ABSTRACT
Twenty-one samples belonging to species of Spyridia Harvey with uncinate (= hook-shaped) spines on determinate lateral branches were investigated for their morphology with a focus on the structure of determinate branches, and for comparative chloroplast-encoded rbcL and nuclear LSU rDNA sequence analysis to elucidate their taxonomy and phylogeny. Currently, four Spyridia species with uncinate spines are recognized worldwide: S. alternans, S. cupressina, S. horridula and S. hypnoides. Of them, S. hypnoides has been recognized as the most common species with uncinate spines. In this study, we show that S. aculeata needs to be resurrected from S. hypnoides, and recognized as a distinct species based on samples from Israel, Red Sea, near the type locality. Spyridia aculeata is characterized by the spiral arrangement of determinate branches, incompletely corticated determinate branches, naked basal segment of determinate branches, by the presence of only acropetal cortication in the nodes of determinate branches, and by uncinate spines at the terminal node and on the first and second nodes of determinate branches. In our molecular analyses based on rbcL and LSU rDNA, although four Spyridia species with uncinate spines are supported on phylogenetic trees, they are not a monophyletic group. The feature of uncinate spines in Spyridia is recognized as a polyphyletic character. Our phylogenetic analysis using rbcL and LSU rDNA sequences reveals high gene sequence divergence (6.8–7.0% for rbcL and 1.2–1.3% for LSU rDNA) between samples of S. aculeata and S. hypnoides. Therefore, the distribution of S. hypnoides may be restricted to the Indian Ocean, whereas S. aculeata is widely distributed in the Atlantic Ocean including the Mediterranean Sea and the Red Sea. Another Spyridia species with uncinate spines, S. alternans, is recognized as a synonym of S. horridula by our detailed morphological observations of its type specimen.
Introduction
The genus Spyridia was established by Harvey in 1833 based on Adriatic Sea collections of Fucus filamentosus Wulfen Citation1803 [= S. filamentosa (Wulfen) Harvey]. Spyridia is characterized by erect and filamentous thalli with numerous radially arranged lateral branches per axial cell, complete elongate cortication in primary axes, determinate lateral branches corticated only at the node, and tetrasporangia sessile on lateral branch cortical nodes. Currently, 15 Spyridia species are distributed worldwide in temperate to tropical seas.
Historically, 11 Spyridia species with uncinate spines have been recorded, S. aculeata (C.Agardh ex Decaisne) Kützing Citation1843, S. alternans Børgesen Citation1933, S. armata Kützing Citation1847, S. berkeleyana Montagne Citation1849, S. complanata J.Agardh Citation1852, S. cupressina Kützing Citation1849, S. ericoides (Hering) Kützing Citation1847, S. hypnoides (Bory) Papenfuss Citation1968a, S. insignis (J.Agardh) J.Agardh Citation1851, S. horridula F.Schmitz ex J.Agardh Citation1897, and S. tetracantha Kützing Citation1862. After re-examination of type material of species having uncinate spines, including S. aculeata, S. insignis, S. ericoides, S. tetracantha and Thamnophora hypnoides Bory in Bélinger, Papenfuss (Citation1968a) synonymized these taxa as Spyridia hypnoides based on the nomenclatural priority of T. hypnoides. In 2004, John et al. (Citation2004) synonymized S. armata under S. hypnoides, and Wynne (Citation1998) transferred S. complanata to Spyridia hypnoides subsp. complanata (J.Agardh) M.J.Wynne. Agardh (Citation1851) reduced S. berkeleyana to variety rank as S. aculeata var. berkeleyana. Currently, four Spyridia species with uncinate spines on determinate lateral branches are recognized: S. alternans, S. cupressina, S. horridula, S. hypnoides. Of them, Spyridia hypnoides is the most widely reported species with uncinate spines in the world (Yoshida et al., Citation1990; Schneider, Citation2003; John et al., Citation2004; Wynne, Citation2017), while S. alternans, S. cupressina and S. horridula are reported from the Indian Ocean and have a simple nomenclatural histories (Silva et al., Citation1996).
Spyridia hypnoides was described based on a specimen from Cape Comorin, southern India (Bory de Saint-Vincent, Citation1834). It (including synonyms) is characterized by (1) uncinate spines near the tip of the determinate branches, (2) isometric nodal and elongated internodal cells in the main axes, (3) a radial arrangement of indeterminate and determinate branches and (4) incomplete cortication of the determinate branches (Hommersand, Citation1963; Papenfuss, Citation1968a). Spyridia hypnoides has been known for inhabiting temperate to tropical seas (Guiry & Guiry, Citation2020): Europe (Gómez Garreta et al., Citation2001), Atlantic Islands (John et al., Citation2004), South-east Atlantic Ocean (Lawson & John, Citation1987), eastern North America (Schneider & Searles, Citation1991), Caribbean islands (Littler & Littler, Citation2000), South America (Ganesan, Citation1990), Africa (De Clerck et al., Citation2005), Indian Ocean Islands (Silva et al., Citation1996), south-west Asia (Silva et al., Citation1996), south-east Asia (Tsutsui et al., Citation2005), and Pacific Islands (South & Skelton, Citation2003). Additionally, S. aculeata was widely recognized as a cosmopolitan taxon until Papenfuss (Citation1968a) synonymized it as S. hypnoides.
We collected 21 worldwide samples of Spyridia species with uncinate spines and observed type specimens including type material of S. alternans. In this study, we resurrect S. aculeata and synonymize S. alternans under S. horridula based on morphological observation and phylogenetic relationships of rbcL and LSU sequence analysis.
Materials and methods
Samples
Twenty-one samples of Spyridia with uncinate spines were collected from South Africa, India, Israel, Brazil and Guadeloupe from 1993 to 2015 (Supplementary table S1). Of them, samples of S. hypnoides were collected from the type locality, and vouchers of S. aculeata, S. cupressina and S. horridula from near their type localities. The type specimen of S. alternans in New York Botanical Garden was also examined.
Morphological observations
All samples were preserved in 4–5% formalin/seawater for the morphological study and in silica gel for the molecular study. Light microscope observations were made from material stained with 1% aqueous aniline blue acidified with 0.1% diluted HCl. Photomicrographs were taken using an Olympus microscope (BX51TRF, Olympus, Tokyo, Japan) equipped with an Olympus DP71 camera. Voucher specimens used in this study were deposited both in the Seaweed Herbarium at UL Lafayette Lafayette, USA (UL) and in the herbarium of Chosun University (CUK), Gwangju, Korea. Herbarium abbreviations follow Thiers (Citation2020).
Molecular phylogenetic analysis
DNA extraction and PCR amplification
Genomic DNA was extracted from samples preserved in silica gel (Supplementary table S1) using the DNeasy Plant Mini Kit (QIAGEN, Valencia, California, USA), following the manufacturer’s instructions. Chloroplast-encoded rbcL was amplified using the primer combinations F7-R753 and F645-RrbcSstart as listed in Lin et al. (Citation2001), and sequenced with the primers F7, F645, F993, R337, R753, R1150 and RrbcSstart (Freshwater & Rueness, Citation1994; Lin et al., Citation2001; Gavio & Fredericq, Citation2002). Partial fragments of nuclear LSU rDNA were also amplified using the X, W, 28D and 28F primers (Freshwater et al., Citation1999). Their PCR and sequencing protocols were described in Cho et al. (Citation2003). Sequences were determined for both forward and reverse strands using the ABI Prism 3100 Genetic Analyzer (PE Applied Biosystems, Foster City, CA) with the ABI Prism BigDyeTM Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems) at UL Lafayette.
Phylogenetic analysis
New rbcL and LSU rDNA sequences and other sequences obtained from GenBank were compiled (Supplementary table S1). These sequences were manually aligned by eye using the Genetic Data Environment (GDE 2.4) program (Smith et al., Citation1994). Clustal W was used for LSU rDNA sequence alignment and trimming extraneous sequence portions. Members of the tribe Ceramieae, Centroceras clavulatum (C.Agardh) Montagne Citation1846 and Ceramium kondoi Yendo Citation1920, were selected as outgroups for phylogenetic analyses of rbcL and the combined dataset of rbcL and LSU rDNA. Maximum likelihood (ML) analyses were conducted with the RAxML HPC-AVX program (Stamatakis, Citation2014) implemented in the RAxML GUI 1.3.1 interface (Silvestro & Michalak, Citation2012) using a GTRGAMMAI model with 1,000 bootstrap replications. Bayesian inference (BI) was performed with MrBayes v. 3.2.6 software (Ronquist et al., Citation2012) using Metropolis-coupled MCMC and the GTR + Γ + I model. We conducted two runs each with four chains (three hot and one cold) for 10 million generations, sampling trees every 1000 generations.
RESULTS
Taxonomic treatments
Spyridia aculeata (C.Agardh ex Decaisne) Kützing Citation1843 ()
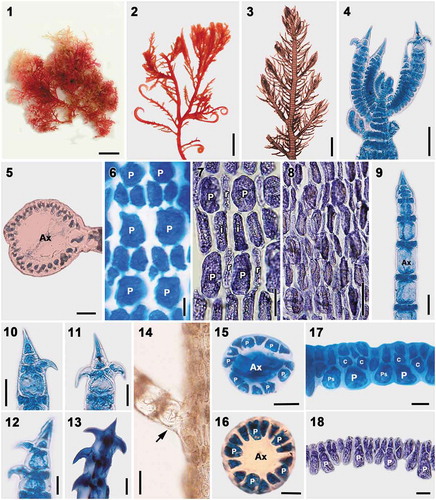
Figs 1–18. Vegetative structures of Spyridia aculeata. Fig. 1. Vegetative thallus. Fig. 2. Upper thallus part showing hooked indeterminate branches. Fig. 3. Upper thallus part showing indeterminate branches pattern. Fig. 4. Apex of young thallus. Fig. 5. Cross-section of main axis. Fig. 6. Cortication pattern of indeterminate branches in upper part of thallus. Fig. 7. Cortication pattern of indeterminate branches in middle part of thallus. Fig. 8. Cortication pattern of indeterminate branches in bottom part of thallus. Figs 9–13. Spine shapes at apex of determinate branches. Fig. 14. Intersection point of determinate branch on main axis lacking cortication (arrow). Figs 15–16. Cross-section of determinate branch. Figs 17–18. Cortication of determinate branch with only acropetal filament. Scale bars: Figs 1, 2 = 0.5 cm; Fig. 3 = 0.5 mm; Fig. 4 = 40 µm; Fig. 5 = 50 µm; Figs 6, 16 = 5 µm; Figs 7–9, 14 = 25 µm; Figs 10–13, 15 = 20 µm; Figs 17–18 = 10 µm
BASIONYM: Ceramium aculeatum C.Agardh ex Decaisne Citation1841.
SYNONYMS: Spyridia berkeleyana Montagne Citation1849, Spyridia armata Kützing Citation1847.
TYPE LOCALITY: Nuweiba, Egypt.
DISTRIBUTION: Brazil, Bermuda, Egypt, Guadeloupe (F.W.I), Gulf of Mexico, Israel, Florida, North Carolina, Spain.
Description
Thalli are epiphytic, erect to entangled, grey-red or brownish, 2–18 cm high, and composed of main axes with indeterminate and determinate branches (), sometimes with hooked apices on indeterminate branches (). Axial cells produce periaxial cells by alternating sequence. All periaxial cells remain at the nodes after axial cell elongation and produce cortical cells (). Cortication in main axes is complete and composed of periaxial cells, internodal cells and rhizoidal filaments. Internodal cells are produced in pairs from each pericentral cell, elongate reaching the next periaxial cells, connecting the periaxial cells in the segment below, and establishing secondary pit connections (). Rhizoidal filaments are derived primarily from internodal cells (). In the basal part of the thallus, rhizoidal cortical cells are cut off from both periaxial and internodal cells, and the cortication of the main axes becomes more complex with inner and outer cortical cells. Indeterminate branches are spirally arranged throughout the axes, produced from periaxial cells developed from each 6–7th segment of the main axes, and have complete cortication. Determinate branches, 377 ± 98 μm long, 59 ± 10 μm wide, are spirally arranged throughout the axes and developed from periaxial cells, terminated with spines, and have incomplete cortication (). Spines are simple or uncinate. One to three uncinate spines are present at the terminal node in determinate branches (–11) with, at times, one or more lateral uncinate spines on the first and second nodes (). The determinate branches have 11–15 cortical nodes only at the segments. Cortication in the determinate branches is 26 ± 5 μm long and composed of periaxial cells and cortical filaments. The basal segment of the determinate branches remains uncorticated (). Five or six periaxial cells are cut off per axial cell (,). Each periaxial cell produces three cortical cells by slightly oblique division in an alternate sequence. The first cortical initial extends longitudinally parallel to the periaxial cells, and becomes a pseudoperiaxial cell that continues to divide in two acropetal cortical filaments (). The other two cortical initials are cut off obliquely from the upper ends of a periaxial cell and develop as acropetal corticating filaments (). Acropetal cortical filaments are 2–3 cells long and lack basipetal cortication ().
Reproductive thalli were not found in our collections.
Spyridia cupressina Kützing Citation1849 ()
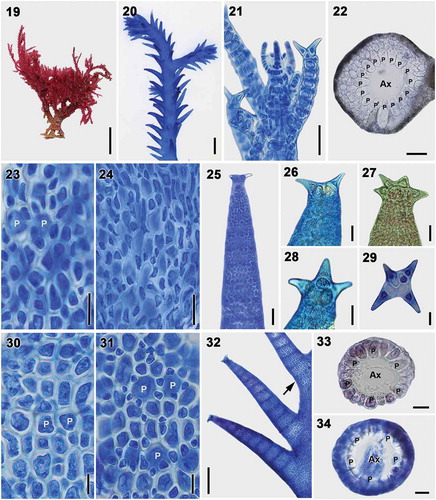
Figs 19–34. Vegetative structures of Spyridia cupressina. Fig. 19. Vegetative thallus. Fig. 20. Upper thallus part showing indeterminate branches. Fig. 21. Apex of young thallus. Fig. 22. Cross-section of the main axis. Fig. 23. Cortication pattern of indeterminate branches in upper part of thallus. Fig. 24. Cortication pattern of indeterminate branches in middle part of thallus. Figs 25–29. Spine shapes at apex of determinate branches. Figs 30–31. Cortication pattern of determinate branches. Fig. 32. Intersection point of determinate branch on main axis having complete cortication (arrow). Figs 33–34. Cross-section of determinate branch. Scale bars: Fig. 19 = 1 cm; Fig. 20 = 500 µm; Figs 21–22, 24–25 = 50 µm; Figs 23, 30–31, 33–34 = 25 µm; Figs 26–29 = 20 µm; Fig. 32 = 200 µm
TYPE LOCALITY: Cape of Good Hope, South Africa.
DISTRIBUTION: Kenya, Madagascar, South Africa.
Description
Thalli are rigid, red or brownish, 2–16 cm high, irregularly branched, composed of main axes with indeterminate and determinate branches (). Axial cells produce 16–17 periaxial cells in alternating sequence. All periaxial cells remain at the nodes after axial cell elongation and produce cortical cells (). Cortication in main axes is complete and composed of periaxial cells, internodal cells and outer cortical cells (). Internodal cells are produced from each periaxial cell, elongate reaching the next periaxial cells, connect with the periaxial cells in the segment below, and develop outer cortical cells. Cortication of the main axes becomes rapidly more complex. Indeterminate branches are irregularly arranged throughout the axes, are produced from periaxial cells, and become completely corticated to immediately below the apices. Determinate branches, 785 ± 107 μm long, 182 ± 41 μm wide, are irregularly arranged in four rows throughout the axes (), develop from periaxial cells, and terminate in spines. Spines are simple or uncinate. Two to four spines are present at the terminal node (). Cortication in determinate branches is complete and composed of periaxial and cortical cells (). The number of segments varies, but mostly consists of 10–13 segments. The basal segments of the determinate branches are always corticated (). Five or six periaxial cells are cut off per axial cell (,). Each periaxial cell produces complete cortication immediately below the apices.
Reproductive thalli were not found in our collections.
Spyridia horridula F.Schmitz ex J.Agardh Citation1897 ()
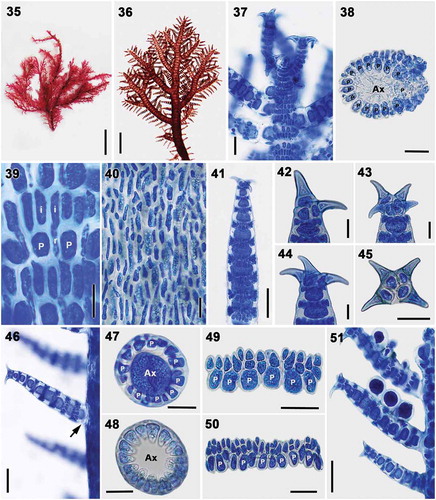
Figs 35–51. Vegetative structures of Spyridia horridula. Fig. 35. Vegetative thallus. Fig. 36. Upper thallus part showing indeterminate branching pattern. Fig. 37. Apex of young thallus. Fig. 38. Cross-section of main axis. Fig. 39. Cortication pattern of indeterminate branches in upper part of thallus. Fig. 40. Cortication pattern of indeterminate branches in middle part of thallus. Figs 41–45. Spine shapes at apex of determinate branches. Fig. 46. Intersection point of determinate branch on main axis lacking cortication (arrow) Figs 47–48. Cross-section of determinate branch. Figs 49–50. Cortication pattern of determinate branches with acropetal filament only. Fig. 51. Determinate branches with tetrasporangia. Scale bars: Fig. 35 = 1 cm; Fig. 36 = 0.5 mm; Figs 37–38, 46 = 50 µm; Figs 39–40, 42–45, 47–50 = 25 µm; Figs 41, 51 = 100 µm; Fig. 32 = 200 µm
TYPE LOCALITY: South Africa (mouth of Kowie River, Port Alfred, Cape Province).
SYNONYM: Spyridia alternans Børgesen Citation1933
DISTRIBUTION: South Africa, Pakistan.
Description
Thalli are epiphytic, erect, grey-red or brownish, mostly compressed, 6–15 cm high, and composed of main axes with indeterminate and determinate branches (). Axial cells produce 14–16 periaxial cells in alternating sequence. All periaxial cells remain at the nodes after axial cell elongation and produce cortical cells (). Cortication in main axes is complete and composed of pericaxial cells, internodal cells and rhizoidal filaments (). The internodal cells are produced in pairs from each pericentral cell, elongate to the level of the next periaxial cells, connect with the periaxial cells in the segment below, and establish secondary pit connections (). Rhizoidal filaments are derived primarily from internodal cells (). In basal part of the thallus, rhizoidal cortical cells are cut off from both periaxial and internodal cells, and the cortication of the main axes becomes more complex. Most indeterminate branches are alternately arranged on each side of the axes, produced from a periaxial cell, and developed from each 6–9th segment of the main axes. Determinate branches are 234 ± 62 μm long, 63 ± 24 μm wide, distichously arranged on each side of the axes, developed from a periaxial cell, and terminated with 2–4 uncinate spines at the terminal node (). Cortication in determinate branches composed of periaxial cells and cortical filaments, encompasses 7–9 cortical nodes, 32 ± 8 μm long, present only at the segments (). The basal segment of the determinate branches remains uncorticated (). Six or seven periaxial cells are cut off each axial cell (). Each periaxial cell produces three cortical cells by slightly oblique division in alternate sequence (). The first cortical initial extends longitudinally parallel to the periaxial cells and becomes a pseudoperiaxial cell that continues to divide in two acropetal cortical filaments. The other two cortical initials are cut off obliquely from the upper ends of periaxial cell and develop as acropetal corticating filaments (). Acropetal cortical filaments are 2–3 cells long and there is no basipetal cortication.
Tetrasporangia are tetrahedral, naked, 40 ± 12 μm wide, and produced from periaxial cells of determinate branches (). Spermatangial and carposporangial thalli were not found in our collections.
Spyridia hypnoides (Bory) Paperfuss 1968a ()
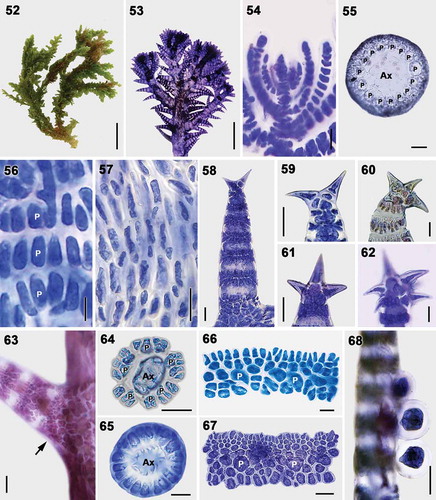
Figs 52–68. Vegetative structures of Spyridia hypnoides. Fig. 52. Vegetative thallus. Fig. 53. Upper thallus part showing indeterminate branching pattern. Fig. 54. Apex of young thallus. Fig. 55. Cross-section of the main axis. Fig. 56. Cortication pattern of indeterminate branches in upper part of thallus. Fig. 57. Cortication pattern of indeterminate branches in middle part of thallus. Figs 58–62. Spine shapes at apex of determinate branches. Fig. 63. Intersection point of determinate branch on main axis becoming completely corticated (arrow). Figs 64–65. Cross-section of determinate branch. Figs 66–67. Cortication pattern of determinate branches with acropetal filament and basipetal filament. Fig. 68. Determinate branches with tetrasporangia. Scale bars: Fig. 52 = 1 cm; Fig. 53 = 0.5 mm; Fig. 54 = 10 µm; Fig. 55 = 100 µm; Fig. 56 = 5 µm; Figs 57, 63 = 50 µm; Figs 58–62, 64–68 = 25 µm
BASIONYM: Thamnophora hypnoides Bory Citation1834.
SYNONYMS: Alsidium ericoides Hering Citation1841, Bindera insignis J.Agardh Citation1841, Spyridia ericoides (Hering) Kützing Citation1847, Spyridia insignis J.Agardh Citation1852, Spyridia tetracantha Kützing Citation1862.
TYPE LOCALITY: Cape Comorin, Tamil Nadu, India.
DISTRIBUTION: Sri Lanka, South Africa, India.
Description
Thalli are epiphytic, erect, grey-red or brownish, bushy, 5–12 cm high, composed of main axes with indeterminate and determinate branches (). Axial cells produce 14–16 periaxial cells in alternating sequence. All periaxial cells remain at the nodes after axial cell elongation and produce cortical cells (). Cortication in main axes is complete and composed of periaxial cells, internodal cells and rhizoidal filaments (). The internodal cells are produced in pairs from each pericentral cell, elongate to the level of the next periaxial cells, connect with the periaxial cells in the segment below, and establish secondary pit connections. Rhizoidal filaments are derived primarily from internodal cells. In basal part of the thallus, rhizoidal cortical cells are cut off from both periaxial and internodal cells, and cortication of the main axes becomes more complex. Indeterminate branches are spirally arranged throughout the axes, produced from periaxial cells in each 6–9th segment of the main axes, and completely corticated to immediately below the apices. Determinate branches are 532 ± 202 μm long, 98 ± 31 μm wide, spirally arranged throughout the axes, developed from periaxial cells, terminating in spines, and with incomplete cortication. Spines are simple or uncinate. Two to five uncinate spines are at the terminal node of determinate branches (). Cortication in determinate branches, composed of periaxial cells and cortical filaments, is 75 ± 15 μm long and encompasses 8–12 cortical nodes only at the segments; however, the basal segment of the determinate branches remains corticated (). Five or six periaxial cells are cut off from axial cell (). Each periaxial cell produces four or five cortical initials by slightly oblique division in alternate sequence. The first cortical initial extends parallel to the periaxial cells where it becomes a pseudoperiaxial cell that continues to divide acropetal and basipetal cortical filaments (). The other cortical initials develop acropetal and basipetal corticating filaments (). Acropetal cortical filaments are small and 4–5 cells long, and basipetal cortical filaments are large and 1–2 cells long ().
Tetrasporangia are tetrahedral, naked, 36 ± 2 μm wide, and produced from periaxial cells of determinate branches (). Spermatangial and carposporangial thalli were not found in our collections.
Molecular phylogenetic analyses
A 1,458 bp portion of the 1,467 bp rbcL gene (99.39% nucleotides sequenced) and partial 840 bp fragment of LSU rDNA were determined from samples of Spyridia species with uncinate spines for assessing their phylogenetic relationships. The interspecific divergences among Spyridia species with uncinate spines for rbcL and LSU rDNA were 4.4–7.7% and 0.2–1.4%, respectively (). The ML and Bayesian tree topologies inferred from the rbcL and combined dataset of rbcL and LSU rDNA sequences were congruent (). Four Spyridia species with uncinate spines were supported on these phylogenetic trees. However, Spyridia species with uncinate spines were not resolved as monophyletic ().
Fig. 69. Phylogenetic tree based on rbcL sequences. Values above branches denote the Maximum likelihood bootstrap values (BS) of > 50% or the Bayesian posterior probabilities (BPP) of > 0.75. BS values of < 50% and BPP values of < 0.75 are indicated by a hyphen (-). BS values of 100 and BPP values of 1 are indicated by *
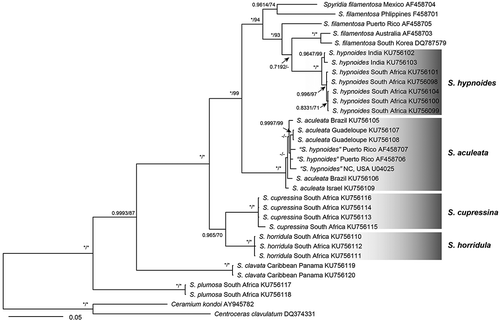
Fig. 70. Phylogenetic tree based on the combined dataset with rbcL and LSU sequences. Values above branches denote the Maximum likelihood bootstrap values (BS) of > 50% or the Bayesian posterior probabilities (BPP) of > 0.75. BS values of < 50% and BPP values of < 0.75 are indicated by a hyphen (-). BS values of 100 and BPP values of 1 are indicated by *
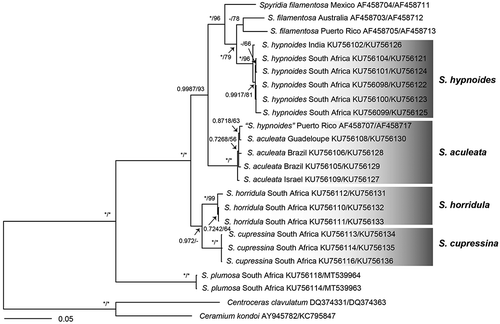
Table 1. Comparisons (rbcL/LSU) of gene sequence divergence value (%) among Spyridia species with uncinate spines
Key to the Spyridia species with uncinate spines
1. Determinate branches completely corticated so that the species name is lined up with others in the keyS. cupressina
1. Determinate branches incompletely corticated2
2. Basal segment of the determinate branches corticated; acropetal and basipetal cortication in determinate branches S. hypnoides
2. Basal segment of the determinate branches naked; acropetal cortication only in determinate branches 3
3. Two-to-four uncinate spines at the terminal node of determinate branches S. horridula
3. One-to-three uncinate spines at the terminal node and additionally one or more lateral uncinate spines on the first and second nodes of determinate branches S. aculeata
DISCUSSION
Spyridia alternans, S. cupressina, S. horridula, and S. hypnoides are accepted in the current literature as species bearing uncinate spines in the terminal portion of their determinate branches; 11 Spyridia species with uncinate spines have been reported worldwide (Guiry & Guiry, Citation2020). Of the synonyms of Spyridia species with uncinate spines, S. aculeata originated as Ceramium aculeatum from Nuweiba, Egypt by Decaisne in 1841, transferred to Spyridia by Kützing in 1843, and considered the same species as S. insignis by Papenfuss (Citation1968b). Spyridia insignis was described as Bindera insignis by J. Agardh in 1841, transferred to Spyridia by J. Agardh in 1852, and included S. ericoides (described as Alsidium ericoides Hering Citation1841) as a synonym (Agardh, Citation1852; Durairatnam, Citation1961). Papenfuss (Citation1968a) agreed that S. insignis and S. ericoides were conspecific after examination of their type materials. Papenfuss (Citation1968a) recognized these species (S. aculeata, S. insignis, S. ericoides) as representing a same species and suggested the combination, Spyridia hypnoides, based on Thamnophora hypnoides, the oldest conspecific taxon of S. insignis. However, we here recognize S. aculeata as a different species from the western Atlantic and the Red Sea. Spyridia aculeata is distinct from clades of S. hypnoides, S. horridula and S. cupressina in our phylogenetic trees based on rbcL and combined data set of rbcL and LSU sequence analyses (). Although Agardh (Citation1897) noted that S. aculeata was similar to S. horridula, it is shown to be distinct from samples of S. horridula by having a spiral arrangement of determinate branches, and uncinate spines at the terminal node and on the first and second nodes of determinate branches. Spyridia aculeata may be distinguished from the other Spyridia species by a spiral arrangement of determinate branches, incompletely corticated determinate branches, a naked basal segment at determinate branches, only acropetal cortication at the nodes of determinate branches, and with uncinate spines at the terminal node and on the first and second nodes of determinate branches. There is also sufficient sequence divergence between S. aculeata and S. hypnoides with regard to rbcL and LSU rDNA (). Therefore, in this study, we resurrect S. aculeata based on both morphology and molecular data.
Cortication has not been known as one of the important characters in the taxonomy of Spyridia because its developmental pattern was recognized as uniform. Vegetative morphological comparisons of S. aculeata, S. cupressina, S. horridula and S. hypnoides () show that these species have complete cortication in the main axes and indeterminate branches. Main axes and indeterminate branches are completely corticated immediately below their apices by small rhizoidal cortical cells covering the internodal corticating cells. However, determinate branches have variable features in the development leading to degree and direction of the corticating filaments, features that may be used as important characters in recognizing species of Spyridia. Spyridia aculeata, S. horridula and S. hypnoides have incomplete cortication by having a cortical band only at the nodes of the determinate branches, whereas S. cupressina has complete cortication in its determinate branches. Among these three species with incomplete cortication, S. hypnoides has cortication in the basal node of the determinate branch, while S. aculeata and S. horridula do not. Also, in the development of cortical filaments in cortical nodes of the determinate branch, S. aculeata and S. horridula have only acropetally corticating filaments, whereas S. hypnoides has predominant acropetal corticating filaments and S. cupressina has acropetal and basipetal corticating filaments equally.
Table 2. Comparison of principal morphological features in determinate branches among Spyridia species with uncinate spines
Although the length, colour, and the number of spines in Spyridia species were used as principal characters to define species by Kützing (Citation1862), these features have been considered as unreliable characters in the literature. In contrast, our observations reveal morphological differences in the arrangement of spines at the nodes with S. aculeata having 1–2 uncinate spines each on the 1st–2nd uppermost nodes of the determinate branch and S. hypnoides, S. horridula and S. cupressina bearing 2–5 uncinate spines only on the first uppermost node of the determinate branch. Although Papenfuss (Citation1968a) synonymized S. aculeata with S. hypnoides after re-examination of type materials, S. aculeata is distinguished from S. hypnoides by the arrangement of spines at the nodes. In our molecular analyses based on rbcL and LSU rDNA, although four Spyridia species with uncinate spines are supported on phylogenetic trees, they are not a monophyletic group (). The phylogeny shows that the feature of uncinate spines in Spyridia is a polyphyletic character.
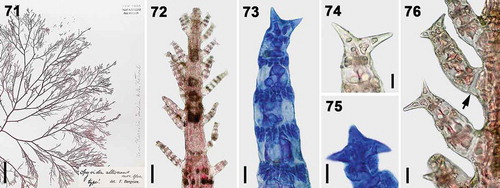
Spyridia alternans was described from Pakistan by Børgesen in 1933 based on the alternating branching feature. In this study, we compared the type material of S. alternans at the New York Botanical Garden with the other Spyridia species with uncinate spines based on the branching pattern and cortication of determinate branches. Spyridia alternans has an alternating branching pattern, incomplete cortication in determinate branches, cortical nodes with only acropetally corticating filaments in the determinate branch, the uncorticated basal node of the determinate branches, and uncinate spines only at the terminal node of the determinate branch (). These features in S. alternans are identical to those in S. horridula. Therefore, although we have not succeeded in sequencing these archival materials for the molecular analysis, S. alternans is here recognized as a synonym of S. horridula based on detailed morphological observations.
Figs 71–76. Vegetative structures of Spyridia alternans from the New York Botanical Garden (NY #922229). Fig. 71. Vegetative thallus. Fig. 72. Apex of young thallus with determinate branches. Fig. 73. Cortication pattern of determinate branch. Figs 74–75. Spine shapes at apex of determinate branches. Fig. 76. Intersection point of determinate branch on main axis lacking cortication (arrow). Scale bars: Fig. 71 = 2 cm; Fig. 72 = 100 µm; Figs 73–74, 76 = 25 µm; Fig. 75 = 20 µm
Spyridia hypnoides has been reported to have a cosmopolitan distribution from warm temperate to tropical oceans (Lawson & John, Citation1987; Yoshida et al., Citation1990; Schneider & Searles, Citation1991; Silva et al., Citation1996; De Clerck et al., Citation2005; Guiry & Guiry, Citation2020). However, morphological features and illustrations of S. hypnoides from the Atlantic and Mediterranean correspond to what we have observed in S. aculeata (Kützing, Citation1862: tab. 50 as S. berkeleyana and S. armata, tab. 51 as S. aculeata; Børgesen, Citation1917: fig. 228 as S. aculeata var. typical, fig. 229 as S. aculeata var. disticha, fig. 230 as S. aculeata f. inermis; Taylor, Citation1960: pl. 66: , as S. aculeata; pl. 71: , as S. aculeata var. hypneoides; Chapman, Citation1963: 179a–b, as S. aculeata var. hypneoides; Schneider & Searles, Citation1991: figs. 485–462). Furthermore, some rbcL GenBank sequences identified as S. hypnoides from Puerto Rico (AF458707 & AF458706) and North Carolina (U04025) are embedded within our findings for S. aculeata. Therefore, the distribution of S. hypnoides appears to be restricted to the Indian Ocean, whereas S. aculeata is widely distributed in the western and eastern Atlantic Ocean, the Caribbean, Mediterranean and Red Sea (). Spyridia aculeata might be widely distributed from the Atlantic to Rea Sea when water masses came from Mediterranean to Red Sea via the Proto-Gulf of Suez between 24 Ma (Chattian–Aquitanian) and 15 Ma (Late Burdigalian–Langhian) (Segev et al., Citation2017; Bialik et al., Citation2019). A population of S. aculeata described in 1843 from Red Sea might be segregated from theMediterranean population by 8 Ma (Tortonian) before the opening of the Suez Canal in 1869.
Fig. 77. Distributions of Spyridia aculeata (![]()
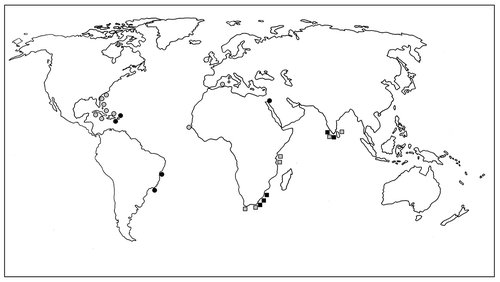
Although S. horridula has been known as an endemic species of South Africa (Silva et al., Citation1996), its distribution is expanded to Pakistan in this study. Spyridia cupressina has been known from the South-west Indian Ocean including South Africa, Kenya and Madagascar (Silva et al., Citation1996).
Supplementary table S1. List of specimen information used in molecular analyses. Additional rbcL and LSU sequences downloaded from GenBank are provided.
Author contributions
B.Y. Won: original concept, experiment, drafting and editing the manuscript; S. Fredericq: original concept, funding and editing the manuscript; T.O. Cho: funding and editing the manuscript.
Supplemental Material
Download MS Word (29 KB)Acknowledgements
We would like to thank Drs Daniela Gabriel and Thomas Sauvage for the collection from the Red Sea and Dr Laura Briscoe for her help communicating type material of S. alternans at the New York Botanical Garden.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2020.1840634.
Additional information
Funding
References
- Agardh, J.G. (1841). In historiam algarum symbolae. Linnaea, 15: 1–50, 443–457.
- Agardh, J.G. (1851). Species genera et ordines algarum, seu descriptiones succinctae specierum, generum et ordinum, quibus algarum regnum constituitur. Volumen secundum: algas florideas complectens. Part 1. pp. [i]–xii, [1]–336 + 337–351 [Addenda and Indices]. Lundae [Lund]: C.W.K. Gleerup.
- Agardh, J.G. (1852). Species genera et ordines algarum, seu descriptiones succinctae specierum, generum et ordinum, quibus algarum regnum constituitur. Volumen secundum: algas florideas complectens. Part 3, fasc.1., pp. 701–786. Lundae [Lund]: C.W.K. Gleerup.
- Agardh, J.G. (1897). Analecta algologica. Continuatio IV. Lunds Universitets Års–Skrift, Andra Afdelningen, Kongl. Fysiografiska Sällskapets i Lund Handlingar, 33(9): 106, 2 plates.
- Bialik, O.M., Frank, M., Betzler, C., Zammit, R. & Waldmann, N.D. (2019). Two-step closure of the Miocene Indian Ocean Gateway to the Mediterranean. Scientific Resports, 9: 8842.
- Børgesen, F. (1917). The marine algae of the Danish West Indies. Part 3: Rhodophyceae (3). Dansk Botanisk Arkiv, 3: 145–240.
- Børgesen, F. (1933). Some Indian Rhodophyceae especially from the shores of the Presidency of Bombay–III. Bulletin of Miscellaneous Information, Royal Botanic Gardens, Kew 1933: 113–142, 20 figs, Pls V–IX.
- Bory de Saint-Vincent, J.B.G.M. (1834). Hydrophytes, Hydrophytae. In Voyage aux Indes-Orientales … pendant les années 1825, 1826, 1827, 1828 et 1829. Botanique. Cryptogamie. (Bélanger, C., editor), 159–178. Paris: Bertrand.
- Chapman, V.J. (1963). The Marine Algae of Jamaica, part 2: Phaeophyceae and Rhodophyceae. Bulletin of the Institute of Jamaica: Science Series, 12: 1–201.
- Cho, T.O., Fredericq, S. & Boo, S.M. (2003). Ceramium inkyuii sp. nov. (Ceramiaceae, Rhodophyta) from Korea; a new species based on morphological and molecular evidence. Journal of Phycology, 39: 237–247.
- De Clerck, O., Tronchin, E.M. & Schils, T. (2005). Red algae. Rhodophyceae. Guide to the seaweeds of KwaZulu-Natal. Scripta Botanica Belgica, 33: 131–267.
- Decaisne, J. (1841). Plantes de l’Arabie Heureuse, recueillies par M.P.-E. Botta et décrites par M.J. Decaisne. Archives du Muséum d’Histoire Naturelle, Paris, 2: 89–199, Pls. V–VII.
- Durairatnam, M. (1961). Contribution to the study of the marine algae of Ceylon. Fisheries Research Station, Ceylon, 10: 181, 32 plates.
- Freshwater, D.W., Fredericq, S. & Bailey, J.C. (1999). Characteristics and utility of nuclear-encoded large-subunit ribosomal gene sequences in phylogenetic studies of red algae. Phycological Research, 47: 33–38.
- Freshwater, D.W. & Rueness, J. (1994). Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia, 33: 187–194.
- Ganesan, E.K. (1990). A Catalog of Benthic Marine Algae and Seagrasses of Venezuela. Caracas: Fondo Editorial Conicit.
- Gavio, B. & Fredericq, S. (2002). Grateloupia turuturu (Halymeniaceae, Rhodophyta) is the correct name of the non-native species in the Atlantic known as Grateloupia doryphora. European Journal of Phycology, 37: 349–359.
- Gómez Garreta, A., Gallardo, T., Ribera, M.A., Cormaci, M., Furnari, G., Giaccone, G. & Boudouresque, C.-F. (2001). Checklist of the Mediterranean seaweeds, III. Rhodophyceae Rabenh., 1: Ceramiales Oltm. Botanica Marina, 44: 425–460.
- Guiry, M.D. & Guiry, G.M. (2020). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. https://www.algaebase.org; searched on 24 March 2020.
- Harvey, W.H. (1833). Div. II. Confervoideae; Div. III. Gloiocladeae. In The English Flora of Sir James Edward Smith. Class XXIV. Cryptogamia. Vol. V. (or Vol. II of Dr. Hooker’s British flora). Part I. Comprising the Mosses, Hepaticae, Lichens, Characeae and Algae (Hooker, W.J., editor), 263–265, 265–266, 326–389, 389–405. London: Longman, Brown, Green & Longmans Paternoster-Row.
- Hering, K. (1841). Diagnoses algarum novarum a cl. Dre. Ferdinand Krauss in Africa Australi lectarum. Annals and Magazine of Natural History [Series 1], 8: 90–92.
- Hommersand, M.H. (1963). The morphology and classification of some Ceramiaceae and Rhodomelaceae. University of California Publications in Botany, 35: 165–366.
- John, D.M., Prud’homme van Reine, W.F., Lawson, G.W., Kostermans, T.B. & Price, J.H. (2004). A taxonomic and geographical catalogue of the seaweeds of the western coast of Africa and adjacent islands. Beihefte zur Nova Hedwigia, 127: 1–139.
- Kützing, F.T. (1843). Phycologia generalis oder Anatomie, Physiologie und Systemkunde der Tange. Mit 80 farbig gedruckten Tafeln, gezeichnet und gravirt vom Verfasser. pp. [part 1]: [i]-xxxii, [1] –142, [part 2:] 143–458, 1, err.], pls 1–80. Leipzig: F.A. Brockhaus.
- Kützing, F.T. (1847). Diagnosen und Bemerkungen zu neuen oder kritischen Algen. Botanische Zeitung, 5: 1–5, 22–25, 33–38, 52–55, 164–167, 177–180, 193–198, 219–223.
- Kützing, F.T. (1849). Species algarum. pp. [i]–vi, [1]–922. Lipsiae [Leipzig]: F.A. Brockhaus.
- Kützing, F.T. (1862). Tabulae phycologicae; oder, Abbildungen der Tange. Vol. XII pp. i–iv, 1–30, 100 pls. Nordhausen: Gedruckt auf kosten des Verfassers (in commission bei W. Köhne).
- Lawson, G.W. & John, D.W. (1987). The marine algae and coastal environment of tropical West Africa (Second Edition). Beihefte zur Nova Hedwigia, 93: 1–415.
- Lin, S.-M., Fredericq, S. & Hommersand, M.H. (2001). Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on large subunit rDNA and rbcL sequences, including the Phycodryoideae, subfam. nov. Journal of Phycology, 37: 881–899.
- Littler, D.S. & Littler, M.M. (2000). Caribbean Reef Plants. An Identification Guide to the Reef Plants of the Caribbean, Bahamas, Florida and Gulf of Mexico. Offshore Graphics, Washington, D.C.
- Montagne, C. (1846). Flore d’Algérie. Ordo I. Phyceae Fries. In: Exploration scientifique de l’Algérie pendant les années 1840, 1841, 1842 … Sciences physiques. Botanique. Cryptogamie, Vol. 1. (Durieu de Maisonneuve, M.C., editor) 1–197. Imprimerie Royale, publiée par ordre du Gouvernement et avec le concours d’une Commission Académique, Paris.
- Montagne, C. (1849). Phyceae. In Exploration scientifique de l’Algérie pendant les années 1840, 1841, … Botanique I(Bory de St Vincent & Durieu de Maisonneuve, editors), fasc. 11, pp. 401–440, pls. 39, 45, 47, 60, 76, 90. Imprimerie Royal, Paris.
- Papenfuss, G.F. (1968a). Notes on South African marine algae. V. Journal of South African Botany, 34: 267–287.
- Papenfuss, G.F. (1968b). A history, catalogue, and bibliography of the Red Sea benthic algae. Israel Journal of Botany, 17: 1–118.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Schneider, C.W. (2003). An annotated checklist and bibliography of the marine macroalgae of the Bermuda Islands. Nova Hedwigia, 76: 275–361.
- Schneider, C.W. & Searles, R.B. (1991). Seaweeds of the Southeastern United States. Cape Hatteras to Cape Canaveral. Duke University Press, Durham, NC.
- Segev, A., Avni, Y., Shahar, J. & Wald, R. (2017). Late Oligocene and Miocene different seaways to the Red Sea-Gulf of Suez rift and the Gulf of Aqaba-Dead Sea basins. Earth-Science Reviews, 171: 196–219.
- Silva, P.C., Basson, P.W. & Moe, R.L. (1996). Catalogue of the benthic marine algae of the Indian Ocean. University of California Publications in Botany, 79: 1–1259.
- Silvestro, D. & Michalak I. (2012). raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution, 12: 335–337.
- Smith, S.W., Overbeek, R., Woese, C.R., Gilbert, W. & Gillevet, P.M. (1994). The genetic data environment: an expandable GUI for multiple sequence analysis. Computer Applications in the Biosciences, 10: 671–675.
- South, G.R. & Skelton, P.A. (2003). Catalogue of the marine benthic macroalgae of the Fiji Islands, South Pacific. Australian Systematic Botany, 16: 699–758.
- Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312–1313.
- Taylor, W.R. (1960). Marine Algae of the Eastern Tropical and Subtropical Coasts of the Americas. University of Michigan Press, Ann Arbor, MI.
- Thiers, B. 2020. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium, Bronx, NY (continuously updated, : http//sweetgum.nybg.org/ih/).
- Tsutsui, I., Huybh, Q.N., Nguyên, H.D., Arai, S. & Yoshida, T. (2005). The Common Marine Plants of Southern Vietnam. Japan Seaweed Association Kochi, Japan.
- Wulfen, F.X. (1803). Cryptogama aquatica. Archiv für die Botanik, 3: 1–64.
- Wynne, M.J. (1998). A checklist of benthic marine algae of the tropical and subtropical western Atlantic: first revision. Nova Hedwigia Beihefte, 116: 1–155.
- Wynne, M.J. (2017). A checklist of benthic marine algae of the tropical and subtropical western Atlantic: fourth revision. Nova Hedwigia Beihefte, 145: 1–202.
- Yendo, K. (1920). Novae algae japoniae. Decas I-III. Botanical Magazine, Tokyo, 34: 1–12.
- Yoshida, T., Nakajima, Y. & Nakata, Y. (1990). Check-list of marine algae of Japan (revised in 1990). Japanese Journal of Phycology, 38: 269–320.
