ABSTRACT
The diatom Torgania coronata gen. et sp. nov. from the Brazilian Amazon is described based on light and scanning electron microscopy. Members of this genus have the raphe system entirely located on the valve surface, needle-shaped, isopolar valves with one rimoportula at each apex, and isovalvar frustules. Under the light microscope, members of Torgania can be confused with some species of Fragilaria, such as F. tenera, due to the aciculiform valve outline and inconspicuous striae. We consider that the presence of the raphe on the valve surface prevents Torgania from being placed in the Eunotiaceae. We also consider that Torgania’s placement within Peroniaceae is inadequate due to its isovalvar frustules and isopolar valves. Therefore, we did not include Torgania in this family either. Fragilaria braunii was recently transferred to the genus Peronia due to the discovery of a vestigial raphe system, along with the presence of rimoportulae, and characteristics of the girdle elements. However, in our opinion, this species better fits in the newly proposed Torgania than in Peronia and we therefore propose the new combination, Torgania braunii comb. nov. The characteristics found in the newly proposed genus indicate that the traditional dichotomy between Eunotiaceae/Peroniaceae is no longer appropriate. We argue that the eunotioid clade should be defined mainly by the raphe system non-integrated with the sternum, and that terms such as ‘simple’ or ‘short’ in reference to the raphe should be avoided. In addition, we provide a morphological key for the identification of Eunotiales genera.
Introduction
In molecular phylogenetic studies of the Bacillario-phyceae, the Eunotiales P.C.Silva seems to be a monophyletic order, occupying a basal position within the raphid diatoms (Mayama & Kuriyama, Citation2002; Sims et al., Citation2006; Theriot et al., Citation2010; Medlin, Citation2017). We adopted the classification system proposed by Kociolek et al. (Citation2018), which does not consider the Subclass Eunotiophycidae D.G.Mann, which is the taxonomic rank used to refer to this clade in most of the references cited in this article. Bacillariophycidae refers to the raphid diatoms.
However, since these studies were based on an extremely limited number of taxa mainly restricted to Eunotia C.G.Ehrenberg, the phylogenetic relationships among the genera and families attributed to this clade remain unclear, as is the relationship between phylogeny and morphology. On the other hand, several studies have inferred the phylogenetic relationships within this order based on morphology, reflecting the author’s choice and understanding of character evolution (primitive vs. derived states), homologies or even biogeographic aspects (Hustedt, Citation1926, Citation1952a; Kolbe, Citation1956; Kociolek & Rhode, Citation1998; Vyverman et al., Citation1998; Kociolek, Citation2000, Citation2018; Novitski & Kociolek, Citation2005; Williams & Reid, Citation2006a, Citation2006b, Citation2008; Williams & Kociolek, Citation2010, Citation2017; Burliga et al., Citation2013; Taylor et al., Citation2014; Siver et al., Citation2015; Liu et al., Citation2018).
The presence of a raphe system is considered a synapomorphy of the Bacillariophycidae. The order Eunotiales is commonly defined by the concomitant presence of rimoportulae and a simple raphe – confined to the valve poles, occasionally extending onto the valve mantle (Round et al., Citation1990; Williams & Reid, Citation2006b, Citation2008). The ontogeny of the eunotioid raphe type is only known for the genus Eunotia, in which the completion of the raphe system occurs only in the final stages of valve formation, and the raphe is not integrated with the sternum (Mayama & Kuriyama, Citation2002; Sims et al., Citation2006; Cox, Citation2012).
The relative position of the raphe and the sternum has traditionally been considered a key feature to delimit the two families in Eunotiales (Round et al., Citation1990; Williams & Reid, Citation2008). In Eunotiaceae Kützing, the raphe system is located mainly in the mantle and is not integrated with the sternum (Round et al., Citation1990; Vyverman et al., Citation1998; Williams & Reid, Citation2008; Burliga et al., Citation2013; Taylor et al., Citation2014), whereas in Peroniaceae (Karsten) Topachevs’kyj & Oksiyuk, the raphe slit is entirely located on the valve surface, more or less aligned with the axial area, and the frustules are heterovalvar (Round et al., Citation1990; Vyverman et al., Citation1998; Taylor et al., Citation2014).
Until 2014, Peronia A.de Brébisson & G.A.W.Arnott ex F.Kitton was the only described genus in Peroniaceae. Since then, Actinellopsis J.C.Taylor, B.Karthick & Kociolek, and Sinoperonia Kociolek, Liu, Glushchenko & Kulikovskiy were proposed (Taylor et al., Citation2014; Liu et al., Citation2018). Recently, ultrastructure analysis of two Amazonian species, originally assigned to Fragilaria H.C.Lyngbye, showed the presence of a vestigial raphe, and these species were transferred to genera in the Eunotiales clade (Costa et al., Citation2017; Wetzel & Kociolek, Citation2018).
The high diversity of Eunotiales in the Neotropical region is noteworthy. The studies have revealed a very peculiar diversity, which is particularly rich in species of Eunotia and Actinella F.W.Lewis and differs from the diatom diversity known to Europe (Patrick Citation1940a, Citationb; Hustedt, Citation1952a, Citation1965). Since then, the samples collected in the Amazon have again received attention, either by the reanalysis of type materials preserved in the Hustedt’s Diatom Collection (Simonsen, Citation1987; Metzeltin & Lange-Bertalot, Citation1998, Citation2007; Kociolek et al., Citation2001) or by recent samples that resulted in new combinations and the description of new genera and species, mainly of Eunotia and Actinella (Wetzel et al., Citation2010, Citation2011, Citation2012; Burliga et al., Citation2013; Costa et al., Citation2017; Wetzel & Kociolek, Citation2018). Also, the comprehensive list of freshwater diatom genera distribution by Kociolek (Citation2018) showed a high level of endemism of Eunotiales genera of South America. These studies have confirmed that the Amazon region is an important diversity hotspot for Eunotiales in the world. Research in other historically under-sampled tropical regions such as Africa and Southeast Asia, suggests that the diversity within this order must be even greater than previously thought (Taylor et al., Citation2014; Liu et al., Citation2018).
In this paper, we describe Torgania coronata gen. et sp. nov., based on a unique set of character states within the Eunotiales. In addition, we discuss phylogenetic aspects of this order based on raphe system morphology and ontogeny and propose the transfer of Peronia braunii (Hustedt) L.F.Costa, C.E.Wetzel & D.M.Williams, a widespread species in Amazonian waters, to this new genus. A morphological identification key for the genera of Eunotiales is also provided.
Materials and methods
Study area
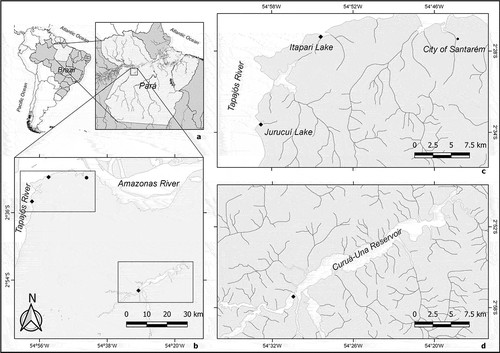
Samples of periphyton were collected from lentic environments, during the execution of two projects performed in a region close to Santarém city, Central Amazon, Pará state, Brazil. One project was conducted in streams (‘igarapés’) and floodplains along the Tapajós riverbanks, such as the Itapari and Jurucuí lakes (Canani et al., Citation2018), located in the ‘Alter do Chão’ environmental preservation area, eastern Santarém (). The other project was carried out in the reservoir of the Curuá-Una hydroelectric plant, south-eastern Santarém, which is elongated and narrow, with an area of 102 km2 and a volume of 400 hm3 (Junk et al., Citation1981). It receives water from three tributary rivers, which have their springs inside or in the surrounding area of the Tapajós National Forest (FLONA Tapajós).
Fig. 1. Study area indicating the location of Pará State in Brazil and in South America (a), the region near the city of Santarém, where the Tapajós River flows into the Amazon River (b), sampling site locations in Jurucuí and Itapari lakes, on the right bank of the Tapajós River (c) and in Curuá-Una reservoir (d). References: •: municipality headquarters; ♦: sampling sites
The type materials of many new araphid taxa and Eunotiales described in the mid-twentieth century (Hustedt, Citation1952a, Citationb, Citation1965) and more recently (Metzeltin & Lange-Bertalot, Citation1998, Citation2007; Kociolek et al., Citation2001; Costa et al., Citation2017; Wetzel & Kociolek, Citation2018) and housed in the Hustedt Collection (BRM, Friedrich Hustedt Diatom Study Center, Alfred-Wegener-Institut Helmholtz-Zentrum für Polar und Meeresforschung, Bremerhaven, Germany), were collected from Lake Jurucuí in the 1940s (Braun, Citation1952).
Sample collection, preparation and analysis
Samples of periphyton were collected from native macrophytes and artificial samplers, placed in the studied sites, by scraping the macrophyte stems and roots and the substrata using a toothbrush. Samples were placed in plastic bottles and fixed with a 4% formaldehyde solution.
Temperature (°C), dissolved oxygen (mg l–1), pH and conductivity (mS cm–1) were measured in the field at the time of sampling using an Oakton® multiparameter probe. Information about the sampling sites is summarized in .
Table 1. Sampling sites, geographic coordinates, sampling dates and substrates sampled
The liquid samples resulting from the scraping of macrophytes and artificial substrata were oxidized with nitric acid (Talgatti et al., Citation2014). Aliquots of the oxidized material were used for preparing permanent slides from each sample with Naphrax® (refractive index 1.74) as a mounting medium, and stubs for scanning electron microscopy (SEM). Permanent slides were observed under a Zeiss Axio Scope and a Zeiss Axio Scope A1 with differential interference contrast (DIC) equipped with a Zeiss Axiocam ERc 5s and a Zeiss Axiocam 506, respectively. The stubs were coated with a 16 nm gold layer using an Emitech K550X sputter coater and observed either under a JEOL JSM-6060 (10 kV, 10 mm WD) or a Zeiss Sigma-VP (10 kV, 8.5 mm WD) scanning electron microscope (SEMs). Plates were prepared using Corel Draw X6 (Corel Corporation, 2013).
Results
The water of sampling sites had high temperatures, were poorly oxygenated with pH acidic to neutral waters, with low electrical conductivity (). The diatom flora found in the samples was typical of acid and oligotrophic waters. Neither of the species addressed in this paper was abundant in the samples. Noteworthy is the high richness of eunotioid taxa in the samples, mainly Eunotia, Actinella and Eunotioforma J.P.Kociolek & A.L.Burliga (three species).
Table 2. Water conditions of sampling sites and their abundant species
Taxonomic account
Torgania L.G.C.Canani & D.M.Talgatti, gen. nov. ()
Figs 2–15. Torgania coronata sp. nov., light microscopy (Figs 9, 11, 15: DIC). Figs 2, 3: girdle view. Figs 4–15: valve view, size diminution series. Scale bar: 10 µm

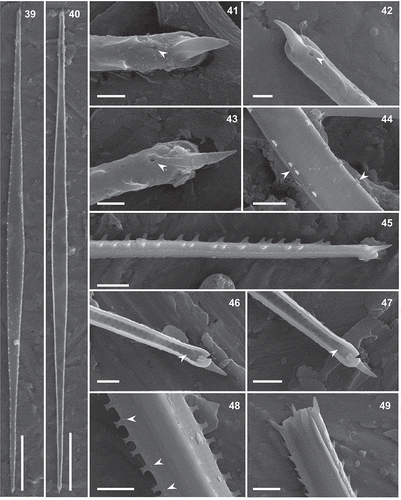
Figs 16–27. Torgania coronata gen. et sp. nov., SEM. Figs 16, 18–22. External view. Figs 17, 23–27. Internal view. Figs 16, 17. Full valves. Fig. 18. Apex with ornamented margin, distal part of raphe slit and external opening of the rimoportula (black arrowhead). Fig. 19. Pole with sigmoid raphe slit entirely located on the valve face and rimoportula (white arrowhead). Fig. 20. Uniseriate striae and ventral sternum (black arrowheads), in the central part of the valve. Fig. 21. Detail of the central part of the valve showing the virgae with wart-shaped structures (white arrowheads) on the valve face and on the valve-mantle junction (black arrowheads), and the striae composed of occluded areolae. Fig. 22. Shallow mantle with one longitudinal row of areolae (arrowhead) and perforated valvocopula (*). Fig. 23. Pole with the valvocopula (*) and two intercalary bands (a, b). Fig. 24. Inner raphe slit and oblique rimoportulae (black arrowhead) at the poles. Fig. 25. Valve apex showing rimoportula (black arrowhead) and the sternum (white arrowhead) Fig. 26. Raised helictoglossa (black arrowhead) at a pole. Fig. 27. Internal view of the ventral sternum and the non-occluded areolae and sternum (white arrowheads). Scale bars: Figs 16, 17: 10 µm, Figs 19, 20, 22, 25, 27: 1 µm, Figs 23, 24, 26: 500 nm, Figs 18, 21: 300 nm
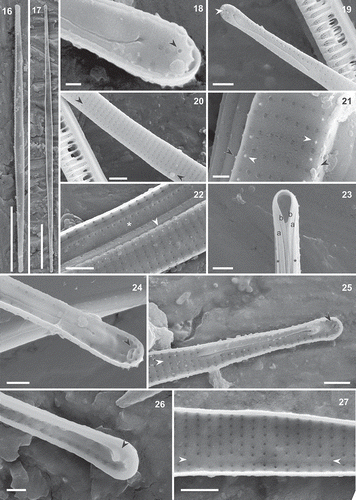
Figs 28–38. Torgania braunii comb. nov., light microscopy (Figs 30, 34, 35: DIC). Figs 28–35. Valve view. Figs 36–38. Girdle view. Figs 31–33 are from the same specimen. Scale bar: 10 µm
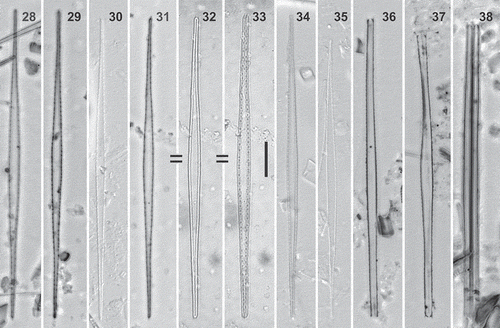
Figs 39–49. Torgania braunii comb. nov., SEM. Figs 39, 41–45, 48, 49t. External valve view. Figs 40, 46, 47. Internal valve view. Figs 48, 49. Girdle views of the same specimen. Figs 39, 40. Full valves. Figs 41–43. Poles bearing a big horn-shaped spine. White arrowheads indicate the vestigial raphe system, which is reduced to a round (Fig. 42) or elongated (Fig. 43) opening, that can be related to a short slit (Fig. 41). Fig. 44. Spatulate spines irregularly spaced in the central valve part (white arrowheads). Ghost striae composed of occluded areolae (see also Fig. 48). Fig. 45. Lateral apex view, showing the aculeate spines on the valve-mantle junction, the big terminal spine and one or two longitudinal rows of pores on the mantle. The vestigial raphe opening is close to the base of the terminal spine. Figs 46, 47. Inner openings of the rimoportula surrounded by conspicuous lips (black arrowheads) and an inner raphe slit. Figs 48, 49. Apparently, this specimen was undergoing cell division; Fig. 49 shows the presence of four terminal spines, the boundaries of the two frustules are hidden under the girdle elements in Fig. 48. Valvocopula with small silica projections along its margins (Fig. 48, white arrowheads), and intercalary bands with tiny protuberances. Scale bars: Figs 39, 40: 10 µm, Figs 44, 45, 48, 49: 2 µm, Figs 46, 47: 1 µm, Figs 41–43: 500 nm
DIAGNOSIS: Frustules biraphid isovalvar. Valves narrowly lanceolate, needle-shaped, isopolar, with subcapitate to narrowly rounded apices. Outer raphe fissure entirely located on the valve face, restricted to the poles. The inner fissure ends in a raised helictoglossa. One rimoportula at each pole. External openings of the rimoportulae round, slit-shaped internally and surrounded by conspicuous lips. Striae parallel, uniseriate, composed of small rounded areolae, which are externally occluded.
GENERITYPE: Torgania coronata L.G.C.Canani & D.M.Talgatti.
ETYMOLOGY: This genus is dedicated to Dr Lezilda Carvalho Torgan, our friend and former advisor, for her dedication in training diatomists and valuable work to improve the knowledge of the Brazilian diatom flora.
Torgania coronata L.G.C.Canani & D.M.Talgatti, sp. nov. ()
DIAGNOSIS and DESCRIPTION: Frustules isovalvar, narrowly rectangular to slightly sinuous in girdle view (, 3). Valves isopolar, needle-shaped, narrowly lanceolate (, 16, 17). Smaller individuals slightly asymmetrical to the apical axis (), larger valves linear (, 5). Margins parallel, with subcapitate to narrowly rounded ends, slightly bent to the dorsal (primary) side of the valve. Length 29.7–159.8 µm; width (at valve centre) 1.4–2.2 µm; length/width ratio 16–80 µm; striae parallel, inconspicuous under the light microscope, 30–40 in 10 µm. Electron microscopy: in external view, there is one small round opening of the rimoportula () at each pole, between the helictoglossa and the valve-mantle edge. Outer raphe fissure sigmoid, entirely located on the valve face, and restricted to the apex. Distal part of the raphe fissure bent to the dorsal side, with an expanded terminal pore placed in the middle of the pole and close to the valve end (, 19). The proximal portion of the raphe fissure is bent to the ventral side, with a simple or slightly expanded central pore (). The sternum runs above the primary side of the raphe slit and becomes more ventral, just after the proximal raphe end, as the number of areolae rows increase towards the middle of the valve. One longitudinal row of areolae between the sternum and the ventral valve margin (). Some virgae have wart-shaped structures (granules) on the valve face and at the valve-mantle edge (). At the valve apices, the projections are longer and sharper (). Striae (60–65 in 10 µm) composed of 1–5 externally occluded areolae (). Mantle shallow, with one longitudinal row of areolae (, 22). Cingulum composed of three bands, valvocopula with one longitudinal row of pores (, 23).
Internal view: rimoportula slit-shaped, one at each apex, oblique to the transapical axis and surrounded by conspicuous lips (). Inner raphe slit almost straight, ending in a raised helictoglossa, flanked by a non-perforated silica fold ventrally and by a longitudinal row of pores dorsally (, 26). Sternum discernible from the proximal raphe end and extending along the ventral valve margin, separated from it by a longitudinal row of pores (, 27). Inner openings of the areolae round; internal occlusions not observed (, 27).
HOLOTYPE: Permanent Slide HSTM-Algas 14332, represented here as , deposited in Herbário HSTM-Algas, Universidade Federal do Oeste do Pará, Pará, Brazil. Periphyton collected from submerged palm tree leafs, Itapari Lake (Santarém, Pará, Brasil, 02°26′32.2′′S, 54°53′58.6′′W), by L.G.C.Canani & R.S.Fraia, on 18 July 2016.
ISOTYPE: Permanent Slide BM 100840, deposited in the Diatom Collection, BM Herbarium, Natural History Museum, London, UK.
TYPE LOCALITY: Brazil. Pará: Santarém, Itapari Lake (right bank of Tapajós River, 02°26′32.2′′S, 54°53′58.6′′W), periphyton on submerged palm tree leafs.
ETYMOLOGY: The epithet coronata refers to the ornamented apices resembling a crown.
Material examined (n = 30): HSTM-Algas 9530, HSTM-Algas 9545, HSTM-Algas 13548.
Torgania braunii (Hustedt) L.G.C.Canani & D.M.Talgatti, comb. nov. ()
BASIONYM: Fragilaria braunii Hustedt, Citation1952, Botaniska Notiser 4: p. 379, .
SYNONYM: Peronia braunii (Hustedt) L.F. Costa, C.E.Wetzel & D.M.Williams, 2017, Fottea, Olomouc, 17(1): –56.
TYPE LOCALITY: Lake Jurucuí, Tapajós River, Pará, Brazil.
DISTRIBUTION: Tapajós River (Pará State, Brazil), Paracuni River (Amazonas State, Brazil) and Curuá-Una River (Pará State, Brazil).
Material examined (n = 26): HSTM-Algas 9530, HSTM-Algas 9545, HSTM-Algas 13548.
Description
Valves linear-lanceolate, needle-shaped, with an expanded central region (, 39, 40). Length 66.3–98.0 µm, width: 2.3–3.6 µm. Valve ends rounded, with one prominent horn-shaped terminal spine at each apex (, 45–47, 49). Valvar margin with spines (, 33–37, 33–37, 39, 44, 45, 48, 49), which are denser and aculeate near the poles (, 43, 45, 49), becoming irregularly spaced and spatulate towards the middle of the valve (, 45, 48). One rimoportula at each pole, with the inner opening slit-shaped, bordered by prominent lips parallel to the apical axis (, 47) and the outer opening hidden by a big terminal spine (, 42, 46). The raphe system at the poles is reduced to a big round pore externally, with or without a small fissure (), and to a short slit, internally (, 47). Striae 30–32 in 10 µm, composed of rounded areolae, externally occluded and often lacking in the middle valve part (ghost striae) (, 48). Frustules rectangular in girdle view, with four or five bands (, 48, 49); mantle with one or two longitudinal row(s) of areolae (). Valvocopulae ornamented with little pores and little projections on the proximal margins (); the intercalary bands with tiny silica protuberances, without pores (, 49). Some aggregate frustules found in the oxidized material (, 49), might correspond to non-separated frustules after vegetative reproduction, or indicate colony formation, with individuals joined by marginal spines as in some species of Fragilaria and Fragilariforma Williams & Round.
Although the apices have a narrowly rounded contour (, 41–43, 46, 47), they seem to be acute under the LM (Costa et al., Citation2017), depending on the focus, due to the presence of terminal spines (). Our specimens have similar morphometry to those described by Costa et al. (Citation2017), but with a slightly wider range of length, width and number of striae in 10 µm. Although the presence of siliceous protuberances in the girdle bands observed herein was not mentioned in the description of Costa et al. (Citation2017), they can be observed in (p. 110).
Discussion
Under the LM, Torgania coronata can easily be mistaken for members of Fragilaria, such as F. tenera (W.Smith) Lange-Bertalot and F. neotropica P.D.Almeida, E.Morales & C.E.Wetzel, due to its needle-shaped outline, inconspicuous striae, and the presence of (sub) capitate apices as in T. coronata. Fragilaria spectra P.D.Almeida, E.Morales & C.E.Wetzel is another morphologically similar taxon but lacks capitate apices. Eunotia enigmatica L.F.Costa & C.E.Wetzel (Costa et al., Citation2017) is also a species with acicular contour. Although large individuals of E. enigmatica are very similar to T. coronata, under the LM the former species has conspicuous striae, which cannot be seen in T. coronata. Moreover, the SEM ultrastructure evidenced that E. enigmatica has an Eunotia-type raphe system (i.e. located on the mantle and with the terminal fissure on the valve surface), whereas the raphe in T. coronata is entirely located on the valve surface ().
Table 3. Main features of Torgania coronata sp. nov., T. braunii comb. nov. and other related taxa with similar valve outlines
Costa et al. (Citation2017) concluded that the individuals illustrated by Metzeltin & Lange-Bertalot (Citation2007, pl 16, ) as Fragilaria cf. nanana belong to E. enigmatica: We consider that the morphometry and valve contour of the specimens illustrated by Metzeltin & Lange-Bertalot (Citation2007) are more related to T. coronata than to E. enigmatica, which has been recorded hitherto only in São Paulo, south-eastern Brazil (Costa et al., Citation2017), whereas the specimens identified by Metzeltin & Lange-Bertalot (Citation2007) as Fragilaria cf. nanana, and T. coronata, were collected at the mouth of the Tapajós River region, in the Lower Amazon River basin.
In their paper, Costa et al. (Citation2017) also reanalysed the type material of T. braunii, which is considered similar to E. enigmatica. Under the LM, the large apical spine of T. braunii gives an acute appearance to the apices and helps its differentiation from T. coronata, in which the apices are narrowly subcapitate and slightly curved toward the dorsal side of the valve. The strongly capitate appearance of the apices in some specimens of T. coronata under the LM (, 8) appears to be a focal artefact, due to the contour of the connective bands that can remain with the valves after the frustule separation in the oxidation process (). Torgania braunii and T. coronata have marginal spines, more developed at the apices. In T. braunii these spines are larger and spatulate and it is not clear if they are homologous structures in those taxa (, 21, 41–45). The vestigial nature of the raphe system in T. braunii hinders its comparison with more developed raphe systems present in Peronia and Torgania, which are respectively straight to slightly curved, and sigmoid, but the isopolar valves (in valve and girdle views) and isovalvar frustules of T. braunni are good arguments in favour of its transfer to Torgania, as these characteristics suggest that this species is unlikely to be a member of Peronia.
The commonly used concepts of the two families of Eunotiales are, in short, that in Eunotiaceae the raphe system is mainly in the mantle, whereas in Peroniaceae the raphe system is positioned on the valve face, and frustules are heterovalvar and heteropolar (Round et al., Citation1990; Williams & Reid, Citation2008; Burliga et al., Citation2013; Taylor et al., Citation2014). The presence of raphe entirely located on the valve surface prevents Torgania from being assigned to Eunotiaceae. In addition, this genus differs from members of Peroniaceae by the presence of isopolar and isovalvar frustules, in valve and girdle view, besides a sigmoid (not straight or arched) outer raphe slit and strongly ventral sternum. The value and/or the use of symmetry as a diagnostic character have been under debate (Sabbe et al., Citation2001; Cox, Citation2012; Siver et al., Citation2015), and we agree that it is not a good one. So, if one emends the diagnosis of the family, taxa that are symmetrical about both axes could be included in Peroniaceae. However, unless the heterovalvar condition is no longer considered also as a diagnostic character, Torgania might not be part of Peroniaceae as well, but is clearly a member of the Eunotiales, because of its raphe system.
Among the Eunotiales, the ontogeny of the raphe system has only been documented for Eunotia (Mayama & Kuriyama, Citation2002; Cox, Citation2012). In relation to the raphe ontogeny in Eunotia, we would like to underline that (1) there is a delay between the formation of the main part of the valve (sternum plus subtended ribs) and the raphe, so (2) the primary side of the raphe system is formed below and by the fusion of a defined (?) number of ventral virgae, which in turn determines the length of the raphe slit, and (3) along its length, the raphe slit will be as far from the sternum as the length of the early formed ventral virgae, and joined to it only by its distal end. Altogether, this will reflect in the typical eunotioid raphe morphology, (4) being the ventral position of the sternum related to the degree of the uneven growth of the pattern centre´s virgae.
Thus we have two possibilities: (1) all the recognized Eunotiales have the same kind of raphe ontogenesis (their raphes are homologous structures) and form a monophyletic clade, or (2) there are misplaced taxa within the Eunotiales and the order (in its current composition) is paraphyletic. Of course, the definitive answer depends on observation, but given the raphe characteristics observed in some genera, such as Actinella and Perinotia D.Metzeltin & H.Lange-Bertalot, it is possible that the same process might occur in other Eunotiaceae genera. For the members of the Peroniaceae this is not so obvious, but it is also plausible, if one interprets the peronioid (or any other eunotioid) raphe and sternum morphologies as a consequence of variation of the steps 1–4 underlined above, that is, temporal separation of sternum and raphe synthesis plus characteristics of the virgae subtended to the sternum and those subtended (when present) to both the raphe rims.
In many publications the Eunotiales are defined by the presence of rimoportulae along with a simple raphe – confined to the valve poles, occasionally extending onto the valve mantle, following Round et al. (Citation1990), sometimes with little modifications. In agreement with the considerations pointed out by Williams & Kociolek (Citation2010), ‘simple raphe’ is rather an imprecise description than a character itself. Moreover, given the documented variation of this set of characters among the Eunotiales, it turns out that not all of its recognized members are encompassed by that definition, such as Eunophora W.Vyverman, K.Sabbe & D.G.Mann or Sinoperonia (Vyvermann et al., Citation1998; Liu et al., Citation2018). Although the presence of rimoportulae along with the raphe system is a remarkable feature of the eunotioids, Kociolek (Citation2000) stated that ‘this suite of features cannot be used to diagnose the Eunotiaceae … ’. Williams & Reid (Citation2006b) also stressed that the concept of the Eunotiales should be expanded, and others mentioned the non-integration of the raphe and the sternum within this group (Sabbe et al., Citation2001; Vyverman et al., Citation1998). Thus, it seems more appropriate to define the Eunotiales, based on the valve ontogeny, as raphid diatoms that have a raphe system non-integrated with the sternum (see Cox, Citation2012), avoiding the use of the terms ‘simple’ or ‘short’ to characterize the raphe slits. This approach clearly separates the Eunotiales from other raphid diatoms (which have the raphe-sternum), merits neither the raphe length (restricted or not to the poles) nor its position (on the mantle or on the valve face), and does not qualify its complexity. The proposed diagnosis for Eunotiales can be applied even in the particular case of Sinoperonia, in which specimens with rapheless frustules occur, since this doesn’t change the fact that the raphe system is still present in the genus (in 75% of the valves; Liu et al., Citation2018). This definition implies in the acceptance of the homology of the eunotioid raphe type, which is the final product of the raphe ontogeny process.
Keeping up with all taxonomic updates is no easy task considering the large number of new taxa and new combinations being proposed every year. Thus, we have worked out an identification key for generaFootnote1 that can be a starting point to those who do not routinely work with the Eunotiales.
(1) Valves symmetrical to the transapical axis ……………………………………………….... 2
(1) Valves asymmetrical to the transapical axis …… ……………………………………….... 12
(2) Valves with subtle dorsiventrality or dorsiventrality absent ………………………………. 3
(2) Valves with marked dorsiventrality……….. 5
(3) Valve width > 15 µm, with cuneate to sub-rostrate ends …………………………………….. Bicudoa
(3) Valve width < 5 µm ………………………… 4
(4) Valves narrowly lanceolate, needle-shaped, width 1.4–3.6 µm, ends narrowly rounded, striae not or barely distinguishable under the LM ………………………….. Torgania
(4) Valves linear-lanceolate, width 3.6–4.5 µm, ends protracted, subcapitate, striae distinguishable under the LM …… Burliganiella
(5) Amphoroid frustule …………………………. 6
(5) Non-amphoroid frustule (dorsal and ventral mantle of equal width) ……………………… 8
(6) Rimoportulae lacking ….Colliculoamphora
(6) Rimoportulae present …………………… 7
(7) Raphe slits long, with a central nodule ……… ………………………………………. Eunophora
(7) Raphe slits short, restricted to the poles …… ……………………………………. Amphorotia
(8) Undulations at the dorsal and ventral margins …………………………. Amphicampa
(8) Otherwise ………………………………... 9
(9) Raphe slits mostly on the valve face, U-shaped ……………………... Eunotioforma
(9) Raphe slits mostly on the mantle ……………10
(10) Strongly arched valves, external ribs developed into spine-like projections, rimoportula absent ………….. Semiorbis
(10) No external ribs developed into spine-like projections ……………………………. 11
(11) Multiseriate striae near the dorsal margin (deltoid dorsal depressions) ……… Perinotia
(11) Uniseriate striae throughout the entire valve surface ………………………………. Eunotia
(12) Raphe slits only at the poles, mainly on the mantle …………………….. Actinella
(12) Raphe slits of different lengths entirely placed on the valve face …………….. 13
(13) Valves asymmetrical to the apical axis ……… …………………………………... Actinellopsis
(13) Valves symmetrical to the apical axis ……. 14
(14) Only biraphid frustules ………… Peronia
(14) Either mono, bi or araphid frustules …. …………………………………. Sinoperonia
We consider that the eunotioid raphe ontogeny, which results in the non-integration of raphe and sternum, is a plausible synapomorphy for the Eunotiales and that it could produce all the variation concerning position and size of those valve elements, but the observation of this process in valves of different genera is necessary. The reduction or complete loss of the raphe found in some eunotioid taxa, understood as derived, indicates that among the ‘araphids’ there are probably hidden members of the Eunotiales clade. Molecular studies will undoubtedly help elucidate phylogenetic relationships within Eunotiales, but the isolation in axenic culture of species belonging to different genera followed by the publication of the results will probably take several years. Until then, the reconstruction of phylogenetic hypotheses based on the morphology, using well-defined and homologous characters, might be a good and reliable alternative to refine the taxonomy within the paraphyletic genus Eunotia and identify clades which may lead to the breakup of traditional dichotomy between Eunotiaceae and Peroniaceae.
Author contributions
L.G.C. Canani: original concept, sample collection; microscopy analysis, drafting and editing the manuscript; D.M. Talgatti: sample collection; microscopy analysis, manuscript editing; S. Melo: provided the structure and conditions for preparing samples, permanent slides, and light microscopy observations.
Acknowledgements
We thank the Vale Institute (ITV) and Microanalysis Laboratory of the Geosciences Institute of Pará Federal University (UFPA) for the SEM analysis. We are also grateful to Dr Patrick Kociolek and two anonymous reviewers for valuable suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
1. The genus Desmogonium C.G.Ehrenberg in R. Schomburgk is not widely accepted, it changed from status, and was transferred to other genera (mainly Eunotia), many times over time. There is no modern study dedicated to its taxonomy, towards a better definition of its exclusive characters. Thus, we think it is better to not include it in this key.
References
- Almeida, P.D., Morales, E.A., Wetzel, C.E., Ector, L. & Bicudo, D.C. (2016) Two new diatoms in the genus Fragilaria Lyngbye (Fragilariophyceae) from tropical reservoirs in Brazil and comparison with type material of F. tenera. Phytotaxa, 243: 163–183.
- Braun, R. (1952) Limnologische Untersuchungen an einigen Seen im Amazonasgebiet. Schweizerische Zeitschrift Fur Hydrologie, 14: 1–128.
- Burliga, A.L., Kociolek, J.P., Salomoni, S.E. & Figueiredo, D. (2013). A new genus and species in the diatom Family Eunotiaceae Kützing (Bacillariophyceae) from the Amazonian hydrographic region, Brazil. Phytotaxa, 79: 47–57.
- Canani, L.G.C, Fraia, R.S. & Melo, S. (2018). Periphytic Actinella Lewis (Ochrophyta, Bacillariophyceae) species from an Environmental Protection Area in the Brazilian Amazon. Acta Limnologica Brasiliensia, 30: e209.
- Costa, L.F., Wetzel, C.E., Ector, L., Williams, D.M. & Bicudo, D.C. (2017). Eunotia enigmatica sp. nov., a new planktonic diatom from Brazil and the transfer of Fragilaria braunii to the genus Peronia (Bacillariophyceae). Fottea, 17: 103–113.
- Cox, E.J. (2012). Ontogeny, homology, and terminology – wall morphogenesis as an aid to character recognition and character state definition for pennate diatom systematics. Journal of Phycology, 48: 1–31.
- Hustedt, F. (1926). Untersuchungen über den Bau der Diatomeen. I. Raphe und Gallertporen der Eunotioideae. Berichte der Deutschen Botanischen Gessellschaft, 44: 142–150.
- Hustedt, F. (1952b). Neue und wenig bekannte Diatomeen. IV. Botaniska notiser, 4: 366–410.
- Hustedt, F. (1952a). Neue und wenig bekannte Diatomeen. III. Phylogenetische Variationen bei den raphidioiden Diatomeen. Berichte der Deutschen botanischen Gesellschaft, 65: 133–145.
- Hustedt, F. (1965). Neue und wenig bekannte Diatomeen. IX. Süßwasserdiatomeen aus Brasilien, insbesondere des Amazonasgebietes. International Review of Hydrobiology, 50: 391–410.
- Junk, W.J., Robertson, B.A., Darwich, A.J. & Vieira, I. (1981). Investigações limnológicas e ictiológicas em Curuá-Una, primeira represa hidrelétrica na Amazônia Central. Acta Amazonica, 11: 689–716.
- Kociolek, J.P. (2000). Valve ultrastructure of some Eunotiaceae (Bacillariophyceae), with comments on the evolution of the raphe system. Proceedings of the California Academy of Sciences, 52: 11–21.
- Kociolek, J.P. (2018). A worldwide listing and biogeography of freshwater diatom genera: a phylogenetic perspective. Diatom Research, 33: 509–534.
- Kociolek, J.P., Balasubramanian, K., Blanco, S., Coste, M., Ector, L., Liu, Y., Kulikovskiy, M., Lundholm, N., Ludwig, T., Potapova, M., Rimet, F., Sabbe, K., Sala, S., Sar, E., Taylor, J., Van de Vijver, B., Wetzel, C.E., Williams, D.M., Witkowski, A. & Witkowski, J. (2018). DiatomBase. Eunotiales. Accessed at: http://www.diatombase.org/aphia.php?p=taxdetails&id=149400 on 2019-11–07
- Kociolek, J.P., Lyon, D. & Spaulding, S. (2001). Revision of the South American species of Actinella. In Studies on Diatoms (Jahn, R., Kociolek, J.P., Witkowski, A & Compère, P., editors), 131–165. Gantner Verlag K.G., Koenigstein.
- Kociolek, J.P. & Rhode, K. (1998). Raphe vestiges in “Asterionella” species from Madagascar: evidence for a polyphyletic origin of the araphid diatoms? Cryptogamie, Algologie, 19: 57–74.
- Kolbe, R.W. (1956). Zur Phylogenie des Raphe-Organs der Diatomeen: Eunotia (Amphicampa) eruca Ehr. Botaniska Notiser, 109: 91–97.
- Lange-Bertalot, H. & Ulrich, S. (2014). Contributions to the taxonomy of needle-shaped Fragilaria and Ulnaria species. Lauterbornia, 78: 1–73.
- Liu, Y., Kociolek, J.P., Gluschenko, A., Kulikovskiy, M. & Fan, Y. (2018). A new genus of Eunotiales (Bacillariophyta, Bacillariophyceae: Peroniaceae), Sinoperonia, from Southeast Asia, exhibiting remarkable phenotypic plasticity with regard to the raphe system. Phycologia, 57: 147–158.
- Mayama, S. & Kuriyama, A. (2002). Diversity of mineral cell coverings and their formation processes: a review focused on the siliceous cell coverings. Journal of Plant Sciences, 115: 289–295.
- Medlin, L.K. (2017). Evolution of the diatoms: IX. Two datasets resolving monophyletic classes of diatoms are used to explore the validity of adding short clone library sequences to the analysis. European Journal of Phycology, 52: 90–103.
- Metzeltin, D. & Lange-Bertalot, H. (1998). Tropical diatoms of South America I. About 700 predominantly rarely known or new taxa representative of the Neotropical flora. Iconographia Diatomologica, 5: 1–695.
- Metzeltin, D. & Lange-Bertalot, H. (2007). Tropical diatoms of South America II. Special remarks on biogeographic disjunction. Iconographia Diatomologica, 18: 1–876.
- Novitski, L. & Kociolek, P. (2005). Preliminary light and scanning electron microscope observations of marine fossil Eunotia species with comments on the evolution of the genus Eunotia. Diatom Research, 20: 137–143.
- Patrick, R. (1940a). Diatoms of northeastern Brazil. Part I. Coscinodiscaceae, Fragilariaceae and Eunotiaceae. Proceedings of the Academy of Natural Sciences of Philadelphia, 92: 191–227.
- Patrick, R. (1940b). Some new diatoms from Brazil. Notulae Naturae, 59: 1–7.
- Round, F.E., Crawford, R.M. & Mann, D.G. (1990). The Diatoms: Biology & Morphology of the Genera. Cambridge University Press, Cambridge.
- Sabbe, K., Vanhoutte, K., Lowe, R.L., Bergey, E.A., Biggs, B.J.F., Francoeur, S, Hodgson, D. & Vyverman, W. (2001). Six new Actinella (Bacillariophyta) species from Papua New Guinea, Australia and New Zealand: further evidence for widespread diatom endemism in the Australasian region. European Journal of Phycology, 36: 321–340.
- Simonsen, R. (1987). Atlas and Catalogue of the Diatom Types of Friedrich Hustedt. J. Cramer, Berlin & Stuttgart.
- Sims, P.A., Mann, D.G. & Medlin, L.K. (2006). Evolution of the diatoms: insights from fossil, biological and molecular data. Phycologia, 45: 361–402.
- Siver, P.A., Bishop, J., Lott, A. & Wolfe, A.P. (2015). Heteropolar eunotioid diatoms (Bacillariophyceae) were common in the North American Arctic during the middle Eocene. Journal of Micropalaeontology, 34: 151–163.
- Siver, P.A., Hamilton, P.B. & Morales, E.A. (2006). Two new planktic species of Eunotia (Bacillariophyceae) from freshwater waterbodies in North Carolina, U.S.A. Algological Studies, 119: 1–16.
- Talgatti, D., Sar, E.A. & Torgan, L.C. (2014). Haslea sigma (Naviculaceae, Bacillariophyta) a new sigmoid benthic species from salt marshes of Southern Brazil. Phytotaxa, 177: 231–238.
- Taylor, I.C., Karthick, B., Kociolek, J.P., Wetzel, C.E. & Cocquyt, C. (2014). Actinellopsis murphy gen. et spec. nov.: a new small celled freshwater diatom (Bacillariophyta, Eunotiales) from Zambia. Phytotaxa, 178: 128–137.
- Theriot, E.C., Ashworth, M., Ruck, E., Nakov, T. & Jansen, R.K. (2010). A preliminary multigene phylogeny of the diatoms (Bacillariophyta): challenges for future research. Plant Ecology and Evolution, 143: 278–296.
- Vyverman, W., Sabbe, K., Mann, D.G., Kilroy, K., Vyverman, R., Vanhoutte, K. & Hodgson, D. (1998). Eunophora gen. nov. (Bacillariophyta) from Tasmania and New Zealand: description and comparison with Eunotia and amphoroid diatoms. European Journal of Phycology, 33: 95–111.
- Wetzel, C.E., Ector, L., Hoffmann, L. & Bicudo, D.C. (2010). Colonial planktonic Eunotia (Bacillariophyceae) from Brazilian Amazon: taxonomy and biogeographical considerations on the E. asterionelloides species complex. Nova Hedwigia, 91: 49–86.
- Wetzel, C.E., Ector, L., Hoffmann, L., Lange-Bertalot, H. & Bicudo, D.C. (2011). Two new periphytic Eunotia species from the neotropical Amazonian ‘black waters’, with a type analysis of E. braunii. Diatom Research, 26: 135–146.
- Wetzel, C.E., Lange-Bertalot, H., Morales, E.A., Bicudo, D.C., Hoffmann, L. & Ector, L. (2012). Bicudoa amazonica gen. nov. et sp. nov. (Bacillariophyta) – a new freshwater diatom from the Amazon basin with a complete raphe loss in the eunotioid lineage. Phytotaxa, 75: 1–18.
- Wetzel, C.E.M. & Kociolek, J.P. (2018). Burliganiella gen. nov. (Bacillariophyta, Eunotiales): another case of raphe reduction based on the type material of Fragilaria siolii Hustedt. Cryptogamie, Algologie, 39: 1–11.
- Williams, D.M. & Kociolek, J.P. (2010). Classifications of convenience: the meaning of names. Diatom Research, 25: 213–216.
- Williams, D.M. & Kociolek, J.P. (2017). Historical biogeography of diatoms in Australasia: a preliminary assessment. In Handbook of Australasian biogeography (Ebach, M.C., editor), 17–46. CRC Press, Boca Raton.
- Williams, D.M. & Reid, G. (2006a). Amphorotia nov. gen., a new genus in the family Eunotiaceae (Bacillariophyceae), based on Eunotia clevei Grunow in Cleve et Grunow. Diatom Monographs, 6: 1–152.
- Williams, D.M. & Reid, G. (2006b). Fossils and the tropics, the Eunotiaceae (Bacillariophyta) expanded: a new genus for the Upper Eocene fossil diatom Eunotia reedii and the recent tropical marine diatom Amphora reichardtiana. European Journal of Phycology, 41: 147–154.
- Williams, D.M. & Reid, G. (2008). Type material of Peronia fibula: morphology, systematics and relationships. In Proceedings of the Nineteenth International Diatom Symposium (Likhoshway, Y., editor), 141–150. Biopress Limited, Bristol.
