?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Cladophora spp. constantly or periodically form floating and benthic mats in Crimean hypersaline lakes and lagoons. In 2017–2018, characteristics of the Cladophora mats and the microalgae on Cladophora filaments were studied employing field research and microscopy. The whole Cladophora mat has a vertical structure, including the upper and lower layers of the floating mat and benthic mat. In total, 50 species of microalgae were found: 40 of them belonged to Bacillariophyta (Bacillariophyceae and Mediophyceae), two to Haptophyta (Coccolithophyceae), four to Miozoa (Dinophyceae), one to Chlorophyta (Zygnematophyceae) and three to Cyanobacteria (Cyanophyceae). Floating layers of the mat differed significantly in species composition from the benthic mat. The total abundance of microalgae and their biomass on Cladophora (per unit area of filaments) varied widely within sampling sites from 3.79 × 106 to 5.04 × 108 cells m–2 and 2.6 to 2197.8 mg m–2. The total mass of microalgae achieved more than 32% of the mass of Cladophora, averaging 7.7% (standard deviation = 13.3).
Introduction
The physical modification of an environment by organisms has been proposed to be among the key mechanisms increasing habitat heterogeneity, inducing and supporting high species richness at different spatial and temporal scales (Jones et al., Citation1994; Wright et al., Citation2002). Some species may significantly directly or indirectly modulate their physical environment, the availability of resources, and/or create habitats for other species (Mills et al., Citation1993; Bond, Citation1994; Zulkifly et al., Citation2012; Graham et al., Citation2015; Shadrin et al., Citation2018). Among these are Cladophora spp., which belong to one of the largest genera of green algae, found in fresh, marine and hypersaline water bodies worldwide (Dodds & Gudder, Citation1992; Higgins et al., Citation2008; Boedeker et al., Citation2016; Prazukin et al., Citation2018). Owing to the morphological features of the Cladophora thallus, as well as the ability of these algae to form extensive dense benthic and floating mats, they can significantly modify the physical and chemical parameters of aquatic environments (Prazukin et al., Citation2008; Zulkifly et al., Citation2012, Citation2013; Graham et al., Citation2015; Shadrin et al., Citation2019a). As an example, Cladophora with epiphyte assemblages, releasing extracellular alkaline phosphatase enzyme into the medium, accelerate and stabilize the phosphorus cycle in water bodies (Young et al., Citation2010; Song et al., Citation2017). Cladophora mats also create habitats for various groups of organisms, which can reach high abundance in the mats (Ivanova et al., Citation1994; Kolesnikova et al., Citation2008; Prazukin et al., Citation2018). Many epibiotic microalgae attach to Cladophora filaments, providing food for different groups of animals (Stevenson & Stoermer, Citation1982; Hardwick et al., Citation1992; Marks & Power, Citation2001; Mpawenayo & Mathooko, Citation2005; Malkin et al., Citation2009; Furey et al., Citation2012; Anufriieva et al., Citation2018). The proportion of epiphytic microalgae can reach up to 67% of the total algal mass of Cladophora mats (Stevenson & Stoermer, Citation1982; Zulkifly et al., Citation2013). Many studies have been conducted on epiphytes on Cladophora in freshwater and marine environments (Ryabushko, Citation2013; Zulkifly et al., Citation2012, Citation2013) but epiphytes on Cladophora in saline and hypersaline lakes have attracted much less attention (Nevrova & Shadrin, Citation2005).
In Crimea, the largest peninsula in the Black Sea, there are a large number of hypersaline lakes and lagoons, including Bay Sivash, the world’s largest hypersaline lagoon (Anufriieva et al., Citation2014; Golubkov et al., Citation2018; Shadrin et al., Citation2018). Cladophora spp. constantly or periodically form floating and benthic mats in these lakes and lagoons, occupying large areas (Ivanova et al., Citation1994; Balushkina et al., Citation2009; Prazukin et al., Citation2018; Shadrin et al., Citation2018). The biomass of these mats can be up to more than 2 kg m–2 (wet weight), creating habitats for many animals, with reports of more than 3 million individuals m−2 (Kolesnikova et al., Citation2008; Prazukin et al., Citation2018; Shadrin et al., Citation2018). Microalgae living on Cladophora filaments are a very important component of the diet of invertebrates inhabiting these mats (Anufriieva et al., Citation2018). Despite this, there are few data available on the taxonomic composition and abundance of microalgae living on the mats of the Crimean hypersaline water bodies (Nevrova & Shadrin, Citation2008; Anufriieva et al., Citation2018), and no data are available on the spatial distribution of microalgae in the mats. It has been hypothesized that the abundance of microalgae in the mats is much higher than in the water column. However, there is currently insufficient information on the potential of Cladophora mats to provide habitat for epiphytic microalgae within Crimean hypersaline waters. The objectives of this work were: (1) to identify the taxonomic composition of the epiphytic unicellular algae inhabiting the Cladophora mats in the hypersaline Chersonesskoye lake and evaluate their absolute and relative abundances, (2) to reveal and discuss patterns in the vertical distribution of these algae in floating and benthic mats, and (3) to quantify the role of the mats as a habitat for microalgae and verify or disprove the hypothesis suggested above that the abundance of microalgae in the mats is much higher than in the water column.
Materials and methods
Study area
Comprehensive studies of Lake Chersonesskoye () located near the city of Sevastopol (44°35′09′′N, 33°23′39′′E) were carried out in 2000–2019, and the main results were published (Prazukin et al., Citation2008, Citation2018; Senicheva et al., Citation2008; Shadrin et al., Citation2019b). The lake is shallow with an average depth of 0.38 m, and a maximum of 1.50 m. It is a small lake of 0.05 km2, and a catchment area of 0.92 km2, which is separated from the Black Sea by a narrow spit. There is some spatial gradient of salinity and seasonal fluctuations; the total observed salinity range was from 27 g l–1 to 340 g l–1. The oxygen concentration is spatially and temporally variable, for example, during the daytime it may exceed 200% saturation in the upper layer of the mats and anoxic below the mats on the benthos. The highest animal species richness and abundance of up to 15 × 107 individuals m–3 was observed in the Cladophora floating mats in the summer to autumn period. Macrophytes were represented by six species, five of which belong to Chlorophyta: Cladophora vadorum, C. siwachensis, C. echinus, Ulothrix implexa, Rhizoclonium tortuosum and one to the marine grass Ruppia cirrhosa. Filamentous green algae often form thick benthic and floating mats, in which the purple bacteria Chromatium and Ectothiorhodospira sometimes play an important role. In the winter months, macrophyte vegetation in the lake exists only as a thin strip of Cladophora spp. along the lake coastline, and clumps of R. cirrhosa. In mid-March, Cladophora mats begin to form along the coastline, which by mid-August may occupy up to 60–90% of the lake area. The lake ecosystem can be in several stable states when different groups of primary producers (phytoplankton, mats of green filamentous algae, etc.) play a leading role. The state with developed mats of green filamentous algae was the most stable with mats absent in the summer only three times over 19 years of observation (Prazukin et al., Citation2018).
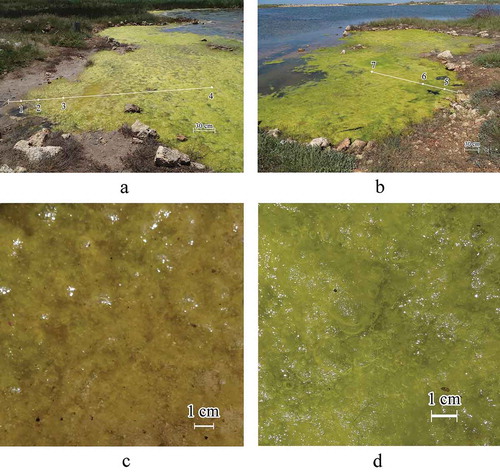
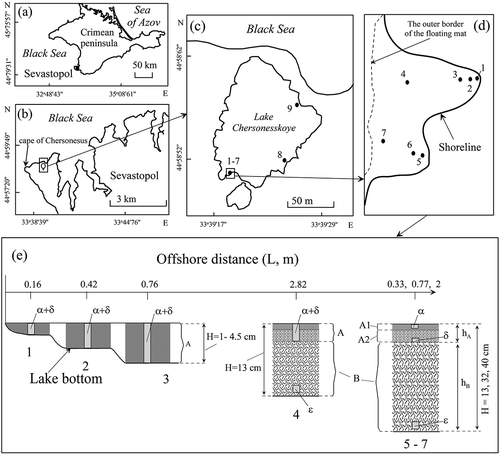
Fig 1. (a, b) The Crimean Lake Chersonesskoye on different scales. (c, d) with the scheme of location of sampling stations where 1–9 is the numbers of sampling stations (stations 1–7 were in 2018 and 8–9 were in 2017). (e) Algal mat layers where A, B are floating and benthic mats, respectively, A1 and A2 are the upper and lower layers of the floating mat, and places of sampling of algae in the mats are defined as: α, in the upper layer of the floating mat; δ, in the lower layer of the floating mat; ε, in the benthic mat. H is the depth in a sampling site where hA, hB represent thickness of the floating and benthic mats, respectively
Sampling and sample processing
To analyse the species structure of epiphytic microalgae, 13 samples of a Cladophora mat were collected at stations at the southern and eastern shores on 26 July 2017, and 13 samples were collected at seven stations in the shallow part of the lake on 29 May 2018 (). Mat samples of 1–2 g (wet weight) were taken with tweezers in triplicate. In 2017, algae samples were taken from the entire depth of the mat without taking into account its vertical structure. In 2018, the vertical structure of the Cladophora mat was considered when taking samples (). At stations 1–3, the thickness of the layered floating Cladophora mat ranged between 1 and 4.5 cm. Algal samples were taken from both layers of the floating mat, the upper (α) and lower (δ) (). At station 4, algal samples were taken from both layers of the floating mat (α + δ) and the benthic mat (ε) (). At stations 5 and 6, a thin 2–3 mm layer of algae were collected from the upper (α) and lower (δ) floating mat and benthic mat near the bottom (ε) (). At station 7, samples were taken only from the upper (α) and lower (δ) layers of the floating mat, because the benthic mat had not formed. On 29 May 2018, samples were taken from stations 1 to 7 to assess the vertical structure of the Cladophora mats. Cladophora was sampled using a cylindrical sampler with a cross-sectional area of Ssect = 0.0452 m2, which made it possible to take algae in layers throughout the water column, as described previously (Prazukin et al., Citation2018). Water temperature and salinity were measured directly in the floating and benthic mats by an electronic thermometer (PHH-830) and refractometer (Kelilong WZ212).
Sample processing in the laboratory
Mat samples were washed in fresh water, dried on filter paper, and weighed on a WT-250 electronic balance (Techniprot, Poland). To determine the dry mass, samples were dried at a temperature of 105°C to a constant weight then weighed on the same balance. Cladophora samples, taken for assessment of epiphytic microalgae, were quickly delivered to the laboratory. The diameter (d, μm) of alive Cladophora filaments was measured using a microscope (Axioskop 40) with eyepiece micrometer at magnifications of 10 × 10 and 10 × 20 (from 50–80 measurements for every sample), and attached microalgae were also examined. After the described operation, microalgae were removed from the Cladophora by a soft synthetic brush. They were washed out into a Petri dish with filtered seawater (membrane filter, pore diameter 1 μm). The entire process was monitored under a microscope. The microalgae from Cladophora filaments were brushed off and washed until a complete absence of microalgae was observed under a microscope on a randomly selected fragment. The resulting suspension of algae was fixed by 96% alcohol, 2 ml per 100 ml of sample. The microalgae were studied using a light microscope (Axioskop 40) at magnifications of 10 × 20, 10 × 40 and 10 × 100. Microphotography of microalgae was carried out using a Canon PowerShot A640 camera and using the AxioVision Rel. 4.6 program at different magnifications. Microalgae abundance values were determined by counting cells using a light microscope in a Goryaev chamber with a volume of 0.9 mm3, in triplicate. To determine the wet weight of Cladophora fragments (Wf) without microalgae, Cladophora fragments were dried on filter paper and weighed on a balance. To determine the dry weight, these samples were dried at a temperature of 105°C to constant weight and then weighed. The authors follow the current classification of algae according to the AlgaeBase website (Guiry & Guiry, Citation2020).
Calculation of values and statistical data processing
Based on the data obtained, the values were calculated in multiple ways. First, as the concentration of algae biomass (Cladophora or microalgae) in water volume occupied by a mat at different sites of sampling according to Equationequation 1(1)
(1) (Prazukin, Citation2015; Prazukin et al., Citation2018),
where CW = the dry algae biomass per volume unit of the mat (mg dry weight m–3), Wf = the dry weight of the mat sample (mg), and Vmat = mat volume (m3). The value of Vmat was calculated by Equationequation 2(2)
(2) ,
where Vmat = the volume of the floating or benthic mat, respectively (m3), Ssect = the cross-sectional area of the cylindrical sampler equal to 0.0452 (m2); H = the thickness of the floating or benthic mat, respectively (m).
Second, the wet Cladophora mass per unit of water surface at the sampling sites was calculated using Equationequation 3(3)
(3) ,
where M = the wet mat mass at the sampling sites, (g wet weight m–2), Wwet = wet weight of mat sample (g), and S0 = surface area from which the sample was taken (m2).
Third, the total surface area of the Cladophora filaments per unit of water surface at the sampling sites was calculated using Equationequation 4(4)
(4) .
where S = the total surface area of the Cladophora filaments per unit of water surface at the sampling sites (m2), SCl = the surface area of the Cladophora filaments in the mat sample (m2), and S0 = water surface area from which the sample was taken (m2). SCl value was calculated using Equationequation 5(5)
(5) ,
where (S/W)Cl = the specific surface area of Cladophora thallus (m2 g–1). The specific surface area of the Cladophora filaments was calculated using Equationequation 6(6)
(6) obtained by simple transformations, provided that the Cladophora mass is equal to its volume, i.e. specific gravity is 1,
where D = the average diameter of the Cladophora filaments (m).
Fourth, the total number of microalgae on Cladophora filaments was determined using Equationequation 7(7)
(7) ,
where N = the total number of microalgae (cells m–2), n = the number of cells in the Goryaev chamber, V = the sample volume (ml), Vk = Goryaev’s chamber volume equal to 0.0009 ml and Sf = total filament area in a sample (m2).
Fifth, the total number of microalgae per unit of water surface at a sampling site was calculated using Equationequation 8(8)
(8) ,
where Nc/S0 = total number of microalgae per unit of water surface at the sampling sites (cells m–2), Nc = the total number of microalgae per unit of Cladophora surface area (cells m–2).
Sixth, the microalgae biomass was calculated using Equationequation 9(9)
(9) ,
where B = biomass (mg m–2), ρ = the specific gravity of microalgae equal to 1.2 × 10–9 mg μm–3 for benthic diatoms (for others it is 1 × 10–9 mg μm–3; Oksiyuk & Yurchenko, Citation1971), and b = the total biological volume of cells in the Goryaev chamber, which were determined following Bryantseva (Citation2005; μm3).
Lastly, the total biomass of microalgae per unit of water surface at a sampling site was calculated using Equationequation 10(10)
(10) ,
where Btot = total microalgae biomass per unit of water surface at a sampling site (g m–2) and Bf = the total biomass of microalgae per unit of Cladophora surface area (g m–2).
Calculation of average values, their standard deviations (SD), correlation (R), and variability (CV) coefficients, as well as parameters of regression equations (Exсel 2007, Microsoft, Washington, USA). Significant differences in means were investigated using Student’s t-test (Müller et al., Citation1979). To calculate a set of the accumulation curves, random permutations of samples were conducted online (http://www.webcalculator.co.uk/statistics/rpermute3.htm). To compare the species composition of unicellular algal communities, the Jaccard and Czekanowski-Sørensen-Dice similarity indices were calculated using Equationequations 11(11)
(11) and Equation12
(12)
(12) (Semkin, Citation2009),
where KJ and KCSD = Jaccard and Czekanowski-Sørensen-Dice similarity coefficients, respectively, c = number of species common to both plots or periods, a = number of species found in the first case, e = number of species found in the second case. The threshold values allowing us to conclude that the species composition is similar are 0.42 (Jaccard) and 0.59 (Czekanowski-Sørensen-Dice) (Semkin, Citation2009). Using data from (Supplementary table 1), a cluster analysis was done by calculating Euclidean distances between samples and creating a dendrogram (STATISTICA 6, TIBCO Software Inc., California, USA).
Results
Temperature and salinity within the mat
In 2017, on 26 July, salinity was 67 g l–1 at one sampling site (station 9) and 71 g l–1 at another one (station 8). The temperature ranged between 31–32°C. In 2018, the air temperature on the mat surface varied between 18–19.8°C at different stations. Within the floating mat, at a depth of 1–1.5 cm, the temperature was slightly higher at 20–20.2°C while at a depth of 15–20 cm it was 18°C. Water salinity within the floating mats was 30.8 g l–1 at a depth of 1.5 cm at station 4 and above 26.5 g l–1 at stations 5 and 6 at a depth of 1.5 cm. Salinity in the benthic mat was 27 g l–1 at a depth of 12–30 cm.
Mat structure
The spatial structure of both the floating and benthic Cladophora mats depends on water depth and distance from the water’s edge () as can be seen from the data. At stations 1–3 where the water was shallow, the 1–4.5 cm Cladophora mat filled the water column with no differentiation between floating and benthic mat (). Dry Cladophora mass per unit of mat volume (Cw) at stations 1–3 was 0.66 kg (dry weight) m–3 (SD = 0.172, CV = 0.260). At stations 4 and 6, there was a distinct division between the floating (A) and benthic (B) mats although they were in contact with each other (). Within the 2–3 cm floating mat (A) there were clear divisions between upper and lower layers with the upper layer (A1) being thin, dense and light green and the lower layer (A2) being thick, loose, and dark green. Cw at station 4 was 3.62 kg (dry weight) m–3 (SD = 0.802, CV = 0.22), and at station 6 it was 6.32 kg (dry weight) m–3 (SD = 2.123, CV = 0.34). The benthic mat was formed by dark-green Cladophora ‘balls’ freely floating in the water space from the lower boundary of the floating mat to the benthos (). Cw in the benthic mat was 0.13 kg (dry mass) m–3 (SD = 0.106, CV = 0.836) at station 4, and it was only 0.07 kg (dry mass) m–3 (SD = 0.004, CV = 0.052) at station 6 (). Within the entire algal mat, the floating mat accounted for 83.5% of the Cladophora biomass at station 4, and 89.8% at station 6. Note that the average diameter of Cladophora filaments did not significantly differ in different samples and amounted to 24.6 μm (SD = 1.774, CV = 0.072) or 2.45 × 10–5 m. The average value of the specific surface area of Cladophora filaments was 0.1626 m2 g–1. The total area of Cladophora filaments in the floating mat per unit of the water surface was significantly larger than in the benthos, averaging 86.4% of the total surface area of the mat (SD = 2.9, CV = 0.034) ().
Table 1. Quantitative characteristics of the Cladophora mat and its epiphytic microalgae in Lake Chersonesskoye during May 2018
Taxonomic composition and spatial variability of microalgae-epibionts
On 26 July 2017, 26 species of microalgae were found on Cladophora at two study sites. There were 23 species of Chromista (Bacillariophyta: Bacillariophyceae and Mediophyceae) and three species of Chromista (Miozoa: Dinophyceae) (). On 29 May 2018, 27 species of unicellular algae were identified on Cladophora filaments, three species of Cyanobacteria (Cyanophyceae), 20 species of Chromista (Bacillariophyta: Bacillariophyceae and Mediophyceae), one species of Chromista (Miozoa: Dinophyceae), two species of Chromista (Haptophyta: Coccolithophyceae) and one species of Plantae (Chlorophyta) (–11, ). The calculated similarity coefficients KJ and KCSD were 0.43 and 0.60, respectively, indicating that there was no significant difference between Bacillariophyta composition in 2017 and 2018. There were only Bacillariophyta and Miozoa in the samples of 2017 and 2018; KJ and KCSD for the sum of these groups were 0.38 and 0.55, respectively, and were lower than the critical values. The total composition of microalgae in these groups differed in 2018 from that in 2017. KJ and KCSD for all microalgae were 0.325 and 0.490, respectively. This indicates that the whole microalgae composition in 2018 differed significantly from that in 2017.
Table 2. Microalgae species composition in the Cladophora mats of Lake Chersonesskoye in different years
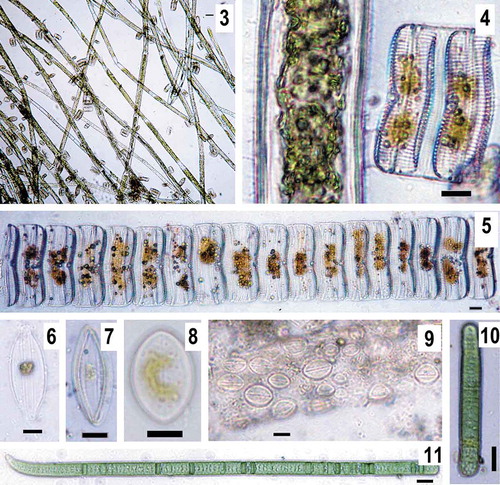
Figs. 3–11. Some abundant species of epiphytic microalgae on Cladophora filaments in Lake Chersonesskoye (May 2018). Fig. 3, 4. Cladophora filaments overgrown with colonies of Achnanthes armillaris. Fig. 5. a colony of A. armillaris. Fig. 6. Halamphora coffeiformis. Fig. 7. Mastogloia braunii. Figs 8, 9. Cocconeis kuyalnitzkensis. Cyanobacteria. Fig. 10. Oscillatoria tenuis. Fig. 11. Phormidium breve. Scale bar for 3 is 50 μm, for 4–11 is 10 μm
In 2018, 13 species (or 48% of the total number of species found) were recorded in one sample only and four species (15% of total species) were found in two samples only (Supplementary table 1). Only two diatom species were present in all samples: Achnanthes brevipes and Cocconeis kuyalnitzkensis. Another diatom species, Halamphora coffeiformis was found in 92% of all samples. Other common species of diatom included Navicula sp. 1 (frequency 77%) and Navicula meniscus (frequency 54%). The dinophyte, Prorocentrum balticum was also a common species (frequency 69%). Only six species of diatoms (A. brevipes, A. armillaris, C. kuyalnitzkensis, H. coffeiformis, N. meniscus, Navicula sp. 1) were found in all the studied sites, regardless of the depth and thickness of the mat. There were 5–15 species per sample (mean = 8). Species richness in the samples taken from the upper layer of the floating mats varied from 5–7, averaging 6.3 (SD = 0.881), and from 6–10, on average 7.7 (SD = 1.770) in the lower layer but was not statistically significant. In the benthic mat, species richness was significantly higher (p = 0.05) than in the floating mat, averaging 10.7 (SD = 4.160) and varying from 5–15. The calculated coefficients of species similarity between different samples are presented in Supplementary table 2. These coefficients showed that the species structure of the microalgae of the upper layer of the floating mat has little variation and could be considered as a single species complex. This was not the case for the lower layer of the floating mat and the benthic mat where there were significant differences between the sampling sites. The species composition of the upper and lower layers of the floating mat was the same at some sites, whereas at others it differed significantly. Both layers of the floating mat were significantly different in species composition from the benthic mat. Vertical heterogeneity was expressed more strongly than horizontal (Supplementary tables 1–2). The results of cluster analysis based on microalgal composition showed that all samples were grouped into two main distant clusters (Supplementary fig. 1). One cluster included two samples taken from the bottom mat at stations 5 and 6. The other cluster was divided into two main subclusters. One of them included both samples from the floating mat at station 7 and the sample from the lower layer of the floating mat at station 6. Another subcluster was composed of all near-shore samples and from the upper layer of the floating mat at station 6. The results of this cluster analysis also support that there were changes in microalgae composition due to depth and distance from shore.
The total abundance and biomass of unicellular algae on Cladophora
The total abundance of microalgae and their biomass on Cladophora (per unit area of filaments) varied widely at sampling sites with abundance from 3.79 × 106 to 5.04 × 108 cells m–2, and biomass from 2.6 to 2197.8 mg m–2 (Supplementary tables 1–3). The average biomass was 6.2 mg m–2 (SD = 53.430) and 21.5 mg m–2 (SD = 193.308) in the upper and lower layers of the floating mat, respectively. In the benthic mat, the average biomass was 2099.5 mg m–2 (SD = 982.745) at stations 5 and 6, but it was significantly lower only with 5.36 mg m–2 at station 4. The proportion of diatoms in the total microalgae biomass varied very little in the upper layer of the floating mat, averaging 98.0% (SD = 0.920, CV = 0.009). In the lower floating mat layer, this fraction was lower and more variable, on average 45.9% (SD = 23.650, CV = 0.515). In the benthic mat at station 4, only diatoms were present, but at stations 5 and 6, the proportion of diatoms in the benthic mat was not high, on average 3.6% (SD = 3.0180, CV = 0.838). The main contribution to the biomass of more than 90%, was by the filamentous cyanobacteria Phormidium breve and Oscillatoria tenuis. In 69% of cases, one diatom species A. brevipes accounted for more than 50% of the total number of microalgae, on average 71% (SD = 9.0), and the most common subdominant was C. kuyalnitzkensis. By biomass A. brevipes dominated in all samples of the upper floating mat layer (75–99% of the total diatom biomass), except for one time when Nitzschia sigma contributed 55% of the total diatom biomass. Among diatoms, A. brevipes also dominated in the lower floating mat layer, accounting for 68–99% of their total biomass. At station 5, in the lower layer of the floating mat, cyanobacterium Gloeocapsa sp. dominated creating 67% of the total microalgae biomass. A. brevipes (89% of the total biomass) also dominated in the benthic mat at station 4. At stations 5 and 6, cyanobacteria O. tenuis dominated (51% of the total microalgae biomass at one station and 78% at the other) and another cyanobacterium P. breve was subdominant (48% and 15% of total biomass), in the benthic mat. A. brevipes dominated at stations 5 and 6, accounting for 56–63% of the total diatom biomass.
The total microalgae abundance on Cladophora per unit of lake surface varied from 0.6 × 108 to 41.5 × 108 cells m–2 (). Their biomass per unit of the benthic area also varied widely from 0.03–14.55 g m–2, and the total mass of microalgae could reach more than 32% of the mass of Cladophora, averaging 7.7% (SD = 13.31, CV = 1.738) ().
Discussion
Cladophora mats
Considering the floating and benthic mats together as a single integrated system, it can be noted that both its various characteristics and the vertical structure are characterized by high spatial and temporal variability in the lake (Prazukin et al., Citation2008, Citation2018). The mat usually begins to form in the spring, gradually increasing its total mass, occupied area, and the degree of vertical differentiation. The general patterns of seasonal changes in the mat in the lake were described earlier (Prazukin et al., Citation2008, Citation2018). During the summer, the area of the projective cover of the mat increases, reaching up to 60–70% of the lake surface, and the total specific biomass of the mat increases reaching up to 4.5–5.5 kg m–2 (wet weight). It should be noted that at the same time in different parts of the lake and in different years, the peak of biomass is reached in different months. In August 2016, in the lake area studied in this research (depth 5–13 cm), the total mass of floating and benthic mats reached 0.7–1.0 kg m–2, although the values obtained in May to June of that year are close to those obtained in May 2018 (). From the compared values, it is seen that from the end of May to August, the total concentration of mat biomass increased by at least 2 to 3 times. In August 2016, the proportion of floating mat varied between 45 and 60%, i.e. it was less than in May 2018. Long-term studies show that there is also high spatial heterogeneity of thickness and density of the floating mat, which could not be explained only by depth and distance from the shoreline.
Taxonomic diversity
In the literature, numbers of microalgal species on Cladophora filaments vary considerably, from 17 to 78 species (Hardwick et al., Citation1992; Mpawenayo & Mathooko, Citation2005; Malkin et al., Citation2009). A year-round study on Lake Huron (USA) revealed 245 species of diatoms on Cladophora (Stevenson & Stoermer, Citation1982). In this study, 40 species of microalgae, including 30 species of diatoms, were identified on Cladophora filaments in 26 samples. Not all species of epibionts that live on Cladophora in the lake have been found. It is well known that the number of species found is largely dependent on the number of samples analysed. This dependence in various taxa may be well approximated by power equations (Shen et al., Citation2003; Brucet et al., Citation2009; Anufriieva et al., Citation2014; El-Shabrawy et al., Citation2018; Sergeeva et al., Citation2019). The equation calculated using obtained (Supplementary fig. 2) data was (R = 0.979, p = 0.0001):
К = 6.246 × N°.564 (13)
where K = the number of species found; N = the number of samples analysed.
It is clear that this sampling order is random, and this equation is true only for this order. Therefore, using our samples, we generated 10 of their random sequences and calculated another 10 equations. Using all these obtained equations, the authors estimated how many species would be found in the analysis of 500 samples. The scatter of the obtained values was wide – from 60 to 301 species, the average predicted value was 164 species (SD = 82.013), which includes more than 100 species of diatoms.
From 2000 to 2001, 79 species of diatoms were found in the analysis of a relatively small number of samples in the hypersaline lakes of Crimea (Nevrova & Shadrin, Citation2008; Anufriieva et al., Citation2017). In that time, in Lake Chersonesskoye, 22 species of diatoms were found on Cladophora at salinity varying from 45–85 g l–1, nine of which were not found in 2017 and 2018 (). The values of the coefficients KJ and KCSD calculated for the pairs ‘2001 vs 2018’ and ‘2001 vs 2018’ were significantly lower than the threshold ones. This means that the species composition of diatoms in different periods was different. Only eight species of diatoms (20% of all found in the lake) were present in all periods (). In 2002, the diatom Thalassionema nitzschioides was the most abundant on Cladophora in the lake (Nevrova & Shadrin, Citation2008). A study between 2016 and 2018 of the intestinal contents of Eucypris mareotica (Crustacea, Ostracoda) from Cladophora mats in Lake Chersonesskoye (Anufriieva et al., Citation2018) revealed 22 microalgal species: four cyanobacteria (three of which were absent from the present study), three Miozoa (none of which were found in the present study), four Haptophyta (three of which were absent from the present study) and 11 Bacillariophyta (five of which were absent from the present study). In autumn 2008, the cyanobacterium Leibleinia willei covered 60% of the Cladophora filament surface (Batogova et al., Citation2009). Approximately 100 species of benthic cyanobacteria were found in the Crimean hypersaline lakes (Shadrin et al., Citation2008; Anufriieva et al., Citation2017). It is assumed that most of these species may be present in the mats of Lake Chersonesskoye at certain periods. In the Black Sea, 37 species of microalgae were found on Cladophora (Ryabushko, Citation2013). In the epiphyton of Cladophora albida 24 diatom species were found in two areas of the sea, while the species composition of the regions varied. In Cossack Bay, next to which there is Lake Chersonesskoye, the diatoms A. armillaris, Berkeleya rutilans, Licmophora abbreviata, Licmophora flabellata, Tabularia tabulata were abundant (Ryabushko et al., Citation2013). In Bay Kalamite, there were Striatella unipunctata, Licmophora gracilis, Craticula halophila, Cylindrotheca closterium, Campylodiscus fastuosus, Nitzschia tenuirostris, Toxonidea insignis (Ryabushko et al., Citation2013). To date, more than 1000 species of benthic microalgae, including ~650 species of diatoms, have been found in the Black Sea (Ryabushko, Citation2013). During severe storms, waves break over into the lake carrying many of these species which then may become epiphytes on Cladophora. Therefore, the mean predicted value of total species richness is most likely to be closest to reality, especially given the wide range of salinity in the lake and the large spotting distribution of most species. Thus, we assume that the 65 species of microalgae found on Cladophora to date are only a proportion of the species that may be found in Cladophora mats in the lake.
An intensive study of the phytoplankton (at three sites monthly) outside the mats from 2000–2006 showed the presence of 61 species of microalgae (Senicheva et al., Citation2008). The two most common species were dinophytes (19 species) and diatoms (15 species). Among the diatoms, benthic forms prevailed and planktonic species were almost completely absent. The comparison indicates that the species composition of microalgae in the mat is fundamentally different from that in the plankton. The species richness of microalgae in mats is higher than that in the plankton.
Quantitative development of microalgae on Cladophora
In the current study, the maximum observed microalgal biomass in Cladophora mats was 14.6 g m–2 of the lake’s surface. Significantly higher values have been noted in this lake, for example, T. tabulata was up to 636.5 g m–2 (water surface) (Nevrova & Shadrin, Citation2005). In other Crimean hypersaline lakes, a high concentration of diatoms on Cladophora mats was also noted, for example in Lake Bakalskoye, Cocconeis euglypta had a biomass of up to 117 g m–2, A. brevipes up to 235 g m–2 and Navicula salinarum up to 65 g m–2 (Nevrova & Shadrin, Citation2005). The relatively high biomass of benthic cyanobacteria noted in this study is not surprising either, with higher numbers previously found on Cladophora in the Crimean hypersaline lakes (Shadrin et al., Citation2008). These values are significantly higher than were previously found in fresh and marine waters (Malkin et al., Citation2009; Gubelit et al., Citation2015). The average biomass of phytoplankton in the lake rarely exceeded 100 g m–3 (Senicheva et al., Citation2008), i.e. at a depth of 0.3 m, this was equal to 33 g m–2 of the lake surface. From a comparison of this value with the maximum biomass of microalgae in Cladophora mats, it can be concluded that microalgae of mats are more abundant and create a greater primary production than phytoplankton. Cladophora mats (Cladophora and its epiphytes) can produce 10–15 times greater primary production than phytoplankton in the Crimean hypersaline lakes (Ivanova et al., Citation1994; Golubkov et al., Citation2018; Prazukin et al., Citation2018). The maximum animal abundance recorded in habitats within this lake was in the Cladophora mats (Kolesnikova et al., Citation2008). This was also found within other hypersaline Crimean water bodies (Ivanova et al., Citation1994; Golubkov et al., Citation2018; Shadrin et al., Citation2019b). The high primary production of Cladophora mats (Cladophora and its epiphytes) creates an energy basis for the existence of a huge number of animals. Perhaps the presence of a large number of microalgae in mats is the main reason for the high concentration of animals in them.
Vertical heterogeneity
Consistent with previous studies (Prazukin et al., Citation2018), we show here that there is a vertically structured complex of epibiotic microalgae in Cladophora mats. Three differentiated layers are apparent: the upper and lower layers of the floating mat and the benthic mat. Vertical zonation in the distribution of epibiotic microalgae on Cladophora was also noted in other studies, for example, diatoms demonstrated this on Cladophora glomerata (Linnaeus) Kützing, 1843 in the Colorado River (Hardwick et al., Citation1992). A developed floating mat, starting in the second half of summer, contributes to the emergence of hard vertical gradients of illumination, temperature, pH, oxygen concentration and other factors (Goldsborough & Robinson, Citation1996; Prazukin et al., Citation2008; Shadrin & Anufriieva, Citation2018). As a result, for example, the temperature at a depth of less than a metre can be more than 5°С lower than the surface temperature. During the daytime, oxygen content may be 200% of saturation in the upper layer of the mat with no oxygen in the benthic mat (Shadrin & Anufriieva, Citation2018). These gradients determine the vertical distribution of microalgae to some extent. Vertical zonation cannot be explained by any one factor although there have been suggestions that this is primarily associated with a change in illumination with depth (Hardwick et al., Citation1992) or the fact that there is a better supply of nutrients near the bottom (Marks & Power, Citation2001; Malkin et al., Citation2009). Various animals, mainly crustaceans and insects, develop in large numbers on the surface and in the depths of the mat (Dodds & Gudder, Citation1992; Salovius & Kraufvelin, Citation2004; Prazukin et al., Citation2018). Selectively eating microalgae, they also affect their quantitative characteristics, species structure, and vertical distribution in a mat (Dodds, Citation1991; Furey et al., Citation2012). The formation of gradients of various factors leads to an increase in the diversity of micro-habitats, thereby contributing to the growth of the species richness of microalgae. The species structure of epibiotic microalgae is therefore characterized by rather high spatial and temporal variability due to the interaction of various abiotic and biotic factors, as well as random causes.
Supplementary fig. 1. The similarity of the samples according to their microalgae composition from different points in Lake Chersonesskoye during May 2018. Position 1 (station 1), the upper layer + lower layer of a floating mat; position 2 (station 2), the upper layer + lower layer of a floating mat; position 3 (station 3), the upper layer + lower layer of a floating mat; position 4 (station 4), the upper layer + lower layer of a floating mat; position 5 (station 4), the benthic mat; position 6 (station 5), the upper layer of the floating mat; position 7 (station 5), the lower layer of the floating mat; position 8 (station 5), the benthic mat; position 9 (station 6), the upper layer of the floating mat; position 10 (station 6), the lower layer of the floating mat; position 11 (station 6), the benthic mat; position 12 (station 7), the upper layer of the floating mat; and position 13 (station 7), the lower layer of the floating mat.
Supplementary fig. 2. The relationship between microalgal species richness and the number of samples assessed for Lake Chersonesskoye during June 2017 and May 2018.
Supplementary table 1. Species composition and abundance of epiphytic microalgae on Cladophora filaments in Lake Chersonesskoye during May 2018.
Supplementary table 2. Similarity coefficients of microalgae species composition on Cladophora filaments between the samples among pairwise comparison, Lake Chersonesskoye during May 2018.
Supplementary table 3. Biomass of epiphytic microalgae on Cladophora filaments in Lake Chersonesskoye during May 2018.
Author contributions
A. Prazukin contributed to design of the study, fieldwork, data analysis, writing of first manuscript draft and editing of the final manuscript; N. Shadrin contributed to the design of the study, data analysis and writing of the first draft and final manuscript; D. Balycheva contributed to fieldwork, microalgae sample processing, their species identification, data analysis and editing of drafts; Yu. Firsov contributed to fieldwork, Cladophora mat sample processing; R. Lee contributed to fieldwork, microalgae sample processing, their species identification; E. Anufriieva contributed to data analysis, and first draft writing and editing of the final manuscript.
TEJP-2019-0151-File009.DOCX
Download MS Word (891.6 KB)TEJP-2019-0151-File008.DOCX
Download MS Word (78 KB)TEJP-2019-0151-File007.DOCX
Download MS Word (20.9 KB)TEJP-2019-0151-File006.DOCX
Download MS Word (14.2 KB)TEJP-2019-0151-File005.DOCX
Download MS Word (20.6 KB)Acknowledgements
The authors are grateful to Dr Bindy Datson (Australia) for her selfless work in improving the English of the manuscript and anonymous reviewers and the editor for helpful suggestions to improve the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2020.1814423
Additional information
Funding
References
- Anufriieva, E., Holynska, M. & Shadrin, N. (2014). Current invasions of Asian Cyclopid species (Copepoda: Cyclopidae) in Crimea, with taxonomical and zoogeographical remarks on the hypersaline and freshwater fauna. Annales Zoologici, 64: 109–130.
- Anufriieva, E.V., Shadrin, N.V. & Shadrina, S. N. (2017). History of research on biodiversity in Crimean hypersaline waters. Arid Ecosystems, 7: 52–58.
- Anufriieva, E.V., Balycheva, D.S., Vdodovich I.V. & Shadrin N.V. (2018). Microalgae in the diet of Eucypris mareotica (Crustacea, Ostracoda) in the hypersaline lake Chersonesskoye (Crimea). Ecologica Montenegrina, 17: 100–104.
- Balushkina, E.V., Golubkov, S.M., Golubkov, M.S., Litvinchuk, L.F. & Shadrin, N.V. (2009). Effect of abiotic and biotic factors on the structural and functional organization of the saline lake ecosystems in Crimea. Zhurnal Obshchei Biologii, 70: 504–514.
- Batogova, E.A., Gerasimova O.V. & Shadrin, N.V. (2009). Cladophora mats as unique communities of hypersaline lakes. In Proceedings of the International Conference of Young Scientists “Actual problems of botany and ecology”. Pidruchniki i posibniki, Ternopil, pp. 17–18 (in Russian).
- Boedeker, C., Leliaert, F. & Zuccarello, G.C. (2016). Molecular phylogeny of the Cladophoraceae (Cladophorales, Ulvophyceae), with the resurrection of Acrocladus Nägeli and Willeella Børgesen, and the description of Lurbica gen. nov. and Pseudorhizoclonium gen. nov. Journal of Phycology, 52: 905–928.
- Bond, W.J. (1994). Keystone species. In Biodiversity and Ecosystem Function (Schulze, E‐D. & Mooney, H.A., editors), 237–253. Springer, Berlin.
- Brucet, S., Boix, D., Gascon, S., Sala, J., Quintana, X.D., Badosa, A., Søndergaard, M., Lauridsen, T.L. & Jeppesen, E. (2009). Species richness of crustacean zooplankton and trophic structure of brackish lagoons in contrasting climate zones: north temperate Denmark and Mediterranean Catalonia (Spain). Ecography, 32: 692–702.
- Bryantseva, Yu.V., Lyakh, A.M. & Sergeeva, A.V. (2005). Calculation of Volumes and Surface Areas of the Black Sea Unicellular Algae. Publishing House of the Institute of Biology of the South Seas, Sevastopol (in Russian).
- Dodds, W.K. (1991). Community interactions between the filamentous alga Cladophora glomerata (L.) Kuetzing, its epiphytes, and epiphyte grazers. Oecologia, 85: 572–580.
- Dodds, W.K. & Gudder, D.A. (1992). The ecology of Cladophora. Journal of Phycology, 28: 415–427.
- El-Shabrawy, G.A., Anufriieva, E. & Shadrin, N. (2018). Tintinnina (Ciliophora) and Foraminifera in plankton of hypersaline Lagoon Bardawil (Egypt): spatial and temporal variability. Turkish Journal of Zoology, 42: 218–229.
- Furey, P.C., Lowe, R.L., Power, M.E. & Campbell-Craven, A.M. (2012). Midges, Cladophora, and epiphytes: shifting interactions through succession. Freshwater Science, 31: 93–107.
- Goldsborough, L.G. & Robinson, G.G. (1996). Patterns in wetlands. In Algal Ecology: Freshwater Benthic Ecosystems (Stevenson, R.J., Bothwell, M.L., Lowe, R.L. & Thorp, J.H., editors), 77–117. Academic Press, New York.
- Golubkov, S.M., Shadrin, N.V., Golubkov, M.S., Balushkina, E.V. & Litvinchuk, L.F. (2018). Food chains and their dynamics in ecosystems of shallow lakes with different water salinities. Russian Journal of Ecology, 49: 442–448.
- Graham, L.E., Knack, J.J., Graham, M.E., Graham, J.M. & Zulkifly, S. (2015). A metagenome for lacustrine Cladophora (Cladophorales) reveals remarkable diversity of eukaryotic epibionts and genes relevant to materials cycling. Journal of Phycology, 51: 408–418.
- Gubelit, Y.I., Makhutova, O.N., Sushchik, N.N., Kolmakova, A.A., Kalachova, G.S. & Gladyshev, M.I. (2015). Fatty acid and elemental composition of littoral “green tide” algae from the Gulf of Finland, the Baltic Sea. Journal of Applied Phycology, 27: 375–386.
- Guiry, M.D. & Guiry, G.M. (2020). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 13 May 2020.
- Hardwick, G.G., Blinn, D.W. & Usher, H.D. (1992). Epiphytic diatoms on Cladophora glomerata in the Colorado River, Arizona: longitudinal and vertical distribution in a regulated river. The Southwestern Naturalist, 37: 148–156.
- Higgins, S.N., Hecky, R.E. & Guildford, S.J. (2008). The collapse of benthic macroalgal blooms in response to self-shading. Freshwater Biology, 53: 2557–2572.
- Ivanova, M., Balushkina, E. & Basova, S. (1994). Structural functional reorganization of ecosystem of hyperhaline Lake Saki (Crimea) at increased salinity. Russian Journal of Aquatic Ecology, 3: 111–126.
- Jones, C.G., Lawton, J.H. & Shachak, M. (1994). Organisms as ecosystem engineers. Oikos, 69: 373–386.
- Kolesnikova, E.A., Mazlumyan, S.A. & Shadrin, N.V. (2008). Seasonal dynamics of meiobenthos fauna from a salt lake of the Crimea (Ukraine). In The Fifth International Conference on Environmental Micropaleontology, Microbiology, and Meiobenthology “EMMM 2008”, Chennai, India, February 17–25, 2008. Abstracts of Papers, Chennai: Univ. of Madras, 2008, pp. 155–158.
- Malkin, S.Y., Sorichetti, R.J., Wiklund, J.A. & Hecky, R.E. (2009). Seasonal abundance, community composition, and silica content of diatoms epiphytic on Cladophora glomerata. Journal of Great Lakes Research, 35: 199–205.
- Marks, J.C. & Power, M.E. (2001). Nutrient induced changes in the species composition of epiphytes on Cladophora glomerata Kütz. (Chlorophyta). Hydrobiologia, 450: 187–196.
- Mills, L.S., Soulé, M.E. & Doak, D.F. (1993). The keystone-species concept in ecology and conservation. BioScience, 43: 219–224.
- Mpawenayo, B. & Mathooko, J.M. (2005). The structure of diatom assemblages associated with Cladophora and sediments in a highland stream in Kenya. Hydrobiologia, 544: 55–67.
- Müller, P.H., Neuman, P. & Storm, R. (1979). Tafeln der mathematischen Statistik. VEB Fachbuchverlag, Leipzig.
- Nevrova, E.L. & Shadrin, N.V. (2005). Benthic diatoms in Crimean saline lakes. Marine Ecological Journal, 4: 61–71 (in Russian).
- Nevrova, E.L. & Shadrin, N.V. (2008). Bottom diatoms of hypersaline reservoirs of Crimea. In Microalgae of the Black Sea: Problems of Biodiversity Preservation and Biotechnological Use (Tokarev, Yu.N., Finenko, Z.Z. & Shadrin, N.V., editors), 112–118. EKOSI-Gidrophyzika, Sevastopol.
- Oksiyuk, O.P. & Yurchenko, V.V. (1971). About the weight of diatoms. Hydrobiological Journal, 7: 116–119 (in Russian).
- Prazukin, A.V. (2015). Ecological Phytosystemology. Pero Press, Moscow (in Russian).
- Prazukin, A.V., Bobkova, A.N., Evstigneeva, I.K., Tankovska, I.N. & Shadrin, N.V. (2008). Structure and seasonal dynamics of the phytocomponent of the bioinert system marine hypersaline lake on cape of Chersonesus (Crimea). Marine Biological Journal, 7: 61–79 (in Russian).
- Prazukin, A.V., Anufriieva, E.V. & Shadrin, N.V. (2018). Cladophora mats in a Crimean hypersaline lake: structure, dynamics, and inhabiting animals. Journal of Oceanology and Limnology, 36: 1930–1940.
- Ryabushko, L.I. (2013). Microphytobenthos of the Black Sea. ECOSI- Gidrophyzika, Sevastopol (in Russian).
- Ryabushko, L.I., Lokhova, D.S. & Strizhak, A.V. (2013). Diatom epiphyton of some species of green algae macrophytes and periphyton of anthropogenic substrates of the Crimean Black Sea coast. Algologia, 23: 419–437 (in Russian).
- Salovius, S. & Kraufvelin, P. (2004). The filamentous green alga Cladophora glomerata as a habitat for littoral macro-fauna in the northern Baltic Sea. Ophelia, 58: 65–78.
- Semkin, B.I. (2009). On the relation between mean values of two measures of inclusion and measures of similarity. Biulleten Botanicheskogo Sada-Instituta DVO RAN, 3: 91–101 (in Russian).
- Senicheva, M.I., Gubelit, Yu.I., Prazukin, A.V. & Shadrin, N.V. (2008). Phytoplankton of hypersaline lakes of Crimea. In Microalgae of the Black Sea: Problems of Biodiversity Preservation and Biotechnological Use (Tokarev, Yu.N., Finenko, Z.Z. & Shadrin, N.V., editors), 93–99. EKOSI-Gidrophyzika, Sevastopol.
- Sergeeva, N.G., Shadrin, N.V. & Anufriieva, E.V. (2019). Long-term changes (1979–2015) in the nematode fauna in Sivash Bay (Sea of Azov), Russia, worldwide the largest hypersaline lagoon, during salinity transformations. Nematology, 21: 337–347.
- Shadrin, N. & Anufriieva, E. (2018). Ecosystems of hypersaline waters: structure and trophic relations. Zhurnal Obshchei Biologii, 79: 418–427 (in Russian).
- Shadrin, N.V., Mikhodyuk, O.S., Naidanova, O.G., Voloshko, L.N. & Gerasimenko, L.M. (2008). Bottom cyanobacteria of the hypersaline lakes of Crimea. In Microalgae of the Black Sea: Problems of biodiversity preservation and biotechnological use (Tokarev, Yu.N., Finenko, Z.Z. & Shadrin, N.V., editors), 100–112. EKOSI-Gidrophyzika, Sevastopol.
- Shadrin, N.V., Anufriieva, E.V., Kipriyanova, L.M., Kolesnikova, Е.А., Latushkin, A.A., Romanov, R.E. & Sergeeva, N.G. (2018). The political decision caused the drastic ecosystem shift of the Sivash Bay (the Sea of Azov). Quaternary International, 475: 4–10.
- Shadrin, N., Kolesnikova, E., Revkova, T., Latushkin, A., Chepyzhenko, A., Drapun, I., Dyakov, N. & Anufriieva, E. (2019a). Do separated taxa react differently to a long-term salinity increase? The meiobenthos changes in Bay Sivash, largest hypersaline lagoon worldwide. Knowledge & Management of Aquatic Ecosystems, 420: 36.
- Shadrin, N., Yakovenko, V. & Anufriieva, E. (2019b). Suppression of Artemia spp. (Crustacea, Anostraca) populations by predators in the Crimean hypersaline lakes: a review of the evidence. International Review of Hydrobiology, 104: 5–13.
- Shen, T.J., Chao, A. & Lin, C.F. (2003). Predicting the number of new species in further taxonomic sampling. Ecology, 84: 798–804.
- Song, C., Cao, X., Zhou, Y. & Shadrin, N.V. (2017). Filamentous green algae, extracellular alkaline phosphatases and some features of the phosphorus cycle in ponds. Marine Biological Journal, 2: 66–78.
- Stevenson, R.J. & Stoermer, E.F. (1982). Seasonal abundance patterns of diatoms on Cladophora in Lake Huron. Journal of Great Lakes Research, 8: 169–183.
- Wright, J.P., Jones, C.G. & Flecker, A.S. (2002). An ecosystem engineer, the beaver, increases species richness at the landscape scale. Oecologia, 132: 96–101.
- Young, E.B., Tucker, R.C. & Pansch, L.A. (2010). Alkaline phosphatase in freshwater Cladophora‐epiphyte assemblages: regulation in response to phosphorus supply and localization. Journal of Phycology, 46: 93–101.
- Zulkifly, S., Hanshew, A., Young, E.B., Lee, P., Graham, M.E., Graham, M.E., Piotrowski, M. & Graham, L.E. (2012). The epiphytic microbiota of the globally widespread macroalga Cladophora glomerata (Chlorophyta, Cladophorales). American Journal of Botany, 99: 1541–1552.
- Zulkifly, S.B., Graham, J.M., Young, E.B., Mayer, R.J., Piotrowski, M.J., Smith, I. & Graham, L.E. (2013). The genus Cladophora Kützing (Ulvophyceae) as a globally distributed ecological engineer. Journal of Phycology, 49: 1–7.