ABSTRACT
The swimming of male gametes of brown algae is controlled by two heterokont flagella, regardless of reproductive system, but the patterns of flagellar movement can vary. We investigated sperm chemotaxis and phototaxis in four oogamous Australasian fucalean species. Sperm morphology was similar to that of male gametes of isogamous and anisogamous species but their chemotactic behaviours were much closer to those of other oogamous species than those of isogamous and anisogamous species. Moreover, unlike anisogamous species, sperm chemotaxis of the oogamous species, Hormosira banksii, was not inhibited by a phosphodiesterase inhibitor, theophylline, suggesting variability in regulatory mechanisms of sperm chemotaxis amongst brown algae. In our previous work, lower extracellular Ca2+, supernatant of female gametes and theophylline induced the reversal of phototactic signs in anisogamous male gametes. In contrast, lower extracellular Ca2+ and theophylline did not significantly affect phototaxis in sperm of the oogamous H. banksii. Moreover, high extracellular Ca2+ and supernatant of eggs disrupted the negative phototaxis but did not reverse their phototactic sign. These results suggest that the regulation of phototaxis also varies amongst brown algal gametes. Further investigation of brown algal species is warranted to assess the generality of these patterns of chemotaxis and phototaxis among anisogamous and oogamous gametes.
Introduction
Sexual reproduction of brown algae is divided into three forms: isogamy, anisogamy and oogamy (Wynne & Loiseaux, Citation1976). The swimming of male gametes of all three forms is controlled by two heterokont flagella, called the anterior flagellum (AF) and the posterior flagellum (PF). The AF is always longer than the PF in the male gametes of isogamous and anisogamous species. In oogamous species, the sperm show three patterns (Kawai, Citation1992): (1) the majority of sperm of Fucales possess longer AF and shorter PF, a pattern similar to the male gametes of isogamous and anisogamous species; (2) sperm of Laminariales, Sporochnales, Desmarestiales and some Fucales have shorter AF and much longer PF; (3) sperm of Dictyotales lack PF. In general, AF waveform is sinusoidal and moves periodically, differing from the ‘rudder-like’ waveform of PF. Using these heterogeneous flagella, male gametes/sperm respond to chemical (chemotaxis) and light (phototaxis) signals, which is crucial for reproductive success (Kinoshita et al., Citation2017a).
Although the various morphologies of heterokont flagella appear related to the phylogenies of oogamous species (Kawai, Citation1992), only a few studies have been carried out on flagellar motility. The locomotion of sperm and their flagellar waveforms during chemotaxis have been reported in three oogamous species with shorter AF and longer PF (pattern 2 above): Fucus distichus, Saccharina japonica and Laminaria digitata (Maier & Müller, Citation1986, Citation1990; Kinoshita et al., Citation2017b). In F. distichus, sperm showed a U-turn movement during chemotaxis. This movement was driven by a rapid and large bending of the PF (Kinoshita et al., Citation2017b) and contrasts with that of the male gametes of isogamous and anisogamous species, in which the change of swimming direction is mainly driven by the AF (Kinoshita et al., Citation2016a, b). Although the swimming trajectories of Hormosira banksii sperm (with longer AF and shorter PF; pattern 1 above) during chemotaxis have been reported (Maier et al., Citation1992), detailed analysis of flagellar waveforms has not been assessed. Moreover, nothing has been reported on the sperm movement in species with only AF (pattern 3 above).
To further explore the relationships between the three types of reproductive systems and the changes in the morphology and motility of male gametes/sperm in brown algae, we analysed the swimming trajectories and flagellar waveforms during chemotaxis and phototaxis in four Australasian species of oogamous fucaleans: H. banksii (Hormosiraceae), Durvillaea potatorum (Durvillaeaceae), Caulocystis uvifera (Sargassaceae) and Cystophora gracilis (Sargassaceae). Moreover, the signalling pathways controlling chemotaxis and phototaxis were examined in detail in sperm of H. banksii.
Materials and methods
Multiple individuals of H. banksii, D. potatorum, C. uvifera and C. gracilis were collected from Port Fairy, Victoria, Australia (38°23’38”S, 142°13’06”E), between 4–14 September 2018, and transported to the laboratory (< 1 h) on ice. Samples of H. banksii and D. potatorum were washed with seawater to remove debris and dried with a piece of gauze. They were kept in the dark at 4°C overnight. Thalli were then rinsed with tap water, dried with a piece of gauze and spread on trays for 20 min at room temperature and standard fluorescent lighting to stimulate spawning. Thalli were then submerged in seawater to collect eggs and sperm. For C. uvifera and C. gracilis, samples were kept in seawater in the dark at 4°C for 1–2 hours, then transferred to an incubator at 15°C under PAR (~35 µmol photons m–2s–1). Eggs and sperm were released under these conditions within 1 hour. Although H. banksii and D. potatorum are dioecious, C. uvifera and C. gracilis are monoecious (Womersley, Citation1987). Eggs and sperm of C. uvifera and C. gracilis were manually separated under a stereoscopic microscope. Chemotaxis assays were carried out quickly after the separation of eggs and sperm. Phototaxis assays were conducted using a plastic 96-well plate (6.45 mm well diameter) or an observation chamber made of a coverslip and slide glass and a silicon sheet (1 mm thickness) by unidirectional illumination of blue light (465–475 nm, 75 µmol photons m–2s–1). Each experiment was repeated at least three times. Sperm motility was recorded in a dark room using an R-60 red cut-off glass filter (HOYA, Tokyo, Japan) and high-speed CCD camera (HAS220, Ditect, Tokyo, Japan) with 200 frames per second (fps) and 1/1000 second shutter speed. Analysis of sperm locomotion was performed using BohBoh software (Bohboh Soft, Tokyo, Japan), according to the method previously described (Kinoshita et al., Citation2017c). The swimming velocity was measured for 28 randomly selected sperm. The beat frequency of AF was estimated from the average of three beat cycles from seven randomly selected sperm. For a quantitative analysis of phototaxis, the angle (θ°) between the light and swimming direction was manually measured from video-recorded, 0.5 s swimming trajectories of randomly selected sperm (following Kinoshita et al., Citation2017c). Data were analysed by one- and two-factor analyses of variance (ANOVA) with subsequent Tukey’s HSD pairwise comparisons where appropriate. Where interaction terms were significant in 2-factor ANOVAs, simple main effects (SME) analyses were conducted using the degrees of freedom and mean square from the residual term in the original ANOVA as the error term (Quinn & Keough, Citation2002). For all ANOVAs, assumptions of normality and homogeneity of variances were checked with box plots and residual plots and either fourth-root or log transformed to improve normality or heterogeneous variances where necessary (Quinn & Keough, Citation2002). All analyses were conducted using SYSTAT version 13.2 and α = 0.05 for all statistical analyses.
Results and discussion
Sperm morphology and motility
Egg sizes of H. banksii, D. potatorum, C. uvifera and C. gracilis varied in diameter among species (~32–126 µm) (Supplementary table 1). Sperm also differed among species with respect to both cell size (3.4–5.2 µm length) and shape (). Only H. banksii and C. uvifera had an eyespot (Supplementary fig.1). AF (8.9–13.9 µm) and PF (5.1–12.1 µm) lengths varied among the four species (Supplementary table 1). All species had shorter PF than AF except C. gracilis (in which PF was slightly longer than AF). The ratio of PF relative to AF (PF : AF) of isogamous and anisogamous species is almost the same (0.4–0.5) (Kinoshita et al., Citation2016a, b). In our previous study of the oogamous species F. distichus, AF was much shorter than PF and the PF : AF ratio was 2.0 (Kinoshita et al., Citation2017b). However, in this study, the PF : AF ratio differed significantly among all four oogamous species examined (1-factor ANOVA: F(3, 24) = 76.24, p< 0.001; Tukey’s HSD: p< 0.05 for all species pairs): 0.7 in H. banksii, 0.5 in D. potatorum, 0.4 in C. uvifera and 1.4 in C. gracilis. The increase of PF:AF ratio tends to generate a more sinuosoidal waveform of PF.
Fig 1–3. Flagellar beat pattern and swimming velocity of four species of fucalean algae. Fig. 1. Variation of sperm cell shapes and beating patterns of flagella in four species of fucalean algae. Fig. 2. Swimming velocities of sperm in four species of fucalean algae in the dark with and without eggs (mean ± SD, n = 28). Fig. 3. Beat frequencies of AF in four species of fucalean algae in the dark with and without eggs (mean ± SD, n = 7). SME (−Egg vs. +Egg), ***p < 0.001 (see text for the detailed description). Scale bars: 10
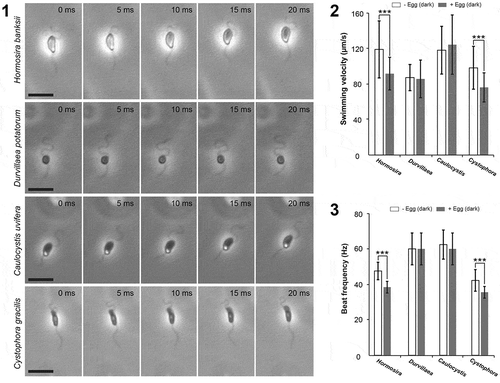
We examined two basic parameters for motility of sperm, the swimming velocity and the beat frequency of AF, in response to the presence and absence of conspecific eggs and the associated sex pheromone cue. In isogamous and anisogamous species, swimming velocities of male gametes commonly decrease when mixed with settled female gametes (Kinoshita et al., Citation2016a, b). Previous studies have shown this is not common in oogamous sperm (Kinoshita et al., Citation2017b), but in this study, we found mixed effects among species. The effects of the presence of eggs on swimming velocities of sperm were not consistent among species (2-factor ANOVA, species × pheromone interaction: F(3, 216) = 5.89, p= 0.001; ). Swimming velocity in both H. banksii and C. gracilis was significantly suppressed in the presence of conspecific eggs (SME: F(1, 216) = 16.46, p< 0.001 and F(1, 216) = 14.86, p< 0.001, respectively; ), whereas suppression was not observed in D. potatorum and C. uvifera (SME: F(1, 216) = 0.19, p= 0.663 and F(1, 216) = 0.53, p= 0.468, respectively; ). For beat frequency of AF, there was a significant difference among species (2-factor ANOVA species: F(3, 48) = 40.62, p< 0.001; Tukey’s HSD: (C. uvifera = D. potatorum) > (H. banksii = C. gracilis); ) and beat frequencies of AF were significantly lower in the presence of eggs (2-factor ANOVA pheromone (F(3, 48) = 7.34, p< 0.009), but the effect was strongest for H. banksii and C. gracilis; ).
Sperm chemotaxis
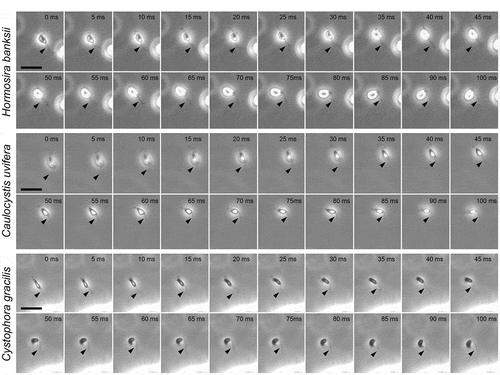
After spawning, male gametes of isogamous and anisogamous species change their motility from free-swimming to two-dimensional movement close to the substratum, regarded as thigmotaxis; a response enhanced by the presence of sex pheromones from female gametes (Müller, Citation1978; Geller & Müller, Citation1981). In the case of oogamous species in this study, H. banksii, D. potatorum, C. uvifera and C. gracilis, sperm thigmotaxis was not observed regardless of the presence or absence of eggs. This difference between isogamous/anisogamous and oogamous species may be related to differences in the size and motility of female gametes compared with oogamous eggs. A circular swimming path is typical of male gametes in isogamous (or near-isogamous; Ahmed et al., Citation2014; Luthringer et al., Citation2014) and anisogamous species when they sense sex pheromones (). However, in the four oogamous species examined, a circular swimming pattern was not observed, instead only a linear spiral swimming trajectory (). In particular, sperm of H. banksii, C. uvifera and C. gracilis showed significant three-dimensional U-turn movements around the eggs, accompanied by large bending of the PF (). These patterns of sperm movement were similar to those previously reported for F. distichus (Fucales) and S. japonica (Laminariales) with long PF and short AF (; Kinoshita et al., Citation2017b), and thus chemotactic movements do not appear to be associated with consistent patterns of PF and AF length (). Moreover, sperm of all species in this study appeared to have a more efficient approach to the eggs, compared with sperm of the oogamous laminarian species S. japonica (Kinoshita et al., Citation2017b). The difference in this efficiency is probably related to the extended length of the PF of S. japonica sperm: shorter PF may contribute to producing more efficient swimming patterns to approach eggs.
Fig 4. Schematic representation of male gametes/sperm morphology and swimming behaviours in response to chemical and light signals. ‘Pattern’ denotes typical swimming patterns during chemotaxis, where the circle represents the egg and the arrow represents the typical path of the male gametes/sperm. ‘Steering’ refers to which flagellum contributes more to switching swim orientation chemotaxis: AF = anterior flagellum, PF = posterior flagellum. The black line (–) indicates the timing of the sharpest bends in PF. + and – denote positive and negative phototaxis, respectively; X indicates no phototaxis and n.i. indicates phototaxis was not investigated
Fig 5. Swimming patterns of sperm in four species of fucalean algae. Left and right pictures show the two-second trajectories of sperm without and with eggs, respectively. * represents the position of eggs. Scale bars: 200 µm
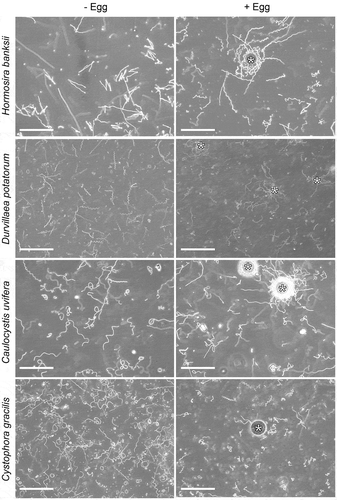
Fig 6. Chemotactic turns of sperm in Hormosira banksii, Caulocystis uvifera and Cystophora gracilis. Arrowheads indicate the position of PF. Scale bars: 10 µm
In the anisogamous species Mutimo cylindricus (Cutleriales), the attraction of male gametes to the settled female gametes is inhibited by the increase of intracellular cyclic nucleotides by the phosphodiesterase inhibitor, theophylline (Kinoshita-Terauchi et al., Citation2019). To compare the regulatory mechanism for sperm chemotaxis between anisogamous and oogamous species, we examined the effect of theophylline on the sperm chemotaxis in H. banksii (as this species exhibited strong chemotaxis () and was easiest to spawn). Theophylline seemed to have no effect on chemotaxis in H. banksii, with the sperm often showing U-turn movements and accumulation around the egg (Supplementary fig. 2). Thus, as well as describing the movements of sperm during chemotaxis, we have also demonstrated that the oogamous sperm of H. banksii had different molecular signalling for chemotaxis than that in the anisogamous species M. cylindricus. It is likely that this difference is due to the thigmotaxis uniquely seen in isogamous/anisogamous sperm during chemotaxis.
Sperm phototaxis
We examined the phototaxis of sperm from four oogamous species and found different phototactic responses to those in published reports for male gametes of anisogamous species. Male gametes of the anisogamous species M. cylindricus show positive phototaxis just after spawning, but as time passes they reverse the phototactic sign and start to show negative phototaxis (Kinoshita-Terauchi et al., Citation2019). In contrast, positive phototaxis was never observed in any of the four oogamous species examined in this study. Negative phototaxis to blue light was only observed in sperm of H. banksii and C. uvifera, which both possess an eyespot, whereas D. potatorum and C. gracilis showed no clear phototactic responses (, Supplementary figs 3, 4).
Fig 7–8. Phototaxis of sperm in Hormosira banksii at different times after spawning. Fig. 7. Blue light (blue arrows) was provided to sperm in dish plates. Dish phototaxis assays showing the appearance before and 3 min after unidirectional blue light provision. Brown colour in the photos represents sperm. Fig. 8. Plots showing the number of the cells moving in a particular direction relative to the blue light direction (θ°) (12 bins of 30°, n = 28 cells per each condition). Data for 1 h, 2 h, 3 h, 4 h and 5 h after spawning are shown without or with blue light
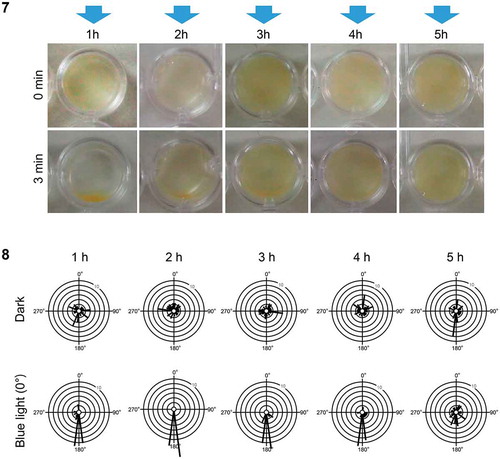
Sperm of H. banksii exhibited negative phototaxis immediately after spawning, consistent with previous reports (Maier et al., Citation1992). Negative phototaxis was observed for at least 4 hours but became weak at 5 hours after spawning (). However, negative phototaxis became weak and finally disappeared within 1 hour after spawning in sperm of C. uvifera (data not shown). In H. banksii, the differences in swimming velocities between light treatments (dark vs. blue light) were not consistent over time (2-factor ANOVA light × time interaction: F(4, 270) = 25.64, p< 0.001). Whilst swimming velocities of sperm gradually decreased over time, swimming velocity was significantly increased by blue light stimulation for the first 2 hours after spawning only (SME:F(1, 270) = 140.66, p< 0.001 and F(1, 270) = 7.27, p= 0.007, respectively; Supplementary figs 3 and 4).
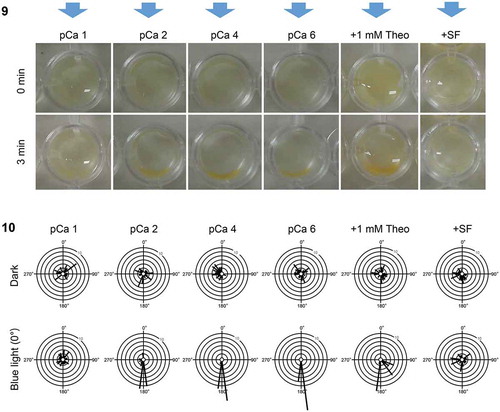
The phototactic sign of male gametes of the anisogamous species M. cylindricus is regulated by extracellular Ca2+, cyclic nucleotides and sex pheromones (Kinoshita et al., Citation2017c; Kinoshita-Terauchi et al., Citation2019). To determine whether a similar mechanism controls the phototaxis in an oogamous species, we examined the effects of extracellular Ca2+ concentration, a phosphodiesterase inhibitor, theophylline and addition of egg supernatant (containing the sex pheromones) on the phototaxis of H. banksii sperm. The methods were consistent with those of Kinoshita et al. (Citation2017c) and Kinoshita-Terauchi et al. (Citation2019). Higher concentration of extracellular Ca2+ (pCa 1; 10–1 M) disrupted negative phototaxis but did not reverse the phototactic sign. Neither lower extracellular Ca2+ concentration (pCa 4; 10–4 M, pCa 6; 10–6 M) nor theophylline (1–5 mM) affected the sign of phototaxis (), but the addition of egg supernatant (SF, supernatant of female gametes; Kinoshita-Terauchi et al., Citation2019) inhibited their phototaxis (). The differences in swimming velocities between light treatments (dark vs. blue light) were not consistent among regulatory treatments (2-factor ANOVA light × regulatory treatment interaction: (F(5, 324) = 12.691, p< 0.001). Increased swimming velocities were induced by blue light under control and low Ca2+ media (SME: F(1, 324) = 74.22, p< 0.001; F(1, 324) = 5.09, p= 0.025 and F(1, 324) = 18.57, p< 0.001 for pCa 2, pCa 4 and pCa 6 respectively), but were not seen under a high Ca2+ medium (pCa 1, SME: F(1, 324) = 1.05, p= 0.305), a theophylline-containing medium (SME: F(1, 324) = 0.783, p= 0.377) or a SF-containing medium (SME: F(1, 324) = 0.088, p= 0.767; Supplementary figs 5 and 6). These results demonstrate that the experimental media used in this study did not induce positive phototaxis of H. banksii sperm. It can thus be assumed that reversal of phototaxis from negative to positive (unlikely to occur naturally in the field), may not be a readily convertible signalling pathway in comparison with positive to negative phototaxis (which promotes initial dispersal then settlement; Reed et al., Citation1988; Santelices, Citation1990). Inhibition of negative phototaxis by SF seems efficient because sperm are already guided by sex pheromones in the vicinity of eggs. In contrast, the negative phototaxis of male gametes is not inhibited by SF in the anisogamous species M. cylindricus. This is probably due to the restriction of male gamete motility by thigmotaxis.
Fig 9–10. Phototaxis of sperm in Hormosira banksii under several conditions of observation medium. Fig. 9. Blue light (blue arrows) was provided to sperm in dish plates. Dish phototaxis assays showing the appearance before and 3 min after unidirectional blue light provision. Brown colour in the photos represents sperm. Fig. 10. Histograms showing the number of the cells moving in a particular direction relative to the blue light direction (θ°) (12 bins of 30°, n = 28 cells per each condition). Data in pCa 1, pCa 2 (control), pCa 4, pCa 6, 1 mM theophylline and + SF are shown without or with blue light
A phylogenetic consideration of sperm morphology and motility in brown algae
The three reproductive forms of brown algae, isogamy, anisogamy and oogamy, provide a great opportunity to study the evolutionary pathways for reproductive systems. We focused on the flagellar morphology and motility of male gametes in brown algae. Male gametes of isogamous/anisogamous species of brown algae have longer AF and shorter PF, whereas sperm of oogamous species have three patterns (). The chemotactic behaviour of male gametes in isogamous/anisogamous species is accompanied by thigmotaxis and a circular moving path (Müller, Citation1978; Geller & Müller, Citation1981; Kinoshita et al., Citation2016a, b). Sperm of oogamous species exhibited straight paths and U-turn movement during chemotaxis, even in the species with longer AF and shorter PF (this study; Maier et al., Citation1992). Sperm of other oogamous species with shorter AF and longer PF also move in the same way during chemotaxis (C. gracilis in this study; Maier & Müller, Citation1986, Citation1990; Kinoshita et al., Citation2017b). Therefore, the chemotactic movement of sperm in oogamous species appears not to be governed by the length of PF but linked to the oogamous reproduction system itself. Future detailed analysis of the movement and flagella in oogamous sperm with only AF, such as Dictyota, may provide support for this hypothesis.
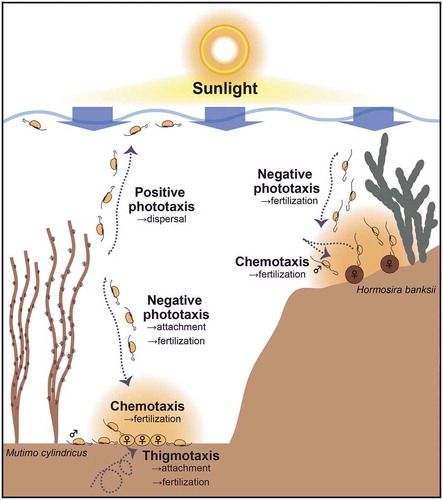
Positive phototaxis is thought to help dispersal of swimming cells (Reed et al., Citation1988; Santelices, Citation1990) and could thus be advantageous for isogamous/anisogamous male gametes, which usually undergo parthenogenesis (). However, it could be a disadvantage for sperm in oogamous species, incapable of parthenogenesis, because of dilution in surface waters away from eggs. However, negative phototaxis could also be advantageous for facilitating fertilization in isogamous/anisogamous species, because the female gametes fuse with male gametes after settlement on the benthos (). In the case of oogamous species, eggs and sperm generally fuse on benthic substrates (or more rarely on the thallus surface as in Seirococcaceae). Therefore, negative phototaxis and chemotaxis could orient the sperm to maximize fertilization success in oogamous species (). Unlike the male gametes of isogamous/anisogamous species, sperm phototaxis of the oogamous H. banksii was not affected by lower extracellular Ca2+ concentration or theophylline. We hypothesize that most oogamous species are likely to (1) have lost Ca2+- and cyclic nucleotide-dependent mechanisms controlling positive phototaxis and retained negative phototaxis or (2) have lost phototaxis. In D. potatorum and C. gracilis, clear phototaxis of sperm was not observed and in C. uvifera, even negative phototaxis of sperm disappeared soon after spawning. In addition, an oogamous species F. serratus was reported to have both positive and negative sperm phototaxis (Müller et al., Citation1987), although another species in the same genus, Fucus distichus, shows only negative phototaxis (Kinoshita et al., Citation2017b). Thus, some of the sperm in oogamous species show different or no phototactic responses, which may reflect the diversified or transient process during the course of oogamy evolution.
Fig 11. Schematic models of the relationships between chemotaxis, thigmotaxis and phototaxis in nature for anisogamous (represented by Mutimo cylindricus) and oogamous (represented by Hormosira banksii) species. Note, these two representative species do not naturally occur in proximity to each other
supplementary-material
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at: https://doi.org/10.1080/09670262.2020.1767307
Supplementary table 1. Diversity of cell size and flagellar length in four species of fucalean algae (mean ± SD, n = 7).
Supplemental figure 1. Diversity of sperm structures. Arrowheads indicate the position of eyespot. Scale bars: 10 µm.
Supplemental figure 2. Chemotactic responses of sperm in Hormosira banksii under addition of 5 mM theophylline. The two seconds trajectories of sperm are shown. * indicates the position of an egg. Scale bar: 200 µm.
Supplemental figure 3–4. Phototaxis of sperm in Hormosira banksii for different times after spawning. . 0.5 s swimming trajectories of sperm without and with blue light (blue arrows). Red and blue dots indicate start and end point of their swimming, respectively. . Mean (± SD) swimming velocities of sperm in the dark and under blue light (n = 28). SME (Dark vs. Blue light), **p<0.01, ***p<0.001. Scale bars: 200 µm.
Supplemental Figs 5–6. Phototaxis of sperm in Hormosira banksii under several conditions of observation medium. . 0.5 s swimming trajectories of sperm without and with blue light (blue arrows). Red and blue dots indicate start and end point of their swimming, respectively. . Mean (± SD) swimming velocities of sperm in the dark and under blue light (n = 28). SME (Dark vs. Blue light), *p<0.05, ***p<0.001. Scale bars: 200 µm.
Author contributions
N. Kinoshita-Terauchi: conceptualization, investigation and formal analysis and drafting and editing manuscript; A. Bellgrove: brown algal sampling, investigation, statistical analysis drafting and editing manuscript; K. Shiba: drafting and editing manuscript; K. Inaba: conceptualization, drafting and editing manuscript.
TEJP-2019-0107-File013.tif
Download TIFF Image (33.4 MB)TEJP-2019-0107-File012.tif
Download TIFF Image (32.9 MB)TEJP-2019-0107-File011.tif
Download TIFF Image (2.3 MB)TEJP-2019-0107-File010.tif
Download TIFF Image (3.9 MB)TEJP-2019-0107-File009.pdf
Download PDF (7.5 KB)Acknowledgements
We thank Jasmine Bursic and Paul Tinkler for assistance with collections.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental Material
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2020.1767307.
Additional information
Funding
References
- Ahmed, S., Cock, J.M., Pessia, E., Luthringer, R., Cormier, A., Robuchon, M., Sterck, L., Peters, A.F., Dittami, S.M., Corre, E., Valero, M., Aury, J.M., Roze, D., Van de Peer, Y., Bothwell, J., Marais, G.A. & Coelho, S.M. (2014). A haploid system of sex determination in the brown alga Ectocarpus sp. Current Biology, 24: 1945–1957.
- Geller, A. & Müller, D.G. (1981). Analysis of the flagellar beat pattern of male Ectocarpus siliculosus gametes (Phaeophyta) in relation to chemotactic stimulation by female cells. Journal of Experimental Biology, 92: 53–66.
- Kawai, H. (1992). A summary of the morphology of chloroplasts and flagellated cells in the Phaeophyceae. Korean Journal of Phycology, 7: 33–43.
- Kinoshita, N., Nagasato, C., Tanaka, A. & Motomura, T. (2016a). Chemotaxis in the anisogamous brown alga Mutimo cylindricus (Cutleriaceae, Tilopteridales). Phycologia, 55: 359–364.
- Kinoshita, N., Shiba, K., Inaba, K., Fu, G., Nagasato, C. & Motomura, T. (2016b). Flagellar waveforms of gametes in the brown alga Ectocarpus siliculosus. European Journal of Phycology, 51: 139–148.
- Kinoshita, N., Nagasato, C. & Motomura, T. (2017a). Phototaxis and chemotaxis of brown algal swarmers. Journal of Plant Research, 130: 443–453.
- Kinoshita, N., Nagasato, C. & Motomura, T. (2017b). Chemotactic movement in sperm of the oogamous brown algae, Saccharina japonica and Fucus distichus. Protoplasma, 254: 547–555.
- Kinoshita, N., Nagasato, C. & Motomura, T. (2017c). Calcium control of the sign of phototaxis in brown algal gametes of Mutimo cylindricus. Photochemistry and Photobiology, 93: 1216–1223.
- Kinoshita-Terauchi, N., Shiba, K., Umezawa, T., Matsuda, F., Motomura, T. & Inaba, K. (2019). A brown algal sex pheromone reverses the sign of phototaxis by cAMP/Ca2+-dependent signaling in the male gametes of Mutimo cylindricus (Cutleriaceae). Journal of Photochemistry and Photobiology B: Biology, 192: 113–123.
- Luthringer, R., Cormier, A., Ahmed, S., Peters, A.F., Cock, J.M. & Coelho, S.M. (2014). Sexual dimorphism in the brown algae. Perspectives in Phycology, 1: 11–25.
- Maier, I. & Müller, D.G. (1986). Sexual pheromones in algae. The Biological Bulletin, 170: 145–175.
- Maier, I. & Müller, D.G. (1990). Chemotaxis in Laminaria digitata (Phaeophyceae) I. Analysis of spermatozoid movement. Journal of Experimental Botany, 41: 869–876.
- Maier, I., Wenden, A. & Clayton, M.N. (1992). The movement of Hormosira banksii (Fucales, Phaeophyta) spermatozoids in response to sexual pheromone. Journal of Experimental Botany, 43: 1651–1657.
- Müller, D.G. (1978). Locomotive responses of male gametes to the species specific sex attractant in Ectocarpus siliculosus (Phaeophyta). Arch. Protistenk. Bd., 120: 371–377.
- Müller, D.G., Maier, I. & Müller, H. (1987). Flagellum autofluorescence and photoaccumulation in Heterokont algae. Photochemistry and Photobiology, 46: 1003–1008.
- Phillips, J.A., Clayton, M.N., Maier, I., Boland, W. & Müller, D.G. (1990). Sexual reproduction in Dictyota diemensis (Dictyotales, Phaeophyta). Phycologia, 29: 367–379.
- Quinn, G.P. & Keough, M.J. (2002). Experimental Design and Data Analysis for Biologists. Cambridge University Press, Cambridge.
- Reed, D.C., Laur, D.R. & Ebeling, A.W. (1988). Variation in algal dispersal and recruitment: the importance of episodic events. Ecological Monographs, 58: 321–335.
- Santelices, B. (1990). Patterns of reproduction, dispersal and recruitment in seaweeds. In Oceanography and Marine Biology Annual Review, 28: 177–276.
- Womersley, H.B.S. (1987). The Marine Benthic Flora of Southern Australia Part II. South Australian Gov. Print, Adelaide.
- Wynne, M. & Loiseaux, S. (1976). Recent advances in life history studies of the Phaeophyta. Phycologia, 15: 435–452.
