ABSTRACT
Background
The variation of serum lipid levels can be part-related to certain genes. One such gene, SLCO1B1, encodes a transporter that may have a role in lipid metabolism. We hypothesised that differences in certain SLCO1B1 genotypes are related to levels of serum lipids.
Materials and methods
We recruited 636 subjects who were genotyped for SLCO1B1 variants *1a, *1b, *5 and *15. Routine liver function tests, renal function tests and routine lipid indices were measured by standard techniques.
Results
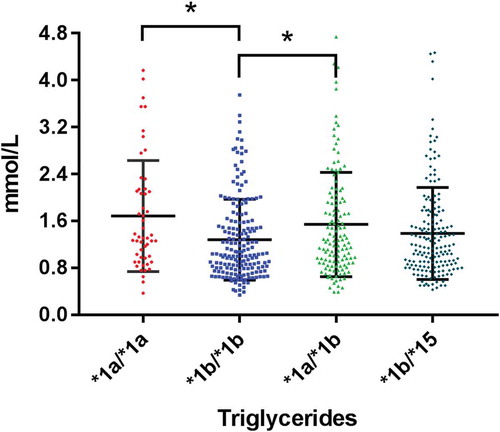
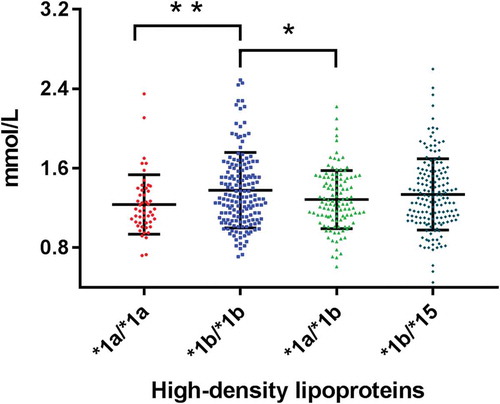
The most frequent genotypes were *1b/*1b (29.3%), *1b/*15 (27.5%), *1a/*1b (21.1%), *1a/*15 and *1b/*5 (10.2%) and *1a/*1a (8.5%). There were significant differences in levels of triglycerides and HDL in the four SLCO1B1 genotypes *1a/*1a, *1b/*1b, *1a/*1b and *1b/*15 (all p < 0.05).
Conclusion
The genotypes *1a/*1a and *1a/*1b indicate a high risk of cardiovascular disease, while the *1b/*1b group may have a relatively low risk. SLCO1B1 may be involved in the metabolism of triglycerides and HDL. We have provided a tool for identifying potentially high-risk groups that could be helpful for early diagnosis and prevention, individualized drug therapy and even gene therapy.
Introduction
Dyslipidaemia is a major risk factor for cardiovascular disease, serum levels being determined by diet and the genetic-controlled production, and accordingly, serum triglyceride, cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) are commonly used in screening. A well-documented lipid-lowering therapy (statins) reduces the risk of death or cardiovascular events in populations with or without a history of coronary artery disease [Citation1,Citation2].
The organic anion-transporting polypeptide 1B1 (OATP1B1), encoded by solute carrier organic anion transporter family member SLCO1B1, is a hepatic uptake transporter predominantly expressed in the basolateral side of hepatocytes [Citation3]. OATP1B1 has a role in the transport of a wide variety drugs, such as statins. Several pharmacogenetic studies and meta-analyses have focused on the potential contribution of SLCO1B1 variations in the response to statins or repaglinide, but little attention has been paid to whether SLCO1B1 is involved in lipoprotein transformation and metabolic process that affects individual lipid levels [Citation4–15]. We therefore hypothesised that common variants of SLCO1B1 have an impact into levels of serum lipids, and so by extension, a potential role in risk assessment.
Materials and methods
We tested our hypothesis in a cohort of 636 subjects (352 men and 284 women) who visited the Physical Examination Centre in Foshan Hospital of Traditional Chinese Medicine from August 2019 to January 2020. We retrospectively reviewed the results of the subjects through laboratory information system. The study was approved by the local ethical committee at Foshan Hospital of Traditional Chinese Medicine and informed consent was obtained from each participant.
Blood samples were collected in 2 mL vacuum tubes containing EDTA. DNA was obtained by an extraction kit and matching NES-32 Nucleic acid extraction instrument (Aikang, Hangzhou, China) following the manufacturer’s instructions. DNA concentration was quantified using Bio Tek Gen5 Spectrophotometer (BioTek Instruments Inc., Vermont, USA). PCR was performed by ABI7500 PCR amplification fluorescence detector (Life Technologies, Waltham, USA) according to the following protocol: 37 °C for 10 minutes, predenaturation at 95 °C for 5 minutes, followed by 40 cycles at 95 °C for 15 seconds, and 60 °C for 45 seconds. The fluorescence signals were collected as FAM (SLCO1B1*1b 388A, SLCO1B1*5 521 T) and VIC (SLCO1B1*1b 388 G, SLCO1B1*5 521 C) and ROX (Internal standard genes) (PCR-fluorescence probe method) (Youzhiyou, Wuhan, China). A second sample of 3 mL was drawn in vacuum tubes containing separation gel, and centrifuged to separate the serum. The levels of glucose, total cholesterol, triglycerides, HDL, LDL, urea, alanine transaminase (ALT), aspartic transaminase (AST), gamma-glutamyl transferase (GGT), creatinine and uric acid were measured by ADVIA 2400 Automatic biochemical analyser (Siemens, Munich, Germany) and these showed that no participant had diabetes or abnormal liver or kidney function.
Data are presented as numbers and frequencies for categorical variables and as means and standard deviation (SD) for continuous variables. A 1-way analysis of variance (ANOVA) test was used in case of parametric data and one-way analyses of variance with multiple comparison post-test (LSD) was used to compare the means between SLCO1B1 genotype groups as indicated. P < 0.05 was considered to be significant. A Kruskal–Wallis test was used in case of nonparametric data. Statistical analysis was performed using the SPSS 21.0 software (SPSS; Inc, Armonk, USA).
Results
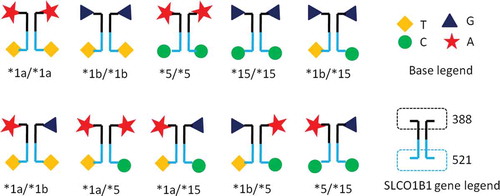
Demographic and laboratory characteristics of the 636 participants are shown in . There was no significant difference in age, sex or any of the biochemical markers when analysed by SLCO1B1 genotypes. A schematic of the various SLCO1B1 alleles is shown in . The most frequent genotypes were *1a/*1a, *1b/*1b, *1a/*1b and *1b/*15. We excluded genotypes *15/*15,*1a/*5 and *5/*5 for further analysis due to their small sample size, and genotypes *1a/*15 and *1b/*5 as for methodological reasons they cannot be separated.
Table 1. Demographic and laboratory characteristics of the study participants
Figure 1. Representation of possible SLCO1B1 allele/genotype combinations and their particular nucleotide composition
Analysis of the remaining 614 subjects is shown in . There were significant differences in serum triglycerides and HDL between the four genotypes. Triglycerides were highest in those genotypes *1a/*1a and *1a/*1b and lowest in *1b/*1b (). Levels of HDL were lowest in *1a/*1a and highest in genotype *1b/*1b (). Thus, genotype *1b/*1b has the preferred profile (lowest triglycerides and highest HDL) whilst genotype *1a/*1a has an unfavourable profile (highest triglycerides and lowest HDL).
Table 2. Association between SLCO1B1 genotypes and serum lipids
Discussion
Variation in numerous genes coding for drug transport and/or metabolic pathways are linked to levels of serum metabolites, including lipids. One of these genes, SLCO1B1 is located on chromosome 12, with single nucleotide polymorphisms (SNPs) (388A>G and 521 T > C) [Citation16,Citation17]. We found significant differences in the frequency of haplotypes SLCO1B1 *1a(388A-521 T), *1b(388 G-521 T), *5(388A-521 C), *15(388 G-521 T): *1b/*1b, *1b/*15, *1a/*1b, *1a/*15 and *1a/*1a are common, together accounting for 96.5% of our cohort. However, these data differ from those of Zhong et al. [Citation18], who found, for example, an *1b/*1b frequency of 40%, whereas in our population the frequency is 29%. The reasons for this are unclear but may reflect difference in recruitment in that their cohort is some five times larger than ours.
Organic anion-transporting polypeptide 1B1 (OATP1B1) is a membrane transport protein of the organic anion-transporting polypeptide (OATP) transporter superfamily, coded for by SLCO1B1 [Citation19–21]. A form of OATP1B1 coded by a mutant SLCO1B1 causes a decreased ability to transport proteins, which decreased ability of liver to ingestion drugs, resulting in increased blood concentration of statins, and increased risk of rhabdomyolysis or myopathy [Citation15,Citation22]. Hepatic uptake transporters such as OATP1B1 are well-recognized determinants of drug disposition. Therefore, it is important to gain insights into protein regions important for substrate recognition and transport [Citation23].
Genotypes of SLCO1B1 influence adverse reactions with statins [Citation18]. SLCO1B1 *5/*5, *5/*15, *15/*15 are high-risk genotypes with adverse reactions to statins. *1a/*5, *1a/*15, *1b/*15 genotypes are at medium risk.*1a/*1a,*1a/*1b,*1b/*1b genotypes are low risk [Citation24,Citation25]. Accordingly, we calculate that 2.7% of our cohort are at high risk for adverse statins reactions, a finding that promotes the view that those at high-risk need additional prevention and control guidance such as diet control and lifestyle improvement, and also regular health care screening, so enabling early diagnosis and targeted treatment.
In addition to raised serum cholesterol, the risk of coronary heart disease is increased in people with mild to moderate elevated serum triglycerides levels [Citation26]. It is now well accepted that triglycerides have independent effects on CHD risk. HDL can transport cholesterol in peripheral tissues to the liver for catabolic metabolism. The reverse transport of cholesterol can reduce the deposition of cholesterol in the vascular wall and play an anti-atherosclerosis role. High triglycerides or low HDL levels were also associated with an increased risk of atherosclerotic cardiovascular disease [Citation27]. The results showed that * 1a and * 1b were the most common haplotypes in SLCO1B1. Those with genotypes *1a/*1a and *1a/*1b were at high risk for cardiovascular disease (CVD) because they had higher triglyceride levels and lower HDL levels. The *1b/*1b group had lower triglycerides levels and higher HDL levels, so the risk of cardiovascular disease might be relatively low.
The important and essential action to prevent cardiometabolic diseases is to promote a healthy lifestyle throughout life. Common recommendations have been developed to guide actions for the primary prevention of cardiometabolic diseases among all populations [Citation26–28]. Should this prove difficult, drugs treatments are required, but these have side effects. SLCO1B1 genotypes affect the incidence of adverse reactions to statin therapy, and SLCO1B1 genotyping can be used to identify high-risk groups so that this knowledge may be useful in patients who are using or considering treatment with statins. We recognise certain limitations: the number of subjects per genotype is not large, and we cannot be certain that some were taking lipid-lowering therapy. Nevertheless, we have identified potentially high-risk SLCO1B1 genotypes that could be helpful for early diagnosis and prevention, individualized drug therapy, and even gene therapy.
This paper represents an advance in biomedical science because it points to a link between SNPs in SLCO1B1 and triglycerides and HDL.
Summary table
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Moran A, Gu D, Zhao D, et al. Future cardiovascular disease in China: markov model and risk factor scenario projections from the coronary heart disease policy Model-China. Circ Cardiovasc Qual Outcomes. 2010;5:243–252.
- Ishihara M. Intensive lipid-lowering therapy with statins for primary prevention. Circ J. 2012;76:55–56.
- Hoosain N, Pearce B, Jacobs C, et al. Mapping SLCO1B1 genetic variation for global precision medicine in understudied regions in Africa: a focus on Zulu and cape admixed populations. OMICS. 2016;20:546–554.
- Zhou S, Xiang Q, Mu G, et al. Effects of CYP2C8 and SLCO1B1 genetic polymorphisms on repaglinide pharmacokinetics: a systematic review and meta-analysis. Curr Drug Metab. 2019;20:266–274.
- Wu X, Gong C, Weinstock J, et al. Associations of the SLCO1B1 polymorphisms with hepatic function, baseline lipid levels, and lipid-lowering response to simvastatin in patients with hyperlipidemia. Clin Appl Thromb Hemost. 2018;24:241S–247S.
- Soko ND, Masimirembwa C, Dandara C. A cost effective RFLP method to genotype Solute carrier organic anion 1B1 (SLCO1B1) c.1929A>C (p.Leu643Phe, rs34671512); a variant with potential effect on rosuvastatin pharmacokinetics. BMC Res Notes. 2018;11:384.
- Wilke RA, Fanciullo J. Point-Counterpoint: SLCO1B1 genotyping for statins. S D Med. 2017;70:102–104.
- Wang Y, Tian Y, Lv P, et al. The effect of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and 2-hydroxyatorvastatin in healthy Chinese people. Pharmazie. 2017;72:365–368.
- Thakkar D, Dash RP. Pharmacokinetic interactions between glimepiride and rosuvastatin in healthy Korean subjects: does the SLCO1B1 or CYP2C9 genetic polymorphism affect these drug interactions? Observations and introspection of the bioanalysis. Drug Des Devel Ther. 2017;11:1263–1265.
- Mladenovska K, Grapci AD, Vavlukis M, et al. Influence of SLCO1B1 polymorphisms on atorvastatin efficacy and safety in Macedonian subjects. Pharmazie. 2017;72:288–295.
- Kitzmiller JP, Luzum JA, Dauki A, et al. Candidate-Gene Study of Functional Polymorphisms in SLCO1B1 and CYP3A4/5 and the Cholesterol-Lowering Response to Simvastatin. Clin Transl Sci. 2017;10:172‐177.
- Kim CO, Oh ES, Kim H, et al. Pharmacokinetic interactions between glimepiride and rosuvastatin in healthy Korean subjects: does the SLCO1B1 or CYP2C9 genetic polymorphism affect these drug interactions? Drug Des Devel Ther. 2017;11:503‐512.
- Jiang F, Choi JY, Lee JH, et al. The influences of SLCO1B1 and ABCB1 genotypes on the pharmacokinetics of simvastatin, in relation to CYP3A4 inhibition. Pharmacogenomics. 2017;18:459‐469.
- Khine H, Yuet WC, Adams-Huet B, et al. Statin-associated muscle symptoms and SLCO1B1 rs4149056 genotype in patients with familial hypercholesterolemia. Am Heart J. 2016;179:1‐9.
- Jiang J, Tang Q, Feng J, et al. Association between SLCO1B1-521T>C and −388A>G polymorphisms and risk of statin-induced adverse drug reactions: A meta-analysis. Springerplus. 2016;5:1368.
- Stojakovic N, Igic R. Simvastatin-lnduced nocturnal leg pain disappears with pravastatin substitution. Srp Arh Celok Lek. 2013;141:387–389.
- Scarpini F, Cappellone R, Auteri A, et al. Role of genetic factors in statins side-effects. Cardiovasc Hematol Disord Drug Targets. 2012;12:35‐43.
- Zhong Z, Wu H, Li B, et al. Analysis of SLCO1B1 and APOE genetic polymorphisms in a large ethnic Hakka population in southern China. J Clin Lab Anal. 2018;32:e22408.
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665.
- Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1‐18.
- Lee HH, Ho RH. Interindividual and interethnic variability in drug disposition: polymorphisms in organic anion transporting polypeptide 1B1 (OATP1B1; SLCO1B1). Br J Clin Pharmacol. 2017;83:1176–1184.
- Kameyama Y, Yamashita K, Kobayashi K, et al. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics. 2005;15:513‐522.
- Gruetz M, Sticht H, Glaeser H, et al. Analysis of amino acid residues in the predicted transmembrane pore influencing transport kinetics of the hepatic drug transporter organic anion transporting polypeptide 1B1 (OATP1B1). Biochim Biophys Acta. 2016;1858:2894‐2902.
- Wilke RA, Ramsey LB, Johnson SG, et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin Pharmacol Ther. 2012;92:112‐117.
- SEARCH Collaborative Group, Link E, Parish S, et al. SLCO1B1 variants and statin-induced myopathy–a genomewide study. N Engl J Med. 2008;359:789‐799.
- Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292‐2333.
- Ren J, Grundy SM, Liu J, et al. Long-term coronary heart disease risk associated with very-low-density lipoprotein cholesterol in Chinese: the results of a 15-year chinese multi-provincial cohort study (CMCS). Atherosclerosis. 2010;211:327‐332.
- Almeida AP, Rocha DMUP, Moreira AVB, et al. Personalized nutrition using PROCARDIO to reduce cardiometabolic risk in the academic community: a study protocol with preliminary results. J Am Coll Nutr. 2020;1‐10.