ABSTRACT
We describe five new araphid diatom species belonging to the genus Pseudostaurosira. These new taxa were found in modern and fossil material collected from five southern Patagonian waterbodies (49–52°S), four from the Santa Cruz province, Argentina and one from the Magallanes Region, Chile. When observed under light and scanning electron microscopy, each species differs by a single or a combination of valve character(s). Pseudostaurosira australopatagonica sp. nov. has unique volae that are profusely, dichotomously branched, internally anastomosed, and affixed to the virgae. Pseudostaurosira hyalopatagonica sp. nov. has fusiform valves with cuneate, elongate ends, narrow valves and round to slightly elliptical areolae. Pseudostaurosira catalinae sp. nov. has broadly fusiform valves with cuneate to subrostrate ends; volae form a circular to elliptical ring at the centre of the areolar opening and, externally covering the mantle areolae, it has a watch glass-like flap. Pseudostaurosira tehuelcheana sp. nov. has lanceolate valves with broadly rounded apices, solid incipient conical spines and marginal, transapically elongate areolae occluded by profusely branched volae. Pseudostaurosira zolitschkae sp. nov. is a relatively large-valve species with subrostrate apices, hollow spines, marginal areolae sometimes separated by a thin vimen. The volae in this latter, new taxon, only originate from a position parallel to the transapical axis of the valve, an origin that is uncommon within the genus. We discuss the validity of describing species based on morphological data and highlight the importance of increasing taxonomic resolution on isolated regions such as Patagonia.
Introduction
Taxonomic concept drift and force-fitting have compounded the taxonomy of non-marine Fragilariaceae Kützing, leading to variations in the original concept and uncertain identification of several taxa (Tyler, Citation1996; Kociolek & Spaulding, Citation2000). The number of publications re-examining type material of species within the family has increased during the last 20 years (Morales & Edlund Citation2003; Edlund et al., Citation2006; Hamilton & Siver, Citation2008; Morales et al., Citation2010, Citation2015, Citation2019; Cejudo-Figueiras et al., Citation2011). These studies have provided detailed morphological data, including character variability and high-quality illustrations of key features under both light (LM) and scanning electron microscopy (SEM). Although those analyses of type material are time-consuming (Morales et al., Citation2013), they are still essential in the resolution of taxonomic problems related to, for example, palaeoenvironmental reconstructions.
The genus Pseudostaurosira was described by Williams & Round (Citation1987) in their revision of Fragilaria Lyngbye. Ever since its description, Pseudostaurosira has been the subject of taxonomic discussion, since it remains difficult to find derived characters that would unify all the species within the genus and, at the same time, separate them from other small araphids without rimoportulae (Morales et al., Citation2019). Morales et al. (Citation2015) re-examined the material of the generitype Pseudostaurosira brevistriata (Grunow) Williams & Round, allowing a more precise diagnosis of the genus. Based on this assessment, several new species have been ascribed to this genus (for example Cejudo-Figuerias et al., Citation2011; Grana et al., Citation2018; Williams & Wetzel, Citation2019). Morales et al. (Citation2019) emended the description of Pseudostaurosira and suggested that its distinguishing characteristic is the possession of wide and short vimines. Other features, which may vary among the different species, are: rectangular frustules in girdle view, formation of chains, cruciform, bigibbous, lanceolate, rhombic or elliptic valves, generally uniseriate striae consisting of wide, round, transapically elliptical areolae, which run from valve face to mantle. The areolae are occluded by delicate volae that are highly dichotomously branched. The areolae can also have flaps covering them externally and there can also be stipulae at the base of spines (Morales et al., Citation2012). Spines can be absent (e.g. Pseudostaurosira parasitica (W.Smith) Morales), or, when present, they can interrupt the striae at the valve face-mantle junction (e.g. Pseudostaurosira microstriata (Marciniak) Flower), or grow on the virgae (e.g. Pseudostaurosira pseudoconstruens (Marciniak) D.M.Williams & Round). The sternum has variable width and shape. The ocellulimbus type apical pore fields may be absent, reduced or more fully developed. In many cases, the apical pore fields are sunken into the apical portion of the valve. Rimoportulae are absent and mantle plaques are present along the mantle edge. The cingulum consists of several unperforated, open, plain, ligulate copulae; the valvocopula is larger than the rest of the elements.
Patagonia is a geographic region that covers the southernmost part of South America, including both Chilean and Argentinian territories between ~37°S and 56°S (Garreaud et al., Citation2013); it has a unique geographic position as the only large continuous continental landmass within this latitudinal range. Patagonia can be divided into two distinct subregions: the Andean Patagonia to the west, comprising the Andean Cordillera, and the Patagonian Steppe to the east, characterized by extensive tablelands (Coronato et al., Citation2008). The mountain range acts as a barrier to the flux of the westerly winds, which carry moist air from the Pacific Ocean, causing abundant precipitation over the windward side of the Cordillera and a major rain-shadow effect on its leeward side. As a result, there is a dramatic W–E mean annual precipitation gradient that decreases from ~1400 mm in the Andean range to less than 200 mm in the steppe (Garreaud et al., Citation2013). Patagonia has a great diversity of wetlands, particularly lakes and peatlands, with unique flora and fauna, which constitute fragile ecosystems, vulnerable to human intervention (Díaz Pardo et al., Citation2008). Diatoms are often used as biological proxies in studies aiming to understand present and past environmental and climate changes, which justifies the importance of investigating the local taxonomy and ecology (Smol, Citation2017).
Previous studies on the diatom flora from the Andean Patagonia reported several ‘fragilarioids’ documented in drawings or species lists (e.g. Frenguelli, Citation1924, Citation1942; Frenguelli & Orlando, Citation1956). Some of those studies do include LM and SEM images, which help in the reconstruction of the local flora (Krasske, Citation1949; Maidana, Citation1996; García et al., Citation2017, Citation2018; Grana et al., Citation2018; Seeligmann et al., Citation2018; Guerrero et al., Citation2019). Rumrich et al. (Citation2000) recorded several unidentified small araphids from this region and described a new species. Considering these recent publications, an updated list of the Pseudostaurosira species and varieties described from the Andes include the following: Pseudostaurosira altiplanensis (Lange-Bertalot & Rumrich) E.Morales, Pseudostaurosira decipiens E.Morales, G.Chávez & Ector, Pseudostaurosira ferrarioae C.Seeligmann, N.Maidana & E.Morales, Pseudostaurosira laucensis (Lange-Bertalot & Rumrich) E.Morales & M.L.Vis, Pseudostaurosira laucensis var. vulpina (Lange-Bertalot & Rumrich) E.Morales, Pseudostaurosira oliveraiana Grana, E.Morales, Maidana & Ector, Pseudostaurosira sajamaensis E.Morales & Ector, Pseudostaurosira santaremensis (Metzeltin & Lange-Bertalot) Grana, E.Morales & Maidana.
In this study, we describe five new species of the genus Pseudostaurosira based on modern and fossil lacustrine Patagonian sediments. We base each description on detailed LM and SEM observations and compare their main morphological and morphometric features to closely related species. We aim to give an overview of the biogeographic status for the genus Pseudostaurosira in the southern Patagonia region.
Materials and methods
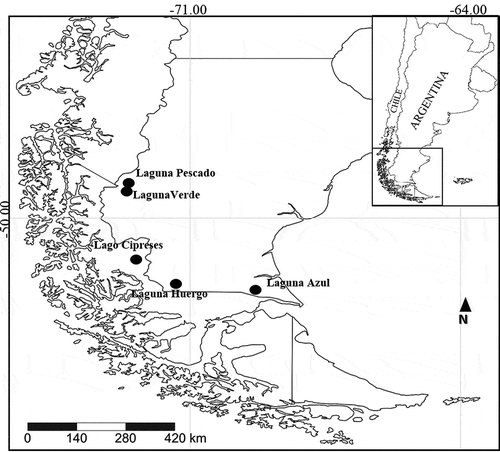
Four shallow lakes from Santa Cruz Province on the east (Argentinian) side of the Andes and one shallow lake from the Magallanes Region on the west (Chilean) side were sampled in 2007, 2013 and 2017 between 49°–52°S, 69°–72°W: Laguna Pescado, Laguna Verde, Lago Cipreses, Laguna Huergo and Laguna Azul (, ).
Table 1. List of studied sites
In Laguna Verde, Laguna Azul, Laguna Huergo and Laguna Pescado, selected limnological variables (pH, electrical conductivity, water temperature, dissolved oxygen) were measured from lake waters with a Hanna HI 9828 Multiparameter Portable Meter (Hanna, USA) ().
Diatom analyses were performed following standard methods described in Battarbee (Citation1986). An aliquot of each sample was dried at 80°C. Each sample was oxidized with H2O2 (30%, 100 Vol.) and heated in a microwave oven for 2 min to eliminate organic material. Samples were then rinsed repeatedly until neutrality with distilled water. Permanent slides were mounted using Naphrax. Light micrographs were captured using a Reichert-Jung Polyvar binocular optical microscope equipped with a Plan Apo 100X, NA 1.32, immersion objective and DIC optics and a Canon EOS 600D digital camera.
For SEM observations, aliquots of the cleaned material were dried on aluminium stubs at room temperature before being coated with gold and examined using a Carl Zeiss SUPRA 40 (15kV) at the Centro de Microscopías Avanzadas (CMA), FCEyN, Universidad de Buenos Aires, Argentina.
Images captured on both LM and SEM were measured using the Zeiss Axiovision 4.8.2 software, plates were edited using Adobe Photoshop® and Illustrator®.
Identification of diatom assemblages was carried out using mainly South American literature (Frenguelli, Citation1924, Citation1942; Metzeltin & Lange-Bertalot, Citation1998, Citation2007; Rumrich et al., Citation2000; Metzeltin et al., Citation2005), and taxonomic articles by Morales (Citation2001), Morales et al. (Citation2010, Citation2013, Citation2019), among others.
Morphological terminology follows Anonymous (Citation1975) and Ross et al. (Citation1979) for striae, areolae and spines; Barber and Haworth (Citation1981) for valve shape and striae pattern, and Williams & Round (Citation1987) and Round et al. (Citation1990) for areolar substructures, apical pore fields, and girdle band features.
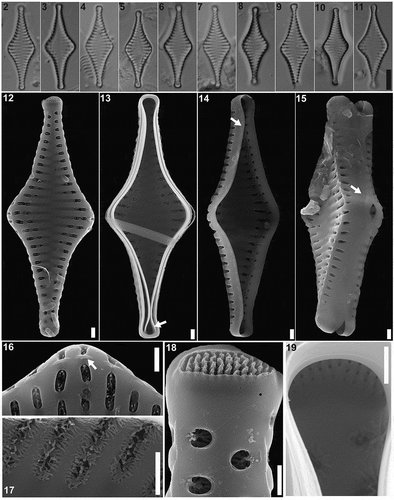
Figs 2–19. Pseudostaurosira australopatagonica sp. nov., type material (LPC 15861a. Laguna Pescado, Santa Cruz Province, Argentina). Figs 2–11. LM of type material, valve views. Fig. 5. Holotype specimen. Figs 12–19. SEM images. Fig. 12. External valve view. Fig. 13. Internal valve view with an open girdle band still attached (arrow). Figs 14–15. Tilted specimens in internal valve view, showing mantle plaques at the abvalvar margin of the valve (arrows). Fig. 16. Detail of areolae with profusely dichotomously branched volae, internal view. Fig. 17. Detail of areolae with profusely dichotomously branched, internal view. Fig. 18. Detail of the apical pore field, external view. Fig. 19. Detail of the apical pore field, internal view. Scale bar: Figs 2–11, 10 µm; Figs 12–17, 10 µm; Figs 18–19, 500 nm
Results
Pseudostaurosira australopatagonica M.L.García, L.A.Villacís, Maidana & E.Morales sp. nov. (LM , SEM )
Description
LM. Valves cruciate to rhomboid, with a broad central inflation, apices capitate to subcapitate (). Axial area widely lanceolate, sometimes with ghost striae, reaching a narrow linear sternum (). Valve dimensions (n = 23): length: 20–25 µm, width: 6.5–9.0 µm, stria density: 10–12 in 10 µm.
SEM. Striae uniseriate, 1 to 3 round to transapically elongate areolae. Each areola partially occluded by profusely dichotomously branched volae. Volae forming a net-like structure, with terminations internally attached to virgae (). Elliptical areolae with volae originating from transapical areolar extremes; round areolae with volae originating from side closer to valve face-mantle junction (). Striae sometimes interrupted on the valve/mantle junction by incipient spines. Apical pore fields of the ocellulimbus type, composed of several parallel rows of 3 to 5 round poroids (). Internally, apical pore fields sunken in semicircular depression. Girdle bands open and smooth (). Mantle plaques located on abvalvar margin of the valve mantle ().
HOLOTYPE: Circled specimen in slide L Pescado 2013, LPC 15861a! (). Herbarium División Ficología ‘Dr. Sebastián A. Guarrera’, Museo de La Plata, Argentina.
TYPE LOCALITY: Argentina, Laguna Pescado (49.125°S, 72.921°W), Santa Cruz Province.
ETYMOLOGY: The epithet refers to the austral part of Patagonia, where it was found.
DISTRIBUTION AND ASSOCIATED TAXA: The new species was found in surface sediments of Laguna Pescado (Santa Cruz Province, Argentina) and in fossil samples in Lago Cipreses (Región de Magallanes, Chile) (). The diatom assemblage in Laguna Pescado was dominated by a still unidentified Sellaphora sp. and several yet unidentified small fragilarioid species. Diatom assemblages from Lago Cipreses include other small fragilarioids, Sellaphora [laevissima Krammer & Lange-Bertalot] Φ ‘normal’ sensu Mann et al. (Citation2008), and Aulacoseira aff. humilis (Cleve-Euler) Genkal & Trifonova.
In the Chilean assemblages, Pseudostaurosira australopatagonica individuals are found all along the core PS0710 and are especially abundant at 29–30 cm depth (), where it attained 7.4% of the total assemblage. There, the valves have a wider size range than the type population (15.5–28.0 µm, n = 57), with similar width (6.5–10.0 µm) and stria density (11–12 in 10 µm). Interestingly, in another sample, at 95–96 cm depth where P. australopatagonica is abundant (11%), 28% of the individuals (n = 149) have some type of deformity (). Additionally, in the populations from Lago Cipreses we note that some individuals have apical pore fields located on the valve face near the apical margins () rather than on the margin itself, as is the case with the population from Laguna Pescado.
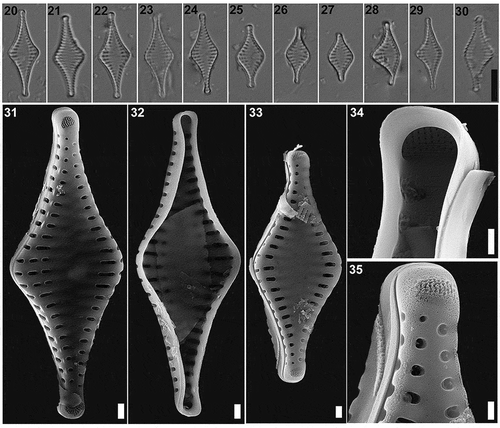
Figs 20–35. Pseudostaurosira australopatagonica sp. nov., population from Lago Cipreses, Chile. Figs 20–30. LM images of valve views. Figs 28–30. Teratological forms. Figs 31–35. SEM images. Fig. 31, 33. External valve view. Fig. 32. Internal valve view. Fig. 34. Detail of the apical pore field, internal view. Fig. 35. Detail of the apical pore field, external view. Scale bar: Figs 20–30, 10 µm; Figs 31–33, 10 µm; Figs 34–35, 500 nm
TAXONOMIC REMARKS: When observed with LM, the valves of Pseudostaurosira australopatagonica are difficult to differentiate from similar taxa within the genus (). Overall appearance of the new species under LM resembles P. decipiens, P. parasitica and P. laucensis.
Table 2. Comparison of morphometric features of the new Pseudostaurosira spp. and related species. n.d. = no data
The valve outline in P. australopatagonica is cruciate to rhomboid whereas the other species have a more lanceolate valve outline, even though the smaller valves from P. decipiens seem somewhat rhomboid. However, all these taxa have a widely lanceolate axial area. Moreover, our new species has a strong resemblance to Staurosira spec. presented by Rumrich et al. (Citation2000, pl. 12, ), from different lakes in Chilean Patagonia, but unfortunately only LM images of this diatom were shown.
Pseudostaurosira australopatagonica is wider than the similar taxa mentioned above, and it is larger than P. parasitica and P. laucensis but overlaps with the larger specimens (uppermost size limit) of P. decipiens. The apices in P. australopatagonica are capitate to subcapitate instead of rostrate like in P. decipiens, P. parasitica and P. laucensis. The stria density in P. australopatagonica (10–12) is similar to P. decipiens (13–15) and P. laucensis (14–15) but lower than P. parasitica (17–18). The areola shape in our new species is elliptical to round with profusely dichotomously branched volae that anastomose internally. The volae structure is certainly unique compared with other Pseudostaurosira species. Similar species also have profusely branched volae but with different appearance on the inner side. For example, P. decipiens has siliceous depositions on outer areolar opening in the form of rounded floating disc and on the inner opening in the form of two superimposed discs (Morales et al., Citation2012). In our species, there is always one areola on the mantle, whereas other species can have one or two. The apical pore fields in P. australopatagonica are very well developed ocellulimbi, externally cavernous, as in P. parasitica. There is no evidence of any developed spine in the observed specimens of P. australopatagonica, however, incipient spines are always present. This differs from the well-developed spines of P. decipiens or the numerous small spines of P. laucensis. Nevertheless, a P. parasitica population was observed without any spines (Rumrich et al., Citation2000). The girdle bands in P. australopatagonica are open and smooth like in P. parasitica, although the latter have perforations which were not observed in our species. Pseudostaurosira australopatagonica has mantle plaques at the abvalvar margin of the valve mantle just like other species in the genus.
Figs 36–51. Pseudostaurosira hyalopatagonica sp. nov., type material (LPC 15862. Laguna Verde, Santa Cruz Province, Argentina). Figs 36–46. LM images of type population, valve views. Fig. 37. Holotype specimen. Figs 47–51. SEM images. Fig. 47. Detail of areolae (arrowhead) and apical pore field (arrow), external view. Fig. 48. Detail of areolae occluded by branched volae (arrowhead) and the apical pore field (arrow), internal view. Fig. 49. External valve view. Fig. 50. Internal valve view. Fig. 51. Tilted specimen in external valve view, showing conical spines on the margins of the broken valve (arrow). Scale bar: Figs 36–46, 10 µm; Figs 47–48, 500 nm; Figs 49–51, 10 µm

Pseudostaurosira hyalopatagonica M.L.García, Maidana & E.Morales sp. nov. (LM , SEM )
Description
LM. Valves fusiform, with a central inflation and cuneate, elongate ends (). Axial area widely lanceolate. Valve dimensions (n = 25): length: 6.0–9.0 µm, width: 2.5–3.0 µm, stria density: 18–20 in 10 µm.
SEM. Striae uniseriate, with 1 to 3 round areolae (). Each areola occluded by profusely branched volae () and internally opening into a shallow depression. Striae interrupted on the valve face-mantle/mantle junction by a small conical spine (). Apical pore fields small, composed of 3 parallel rows of 2 to 3 poroids (). Internally, each apical pore field lies in an almost circular depression ().
HOLOTYPE: Circled specimen in slide VER17–1 31–32 cm, LPC 15862! (). Herbarium División Ficología ‘Dr. Sebastián A. Guarrera’, Museo de La Plata, Argentina.
TYPE LOCALITY: Argentina, Laguna Verde (49.210°S, 72.973°W), Santa Cruz, Argentina.
ETYMOLOGY: The epithet refers to the delicate aspect, almost hyaline valves, of this diatom, thus far only found in Patagonia.
DISTRIBUTION AND ASSOCIATED TAXA: This species was found in modern and fossil samples from Laguna Verde. This species was rare to very rare in the studied samples (0.1–0.9% abundance). The diatom assemblage was dominated by Aulacoseira lauquenensis M.L.García, J.M.Guerrero, Tremarin, Maidana & E.Morales and A. liucoensis M.L.García, J.M.Guerrero, Tremarin, Maidana & E.Morales.
TAXONOMIC REMARKS: Under LM, our species resembles Pseudostaurosira parasitoides (Lange-Bertalot, Rol.Schmidt & Klee) E.Morales, M.L.García & Maidana and P. pseudoconstruens (). All these three taxa are small (<10 µm) representatives of Pseudostaurosira and they have similar valve outlines. However, P. pseudoconstruens is cruciate, whereas P. hyalopatagonica is fusiform, like P. parasitoides. Our new species is the narrowest compared with the other two taxa, for example, in P. parasitoides the central area is inflated to a higher degree than in our new species. The apices in P. hyalopatagonica are cuneate elongated ends, different from the apices in P. parasitoides (round and protracted, and on larger specimens, protracted to subcapitate) and the apices in P. pseudoconstruens (rostrate to capitate). The axial area of our new species is lanceolate just like the ones from P. parasitoides and P. pseudoconstruens and the stria density is also similar. Pseudostaurosira hyalopatagonica has round to slightly elliptical areolae while P. parasitoides has short to elongate elliptical areolae and P. pseudoconstruens have round to elliptical areolae. Another difference is the internal depression into which the areolae open. Our new species has a short depression whereas in P. parasitoides and P. pseudoconstruens is long. There is almost no information about the volae on the two latter species and in our new species the material was so eroded that we found only one individual with part of the volae. The apical pore fields are small and present in both poles in P. hyalopatagonica and resemble the one in P. parasitoides. Internally, in P. hyalopatagonica, the apical pore field is similar to a small ocellulimbus (sunken into a round depression), but unfortunately the internal view is not available for the other taxa. The spines in P. hyalopatagonica are small, solid and conical, one on each striae, while P. parasitoides has numerous small linking spines. Girdle bands and mantle plaques were not observed in our material, probably due to poor preservation of the valves in the sediments.
Pseudostaurosira hyalopatagonica also resembles another two small taxa within the genus, Pseudostaurosira microstriata var. microstriata (Marciniak) Flower and Pseudostaurosira microstriata var. spinosa Flower, and both species overlap in size range with our new taxon (Marciniak, Citation1982; Flower, Citation2005). The valve shape of these two varieties is more lanceolate, while P. hyalopatagonica has a fusiform valve outline. The stria density of P. microstriata var. microstriata and P. hyalopatagonica are similar (18–20, for our new species, 18–22 for var. microstriata), whereas the stria density is lower in P. microstriata var. spinosa (17). Another difference among these taxa is that, while P. microstriata var. miscrostriata is spineless, spines are present on the other two taxa. However, P. microstriata var. spinosa has well developed short, truncated and apically flattened spines while P. hyalopatagonica has conical spines.
Figs 52–66. Pseudostaurosira catalinae sp. nov., type material (LPC 15861b. Laguna Pescado, Santa Cruz Province, Argentina). Figs 52–61. LM images of population on type material, valve views. Fig. 58. Holotype specimen. Figs 62–66. SEM images. Fig. 62. External valve view. Fig. 63. Internal valve view, showing areolae (arrow) and the apical pore field (arrowhead). Fig. 64. Detail of an external view of the areolae occluded by branched volae (arrow) and the structure over the mantle areolae (arrowhead). Fig. 65. Girdle view showing the mantle plaques on the abvalvar margin (black arrow), open girdle bands (white arrow) and the spatulate spines (arrowhead). Fig. 66. Detail of the apical pore field (arrow), external view. Scale bar: Figs 52–61, 10 µm; Figs 62–63, 65, 10 µm; Figs 64, 66, 500 nm
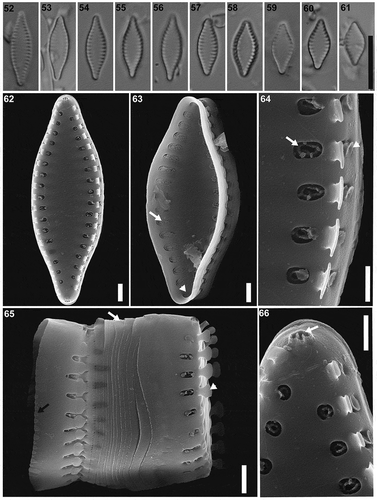
Pseudostaurosira catalinae M.L.García, Maidana & E.Morales sp. nov. (LM Figs , SEMFigs )
Description
LM. Valves broadly fusiform, in some cases lanceolate. Apices cuneate to subrostrate. Striae very short and marginal, widely lanceolate axial area (). Valve dimensions (n = 32): length: 6.0–12.5 µm, width: 3.0–4.5 µm, stria density: 13–18 in 10 µm.
SEM. Striae uniseriate, one round to elliptical areolae on the valve face and one transapically elongated areolae on the valve mantle (). Each areola occluded by profusely branched volae. Volae originating from the inner areolar periphery next to the spine () or have at least two points of origin opposed to each other (), forming a circular to elliptic ring at the centre of the areolar opening (). Watch glass-like flaps externally, cover the areolae on the valve mantle (). Internally, profuse sieve-like volae cover the areolar openings (). Striae interrupted at the valve face-mantle junction by solid spines, with apically flattened base and broadly spatulate apex (). Apical pore fields of the ocellulimbus type, externally with cavernous appearance, composed by 2 to 3 parallel rows of 2 to 3 poroids and located on valve mantle at the valve apex (). Internally, apical pore fields sunken into circular depressions (). Girdle bands open and lacking perforations. Ligulae and antiligulae not evident. Mantle plaques located on abvalvar margin of the valve mantle ().
HOLOTYPE: Circled specimen in slide L Pescado, LPC 15861b! (). Herbarium División Ficología ‘Dr. Sebastián A. Guarrera’, Museo de La Plata, Argentina.
TYPE LOCALITY: Argentina, Laguna Pescado (49.125°S, 72.921°W), Santa Cruz Province.
ETYMOLOGY: This species is named after Catalina Vega Bustos on the occasion of her first birthday.
DISTRIBUTION AND ASSOCIATED TAXA: The new species was found in surface sediment samples of Laguna Pescado. The diatom assemblages were dominated by a still unidentified Sellaphora sp. and several small fragilarioids.
TAXONOMIC REMARKS: Our new species resembles Pseudostaurosira brevistriata (Williams & Round, Citation1987, ). However, Morales et al. (Citation2015, –143), in their re-examination of the type material, state that P. brevistriata has lanceolate valves, whereas our new taxon has broadly fusiform valves. The apices of P. brevistriata have variable shape while in P. catalinae they are consistently subrostrate. The stria density is higher on our new species (13–18, while 13–14 in P. brevistriata). Both taxa have striae interrupted at the valve face-mantle junction by solid spines with a flattened base and spatulate apex, even though they have a different apex shape. In P. brevistriata the apex is mostly dichotomously branched (Morales et al., Citation2015), whereas in our new species the apex is broadly spatulated. The areolae are round to elliptical in both species and occluded by profusely branched volae. The volae in P. brevistriata originate from the inner periphery of the areolae while in P. catalinae they originate from the outer side, forming a circular to elliptic ring at the centre of the areolar opening. Pseudostaurosira brevistriata has two flaps growing, opposite to each other, over the volae on the valve mantle areolae while P. catalinae has watch glass-like flaps. Both species have the ocellulimbi apical pore fields, however, in our new taxon they have a cavernous appearance in external view. The girdle bands (open and without perforations) and mantle plaques are similar in both species.
Pseudostaurosira catalinae also resembles Pseudostaurosira elliptica (Schumann) Edlund, E.Morales & Spaulding, it has similar sizes, however, the latter can have elliptical valve outline, whereas our new species is consistently broadly fusiform. The areolae in P. elliptica is markedly coarsely punctate that differs from the round to elongated ones from our new species. The structure of the volae between these two species is also different. In our new species volae form a circular to elliptic ring at the centre of the areolar opening, whereas on P. elliptica volae are dichotomously branched. Moreover, P. elliptica has two flaps growing over the mantle areolae like our new species but the shape of these flaps have a different shape from those in P. catalinae (Morales, Citation2011; Li et al., Citation2018). Both species have spines interrupting the striae, while our new species has solid spines with flattened base and broadly spatulate apex, P. elliptica has spines with hollow base and flattened distal ends. The apical pore fields in P. elliptica are less developed than the ones in P. catalinae.
Pseudostaurosira catalinae looks very similar to another Pseudostaurosira species described for northern Argentina, P. ferrarioae. Our new species overlaps on its length and width with P. ferrarioae but it has higher stria density (13–18 for P. catalinae, 13–15 for P. ferrarioae). Moreover, the valve outline in P. ferrarioae is rhomboid with elongate subrostrate ends, which differs from P. catalinae that has broadly fusiform valve outline with cuneate to subrostrate ends. Also, volae are different between these two species. Pseudostaurosira ferrarioae has dichotomously branched volae, meanwhile P. catalinae has profusely branched volae, forming a circular to elliptic ring at the centre of the areolar opening. Albeit both species have spines, in P. ferrarioae they are short and conical while in P. catalinae they are more developed and spatulate. The apical pore fields are developed in both species. Pseudostaurosira ferrarioae has more rows of poroids (6 to 8) than our new species (2 to 3), however, the apical pore fields in P. catalinae have an externally cavernous appearance, which is not the case in P. ferrarioae.
Pseudostaurosira tehuelcheana M.L.García, Maidana & E.Morales sp. nov. (LM , SEM )
LM. Valves lanceolate with broadly rounded apices. Axial area widely lanceolate, with marginal striae (). Valve dimensions (n = 12): length: 5.0–12.0 µm, width: 2.0–4.5 µm, stria density: 12–13 in 10 µm.
Figs 67–80. Pseudostaurosira tehuelcheana sp. nov., type material (LPC 15378b. Laguna Azul, Santa Cruz Province, Argentina). Figs 67–76. LM images of population on type material, valve views. Fig. 70. Holotype specimen. Figs 77–80. SEM images. Fig. 77. External valve view, showing the apical pore field (arrow). Fig. 78. Girdle view, showing open girdle bands (arrow). Fig. 79. Detail of an external view of the areolae occluded by branched volae (arrow) and the conical spines (arrowhead). Fig. 80. Tilted frustules, showing several open girdle bands. Scale bar: Figs 67–76, 10 µm; Figs 77–78, 80, 10 µm; Figs 79, 500 nm
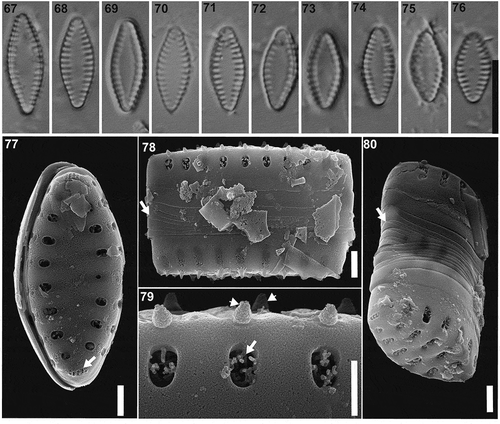
SEM. Striae uniseriate, with one transapically elongate areola on the valve face and one elongate areola on the valve mantle (). Each areola occluded by profusely branched volae (). Striae interrupted at the valve face-mantle junction by solid conical incipient spines (). Apical pore fields of the ocellulimbus type, composed of 5 parallel rows of 2 to 3 poroids (). Girdle bands open and lacking perforations (). Mantle plaques absent.
HOLOTYPE: Circled specimen in slide Azul 2013, LPC 15378b! (). Herbarium División Ficología ‘Dr. Sebastián A. Guarrera’, Museo de La Plata, Argentina.
TYPE LOCALITY: Argentina, Laguna Azul (52.079°S, 69.582°W), Santa Cruz Province.
ETYMOLOGY: The specific epithet refers to the Amerindian ethnic group Tehuelche, who formerly occupied the area where the lake is located.
DISTRIBUTION AND ASSOCIATED TAXA: The new taxon was observed in modern and fossil samples from Laguna Azul. The diatom assemblages in this lake were dominated by different species of Fragilariaceae, represented by Staurosirella andino-patagonica J.M.Guerrero, M.L.García & E.Morales, Pseudostaurosira zolitschkae sp. nov. (this work), Pseudostaurosira aff. sopotensis (Witkowski & Lange-Bertalot) E.Morales, Wetzel & Ector, two unidentified Staurosira spp. and one Punctastriata sp.
TAXONOMIC REMARKS: Our new species resembles P. elliptica on its valve outline and morphometric data. Pseudostaurosira elliptica has cuneate apices while our new species has broadly rounded apices, however, both species have widely lanceolate axial area. The stria density is lower on our new species (12–13) than in P. elliptica (14–16). In both taxa striae are composed by marginal areolae, meanwhile the shape of the areola is widely punctate in P. elliptica, in our new taxon areolae are transapically elongate. The volae in both species are profusely branched but in P. elliptica they are dichotomously branched. The spines lie on the valve face-mantle/mantle junction in both species, but in P. elliptica, the spines are hollow at the base and have flattened distal ends, whereas our new species has solid and conical spines. Pseudostaurosira tehuelcheana has ocellulimbus type apical pore fields in contrast to P. elliptica which are less developed. Both species have a cingulum with several copulae without perforations and lack mantle plaques.
Pseudostaurosira tehuelcheana also resembles P. ferrarioae, the two species having somewhat overlapping morphometric data, but our new species has lower stria density (12–13 to 13–15 for P. ferrarioae) and the valve outline is lanceolate with broadly rounded ends whereas in P. ferrarioae it is rhomboid with elongate subrostrate ends. The spines are solid and conical in both species. The apical pore fields are developed in both taxa, but in P. ferrarioae they have more rows of poroids (6–8) than in our species (5).
LM. Valves linear to lanceolate, apices subrostrate, axial area widely lanceolate, with marginal striae (). Valve dimensions (n = 92): length: 8.5–28.0 µm, width: 3.5–5.0 µm, stria density: 11–14 in 10 µm.
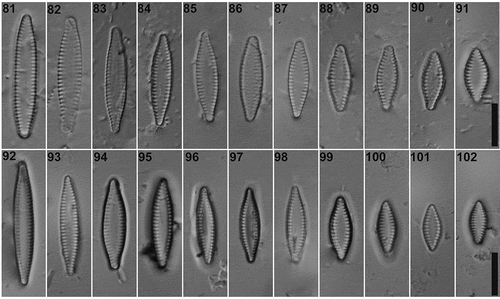
Figs 81–102. LM images of Pseudostaurosira zolitschkae sp. nov. Figs 81–91. Valve views of type material (LPC 15378c. Laguna Azul, Santa Cruz Province, Argentina). Fig. 86. Holotype specimen. Figs 92–102. Pseudostaurosira zolitschkae sp. nov. from Laguna Huergo, Santa Cruz Province, Argentina. Scale bar: Figs 81–102, 10 µm
Figs 103–109. SEM images of Pseudostaurosira zolitschkae sp. nov., type material (LPC 15378c, Laguna Azul, Santa Cruz Province, Argentina). Figs 103–104. External valve view. The arrows mark the thin vimen. Fig. 105. Girdle view, eroded mantle plaques (arrow). Fig. 106. Detail of the spines with lobulated flaps (arrows). Fig. 107. Internal valve view. Fig. 108. Detail of the apical pore field (arrow), areolae (black arrowhead) and hollow spines (white arrowhead), external view. Fig. 109. Detail of the apical pore field (arrowhead) and areolae (arrow), internal view. Scale bar: Figs 103–105, 107–109, 10 µm; Fig 106, 500 nm

SEM. Striae uniseriate, with one transapically elongate areola on the valve face and one elongate on the valve mantle (). Each areola occluded by profusely branched volae, originating at the larger sides (). Exceptionally, two areolae may be separated by a relatively thin vimen (). Two lobulated flaps grow opposite to each other (), covering the mantle areolae externally. Striae interrupted at the valve face-mantle junction by hollow spatulate spines (). Apical pore fields of the ocellulimbus type, composed of 3 to 4 parallel rows of 2 to 3 poroids (). Girdle bands open and lacking perforations (). Mantle plaques located on abvalvar margin of the valve mantle ().
HOLOTYPE: Circled specimen in slide Azul 2013, LPC 15378c! (). Herbarium División Ficología ‘Dr. Sebastián A. Guarrera’, Museo de La Plata, Argentina.
TYPE LOCALITY: Argentina, Laguna Azul (52.079°S, 69.582°W), Santa Cruz Province.
ETYMOLOGY: The new species is named after Prof. Dr. Bernd Zolitschka in recognition for his valuable palaeolimnological research in southern Patagonia.
DISTRIBUTION AND ASSOCIATED TAXA: The new taxon was observed in modern and fossil samples from Laguna Azul and modern samples from Laguna Huergo (). The diatom assemblage in this lake was dominated by different species of Fragilariaceae, represented by Staurosirella andino-patagonica J.M.Guerrero, M.L.García & E.Morales, Pseudostaurosira tehuelcheana sp. nov. (this work), Pseudostaurosira aff. sopotensis (Witkowski & Lange-Bertalot) E.Morales, Wetzel & Ector, two unidentified Staurosira spp. and one unidentified Punctastriata sp. The diatom assemblage of Laguna Huergo is dominated by Amphora pediculus (Kützing) Grunow, Cocconeis neuquina Frenguelli, Karayevia clevei (Grunow) Bukhtiyarova, Cyclotella ocellata Pantocsek and several small fragilarioid species.
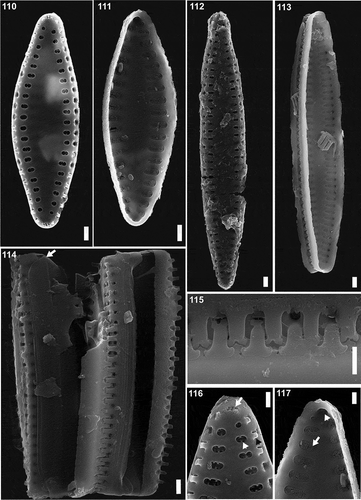
Figs 110–117. SEM images of Pseudostaurosira zolitschkae sp. nov., population from Laguna Huergo, Santa Cruz Province, Argentina LPC 15255. Figs 110, 102. External valve view. Figs 111, 113. Internal valve view. Fig. 114. Girdle view, showing open girdle bands (arrow). Fig. 115. Detail of the spines. Fig. 116. Detail of the apical pore field (arrow), areolae (white arrowhead) and hollow spines (black arrowhead), external view. Fig. 117. Detail of the apical pore field (arrowhead) and areolae (arrow), internal view. Scale bar: Figs 110–115, 10 µm; Figs 116–117, 500 nm
TAXONOMIC REMARKS: In LM, Pseudostaurosira zolitschkae resembles P. subsalina (Hustedt) E.Morales (Morales, Citation2005) illustrated by Cejudo-Figueiras et al. (Citation2011, see ) and the morphometric data and the valve outline are quite similar. However, the axial area of P. subsalina is variable (narrow to broadly lanceolate), while in P. zolitschkae it is consistently broadly lanceolate, which is related to the striae being composed by marginal areolae on the latter. Moreover, in SEM, the areolae shape of our new species are more rounded and transapically elongate while in P. subsalina they are more squared and transapically elongate; volae in P. zolitschkae feature sieve-like formations while in P. subsalina they are profusely branched; and spines look completely different (see ).
Table 3. Comparison of morphometric features of the new Pseudostaurosira spp. and related species. n.d. = no data
Other large-valved Pseudostaurosira, such as Pseudostaurosira polonica (Witak & Lange-Bertalot) E.Morales & Edlund (Morales & Edlund, Citation2003) and Pseudostaurosira americana E.Morales (Cejudo-Figueiras et al., Citation2011) share similar morphometric data to our new species but differ markedly on their valve outline (broadly elliptical to linear for P. polonica, lanceolate for P. americana, meanwhile our new species is linear to lanceolate) and apices shape (tapering apices to broadly rounded for P. polonica, cuneate for P. americana, meanwhile our new species is subrostrate), both have higher stria density than P. zolitschkae and different areolae shape (more rounded).
In SEM, P. zolitschkae is similar to P. oliveraiana (Grana et al., Citation2018), mainly in the areola shape and structure, including the rare areolae separated by a relatively thinner vimen. The spines look similar but in P. oliveraiana they are solid, whereas on P. zolitschkae they are hollow. However, the apices of P. oliveraiana are not so similar to P. zolitschkae, as they are subcapitate in the former and subrostrate in the latter. Pseudostaurosira oliveraiana has a more linear outline, resembling that of the largest individuals in the population of P. zolitschkae.
The volae structure of P. zolitschkae is uncommon within the genus and has been reported only for P. elliptica, P. oliveraiana, P. tenuis and P. brevistriata var. trigona Lange-Bertalot. In all these taxa, the volae originate from a position parallel to the transapical axis of the valve.
Some of the smaller valves of P. zolitschkae are similar to those of smaller individuals of P. brevistriata and P. elliptica. However, the valve outline of our new species is linear to lanceolate with subrostrate ends, meanwhile P. brevistriata has lanceolate to rhomboid, sometimes elliptical valves with subrostrate to cuneate ends and P. elliptica has elliptical to lanceolate valves with cuneate to round ends. The stria density is higher in P. brevistriata (13–14) and in P. elliptica (14–16) than in our new species (11–14). The areolae in P. zolitschkae are transapically elongate while in P. brevistriata they are elliptical to round and in P. elliptica they are widely punctate. Despite both species having areolae occluded by profusely branched volae, neither P. elliptica nor P. brevistriata have the uncommon volae structure of P. zolitschkae. Another difference between these three taxa is the spines, P. zolitschkae has hollow and spatulate spines, similar to P. elliptica, whereas P. brevistriata has solid ones. Our new taxon and both P. brevistriata and P. elliptica share the characteristic of having two flaps growing over the mantle areolae (Morales, Citation2011; Morales et al., Citation2015; Li et al., Citation2018) as well as featuring mantle plaques.
Discussion
Recent studies in southernmost Patagonia have revealed highly diverse diatom communities, often including new species, that are currently only known from this region (e.g. García et al., Citation2017, Citation2019, Citation2020; Guerrero et al., Citation2019). Our own observations also yielded several new taxa, of which the five new species presented herein are only a small fraction. This suggests that in South American regions such as Patagonia, non-marine diatoms remain severely understudied, and hence species richness is underestimated. Morales et al. (Citation2012, Citation2014) also showed this for other Andean ecosystems, from which the diatom flora is mostly known from easily accessible localities, generally affected by contamination and eutrophication. Of all potentially new species observed in these habitats, small araphid diatoms seem to be particularly specious. Most papers from Patagonia that list diatom taxa mainly include species names from specimens originally described from Europe or other continents (e.g. Díaz Villanueva & Maidana, Citation1999; Maidana & Díaz Villanueva, Citation2001). The lack of illustrations in those references hinder any revision, so the information is of limited value in the construction of taxonomically sound diatom floras. Force-fitting has indeed been a major factor that has shrouded our knowledge of diatom species distribution and their correct identity (Tyler, Citation1996; Kociolek & Spaulding, Citation2000, Morales et al., Citation2014). Working on diatom floras is a time-consuming endeavour that will take many years to produce a comprehensible catalogue of species for different regions of South America.
The books by Rumrich et al. (Citation2000), Metzeltin et al. (Citation2005) and Metzeltin & Lange-Bertalot (Citation2007) remain the largest publications on South American diatoms, but they seem to have reported only a low number of local species in comparison to a rather high number of European taxa (see discussion in Morales et al., Citation2012). Thus, the relatively poor knowledge of diatom biodiversity of the South American flora hampers the information of the occurrence and distribution of taxa with the consequence that fair comparisons among different biomes of the continent is unpractical at the moment (Wetzel et al., Citation2011; Costa et al., Citation2017). Therefore, a major effort is needed to increase the geographic coverage, especially in remote regions such as southernmost Patagonia. Detailed taxonomic studies will allow the improvement of taxonomic resolution, which is crucial to advance beyond the current foreign bias and to generate a genuine knowledge of South American diatom diversity (Metzeltin & Lange-Bertalot, Citation2007).
Small araphid diatoms are common components of the plankton and benthos of freshwater environments (Finkelstein & Gajewski, Citation2008). This group is often very abundant in both recent and postglacial lacustrine sediments, particularly in temperate and alpine regions of the northern hemisphere (Smith, Citation2002), and across arctic and subarctic regions (LeBlanc et al., Citation2004; Paull et al., Citation2008). Although the five species presented herein almost double the number of Pseudostaurosira species described from the Andes, we consider that the assessment of the species richness of Pseudostaurosira and other small araphid genera is still far from being complete. This fact constitutes a severe limitation in ecological and palaeoecological studies where fragilarioids are often used as bioindicators of water quality or past environmental conditions (Díaz Pardo et al., Citation2008).
Pseudostaurosira australopatagonica sp. nov. assemblages recovered from the sediment core PS0710 in Lago Cipreses have up to 28% of teratological valves. Teratologies in diatom populations are commonly observed, but they generally have low frequencies (<0.5%, according to Arini et al., Citation2012). The presence of multiple stressors, however, can significantly increase the proportion of teratological individuals. Lavoie et al. (Citation2017) showed that the character most frequently affected is the valve outline, and that araphids are more ‘prone’ to show these abnormal valve outlines in comparison to raphid or centric diatoms. In the case of P. australopatagonica sp. nov. it was very important to have more than one population in order to better delimit its morphometric data, as well as to point out that the readily visible abundance of teratological forms found might assist future environmental inferences by means of registering this particular species as sensitive to potential stressors, or alternatively, showing error prone replication under productive conditions.
Even though the definition of a species should be based on the study of specimens belonging to more than one population (the species type) (Williams & Blanco, Citation2020), the availability of detailed studies of type material already allows a thorough comparison with newly found populations and the description of new variants at the species level, as is the case of our study. Therefore, how do we know the amount of data that is required to recognize a new species? Describing taxa is a scientific question which involves the discovery of defining character(s) (Kociolek & Williams, Citation2015). Round (Citation1996) stated that very narrow circumscriptions of diatom genera (also applicable to the species level) would lead to homogeneity within a genus and would therefore give origin to a more ‘natural’ classification. Morales et al. (Citation2019) define wide and short vimines as the main diagnostic feature of Pseudostaurosira, leaving a wide range of variations and combinations for the rest of the valve features. Thus, some species within this genus can be distinguished by a single defining character (for example the structure of the volae in P. australopatagonica), whereas other species might be distinguished by the combination of several characters (e.g. morphometric data, valve outline, spine structure) to validate their description as new species.
Despite the growing acceptance of molecular analyses, that have unveiled a hitherto unknown diversity in several diatom genera (e.g. Eunotia, Sellaphora, Pinnularia) morphological data continue to be the most used basis for identifying diatom species (Morales et al., Citation2019), in particular the small fragilarioids. This is because taxon sampling in molecular studies is still small and unrepresentative of the great diversity found among araphids, and because, to date, molecular data have only resolved convincingly the classification of araphids at levels higher than genera and species (Medlin et al., Citation1993, Citation2008, Citation2012). Therefore, our study confirms the validity of valve morphological characters to recognize unique diagnostic characteristics or a combination of features that fall apart from the descriptions of other species within Pseudostaurosira.
Author contributions
M.L.García: research concept, drafting and editing the manuscript, producing figures, drafting and editing all species descriptions, processing samples, performing microscopy observations and producing LM and SEM images of all species, analysing data; S.Bustos: sample processing, performing microscopy observations and producing LM images, analysing data of P. zolitschkae, editing the manuscript; L.A.Villacís: processing samples, performing microscopy observations and producing LM and SEM images, analysing data of P. australopatagonica, editing the manuscript; N. I. Maidana and E. A. Morales: research concept, funding, editing the manuscript and species descriptions; C. Laprida, C. Mayr, P. I. Moreno: providing materials, fieldwork, stratigraphy and chronology, funding, editing the manuscript.
Acknowledgements
EAM, MLG and NIM thank Dr Saúl Blanco for his guidance regarding nomenclature. MLG thanks Lic. José María Guerrero, Curator of the Herbarium ‘Dr Sebastián A. Guarrera’, Museo de La Plata, Argentina for helping with the formalities and type material deposit. We thank the two anonymous reviewers and the editors who greatly contributed to improving the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anonymous (1975). Proposals for the standardization of diatom terminology and diagnoses. Nova Hedwigia Beihefte, 53: 323–354.
- Arini, A., Feurtet-Mazel, A., Morin, S., Maury-Brachet, R., Coste, M. & Delmas, F. (2012). Remediation of a watershed contaminated by heavy metals: a 2-year field biomonitoring of periphytic biofilms. Science of the Total Environment, 425: 242–253.
- Barber, H.G. & Haworth, E.Y. (1981). A guide to the morphology of the diatom frustule with a key to the British freshwater genera. Freshwater Biology Association, 44: 1–112.
- Battarbee, R.W. (1986). Diatom analysis. In Handbook of Holocene Palaeoecology and Palaeohydrology (Berglund, B.E., editor), 527–570. John Wiley & Sons, Chichester.
- Cejudo-Figueiras, C., Morales, E.A., Wetzel, C.E., Blanco, S., Hoffmann, L. & Ector, L. (2011). Analysis of the type of Fragilaria construens var. subsalina (Bacillariophyceae) and description of two morphologically related taxa from Europe and the United States. Phycologia, 50: 67–77.
- Coronato, A.M., Coronato, F., Mazzoni, E. & Vázquez, M. (2008). The physical geography of Patagonia and Tierra del Fuego. In Late Cenozoic of Patagonia and Tierra del Fuego (Rabassa, J., editor). Developments in Quaternary Sciences, 11: 13–55.
- Costa, L.F., Wetzel, C.E., Lange-Bertalot, H., Ector, L. & Bicudo, D.C. (2017). Taxonomy and ecology of Eunotia species (Bacillariophyta) in southeastern Brazilian reservoirs. Bibliotheca Diatomologica, 64: 1–302.
- Díaz Pardo, C.A., Echazú, D.M. & Maidana, N.I. (2008). Diatomeas continentales como indicadoras de cambios climáticos en Patagonia. In Efecto de los cambios globales sobre la biodiversidad (Volpedo, A.V. & Fernández Reyes, L., editors), 233–246. Red 406RT0285, CYTED – Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo.
- Díaz Villanueva, V. & Maidana, N.I. (1999). Diatoms (Bacillariophyceae) from Pulmari Lake (Neuquen, Argentina). Biologia Bratislava, 54: 1–10.
- Edlund, M.B, Morales, E.A. & Spaulding, S.A. (2006). The type and taxonomy of Fragilaria elliptica Schumann, a widely misconstrued taxon. In Proceedings of the 18th International Diatom Symposium (Witkowski, A., editor), 53–59. Miedzyzdroje, Poland. Biopress Limited, Bristol.
- Finkelstein, S.A. & Gajewski, K. (2008). Responses of Fragilarioid-dominated diatom assemblages in a small Arctic lake to Holocene climatic changes, Russell Island, Nunavut, Canada. Journal of Paleolimnology, 40: 1079–1095.
- Flower, R.J. (2005). A taxonomic and ecological study of diatoms from freshwater habitats in the Falkland Islands, South Atlantic. Diatom Research, 20: 23–96.
- Frenguelli, J. (1924). Resultados de la Primera Expedición a Tierra del Fuego (1921) Diatomeas de Tierra de Fuego. Anales de la Sociedad Científica Argentina, 98: 5–63.
- Frenguelli, J. (1942). Diatomeas del Neuquén (Patagonia). Revista del Museo de La Plata, Nueva Serie, Sección Botánica, 5: 73–219.
- Frenguelli, J. & H. A. Orlando. (1956). Diatomeas y Silicoflagelados del Sector Antártico Sudamericano. Publicación Instituto Antártico Argentino, 5: 1–191.
- García, M.L., Maidana, N.I., Ector, L. & Morales, E. (2018). Staurosira magallanesica, a replacement name for Staurosira patagonica M.L.Garcia, Maidana, Ector & E.Morales, nom. illeg. (non Staurosira patagonica Cleve) (Bacillariophta, Staurosiraceae). Notulae Algarum, 51: 1–2.
- García, M.L., Maidana, N.I., Ector, L. & Morales, E.A. (2017). Staurosira patagonica sp. nov., a new diatom (Bacillariophyta) from southern Argentina, with a discussion on the genus Staurosira Ehrenberg. Nova Hedwigia, Beihefte, 146: 103–123.
- García, M.L., Morales, E.A., Guerrero, J.M., Tremarin, P.I. & Maidana, N.I. (2019). New Aulacoseira species (Bacillariophyta) from the Argentinian Patagonia and re-examination of type material of Melosira perpusilla Frenguelli. Phytotaxa, 408: 161–177.
- García, M.L., Morales, E.A., Mann, D.G. & Maidana, N.I. (2020). Sellaphora mayrii (Bacillariophyceae), a new diatom from the Argentinian Patagonia. Phytotaxa, 437: 135–146.
- Garreaud, R., Lopez, P., Minvielle, M. & Rojas, M. (2013). Large-scale control on the Patagonian climate. Journal of Climate, 26: 215–230.
- Grana, L., Morales, E.A., Maidana, N.I. & Ector, L. (2018). Two new species of Staurosira and Pseudostaurosira (Bacillariophyta) from the highlands of Argentina (south-central Andes) and two new nomenclatural combinations. Phytotaxa, 365: 60–72.
- Guerrero, J.M., García, M.L. & Morales, E.A. (2019). Staurosira andino-patagonica sp. nov. (Bacillariophyta) from lake sediments in Patagonia, Argentina. Phytotaxa, 402: 131–144.
- Hamilton, P.B. & Siver, P.A. (2008). The type for Fragilaria lancettula Schumann 1867 and transfer to the genus Punctastriata as Punctastriata lancettula (Schum.) Hamilton & Siver nov. comb. Diatom Research, 23: 355–365.
- Kociolek, J.P. & Spaulding, S.A. (2000). Freshwater diatom biogeography. Nova Hedwigia, 71: 223–241.
- Kociolek, J.P. & Williams, D.M. (2015). How to define a diatom genus? Notes on the creation and recognition of taxa, and a call for revisionary studies of diatoms. Acta Botanica Croatica, 74: 195–210.
- Krasske, J. (1949). Subfossile Diatomeen aus den Mooren Patagoniens und Feuerlands. Suomalaisen Tiedeakatemian Toimituksia Annales Academiae Scientiarum Fennicae, Sarja Series A. IV, Biologica, 14: 1–92.
- Lavoie, I., Hamilton, P.B., Morin, S., Kim Tiam, S., Kahlert, M., Kahlert, M., Gonçalves, S., Falasco, E., Fortin, C., Gontero, Heudre, D., Kojadinovic-Sirinellii, M., Manoylov, K., Pandey, L.K. & Taylor, J.C. (2017). Diatom teratologies as biomarkers of contamination: are all deformities ecologically meaningful? Ecological Indicators, 82: 539–550.
- LeBlanc, M., Gajewski, K. & Hamilton, P.B. (2004). A diatom-based Holocene paleoenvironmental record from a mid-arctic lake on Boothia Peninsula, Nunavut, Canada. The Holocene, 14: 417–425.
- Li, C.L., Witkowski, A., Ashworth, M.P., Dąbek, P., Sato, S., Zgłobicka, I., Witak, M., Khim, J.S. & Kwon, C.J. (2018). The morphology and molecular phylogenetics of some marine diatom taxa within the Fragilariaceae, including twenty undescribed species and their relationship to Nanofrustulum, Opephora and Pseudostaurosira. Phytotaxa, 355: 1–104.
- Maidana, N.I. (1996). Diatomeas fósiles nuevas o poco conocidas para la Argentina: Lago Nahuel Huapi (Brazo Campanario), Prov. de Río Negro. Boletín de la Sociedad Argentina de Botánica, 31: 177–199.
- Maidana, I.N. & Díaz Villanueva, V. (2001). Diatomeas de lagos oligotr6ficos Andinos, (Provincia de Neuquén, Argentina. Boletin de la Sociedad Argentina de Botanica, 36: 15–27.
- Mann, D.G., Thomas, S.J. & Evans, K.M. (2008). Revision of the diatom genus Sellaphora: A first account of the larger species in the British Isles. Fottea, 8: 15–78. doi:10.5507/fot.2008.002
- Marciniak, B. (1982). Late glacial and Holocene new diatoms from a glacial Lake Przedni Staw in the Piec Stawów Polskich Valley, Polish Tatra Mts. Acta Geologica Academiae Scientiarum Hungaricae, 25: 161–171.
- Marciniak, B. (1988). Late glacial Fragilaria flora from lake sediments of the Tatra Mts. and the Alps. In Proceedings of the 9th International Diatom Symposium (Round, F.E., editor), 233–245. Biopress, Bristol and Koeltz Scientific Books, Koenigstein.
- Mayr, C., Smith, R.E., García, M.L., Massaferro, J., Dubois, N., Lücke, A., Maidana, N.I., Meier, W. J-H., Wissel, H. & Zolitschka, B. (2019). Historical eruptions of Lautaro volcano and their impacts on lacustrine ecosystems in southern Argentina. Journal of Paleolimnology, 62: 205–221.
- Medlin, L., Jung, I., Bahulikar, R., Mendgen, K., Kroth, P. & Kooistra, W.H.C.F. (2008). Evolution of the diatoms. VI. Assessment of the new genera in the araphids using molecular data. Nova Hedwigia Beihefte, 133: 81–100.
- Medlin, L., Williams, D.M. & Sims, P.A. (1993). The evolution of the diatoms (Bacillariophyta). I. Origin of the group and assessment of the monophyly of its major divisions. European Journal of Phycology, 28: 261–275.
- Medlin, L., Yang, I. & Sato, S. (2012). Evolution of the diatoms. VII. Four gene phylogeny assesses the validity of selected araphid genera. Nova Hedwigia Beihefte, 141: 505–514.
- Metzeltin, D. & Lange-Bertalot, H. (1998). Tropical diatoms of South America I: About 700 predominantly rarely known or new taxa representative of the neotropical flora. In Annotated Diatom Micrographs, Iconographia Diatomologica 5 (Lange-Bertalot, H., editor), 1–695. Koeltz Scientific Books, Königstein.
- Metzeltin, D. & Lange-Bertalot, H. (2007). Tropical diatoms of South America II. Special remarks on biogeography disjunction. In Annotated Diatom Micrographs, Iconographia Diatomologica 18 (Lange-Bertalot, H., editor), 1–877. Koeltz Scientific Books, Königstein.
- Metzeltin, D., Lange-Bertalot, H. & García-Rodríguez, F. (2005). Diatoms of Uruguay. In Annotated Diatom Micrographs, Iconographia Diatomologica 15 (Lange-Bertalot, H., editor), 1–737. Koeltz Scientific Books, Königstein.
- Morales, E. (2011). Pseudostaurosira elliptica. In Diatoms of North America. Retrieved 11 June 2020, from https://diatoms.org/species/pseudostaurosira_elliptica
- Morales, E.A. (2001). Morphological studies in selected fragilarioid diatoms (Bacillariophyceae) from Connecticut waters (U.S.A.). Proceedings of the Academy of Natural Sciences of Philadelphia, 151: 105–120.
- Morales, E.A. (2005). Observations of the morphology of some known and new fragilarioid diatoms (Bacillariophyceae) from rivers in the USA. Phycological Research, 53: 113–133.
- Morales, E.A. & Edlund, M.B. (2003). Studies in selected fragilarioid diatoms (Bacillariophyceae) from Lake Hovsgol, Mongolia. Phycological Research, 51: 225–239.
- Morales, E.A., Edlund, M.B. & Spaulding, S.A. (2010). Description and ultrastructure of araphid diatom species (Bacillariophyceae) morphologically similar to Pseudostaurosira elliptica (Schumann) Edlund et al. Phycological Research, 58: 97–107.
- Morales, E.A., Guerrero, J.M., Wetzel, C.E., Sala, S. & Ector, L. (2013). Unraveling the identity of Fragilaria pinnata Ehrenberg and Staurosira pinnata Ehrenberg: research progress on a convoluted story. Cryptogamie, Algologie, 34: 89–102.
- Morales, E.A., Novais, M.H., Chávez, G., Hoffmann, L. & Ector, L. (2012). Diatoms (Bacillariophyceae) from the Bolivian Altiplano: three new araphid species from the Desaguadero River draining Lake Titicaca. Fottea, 12: 41–58.
- Morales, E.A. & Vis, M.L. (2007). Epilithic diatoms (Bacillariophyceae) from cloud forest and alpine streams in Bolivia, South America. Proceedings of the Academy of Natural Sciences of Philadelphia, 156: 123–155.
- Morales, E.A., Wetzel, C., Novais, M., Buczkó, K., Morais, M. & Ector, L. (2019). Morphological reconsideration of the araphid genus Pseudostaurosira (Bacillariophyceae), a revision of Gedaniella, Popovskayella and Serratifera, and a description of a new Nanofrustulum species. Plant Ecology and Evolution, 152: 262–284.
- Morales, E.A., Wetzel, C. E., Van de Vijver, B. & Ector, L. (2015). Morphological studies on type material of widely cited araphid diatoms (Bacillariophyta). Phycologia, 54: 455–470.
- Morales, E.A., Wetzel, C.E., Rivera, S.F., Van de Vijver, B. & Ector, L. (2014). Current taxonomic studies on the diatom flora (Bacillariophyceae) of the Bolivian Altiplano, South America, with possible consequences for palaeoecological assessments. Journal of Micropalaeontology, 33: 121–129.
- Moreno, P., Vilanova, I., Villa-Martínez, R., Garreaud, R.D., Rojas, M., & De Pol-Holz, R. (2014). Southern Annular Mode-like changes in southwestern Patagonia at centennial timescales over the last three millennia. Nature Communications, 5: 1–7.
- Moreno, P.I., Vilanova, I., Villa-Martínez, R., Dunbar, R.B., Mucciarone, D.A., Kaplan, M.R., Garreaud, R.D., Rojas, M., Moy, C.M., De Pol-Holz, R. &. Lambert, F. (2018). Onset and evolution of Southern Annular Mode-like changes at centennial timescale. Scientific Reports, 8: 1–9.
- Paull, T.M., Hamilton, P.B., Gajewski, K. & LeBlanc, M. (2008). Numerical analysis of small Arctic diatoms (Bacillariophyceae) representing the Staurosira and Staurosirella species complexes. Phycologia, 47: 213–224.
- Ross, R., Cox, E.J., Karayeva, N.I., Mann, D.G., Paddock, T.B., Simonsen, R. & Sims, P.A. (1979). An amended terminology for the siliceous components of the diatom cell. Nova Hedwigia, Beihefte, 64: 513–533.
- Round, F.E. (1996). What characters define diatom genera, species and infraspecific taxa? Diatom Research, 11: 203–218.
- Round, F.E., Crawford, R.M. & Mann, D.G. (1990). Diatoms: Biology and Morphology of the Genera. Cambridge University Press, Cambridge.
- Rumrich, U., Lange-Bertalot, H. & Rumrich, M. (2000). Diatomeen der Anden. Von Venezuela bis Patagonien/Feurland. In Annotated Diatom Micrographs, Iconographia Diatomologica 9 (Lange-Bertalot, H., editor), 1–673. Koeltz Scientific Books, Königstein.
- Schmidt, R., Kamenik, C., Lange-Bertalot, H. & Rolf, K.L.E.E. (2004). Fragilaria and Staurosira (Bacillariophyceae) from sediment surfaces of 40 lakes in the Austrian Alps in relation to environmental variables, and their potential for palaeoclimatology. Journal of Limnology, 63: 171–189.
- Seeligmann, C.T., Maidana, N.I. & Morales, E.A. (2018). Fragilariaceae (Bacillariophyta) en humedales de altura de Catamarca (Argentina). Boletín de la Sociedad Argentina de Botánica, 53: 507–519.
- Smith, R.I. (2002). Diatom-based Holocene paleoenvironmental records from continental sites on northeastern Ellesmere Island, high Arctic, Canada. Journal of Paleolimnology, 27: 9–28.
- Smol, J.P. (2017). Paleolimnology. In Reference Module in Earth Systems and Environmental Sciences. Elsevier Publishers, Amsterdam.
- Tyler, P.A. (1996). Endemism in freshwater algae. Hydrobiologia, 336: 127–135.
- Wetzel, C.E., Ector, L., Hoffmann, L., Lange-Bertalot, H. & Bicudo, D.C. (2011). Two new periphytic Eunotia species from the neotropical Amazonian ‘black waters’, with a type analysis of E. braunii. Diatom Research, 26: 135–146.
- Williams, D.M. & Blanco, S. (2020). Studies on type material from Kützing’s diatom collection II: Synedra acus Kützing, Synedra arcus Kützing, their morphology, types and nomenclature. Diatom Research, 34: 1–14.
- Williams, D.M. & Round, F.E. (1987). Revision of the genus Fragilaria. Diatom Research, 2: 267–288.
- Williams, D.M & Wetzel, C.E. (2019). Description of a new Pseudostaurosira based on “Fragilaria virescens f. parva” from Erbario Crittogamico Italiano. Botany Letters, 167: 1–9.
- Witkowski, A., Lange-Bertalot, H. & Witak, M. (1995). Diatom taxa of unusual frustule structure belonging to the genus Fragilaria. Fragmenta Floristica et Geobotanica Polonica, 40: 729–741.