ABSTRACT
Coralline red algae in the non-geniculate genera Clathromorphum, Phymatolithon and Lithothamnion are important benthic ecosystem engineers in the photic zone of the Arctic and Subarctic. In these regions, the systematics and biogeography of Clathromorphum and Phymatolithon have mostly been resolved whereas Lithothamnion has not, until now. Seventy-three specific and infraspecific names were given to Arctic and Subarctic Lithothamnion specimens in the late 19th and early 20th century by Frans R. Kjellman and Mikael H. Foslie. DNA sequences from 36 type specimens, five historical specimens, and an extensive sampling of recent collections resulted in the recognition of four Arctic and Subarctic Lithothamnion species, L. glaciale, L. lemoineae, L. soriferum and L. tophiforme. Three genes were sequenced, two plastid-encoded, rbcL and psbA, and the mitochondrial encoded COI-5P; rbcL and COI-5P segregated L. glaciale from L. tophiforme but psbA did not. Partial rbcL sequences obtained from type collections enabled us to correctly apply the earliest available names and to correctly place the remainder in synonymy. We were unable to sequence another 22 type specimens, but all of these are more recent names than those that are now applied. It is difficult to identify these species solely on morpho-anatomy as they can all occur as encrusting corallines or as maerl (rhodoliths). We demonstrate the importance of sequencing historical type specimens by showing that the recently proposed North-east Atlantic L. erinaceum is a synonym of one of the earliest published Arctic species of Lithothamnion, L. soriferum, itself incorrectly placed in synonymy under L. tophiforme based on morpho-anatomy. Based on sequenced specimens, we update the distributions and ecology of these species.
Introduction
Coralline algae are important ecosystems engineers worldwide through the formation of extensive and biodiverse cover on hard substrata and as unattached maerl (rhodoliths, Freiwald & Henrich, Citation1994; Foster, Citation2001; Amado-Filho et al., Citation2010; Riosmena-Rodriguez et al., Citation2017). Approximately one-third of the total continental carbonate production takes place in temperate and polar coastal waters with a significant amount coming from coralline algae (Nelson, Citation2009). In cold-water habitats, coralline algae can live for hundreds of years (Freiwald & Henrich, Citation1994; Halfar et al., Citation2013; Adey et al., Citation2015a), providing habitats for other seaweeds (Peña et al., Citation2014a) and for many epibenthic and cryptic macrofauna (Gagnon et al., Citation2012; Teichert, Citation2014). Over the past two decades, surveys have shown that rhodolith beds are widespread in the NE Pacific (Robinson et al., Citation2017), NW Atlantic (Gagnon et al., Citation2012; Copeland et al., Citation2013; Adey et al., Citation2015a), Labrador Sea and Western Greenland (Jørgensbye & Halfar, Citation2017; Schoenrock et al., Citation2018a, b) and the Arctic (Teichert et al., Citation2012, Citation2014); this habitat is clearly much more abundant in Arctic environments than was previously assumed.
Adey & Steneck (Citation2001) identified as Arctic those marine habitats ranging in temperature from ≤ 5°C in summer to ~ −1.5°C in winter, and as subarctic, those experiencing 5–15°C in summer and −1.5 to +1°C in winter. This characterization also applies to the NW Pacific subarctic, but in the NE Pacific subarctic, summer temperatures range from 10–15°C and winter temperatures are −1.5 to ~5°C (based on oceanographic conditions where the species occur). The Arctic and subarctic are warming faster than most of the world’s oceans, but the impact this will have on marine photosynthetic organisms is largely unknown. Wassmann et al. (Citation2011) cited 51 reports of documented changes in the Arctic marine biota in response to ocean warming, but most focused on marine mammals and fish. Two of these papers focused on benthic marine algae, but not on the corallines, the group that provides the dominant benthic cover of seabed habitats in the photic zone (Adey & Hayek, Citation2011). Brodie et al. (Citation2014) projected a significant decrease of coralline algae in the Arctic because anthropogenic carbon dioxide emissions are causing ocean acidification, which in turn results in waters in the photic zone of the Arctic becoming undersaturated with aragonite. Many coralline algae are susceptible to reductions in the concentration of aragonite as this can make seawater corrosive to their high magnesium calcite skeletons, a response that is mediated by the rate of environmental change (Kamenos et al., Citation2013, Citation2016; Martin & Hall-Spencer, Citation2017; Chan et al., Citation2020). Climate-change induced permafrost thawing and snow melting at high latitudes also increase freshwater runoff and coastal nutrient inputs (Walvoord & Striegl, Citation2007; Kendrick et al., Citation2018), which in turn can alter calcification rates and subsequent coralline growth (McCoy & Kamenos, Citation2018; Bélanger & Gagnon, Citation2020) and photophysiology (Schoenrock et al. Citation2018a). In this regard, Williams et al. (Citation2020) observed different responses among species of Clathromorphum Foslie related to their sensitivity to environmental change; thus, the widely distributed C. compactum (Kjellman) Foslie might expand its northern limit whereas the narrow-range C. nereostratum Lebednik is expected to decline.
In Arctic and subarctic regions, Lithothamnion Heydrich species often dominate coralline algal assemblages from the low intertidal to the lower limit of the photic zone, contributing significantly to shelf carbonate budgets (Freiwald & Henrich, Citation1994; Nelson, Citation2009; Adey & Hayek, Citation2011; Teed et al., Citation2020). Several Lithothamnion species form maerl, or branched crusts, and these structures significantly increase benthic habitat complexity and biodiversity (Gagnon et al., Citation2012; Teichert et al., Citation2014; Jørgensbye & Halfar, Citation2017; Schoenrock et al., Citation2018b). Because several Lithothamnion species (like most coralline algae) also induce larval settlement and metamorphosis in invertebrates with important functional roles, the genus is considered an ecosystem engineer (Steneck, Citation1982; Rowley, Citation1989; Pearce & Schiebling, Citation1990; Nelson, Citation2009).
Adey and co-workers have studied Arctic and subarctic subtidal benthic non-geniculate coralline communities for the past 50+ years, publishing on the ecology (Adey, Citation1964, Citation1965, Citation1966a, Citationb, Citation1970a, Citation1971; Adey & McKibbin, Citation1970; Adey & Adey, Citation1973; Adey et al., Citation2005), physiology (Adey, Citation1970a, Citationb, Citation1973; Adey et al., Citation2013, Citation2015a) and biogeography (Adey, Citation1966a, Citationb; Adey et al., Citation1976, Citation2008; Adey & Steneck, Citation2001) of these algae, and recently have added DNA-based taxonomic and phylogenetic studies. Thus, Arctic and Subarctic species of Clathromorphum (Adey et al., Citation2015a, b), Neopolyporolithon W.H.Adey & H.W.Johansen (Gabrielson et al., Citation2019) and Phymatolithon Foslie (Adey et al., Citation2018) have largely been resolved, but Lithothamnion species still need clarification. This is mainly due to the large number of species and infraspecific taxa named in the late 19th and early 20th century, primarily by the Norwegian corallinologist Mikael Heggelund Foslie, but also by the Swedish phycologist Frans Reinhold Kjellman. Some of these taxa have been placed in synonymy based on morpho-anatomy, but many are still recognized (Guiry & Guiry, Citation2020) or are considered incertae sedis (Athanasiadis, Citation2016). Studies of other coralline genera have shown that morpho-anatomy alone cannot distinguish species (Sissini et al., Citation2014; Peña et al., Citation2014b, Citation2015a; Hernández-Kantún et al., Citation2016; Gabrielson et al., Citation2018). Here, we assess many of the unresolved species and infraspecific taxa of Arctic and subarctic Lithothamnion to provide fundamental taxonomic, ecological and biogeographic knowledge of these species in the face of the anticipated but unknown effects of climate change on the marine flora of these regions.
Materials and methods
Collections studied
Fifty-eight type specimens of Lithothamnion species and infraspecific taxa housed in TRH and UPS as well as 11 historical specimens in TRH (herbarium acronyms follow Thiers, Citation2021) were considered for DNA analysis (Supplementary table S1, Supplementary note S1). One of us (SCL), as a guest of the Department of Botany, Stockholm University, located Kjellman’s type specimens in UPS housed in a room separate from the main algal collection. This may explain why earlier investigators were unable to locate them. These specimens, described by F.R. Kjellman between 1877 and 1889, were later received on loan by PWG; specimens described by M.H. Foslie between 1891 and 1908 were examined by VP or PWG. Most of the specimens had type localities along the Norwegian coast but some were described from Svalbard, Scotland, Greenland, Canada and USA (Kjellman, Citation1883, Citation1889; Foslie, Citation1891, Citation1895, Citation1896, Citation1900; Citation1905a, Citationb, Citation1908). In addition, 440 recent collections from Norway, Svalbard, Greenland, and the Atlantic and Pacific coasts of Canada and USA were also sequenced (Supplementary table S2). Most of these specimens were collected subtidally in coralline algal beds (maerl or rhodolith beds) or as crusts, and are preserved in NCU, TRH, SANT, UBC and UNB (see collection details in Supplementary table S2).
DNA sequencing and analyses
Herbarium material was extracted and amplified at five institutions: the Muséum National d’Histoire Naturelle, Paris (MNHN), the University of North Carolina, Chapel Hill (UNC), Hartnell College (HC), the University of British Columbia (UBC) and the University of New Brunswick (UNB). Extractions and amplifications of types and historical collections were accompanied by negative controls at every step, separate from recent collections. At the MNHN, DNA of type specimens and historical collections was extracted using QIAamp®DNA Micro Kit (Qiagen SAS, Les Ulis, France) following the manufacturer’s protocol for tissues; DNA of recent collections was extracted using a NucleoSpin® 96 Tissue kit (Macherey-Nagel, GmbH and Co. KG, Germany). At UNC type material and recent collections were extracted following Gabrielson et al. (Citation2011); at HC type material was extracted according to Hernández-Kantún et al. (Citation2016) following the precautionary guidelines proposed by Hughey & Gabrielson (Citation2012); at UNB recent collections were extracted following Saunders & McDevit (Citation2012); at UBC recent collections were extracted following Lindstrom & Fredericq (Citation2003). Three genes (rbcL, psbA and COI) were amplified in this study. For type specimens and historical collections, rbcL sequences were obtained with two primer combinations, F1150Cor-R1460 or F1150Cor-RbcS-Start, yielding a fragment trimmed to 263 bp (1172–1434) or 293 bp (1172–1464), respectively; for recent collections, rbcL sequences of 1383 bp were obtained with overlapping primer combinations F57-R1150 and F753-RrbcS, with primer combination F753/RrbcS-Start trimmed to 691 bp (772–1464), or followed Saunders & Moore (Citation2013) for amplifications completed at UNB. For recent collections and for some type specimens and historical collections, psbA sequences were obtained by the institutions mentioned above, using the primer pairs psbA-F1/psbA-R2 and psbA-F1/psbA-600R (Yoon et al., Citation2002), following Peña et al. (Citation2015b) or Adey et al. (Citation2015b). COI-5P sequences were obtained only for recent collections using the primer pairs Gaz-F1/Gaz-R2 and Gaz-F1/GCorR3, following Saunders & Moore (Citation2013) or Peña et al. (Citation2015b). PCR products were purified and sequenced at MNHN by Eurofins (Eurofins Scientific, Nantes, France); at UNC according to Hughey et al. (Citation2001) and sequenced at the DNA Analysis Core Facility, Center for Marine Sciences, University of North Carolina, Wilmington; and at HC by Functional Biosciences, Inc. (Madison, Wisconsin, USA). Sequences were assembled and aligned with the assistance of CodonCode Aligner® (CodonCode Corporation, USA) or with Sequencher (Gene Codes Corp., Ann Arbor, Wisconsin, USA) and adjusted manually using SeaView version 4 (Gouy et al., Citation2010) or using Sequence Alignment Editor (http://tree.bio.ed.ac.uk/software/seal/); sequences were submitted to the Barcode of Life Data Systems (BOLD projects ‘NCCAB’, ‘NGCOR’ and dataset ‘LITHOTH1’, http://www.boldsystems.org; Ratnasingham & Hebert, Citation2007) and/or to GenBank (accession numbers listed in Supplementary tables S1 and S2).
DNA sequencing and analyses. Three data sets were built, one for each gene (rbcL, psbA and COI-5P), comprising ~526 sequences obtained in this study (Supplementary tables S1 and S2) and supplemented with GenBank sequences publicly available for Arctic and Subarctic collections of Lithothamnion as well as for other Hapalidiales genera (Clathromorphum and Phymatolithon) for which relevant matches were found (Supplementary table S3). As outgroup we used rbcL and psbA sequences linked to the generitype Lithophyllum incrustans Philippi, order Corallinales; for COI-5P we used a sequence generated from the neotype of Phymatolithon calcareum (Pallas) Adey & McKibbin, order Hapalidiales (Supplementary table S3). Phylogenetic relationships were inferred with maximum likelihood (RAxML) and Bayesian inference (BI) using Mega 6.06 (Tamura et al., Citation2013), RAxML 8.1.11 (Stamatakis, Citation2014; available in CIPRES Science Gateway, Miller et al., Citation2010) and MrBayes 3.2.1 (Ronquist & Huelsenbeck, Citation2003). Models of sequence evolution were estimated using the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) obtained in jModeltest 2.1.3 (Darriba et al., Citation2012). Maximum likelihood for the rbcL, COI-5P and psbA alignments were performed under a generalized time-reversible with invariant sites heterogeneity model (GTR+I+G). The Bayesian analyses for the rbcL and psbA alignments were performed under the same model (GTR+I+G) with four Markov chain Monte Carlo method for 10 million generations, and tree sampling every 1000 generations.
Distribution of Arctic/subarctic Lithothamnion species
Geographic coordinates were obtained by GPS for each sequenced collection and were estimated for types and historical specimens using Google Earth Pro 7.3.3.7786 (© 2020 Google LLC). Distribution maps were created by projecting latitude and longitude of all specimens delimited for each species using QGIS3.10 (QGIS.org, Citation2020) with North Pole Lambert Azimuthal Equal Area projection. The following shape file was used for the map background: https://www.data.gouv.fr/fr/datasets/continents/.
Results
Of the type specimens (58) and historical collections (11) that were attempted to sequence, we successfully amplified and sequenced 62% for rbcL (36 types and five historical specimens) and psbA (5 types) (Supplementary table S1).
The rbcL alignment comprised 121 sequences of variable length resulting in 81 unique DNA sequences ranging from 205 to 313 bp, with 114 variable sites. Both ML and Bayesian analyses resolved the type specimens and historical collections with moderate to full support in different Hapalidiales lineages encompassing species of Lithothamnion, Phymatolithon and Clathromorphum (). Most of the type specimens and historical collections sequenced were within lineages that included the lectotype of Lithothamnion glaciale Kjellman (0–3 bp differences, up to 1.14% divergence (uncorrected p-distance)), the lectotype of L. soriferum Kjellman (0–1 bp differences, up to 0.41% divergence) and the neotype of L. tophiforme (0–2 bp, up to 0.68% divergence) (). Three type specimens were placed in the genus Phymatolithon (, Supplementary table S1): the holotype of Lithothamnion scabriusculum Foslie was positioned within a clade encompassing collections of P. rugulosum W.H.Adey (1–3 bp differences; 0.3–1% divergence); the lectotype of L. squarrulosum f. palmatifidum Foslie was resolved within a clade represented by the neotype of P. calcareum (2 bp differences, 0.9% divergence); the holotype of Lithothamnion lenormandii f. squamulosum (Foslie) Cotton was identical in sequence to the isotype of P. squamulosum (Foslie) W.H.Adey, Hernández-Kantún & P.W.Gabrielson. Another two lectotypes (L. coalescens Foslie and L. evanescens Foslie) and one historical collection of Clathromorphum circumscriptum (Strömfelt) Foslie from Norway were identical in sequence to the epitype of C. circumscriptum (0 bp, ); the infraspecific variation within C. circumscriptum ranged up to 13 bp (1.3% uncorrected p-distance) and to 14 bp (1.4%) including two further rbcL sequences obtained from recent Alaskan collections (UBC A92115 and UBC A94120, not included in ). None of the types and historical collections sequenced were resolved within the lineage of Lithothamnion lemoineae W.H.Adey; only three recent collections (UBC A94112, , together with UBC A94113 and UBC A94121, as Lithothamnion sp., not included in , Supplementary table S2) appeared to be closely related to L. lemoineae, showing at minimum 11 bp differences (1.15% of divergence) between the taxa.
Fig. 1. Phylogenetic tree inferred from maximum likelihood (ML) analyses of rbcL sequences included in the present study. Bold formatted font represent sequences generated from type materials. Bootstrap ML values > 60% and posterior probabilities > 0.60 from Bayesian inference shown for each node. Scale bar: 0.05 substitutions per site
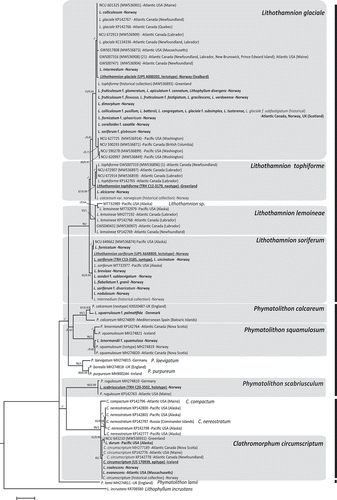
The COI-5P alignment comprised 151 sequences that included 81 unique sequences ranging from 518 to 579 bp, with 153 variable sites. The ML phylogenetic tree resolved seven fully and two moderately supported lineages (Supplementary fig. S1). Four of these lineages are represented by recent collections of L. glaciale, L. tophiforme (Esper) Unger, L. lemoineae and L. erinaceum Melbourne & J.Brodie (herein proposed as a synonym of L. soriferum Kjellman, see next section below). The remaining three fully supported lineages (as Lithothamnion sp. 2 to L. sp. 4) and another two moderately supported lineages (Lithothamnion sp.1, L. sp. 5) corresponded to recent collections pertaining to five Lithothamnion species that did not return any relevant match with publicly available GenBank sequences (Supplementary fig. S1, Supplementary tables S2 and S3). The highest infralineage variation (uncorrected p-distance) was recorded in L. glaciale (up to 2.07%).
The psbA alignment comprised 421 sequences that resulted in 124 unique sequences, ranging from 382 to 851 bp with 249 variable sites. The alignment encompassed recent collections, five type collections generated in the present study and publicly available sequences from GenBank such as the holotypes of L. erinaceum and L. lemoineae, and the isotype of P. rugulosum (herein as P. scabriusculum, see next section of taxonomic proposals) (Supplementary tables S1–S3). Both RAxML and Bayesian analyses (Supplementary fig. S2) resolved our recent collections in different lineages pertaining to the genera Clathromorphum (C. circumscriptum), Phymatolithon (P. squamulosum) and Lithothamnion (L. lemoineae, L. tophiforme, L. glaciale and L. erinaceum (herein as L. soriferum, see next paragraph of taxonomic proposals)). In agreement with results obtained for rbcL, both RAxML and Bayesian analyses of psbA resolved these type collections in three different genera (Supplementary fig. S2): Lithothamnion (neotype of L. glaciale f. subsimplex Foslie), Clathromorphum (lectotypes of L. coalescens and L. evanescens) and Phymatolithon (holotype of L. lenormandii f. squamulosa and lectotype of L. squarrulosum f. palmatifida). However, one recent collection (as Lithothamnion sp., Supplementary fig. S2, Supplementary table S2) had a sequence distinct from all other analysed taxa, with no relevant match with publicly available GenBank sequences. In contrast to rbcL and COI analyses, the support values obtained were generally lower, particularly for L. glaciale with weak support (Supplementary fig. S2).
Given the molecular evidence noted above, and in accordance with Article 11.4 of the International Code of Nomenclature for algae, fungi and plants (ICN, Turland et al., Citation2018), we present the following taxa with the corresponding heterotypic synonyms from the present study. Note that for each taxon below where it is stated “…the lectotype is narrowed…” we are invoking Article 9.17 of the ICN (Turland et al. Citation2018).
Clathromorphum circumscriptum (Strömfelt) Foslie, Citation1898a: 5
BASIONYM: Lithothamnion circumscriptum Strömfelt, Citation1886: 20, pl. 1, figs 4–8.
HOMOTYPIC SYNONYMS: Phymatolithon compactum f. circumscriptum (Strömfelt) Foslie, Citation1905a: 88.
Clathromorphum compactum f. circumscriptum (Strömfelt) Foslie, Citation1908: 11.
Lithothamnion compactum f. circumscriptum (Ström felt) Lund, Citation1959: 200.
LECTOTYPE: S; seven microscope slides apparently from the original material designated by Athanasiadis (Citation2016: 251) as the holotype, but as Strömfelt (Citation1886) designated syntype localities, this material is better called a lectotype. This is a correctible error in accordance with Art. 9.10 of the ICN (Turland et al., Citation2018).
Comment: Adey et al. (Citation2015b), while designating an epitype for C. circumscriptum, inadvertently omitted submitting this epitype sequence to GenBank. This has now been rectified: GenBank MW536846, an rbcL-263 (bp 1172–1434) sequence.
HETEROTYPIC SYNONYMS:
Lithothamnion coalescens Foslie, Citation1895: 162 (reprint 134), pl. 19, figs. 15–20.
Clathromorphum coalescens (Foslie) Foslie, Citation1898b: 8.
Phymatolithon compactum f. coalescens (Foslie) Foslie, Citation1905a: 8.
LECTOTYPE: TRH C21-3503; 12.viii.1893, leg. unknown.
TYPE LOCALITY: Inderøen, Strømmen, Trondheimsfjorden, Norway.
Lectotype DNA sequences: psbA and rbcL-263 (bp 1172–1434), GenBank MW564561 and MW536929.
Comment: Following his description, Foslie (Citation1895) transferred the species without comment to Clathromorphum (Foslie, Citation1898b). Later, Foslie (Citation1905a) reduced Clathromorphum to a subgenus of Phymatolithon and reduced C. coalescens to a form of Phymatolithon, as P. compactum f. coalescens. This name was not treated by Lebednik (Citation1977) nor by Adey et al. (Citation2015b), but was listed by Athanasiadis (Citation2016) as a synonym of C. compactum (Kjellman) Foslie. According to Woelkerling et al. (Citation2005), the lectotype of L. coalescens is a blue box with five specimens designated by Woelkerling (Citation1993: 52) as the lectotype, noting that Foslie (Citation1895: 163) had cited two syntype localities. Upon examination, the box contained four specimens illustrated in Foslie (Citation1895: pl. 19, figs 15–20), one of which was sequenced, and herein the lectotype is narrowed to that sequenced specimen (GenBank MW564561 and MW536929). Both the rbcL and psbA sequences obtained are identical to GenBank sequences of C. circumscriptum (voucher US 169083), which were confirmed as identical to the epitype (voucher US 170939, Adey et al., Citation2015a, b: 195).
Lithothamnion durum Kjellman, Citation1889: 22, pl. 1, figs 3–5.
Clathromorphum durum (Kjellman) Foslie, Citation1898b: 8.
HOLOTYPE: UPS A-000297, vii.1877, leg. F. R. Kjellman.
TYPE LOCALITY: Port Clarence, Alaska, USA.
Holotype DNA sequence: The rbcL-263 (bp 1172–1434) sequence was obtained from the holotype specimen, and over this sequence length differed by 1 bp from the epitype of Clathromorphum circumscriptum. This position is variable in C. circumscriptum, with specimens from Iceland, Labrador, Newfoundland and Maine sharing the same single nucleotide polymorphism (SNP) and likewise those from Greenland and Alaska (Port Clarance and Juneau) sharing the same SNP.
Comment: This synonymy was first proposed by Foslie (Citation1900: 10) and accepted by Lebednik (Citation1977: 64), who noted that a fragment, apparently from the holotype, was in TRH. This fragment is now considered an isotype (Art. 8.3, Turland et al. Citation2018). The holotype illustrated by Kjellman (Citation1889: pl. 1, fig. 3), was found in UPS and the DNA sequence was obtained from the specimen labelled ‘b‘ (Kjellman, Citation1889: pl. 1, fig. 3). We did not sequence the fragment in TRH.
Lithothamnion evanescens Foslie, Citation1895: 137.
Clathromorphum evanescens (Foslie) Foslie, Citation1898b: 8.
Phymatolithon evanescens (Foslie) Foslie, Citation1905a: 92.
LECTOTYPE: TRH C21-3518, iv.1889, leg. F.S. Collins.
TYPE LOCALITY: Marblehead, Massachusetts, USA.
Lectotype DNA sequences: psbA and rbcL-263 (bp 1172–1434), GenBank MW564560 and MW536911.
Comment: Foslie (Citation1895: 137, pl. 22, figs 6, 7) cited and illustrated specimens from two syntype localities, Marblehead, Massachusetts, USA collected by F. S. Collins and from Mastervik, Malangen, Norway collected by himself. Foslie transferred the species twice, first, without comment, to Clathromorphum (Foslie Citation1898b), and later to Phymatolithon after admitting that Clathromorphum should be considered a subgenus of the latter (Foslie, Citation1905a: 87). Lebednik (Citation1977) first proposed that this species was a synonym of C. circumscriptum, and this was accepted by Athanasiadis (Citation2016). Woelkerling (Citation1993: 87) designated as lectotype a single specimen collected by Collins in Marblehead, Massachusetts and illustrated by both Foslie (Citation1895: pl. 22, fig 6) and Printz (Citation1929: pl. 41, fig. 13). Woelkerling (Citation1993) justified the selection of this specimen as lectotype because ‘it was in better condition and had numerous conceptacles’. Both rbcL and psbA sequences obtained for this lectotype specimen are identical to GenBank sequences of C. circumscriptum (voucher US 169083), which was confirmed as identical to the epitype (voucher US 170939, Adey et al., Citation2015b: 195).
Historical collection:
TRH C20-3495, as Lithothamnion circumscriptum. Tamsøya, Finnmark, Norway, 31.vii.1897, no habitat data, leg. M. H. Foslie. DNA sequence: rbcL-263 (bp 1172–1434), GenBank MW536916 (Supplementary table S1). The largest fragment of the four in the box was sequenced.
Recent collections:
Norway: Porsangerfjorden (Finnmark) and Krøttøya (Troms). Intertidal to subtidal (6 m depth), encrusting pebbles and pottery, on hard substrata and associated with maerl beds. One specimen collected in Krøttøya had uniporate conceptacles (gametangial or carposporangial). DNA sequences: psbA (Supplementary table S2).
Lithothamnion glaciale Kjellman, Citation1883: 123–127, pls 2, 3.
LECTOTYPE, herein designated: UPS A-000202, xi–xii.1872, leg. F. R. Kjellman.
TYPE LOCALITY: Mosselbay, Spitsbergen.
Lectotype DNA sequence: rbcL-263 (bp 1172–1434), GenBank MW536933.
Comment: Adey (Citation1970a) made a provisional lectotypification based on ‘a Spitzbergen specimen (No. 241, Institute of Taxonomy, Uppsala) collected by Kjellman in 1872–1873’. Adey (Citation1970a) further stated that this specimen was not one illustrated by Kjellman (Citation1883) that accompanied the original description. The ICN does not accept provisional lectotypes (Art. 7.11, Turland et al. Citation2018). Chamberlain & Irvine (Citation1994) repeated Adey’s (Citation1970a) lectotypification, thus making it acceptable, but stated that they did not see the specimen.
Among Kjellman’s type collections was the single individual rhodolith of L. glaciale illustrated by Kjellman (Citation1883: pls 2, 3), with some artistic licence (Supplementary fig. S3A), along with a collection label stating the type locality of Mosselbay on the island of Spetsbergen (Spitzbergen) and dated November and December 1872, collected while the expedition aboard the Polhem was iced in until August 1873 (Wynne, Citation1995). We here designate this specimen from which we obtained a partial rbcL sequence as the lectotype of L. glaciale. All other sequences of L. glaciale differ by 1 bp from the lectotype sequence, including all the type sequences of synonyms listed below.
Lectotype SEM observations: A cross-section through a protuberance showed radial construction and a buried conceptacle (Supplementary fig. S3B). Thallus construction was monomerous with elongate hypothallial cells (Supplementary fig. S3C–D). Abundant fusions linked cells from adjacent perithallial filaments (Supplementary fig. S3E) and secondary pit connections were absent. The epithallus was single layered and epithallial cells were flared; intercalary meristematic cells (subepithallial initials) were shorter or about the same length as subtending perithallial cells (Supplementary fig. S3F).
HETEROTYPIC SYNONYMS:
Lithothamnion apiculatum f. connatum Foslie, Citation1895: 54, pl. 15, figs 9–13 (as ‘connata’).
LECTOTYPE: TRH B20-2669, 12.vii.1893, no habitat data, leg. H.H. Gran.
TYPE LOCALITY: Drøbak, Norway.
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536892.
Comment: Woelkerling (Citation1993) located four of five specimens as part of the protologue of L. apiculatum f. connatum and designated these as lectotype. They were illustrated by Foslie (Citation1895: pl. 15, figs 9–12) and by Printz (Citation1929: pl. 21, figs 11–15) under the name L. colliculosum f. pusilla. One of these four specimens, branched and epilithic on a pebble (among the specimens illustrated as figs 9–11, Foslie Citation1895: pl. 15) was sequenced, and herein this lectotype is narrowed to that specimen.
Lithothamnion battersii Foslie, Citation1896: 1, pl. 1, figs 1–5.
HOLOTYPE: TRH C10-3098, viii.1891, leg. E. Batters.
TYPE LOCALITY: Cumbrae, Scotland.
Holotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536898.
Comment: The holotype collection comprises five individuals illustrated by Foslie (Citation1896: pl. 1, figs 1–5); the specimen sequenced is depicted in Foslie’s (Citation1896) fig. 2. Based on morpho-anatomy, Chamberlain & Irvine (Citation1994: 182) and Athanasiadis (Citation2016: 217) correctly listed L. battersii as a synonym of L. glaciale.
Lithothamnion colliculosum Foslie, Citation1891: 43, pl. 3, fig. 1.
LECTOTYPE: TRH B11-2311, 8.ix.1890, leg. M. H. Foslie (designated by Woelkerling, Citation1993: 53).
TYPE LOCALITY: Skorpen, Kvænangen, Norway.
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536855.
Comment: Foslie (Citation1891: 43–45, pl. 3, fig. 1) described and illustrated eight individual specimens that he ascribed to this species. Adey & Lebednik (Citation1967: 71) could not locate any of these specimens, and therefore Adey (Citation1970c) designated as neotype one specimen from Kragerø collected in 1890. Later, Woelkerling (Citation1993: 54) located in TRH numerous specimens with the original collection data of L. colliculosum, including four of the eight individuals comprising the holotype and depicted by Foslie (Citation1891, pl. 3, fig. 1). Because the other four individuals comprising the holotype remain missing, Woelkerling designated the found specimens as the lectotype of L. colliculosum, superseding Adey’s neotype. The lectotype is narrowed herein to the individual sequenced crust among the original specimens depicted by Foslie (Citation1891: pl. 3, fig. 1, bottom row, second from right). Based on morpho-anatomy, Athanasiadis (Citation2016: 224) listed L. colliculosum as incertae sedis; DNA sequence data has confirmed the placement of the species in L. glaciale.
Lithothamnion colliculosum f. pusillum Foslie, Citation1905a: 35 (as ‘pusilla’).
LECTOTYPE: TRH B20-2706, 12.vii.1898, leg. H. H. Gran (designated by Woelkerling Citation1993: 185).
TYPE LOCALITY: Drøbak, Norway.
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536921.
Comment: We sequenced one of the 14 specimens comprising the lectotype, which is located separately within a blue box with label ‘Prep. 76-77’. The lectotype is narrowed herein to the sequenced specimen. Based on morpho-anatomy, Athanasiadis (Citation2016: 224) listed L. colliculosum f. pusillum as incertae sedis; DNA sequence data has confirmed the placement of the species in L. glaciale.
Lithothamnion congregatum Foslie, Citation1895: 142, pl. 20, figs 1–6.
HOMOTYPIC SYNONYM: Lithothamnion nodulosum f. congregatum (Foslie) Foslie, Citation1900: 13.
LECTOTYPE: TRH C7-3062, 20.vii.1894, leg. M. H. Foslie (designated by Woelkerling, Citation1993: 60–61).
TYPE LOCALITY: Skjørn (now Stjørna), Trondheimsfjord, Norway (Woelkerling et al., Citation2005: 424).
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536865.
Comment: Foslie (Citation1895: 142–144, pl. 20, figs 1–6) described and illustrated this species based on six individuals from a single locality, and compared it with two other species named in the same publication, L. dehiscens Foslie and L. nodulosum Foslie. Later, Foslie (Citation1900) reduced L. congregatum to a form of L. nodulosum. The sequenced specimen is illustrated in Foslie (Citation1895: pl. 20, fig. 2), and the lectotype is here narrowed to that sequenced specimen. Based on morpho-anatomy Athanasiadis (Citation2016: 224) listed L. congregatum as incertae sedis; DNA sequence data has confirmed the placement of the species in L. glaciale.
Lithothamnion corallioides f. saxatile Foslie, Citation1895: 90, pl. 16, figs 12–23 (as ‘saxatilis’).
LECTOTYPE: TRH C9-3097, 1.viii.1894, leg. M. H. Foslie (designated by Woelkerling, Citation1993: 195).
TYPE LOCALITY: Røberg (now Raudberget), Norway (Woelkerling et al., Citation2005: 413).
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536900.
Comment: Woelkerling (Citation1993: 195–196) located and designated as the lectotype four of the original 12 specimens included by Foslie (Citation1895: pl. 16, figs 14–17) in the original protologue of this form. The specimen sequenced had a green label ‘Prep. 100-101’; it resembled the specimen illustrated in Foslie (Citation1895: pl. 16, fig. 16). The lectotype is here narrowed herein to this single sequenced specimen.
Lithothamnion dimorphum Foslie, Citation1895: 68, pl. 10, figs 1–6.
HOMOTYPIC SYNONYM: Lithothamnion fornicatum f. dimorphum (Foslie) Foslie, Citation1905a: 38.
LECTOTYPE: TRH B25-2773, 10.vii.1894, 0–5.5 m depth on sandy and stony bottom, leg. M. H. Foslie (designated by Woelkerling, Citation1993: 75).
TYPE LOCALITY: Frøjen (now Frøya), Rottingsundet, Trondeland, Norway (Woelkerling et al., Citation2005: 375).
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536934.
Comment: Foslie (Citation1895: pl. 10, figs 1–6) illustrated six specimens belonging to this species, but did not designate a holotype. Woelkerling (Citation1993: 74–75) designated as the lectotype four of the six specimens depicted in figs 1, 3, 5 and 6 (Foslie, Citation1895: pl. 10). The lectotype is narrowed herein to the Foslie Citation1895: pl. 10, fig. 3 specimen that we sequenced. Based on morpho-anatomy, Athanasiadis (Citation2016: 225) listed L. dimorphum as incertae sedis; DNA sequence data have placement of the species in L. glaciale.
Lithothamnion divergens Foslie, Citation1895: 96, pl. 16, figs 43–50.
HOMOTYPIC SYNONYMS: Lithothamnion ungeri f. divergens (Foslie) Foslie, Citation1900: 11; Lithothamnion tophiforme f. divergens (Foslie) Foslie, Citation1905a: 51.
HOLOTYPE: C11-3167, 8.ix.1890, leg. M. H. Foslie.
TYPE LOCALITY: Kvaenangen, Skørpen (now Skorpa), Norway (Woelkerling et al., Citation2005: 438).
Holotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536922.
Comment: The holotype material comprised two boxes (one round and one square) with seven specimens and fragments (Woelkerling, Citation1993: 80; Woelkerling et al., Citation2005: 438). The sequenced specimen is located in the round box with the green tag ‘Lith. Mon. pl. 20, f. 8’ and illustrated in Foslie (Citation1895: pl. 16, fig. 48) and Printz (Citation1929: pl. 20, fig. 8). Based on morpho-anatomy, Athanasiadis (Citation2016: 226) listed L. divergens as incertae sedis; DNA sequence data confirm the placement of the species in L. glaciale.
Lithothamnion fornicatum f. sphaericum Foslie, Citation1900: 12 (as ‘sphaerica’).
HOLOTYPE: TRH B26-2789, 20.vii.1894, no habitat data, leg. M. H. Foslie.
TYPE LOCALITY: Skjørn, Dalsøren (now Stjørna, Daleøra), Trondheimsfjorden, Norway (Woelkerling et al., Citation2005: 380).
Holotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536918.
Comment: Foslie (Citation1900) only provided a reference to a previously published figure (Foslie Citation1895: pl. 12, fig. 1) for the protologue of this form, but Woelkerling (Citation1993: 205) considered this a validly published name. We sequenced the same individual rhodolith cited by Foslie (Citation1900) and illustrated in Foslie (Citation1895: pl. 12, fig. 1).
Lithothamnion fruticulosum f. fastigiatum Foslie, Citation1895: 46, pl. 5.
LECTOTYPE: TRH B25-2777, 6.vii.1894, leg. M. H. Foslie (designated by Woelkerling, Citation1993: 91).
TYPE LOCALITY: Bejan (now Beian), Beiskjaeret, Norway (Woelkerling et al., Citation2005: 377).
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536847.
Comment: Foslie (Citation1895: pl. 5, figs 1–7) illustrated seven individual rhodoliths that according to Woelkerling (Citation1993) came from two collections from neighbouring localities on successive days. Woelkerling (Citation1993: 91) designated as the lectotype collection nine specimens comprising two of those illustrated in the protologue (Foslie, Citation1895: pl. 5, figs 5 and 7) and seven other specimens. The lectotype is narrowed herein to the sequenced specimen that is marked with label ‘nr. 2’, which is cited as part of the lectotype in Woelkerling et al. (Citation2005: 377).
Lithothamnion fruticulosum f. flexuosa Foslie, Citation1895: pl. 7, figs 1–3.
LECTOTYPE: TRH B27-2805, 15.viii.1890, leg. M. H. Foslie (designated by Woelkerling, Citation1993: 95).
TYPE LOCALITY: Tromsø, Norway.
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536923.
Comment: Foslie (Citation1895) based this form on collections from several localities in Norway, but did not designate a type. Woelkerling (Citation1993) designated the specimen illustrated by Foslie (Citation1895: pl. 7, fig. 3) as the lectotype of this form. According to Woelkerling et al. (Citation2005), the lectotype has two green tags, and this was the specimen from which the DNA sequence was obtained.
Lithothamnion fruticulosum f. glomeratum Foslie, Citation1895: 46, pl. 4, fig. 3 (as ‘glomerata’).
LECTOTYPE: TRH B8-2153, 12.vi.1892, leg. unknown (designated by Printz, Citation1929: pl. 22, fig. 5 legend).
TYPE LOCALITY: Lyngø (now Lyngøya), near Tromsø, Norway (designated by Woelkerling, Citation1993: 108, further information in Woelkerling et al. Citation2005: 288).
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536882.
Comment: Foslie (Citation1895) cited specimens from two localities in Norway, Lyngø and Vardø, but did not designate a type. Printz (Citation1929) designated as lectotype the one rhodolith illustrated by Foslie (Citation1895: 46, pl. 4, fig. 3) and this is the specimen sequenced in the current study.
Lithothamnion gracilescens Foslie, Citation1895: 87, pl. 15, figs 20–27, nom. illeg.
HOMOTYPIC SYNONYM: Lithothamnion nodulosum f. gracilescens Foslie, Citation1900: 13, nom. illeg.
LECTOTYPE: TRH C6-3037, 6.vi.1894, leg. M. H. Foslie (designated by Woelkerling, Citation1993: 109; further information in Woelkerling et al., Citation2005: 419).
TYPE LOCALITY: Rotvold (now Rotvoll), Trondsheimsfjord, Norway (Woelkerling et al., Citation2005: 419).
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536881.
Comment: Foslie (Citation1895) proposed Lithothamnion gracilescens for the coralline that Unger (Citation1858: 19) had called Lithothamnion byssoides, but Kjellman (Citation1883: 120) already had named this entity Lithothamnion ungeri. Foslie (Citation1895: 90) listed three syntype localities, Madal, Dröbak and Rotvold, and from the last location illustrated eight individuals (Foslie Citation1895: pl. 15, figs 20–27). Later, Foslie (Citation1900) without comment reduced L. gracilescens to a form of L. nodulosum as L. nodulosum f. gracilescens. The specimen sequenced corresponds to Foslie (Citation1895: pl. 15, fig. 24), to which the lectotype is narrowed as allowed. Based on morpho-anatomy Athanasiadis (Citation2016: 228) listed L. gracilescens as incertae sedis; DNA sequence data have confirmed the placement of the species in L. glaciale.
Lithothamnion intermedium Kjellman, Citation1883: 127, pl. 4, figs 1–10.
HOMOTYPIC SYNONYMS: Lithothamnion fruticulosum f. intermedium (Kjellman) Foslie, Citation1895: 46; Lithothamnion ungeri f. intermedium (Kjellman) Foslie, Citation1898b: 5.
LECTOTYPE herein designated: UPS A648805, vi.1875, leg. F.R.Kjellman.
TYPE LOCALITY: Carlsö (now Karlsøy), Tromsø, Norway.
Lectotype DNA sequence: rbcL-263 (bp 1172–1434), GenBank MW536854.
Comment: Kjellman (Citation1883) did not designate a type specimen for his new species. In UPS there appears to be only one individual rhodolith with a label in Kjellman’s hand and with the specific locality of Karlsøy cited in the protologue of L. intermedium, although two individuals are illustrated by Kjellman (Citation1883: pl. 4, figs 1, 2). Thus, we designate UPS A648805 as the lectotype.
Lithothamnion soriferum f. globosum Foslie, Citation1891: 41, pl. 3, fig. 3 (as ‘globosa’).
LECTOTYPE: C11-3142, 20.vi.1882, no habitat data, leg. M. H. Foslie (designated by Woelkerling, Citation1993: 107).
TYPE LOCALITY: Honningsvaag (now Honningsvåg), Finnmark, Norway (Woelkerling et al., Citation2005: 434).
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536925.
Comment: Woelkerling (Citation1993: 107) located two of the four specimens illustrated by Foslie (Citation1891: pl. 3, fig. 3). The specimen sequenced is illustrated in Foslie (Citation1891: pl. 3, fig. 3, second specimen from the left), and it is this specimen to which the lectotype is narrowed herein. Athanasiadis (Citation2016: 234) lists as incertae sedis; DNA sequence data have confirmed the placement of the species in L. glaciale.
Lithothamnion tusterense Foslie, Citation1905a: 65.
HOLOTYPE: TRH C9-3089, 10.viii.1898, leg. M. H. Foslie.
TYPE LOCALITY: Tusteren (now Tustna), Kristiansund, Norway (Woelkerling et al., Citation2005: 428).
Holotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536924.
Comment: Woelkerling (Citation1993: 229) considered the material in the Foslie herbarium illustrated by Printz (Citation1929: pl. 22, figs 6–13) to be the holotype, and we agree. The specimen sequenced is marked with a green tag ‘pl. 27, fig. 9’, but the specimen corresponds to the plate 22, fig. 9 in Printz (Citation1929), as was noted by Woelkerling et al. (Citation2005: 428). Based on morpho-anatomy, Athanasiadis (Citation2016: 235) listed L. tusterense as incertae sedis; DNA sequence data have confirmed the placement of the species in L. glaciale.
Lithothamnion vardoeense Foslie, Citation1905b: 3 (as vardöense).
LECTOTYPE: TRH C8-3077, 6.ix.1897, leg. M. H. Foslie. Woelkerling (Citation1993: 233) designated as lectotype the specimens illustrated by Printz (Citation1929: pl. 33, figs 12, 13, 15).
TYPE LOCALITY: Svolvær, Lofoten, Norway.
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536872.
Comment: Foslie (Citation1905b) based this species on two collections, one dead, excavated from Vardø, Norway, the other living from Lofoten, Svolvær, Norway. Adey & Lebednik (Citation1967: 77) examined material in TRH under this name, but did not designate a lectotype; this was done by Woelkerling (Citation1993: 233). The specimen sequenced corresponds to the one depicted in Printz (Citation1929: pl. 32, fig. 12), and the lectotype is narrowed herein to this specimen. Based on morpho-anatomy, Athanasiadis (Citation2016: 223) listed L. vardoense as incertae sedis; DNA sequence data have confirmed the placement of the species in L. glaciale.
Historical collections
TRH C12-3177, as Lithothamnion tophiforme. Sukkertoppen, Greenland, no date, leg. Petersen. DNA sequence: rbcL-263 (bp 1172–1434), GenBank MW536893.
TRH B10-2305, as Lithothamnion glacialef. subfastigiatum (as ‘subfastigiata’). Bekkarfjord, Alten (now Alta), Norway, 21.viii.1897, leg. M. H. Foslie. DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536930. Comment: The largest fragment in the collection was sequenced. The sequence was identical to the lectotype sequence of Lithothamnion colliculosum f. pusillum, shown herein as a heterotypic synonym of L. glaciale. L. glaciale f. subfastigiatum is considered a superfluous substitute name for Lithothamnion varians f. varians Foslie (Woelkerling et al., Citation2005: 306); the lectotype of L. varians f. varians (TRH C23-3649) was DNA-extracted but failed to amplify (see Supplementary note S1).
Infraspecific taxa of Lithothamnion glaciale confirmed as L. glaciale.
Lithothamnion glaciale f. subsimplex Foslie, Citation1905a: 27.
NEOTYPE: TRH B9-2255, 22.vi.1900, leg. E. Bay. Woelkerling (Citation1993: 215) designated as neotype the specimen illustrated by Printz (Citation1929: pl. 23, fig. 5).
TYPE LOCALITY: The southern coast of Ellesmereland, Havnefjorden; Northwest Territories, Canada (Woelkerling, Citation1993: 215; Woelkerling et al., Citation2005: 300).
Neotype DNA sequences: psbA and rbcL-263 (bp 1172–1434), GenBank MW56455 and MW536844.
Comment: Athanasiadis (Citation2016) listed this taxon as a synonym of L. glaciale, but with a query (?); DNA sequence data have confirmed this synonymy. The neotype sequence was identical to the lectotype sequence of Lithothamnion colliculosum f. pusillum, shown herein as a heterotypic synonym of L. glaciale.
Lithothamnion soriferum Kjellman, Citation1883: 117, pl. 1, figs 1–19.
LECTOTYPE: herein designated, UPS A648809, viii.1876, leg. F. R. Kjellman.
TYPE LOCALITY: Maasö (now Måsøy), Finnmark, Norway.
Lectotype DNA sequence: rbcL-263 (bp 1172–1434), GenBank MW536886.
Comment: Kjellman (Citation1883) did not designate a type specimen for his new species. As with L. glaciale, one of us (SCL) located in UPS type material of L. soriferum that agrees, with some artistic licence, to the three entire specimens illustrated by Kjellman (Citation1883: pl. 1, figs 1–4). In the figure legends, Kjellman refers to the fig. 1 specimen as young (ungt), the fig. 2 specimen as older (äldre) and seen from above (ofvanifrån), the fig. 3 specimen, the same (samma) specimen (as in fig. 2) seen from below (underifrån), and the fig. 4 specimen as full grown (fullvuxet). An rbcL-263 sequence was obtained from each of these specimens, and the sequences are identical to each other. Kjellman (Citation1883: 120) listed six syntype localities, Tromsö, Carlsö, Maasö, Magerö, Honningsvaag and Lebesby, the last two localities based on specimens sent to Kjellman by Foslie; the syntype corresponding to the latter locality – Lebesby – was also sequenced (TRH C13-3185, see below). The three specimens in UPS were found in a single envelope (Supplementary fig. S4A) with the locality ‘Norway. Finnmark: Hammerfest, Måsö (Måsöya)’. Following Article 9.3 (Turland et al., Citation2018) we herein designate these three specimens as the lectotype of L. soriferum.
The identical rbcL-263 sequences of all three UPS specimens are an exact match to GenBank sequences of the recently described Lithothamnion erinaceum (Melbourne et al., Citation2017). Following Article 11.4 of the ICN (Turland et al., Citation2018), the correct name is the combination of the final epithet of the earliest legitimate name of the taxon at the same rank. Consequently, L. soriferum has nomenclatural priority over L. erinaceum, and it is the correct name for this taxon. Further information about the heterotypic synonym L. erinaceum is in the entry below.
SYNTYPE: TRH C13-3185, 2.viii.1882, leg. M. H. Foslie (identified by Kjellman, Woelkerling et al., Citation2005: 440).
TYPE LOCALITY: Lebesby, Finmarken (now Finnmark), Norway (Woelkerling et al., Citation2005: 441).
Syntype DNA sequence: rbcL-263 (bp 1172–1434), GenBank MW536919.
Comment: The specimen sequenced is the one that according to Woelkerling et al. (Citation2005: 441) is illustrated in Foslie (Citation1891: pl. 3, fig. 3, as Lithothamnion soriferum f. globosa).
Lectotype SEM observations: A vertical section through a protuberance showed the radial construction (Supplementary fig. S4B–D) and an extensive perithallus. Thallus construction was monomerous with elongate hypothallial cells (Supplementary fig. S4E). Fusions occurred between cells of adjacent perithallial filaments (Supplementary fig. S4F), and secondary pit connections were absent. There was a single layer of epithallial cells, and each epithallial cell had flared walls (Supplementary fig. S4F–H). A single layer of intercalary meristematic cells (subepithallial initials) was composed of cells shorter than or as long as subtending perithallial cells (Supplementary fig. S4F–H).
HETEROTYPIC SYNONYMS:
Lithothamnion breviaxe Foslie, Citation1895: 44, pl. 2, figs 1–2.
LECTOTYPE: TRH B12-2327, 3.viii.1887, leg. M. H. Foslie (designated by Adey & Lebednik Citation1967: 63).
TYPE LOCALITY: Kjelmø (now Sør-Varanger, Kjelmøya), Sydvaranger, Finnmark, Norway (Woelkerling et al. Citation2005: 310).
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536888.
Comment: Foslie (Citation1895: 44, pl. 2, figs 1–2) described this species based on several specimens collected at one locality (Kjelmø, Norway), but illustrated only two. We obtained an rbcL sequence from the designated lectotype (Foslie Citation1895: pl. 2, fig. 1) and it was identical to the lectotype sequence of L. soriferum. Based on morpho-anatomy, Athanasiadis (Citation2016: 223) listed L. breviaxe as incertae sedis; DNA sequence data have confirmed the placement of the species in synonymy with L. soriferum.
Lithothamnion erinaceum Melbourne & J.Brodie in Melbourne et al., Citation2017: 7, figs 3, 7–9, 11.
HOLOTYPE: BM 001150576, 13.x.2014, leg. A. Mogg.
TYPE LOCALITY: Loch Creran, Oban, Scotland (Melbourne et al., Citation2017).
Holotype DNA sequences: GenBank KX828452 (psbA) and KX828509 (COI-5P) (Melbourne et al., Citation2017); GenBank MH697546 and MH697547 (rbcL, Hofmann & Heesch, Citation2018).
Comment: According to Melbourne et al. (Citation2017), collections from Northern Ireland, Iceland, Norway and British Columbia provided in Pardo et al. (Citation2014) as Lithothamnion sp. 2 corresponded to L. erinaceum. Based on DNA sequences, these collections are also assigned to L. soriferum, as well as the remaining specimens from the UK identified as Lithothamnion sp. in Melbourne et al. (Citation2017, table S1). Additionally, GenBank records from Norway identified as L. erinaceum (specimens ‘NCCA’ in Supplementary table S3) correspond to L. soriferum (Anglés d´Auriac et al., Citation2019).
Lithothamnion fornicatum Foslie, Citation1891: 38, pl. 2 (bottom specimen).
LECTOTYPE: TRH B21-2712, 20.ix.1890, leg. unknown (designated by Adey & Lebednik, Citation1967: 71).
TYPE LOCALITY: Melangen (now Malangen), Mestervik, Tromsø county, Norway (Woelkerling et al., Citation2005: 366).
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536912.
Comment: Foslie (Citation1891) described and illustrated three specimens from the same locality. Adey (Citation1970c) noted that the lectotype, designated by Adey & Lebednik (Citation1967: 71), comprised two sets of specimens and that the selected set had a specimen pictured in the original description, but Adey (Citation1970c) did not indicate which of the three originally pictured specimens is the designated lectotype. Woelkerling (Citation1993: 97) and Woelkerling et al. (Citation2005: 366) provided information about the lectotype specimen illustrated in Foslie (Citation1891: pl. 2, bottom specimen) and marked with green tag ‘Præp. 151’. Based on morpho-anatomy, Athanasiadis (Citation2016: 228) listed L. fornicatum as incertae sedis; DNA sequence data have confirmed the placement of the species in synonymy with L. soriferum.
Lithothamnion granii (Foslie) Foslie, Citation1900: 11.
BASIONYM: Lithothamnion flabellatum f. granii Foslie, Citation1895: 98, pl. 17, figs 1–7, pl. 22, fig. 1.
HOMOTYPIC SYNONYMS: Lithothamnion glaciale var. granii (Foslie) Rosenvinge, Citation1917: 222, figs 138–142, pl. 3, fig. 4; pl. 4: figs 1–4; Lithothamnion glaciale f. granii (Foslie) Foslie Citation1905a: 10.
LECTOTYPE: TRH C10-3114, 12.vii.1893, no habitat data, leg. H. H. Gran (designated by Adey & Lebednik, Citation1967: 78).
TYPE LOCALITY: Drøbak, Norway.
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536917.
Comment: The specimen sequenced is located in a large, round blue box; the label indicates illustrations in Foslie (Citation1895: pl. 17, fig. 3 and pl. 22, fig. 1) and Printz (Citation1929: pl. 18, fig. 15). Foslie (Citation1895: 98, pl. 17, figs 1–7) described this new form, listed numerous syntype localities and illustrated seven specimens. Later, Foslie (Citation1900) elevated this form to specific rank. Adey & Lebednik (Citation1967) lectotypified the specimens collected by Gran, who is honoured by the form name. Subsequent to the original description, Foslie named five additional forms, f. robustum (Foslie, Citation1895), f. grandifrons, f. sphaericum (Foslie, Citation1900), and f. obcrateriforme and f. tuberculatum (Foslie, Citation1905a). We have not sequenced type material of any of these forms, hence they are not listed as synonyms. Based on morpho-anatomy, Athanasiadis (Citation2016: 228) listed L. granii as incertae sedis; DNA sequence data have confirmed the placement of the species in synonymy with L. soriferum.
Lithothamnion nodulosum Foslie, Citation1895: 144, pl. 21, figs 1–6.
HOMOTYPIC SYNONYM: Lithothamnion nodulosum f. typicum Foslie, Citation1905a: 62, nom. inval.
LECTOTYPE: TRH C5-2999, 18.vii.1894, leg. M. H. Foslie (designated by Woelkerling, Citation1993: 158).
TYPE LOCALITY: Brækstad (now Brekstad), Trond-heimsfjorden, Norway (Woelkerling et al., Citation2005: 414).
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536857.
Comment: We sequenced one of the three rhodoliths comprising the lectotype from Brekstad, Norway, and the sequence was identical to L. soriferum. The specimen sequenced is preserved in a round, red box; it is illustrated in Printz (Citation1929: pl. 25, fig. 2) and not in pl. 21 as marked on the box (see Woelkerling et al., Citation2005: 414). The lectotype is narrowed herein to the sequenced specimen. Based on morpho-anatomy, Athanasiadis (Citation2016: 228) listed L. nodulosum as incertae sedis; DNA sequence data have confirmed the placement of the species in synonymy with L. soriferum.
Lithothamnion sonderi f. sublaevigatum Foslie, Citation1905a: 24.
HOLOTYPE: TRH B15-2426, 21.vii.1902, leg. M. H. Foslie.
TYPE LOCALITY: The islet in front of the lighthouse, Røvær, Norway (Woelkerling, et al., Citation2005: 323).
Holotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536887.
Comment: The partial rbcL sequence of the holotype is identical over its entire length to the corresponding type sequences of L. soriferum. This is the only type specimen of a synonym of L. soriferum that is an encrusting, epilithic coralline; all others are rhodoliths. The holotype material comprised six epilithic crusts, with the piece illustrated in Printz (Citation1929: pl. 4, fig. 8) in a separate box (Woelkerling, Citation1993: 211). We sequenced the specimen located separately within a blue, round box that corresponds to the piece illustrated in plate 4, fig. 8 (Printz, Citation1929).
Lithothamnion soriferum f. divaricatum Foslie, Citation1891: 41, pl. 3, fig. 2.
LECTOTYPE: TRH C11-3161, 5.viii.1882, leg. M. H. Foslie (designated by Woelkerling, Citation1993: 79).
TYPE LOCALITY: Tromsø, Norway.
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536891.
Comment: Woelkerling et al. (Citation2005: 437) noted that there was one specimen in the lectotype collection with a green tag: ‘Lith. Mon. pl. 20, f. 9’ (corresponding to Printz, Citation1929: pl. 20, fig. 9), and another specimen with the annotation, ‘Specimen matches Foslie Citation1891a, pl. 3, fig. 2, lower left’. Previously, Woelkerling, (Citation1993: 79) designated as lectotype element the collection containing this latter specimen because no further type material had been found at that time. The specimen selected for sequencing was the specimen with the green tag ‘Lith. Mon. pl. 20, f. 9’ (corresponding to Printz, Citation1929: pl. 20, fig. 9) among the five rhodoliths contained in the lectotype collection. The lectotype is narrowed herein to the sequenced specimen. Based on morpho-anatomy, Athanasiadis (Citation2016: 228) listed L. soriferum f. divaricatum as incertae sedis; DNA sequence data have confirmed the placement of the taxon in L. soriferum.
Lithothamnion uncinatum Foslie, Citation1895: 154, pl. 19, figs 11–14.
HOMOTYPIC SYNONYMS: Lithothamnion calcareum f. uncinatum (Foslie) Foslie, Citation1897: 9; Lithothamnion norvegicum f. uncinatum (Foslie) Foslie, Citation1900: 13.
HOLOTYPE: TRH C3-2998, 1890, leg. unknown.
TYPE LOCALITY: Kragerø, Norway.
Holotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536848.
Comment: According to Woelkerling (Citation1993: 231), the holotype collection comprises several rhodolith specimens illustrated in Foslie (Citation1895: pl. 19, figs 11–14). The specimen depicted in Foslie (Citation1895: pl. 19, fig. 11) was sequenced, and is identical to L. soriferum. Athanasiadis (Citation2016: 231) listed this name as a synonym of Lithothamnion norvegicum, which he considers incertae sedis (see below).
Historical collection
UPS A648806, as Lithothamnion intermedium, Mestervik, Tromsø, Norway, 20.ix.1890, leg. Foslie. DNA sequence: rbcL-263 (bp 1172–1434), GenBank MW536906 (Supplementary table S1).
Lithothamnion tophiforme (Esper) Unger, Citation1858: 21, pl. 5, fig. 14.
BASIONYM: Millepora polymorpha f. tophiformis Esper, Citation1789: pl. XV (Millepora).
NEOTYPE: TRH C12-3179, no date, no habitat data, leg. C. Ryberg (designated by Adey, Citation1970c).
TYPE LOCALITY: Julianehaab, Greenland.
Neotype DNA sequence: rbcL-263 (bp 1172–1464), GenBank MW536858.
Comment: We sequenced the neotype designated by Adey et al. (Citation2005), and the GenBank sequences used in Adey et al. (Citation2015b) are in agreement with the neotype sequence.
HETEROTYPIC SYNONYMS:
Lithothamnion alcicorne Kjellman, Citation1883: 121, pl. 5, figs 1–8.
HOMOTYPIC SYNONYMS:
Lithothamnion soriferum f. alcicorne Foslie, Citation1891: 41, pl. 3, fig. 4; Lithothamnion tophiforme f. alcicorne Foslie, Citation1895: 147 (as ‘alcicornis’).
LECTOTYPE: TRH C13-3203 (designated herein), 5.viii.1882, leg. M. H. Foslie.
TYPE LOCALITY: Tromsø, Norway.
Lectotype DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536851.
Comment: The rbcL sequence obtained is identical to the neotype sequence of L. tophiforme. Kjellman (Citation1883: 122) stated that this species was from Tromsø and that Foslie was the collector. Woelkerling et al. (Citation2005: 443) called TRH C13-3203 syntype material. The specimen selected for sequencing and designated herein as lectotype was illustrated by Printz (Citation1929: pl. 19, fig. 11). Athanasiadis (Citation2016: 221) cited this species as incertae sedis; DNA sequence data have confirmed the synonymy of the species with L. tophiforme.
Historical collection
TRH C3-2948, Viprandsund, Haugesund, Norway, 17.v.1897, leg. M. H. Foslie.
DNA sequence: rbcL-293 sequence (bp 1172–1464), GenBank MW536878; identical to the neotype sequence of L. tophiforme.
Comment: This collection is topotype material of Lithothamnion norvegicum (Areschoug) Kjellman (Citation1883: 122, basionym: Lithothamnion calcareum var. norvegicum Areschoug, Citation1875: 4). The box contains a large collection of rhodoliths but the specimen sequenced is located separately within a small box marked ‘Prep. 522’.
Areschoug (Citation1875: 4) cited Wittrock as the collector of L. calcareum var. norvegicum, but he did not illustrate material, nor indicate a type. We have been unable to locate any material in either L or UPS where other Areschoug material is located. Eleven of Foslie’s collections in TRH are listed under L. norvegicum (Woelkerling et al., Citation2005: 407–408), but only the sequenced specimen is topotype material. A collection by Wittrock is in TRH (C3-2985) and illustrated in Printz (Citation1929: pl. 16, figs 37–38) but the date of collection and habitat data are lacking, making it difficult to know if this is type material. Woelkerling & Verheij (Citation1995: 67) noted syntype material is present in L. The material in TRH and in L needs to be sequenced to understand the correct application of L. norvegicum.
‘Lithothamnion’ species and infraspecific taxa that belong in Phymatolithon
Phymatolithon calcareum (Pallas) W.H.Adey & McKibbin
Lithothamnion squarrulosum f. palmatifida Foslie, Citation1899: 6.
LECTOTYPE: TRH C1-2892, 12.v.1893, leg. L. K. Rosenvinge; designated by Woelkerling et al. (Citation2005: 398).
TYPE LOCALITY: Fladen, østl. (= Eastern) Kattegat, Denmark (Woelkerling et al., Citation2005: 398).
DNA sequences: psbA and rbcL-293 (bp 1172–1464), GenBank MW564559 and MW536910, respectively.
Comment: Collection consisted of several rhodoliths. The specimen tagged with a blank green label was selected for DNA sequencing. The rbcL sequence differed by 2 bp from the neotype sequence of P. calcareum, while the psbA sequences were identical (neotype specimen BM000712373, Supplementary table S3).
Phymatolithon scabriusculum (Foslie) V.Peña, P.W.Gabrielson & Hughey, comb. nov.
BASIONYM: Lithothamnion scabriusculum Foslie, Citation1895: 170.
HOLOTYPE: TRH C20-3502, 5-10 fathoms, 2.viii.1887, leg. M. H. Foslie.
Type locality: Kjelmø (now Kjelmøya), Finnmark, Norway (Woelkerling et al., Citation2005: 493).
DNA sequence: rbcL-293 (bp 1172–1464), GenBank MW536920.
Comment: The largest fragment preserved in a small box marked ‘520’ (further information in Woelkerling et al., Citation2005: 493) was selected for DNA sequencing. Identical rbcL sequences were obtained independently by VP and by PWG/JRH, which differed by 1–3 bp from the GenBank sequences of P. rugulosum (vouchers US 170942 and BM000659095) collected in Gulf of Maine and Helgoland, Germany, respectively (Adey et al., Citation2015b). By the rule of priority (Art. 11.4 of the ICN, Turland et al., Citation2018), this name must be adopted for P. rugulosum.
HETEROTYPIC SYNONYM:
Phymatolithon rugulosum W.H.Adey, Citation1964: 381, figs 15–20, 27–29, 35–36, 39–44, 51–64.
HOLOTYPE: Adey 61-41A-3 in MICH, 2.xi.1961, 3–5 m depth, leg. W. H. Adey.
TYPE LOCALITY: Merchant Island, East Penobscot Bay, Maine.
Phymatolithon squamulosum (Foslie) W.H.Adey, Hernandez-Kantun & P.W.Gabrielson
BASIONYM: Lithothamnion squamulosum Foslie, Citation1895: 183.
HOLOTYPE: TRH B5-1962, vii. 1894, leg. P. Boye.
TYPE LOCALITY: Sogn, Sulen (now Sula), indre (= inner), Stensund (now Steinsund), Norway (Woelkerling et al., Citation2005: 265).
Holotype DNA sequences: psbA and rbcL-293 (bp 1172–1464), GenBank MW564558 and MW536870, respectively.
Comment: The collection comprises a larger box with two smaller boxes, one square and one round (Woelkerling et al., Citation2005: 265). The specimen in the round box with the annotation ‘Prep. 65’ was sequenced. Recently this taxon was transferred to Phymatolithon according to the molecular data obtained from an isotype preserved in BM (BM000044670, Box 434, Adey et al., Citation2018). The DNA sequencing of the holotype confirms this result; the rbcL sequences of both type collections were identical.
Recent collections
Norway: Krøttøya (Troms) and Averøya. Intertidal to subtidal (9 m depth), encrusting on cobble, pebbles and shells, and on hard substrata. Specimens with uniporate (gametangial or carposporangial) and multiporate conceptacles. DNA sequences: psbA (Supplementary table S2).
N.B. We have not dealt with Lithothamnion sonderi Hauck in this paper as we have not sequenced the type material, even though the species is thought to be widespread in crustose forms at low light levels in the NE Atlantic, from Nordland (Norway) to northern Spain (Chamberlain & Irvine, Citation1994).
Discussion
Since the first DNA sequences from type specimens of geniculate (Gabrielson et al., Citation2011) and non-geniculate (Sissini et al., Citation2014) corallines were published, it has become increasingly clear that the primary method to unequivocally apply a historical name is to obtain DNA sequences from the type material to compare with sequences from other historical or more recently collected specimens (Martone et al., Citation2012; Hind et al., Citation2014a, b, Citation2015; Hernández-Kantún et al., Citation2015a, Citation2016; Richards et al., Citation2017, Citation2018; Gabrielson et al., Citation2018, Citation2019; Peña et al., Citation2018; Jeong et al., Citation2020; Maneveldt et al., Citation2020; Puckree-Padua et al., Citation2020). Herein, we have applied that methodology to the numerous species and infraspecific names of Arctic and subarctic non-geniculate corallines published by Kjellman (Citation1883, Citation1889) and later by Foslie (Citation1891, Citation1895, Citation1896, Citation1899, Citation1900, Citation1905a, b, Citation1908) and others.
With the exception of Lithothamnion tophiforme (Unger, Citation1858), first published as Millepora polymorpha var. tophiformis Esper (Citation1789), the oldest names applicable to Arctic and Subarctic non-geniculate corallines are those of Kjellman (Citation1877, Citation1883, Citation1889). By sequencing type material, Adey et al. (Citation2015b) confirmed the application of two of Kjellman’s names, L. compactum Kjellman (Citation1883) to Clathromorphum, and L. loculosum Kjellman (Citation1889) to Neopolyporolithon, and showed that a third, Lithothamnion foecundum Kjellman (Citation1883), currently placed in Leptophytum W.H.Adey, does not belong in that genus, but its generic position remains unresolved. Lectotype material of the oldest name, Lithophyllum arcticum Kjellman (Citation1877), collected at Uddebay, Novaya Zemlya, Russia, provided an earlier available name for Neopolyporolithon loculosum (Kjellman) W.H.Adey, P.W.Gabrielson, G.P.Johnson & Hernández-Kantún, namely N. arcticum (Kjellman) P.W.Gabrielson, S.C.Lindstrom & Hughey (Gabrielson et al., Citation2019). Lithothamnion flavescens Kjellman (Citation1883) was transferred to Leptophytum by Athananasiadis (Citation2016), based on morpho-anatomy, which has been shown to be problematic in correct generic placement of non-geniculate species (Hind et al., Citation2016, Citation2018; Gabrielson et al., Citation2019). Of the remaining six Kjellman names, five are treated herein, L. alcicorne, L. durum, L. glaciale, L. intermedium and L. soriferum. Attempts to amplify L. ungeri Kjellman (Citation1883) were unsuccessful. We sequenced 35 type specimens of Lithothamnion species and infraspecific taxa described by Foslie. Below we discuss the systematics of the Arctic and Subarctic Lithothamnion species that we recognize, including L. lemoineae, and one of the species of Clathromorphum, C. circumscriptum, for which we found an additional synonym. Distributions, habits and habitat data for each species are updated, including range maps for the Lithothamnion species.
Clathromorphum circumscriptum. DNA sequencing confirmed two earlier proposed heterotypic synonyms for this species, L. durum and L. evanescens, the first proposed by Foslie (Citation1900) and the second by Lebednik (Citation1977). Added to these is L. coalescens, which had been considered a synonym of C. compactum by Foslie (Citation1905a) and recently by Athanasiadis (Citation2016).
The habit and habitat of C. circumscriptum are provided by Adey et al. (Citation2015b), and the sequenced specimens confirm this information. The species is reported to be circum-Arctic ranging south to the Subarctic in both the Atlantic and Pacific Oceans based on morpho-anatomy and its distinct habit (Adey, Citation1965; Adey et al., Citation2013, Citation2015a, Citationb). In the NW Atlantic Subarctic, C. circumscriptum is generally more abundant at depths ≤10 m on moderately exposed rocky shores (Steneck, Citation1978; Adey & Hayek, Citation2011). DNA sequences confirm its presence in Greenland, but material from the Russian Arctic and NW Pacific Ocean has not been sequenced. DNA sequences from the western Gulf of Alaska are from specimens collected in mid- (GenBank MT732997) and high intertidal pools (MT733001). Other mid-pool (MT732990) and low intertidal collections (MT732992, MT732993, MT732996) from this area represent an undescribed species of Clathromorphum. Records based on morpho-anatomy from SE Alaska may also represent an undescribed species.
Lithothamnion glaciale. There is no doubt that the lectotype specimen designated herein is the one illustrated by Kjellman (Citation1883: pls 2, 3), despite multiple listed syntype localities. The partial rbcL sequence from the lectotype differs by 1 bp from all other sequences, including one from Spitzbergen. DNA sequencing also shows that 18 specific and infraspecific Lithothamnion taxa later named by Foslie are heterotypic synonyms of L. glaciale. Lithothamnion intermedium, described in the same publication as L. glaciale (Kjellman, Citation1883), was listed most recently by Athanasiadis (Citation2016) as incertae sedis. Kjellman provided three syntype localities for L. intermedium, but he himself only collected the specimen at Karlsøy (Carlsö); specimens from the other two localities (Tromsø and Vadsø) were collected by Foslie. In UPS only two specimens could be located, one from Karlsøy, collected by Kjellman and with a label in his handwriting, and the other collected by Foslie from Mestervik, Tromsø. The Kjellman specimen from Karlsøy is designated as the lectotype; its sequence differs by 1 bp from the L. glaciale lectotype. From its DNA sequence the specimen collected by Foslie is L. soriferum. Lithothamnion glaciale and L. intermedium were published at the same time and are the same species so either name can be used for this species. We selected L. glaciale because of its long-standing use by the coralline research community and because the lectotype material is homotypic, whereas L. intermedium mostly has been ignored.
The habit and habitat of L. glaciale were described by Adey (Citation1966a) and Adey et al. (Citation2005) based primarily on NW Atlantic material identified by morpho-anatomy. Specimens ranged from epilithic crusts to free living rhodoliths. More recently, encrusting epilithic forms of L. glaciale have been reported to be very common from the low intertidal to the photic limit (Adey & Hayek, Citation2011). Most collections, however, are from the low intertidal to a depth of ~15 m, more a reflection of collecting limits than the species’ true vertical distribution. Numerous studies over the past two decades have documented the presence of rhodoliths throughout the North Atlantic and Arctic at depths of ~3 to 50 m, and while most studies have assumed L. glaciale is the main species, little to no corroborative DNA sequencing work has been carried out (Halfar et al., Citation2000; Blake & Maggs, Citation2003; Kamenos & Law, Citation2010; Gagnon et al., Citation2012; Teichert et al., Citation2012, Citation2014; Adey et al., Citation2015a; Millar & Gagnon, Citation2018; Schoenrock et al., Citation2018b; Bélanger & Gagnon, Citation2020; Teed et al., Citation2020). Although this assumption is legitimate given the ubiquity of L. glaciale in both oceans, morphological deviations from the norm in a few L. glaciale rhodoliths from Newfoundland and Labrador suggests that rhodoliths may also include other species of corallines such as L. tophiforme and C. compactum (D. Bélanger & P. Gagnon, unpublished data). In Norway, Anglés d´Auriac et al. (Citation2019) reported multi-species maerl beds mainly composed of L. glaciale and species of Phymatolithon and Lithophylllum.
We confirm by DNA sequencing the presence of L. glaciale throughout the North Atlantic (). We have not confirmed many of the Arctic Ocean reports. The observation of uniporate conceptacles (mostly carposporangial) and multiporate tetra/bisporangial conceptacles in our collections from Norway confirmed the common occurrence of gametophytes and tetra/bisporophytes for this species, as suggested in the literature (e.g. Chamberlain & Irvine, Citation1994).
Fig. 2: Distribution of Lithothamnion glaciale obtained for collections analysed in the molecular studies, type collections and historical specimens
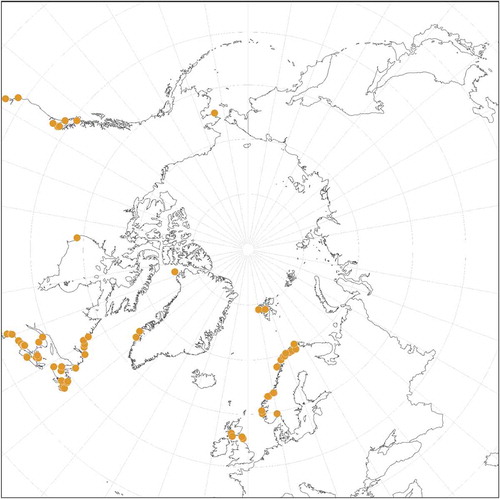
In the North Pacific L. glaciale was first reported by Saunders (Citation1901: 442) from Prince William Sound, Kukak Bay and the Shumagin Islands, identified by Kjellman. Although we have not confirmed the identity of these specimens, Bringloe & Saunders (Citation2019) reported this species from Nome, Alaska, USA (Bering Strait), and we have sequenced specimens from Malcolm Island, central British Columbia, Canada, southwards to Monterey County, California, USA (). In the NE Pacific, only encrusting epilithic specimens were found, although they may completely cover pebbles so that they appear to be rhodoliths. We cannot confirm reports based on morpho-anatomy from Japan and Arctic Russia, but L. glaciale is probably present in those areas as well. Reports of this species from any tropical and warm temperate regions (see AlgaeBase; Guiry & Guiry, Citation2020) are highly improbable. Reports from the Subantarctic (Heydrich, Citation1900; Lemoine, Citation1913) need to be confirmed.
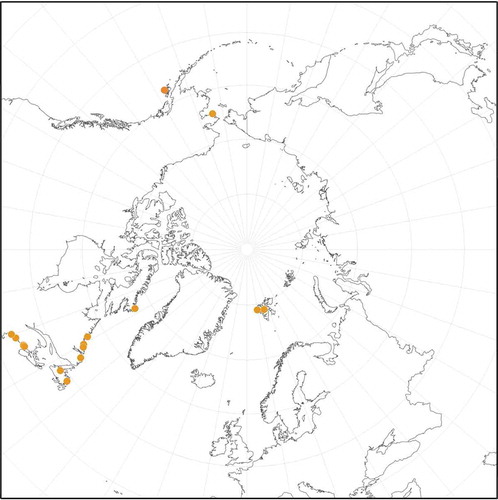
Lithothamnion lemoineae. Melbourne et al. (Citation2017) provided a psbA sequence from the holotype of L. lemoineae from the NW Atlantic (Maine, USA) to unequivocally link DNA sequences from field-collected material to the name. Based on DNA sequenced specimens, we expand the habit of L. lemoineae from encrusting epilithic to epiphytic, to epizoic on shells, and as rhodoliths. The species can also occur in the intertidal, as well as in the shallow subtidal to 12 m depth. In the NW Atlantic Subarctic, L. lemoineae often dominates rock on exposed shores (Adey & Hayek, Citation2011), particularly at 10–15 m depth (R. Steneck, pers. obs.).
Melbourne et al. (Citation2017) found that specimens from England, thought to be L. lemoineae based on morpho-anatomy (Chamberlain & Irvine, Citation1994), were not that species based on DNA sequencing. However, based on DNA sequenced material, we corroborated a recent record of L. lemoineae from the same region in the NE Atlantic (Svalbard, Norway; Hofmann & Heesch, Citation2018), and likewise Bringloe & Saunders (Citation2019) have the first confirmed record from the NE Pacific at Nome, Alaska, USA. Here, we confirm its occurrence on Kodiak Island, Gulf of Alaska (GenBank MT733005), on low intertidal bedrock. Its report from the NW Pacific by Lee (Citation2008) needs to be verified by DNA sequencing. We also confirm its distribution in the NW Atlantic from Labrador, Canada to Maine, USA ().
Fig. 3: Distribution of Lithothamnion lemoineae obtained for collections analysed in the molecular studies, type collections and historical specimens
Lithothamnion soriferum. We located in UPS three specimens of L. soriferum in an envelope with a label in Kjellman’s handwriting including from one of the cited localities, Carlsö (now Karlsøy). These appear to have been illustrated and published by Kjellman (Citation1883: pl. 1, figs 1–4) with some artistic licence. This is similar to what we found for L. durum (Kjellman, Citation1889) and L. glaciale (Kjellman, Citation1883); the specimens are convincing matches to the illustrations. DNA sequences from type specimens of seven species and infraspecific taxa later named by Foslie are all exact matches to the lectotype specimens of L. soriferum. The recently described L. erinaceum (Melbourne et al., Citation2017) also is a heterotypic synonym of L. soriferum, which has had a chequered history, sometimes recognized as a distinct species (Foslie, Citation1905a; Zinova, Citation1955) but mostly being considered a synonym of L. tophiforme (Foslie, Citation1895; Lund, Citation1959; Jaasund, Citation1965; Lee, Citation1969; Adey, Citation1970a; Vinogradova, Citation2010). Interestingly, Adey et al. (Citation2005), in a detailed examination of L. tophiforme, did not mention L. soriferum, and, most recently, Athanasiadis (Citation2016) treated the species as incertae sedis.
Based on DNA sequences, L. soriferum is a distinct species and occurs as an epilithic, epiphytic or epizoic (specimen on a worm tube) crust or as a free-living rhodolith. Specimens are primarily subtidal to 27 m depth, but can be intertidal. In contrast to L. glaciale, gametangial plants have not been observed in any of the collections. Only multiporate tetra/bisporangial conceptacles were observed, as indicated in the type collections of two heterotypic synonyms (L. breviaxe and L. granii, Woelkerling et al., Citation2005) and in the literature (Melbourne et al., Citation2017).
The species is widespread in the central and eastern North Atlantic (Greenland, Iceland, UK, Norway), but there is no evidence of its occurrence in the NW Atlantic; in the NE Pacific there are sporadic records from the Aleutian Islands (Robinson et al., Citation2017) and Prince William Sound (Konar et al., Citation2006), Alaska, USA south to Gwaii Haanas, British Columbia, Canada (). For such a widespread distribution, this species appears uncommon compared with L. glaciale. In the first report of L. soriferum from the NE Pacific (Konar et al., Citation2006), this species was misidentified as Phymatolithon calcareum based on morpho-anatomy. This appears to be because the cell types in the cross-sectional image (Konar et al., Citation2006: fig. 3B) were misidentified. The figure clearly shows flared epithallial cells characteristic not of any Phymatolithon species, but of Lithothamnion species. All reports of P. calcareum outside boreal NE Atlantic and the Mediterranean Sea waters based on morpho-anatomy are doubtful and need to be confirmed by DNA sequences.
Fig. 4: Distribution of Lithothamnion soriferum obtained for collections analysed in the molecular studies, type collections and historical specimens
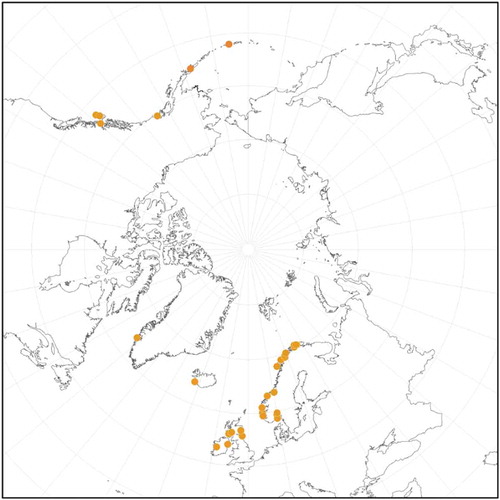
Lithothamnion tophiforme. The sequence of the neotype specimen, TRH C12-3179, designated by Adey et al. (Citation2005), confirms the identity of the specimen used by Adey et al. (Citation2015b) to represent this species. Adey (Citation1970a) reported this species to be encrusting, especially on shells or shell fragments, and also growing as free-living rhodoliths. All of the sequenced specimens to date have been rhodoliths. Adey et al. (Citation2005) considered L. tophiforme an Arctic species, but also stated that its abundance in the high Arctic is unknown, a situation that remains unchanged. They also noted that it is found only in colder waters below 10 m depth and at temperatures below 10°C, and the sequenced specimens confirm this pattern as all were collected below 15 m depth. All recently collected sequenced specimens are from the NW Atlantic, from Newfoundland, Canada northward (). The neotype specimen is the only confirmed specimen from Greenland, and only two historical specimens from the 19th century are from Norway, where the species was not found recently in an extensive collection effort presented herein.
Fig. 5: Distribution of Lithothamnion tophiforme obtained for collections analysed in the molecular studies, type collections and historical specimens
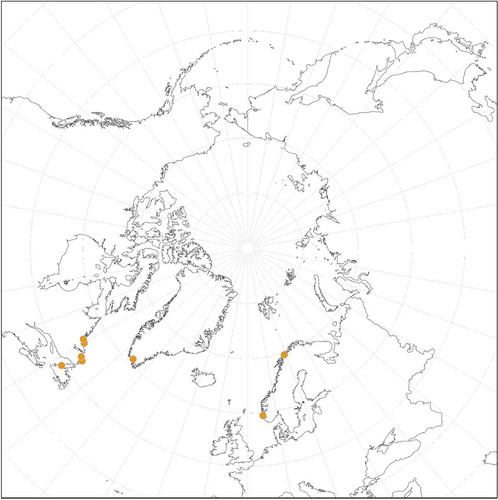
In the NW Atlantic Subarctic, L. tophiforme rhodoliths commonly co-occur with L. glaciale rhodoliths, dominating at depths >25 m (Adey et al., Citation2015a). In rhodolith beds of both species, thalli of L. tophiforme and L. glaciale sometimes merge, forming multi-species rhodoliths with a characteristic colour mosaic with L. tophiforme generally more brownish-orangy in colour than L. glaciale (D. Bélanger & P. Gagnon, unpublished data). Our sequenced specimens of L. tophiforme show a variety of shapes and sizes, from a few cm-long twig-like thalli, to large (>10 cm across) branching spheroidal rhodoliths. A few specimens presented a distinct growth form with fanned branches. The phenotypic plasticity of L. tophiforme highlights the importance of DNA sequencing for identification.
Identifying non-geniculate coralline algae to species
These Arctic and Subarctic Lithothamnion species exemplify the difficulties of identifying non-geniculate coralline species using morpho-anatomy. DNA sequencing has revealed that by the first decade of the 20th century, 26 specific and infraspecific names had been given to three species: L. glaciale, L. soriferum and L. tophiforme. Recently, based on morpho-anatomy, the vast majority of these were listed as incertae sedis by Athanasiadis (Citation2016) including, for example L. alcicorne, L. breviaxe and L. soriferum. In the >100 years since these had been named and examined by coralline morpho-anatomists, their identity could not be determined with any certainty. There remain another 21 species or infraspecific taxa of Lithothamnion named by Foslie, 20 from Norway and one from Scotland, from which we were unable to amplify DNA using PCR. It is highly unlikely, however, that any of these names would apply, due to the extensive sequencing of Norwegian specimens reported herein and the rule of priority. With the exception of L. lemoineae, which has never been recorded from Norway, the applied Lithothamnion names “predate” any of those published by Foslie.
Further complicating the naming of specimens without DNA sequencing is that all of these species can occur either as encrusting corallines attached to a substrate or as unattached rhodoliths – encrusting a core or not – sometimes occurring singly, but also in beds. Lithothamnion lemoineae previously had only been reported to occur as an epilithic crust, whereas L. soriferum (also as L. erinaceum) had not been known as an epilithic crust. In some regions, species can have restricted morphologies, for example in Norway L. glaciale is found as an epilithic crust, or as free-living maerl, whereas in the NE Pacific it has so far only been reported as an epilithic crust. And three of the four species, L. glaciale, L. lemoineae and L. soriferum, can occur from the intertidal to, at minimum, 12 m depth.
The finding by DNA sequencing that four Arctic and Subarctic Lithothamnion species had been named as multiple specific and infraspecific taxa using morpho-anatomy is clearly opposed to the cryptic diversity commonly recorded in temperate corallines (e.g. Pardo et al., Citation2014, Citation2017; Hernández-Kantún et al., Citation2015a, b; Peña et al., Citation2015a, b; Richards et al., Citation2018; Pezzolesi et al., Citation2019). However, this plethora of specific and infraspecific names in the Arctic and Subarctic regions was primarily the work of Foslie, who, as illustrated in the taxonomic results, changed his mind numerous times about which taxa should be recognized and at what rank. In tropical regions, DNA sequencing of non-geniculate corallines has shown that some species are widely distributed, whereas most have local distributions (Sissini et al., Citation2014; Peña et al., Citation2014b; Hernández-Kantún et al. Citation2016; Gabrielson et al., Citation2018; Maneveldt et al., Citation2019).
In the Arctic and Subarctic additional species of Lithothamnion and Clathromorphum need to be recognized based on the DNA sequencing reported herein (Supplementary table S2). It is also likely that the Arctic and Subarctic Lithothamnion species will need to be transferred to a new genus, as the generitype of Lithothamnion, L. muelleri Lenormand ex Rosanoff, belongs in a different clade (Jeong et al., Citation2020).
It is critical in this time of rapid ocean warming and acidification, particularly in polar regions, that we have a firm understanding of the taxa currently present in order to document future changes in their habitats and distributions. Importantly, the biogeography of coralline algae appears especially sensitive to ocean thermogeography (Adey & Steneck, Citation2001; Adey & Hayek, Citation2011). The relevance of non-geniculate coralline algae in these regions as ecosystem engineers cannot be overstated, whether occuring as encrusting species attached primarily to rock substratum (Freiwald & Henrich, Citation1994; Adey et al., Citation2005, Citation2015a) or as free-living maerl (Pardo et al., Citation2014; Teichert, Citation2014; Teed et al., Citation2020). As polar seas warm and become increasingly acidified, these coralline algal species will either be forced to live at lower depths, where they will be limited by the availability of photosynthetically active radiation through the water column, or they will become extinct. DNA barcoding of organisms in these habitats, coupled with DNA sequencing of type and historical specimens, provides the foundation to document these imperiled species.
Supplementary note 1. Type collections and historical specimens DNA-extracted but unsuccessfully sequenced for psbA and rbcL.
Supplementary table S1. Type collections and historical specimens sequenced in the study.
Supplementary table S2. Collection and vouchering details for the recent specimens sequenced in the study. For each species, specimens are sorted by collection areas: NE Atlantic, NW Atlantic and NE Pacific.
Supplementary table S3. List of GenBank accession numbers used in this study with details of locality information and references.
Supplementary fig. S1. Maximum likelihood (ML) tree of COI-5P included in the present sudy. Bootstrap ML values >50% shown for each node. Scale bar: 0.05 substitutions per site.
Supplementary fig. S2. Phylogenetic tree inferred from RAxML analyses of psbA sequences included in the present study. Bootstrap ML values >50% and posterior probabilities >0.50 from Bayesian inference shown for each node. Scale bar: 0.02 substitutions per site.
Supplementary fig. S3. Morpho-anatomy of Lithothamnion glaciale, UPS A-000202. Fig. A. Thallus habit and herbarium labels including label handwritten by Kjellman. Fig. B. Vertical fracture of protuberance showing radial construction and location of overgrown and buried conceptacle (black arrow). Fig. C. Vertical fracture of protuberance with conceptacle (black arrow) overgrown by a secondary hypothallus (arrowheads). Fig. D. Magnified view of secondary hypothallus (bracket) over conceptacle roof. Fig. E. Perithallus with cell fusions (white arrows). Fig. F. Perithallus (lower bracket), intercalary meristem (middle bracket, *), and a single-layered epithallus (upper bracket) of flared cells (e), one with cell roof intact (white arrow). Scale bars: Figs B–C = 100 µm; Figs D–F = 10 µm.
Supplementary fig. S4. Morpho-anatomy of Lithothamnion soriferum, UPS A-648809. Fig. A. Thallus habit of specimens and herbarium label handwritten by Kjellman. Figs B–D. Vertical fractures of protuberance showing radial construction. Fig. E. Magnified view of secondary hypothallus (arrowhead, bracket) over the thallus surface of the older growth layer. Fig. F. Perithallus (lower bracket) with cell fusions (white arrows), intercalary meristem (middle bracket, *), and epithallus (upper bracket). Figs G–H. Magnified view of meristematic cells (*) and a single-layered epithallus of flared cells (e), one with cell roof intact (white arrow, Fig. H). Scale bars: Fig. A = 2 cm. Figs B–D = 100 µm. Figs E–H = 10 µm.
Author contributions
V. Peña: original concept, type sequencing, writing, contemporary collections; D. Bélanger: writing, contemporary collections; P. Gagnon: writing, contemporary collections; J.L. Richards: morpho-anatomy of type specimens, writing; L. Le Gall: type sequencing, distribution maps, writing, contemporary collections; J. R. Hughey: type sequencing, writing; G.W. Saunders: sequencing of contemporary collections, contemporary collections; S.C. Lindstrom: sequencing of contemporary collections, writing, contemporary collections; E. Rinde: led the field sampling in the CoralAlg project that funded the analysis of TRH specimens, writing, contemporary collections; V. Husa: sampling of type collections at the TRH, led the field sampling in the AKVAKYST III project, contemporary collections; H. Christie: contemporary collections; S. Fredriksen: writing, contemporary collections; J.M. Hall-Spencer: writing, contemporary collections; R.S. Steneck: writing, contemporary collections; K.M. Schoenrock: contemporary collections; J. Gitmark: contemporary collections; E.S. Grefsrud: contemporary collections; M.B. Anglès d’Auriac: writing, contemporary collections; E. Legrand: contemporary collections; J. Grall: contemporary collections; T.F. Mumford: contemporary collections; N.A. Kamenos: writing, contemporary collections; P.W. Gabrielson: original concept, type sequencing, writing, contemporary collections.
Supplemental Material
Download PDF (194.8 KB)Supplemental Material
Download PDF (146.6 KB)Supplemental Material
Download MS Word (101.1 KB)Supplemental Material
Download MS Word (22 KB)Supplemental Material
Download MS Word (25.4 KB)Supplemental Material
Download TIFF Image (8 MB)Supplemental Material
Download TIFF Image (6 MB)Supplemental Material
Download MS Word (29.8 KB)Acknowledgements
VP and PWG are very grateful to the following curators and institutions for the loan of type specimens critical to this study: Tommy Presto and Kristian Hassel (TRH) for Foslie specimens; Stefan Ekman (UPS) for Kjellman specimens. VP thanks L. Hoffman and J. Büdenbender for providing specimens from Svalbard; PWG thanks B. Konar for providing specimens from Prince William Sound, Alaska. Acquisition of part of the molecular data was carried out at the Service de Systématique Moléculaire of the Muséum National d’Histoire Naturelle (CNRS - UMS 2700). JLR acknowledges NSF grant DEB–1754504 for rhodolith research to Suzanne Fredericq that funded a portion of this research. PWG thanks Wilson Freshwater, DNA Analysis Core Facility, University of North Carolina, Wilmington for sequencing support and Todd Vision, University of North Carolina, Chapel Hill for research space and equipment. A portion of this research was completed while PWG was a visiting professor at the Friday Harbor Labs, University of Washington. We are grateful to the anonymous reviewers for their helpful comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2021.1880643
Additional information
Funding
References
- Adey, W.H. (1964). The genus Phymatolithon in the Gulf of Maine. Hydrobiologia, 24: 377–420.
- Adey, W.H. (1965). The genus Clathromorphum in the Gulf of Maine. Hydrobiologia, 26: 539–573.
- Adey, W.H. (1966a). The genera Lithothamnion, Leptophytum (nov. gen.) and Phymatolithon in the Gulf of Maine. Hydrobiologia, 28: 321–370.
- Adey, W.H. (1966b). Distribution of saxicolous crustose corallines in the northwestern North Atlantic. Journal of Phycology, 2: 49–54.
- Adey, W.H. (1970a). The crustose corallines of the northwestern North Atlantic, including Lithothamnium lemoineae n. sp. Journal of Phycology, 6: 225–229.
- Adey, W.H. (1970b). Some relationships between crustose corallines and their substrate. Soc. Sciencia Islandica, 2: 21–25.
- Adey, W.H. (1970c). A revision of the Foslie crustose coralline herbarium. Det Kongelige Norske Videnskabers Selskabs Skrifter, 1: 1–46.
- Adey, W.H. (1971). The sublittoral distribution of crustose corallines on the Norwegian coast. Sarsia, 46: 41–58.
- Adey W.H. (1973). Temperature control of reproduction and productivity in a subarctic coralline alga. Phycologia, 12: 111–118.
- Adey, W.H. & Adey, P.J. (1973). Studies of the biosystematics and ecology of the epilithic crustose Corallinaceae of the British Isles. British Phycological Journal, 8: 343–407.
- Adey, W.H., Chamberlain, Y.M. & Irvine, L.M. (2005). An SEM-based analysis of the morphology, anatomy and reproduction of Lithothamnion tophiforme (Esper) Unger (Corallinales, Rhodophyta), with a comparative study of associated North Atlantic Arctic/Subarctic Melobesioideae. Journal of Phycology, 41: 1010–1024.
- Adey, W.H., Halfar, J., Humphreys, A., Suskiewicz, T., Belanger, D., Gagnon, P. & Fox, M. (2015a). Subarctic rhodolith beds promote longevity of crustose coralline algal buildups and their climate archiving potential. Palaios, 30: 281–293.
- Adey, W.H., Halfar, J. & Williams, B. (2013). Biological, physiological and ecological factors controlling high magnesium carbonate formation and a precision Arctic/Subarctic marine climate archive: the coralline genus Clathromorphum emend Adey. Smithsonian Contributions to Marine Science, 40: 1–41.
- Adey, W.H. & Hayek, L.-A.C. (2011). Elucidating marine biogeography with macrophytes: quantitative analysis of the North Atlantic supports the thermogeographic model and demonstrates a distinct Subarctic region in the Northwestern Atlantic. Northeastern Naturalist, 18: 1–128.
- Adey, W.H., Hernández-Kantún, J.J., Gabrielson. P.W., Nash, M.C. & Hayek, L.-A.C. (2018). Phymatolithon (Melobesioideae, Hapalidiales) in the Boreal–Subarctic Transition Zone of the North Atlantic: A Correlation of Plastid DNA Markers with Morpho-Anatomy, Ecology, and Biogeography. The Smithsonian Institution, Washington, DC.
- Adey, W.H., Hernández-Kantún, J.J., Johnson, G. & Gabrielson. P.W. (2015b). DNA sequencing, anatomy and calcification patterns support a monophyletic, subarctic, carbonate reef-forming Clathromorphum (Hapalidiaceae, Corallinales, Rhodophyta). Journal of Phycology, 51: 189–203.
- Adey, W.H. & Lebednik, P. (1967). Catalog of the Foslie Herbarium. Kongelige Norske Videnskabers Selskab Museet, Trondheim.
- Adey, W.H., Lindstrom, S.C., Hommersand, M.H. & Müller, K.M. (2008). The biogeographic origin of Arctic endemic seaweeds: a thermogeographic view. Journal of Phycology, 44: 1384–1394.
- Adey W.H., Masaki, T. & Akioka, H. (1976). The distribution of crustose corallines in eastern Hokkaido and the biogeographic relationships of the flora. Bulletin of the Faculty of Fisheries, Hokkaido University, 4: 303–313.
- Adey, W.H. & McKibbin, D. (1970). Studies of the maerl species of the Ria de Vigo. Botanica Marina, 8: 100–106.
- Adey, W.H. & Steneck, R. (2001). Thermogeography over time creates biogeographic regions: a temperature/space/time-integrated model and an abundance-weighted test for benthic marine algae. Journal of Phycology, 37: 677–698.
- Amado-Filho, G.M., Maneveldt, G.W., Pereira-Filho, G.H., Manso, R.C.C., Bahia, M.B., Barros-Barreto, M.B. & Guimaraes, S.M.P.B. (2010). Seaweed diversity associated with a Brazilian tropical rhodolith bed. Ciencias Marinas, 36: 371–391.
- Anglés d’Auriac, M.B., Le Gall, L., Peña, V., Hall-Spencer, J.M., Steneck, R.S., Fredriksen, S., Gitmark, J., Christie, H., Husa, V., Grefsrud, E.S. & Rinde, E. (2019) Efficient coralline algal psbA mini barcoding and High Resolution Melt (HRM) analysis using a simple custom DNA preparation. Scientific Reports, 578: 578.
- Areschoug, J.E. (1875). Observationes phycologicae. Particula tertia. De algis nonnullis scandinavicis et de conjuctione Phaeozoosporarum Dictyosiphonis hippuroidis. Nova Acta Regiae Societatis Scientiarum Upsaliensis, Series 3, 10(1): 1–36.
- Athanasiadis, A. (2016). Phycologia Europaea Rhodophyta Vol. I. Thessaloniki: Published and distributed by the author.
- Bélanger, D. & Gagnon, P. (2020) Low growth resilience of subarctic rhodoliths (Lithothamnion glaciale) to coastal eutrophication. Marine Ecology Progress Series, 642: 117–132.
- Blake, C. & Maggs, C. (2003). Comparative growth rates and internal banding periodicity of maerl species (Corallinales, Rhodophyta) from northern Europe. Phycologia, 42: 606–612.
- Bringloe, T.T. & Saunders, G.W. (2019). DNA barcoding of the marine macroalgae from Nome, Alaska (Northern Bering Sea) reveals many trans-Arctic species. Polar Biology, 42: 851–864.
- Brodie, J., Williamson, C., Smale, D., Kamenos, N., Mieszkowska, N., Santos, R., Cunliffe, M., Steinke, M., Yesson, C., Anderson, K., Asnaghi, V., Brownlee, C., Burdett, H., Burrows, M., Collins, S., Donohue, P., Harvey, B., Foggo, A., Noisette, F., Nunes, J., Ragazzola, F., Raven, J., Schmidt, D., Suggett, D., Teichberg, M. & Hall-Spencer, J. (2014). The future of the northeast Atlantic benthic flora in a high CO2 world. Ecology and Evolution, 4: 2787–2798.
- Chamberlain, Y.M. & Irvine, L.M. (1994). Melobesioideae Bizzozero. In Seaweeds of the British Isles. Volume 1. Rhodophyta Part 2B Corallinales, Hildenbrandiales (Irvine, L.M. & Chamberlain, Y.M., editors), 159–234. London: HMSO.
- Chan, P., Halfar, J., Adey, W.H., Lebednik, P.A., Steneck, R.S., Norley, C.J.D. & Holdsworth, D.W. (2020). Recent density decline in wild-collected subarctic crustose coralline algae reveals climate change signature. Geology, 48: 226–230.
- Copeland, A., Edinger, E., Devillers, R., Bell, T., LeBlanc, P. & Wroblewski, J. (2013) Marine habitat mapping in support of Marine Protected Area management in subarctic fjord: Gilbert Bay, Labrador, Canada. Journal of Coastal Conservation, 17: 225–237.
- Darriba, D., Taboada, G.L., Doallo, R. & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9: 772.
- Esper, E.J.C. (1789). Die Pflanzenthiere. In Abbildungen nach der Natur mit Farben erleuchtet nebst Beschreibungen. Erster Theil. Part 4: 169–196. Nürnberg: Raspe.
- Foslie, M. (1891). Contribution to the knowledge of the marine algae of Norway. II. Species from different tracts. Tromsø Museums Aarshefter, 14: 36–58.
- Foslie, M. (1895). The Norwegian forms of Lithothamnion. Det Kongelige Norske Videnskabers Selskabs Skrifter, 1894: 29–208.
- Foslie, M. (1896). New or critical Lithothamnia. Det Kongelige Norske Videnskabers Selskabs Skrifter, 1895: 1–10.
- Foslie, M. (1897). On some Lithothamnia. Det Kongelige Norske Videnskabers Selskabs Skrifter, 1897: 1–20.
- Foslie, M. (1898a). Systematical survey of the Lithothamnia. Det Kongelige Norske Videnskabers Selskabs Skrifter, 1898(2): 1–7.
- Foslie, M. (1898b). List of species of the Lithothamnia. Det Kongelige Norske Videnskabers Selskabs Skrifter, 1898(3): 1–11.
- Foslie, M. (1899). Some new or critical Lithothamnia. Det Kongelige Norske Videnskabers Selskabs Skrifter, 1898(6): 1–19.
- Foslie, M. (1900). Revised systematical survey of the Melobesieae. Det Kongelige Norske Videnskabers Selskabs Skrifter, 1900: 1–22.
- Foslie, M. (1905a). Remarks on northern lithothamnia. Det Kongelige Norske Videnskabers Selskabs Skrifter, 1905 (3): 1–138.
- Foslie, M. (1905b). Lithothamnion vardöense a new alga. Det Kongelige Norske Videnskabers Selskabs Skrifter, 1905: 1–4.
- Foslie, M. (1908). Algologiske notiser V. Det Kongelige Norske Videnskabers Selskabs Skrifter, 1908: 1–20.
- Foster, M. (2001). Rhodoliths: between rocks and soft places. Journal of Phycology, 37: 659–667.
- Freiwald, A. & Henrich, R. (1994). Reefal coralline algal build-ups within the Arctic Circle: morphology and sedimentary dynamics under extreme environmental seasonality. Sedimentology, 41: 963–984.
- Gabrielson, P.W., Hughey, J.R. & Diaz-Pulido, G. (2018). Genomics reveals abundant speciation in the coral reef building alga Porolithon onkodes (Corallinales, Rhodophyta). Journal of Phycology, 54: 429–434.
- Gabrielson, P.W., Lindstrom, S.C. & Hughey, J.R. (2019). Neopolyporolithon loculosum is a junior synonym of N. arcticum comb. nov. (Hapalidiales, Rhodophyta), based on sequencing type material. Phycologia, 58: 229–233.
- Gabrielson, P.W., Miller, K.A. & Martone, P.T. (2011). Morphometric and molecular analyses confirm two species of Calliarthron (Corallinales, Rhodophyta), a genus endemic to the northeast Pacific. Phycologia, 50: 298–316.
- Gagnon, P., Matheson, K. & Stapleton, M. (2012). Variation in rhodolith morphology and biogenic potential of newly discovered rhodolith beds in Newfoundland and Labrador (Canada). Botanica Marina, 55: 85–99.
- Gouy, M., Guindon, S. & Gascuel, O. (2010). SeaView Version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution, 27: 221–224.
- Guiry, M.D. & Guiry, G.M. (2020). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 6 October 2020.
- Halfar, J., Adey, W.H., Kronz, A., Edinger, E. & Fitzhugh, W. (2013). Arctic sea-ice decline archived by muticentury annual resolution record from crustose coralline algal proxy. Proceedings of the National Academy of Sciences USA, 110: 19737–19741.
- Halfar, J., Zack, T., Kronz, A. & Zachos, C. (2000). Growth and high-resolution paleoenvironmental signals of rhodoliths (coralline red algae): a new biogenic archive. Journal of Geophysical Research, 105: 107–116.
- Hernández-Kantún, J.J., Gabrielson, P.W., Hughey, J.R., Pezzolesi, L., Rindi, F., Robinson, N.M., Peña, V., Riosmena-Rodriguez, R., Le Gall, L. & Adey, W.H. (2016). Reassessment of branched Lithophyllum spp. (Corallinales, Rhodophyta) in the Caribbean Sea with global implications. Phycologia, 55: 609–635.
- Hernández-Kantún, J.J., Rindi, F., Adey, W.H., Heesch, S., Peña, V., Le Gall, L. & Gabrielson, P.W. (2015a). Sequencing type material resolves the identity and distribution of the generitrype Lithophyllum incrustans, and related European species L. hibernicum and L. bathyporum (Corallinales, Rhodophyta). Journal of Phycology, 51: 791–807.
- Hernández-Kantún, J.J., Riosmena-Rodriguez, R., Hall-Spencer, J.M., Peña, V., Maggs, C.A. & Rindi, F. (2015b). Phylogenetic analysis of rhodolith formation in the Corallinales (Rhodophyta). European Journal of Phycology, 50: 46–61.
- Heydrich, F. (1900). Les Lithothamniées de l’Expédition Antarctique. Bulletin de la Classes des Sciences de l’Académie royale de Belgique, 1900: 560–566.
- Hind, K.R., Gabrielson, P.W., Jensen, C.P. & Martone, P.T. (2016). Crusticorallina gen. nov., a non-geniculate genus in the subfamily Corallinoideae (Corallinales, Rhodophyta). Journal of Phycology, 52: 929–941.
- Hind, K.R., Gabrielson, P.W., Jensen, C.P. & Martone, P.T. (2018). Evolutionary reversals in Bossiella (Corallinales, Rhodophyta): first report of a coralline genus with both geniculate and nongeniculate species. Journal of Phycology, 54:788–798.
- Hind, K.R., Gabrielson, P.W., Lindstrom, S.C. & Martone, P.T. (2014a). Misleading morphologies and the importance of sequencing type specimens for resolving coralline taxonomy (Corallinales, Rhodophyta): Pachyarthron cretaceum is Corallina officinalis. Journal of Phycology, 50: 760–764.
- Hind, K.R., Gabrielson, P.W. & Saunders, G.W. (2014b). Molecular-assisted alpha taxonomy reveals pseudocryptic diversity among species of Bossiella (Corallinales, Rhodophyta) in the eastern Pacific Ocean. Phycologia, 53: 443–456.
- Hind, K.R., Miller, K.A., Young, M., Jensen, C., Gabrielson, P.W. & Martone, P.T. (2015). Resolving cryptic species of Bossiella (Corallinales, Rhodophyta) using contemporary and historical DNA. American Journal of Botany, 102: 1–19.
- Hofmann, L.C. & Heesch, S. (2018). Latitudinal trends in stable isotope signatures and carbon-concentrating mechanisms of northeast Atlantic rhodoliths. Biogeosciences, 15: 6139–1649.
- Hughey, J. & Gabrielson, P.W. (2012). Comment on “Acquiring DNA sequence data from dried archival red algae (Florideophyceae) for the purpose of applying available names to contemporary genetic species: a critical assesssment”. Botany, 90: 1191–1194.
- Hughey, J.R., Silva, P.C. & Hommersand, M.H. (2001). Solving taxonomic and nomenclatural problems in Pacific Gigartinaceae (Rhodophyta) using DNA from type material. Journal of Phycology, 37: 1091–1109.
- Jaasund, E. (1965). Aspects of the marine algal vegetation of North Norway. Botanica Gothoburgensia, 4: 5–174.
- Jeong, S.Y., Nelson, W., Sutherland, J.E., Peña, V., Le Gall, L., Díaz-Pulido, G., Won, B.Y. & Cho, T.O. (2020). Corallinapetrales and Corallinapetraceae: a new order and family of coralline red algae including Corallinapetra gabrieli comb. nov. Journal of Phycology (in press). doi: https://doi.org/10.1111/jpy.13115.
- Jørgensbye, H.I.Ø. & Halfar, J. (2017). Overview of coralline red algal crusts and rhodolith beds (Corallinales, Rhodophyta) and their possible ecological importance in Greenland. Polar Biology, 40: 517–531.
- Kamenos, N.A., Burdett, H.L., Aloisio, E., Findlay, H.S., Martin, S., Longbone, C., Dunn, J., Widdicombe, S. & Calosi, P. (2013). Coralline algal structure is more sensitive to rate, rather than the magnitude, of ocean acidification. Global Change Biology, 19: 3621–3628.
- Kamenos, N.A. & Law, A. (2010). Temperature controls on coralline algal skeletal growth. Journal of Phycology, 46: 331–335.
- Kamenos, N.A., Perna, G., Gambi, M.C., Micheli, F. & Kroeker, K.J. (2016). Coralline algae in a naturally acidified ecosystem persist by maintaining control of skeletal mineralogy and size. Proceedings of the Royal Society B: Biological Sciences, 283: 20161159.
- Kendrick, M.R., Huryn, A.D., Bowden, W.B., Deegan, L.A., Findlay, R.H., Hershey, A.E., Peterson, B.J., Beneš, J.P. & Schuettet, E.B. (2018). Linking permafrost thaw to shifting biogeochemistry and food web resources in an arctic river. Global Change Biology, 24: 5738–5750.
- Kjellman, F.R. (1877). Bidrag till Kännedomen om Kariska hafvets Algvegetation. Öfversigt af Kongl. Vetenskaps-Akademiens Forhandlingar, 2: 3–30.
- Kjellman, F.R. (1883). Norra Ishafvets algflora. Vega-expeditionens Vetenskapliga Iakttagelser, 3: 1–431.
- Kjellman, F.R. (1889). Om Beringhafvets algflora. Kongl. Svenska Vetenskaps-Akademiens Handlingar, 23: 1–58.
- Konar, B., Riosmena-Rodriguez, R. & Iken, K. (2006). Rhodolith bed: a newly discovered habitat in the North Pacific Ocean. Botanica Marina, 49: 355–359.
- Lebednik, P. (1977 ‘1976’). The Corallinaceae of Northwestern North America. I. Clathromorphum Foslie emend. Adey. Syesis, 9: 59–112.
- Lee, R.K.S. (1969). A collection of marine algae from Newfoundland. II. Le Naturaliste Canadien, 96: 123–145.
- Lee, Y.P. (2008). Marine algae of Jeju. Academy Publication, Seoul.
- Lemoine, M. (1913). Mélobésiées. Revision des Mélobésiées antarctiques. In Deuxième Expédition Antarctique Française (1908–1910) commandée par le Dr. Jean Charcot, Sciences Naturelles: Documents Scientifiques, Botanique (Masson et Cie, editors), vol. 1, 1–67. Paris.
- Lindstrom, S.C. & Fredericq, S. (2003). rbcL gene sequences reveal relationships among north-east Pacific species of Porphyra (Bangiales, Rhodophyta) and a new species, P. aestivalis. Phycological Research, 51: 211–224.
- Lund, S. (1959). The marine algae of East Greenland. I. Taxonomic Part. Meddelser om Grønland, 156: 1–247.
- Maneveldt, G.W., Gabrielson, P.W., Townsend, R.A. & Kangwe, J. (2019). Lithophyllum longense (Corallinales, Rhodophyta): a species with a widespread Indian Ocean distribution. Phytotaxa, 419: 149–168.
- Maneveldt, G.W., Jeong, S.Y., Cho, T.O., Hughey, J.R. & Gabrielson, P.W. (2020). Reassessment of misapplied names, Phymatolithon ferox and P. repandum (Hapalidiales, Corallinophycidae, Rhodophyta) in South Africa, based on DNA sequencing of type and recently collected material. Phycologia, 59: 449–455.
- Martin, S. & Hall-Spencer, J.M. (2017). Effects of ocean warming and acidification on rhodolith/maërl beds. In Rhodolith/Maërl Beds: A Global Perspective (Riosmena-Rodríguez, R., Nelson, W. & Aguirre, J., editors), 55–85. Springer International Publishing, Cham.
- Martone, P.T., Lindstrom, S.C., Miller, K.A. & Gabrielson, P.W. (2012). Chiharaea and Yamadaia (Corallinales, Rhodophyta) represent reduced and recently derived articulated coralline morphologies. Journal of Phycology, 48: 859–868.
- McCoy, S.J. & Kamenos, N.A. (2018). Coralline algal skeletal mineralogy affects grazer impacts. Global Change Biology, 24: 4775–4783.
- Melbourne, L.A., Hernández-Kantún, J.J., Russell, S. & Brodie, J. (2017). There is more to maerl than meets the eye: DNA barcoding reveals a new species in Britain, Lithothamnion erinaceum sp. nov. (Hapalidiales, Rhodophyta). European Journal of Phycology, 52: 166–178.
- Millar, K. & Gagnon, P. (2018). Mechanisms of stability of rhoodlith beds: sedimentological aspects. Marine Ecology Progress Series, 594: 65–83.
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environment Workshop (GCE), 1–8.
- Nelson W.A. (2009). Calcified macroalgae – critical to coastal ecosystems and vulnerable to change: a review. Marine and Freshwater Research, 60: 787–801.
- Pardo, C., Barbara, I., Barreiro, R. & Peña, V. (2017). Insights into species diversity of associated crustose coralline algae (Corallinophycidae, Rhodophyta) with Atlantic European maerl beds using DNA barcoding. Anales del Jardín Botánico de Madrid, 74: e059.
- Pardo, C., Lopez, L., Peña, V., Hernández-Kantún, J., Le Gall, L., Bárbara, I. & Barreiro, R. (2014). A multilocus species delimitation reveals a striking number of species of coralline algae forming maerl in the OSPAR maritime area. PLoS ONE, 9: e104073.
- Pearce, C.M. & Scheibling, R.E. (1990). Induction of metamorphosis of larvae of the green sea urchin, Strongylocentrotus droebachiensis, by coralline red algae. Biological Bulletin, 179: 304–3011.
- Peña, V., Bárbara, I., Grall, J., Maggs, C.A. & Hall-Spencer, J.M. (2014a). The diversity of seaweeds on maerl in the NE Atlantic. Marine Biodiversity, 44: 533–551.
- Peña, V., De Clerck, O., Afonso-Carrillo, J., Ballesteros, E., Bárbara, I., Barreiro, R. & Le Gall, L. (2015b). An integrative systematic approach to species diversity and distribution in the genus Mesophyllum (Corallinales, Rhodophyta) in Atlantic and Mediterranean Europe. European Journal of Phycology, 50: 20–36.
- Peña, V., Hernández-Kantún, J.J., Adey, W.H. & Le Gall, L. (2018). Assessment of coralline species diversity in the European coasts supported by sequencing of type material: the case study of Lithophyllum nitorum (Corallinales, Rhodophyta). Cryptogamie Algologie, 39: 123–137.
- Peña, V., Pardo, C., López, L., Carro, B., Hernández-Kantún, J., Adey, W.H., Bárbara, I., Barreiro, R. & Le Gall, L. (2015a). Phymatolithon lusitanicum sp. nov. (Hapalidiales, Rhodophyta): the third most abundant maerl-forming species in the Atlantic Iberian Peninsula. Cryptogamie, Algologie, 36: 429–459.
- Peña, V., Rousseau, F., De Reviers, B. & Le Gall, L. (2014b). First assessment of the diversity of coralline species forming maerl and rhodoliths in Guadeloupe, Caribbean using an integrative systematic approach. Phytotaxa, 190: 190–215.
- Pezzolesi, L., Peña, V., Le Gall, L., Gabrielson, P.W., Kaleb, S., Hughey, J.R., Rodondi, G., Hernández-Kantún, J., Falace, A., Basso, D., Cerrano, C. & Rindi, F. (2019). Mediterranean Lithophyllum stictiforme (Corallinales, Rhodophyta) is a genetically diverse species complex: implications for species circumscription, biogeography and conservation of coralligenous habitats. Journal of Phycology, 55: 473–492.
- Printz, H. (1929). Contributions to a Monograph of the Lithothamnia. After the author’s death collected and edited by Henrik Printz. Aktietrykkeriet, Trondhjem [Trondheim].
- Puckree-Padua, C.A., Gabrielson, P.W., Hughey, J.R. & Maneveldt, G.W. (2020). DNA sequencing of type material reveals Pneophyllum marlothii comb. nov. from South Africa and P. discoideum comb. nov. (Chamberlainoideae, Corallinales, Rhodophyta) from Argentina. Journal of Phycology (in press). doi: 10.1111/jpy.13047‐20‐081.
- QGIS.org (2020). QGIS Geographic Information System. QGIS Association.:http//www.qgis.org.
- Ratnasingham, S. & Hebert, P.D.N. (2007). BOLD: The Bbarcode of Life Ddata Ssystem (:http//www.barcodinglife.org). Molecular Ecology Notes, 7: 355–364.
- Richards, J.L., Gabrielson, P.W., Hughey, J.R. & Freshwater, D.W. (2018). A re-evaluation of subtidal Lithophyllum species (Corallinales, Rhodophyta) from North Carolina, USA, and the proposal of L. searlesii sp. nov. Phycologia, 57: 318–330.
- Richards, J.L., Sauvage, T., Schmidt, W.E., Fredericq, S., Hughey, J.R. & Gabrielson, P.W. (2017). The coralline genera Sporolithon and Heydrichia (Sporolithales, Rhodophyta) clarified by sequencing type material of their generitypes and other species. Journal of Phycology, 53: 1044–1059.
- Riosmena-Rodriguez, R., Nelson, W. & Aguirre, J. (2017). Rhodolith/Maërl Beds: A Global Perspective. Springer International Publishing, Cham.
- Robinson, N.M., Fernández-García, C., Riosmena-Rodríguez, R., Rosas-Alquicira, E.F., Konar, B., Chenelot, H., Jewett, S.C., Melzer, R.R., Meyer, R., Försterra, G., Häussermann, V. & Macaya, E.C. (2017). Eastern Pacific. In Rhodolith/Maërl Beds: A Global Perspective (Riosmena-Rodríguez, R., Nelson, W. & Aguirre, J., editors), 319–333. Springer International Publishing, Cham.
- Ronquist, F. & Huelsenbeck, J. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572–1574.
- Rosenvinge, L.K. (1917). The marine algae of Denmark. Part II. Rhodophyceae II (Cryptonemiales). Kongelige Danske Videnskabernes Selskabs Skrifter, 7. Række, Naturvidenskabelig og Mathematisk Afdeling, 7: 153–284.
- Rowley, R.J. (1989). Settlement and recruitment of sea urchins (Strongylocentrotus spp.) in a sea-urchin barren ground and a kelp bed: are populations regulated by settlement or post-settlement processes? Marine Biology, 100: 485–494.
- Saunders, De A. (1901). Papers from the Harriman Alaska Expedition XXV. The algae. Proceedings of the Washington Academy of Sciences, 3: 391–486.
- Saunders, G.W. & McDevit, D.C. (2012). Methods for DNA barcoding photosynthetic protists emphasizing the macroalgae and diatoms. In DNA Barcodes: Methods and Protocols (Kress, W.J. & Erickson, D.L., editors), 207–222. Humana Press, Totowa, NJ.
- Saunders, G.W. & Moore, T.E. (2013). Refinements for the amplification and sequencing of red algal DNA barcode and RedToL phylogenetic markers: a summary of current primers, profiles and strategies. Algae, 28: 31–43.
- Schoenrock, K.M., Bacquet, M., Pearce, D., Rea, B.R., Schofield, J.E., Lea, J., Mair, D. & Kamenos, N. (2018a). Influences of salinity on the physiology and distribution of the Arctic coralline algae, Lithothamnion glaciale (Corallinales, Rhodophyta). Journal of Phycology, 54: 690–702.
- Schoenrock K.M., Vad, J., Muth, A., Pearce, D.M., Rea, B.R., Schofield, J.E. & Kamenos, N.A. (2018b). Biodiversity of kelp forest and coralline algae habitats in southwestern Greenland. Diversity, 10: 117.
- Sissini, M.N., Oliveira, M.C., Gabrielson, P.W., Robinson, N.M., Okolodkov, Y.B., Riosmena-Rodriguez, R. & Horta, P.A. (2014). Mesophyllum erubescens (Corallinales, Rhodophyta) – so many species in one epithet. Phytotaxa, 190: 299–319.
- Stamatakis, A. (2014). RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312–1313.
- Steneck, R.S. (1978). Factors influencing the distribution of crutose coralline algae (Rhodophyta, Corallinaceae) in the Damariscotta River Estuary, Maine. Thesis. University of Maine, USA.
- Steneck, R.S. (1982). A limpet-coralline alga association: adaptations and defenses between a selective herbivore and its prey. Ecology, 63: 507–522.
- Strömfelt, H.F.G. (1886). Einige für die Wissenschaft neue Meeresalgen aus Island. Botanisches Zentralblatt, 26: 172–173.
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution, 30: 2725–2729.
- Teed, L., Bélanger, D., Gagnon, P. & Edinger, E. (2020). Calcium carbonate (CaCO3) production of a subpolar rhodolith bed: methods of estimation, effect of bioturbators, and global comparisons. Estuarine and Coastal Shelf Science, 242: 106822.
- Teichert, S. (2014). Hollow rhodoliths increase Svalbard’s shelf biodiversity. Scientific Reports, 4: 6972.
- Teichert, S., Woelkerling, W., Rüggeberg, A., Wisshak, M., Piepenburg, D., Meyerhöfer, M., Form, A., Büdenbender, J. & Freiwald, A. (2012). Rhodolith beds (Corallinales, Rhodophyta) and their physical and biological environment at 80° 31’ in Nordkappbukta (Nordaustlandet, Svalbard Archipelago, Norway). Phycologia, 51: 371–390.
- Teichert, S., Woelkerling, W., Rüggeberg, A., Wisshak, M., Piepenburg, D., Meyerhöfer, M., Form, A. & Freiwald, A. (2014). Arctic rhodolith beds and their environmental controls (Spitsbergen, Norway). Facies, 60: 15–37.
- Thiers, B. (2021). Index Herbariorum: A global directory of public herbaria and associated staff. New York: Botanical Garden’s Virtual Herbarium. available at: http://sweetgum.nybg.org/ih/.
- Turland, N.J., Wiersema, J.H., Barrie, F.R., Greuter, W., Hawksworth, D.L., Herendeen, P.S., Knapp, S., Kusber, W.-H., Li, D.-Z., Marhold, K., May, T.W., McNeill, J., Monro, A.M., Prado, J., Price, M.J. & Smith, G.F. (eds.) (2018). International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten.
- Unger, F. (1858). Beiträge zur näheren Kenntniss des Leithakalkes, namentlich der vegetabilischen Einschlüsse und der Bildungsgeschichte desselben. Denkschriften der Kaiserlichen Akademie der Wissenschaften [Wein], Mathematisch-naturwissenschaftliche Klasse, 14: 13–35.
- Vinogradova, K.L. (2010). Taxonomic review of the Corallinales (Rhodophyta) in the northern Russian seas. Botanicheskii Zhurnal (St. Petersburg), 95: 667–681.
- Walvoord, M.A. & Striegl, R.G. (2007). Increase groundwater to stream discharge fom permafrost thawing in the Yukon River Basin: Potential impacts on lateral export of carbon and nitrogen. Geophysical Research Letters, 34: L12402.
- Wassmann, P., Duarte, C.M., Agustí, S. & Sejr, M.K. (2011). Footprints of climate change in the Arctic marine ecosystem. Global Change Biology, 17: 1235–1249.
- Williams, B., Chan, P.T.W., Halfar, J., Hargan, K. & Adey, W. (2020). Arctic crustose coralline alga resilient to recent environmental change. Limnology and Oceanography. (in press). doi: https://doi.org/10.1002/lno.11640.
- Woelkerling, W.J. (1993). Type collections of Corallinales (Rhodophyta) in the Foslie Herbarium (TRH). Gunneria, 67: 1–289.
- Woelkerling, W.J., Gustavsen, G., Myklebost, H.E., Prestø, T. & Såstad, S.M. (2005). The coralline red algal herbarium of Mikael Foslie: revised catalogue with analyses. Gunneria, 77: 1–625.
- Woelkerling, W.J. & Verheij, E. (1995). Type collections of nongeniculate corallines (Rhodophyta) in the Rijksherbarium (L), Leiden University, The Netherlands. Blumea, 40: 33–90.
- Wynne, M.J. (1995). F. R. Kjellman. Phycological Newsletter, 31: 2–3, available at www.psaalgae.org.
- Yoon, S. H., Hackett, J. D. & Bhattacharya, D. (2002). A single origin of the peridinin- and fucoxanthin- containing plastids in dinoflagellates through tertiary endosymbiosis. Proceedings of the National Academy of Sciences, 99: 11724–11729.
- Zinova, A.D. (1955). Opredelitel burykh vodoroslej severnykh morej SSSR [Determination Book of the Red Algae of the Northern Seas of the USSR]. Akad. Nauk SSSR, Moscow & Leningrad.
