Abstract
The substrate specificity of the facilitated hexose transporter, GLUT, family, (gene SLC2A) is highly varied. Some appear to be able to translocate both glucose and fructose, while the ability to handle 2-deoxyglucose and galactose does not necessarily correlate with the other two hexoses. It has become generally accepted that a central substrate binding/translocation site determines which hexoses can be transported. However, a recent study showed that a single point mutation of a hydrophobic residue in GLUTs 2, 5 & 7 removed their ability to transport fructose without affecting the kinetics of glucose permeation. This residue is in the 7th transmembrane helix, facing the aqueous pore and lies close to the opening of the exofacial vestibule. This study expands these observations to include the other class II GLUTs (9 & 11) and shows that a three amino acid motif (NXI/NXV) appears to be critical in determining if fructose can access the translocation mechanism. GLUT11 can also transport fructose, but it has the motif DSV at the same position, which appears to function in the same manner as NXI and when all three residues are replaced with NAV fructose transport lost. These results are discussed in relation to possible roles for hydrophobic residues lining the aqueous pore at the opening of the exofacial vestibule. Finally, the possibility that the translocation binding site may not be the sole determinant of substrate specificity for these proteins is examined.
Introduction
The human facilitated hexose transporter gene family (SLC2A) is made up of at least 13 proteins (GLUTs), which share a high degree of homology Citation[1]. Based upon sequence alignments they have been grouped into three classes, of which the class I proteins are the best characterized, GLUTs 1, 2, 3, & 4 Citation[2]. Hydrophobicity analysis predicts that the secondary structure of all GLUT proteins consists of 12 transmembrane α-helices linked by loops of varying length. A key feature of this arrangement is the very long intracellular loop connecting α-helices 6 & 7, which is believed to allow for flexibility in the overall structure Citation[3]. Several attempts have been made to gain a better understanding of the tertiary structure of these proteins with the use of cysteine scanning mutagenesis. This type of analysis has led to progressively more refined models of GLUT1 and when combined with energy minimization computer modeling has led to the conclusion that up to eight α-helices contribute to the walls of the exofacial side of the transport pore, a structure which is stabilized by an outer set of 4 helices Citation[4]. The pore forming helices have been proposed to be No.'s 1, 2, 4, 5, 7, 8, 10 & 11, with the remaining helices 3, 6, 9 & 12 on the outside. However, this approach cannot predict more subtle elements of the structure such as how the helices may be tilted relative to each other and the plane of the lipid bilayer. Thus, it is possible that this predicted organization represents either, an average of the outward and inward facing conformations of the protein, or only the outward facing conformation. Additional structural analyses employing mathematical algorithms predict quite a complex arrangement of the helices, while still agreeing with some aspects of the model resulting from the cysteine scanning work. In addition, the resolution of two independent crystal structures for the bacterial transporters proteins Lac Y and GlpT have reinforced the view that there may exist quite a conserved arrangement within the membrane facilitator superfamily (MFS) Citation[5], Citation[6]. The application of the crystal structure coordinates for Lac Y and GlpT led to a recently proposed model for GLUT1 which again indicated that α-helix 7 does form part of the pore lining at the exofacial side Citation[7]. In a recent paper we investigated the role of the hydrophobic residue isoleucine (I314) within helix 7 of the human GLUT7 protein, which is predicted to lie just at the opening of the exofacial vestibule. When this residue was mutated to a valine, which is also hydrophobic but has a smaller molecular volume, the result was to abolish the ability of the protein to transport fructose, while the capacity to translocate glucose was fully retained Citation[8]. Two other members of the GLUT family which also transport fructose have an isoleucine at the position equivalent to I314 of GLUT7 and when mutated to valine these too lost the ability to transport fructose. It has largely been assumed that the specificity of facilitated hexose transporters is primarily conferred by the structure of a single binding pocket believed to lie within the core of the aqueous pore. However, when the mutant hGLUT7 was modelled in comparison with the GLUT1, which has a valine in the position equivalent to I314 of GLUT7, it appeared that there is the potential for a hydrophobic interaction between the isoleucine or valine and a tryptophan residue in helix 2 on the other side of the pore. This interaction appears to narrow the entrance to the pore and the valine induces more narrowing than the isoleucine at the same position. This led us to propose that these two hydrophobic residues formed part of a substrate selectivity filter analogous to those found in ion channels.
Therefore, the role of the hydrophobic residues expressed at the position ‘314’ of hGLUT7 has been investigated in more detail for all of the class II GLUTs; hGLUTs 5, 9 & 11. Comparison of the sequence alignments for these proteins indicated that while all of the class I & class II members have either an isoleucine or a valine, GLUT11 does not fit this structure/function pattern. hGLUT 11 was recently shown to transport both glucose and fructose Citation[9], yet it has a valine not an isoleucine at this position and further this residue is located within a different primary sequence. In all of the other class I & II GLUTs the valine/ isoleucine is preceded by an asparagine and an alanine (NAI/NAV), however GLUT11 has an aspartic acid and a serine (DSV) (). Therefore, the role of all three residues was investigated in hGLUT11 and compared with a more detailed analysis of the effect of replacing the isoleucine with valine in GLUTs 5 & 9. The results confirm that the hydrophobicity of this region of helix 7 in class II GLUTs plays a significant role in determining the ability of fructose to access the translocation pathway in these proteins.
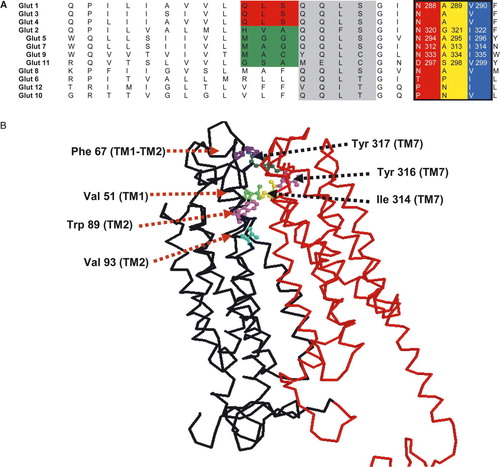
Figure 1. Sequence alignment of the predicted helix 7 for the hGLUT proteins and 3D map of hydrophobic residues lining the exofacial vestibule of GLUT7. Panel A: Sequences were aligned using Clustal X software and written from N to C-termini. Based on the putative membrane topology of this helix, its C-terminus end faces the extracellular side of the membrane. Numbers refer to the amino acid positions in the respective GLUT isoforms. Panel B: 3D model of hGLUT7 showing the position of hydrophobic residues at the opening of the exofacial vestibule. The model was created using RasMol and the structural model obtained using the coordinates from the crystal structure of Glp T and Lac Y.
Material and methods
Site-directed mutagenesis
Plasmids were created using human isoforms of GLUTs 5, 9 & 11 and a pGEM-HE vector to use as templates for creating the GLUT5, 9 & 11 mutants. In GLUT5 & 9 isoleucine was substituted for valine at positions 296 or 335 respectively, (I296V/ I335V). For the GLUT5 mutant we used the forward primer 5′-CGG GCG TCA ACG CTG TCT ACT ACT ACG C-3′ and the reverse primer 5′-GCG TAG TAG TAG ACA GCG TTG ACG CCC G-3′. The GLUT9 mutant was created using the forward primer 5′- GTG GCC TCA ATG CAG TTT GGT TCT ATA CCA ACA GC-3′ and the reverse primer 5′- GCT GTT GGT ATA GAA CCA AAC TGC ATT GAG GCC AC-3′.
Five different mutants were generated in GLUT11; D297N, S298A, D297N-S298A-I299V & D297N-S299A. The primers were as follows: D297N 5′-GAG CTC TGC GGG AAT GAC TCG GTG TAC GCC TAC-3′, for S298A 5′-CTC TGC GGG AAT GAC TCG GTG TAC GCC TAC-3′, and for D297N-S298A-V299I 5′-GAG CTC TGC GGG AAT GAC GCG ATA TAC GCC TAC-3′. In all cases the resulting plasmids were transformed in E.coli DH-5 electro competent cells, which were incubated for 16–18 h on agar plates enriched with ampicillin.
mRNA preparation and Xenopus laevis oocyte microinjection
Plasmids containing each of the wild type and mutant GLUT isoforms (hGLUT5/ hGLUT9/hGLUT11 and hGLUT5 I296V/ hGLUT9 I335V/) were linearized with Nhe I and transcribed in vitro with T7 polymerase mMESSAGE mMACHINE™ (Ambion). Adult female Xenopus laevis oocytes (prepared as previously described Citation[8]) were injected with 10–50 nl (∼20 ng) GLUT7 or the mutant synthetic mRNA transcript and incubated for 3–5 days at 16–18°C prior to functional uptake assays. The concentration of mRNA was determined using a Bio-Rad SmartSpec TM3000 machine.
Determination of the functional activity by radiotracer flux assays
The influx experiments were performed at 22°C using Citation[10–12] oocytes for each condition and 14C or 3H labelled hexose at a specific activity of 4 µCi/ml. Oocytes were washed with ice cold MBM to stop the incubation and then individual oocytes were placed in vials and dissolved in 0.5ml 5% SDS for 30 min. Finally, scintillation fluid (5ml) was added to each vial and radio activity measured using a Beckman LS6500 liquid scintillation counter. All experiments were performed 3–5 times and the results were compared to the influx values obtained with water-injected oocytes.
Kinetic analysis
Hexose uptake into oocytes expressing GLUTs 5, 9, 11 or their mutants was measured over a range of concentrations from 0.05–6 mM depending on their WT kinetic characteristics using 30 min incubations, which had been determined to be within the linear slope of uptake. Uptake was corrected for non-specific entry using water-injected eggs from the same batch in each experiment. SIGMAPLOT 6 software was used to determine the transport kinetics for the GLUT7 & mutant mediated hexose uptake by non-linear regression analysis.
Western blotting and immunohistochemistry
The GLUT5 antibody raised in rabbits against the C –terminal amino acid sequence, was obtained from Chemicon (AB1348, lot 23070390). The purified hGLUT9 specific antibody was raised in sheep against the C-terminal 15-amino acid peptide (KIDSAVTDGKINGRP) of the human sequence Citation[10]. The GLUT 11 polyclonal antibody was raised against a peptide corresponding with the C-terminus, QGPTWRSLEVIQSTEL Citation[12]. Oocytes were embedded in OCT (10% polyvinyl alcohol, 4% polyethylene glycol) embedding media (Shandon) and flash frozen in liquid nitrogen. Ten micrometer thick sections were cut on a cryostat (Leica cryostat, Richmond Hill, Ontario, Canada), mounted on slides and stored at -20°C. On the day of use, sections were brought to room temperature and fixed with methanol for 90 s. Following a 5 min wash with PBS, the sections were treated with 1% SDS for 5 min to increase antigen exposure, and then washed with PBS for 5 min three times. The sections were then treated with 10% goat serum (0.1% Tween 20) to decrease nonspecific binding of the secondary antibody, followed by a rapid rinse with PBS and incubation with primary antibody. The primary anti-GLUT5, 9 or 11 antibodies were diluted 1:1000, 1:200, 1:2000 respectively in a 25% w/v milk solution (25 g powdered milk in 100 ml PBS with 0.05% Tween 20) and placed incubated with the slides for 24 h at 4°C, followed by washes with high salt (3 times normal NaCl) PBS and then PBS. The slides were then incubated with 1 mg/ml biotinylated goat anti-rabbit or anti-sheep secondary antibody (Chemicon) diluted 1:200 in PBS, for 1 h at room temperature. The sections were washed in high salt PBS followed by normal PBS and then treated with streptavidin-conjugated FITC (Amersham) for 30 min in the dark. After three 5 min washes with PBS in the dark, the slides were mounted and sealed with nail polish. They were then viewed and photographed under a confocal microscope (Zeiss LSM510).
Multiple alignments of GLUT sequences
The sequences of GLUTs 1-13 were analyzed by CLUSTALX software and the conserved residues of interest were identified.
Statistical analysis
Uptake data were analysed for statistical significance using two tailed unpaired Student's t-test. Kinetic analyses were done using SIGMAPLOT 6 software which employed non-linear regression analysis to fit the curves and estimate the errors.
Results
We have previously shown that Ile 314 in human GLUT7 and the equivalent residue in GLUTs 2 & 5 (I322 & I296 respectively) play a role in determining substrate selectivity Citation[8]. The GLUT7 wild type protein can transport both glucose and fructose with high affinity, but the I314V mutant cannot handle fructose, while glucose kinetics are unaffected. We also reported that I322V GLUT2 lost the ability to transport fructose, but retained its glucose transport capacity and I296V GLUT5 also lost fructose transport. We have now extended our analysis to include GLUT9 and 11 and extended the analysis of GLUT5. Comparison of the sequence alignments of GLUTs 2, 5, 7 & 9 indicated that they all share an Ile residue at positions 322, 296, 314 and 335 respectively, A. GLUTs 2 and 5 are known to be fructose transporters Citation[13], Citation[14] however, nothing has been published on the substrate specificity of GLUT9 other than both transcripts a & b can transport glucose. In contrast, GLUTs 1, 3, & 4 all have a valine residue at the equivalent positions – 290, 288, and 306 and are unable to mediate fructose translocation, but can transport glucose, 2DOG and 3-O-MG. Therefore, we constructed mutants of GLUT5 & 9 in which the equivalent isoleucine residue was changed to a valine and compared the substrate specificity with that of the WT proteins.
GLUT5
When hGLUT5 was expressed in oocytes it was found to transport glucose as well as fructose and kinetic analysis showed that the Km for glucose was 0.36±0.09 mM and a Vmax of 18.14±1.01 pmol, oocytes−1, 30min−1. In comparison, the GLUT5 I296V mutant, which had previously been shown to lose its ability to transport fructose Citation[8], still maintained its glucose transport with kinetic constants of Km = 0.63±0.19 mM and a Vmax of 22.68±2.0 pmoles, oocyte−1, 30 min−1 (). These data were not significantly different.
Figure 2. Kinetics of glucose transport in the WT and I296V GLUT5 mutant. [14C] D-Glucose uptake (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with mRNA (20 ng). Data represent mean uptake into 10–12 individual oocytes corrected for uptake into water-injected oocytes from a representative experiment. Curves were fitted by non-linear regression for a single Michaelis-Menten component with the kinetic constants for the WT (○) and I296V (•) of Kms = 0.63±19 and 0.36±0.09 mM, and Vmaxes = 22.7±2.0 and 18.14±1.01 pmol, oocytes−1, 30min−1, respectively. The fitted curves for the two sets of data were not significantly different (ANOVAR p>0.05).
![Figure 2. Kinetics of glucose transport in the WT and I296V GLUT5 mutant. [14C] D-Glucose uptake (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with mRNA (20 ng). Data represent mean uptake into 10–12 individual oocytes corrected for uptake into water-injected oocytes from a representative experiment. Curves were fitted by non-linear regression for a single Michaelis-Menten component with the kinetic constants for the WT (○) and I296V (•) of Kms = 0.63±19 and 0.36±0.09 mM, and Vmaxes = 22.7±2.0 and 18.14±1.01 pmol, oocytes−1, 30min−1, respectively. The fitted curves for the two sets of data were not significantly different (ANOVAR p>0.05).](/cms/asset/a999e2cf-b9e5-41c8-aadc-6f474eac89e6/imbc_a_229718_f0002_b.gif)
GLUT9
WT hGLUT9 expression in oocytes showed that this class II GLUT can also transport glucose and fructose, but not galactose (.). Having established that both fructose and glucose were transported at similar rates, we then determined their kinetic characteristics, which indicated a Km of 0.42±0.09 mM (n=4) for fructose and 0.61±0.16 mM (n=3) for glucose. We then determined the effect of the I335V mutation on substrate specificity and kinetics. As with the other class II GLUTs, glucose transport was maintained with unaltered kinetics, while fructose transport was significantly reduced ().
Figure 3. Substrate specificity of WT hGLUT9 and the effect of the mutation I335V. Uptake of [14C] labelled hexoses (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with WT hGLUT9 mRNA (20 ng), panel A or I335V mutant mRNA, panels B & C. In all cases data represent mean uptake into 10–12 individual oocytes corrected for uptake into water-injected oocytes from a representative experiment. Cytochalasin B (CB) was added at a concentration of 100 µM. Error bars represent the standard error of the mean. For the kinetic analysis of glucose uptake into oocytes expressing I335V GLUT9 shown in panel C a curve was fitted by nonlinear regression for a single Michaelis-Menten component with a Km of 0.116±mM and a Vmax of 7.2±pmol, oocytes−1, 30 min−1.
![Figure 3. Substrate specificity of WT hGLUT9 and the effect of the mutation I335V. Uptake of [14C] labelled hexoses (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with WT hGLUT9 mRNA (20 ng), panel A or I335V mutant mRNA, panels B & C. In all cases data represent mean uptake into 10–12 individual oocytes corrected for uptake into water-injected oocytes from a representative experiment. Cytochalasin B (CB) was added at a concentration of 100 µM. Error bars represent the standard error of the mean. For the kinetic analysis of glucose uptake into oocytes expressing I335V GLUT9 shown in panel C a curve was fitted by nonlinear regression for a single Michaelis-Menten component with a Km of 0.116±mM and a Vmax of 7.2±pmol, oocytes−1, 30 min−1.](/cms/asset/9dc885e2-c567-4241-97a1-195006d3eca4/imbc_a_229718_f0003_b.gif)
GLUT11
WT hGLUT11 can also transport glucose and fructose and shows high affinity with Km's for each hexose of 0.16 and 0.06 mM respectively ().
Figure 4. Kinetics of glucose and fructose transport in GLUT11A wild type. [14C] D-Hexose uptake (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with mRNA (20 ng). Data represent mean uptake into 10–12 individual oocytes corrected for uptake into water injected oocytes from a representative experiment which was repeated three times. Curves were fitted by non-linear regression for a single Michaelis-Menten component with a Km of 0.116±mM and a Vmax of 7.2±pmol, oocytes−1, 30 min−1 for glucose (panel A) and Km of 60±µM and a Vmax of 11±pmol, oocyte−1, 30min−1 for fructose (panel B). In each case the experiments were repeated three times with very similar results.
![Figure 4. Kinetics of glucose and fructose transport in GLUT11A wild type. [14C] D-Hexose uptake (30 min at 22°C) was determined 3 days after injection of Xenopus laevis oocytes with mRNA (20 ng). Data represent mean uptake into 10–12 individual oocytes corrected for uptake into water injected oocytes from a representative experiment which was repeated three times. Curves were fitted by non-linear regression for a single Michaelis-Menten component with a Km of 0.116±mM and a Vmax of 7.2±pmol, oocytes−1, 30 min−1 for glucose (panel A) and Km of 60±µM and a Vmax of 11±pmol, oocyte−1, 30min−1 for fructose (panel B). In each case the experiments were repeated three times with very similar results.](/cms/asset/b16e91d9-7c62-4126-b191-627d1775163f/imbc_a_229718_f0004_b.gif)
However, unlike GLUTs 5 & 9 this protein has a valine in TM7 where the others express an isoleucine (A). But, the preceding two amino acids are also different (DSV as opposed to NAI), so each element of this motif was mutated to that of the other GLUTs. The GLUT11 D297N (NSV) and S298A (DAV) mutants both retained glucose and fructose transport (, panel A) however, replacement of the valine with isoleucine (V299I) to give DSI abolished the transport of both hexoses (, panel A). This did not result from a failure to express the protein, as immunohistochemistry indicated the protein was expressed in the oocyte membrane (data not shown). Finally, all three amino acids were changed simultaneously to give the motif NAI or NAV. In both cases this rescued the transport of both glucose and fructose (, panel B).
Figure 5. Glucose and fructose transport mediated by GLUT11 wild type and the mutants D297N, S298A, V299I, and those with DSV replaced with NAI or NAV. Stage 5–6 Xenopus oocytes were injected with WT hGLUT11 or mutant mRNA (20 ng) or water 3 days before determinations of uptake of [14C] D-fructose or D-glucose (100 µM). Bars represent mean net uptake (30 min at 22°C) into 10–12 oocytes injected with mRNA corrected for the uptake into water injected oocytes under identical conditions. Panel A shows a representative experiment which was performed twice. Panel B shows a typical experiment for fructose and glucose uptake into oocytes expressing WT, NAI or NAV replacing the entire DSV motif. These experiments were repeated five times.
![Figure 5. Glucose and fructose transport mediated by GLUT11 wild type and the mutants D297N, S298A, V299I, and those with DSV replaced with NAI or NAV. Stage 5–6 Xenopus oocytes were injected with WT hGLUT11 or mutant mRNA (20 ng) or water 3 days before determinations of uptake of [14C] D-fructose or D-glucose (100 µM). Bars represent mean net uptake (30 min at 22°C) into 10–12 oocytes injected with mRNA corrected for the uptake into water injected oocytes under identical conditions. Panel A shows a representative experiment which was performed twice. Panel B shows a typical experiment for fructose and glucose uptake into oocytes expressing WT, NAI or NAV replacing the entire DSV motif. These experiments were repeated five times.](/cms/asset/587b9b63-8238-439f-a06c-d1cb7282358f/imbc_a_229718_f0005_b.gif)
Discussion
Numerous studies attempting to define the basic structure of the GLUT proteins have indicated a monomeric unit with a central aqueous pore Citation[15–17]. The most recent models suggest that the protein is folded such that the aqueous pore that is formed by 8 of the 12 α-helices (No.'s 1, 2, 4, 5, 7, 8, 10 & 11) which provides the hexose translocation pathway Citation[4]. The structures of the recently crystallized proteins GlpT and Lac Y resolved to ∼3.3 Å for their cytoplasmic facing conformations have been used as templates for additional GLUT 1 modelling Citation[7]. This model suggests that the transport cycle for GLUT proteins evolves through important conformational transition states such that the ligand binding site/s is/are consequently presented to the substrate. While this approach is very powerful it requires functional correlates in order to be validated. There is an impressive body of work of functional and structural information on class I of GLUTs obtained by cysteine scanning mutagenesis studies, use of inhibitors for substrate influx and efflux, labelled metabolites, protease digestion analyses, antibodies or labeling with mercurial agents Citation[18]. Based on this information it has been shown that the putative helical regions of GLUT1 contain a number residues which are crucial for transport function: G75, G76, G79, N288, and A289 Citation[19]; Q161 Citation[20]; V165 Citation[21]; N317, T321, and P387 Citation[22]; Q282 Citation[18], Citation[21]; I287 Citation[23]; W388 Citation[24] and W412 Citation[25]. Interestingly, six of these residues are hydrophobic and their mutation to neutral residues such as glycine, produced significant disruption of the protein's function. Until recently, this observation could only be explained by their contribution to the structurally active conformation of the protein, as they cannot be involved in directly establishing hydrogen bonds with the substrate.
The role of hydrophobic residues in the structural functionality of the membrane proteins has been extensively studied in regard to conformational stabilization. Hydrophobic residues could create the so-called ‘hydrophobic effect’ or ‘hydrophobic interaction’ exerted by or between the side chains of the non-polar residues that are responsible for the folding process. This theory Citation[26] assumes that the non-polar residues, through side-chain-side-chain hydrophobic interactions, get ‘buried’ in the 3-D structure of the protein and the water molecules are cooperatively squeezed out from the hydrophobic core region and ordering in the exterior making them available for future hydrogen bonds Citation[27] at the expense of a loss of randomness in the system. The burying of hydrophobic groups within a folded protein molecule produces, therefore, a stabilizing decrease in the entropy of the ‘dry’ packed protein.
Glucose has been considered the primary substrate for SLC2A proteins and the properties of the transport of this substrate have been researched extensively, whereas the transport of fructose has received less attention. Until recently, only GLUTs 2 & 5 were confirmed as fructose transporters Citation[13], Citation[14], Citation[28], although inhibition studies and the localization of this transporter to the sperm acrosome have suggested that fructose might also be a substrate for the class III member GLUT8 (originally termed GLUTX1) Citation[30], Citation[31]. In addition, we have now shown that GLUTs 7 & 9 can also mediate the movement of this hexose. The initial observation that the hydrophobic residue I314 in hGLUT7 plays a role in determining the ability to recognize fructose has now been extended to the other class II GLUT proteins. Molecular modelling of the protein suggested that this residue faces the aqueous pore within the outer facing vestibule placing it in a position where it could interact with substrate molecules as they enter the pore (B). Employing a similar strategy by mutating Ile to Val in GLUTs 2, 5 & 9 we have confirmed the significance of this residue in determining the ability of these isoforms to transport fructose, while having no effect on their glucose permeation. However, the data reported here extend these observations to indicate that it is not just the valine or isoleucine in helix 7 which creates this hydrophobic region lining the pore. It seems that the preceding two amino acids also contribute to this effect such that NAI or NAV are the motif in class I and II GLUTs with the exception of GLUT11 which has DSV. Replacement of DSV with NAI or NAV in GLUT11 restored both glucose and fructose transport which would appear to contradict the initial conclusions reached with hGLUT7. We had initially proposed that the exofacial hydrophobic residues isoleucine or valine might form some kind of selectivity filter through a hydrophobic interaction with a tryptophan residue in helix 2 on the other side of the vestibule. Such a filter could then determine which hexoses could access the aqueous pore and the transport mechanism Citation[8]. Also, our in silico docking study, using the predicted 3D structures for GLUT1 and GLUT7 based upon the GlpT and LacY crystal structures, indicated that the core binding sites for both glucose and fructose significantly overlapped, but were not identical. This indicated that substrate specificity might not be determined by a single central binding/ translocation site but rather in the vestibule leading to the pore.
A recent modelling study by Cunningham et al. employing a docking algorithm for hexoses within the SUK1 structure for GLUT1 found not one but up to ten cluster sites along the pore Citation[29]. The affinity of these sites for the substrate appears to increase from the external opening of the vestibule down the pore into the central core of the protein where the highest affinity cluster corresponded with the central hexose binding site we had previously identified. In addition, Cunningham et al. found that fructose and L-glucose did not bind to the first cluster in the outer vestibule of GLUT1 and were similarly excluded from the inner vestibule. Neither of these hexoses is transported by GLUT1 and they concluded that this region of the protein must play a role in substrate recognition. Interestingly, the cluster sites for glucose, galactose and mannose binding in GLUT1 appear to involve a significant number of hydrophobic residues, with up to 50% of the total number predicted to interact with substrate being either non polar or having an aromatic side chain.
Our observations demonstrate that the presence of hydrophobic residues in a motif lining the entrance to the exofacial vestibule in a total of five different GLUT proteins (GLUTs 2, 5, 7, 9 & 11) influences their substrate specificity. This provides experimental evidence in support of the concept that the binding of hexoses at the mouth of the exofacial vestibule in GLUTs can determine which substrates can access the translocation mechanism. However, we cannot completely exclude, at this stage, the possibility that these residues may in some way influence the conformation of the protein during the transport cycle such that the interaction with select hexoses is affected while allowing for the translocation of others (see B). It is clear that hydrophobic residues play a significant role in this process, but the three amino acid region we have identified cannot be the sole determinant of substrate selectivity because mutations in this region affected only fructose and not glucose selectivity, with the exception of GLUT11. This isoform could transport both glucose and fructose when it had the DSV motif replaced with NAV or NAI. Similarly, it has been postulated that hGLUT8 with the NAV motif can also transport both glucose and fructose however, the rat and mouse orthologues have the NAI motif and to date fructose transport has only been reported for the rat orthologue Citation[30]. Thus, it is highly likely that other, yet to be identified, residues lining the exofacial vestibule contribute to these binding/selectivity sites. In B we show a number of additional hydrophobic residues in helices 1 and 2 which also line the vestibule and could contribute to these substrate interactions. Future studies are now needed to determine if this principle also applies to class I & III GLUTs and if a similar mechanism operates at the opening of the endofacial vestibule controlling access of substrates for efflux.
Acknowledgements
This work was supported by a grant from the Canadian Institutes of Health Research. We would like to thank Deb O'Neill for her excellent technical support.
References
- Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members. Mol Membr Biol 2001; 18: 247–256
- Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schurmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab 2002; 282: E974–976
- Hruz PW, Mueckler MM. Structural analysis of the GLUT1 facilitative glucose transporter (review). Mol Membr Biol 2001; 18: 183–193
- Mueckler M, Makepeace C. Transmembrane segment 12 of the glut1 glucose transporter is an outer helix and is not directly involved in the transport mechanism. J Biol Chem 2006; 281: 36993–36998
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science 2003; 301: 610–615
- Lemieux MJ, Song J, Kim MJ, Huang Y, Villa A, Auer M, Li XD, Wang DN. Three-dimensional crystallization of the Escherichia coli glycerol-3-phosphate transporter: a member of the major facilitator superfamily. Protein Sci 2003; 12: 2748–2756
- Salas-Burgos A, Iserovich P, Zuniga F, Vera JC, Fischbarg J. Predicting the three dimensional structure of the human facilitative glucose transporter glut1 by a novel evolutionary homology strategy: insights on the molecular mechanism of substrate migration, and binding sites for glucose and inhibitory molecules. Biophys J 2004; 87: 2990–2999
- Manolescu A, Salas-Burgos AM, Fischbarg J, Cheeseman CI. Identification of a hydrophobic residue as a key determinant of fructose transport by the facilitative hexose transporter SLC2A7 (GLUT7). J Biol Chem 2005; 280: 42978–42983
- Scheepers A, Schmidt S, Manolescu A, Cheeseman CI, Bell A, Zahn C, Joost HG, Schurmann A. Characterization of the human SLC2A11 (GLUT11) gene: alternative promoter usage, function, expression, and subcellular distribution of three isoforms, and lack of mouse orthologue. Mol Membr Biol 2005; 22: 339–351
- Yao SYM, Cass CE, Young JD. In: Baldwin SA, editor. Membrane transport: a practical approach. London: Oxford University Press 2000; 2000: 47–48
- Augustin R, Carayannapoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem 2004; 279: 16229–16236
- Doege H, Bocianski A, Scheepers A, Axer H, Eckel J, Joost HG, Schürmann A. Characterization of human glucose transporter (GLUT) 11 (encoded by SLC2A11), a novel sugar-transport facilitator specifically expressed in heart and skeletal muscle. Biochem J 2001; 359: 443–449
- Cheeseman CI. GLUT2 is the transporter for fructose across the rat intestinal basolateral membrane. Gastroenterology 1993; 105: 1050–1056
- Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem 1992; 267: 14523–14526
- Zuniga FA, Shi G, Haller JF, Rubashkin A, Flynn DR, Iserovich P, Fischbarg J. A three-dimensional model of the human facilitative glucose transporter Glut1. J Biol Chem 2001; 276: 44970–44975
- Olsowski A, Monden I, Krause G, Keller K. Cysteine scanning mutagenesis of helices 2 and 7 in GLUT1 identifies an exofacial cleft in both transmembrane segments. Biochemistry 2000; 39: 2469–2474
- Dwyer DS. Model of the 3-D structure of the GLUT3 glucose transporter and molecular dynamics simulation of glucose transport. Proteins 2001; 42: 531–541
- Hruz PW, Mueckler MM. Structural analysis of the GLUT1 facilitative glucose transporter (review). Mol Membr Biol 2001; 18: 183–193
- Olsowski A, Monden I, Krause G, Keller K. Cysteine scanning mutagenesis of helices 2 and 7 in GLUT1 identifies an exofacial cleft in both transmembrane segments. Biochemistry 2000; 39: 2469–2474
- Seatter M J, De la Rue SA, Porter LM, Gould GW. QLS motif in transmembrane helix VII of the glucose transporter family interacts with the C-1 position of D-glucose and is involved in substrate selection at the exofacial binding site. Biochemistry 1998; 37: 1322–1326
- Mueckler M, Makepeace C. Identification of an amino acid residue that lies between the exofacial vestibule and exofacial substrate-binding site of the Glut1 sugar permeation pathway. J Biol Chem 1997; 272: 30141–30146
- Mueckler M, Makepeace C. Analysis of transmembrane segment 10 of the Glut1 glucose transporter by cysteine-scanning mutagenesis and substituted cysteine accessibility. J Biol Chem 2002; 277: 3498–3503
- Hruz PW, Mueckler MM. Cysteine-scanning mutagenesis of transmembrane segment 7 of the GLUT1 glucose transporter. J Biol Chem 1999; 274: 36176–36180
- Kasahara T, Kasahara M. Tryptophan 388 in putative transmembrane segment 10 of the rat glucose transporter Glut1 is essential for glucose transport. J Biol Chem 1998; 273: 29113–29117
- Garcia JC, Strube M, Leingang K, Keller K, Mueckler MM. Amino acid substitutions at tryptophan 388 and tryptophan 412 of the HepG2 (Glut1) glucose transporter inhibit transport activity and targeting to the plasma membrane in Xenopus oocytes. J Biol Chem 1992; 267: 7770–7776
- Hartley, G, 1936. Aqueous solutions of paraffin-chain salts, Hermann & Cie., Paris, as quoted in Tanford C. (1973) The hydrophobic effect: formation of micelles and biological membranes, p viii. New York: John Wiley & Sons.
- Kauzmann W. Factors in interpretation of protein denaturation. Adv Prot Chem 1959; 14: 1–59
- Davidson NO, Hausman AM, Ifkovits CA, Buse JB, Gould GW, Burant C F, Bell GI. Human intestinal glucose transporter expression and localization of GLUT5. Am J Physiol 1992; 262(3 Pt 1)C795–800
- Cunningham P, Afzal-Ahmed I, Naftalin RJ. Docking studies show that D-glucose and quercetin slide through the transporter GLUT1. J Biol Chem 2006; 281: 5797–5803
- Ibberson M, Uldry M, Thorens B. GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. J Biol Chem 2000; 275: 4607–4612
- Schurmann A, Axer H, Scheepers A, Doege H, Joost HG. The glucose transport facilitator GLUT8 is predominantly associated with the acrosomal region of mature spermatozoa. Cell Tissue Res 2002; 307: 237–242