotic medullary cells that form carposporangia, secondary medullary cells around the cystocarp, and terminal tubular gonimoblasts penetrating the cystocarp boundary and linked to cortical cells (Calderon & Boo, Citation2016; Calderon et al., Citation2016). Besides the generitype species Ahnfeltiopsis linearis (C.Agardh) P.C.Silva & DeCew, the genus Ahnfeltiopsis includes A. chnoosporoides (T.Tanaka & Pham-Hoàng Hô) Masuda (Calderon & Boo, Citation2016). However, the taxonomic position of many species, which traditionally were attributed to Ahnfeltiopsis, remains uncertain. For example, according to phylogenetic data, one of the widely distributed species, A. flabelliformis (Harvey) Masuda, is distant from other species of Ahnfeltiopsis (including the generitype A. linearis) and instead is closely related to Gymnogongrus griffithsiae (Turner) C.Martius, the type species of Gymnogongrus (Calderon et al., Citation2016; Calderon & Boo, Citation2017).
The revolutionary changes to Ahnfeltiopsis and Besa necessitate reassessment of these genera in local floras. For the Russian Far Eastern seas, only A. flabelliformis is reported (Perestenko, Citation1994). In this region, the species is distributed along the continental coast of the Sea of Japan, from Peter the Great Bay in the south to Cape Zolotoi in the north, and to Sakhalin Island in the west; in the Sea of Okhotsk, it occurs only in Aniva Bay (Sakhalin Island) and off the north-western coast of Kunashir Island (Perestenko, Citation1994; Klochkova, Citation1996; Kusakin et al., Citation1997; Ivanova et al., Citation2008). The vegetative morphology of A. flabelliformis was noted as quite variable, and it possibly included several species (Masuda et al., Citation1994). In Russian waters, A. flabelliformis is represented by two ecologically and morphologically differentiated forms: attached and unattached (Perestenko, Citation1980, Citation1994). Besides A. flabelliformis, according to Calderon et al. (Citation2016), two species of the genus Besa may also be found in Russian waters: B. divaricata and B. catenata, which previously belonged to the genus Ahnfeltiopsis (as A. divaricata (Holmes) Masuda and A. catenata (Yendo) Masuda, respectively). Specimens of these algae were collected from two areas of Peter the Great Bay (see Calderon et al., Citation2016, supplemental materials). It should be noted that A. flabelliformis, a common species in the flora of Peter the Great Bay, was not sampled by Calderon et al. (Citation2016) in the Bay. However, Russian researchers have until recently considered ‘A. flabelliformis’ to be the only representative of the genus Ahnfeltiopsis sensu lato in Russia.
The aim of our present study is a revision of ‘A. flabelliformis’ along the Russian coast of the North-west Pacific to clarify the taxonomic position of the Russian alga and to ascertain whether Besa occurs in Russian waters. For this purpose, we analysed and compared specimens collected from several localities along the Russian coast of the Sea of Japan and the Sea of Okhotsk with other morphologically close species of Besa, Ahnfeltiopsis and Gymnogongrus previously described from the North-west Pacific. Also, we assessed the phylogenetic relationships of ‘A. flabelliformis’ from the Russian Far Eastern seas based on sequences of plastid (rbcL) and mitochondrial (COI-5P) genes.
Materials and methods
Seaweed collection
Specimens of ‘A. flabelliformis’ for this study were collected from nine localities in marine waters of the Russian Far East. Samples of the attached form were collected from the subtidal zone at a depth of 0.5–1 m, a typical habitat for this species. Samples of the unattached form were taken from the stratum of Ahnfeltia tobuchiensis (Kanno & Matsubara) Makijenko in Peter the Great Bay, at a depth of 7–8 m. In addition, we collected A. flabelliformis from the waters off Jeju Island (South Korea) and off Amakusa Island (Japan), and B. catenata from the waters off Shima peninsula (Mie Pref., Honshu, Japan), a site close to Ijika – the type locality of the species (Masuda, Citation1987). The specimens and the details of their collection are provided in Supplementary table 1.
Specimens for molecular genetic analysis were frozen or dried in silica gel; voucher specimens of the same thalli were preserved and deposited in the herbarium at the A.V. Zhirmunsky National Scientific Center of Marine Biology, FEB RAS (Vladivostok, Russia). The voucher specimen of B. catenata from Japan is deposited in the National Museum of Nature and Science (Tsukuba, Japan) with number TNS-AL-209180. This voucher specimen was also used for the morphological analysis.
In addition we analysed samples of B. catenata from Hiroshima (Hiroshima Pref., TNS-AL 182050, 182051), Kojuki (Oita Pref., TNS-AL 191062), Hotozu (Oita Pref., TNS-AL 209183), Shima (Mie Pref., TNS-AL-209181, 209182) and Waji (Aichi Pref., TNS-AL 209820), Japan, all deposited in the National Museum of Nature and Science, Tsukuba, Japan.
Morphological observations
The anatomy of specimens was studied using manual longitudinal sections and cross-sections made with a safety-razor blade in the basal, middle and apical parts of the thallus, as well as through cystocarps. The cross-sections were stained with 1% aqueous solution of aniline blue acidified with 1% HCl solution. A Carl Zeiss AxioVert 200M microscope was used for analysis and microphotographs. For the analysis of the anatomy, dimensions of the cortical and medullary cells were measured, the number of the cell layers in the cortex and medulla counted, the thickness of the cell walls measured, and details of cystocarps observed.
DNA preparation, PCR amplification, sequence editing and phylogenetic analysis
Genomic DNA was extracted from silica-gel-dried or frozen apical fragments of thalli by the CTAB method (Wang et al., Citation2006). A 550–600 bp fragment of the COI-5P region was amplified with the primers GazF1 (5’-TCAACAAATCATAAAGATATTGG-3’) and GazR1 (5’-ACTTCTGGATGTCCAAAAAAYCA-3’) as described by Saunders (Citation2005). We also used the forward PCR primer F57 (5’-GTAATTCCATATGCTAAAATGGG-3’) in Freshwater & Rueness (Citation1994) and rbcLrevNew (5’-ACATTTGCTGTTGGAGTYTC-3’) of Saunders & Moore (Citation2013) to amplify a 1250–1300 bp fragment of the rbcL. PCR amplification was done in a 10 µl reaction mixture containing 5 µl GoTaq Green Master Mix (Promega Corp., Madison, Wisconsin, USA), 0.5 µM of each primer (10 pg μl–1), 3 µl nuclease-free water, and 1 µl purified DNA. COI and rbcL were amplified according to the protocols described by Saunders & Moore (Citation2013) and Saunders (Citation2005), respectively. All PCR products were checked using electrophoresis on 1.5% TBE agarose by visualizing on GelDoc XR+ imaging systems (Bio-Rad). Each positive PCR product was purified for cycle sequencing using Exonuclease I (ExoI) and Thermosensitive Alkaline Phosphatase (FastAP; ThermoFisher Scientific, Waltham, Massachusetts, USA). DNA sequencing was performed on both strands using a BigDye 3.1® sequencing kit (ThermoFisher Scientific), in a 10 µl reaction mixture including 1.25 µl ABI 5× dilution buffer, 1 µl Big Dye, 0.5 µM primer, 0.5 µl PCR product and 6.75 µl nuclease-free water. Each sequencing reaction was purified by ethanol precipitation as described in the protocol. The PCR products were bidirectionally sequenced on an ABI 3130x sequencer (Applied Biosystems) and aligned in MEGA7 (Kumar et al., Citation2016) using the MUSCLE algorithm (Edgar, Citation2004). Also, MEGA7 was used for calculation of inter- and intraspecific COI-5P and rbcL distances using the Pairwise distance model (P-distance). ABGD analysis (www.abi.snv.jussieu.fr/public/abgd/abgdweb.html; Puillandre et al., Citation2012) was used for species delimitation and establishing taxonomic status of sequenced specimens, using the values of relative gap width (X = 1.0) and intraspecific divergence (P) between 0.5% and 10% using P-distances.
Bayesian phylogenetic analyses were conducted in MrBayes v. 3.2.7 (Ronquist & Huelsenbeck, Citation2003). PartitionFinder 2.1.1 (Lanfear et al., Citation2012) was used to select the best-fit partitioning scheme and models separately for each codon position of COI and rbcL genes, using the greedy algorithm with linked branch lengths for the corrected Bayesian Information Criterion as the optimality criterion for model selection. The best model for the first codon position of both genes was GTR + I + G (Tavaré, Citation1986); for the second codon position, F81 + I (Felsenstein, Citation1981); and for the third codon position, GTR + I + G was the best model for COI and HKY + I + G (Hasegawa et al., Citation1985) for rbcL. Bayesian inference was performed with two independent runs of Metropolis-coupled Markov chain Monte Carlo analyses. The chains were run for 5 million generations and sampled every 100 generations. A burn-in of 100,000 generations (or 2% of the sampled trees) was used. Moreover, trace files were visually inspected in Tracer v. 1.7 (Rambaut et al., Citation2018). Bayesian tree topology was validated by Maximum likelihood (ML) analysis; however, we show only a Bayesian tree with the addition of bootstrap support from a ML tree with the same topology. RAxML v. 8.2.4 (Stamatakis, Citation2006) was used to conduct a ML and bootstrap analysis (1000 replications) using the GTR + G model. FigTree v. 1.4.4 was used to visualize phylogenetic trees after analysis. Chondrus crispus and Mazzaella japonica (Gigartinaceae) were used as outgroup. All sequences have been deposited in GenBank (accession numbers MN694888–MN694916, MT188618 for COI-5P and MN694845–MN694887, MT188617 for rbcL).
Results
Morphological analysis
The comparative morpho-anatomical analysis of specimens of ‘A. flabelliformis’ collected along the Russian coast of the North-west Pacific revealed at least two morphologically close species that differed mainly in texture, width of axes and type of apices. Some were most similar to Ahnfeltiopsis divaricata (Supplementary table 2) in the classical description by M. Masuda (Masuda, Citation1987; Masuda et al., Citation1994), now B. divaricata (Calderon et al., Citation2016). The other specimens differed considerably from B. divaricata and were morphologically similar to Gymnogongrus japonicus as reported by Masuda (Citation1987) and Makijenko (Citation1970) (Supplementary table 2). Both species shared some characteristics with A. flabelliformis but differed from it by having fewer rows of cells in cortex and medulla, a lighter colour of apices compared with other parts of the thallus, and by the equal number of cell rows in the cortex throughout the thallus (Supplementary table 2). We did not find in Russian waters any specimens identical to A. flabelliformis or B. catenata (= A. catenatus) according to descriptions (Masuda, Citation1987; Masuda et al., Citation1994). The specimens of B. catenata from Shima (Japan), the closest site to the type locality of the species, matched the classical descriptions (Masuda, Citation1987; Masuda et al., Citation1994).
A detailed analysis of the cystocarp structure in specimens of both species from the Russian coast, as well as B. catenata from Shima, showed the medullary cells laterally connected to clusters of carposporangia and the fusiform gonimoblast conjunctor cells penetrating the cystocarp boundary. There were no secondary medullary cells around the cystocarp. These features indicate that all specimens from the Russian coast belong to the genus Besa (Calderon et al., Citation2016) and the species B. divaricata and B. japonica (Suringar) A.V. Skriptsova & S.Y.Shibneva, comb. nov.
Besa divaricata (Holmes) M.S.Calderon & S.M.Boo ()
Figs 1–10. Besa divaricata, collected from Sukhoputnaya Bay, Sea of Japan, Russia. Fig. 1. Habit. Scale bar = 1 cm. Fig. 2. Details of apices. Scale bar = 5 mm. Fig. 3. Cross-section showing the mature cystocarp loosely immersed within the medulla. Scale bar = 200 µm. Fig. 4. Longitudinal section of a branch in the middle part of a thallus, showing cortex (c) and pseudoparenchymatous medulla (m). Scale bar = 100 µm. Fig. 5. Cross-section of a branch in the middle part of a thallus. Scale bar = 100 µm. Fig. 6. Cross-section showing details of 4–5 layered cortex. Scale bar = 40 μm. Fig. 7. Close-up view showing the absence of secondary medullary cells around the cystocarp (cys). Note the vegetative medullary cells with unmodified cell walls. Scale bar = 100 µm. Fig. 8. Close-up view showing a carpostome, a cavity composed of periclinal filaments (arrow) emerging from cortical cells. Scale bar = 20 µm. Fig. 9. Medullary cell (arrow) laterally connected to clusters of carposporangia. Scale bar = 20 µm. Fig. 10. Detail showing the fusion of fusiform conjunctor cells (black arrow) penetrating the cystocarp boundary to nearby vegetative cells (white arrow). Scale bar = 20 µm
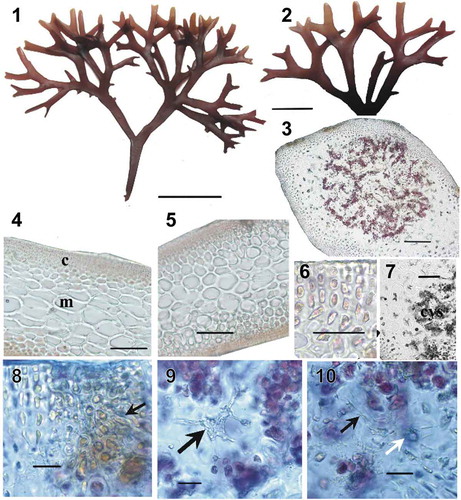
Figs 11–15. Besa divaricata f. ahnfeltioides collected from Amur Bay, Sea of Japan, Russia. Fig. 11. Habit. Scale bar = 2 cm. Fig. 12. Details of apices. Scale bar = 5 mm. Fig. 13. Longitudinal section of a branch in the middle part of a thallus. Scale bar = 200 µm. Fig. 14. Cross-section of a branch in the middle part of a thallus. Scale bar = 200 µm. Fig. 15. Cross-section showing details of 4–5 layered cortex. Scale bar = 20 μm
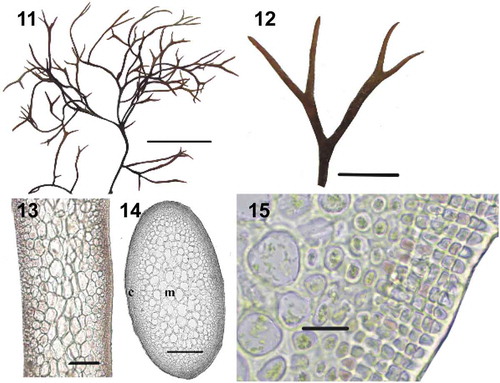
Figs 16–26. Besa japonica collected from Sukhoputnaya Bay, Sea of Japan, Russia. Fig. 16. Habit. Scale bar = 1 cm. Fig. 17. Details of apices. Scale bar = 5 mm. Fig. 18. Cystocarps in catenate series. Scale bar = 5 mm. Fig. 19. Cross-section showing the mature cystocarp loosely immersed within the medulla. Scale bar = 200 µm. Fig. 20. Longitudinal section of a branch in the middle part of a thallus, showing cortex (c) and pseudoparenchymatous medulla (m). Scale bar = 200 µm. Fig. 21. Cross-section of a branch in the middle part of a thallus. Scale bar = 200 µm. Fig. 22. Cross-section showing details of 4–5 layered cortex. Scale bar = 40 μm. Fig. 23. Close-up view showing absence of secondary medullary cells around the cystocarp (cys). Note the vegetative medullary cells with unmodified cell walls. Scale bar = 40 µm. Fig. 24. Close-up view showing a carpostome, a cavity composed of periclinal filaments (arrow) emerging from cortical cells. Scale bar = 20 µm. Fig. 25. Medullary cell (arrow) laterally connected to clusters of carposporangia. Scale bar = 40 µm. Fig. 26. Detail showing the fusion of fusiform conjunctor cells (black arrows) penetrating the cystocarp boundary to nearby vegetative cells (white arrows). Scale bar = 40 µm
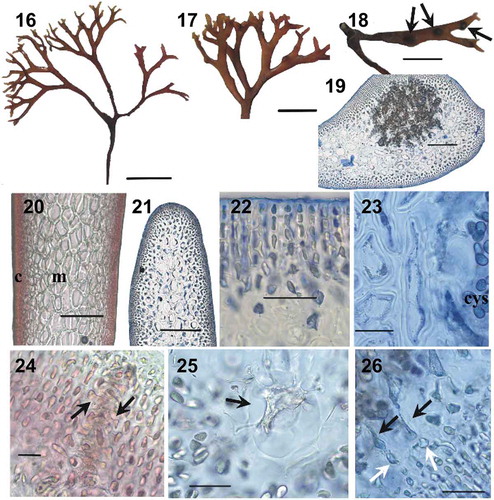
Figs 27–37. Besa catenata, collected from Shima, Mie Pref., Japan. Fig. 27. Habit. Scale bar = 2 cm. Fig. 28. Details of apices. Scale bar = 5 mm. Fig. 29. Cystocarps in catenate series. Scale bar = 5 mm. Fig. 30. Cross-section of a branch in the middle part of a thallus. Scale bar = 200 µm. Fig. 31. Longitudinal section of a branch in the middle part of a thallus showing cortex and pseudoparenchymatous medulla. Scale bar = 1200 µm. Fig. 32. Cross-section showing details of multilayered layered cortex in middle part of the thallus. Scale bar = 20 μm. Fig. 33. Cross-section showing the mature cystocarp loosely immersed within the medulla. Scale bar = 200 µm. Fig. 34. Close-up view showing absence of secondary medullary cells around the cystocarp (cys). Note the vegetative medullary cells with unmodified cell walls. Scale bar = 50 µm. Fig. 35. Close-up view a carpostome, a cavity composed of periclinal filaments (arrows) emerging from cortical cells. Scale bar = 50 µm. Fig. 36. Medullary cell (arrow) laterally connected to clusters of carposporangia. Scale bar = 50 µm. Fig. 37. Detail showing the fusion of fusiform conjunctor cells (black arrow) penetrating the cystocarp boundary to nearby vegetative cells (white arrow). Scale bar 20 µm
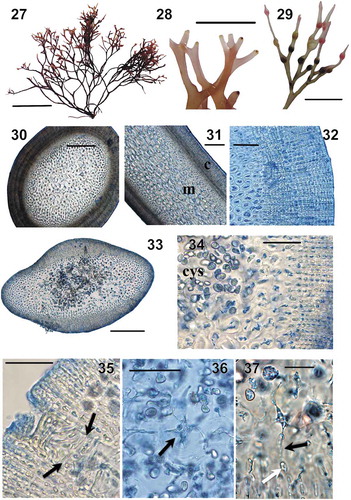
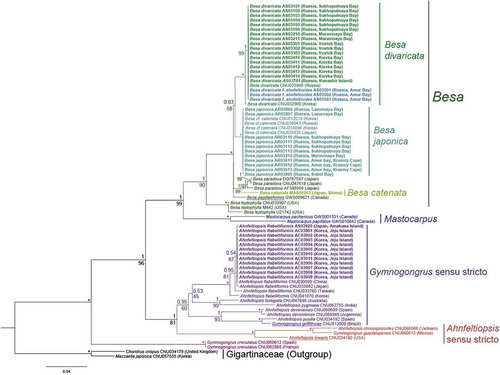
Fig. 38. Bayesian tree of four genera of Phyllophoraceae based on the mitochondrial COI and plastid rbcL sequences (1866 bp). Numerals above tree nodes are Bayesian posterior probabilities; below nodes, ML bootstrap values. Asterisk (*) indicates posterior probabilities of 1.00 and bootstrap support of 100%. Generitypes are underlined. Specimens obtained in this study are highlighted in bold
Basionym: Gymnogongrus divaricatus Holmes 1896: New Marine Algae from Japan. Journal of the Linnean Society of London, Botany, 31: 248–260, pls VII–XII.
Homotypic synonym: Ahnfeltiopsis divaricata (Holmes) Masuda Citation1993: 2.
Holotype: March 1894, collected by Saida; BM.
Type locality: Shimoda, Shizuoka Prefecture, central Japan.
Description: Upright thalli dark-red, red-brown, coriaceous, semi-rigid, 3–5 cm tall (). Erect axes developing from small basal disc. Axes terete below (450–600 µm in diameter), flattened from 3–5 mm above basal disc; narrowly linear, 0.8–1.6 mm wide and 300–400 µm thick in lower and middle parts, broadening to 2–4 mm at the forks. Axes dichotomously or subdichotomously to palmately divided 5–6 times every 0.5–1 cm at angles of 45–60°, rarely up to 90°. Branch apices bifurcate, flattened, 0.7–1.7 mm wide and 200–350 µm thick (). Proliferations rare, mostly in lower part of thallus.
In longitudinal section, medulla throughout thallus consisting of 10–12 rows of oval to angular elongated cells, 55–165 µm long and 20–50 µm thick (length/thickness ratio 1.5–3) (). In cross-section, cells oval, 33–82 × 20–55 µm (). Cell walls hyaline, 3–5 µm. Medullary cells becoming gradually more slender and shorter towards anticlinal cortical filaments. No hypha-like filaments evident in the medulla. Cortex throughout thallus consisting of 3–6 rows of cells, 3.3–5.3 µm thick, with frequent secondary pit-connections between adjacent cells ().
Internal cystocarps 0.5–1.2 mm in diameter, up to 0.5 mm high, developing in series of 2–4 on upper branches, protruding from one side of the axis more than from the other side. Cystocarps loosely immersed within the medulla (); secondary medullary cells around cystocarp not developed (); fusiform gonimoblast cells present, penetrating the cystocarp boundary and connecting with vegetative cells (). Cortex around cystocarps thickened, with carpostomes (). Carpospores ovate, 14–20 × 12–14 µm, often in pairs. Rounded cavities remaining in branches after spore release.
Distribution in the Far Eastern Seas: Sea of Japan (Peter the Great Bay, Kievka Bay), Sea of Okhotsk (Kunashir Island). Represented by two forms: attached and unattached (f. ahnfeltioides).
Besa divaricata (Holmes) M.S.Calderon & S.M.Boo f. ahnfeltioides (Makijenko) A.V.Skriptsova et S.Y.Shibneva, comb. nov. ()
Basionym: Gymnogongrus japonicus f. ahnfeltioides Makjienko Novites Systematicae Plantarum Non Vascularum, Vol. 7: p. 95, , 5, 6: 2, 3, 1971 (‘1970’).
Holotype: LE.
Type locality: Stratum of Ahnfeltia tobuchiensis, Perevoznaya Bay, Peter the Great Bay, Sea of Japan.
Description: Thalli dark-brown, semi-rigid, without attachment organ, 8–11 cm high (). Main axis absent; branches cylindrical (0.7–1 mm in diameter), thin, elongate; branch apices slightly flattened, 200–450 µm thick and 550–700 µm wide (). Ramification rare, dichotomous or subdichotomous, uniform over thallus. Thalli ramifying 7–9 times each 1–3.5 cm at angles of 30–60°, rarely up to 90°.
In longitudinal section, medulla consisting of 8–11 cell rows of oval to angular elongated cells, 70–120 µm long and 30–60 µm thick (length/thickness ratio 2–3) (). In cross-section, cells oval, 25–50 × 20–30 µm (). Medullary cells becoming gradually narrower and shorter towards anticlinal cortical filaments. Hypha-like filaments in medulla absent. Cortex throughout thallus consisting of 4–5 rows of cells, 3–6 µm thick, with pit-connections between cells ().
Cystocarps and spermatangia unknown.
Distribution in the Far Eastern seas: Peter the Great Bay, unattached, in Ahnfeltia tobuchiensis stratum at a depth of 3–12 m.
Comments: This form was described earlier based on obvious morphological and ecological differences from attached specimens of ‘A. flabelliformis’ from Peter the Great Bay (Sea of Japan). Previously, this form was attributed to Gymnogongrus japonicus as f. ahnfeltioides (Makijenko, Citation1970), then it was referred to Gymnogongrus flabelliformis, which Masuda (Citation1987) transferred to Ahnfeltiopsis. Although f. ahnfeltioides is morphologically distinct from B. divaricata, it is genetically identical to it (see below).
Besa japonica (Suringar) A.V.Skriptsova et S.Y.Shibneva, comb. nov.()
Basionym: Gymnogongrus japonicus Suringar 1867: Algarum iaponicarum Musei Botanici L.B. index praecursorius. Annales Musei Botanici Lugduno-Batavi 3: 256–259.
Lectotype: Leiden herbarium, sheet No 943. 85. 36.
Type locality: Japan, the locality not defined.
Description: Upright thalli dark-red, red-brown, coriaceous, rigid, fragile, 3–5 cm tall (). Upright axes arise from small basal disc. Axes terete in lower portion (450–600 µm in diameter), flattened above 3–5 mm from basal disc; narrowly linear, 0.5–1 mm wide and 400–500 µm thick in lower and middle part, broadening up to 2–2.5 mm at the forks. Axes dichotomously or subdichotomously dividing 7–10 times for each 0.3–0.7 cm at angles of 30–60°. Branch apices bifurcate, terete or compressed, rarely flattened, 0.3–1 mm wide and 350–450 µm thick (). Proliferations unknown.
In longitudinal section, medulla throughout thallus consists of 7–12 rows of elliptical, rounded or angular, almost isodiametric cells, 50–175 µm long and 35–90 µm thick (length/thickness ratio 1–2) (). In cross-section, cells are elliptical, 33–82 × 20–55 µm (). Cell walls hyaline, thickened to 7–10 µm. Medullary cells decrease in size to surface. No hypha-like filaments evident in the medulla. Cortex throughout thallus consisting of 2–6 rows of cells, 3–7.5 µm thick, with frequent secondary pit-connections between adjacent cells ().
Internal cystocarps 0.6–1.2 mm in diameter, up to 0.6 mm tall, developing into catenate series of 2–4 on upper branches (), protruding from one side more than the other; cystocarpic part not broader than adjacent vegetative part of branch. Cystocarps loosely immersed in the medulla (); secondary medullary cells around cystocarp not developed (); fusiform gonimoblast cells present, penetrating the cystocarp boundary and connecting with vegetative cells (). Cortex around cystocarps thickened, with carpostomes (). Carpospores ovate, 20–26 × 12–15 µm, often in pairs. Rounded cavities remain on branches after spore release.
Distribution in the Far Eastern Seas: Peter the Great Bay, Sea of Japan.
Besa catenata (Yendo) M.S.Calderon & S.M.Boo ()
Basionym: Gymnogongrus catenatus Yendo Citation1920: 4.
Homotypic synonym: Ahnfeltiopsis catenata (Yendo) Masuda Citation1993.
Lectotype: Ijika, Mie Prefecture, Yendo; 22 March 1894; TI.
Description: Upright thalli dark-red, red-brown, coriaceous, semi-rigid or rigid, 5–12 cm tall (). Erect axes developing from small basal disc. Axes terete in lower portion (up to 640 µm in diameter), gradually becoming compressed upward; narrowly linear, 0.7–1 mm wide and 500–650 µm thick in lower and middle parts of axes, sometimes slightly broader at the forks. Axes dichotomously, irregularly or palmately divided 7–12 times each 0.5–2.5 cm with branching angles of 45–60(–90)°. Apical branchlets compressed to flattened, 0.6–1.2 mm wide and 400–450 µm thick (). Lacking proliferations.
Medulla throughout the thallus in longitudinal section consists of 20–25 rows of oval elongated cells, 55–95 µm long and 25–30 µm wide (length/width ratio 2–4) with smaller cells scattered among them (). In cross-section cells are round, 28–35 µm (). Cell walls hyaline, 3–4 µm. Medullary cells decreasing in size towards the surface. No hypha-like filaments evident in the medulla. Cortex consists of 13–24 rows at base of thallus, 6–18 rows in middle, and 4–7 rows in upper portion of the thallus. Cortical cells rectangular, closely packed, 7–10 μm long and 3.1–3.5 µm thick, with rare secondary pit-connections between adjacent cells ().
Internal cystocarps 0.7–1.0 mm in diameter, up to 0.5 mm high, developing in series of 2–5 on upper and penultimate branches, protruding more from one side of branch or almost central in the medulla. Cystocarpic parts of the branch are broader and thicker than adjacent vegetative parts (). Cystocarps loosely immersed within the medulla (); secondary medullary cells not developing around cystocarp (), fusiform gonimoblast cells penetrating the cystocarp boundary and connected to vegetative cells rare (). Cortex around the cystocarps thickened, with carpostomes (). Carpospores ovate, 6.5–12 × 10–16.5 µm.
This species does not occur along the Russian coast. It occurs along the east coast of Honshu (Shima Peninsula) and Kyushu.
Molecular analysis
A total of 44 specimens of four species, Besa divaricata, B. japonica, B. catenata and A. flabelliformis were sequenced for COI-5P and rbcL genes. The final alignment of the COI fragment was 589 bp (base pairs) in length and the rbcL alignment was 1277 bp long. Attempts to sequence the COI of A. flabelliformis from Jeju (South Korea) and Amakusa (Japan) were unsuccessful.
We performed Bayesian phylogenetic analyses of the genera Besa, Ahnfeltiopsis and Gymnogongrus (Phyllophoraceae) using a two-gene dataset (1866 bp). A comparison of the Bayesian inference (BI) topologies separately for COI and rbcL confirmed their concordance, as well as topologies obtained by ML and BI. The saturation test revealed no evidence for saturation of substitution at any codon positions for COI and rbcL or their combination.
The phylogenetic analyses revealed that the genera Ahnfeltiopsis sensu lato and Gymnogongrus sensu lato are not monophyletic. Gymnogongrus crenulatus from Europe was quite distant from the other clades (). G. guadalupensis formed a clade with A. chnoosporoides and A. linearis (Ahnfeltiopsis sensu stricto clade), while G. griffithsiae, the generitype of Gymnogongrus, was closely related to A. pusilla in a clade with other members of the genus Ahnfeltiopsis including A. flabelliformis (Gymnogongrus sensu stricto clade). These two clades were sister to each other (PP = 1, ML = 82). Sequences of A. flabelliformis from Jeju (South Korea) and Amakusa Islands (Japan) grouped with GenBank sequences from South Korea, China, Taiwan and Japan. All the Besa species were placed in a single well-supported (PP = 1, ML = 100) monophyletic clade (Besa clade), where both attached and unattached samples of B. divaricata from the Russian coast nested with specimens of the species from Korea. B. japonica grouped with previously sequenced samples of Besa cf. catenata (Calderon et al., Citation2016). The sample of B. catenata from Shima (Japan), a site close to the type locality of the species, was distant from both B. divaricata and B. japonica. The Besa clade was clearly separated from both the Gymnogongrus sensu stricto and Ahnfeltiopsis sensu stricto clades. Mastocarpus pacificus was placed as sister to the Besa clade (PP = 1, ML = 99).
The intraspecific P-distances for all the species were low and did not exceed 0.36% for COI and rbcL. Thus, most samples of each species belonged to the same haplotype. Most intraspecific substitutions were at third codon-positions.
The average interspecific distance for the genus Besa was 3.12–6.22% for COI and 0.91–1.79% for rbcL. The P-distance between B. divaricata and B. japonica was 3.56% (COI) and 1.17% (rbcL). The interspecific distance between Besa catenata from the Shima Peninsula (Japan) and B. divaricata was 1.82% for COI and 6.04% for rbcL. The distances between B. catenata and B. japonica were 1.67% and 4.97% for rbcL and COI, respectively. These data indicate that B. divaricata, B. catenata and B. japonica are distinct, also confirmed by ABGD analysis using COI, where intraspecific divergence was 0.5–2.6%. Attached and unattached forms of B. divaricata were identical in COI and rbcL genes.
We observed 20 synonymous and one non-synonymous substitutions at position 76 of COI that results in replacement of a serine in B. divaricata by alanine in B. japonica. The rbcL amino acid sequences of B. divaricata and B. japonica species differed in two substitutions: one was at position 192 resulting in replacement of valine by isoleucine, and the other was at position 248 being responsible for the replacement of lysine by arginine, respectively, in B. divaricata and B. japonica. Besa catenata, as well as B. divaricata, has serine at position 76 of COI, and B. divaricata and B. japonica have serine instead of proline at position 196. B. catenata has a unique non-synonymous substitution at position 212 of rbcL, which results in replacement of glutamic acid in B. divaricata and B. japonica by aspartic acid in B. catenata. Position 248 of rbcL in B. catenata, the same as in B. divaricata, has lysine instead of arginine in B. japonica.
Discussion
According to the present results, species of the genus Ahnfeltiopsis sensu stricto do not occur in the seas of the Russian Far East, and the algae previously identified as ‘A. flabelliformis’ (Zinova, Citation1940; Makijenko, Citation1970; Perestenko, Citation1980, Citation1994) should be attributed to the genus Besa as B. japonica and B. divaricata, including the intraspecific form B. divaricata f. ahnfeltioides. These species are transferred to the genus Besa on the basis of our genetic analysis and the cystocarp structure. B. divaricata and B. japonica are sufficiently similar in vegetative morphology to A. flabelliformis, that, evidently, researchers (Zinova, Citation1940; Perestenko, Citation1980) referred to the Russian specimens as ‘A. flabelliformis’. At the same time, V.F. Makijenko (Citation1970) mentioned G. japonicus along with ‘A. flabelliformis’ (as Gymnogongrus flabelliformis) for the Russian Far Eastern Seas and differentiated this species from ‘A. flabelliformis’ based on its irregular branching and the acute form of apical branchlets. Subsequently, Masuda (Citation1987), when revising the genus Gymnogongrus from Japan and adjacent waters, noted that G. japonicus and G. flabelliformis are similar in most morphological characters such as branch width, medullary cell dimensions and number of rows of cortical cells. Such typical characteristics for G. japonicus as more slender branches and narrow angles between branches, which are irregularly dichotomous and congregated, are within the range of variability of G. flabelliformis and are not sufficient for species discrimination. Consequently, G. japonicus was reduced to a synonym of G. flabelliformis (Masuda, Citation1987). L.P. Perestenko expressed the same opinion (Perestenko, Citation1980). The phylogenetic analysis conducted in the present study confirms that the specimens from the Russian coast, having the morphological characteristics of G. japonicus as reported by Masuda (Citation1987) and Makijenko (Citation1970), belong to a separate species in the genus Besa, B. japonica.
The main differences in vegetative anatomy between both B. japonica and B. divaricata and A. flabelliformis are the uneven thallus colour (with branch apices lighter (to rose-coloured) than the middle and basal parts of thallus) and the almost uniform thickness of the thallus cortex (consisting of 3–6 cell rows, whereas A. flabelliformis is characterized by an increasing number of cortex rows from 4–7 in apical branches to 8–10 in the lower flattened part of thallus). B. japonica differs from B. divaricata by rigid fragile thalli, slenderer branches and terete to compressed apical branchlets, and the width/length ratio (in cross-section) being 1:1–2.2; B. divaricata has flat apical branchlets (with width/length ratio of 1:2–4.5) ().
Table 1. The main distinguishing features of Besa japonica, B. divaricata and B. catenata
It should be noted that the high morphological similarity of Ahnfeltiopsis species with flat or flattened thalli, according to traditional circumscription, has previously been recorded (Masuda, Citation1987; Masuda et al., Citation1994), and the branch width, cell size and number of cell rows in cortex and medulla were pointed out as diagnostic for species delineation (Masuda, Citation1987).
It is difficult to attribute the unattached form to B. divaricata or B. japonica when based solely on morphological data (). However, in the phylogram it is clearly identified as B. divaricata. Earlier, this form was attributed to Gymnogongrus japonicus as f. ahnfeltioides (Makijenko, Citation1970). The differences in morphological and anatomical characteristics, the absence of sexual and asexual reproduction, and the ecological features (living unattached at relatively great depths of 3–12 m), justify regarding this as an intraspecific form of B. divaricata.
Our research has not confirmed the occurrence of B. catenata in Russian waters. This species was mentioned by Calderon et al. (Citation2016, supplemental materials), who identified specimens collected from Sobol Bay (Peter the Great Bay, Sea of Japan) as B.‘catenata’ (= Besa cf. catenata in our phylogenetic analysis). The specimens from Peter the Great Bay, which are clustered with Besa cf. catenata, do not match the original description of this species or more recent reports (Yendo, Citation1920; Masuda, Citation1987; Masuda et al., Citation1994) but agree with B. japonica (as G. japonicus) in Masuda’s (Citation1987) description. The typical features of A. catenata are the rigid thalli and the thick cortex (6–10 cell rows in the upper part, 16–18 rows in the middle part, and 20–30 rows in the lower part of thallus) and medulla, consisting of 18–22 rows of narrow (15–40 µm; length/thickness ratio of 1.6–6.6) cells (Masuda, Citation1987; Masuda et al., Citation1994). Morphologically, this species is well distinguished by the broader cystocarpic parts of a branch than the adjacent vegetative parts (Masuda, Citation1987; Masuda et al., Citation1994). The differences between the samples of Besa cf. catenata analysed by Calderon et al. (Citation2016) from topotype material of B. catenata, which completely matched the description of the species both morphologically and anatomically (Masuda et al., Citation1994), was supported by our phylogenetic analysis. Obviously, B.‘catenata’ (= A. catenata) was misidentified in previous lists of Korean marine algae (Lee et al., Citation1998; Lee & Kang, Citation2001; Calderon et al., Citation2016; Guiry & Guiry Citation2019) and algae from the Russian coast (Calderon et al., Citation2016); the sequences deposited in GenBank/NCBI under numbers KU749574, KU749557 and KU749582 belong to B. japonica.
B. japonica and B. catenata have different distributions along the coast of Japan: B catenata is found in the Pacific Ocean along the eastern coasts of Honshu and Kyushu, in agreement with Masuda (Citation1987). According to our data, as well as to data published in literature, B. japonica is more widely distributed than B. catenata, occurring along the coast of the Sea of Japan, in the Yellow Sea along the western coast of Korea, and off Vietnam (Pham-Hoàng, Citation1969).
Previous studies are concordant with our data in showing that the genus Ahnfeltiopsis as currently circumscribed is an unresolved group including phylogenetically divergent species (Calderon et al., Citation2016; Calderon & Boo, Citation2017). In all analyses, representatives of Ahnfeltiopsis belong to two clades, with either the type species of Ahnfeltiopsis (A. linearis) or Gymnogongrus (G. griffithsiae). Thus, the genus Ahnfeltiopsis, as presently constituted, should be split into at least two genera. At the same time, A. flabelliformis should be restored to Gymnogongrus, as Gymnogongrus flabelliformis Harvey as previously suggested (Calderon et al. Citation2016; Calderon & Boo, Citation2017). Because, recently, cystocarpic developmental features have been suggested for delimitation of genera in the Phyllophoraceae (Calderon & Boo, Citation2016, Citation2017; Calderon et al. Citation2016), the phylogenetic data should be supported by data on anatomy of cystocarps before drawing a conclusion about the species in the genera. Unfortunately, researchers have not provided detailed characterization of the cystocarp structure of A. flabelliformis in comparison with Gymnogongrus; no reproductive structures have been found on the analysed algal thalli in our study either. We hope that the taxonomic transfer of A. flabelliformis to Gymnogongrus will be published elsewhere after the further anatomical study of cystocarp development features of samples from the North-west Pacific including type locality. Beside this species, A. pygmaea, A. fastigiata, A. pusilla and A. devoniensis are more closely related to the generitype of Gymnogongrus than to A. linearis (Maggs et al., Citation2013; Calderon & Boo, Citation2017), and there is no justification for retaining them in Ahnfeltiopsis. Instead, they should be restored to Gymnogongrus in the future.
Сurrently, the genus Ahnfeltiopsis includes 28 species; molecular data are available for only 15 species; cystocarp development has been studied in detail only for four species among them. Most species distributed in the North-west Pacific remain unstudied from the standpoint of molecular systematics and detailed anatomy of cystocarp. Resolving the taxonomic and nomenclatural problems in Ahnfeltiopsis sensu lato from this region requires further attention hereafter.
Author contributions
S.Y. Shibneva: analysis of molecular data, original concept, manuscript preparation; A.V. Skriptsova: seaweed collection, light microscopy, manuscript preparation; A.A. Semenchenko: analysis of molecular data, manuscript preparation; M. Suzuki: seaweeds collection, analysis of molecular data.
TEJP-2019-0148-File009.docx
Download MS Word (20.5 KB)Acknowledgments
We are extremely grateful to Dr Taiju Kitayama, phycological curator of the Department of Botany of National Museum of Nature and Science, Tsukuba, Japan for his assistance with sending herbarium specimens of B. catenata. We also thank Prof. Sung Min Boo for his valuable recommendations, and Prof. Michael D. Guiry for nomenclatural advice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at: https://doi.org/10.1080/09670262.2020.1765025
Supplementary Table 1. List of the species used in DNA analysis: voucher, location, date, collector, and accession numbers in GenBank.
Supplementary Table 2. Comparison of Besa japonica, B. divaricata and B. catenata with their first descriptions (as Gymnogongrus japonicus, G. divaricatus, G. catenatus, and G. flabelliformis).
References
- Calderon, M.S. & Boo, S.M. (2016). Phylogeny of Phyllophoraceae (Rhodophyta, Gigartinales) reveals Asterfilopsis gen. nov. from the southern hemisphere. Phycologia, 55: 543–554.
- Calderon, M.S. & Boo, S.M. (2017). The Phyllophoraceae (Gigartinales, Rhodophyta) from Peru with descriptions of Acletoa tarazonae gen. & sp. nov. and Gymnogongrus caespitosus sp. nov. Phycologia, 56: 686–696.
- Calderon, M.S., Miller, K.A., Seo, T.H. & Boo, S.M. (2016). Transfer of selected Ahnfeltiopsis (Phyllophoraceae, Rhodophyta) species to the genus Besa and description of Schottera koreana sp. nov. European Journal of Phycology, 51: 431–443.
- Edgar, R.C. (2004). MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics, 5: 113.
- Felsenstein, J. (1981). Evolutionary trees from gene frequencies and quantitative characters: finding maximum likelihood estimates. Evolution, 35: 1229–1242.
- Fredericq, S. & Lopez-Bautista, J.M. (2002). Characterization and phylogenetic position of the red alga Besa papillaeformis Setchell: an example of progenetic heterochrony? Constancea, 83: 1–12.
- Fredericq, S., Anderson, R.J. & Lopez-Bautista, J.M. (2003). Circumscription of some Phyllophoraceae (Gigartinales, Rhodophyta) from the Cape region, South Africa, based on molecular evidence. In Proceedings of the XVIIth International Seaweed Symposium (Chapman, A.R.O., Anderson, R.J., Vreeland, V.J. & Davison, I.R., editors), pp. 263–273. Oxford University Press, Oxford.
- Freshwater, D.W. & Rueness, J. (1994). Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia, 33: 187–194.
- Guiry, M.D. & Guiry, G.M. (2019). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 30 April 2019.
- Hasegawa, M., Kishino, H. & Yano, T. (1985). Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution, 22: 160–174.
- Ivanova, V.D., Dziurov, V.N., Kulepanov, T.V., Shaposhnikova, M.V., Sukhoveeva, I.S. & Gusarova, N.V. (2008). Atlas massovikh vidov vodorosley i morskikh trav rossiyskogo Dalnego Vostoka [Atlas of common species of algae and seagrasses from the Russian Far East]. Pacific Research Fisheries Centre (TINRO-Centre). Vladivostok, TINRO-Centre. 327 pp. (In Russian)
- Klochkova, N.G. (1996). Flora vodoroslei-makrofitov Tatarskogo proliva (Yaponskoye more) i osobennosti yeyo formirovaniya [The Marine algal flora of the Strait of Tartary (Sea of Japan) and characteristics of its formation]. Russian Academy of Sciences, Vladivostok, Dalnauka. 292 pp. (In Russian)
- Kumar, S., Stecher, G. & Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33: 1870–1874.
- Kusakin, O.G., Ivanova, M.B. & Tsurpalo, A.P. (1997). A check-list of animals, plants and fungi from the intertidal zone of the Far Eastern seas of Russia. Vladivostok, Dalnauka. 168 pp.
- Lanfear, R., Calcott, B., Ho, S.Y.W. & Guindon, S. (2012). PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution, 29: 1695–1701.
- Lee, H.B., Kim, J.I., Lee, J.W. & Oh, B.G. (1998). Notes on little known algae from Korea. Algae, 13: 165–172.
- Lee, Y.P. & Kang, S.Y. (2001). A Catalogue of the Seaweeds in Korea. Jeju National University Press, Jeju.
- Le Gall, L. & Saunders, G.W. (2010). DNA barcoding is a powerful tool to uncover algal diversity: a case study of the Phyllophoraceae (Gigartinales, Rhodophyta) in the Canadian flora. Journal of Phycology, 46: 374–389.
- Maggs, C.A., Le Gall, I., Mineur, F., Provan, J. & Saunders, G.W. (2013). Fredericqia deveauniensis, gen. et sp. nov. (Phyllophoraceae, Rhodophyta), a new cryptogenic species. Cryptogamie Algologie, 34: 273–296.
- Makijenko, V.F. [Makienko, V.F.] (1971 ‘1970’). Species generis Gymnogongrus Mart. ad oram Sovjeticam marium orientis extreme inventae. Novitates Systematicae Plantarum non Vascularium 7: 91–99, 6 figs. (In Russian)
- Masuda, M. (1987). Taxonomic notes on the Japanese species of Gymnogongrus (Phyllophoraceae, Rhodophyta). Journal of the Faculty of Science, Hokkaido University, Series V (Botany), 14: 39–72.
- Masuda, M. (1993). The taxonomic status of the western Pacific species of Gymnogongrus and Ahnfeltia (Gigartinales, Rhodophyta). Japanese Journal of Phycologia, 41: 1–6.
- Masuda, M., Zhang, J.F. & Xia, B.M. (1994). Ahnfeltiopsis from the Western Pacific: key, description and distribution of the species. In Taxonomy of Economic Seaweeds, Vol. 4 (Abbott, I.A., editor), 159–183. University of California, La Jolla, CA.
- Perestenko, L.P. (1980). Vodorosli Zaliva Petra Velikogo [Marine Algae of Peter the Great Bay]. Nauka, Leningrad. 232 pp. (In Russian)
- Perestenko, L.P. (1994). Krasnyie vodorosly Dalnevostochnykh morey Rossii [Red Seaweeds of Russian Far Eastern Seas]. Olga, St. Petersburg. 332 pp. (In Russian)
- Pham-Hoàng, H. (1969). Rong biên Viêtnam. Marine algae of South Vietnam, pp. [i]–vi, 1–558. Saigon.
- Puillandre, N., Lambert, A., Brouillet, S. & Achaz, G. (2012). ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology, 21: 1864–1877.
- Rambaut, A., Drummond, A.J., Xie, D., Baele, G. & Suchard, M.A. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67: 901–904.
- Ronquist, F. & Huelsenbeck, J.P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572–1574.
- Saunders, G.W. (2005). Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philosophical Transactions of the Royal Society, 360: 1879–1888.
- Saunders, G.W. & Moore, T.E. (2013). Refinements for the amplification and sequencing of red algal DNA barcode and RedToL phylogenetic markers: a summary of current primers, profiles and strategies. Algae, 28: 31–43.
- Silva, P.C. & DeCew, T.C. (1992). Ahnfeltiopsis, a new genus in the Phyllophoraceae (Gigartinales, Rhodophyceae). Phycologia, 31: 576–580.
- Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22: 2688–2690.
- Tavaré, S. (1986). Some probabilistic and statistical problems in the analysis of DNA sequences. Lectures on Mathematics in the Life Sciences, 17: 57–86.
- Wang, D., Wang, X.L., Li, D.P., Wang, F.J. & Duan, D.L. (2006). The genetic analysis and germplasm identification of the gametophytes of Undaria pinnatifida (Phaeophyceae) with RAPD method. Journal of Applied Phycology, 18: 801–809.
- Yendo, K. (1920). Novae algae japoniae. Decas I–III. Botanical Magazine, Tokyo, 34: 1–12.
- Zinova, E.S. (1940). Algae of the Sea of Japan. Red Algae (Rhodophyta). Transactions of the Pacific Committee of the Academy of Science of the USSR, 5: 7–164. (In Russian)
