Abstract
In Saccharomyces cerevisiae Jen1p is a lactate/proton symporter belonging to the lactate/pyruvate:H+ symporter subfamily (TC#2.A.1.12.2) of the Major Facilitator Superfamily. We investigated structure-function relationships of Jen1p using a rational mutational analysis based on the identification of conserved amino acid residues. In particular, we studied the conserved sequence 379NXX[S/T]HX[S/T]QDXXXT391. Substitution of amino acid residues N379, H383 or D387, even with very similar amino acids, resulted in a dramatic reduction of lactate and pyruvate uptake, but conserved measurable acetate transport. Acetate transport inhibition assays showed that these mutants conserve the ability to bind, but do not transport, lactate and pyruvate. More interestingly, the double mutation H383D/D387H, while behaving as a total loss-of-function allele for lactate and pyruvate uptake, can fully restore the kinetic parameters of Jen1p for acetate transport. Thus, residues N379, H383 or D387 affect both the transport capacity and the specificity of Jen1p. Substitutions of Q386 and T391 resulted in no or moderate changes in Jen1p transport capacities for lactate, pyruvate and acetate. On the other hand, Q386N reduces the binding affinities for all Jen1p substrates, while Q386A increases the affinity specifically for pyruvate. We also tested Jen1p specificity for a range of monocarboxylates. Several of the mutants studied showed altered inhibition constants for these acids. These results and 3D in silico modelling by homology threading suggest that the conserved motif analyzed is part of the substrate translocation pathway in the lactate/pyruvate:H+ symporter subfamily.
Introduction
The study of monocarboxylates transporters (MCT) is of great importance, since uptake of these nutrients across the plasma membrane plays a crucial role in the metabolism of most cells and the acid–base equilibrium status in many tissues (Garcia et al. Citation1994). The family of proton-linked monocarboxylate transporters includes mainly mammalian members (Halestrap & Price Citation1999). In Saccharomyces cerevisiae, activities for at least two monocarboxylate-proton symporters have been found: one is encoded by the JEN1 gene (Casal et al. Citation1999) and the second by the gene ADY2 (Paiva et al. Citation2004). JEN1 encodes the only S. cerevisiae member of the Sialate:H+ Symporter (SHS) Family (TC 2.A.1.12.), belonging to the Major Facilitator Superfamily. The only other member of known function in this family is the Escherichia coli NanT gene (Vimr & Troy Citation1985). Jen1p is capable of binding and transporting lactate, pyruvate, acetate and propionate (Casal et al. Citation1999). The Ady2p permease, which is involved in the uptake of acetate, propionate and formate, belongs to an evolutionary distinct family (YaaH; TC 9.B.33.)
Our group has carried out considerable research in the attempt to characterize Jen1p. We have previously reported work on regulation of expression, protein turn-over, mRNA decay, heterologous expression in Pichia pastoris and recovery of permease activity in hybrid vesicles, which was the final proof that the Jen1p was indeed the lactate transporter, and not a regulator of lactate transport (Andrade & Casal Citation2001, Paiva et al. Citation2002, Soares-Silva et al. Citation2003, Andrade et al. Citation2005). It had already been known that in the presence of glucose there was no activity for the lactate permease in S. cerevisiae (Casal et al. Citation1999). Recent work showed that glucose regulates the mRNA decay of the JEN1 gene, as well as the protein localization in the plasma membrane, thus being a major regulation factor both at gene expression level and protein turn-over (Andrade et al. Citation2005, Paiva et al. Citation2002).
JEN1 homologues have recently been described in Candidaalbicans,Kluyveromyces lactis, Metharizyum anisopliae and Beuvaria bassiana (Soares-Silva et al. Citation2004, Lodi et al. Citation2004, Fang et al. Citation2003). In K. lactis, there are two carboxylic permeases with different specificities, KlJEN1 a monocarboxylate permease that transports lactate and pyruvate, and KlJEN2 a dicarboxylate permease that transports malate and succinate (Lodi et al. Citation2004). The C. albicans homologue proved to be also a lactate/proton symporter, and inhibition studies indicated that CaJEN1 shares similar specificity as the ScJEN1, being inhibited by D and L-lactate, pyruvate and propionate. During the expression of the CaJEN1 in S. cerevisiae it was found that a serine located in the fifth transmembrane domain is essential for the uptake of lactate (Soares-Silva et al. Citation2004).
In this work we investigate structure-function relationships in ScJen1p by using a mutational analysis of absolutely conserved residues located in a consensus motif of the lactate/pyruvate:H+ symporter subfamily. Our results and homology threading modelling strongly suggest that we have identified an essential part of the substrate translocation pathway.
Material and methods
Yeast strains, plasmids and growth conditions
The yeast strain used in this work to express the JEN1 mutants was the Saccharomyces cerevisiae W303-1A jen1Δady2Δ lacking monocarboxylate uptake capacity under the conditions tested. The cultures were maintained on slants of yeast extract (1%, w/v), peptone (1%, w/v), glucose (2%, w/v) and agar (2%, w/v) or minimal media with the required supplements for growth of the strains with auxotrophies. Yeast cells were grown in yeast nitrogen base (Difco), 0.67%, w/v (YNB medium), supplemented with adequate requirements for prototrophic growth or in yeast extract (1%, w/v), peptone (1%, w/v) (YP medium). Carbon sources were glucose (2%, w/v), or lactate (0.5%, v/v, pH 5.0). Solid media were prepared adding agar (2%, w/v) to the respective liquid media. Growth was carried out at 30°C, both in solid or liquid media. Cultures were always harvested during the exponential phase of growth. YNB glucose-containing media was used for growth of yeast cells under repression conditions. For derepression conditions glucose-grown cells were centrifuged, washed twice in ice-cold deionized water and cultivated into fresh YNB medium supplemented with lactate for 4 hours.
Construction of JEN1/ADY2 double knockout
The HphMX4 cassette vector pAG32 (Goldstein & McCusker Citation1999) was digested with BglII and EcoRV. This released a 1.7 kb DNA fragment with Ashbya gossypii TEF promoter, the hygromycin B phosphotransferase gene and Ashbya gossypii TEF terminator. S. cerevisiae Y03490 (EUROSCARF ady2Δ) was transformed with the DNA fragment, resulting in marker switch from KanMX4 (G418 resistance) to HphMX4 (Hygromycin resistance). One hygromycin resistant and G418 sensitive colony was used to prepare chromosomal DNA (Ausubel et al. Citation2002). The chromosomal DNA was used as template to amplify the ADY2::HphMX4 locus using the primers A-ADY2 (AGACTGCATTTTCTTACAGCTTTTT) and D-ADY2 (AGACAAGTAAAGGAGTCAGCAAAAA). The S. cerevisiae W303-1A jen1Δ (Paiva et al. Citation2004) was transformed with the PCR product and transformants were selected on medium containing G418 and hygromycin resulting in a jen1Δady2Δ double mutant. The correct deletion of both loci was confirmed with PCR.
Construction of the JEN1 mutations by site-directed mutagenesis
Plasmid isolation from E. coli strains and DNA manipulations were performed as described by Sambrook et al. (Citation1989). JEN1 mutations were constructed in the plasmid pDS-1 (Soares-Silva et al. Citation2003), with the oligonucleotide-directed mutagenesis technique (Ansaldi et al. Citation1996). The mutagenesis was performed using the DNA Polymerase ACCUZYME (Bioline) with proofreading activity as follows: as a template, 20 ng of the plasmid pDS-1 were utilized, and the complementary oligonucleotides (20 pmol) listed in containing the desired substitution were used in the following PCR reaction-30 s at 95°C followed by 18 cycles of 30 s at 95°C, 60 s at the oligonucleotide Tm, 8 min at 68°C, and a final extension step of 10 min at 68°C. In order to destroy the parental strands the PCR reaction was incubated with the restriction enzyme DpnI (NEB) for 2 h at 37°C. This mixture was then used to transform E. coli and plasmid extraction was performed on several clones with the Gene Elute™ Plasmid miniprep Kit (SIGMA). Mutations were confirmed by sequencing using appropriate oligonucleotides for both DNA strands. The genes containing the desired mutations were introduced in the S. cerevisiae jen1Δady2Δ strain by the High Efficiency Transformation Method (Gietz & Woods Citation2002) and the transformant selection was based on complementation of a uracil auxotrophy. As a control the strain was also transformed with the original vector p416GPD. Using plasmid pDS-1-GFP (see below), the same mutagenesis approach was employed for making Jen1p-GFP versions of mutants D387H, D387A, H383K, H383D, N379A, N379Q.
Table I. Sequence of the forward primers used in the JEN1 gene mutagenesis.
Transport assays
Cells incubated under derepression conditions were harvested by centrifugation, washed twice in ice-cold deionized water and resuspended in ice-cold deionized water to a final concentration of about 15–30 mg dry wt. ml−1. 30 µl of yeast cell suspension were mixed in microtubes with 60 µl of 0.1 M potassium phosphate buffer, pH 5.0. After 2 min of incubation at 26°C, the reaction was started by the addition of 10 µl of an aqueous solution of the labelled acid at 60 µM concentration and pH 5.0, and stopped by the addition of cold 120 mM non-labelled acid, pH 5.0. The reaction mixtures were centrifuged for 3 min at 13200 rpm, the pellet was resuspended by vortex in 1 ml of deionized cold water and centrifuged again for 3 min at 13200 rpm. The pellet was finally resuspended in 1 ml of scintillation liquid (Opti-Phase HiSafe II; LKB FSA Laboratory Supplies, Loughborough, UK). Radioactivity was measured in a Packard Tri-Carb 2200 CA liquid scintillation spectrophotometer with disintegrations per minute correction. The inhibition effect of non-labelled substrates on the initial uptake velocities of labelled acid was assayed by adding simultaneously the labelled and non-labelled substrate. The inhibition constant (Ki) was determined by measuring the uptake rates of labelled acid in the presence of the non-labelled inhibitor at the desired concentration. The following radioactive labelled substrates were utilized, D,L-[U-14C] lactate (specific activity [S.a.] 13000 dpm/nmol), sodium salt, [1-14C] acetate (S.a. 13000 dpm/nmol), sodium salt and [1-14C] pyruvate (S.a. 6000 dpm/nmol), sodium salt, all purchased from Amersham Biosciences. Non-specific 14C adsorption to the cells, as well as the diffusion component, was determined by adding a mixture of labelled acid and unlabelled acid 1000-fold concentrated. The values estimated represent less than 5% of the total incorporated radioactivity. The transport kinetics best fitting the experimental initial uptake rates and the kinetic parameters, as well as the inhibition constant were determined by a computer-assisted non-linear regression analysis (GraphPAD Software version 4.00, San Diego, CA, USA). The data shown are mean values of at least three independent experiments, with three replicas of each one.
Construction of the pDS-1-GFP plasmid
The Jen1-GFP fusion was constructed in the plasmid pDS-1 (Soares-Silva et al. Citation2003), that contains the JEN1 gene cloned under the control of the Glyceraldehyde-3-Phosphate Dehydrogenase promoter, with the gap repair technique (Ma et al. Citation1987). The fragment corresponding to the GFP open reading frame was amplified from the pFA6a-GFPS65T-KanMX6A plasmid (Wach et al. Citation1997) with the following primers: S1GFP -5′-gattcgaacgtctcaaagacaatagaggagcatattgagaccgttagtaaaggagaagaacttttc-3′ and GFPrev-5′-gtgaatgtaagcgtgacataactaattacatgatatcgacaaaggaaaaggggcctgttaaacagatctatattaccctg-3′. The GFP fragment was amplified using the DNA Polymerase ACCUZYME (Bioline) with proofreading activity as follows: as a template 200 ng of the plasmid pDS-1 were utilized and the complementary oligonucleotides (20 pmol) S1GFP and GFPrev were used in the following PCR reaction: 3 min at 95°C followed by 35 cycles of 1 min at 95°C, 1 min at 60°C, 1 min at 72°C, and a final extension step of 10 min at 72°C. The resulting PCR fragment was purified from an agarose gel with the QIAquick Gel Extraction Kit (Qiagen). The plasmid pDS-1 was digested with the EcoRI restriction enzyme (Roche) according to the manufacturer's instructions, and the enzyme was inactivated at 65°C for 15 min. The S. cerevisiae W303-1A jen1Δady2Δ strain was co-transformed by the High Efficiency Transformation Method (Gietz & Woods Citation2002) with the purified PCR fragment and the digested pDS-1 plasmid. The transformants selection was based on complementation of a uracil auxotrophy and potential positive clones were verified by colony PCR using the primers S1 and GFP_rev. A clone named S. cerevisiaejen1Δady2Δ pDS-1-GFP was selected, and the plasmid pDS-1-GFP was rescued from this strain (Ausubel et al. Citation2002) and used as a template for the construction of site directed mutants on specific amino acid residues. The plasmid pUG35 (Güldener & Hegemann, unpublished) was used as a control for GFP localization, resulting strain S. cerevisiaejen1Δady2Δ pUG35.
Bioinformatic and homology threading tools for topological analysis
The TMHMM software (http://www.cbs.dtu.dk/services/TMHMM-2.0/) was used for topological prediction of transmembrane helices in Jen1p (Krogh et al. Citation2001). Secondary structure prediction was also performed with the HHpred (http://toolkit.tuebingen.mpg.de/hhpred), a sensitive protein homology detection and structure prediction by HMM-HMM-comparison (Söding et al. Citation2005), that is part of the MPI Toolkit (Biegert et al. Citation2006). The modelling of Jen1p was achieved with the Modeller (http://toolkit.tuebingen.mpg.de/modeller) a program for comparative protein structure modelling (Sali & Blundell Citation1993). Visualization and 3D model analysis were preformed with the PdbViewer (http://www.expasy.org/spdbv/) (Guex & Peitsch Citation1997).
Results
A motif highly conserved in the lactate/pyruvate:H+ symporter subfamily
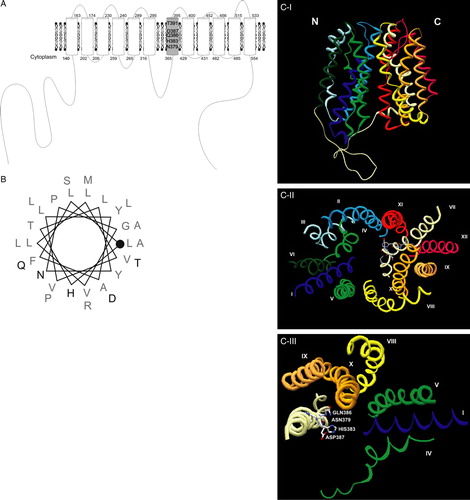
A multiple alignment of several Jen1p homologues described in the literature, as well as with homologous sequences present in databases was performed (A). In this alignment the NanT protein from E. coli, a sialate-specific transporter, was included as well (see introduction). The Jen1p protein belongs to the Major Facilitator Superfamily (MFS) and has a predicted topology of 6 + 6 transmembrane domains, which is a characteristic of this group of proteins (Marger & Saier Citation1993). Various conserved regions were identified, but the conserved motif 379NXX[S/T]HX[S/T]QDXXXT391 (X represents hydrophobic amino acids and numbering refers to ScJEN1p) exhibited, by far, the most impressive conservation with all the conserved amino acids being polar or charged, therefore with the potential to interact with the charged proton or dissociated monocarboxylates (A). In silico analysis of this motif (http://biophysics.biol.uoa.gr/SecStr/) showed that it should form an α-helix possibly followed by a short loop (results not shown). Topology prediction using the program TMHMM, but also other algorithms available for predicting transmembrane domains, suggested that this motif should be located at the end of the seventh α-helical transmembrane segment (TMS7; B). We also utilized the HHpred software (Biegert et al. Citation2006), a sensitive protein homology detection and structure prediction tool (homology threading). This algorithm builds a multiple alignment of the query protein with a set of templates selected from the search results. For Jen1p we selected the alignment with LacY permease from E. coli to build another topological model, where all the conserved amino acids are located within the seventh transmembrane domain (see A). The significance of this is discussed later.
Figure 1. (A) Identification of the conserved sequence 379NXX[S/T]HX[S/T]QDXXXT391 (numbering refers to Jen1p) in the lactate/pyruvate:H+ symporter subfamily (TC#2.A.1.12.2). Multiple sequence alignment of Jen1p homologues available in the databases and the E. coli sialate transporter (NanT) was build by the Multalin (INRA) bioinformatics tool. (http://prodes.toulouse.inra.fr/multalin/multalin.html) (Corpet, Citation1988). (B) Predicted topology of Jen1p, built by the TMHMM software (http://www.cbs.dtu.dk/services/TMHMM-2.0/), with the location of the identified motif highlighted. The protein topology shown is composed of 12 transmembrane helices and cytoplasmic N- and C-tails.
![Figure 1. (A) Identification of the conserved sequence 379NXX[S/T]HX[S/T]QDXXXT391 (numbering refers to Jen1p) in the lactate/pyruvate:H+ symporter subfamily (TC#2.A.1.12.2). Multiple sequence alignment of Jen1p homologues available in the databases and the E. coli sialate transporter (NanT) was build by the Multalin (INRA) bioinformatics tool. (http://prodes.toulouse.inra.fr/multalin/multalin.html) (Corpet, Citation1988). (B) Predicted topology of Jen1p, built by the TMHMM software (http://www.cbs.dtu.dk/services/TMHMM-2.0/), with the location of the identified motif highlighted. The protein topology shown is composed of 12 transmembrane helices and cytoplasmic N- and C-tails.](/cms/asset/34073c92-afda-4be0-b8d8-7555b00f4aa6/imbc_a_234160_f0001_b.gif)
Design and construction of Jen1p mutations
The conserved amino acids N379, H383, Q386, D387 and T391 were selected to be mutated. Two sets of mutations were done following two criteria: substitutions by isofunctional amino acids, (N379Q, H383K, Q386N, D387E and T391S) and substitutions by alanines (N379A, H383A, Q386A, D387A and T391A). The amino acids H383 and D387 present in this domain are oppositely charged, and it is possible that an interaction is established between them that might contribute in Jen1p protein structure. In order to evaluate this hypothesis the double mutant H383D/D387H was constructed, as well as the corresponding single mutants H383D and D387H. The design of all the mutations took into account the S. cerevisiae codon usage. All the mutations were performed in the plasmid pDS-1 that contains the JEN1 sequence under the control of the GPD promoter. This plasmid was previously used to constitutively express the JEN1 gene in S. cerevisiae (Soares-Silva et al. Citation2003). The presence of the mutations was confirmed by sequencing, as described in the Materials and Methods section. The plasmids were introduced in the strain S. cerevisiae jen1Δ ady2Δ, which lacks any activity for both S. cerevisiae plasma membrane monocarboxylate permeases described so far in the literature (Casal et al. Citation1999, Paiva et al. Citation2004).
Conditions for measurement of initial uptake rates of monocarboxylates
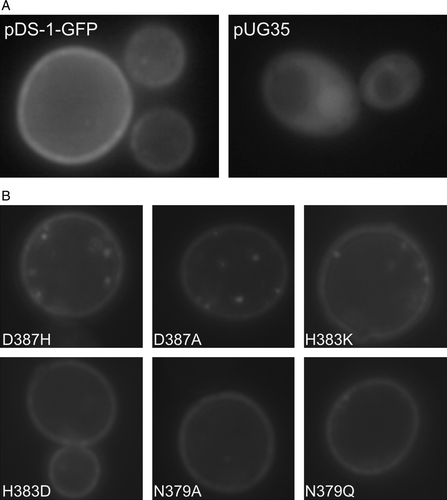
A time assay was carried out for pyruvate, acetate or lactate uptake. As can be seen in , for all the substrates tested, the initial uptake rate of labelled monocarboxylates was linear for at least 60 sec. This was the incubation time chosen for the transport kinetics determination performed in this work. Growth conditions were also optimized, since the conditions previously described to express JEN1 gene were not very efficient, due to the presence of glucose in the growth medium (see references in introduction). In the presence of glucose, the transport capacity obtained for monocarboxylates is very low and would not enable the differentiation of mutants, if they were slightly affected in transport kinetics. In this work, exponentially grown cells on glucose were shifted to minimal medium containing lactic acid (0.5% v/v, pH 5.0) as a sole carbon and energy source. This resulted in a 10-fold increase in the transport capacity for monocarboxylates (results not shown). The negative effect of glucose on Jen1p was further confirmed in a strain expressing a Jen1p-GFP fusion from plasmid pDS-1-GFP. After derepression in lactic acid (3–5 h), it was possible to clearly visualize the protein in the plasma membrane (A). In contrast, on glucose most of the Jen1p was localized inside the cell (not shown). The strain S. cerevisiaejen1Δady2Δ pUG35, used as a control for GFP localization, exhibited fluorescence in the cytoplasm (A), while in S. cerevisiaejen1Δady2Δ cells transformed with pDS-1 plasmid no fluorescence was visualized (results not shown).
Figure 2. Evaluation of the uptake rate of labelled lactate (▴), pyruvate (▪) and acetate (▾) (60 µM final concentration), in the strain S. cerevisiae W303-1A pDS-1. Cells were cultivated in glucose until mid-exponential growth phase, washed and transferred to YNB lactic acid for 4 h.
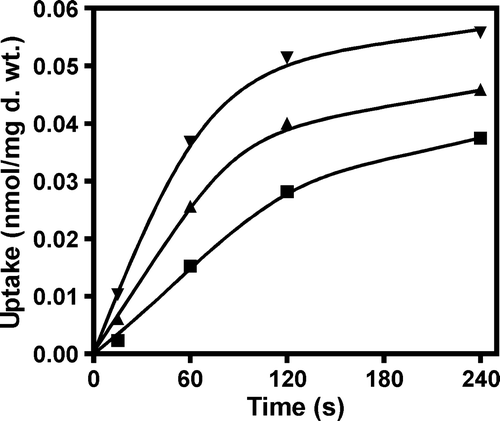
Figure 3. Epifluorescence microscopy localization of Jen1-GFP fluorescence in (A) strain jen1Δ ady2Δ transformed with the plasmids pDS-1-GFP or pUG35 and (B) isogenic Jen1 mutant strains D3987H, D387A, H383K, H383D, N379A and N379Q. S. cerevisiae strains at mid-exponential growth phase were shifted under derepressed conditions for Jen1p expression (YNB lactate 0.5% (v/v), pH 5.0) for 4 h and observed under an epifluorescence microscope and in bright field. Additionally, for S. cerevisiaejen1Δ ady2Δ pUG35 strain methionine was added for repression conditions.
Lactate uptake is severely affected in several of the mutants studied
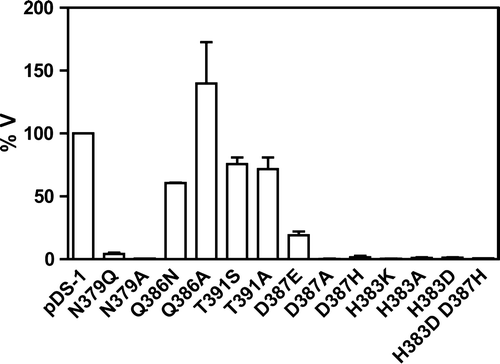
The different mutants were tested for the initial uptake rates of labelled lactate, at pH 5.0, as mentioned in the Material and Methods. In we can observe that the substitution of amino acids N379, H383 and D387, even with very similar amino acids, resulted in a very dramatic reduction of lactate uptake. In fact, only conserved substitutions show practically measurable transport activities (N379Q, D387E). Other replacements resulted in total loss of transport activity. Substitution Q386A was the only one that led to a slightly increased uptake rate of lactate when compared to the wild-type, while substitution by an isofunctional residue (N) decreased the uptake rate to about 50%. Regarding both T391 substitutions, Jen1p transport capacity was slightly decreased (75% of the wild-type). The double mutant H383D/D387H, as well as the corresponding single mutants, presented practically no uptake rate.
Pyruvate and acetate uptake is differentially affected in Jen1p mutants
Jen1p permease was also tested for pyruvate and acetate transport (). The results obtained for the pyruvate uptake exhibited a very similar pattern to the lactate uptake for all the mutants studied. According to the results, all substitutions in H383, N379 and in D387 resulted in total or dramatically reduced Jen1p function. On the other hand substitutions in Q386 and T391 resulted in none or moderate changes in Jen1p transport capacities. Jen1p-mediated acetate transport capacity had a more complex profile. Several substitutions considered to inactivate or dramatically reduce lactate or pyruvate transport, led to measurable acetate transport (N379Q, D387A, D387H, H383D, H383A). On the other hand, mutation Q386A, which allowed maximal lactate or pyruvate transport capacity, led to significantly reduced acetate transport (26%). Substitutions in T391 had the same minor negative effect on acetate transport, as for lactate or pyruvate transport. Most interestingly, while the double substitution H383D/D387H is considered as a total loss of-function allele for lactate or pyruvate transport, it completely restores acetate transport, which was however compromised in the corresponding single mutants. In other words, residues N379, Q386 and especially D387 and H383 are critical for Jen1p substrate specificity.
Table II. Relative transport capacity (%) of lactate, pyruvate and acetate of the Jen1p mutants, compared to the wild-type (pDS-1).
Jen1p expression in the plasma membrane is not impaired by mutations in the motif
Several of the mutations studied, especially those concerning residues N379 and D387, led to a dramatic reduction or lack of Jen1-mediated monocarboxylate transport activity. To formally distinguish whether this is due to problematic plasma membrane expression or Vmaxper se, we re-constructed mutations D3987H, D387A, H383K, H383D, N379A, N379Q, in a plasmid expressing a functional Jen1p-GFP chimeric transporter (see Materials and methods). Mutations were introduced into the jen1Δ ady2Δ strain as described above and previously (Casal et al. Citation1999, Paiva et al. Citation2004). Jen1p-GFP localization was followed in selected transformants, by fluorescence microscopy. In nearly all cases Jen1p was properly located at the plasma membrane level after 4 h induction in YNB lactic acid as it was found for the wild-type (B). Only mutation N379Q seems to exhibit a somehow problematic insertion in the plasma membrane, revealed by lower peripheral fluorescence and increased fluorescence in vacuole, at least in some cells. On the whole, however, these results strongly suggest that in the mutants that present low or no transport capacity this is not a consequence of stability or trafficking, but rather due to an effect on the mechanism of substrate catalysis per se.
Jen1p mutants show altered specificity profiles
In the mutants with a sufficient uptake rate (>10% of the wild-type) the kinetic parameter for substrate affinity (Km) was determined for the three principal Jen1p substrates, lactate, pyruvate and acetate (Materials and methods). The results are presented in . The mutant Q386N has a decreased affinity for all tested substrates but this was much more significant for lactate binding (a 10-fold reduction) than for pyruvate or acetate (3–4-fold). Given that the activities are measured at a concentration 10-fold lower than the wild-type Km this reduction in the binding affinities for pyruvate and lactate should account for the moderate reduction in the transport capacities measured in . Alanine substitution (Q386A) resulted in a minor increase (≤3-fold) in the Km for pyruvate. The results suggest that Q386 affects substrate binding but not transport capacity per se. Mutation in T391 had a minor negative effect (2–6 fold) in the binding affinity for all substrates. This reduction in Km might account for a minor reduction in transport capacity of T391 mutations. Thus, T391 seems to have only minor importance to substrate binding. Among D387 substitutions, only the mutant with the isofunctional change D387E has sufficient transport capacity for performing kinetics. D387E was found to have a minor negative effect (2–3-fold) on both the binding and transport of acetate ( and ). The double mutant H383D/D387H showed an affinity for acetate nearly identical to the wild-type allele. As shown in , the same mutant restores transport capacity for acetate. These results further confirm that the double mutant fully restores Jen1p function, specifically for acetate binding and transport.
Table III. Km values (mM) of Jen1p mutants for the uptake of lactate, acetate, and pyruvate.
Table IV. Ki values (mM) of Jen1p mutants for propionate, benzoate, salicylate and butyrate.
Additional monocarboxylates were tested for their capacity to inhibit Jen1p-mediated uptake of labelled lactate (Q386 and T391 mutations) or acetate (H383D/D387H). Butyrate, benzoate, propionate and salicylate, were initially tested at 1000-fold excess, for competition of the uptake of lactate or acetate. All tested substances inhibited the uptake of lactate or acetate by > 80% (not shown). To further characterize wild-type Jen1p and kinetically measurable mutants, we estimated the Ki values for these monocarboxylates (). Our results showed that wild-type Jen1p binds all tested monocarboxylates, at concentration ranging from 0.2–7.2 mM, with an affinity order: lactate > pyruvate, benzoate > propionate > acetate, butyrate > salicylate. Most mutants tested alter the binding affinities of monocarboxylates in non-hierarchical pattern. In particular, the conserved change Q386N results in an identical affinity (2.2–2.9 mM) for all substrates, with the exception of acetate. In other words, Q386N seems to have lost the ability to discriminate several monocarboxylates. On the other hand, Q386A has also lost the ability to discriminate benzoate, propionate, butyrate and salicylate, but unlike Q386N, it can still bind lactate and pyruvate with much higher affinities. T391A can affect substrate binding both positively (salicylate, butyrate) or negatively (lactate, propionate, benzoate), while T391S has a more subtle effect.
Discussion
The Jen1p N379XX[S/T]H383X[S/T]Q386D387XXXT391 motif that was identified and studied in this work is by far the most conserved sequence in the lactate/pyruvate:H+ symporter subfamily, and given the results presented herein, should be considered as a ‘signature’ motif of this group of transporters. The TMHMM prediction tool for secondary structure showed that this sequence probably forms an α-helix overlapping with the end of TMS7 and the beginning of the following hydrophilic segment. However, the homology threading approach shows that the motif might not extend outside TMS7 (A and 5B). Since this alignment shows 100% of probability of a common topology with the LacY permease, we created a 3D structural model for Jen1p using the MODELLER software (B). This model is consistent with the results obtained; the amino acids N379, H383, Q386 and D387 are facing the internal pore confirming the potential to interact directly with the substrate; the T391 residue is the only one facing outside the pore being the one that less affects binding and transport capacity. Comparing the topology of Jen1p with that of the LacY permease we also observed that the residue N379 of Jen1p could correspond to residue D240 in the TMS7 of LacY. In the solved structure, D240 was shown to interact with LacY substrates (Abramson et al. Citation2003). Based on our kinetic analysis and the fact that 379 is an irreplaceable residue, we propose that N379 might also bind Jen1p substrates. It is possible that, as in the case of LacY and GlpT, the two MFS proteins with solved structures (Abramson et al. Citation2003, Huang et al. Citation2003), TMS7 of Jen1p is part of the substrate translocation pathway.
Figure 5. (A) Jen1p topology based on the HHpred (http://protevo.eb.tuebingen.mpg. de/toolkit/index.php?view=hhpred), showing highlighted the location of the identified motif within TMS7. (B) Schematic representation of TMS7 (LLFAYLVVLLVGPNYLTHASQDLLPTMLRA), illustrating the position of the amino acids in the helix; the beginning of TMS7 is represented with a closed circle and the studied domain is represented in bold (C) Ribbon representation of Jen1p structure modelled to the LacY permease (HHpred Modeller), (I) View parallel to the membrane; (II) View from the cytosol side (the loop regions are not represented); (III) simplified II view with indication of the residues studied in this work. Transmembrane domains are indicated from I to XII and from N to C terminus. The colour of each transmembrane domain is maintained in all of the Figures shown in colour in Molecular Membrane Biology online. Figures were prepared with program Swiss PDB viewer (Guex & Peitsch Citation1997).
An in silico analysis using SecStr, a program which combines six different secondary structure prediction methods (http://biophysics.biol.uoa.gr/SecStr/), was performed for the mutant versions of the N379XX[S/T]H383X[S/T]Q386D387XXXT391 motif. This analysis (not shown) showed that Q386A, and to a lesser degree T391A and D387A, affect the putative local architecture of this region. In particular, the presence of Ala residues significantly increases the probability for α-helix formation. Substitutions of Q386 and T391 resulted in no or moderate changes in Jen1p transport capacity but affected binding affinities for all Jen1p substrates. Based also on these observations, we propose that these residues indirectly affect substrate binding by determining the local architecture and flexibility of a segment close to the binding site.
The role of H383 and D387 is more complex and seems to be critical, determining the specificity of Jen1p. Single mutants lose the ability to transport lactate and pyruvate, the principal Jen1p substrates, but most conserve significant acetate uptake. Moreover, while the double mutant H383/D387 lacks any lactate and pyruvate transport capacity it has wild-type kinetics for acetate transport. This is highly suggestive of a charged interaction between these two residues. The model presented in B supports this assumption, showing that both amino acids are facing the central pore and spatially closely located to each other. This interaction, independently of the position of the positive and negative charges, is necessary and sufficient for acetate binding and transport. In contrast, for lactate or pyruvate binding and transport, the individual positions of the charged residues are critical. This shows that acetate binds using different interactions from those involved in lactate or pyruvate binding. This is not uncommon for enzymes, as well as, for transporters (Balendiran et al. Citation1999, Koukaki et al. Citation2005, Pantazopoulou & Diallinas Citation2006).
We also tested Jen1p specificity for a range of monocarboxylates. Jen1p can bind most monocarboxylates tested (lactate, pyruvate, acetate, benzoate, propionate, butyrate and salicylate). Several of the mutants studied showed non-hierarchical changes in inhibition constants for these acids. These results further suggest that the conserved motif analyzed is critical for substrate binding and translocation. Interestingly, one recently characterized member of the lactate/pyruvate:H+ symporter subfamily, KlJen2p, is specific for dicarboxylates. This transporter conserves the residues studied herein, but is the only one, which has a polar residue (Ser), instead of a hydrophobic residue, at position 381 (numbering of ScJen1p). Such changes, but also residues other than the ones studied here could contribute to the ability to bind dicarboxylates rather than monocarboxylates
Acknowledgements
We thank A. Vlanti for critically reading the manuscript. This work was supported by Portuguese grant POCTI/1999/BME/36625 and POCI/BIA-BCM/57812/2004 (Eixo 2, Medida 2.3, QCAIII-FEDER). I.S-S. received a fellowship from the Portuguese government: SFRH/BD/4699/2001.
References
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback H R, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science 2003; 301: 610–615
- Andrade RP, Casal M. Expression of the lactate permease gene JEN1 from the yeast Saccharomyces cerevisiae. Fungal Genet Biol 2001; 32: 105–111
- Andrade RP, Kotter P, Entian KD, Casal M. Multiple transcripts regulate glucose-triggered mRNA decay of the lactate transporter JEN1 from Saccharomyces cerevisiae. Biochem Biophys Res Commun 2005; 332: 254–262
- Ansaldi M, Lepelletier M, Mejean V. Site-specific mutagenesis by using an accurate recombinant polymerase chain reaction method. Anal Biochem 1996; 234: 110–111
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. Wiley Interscience, New York 2002
- Balendiran GK, Molina JA, Xu Y, Torres-Martinez J, Stevens R, Focia PJ, Eakin AE, Sacchettini JC, Craig SP. Ternary complex structure of human HGPRTase, PRPP, Mg2+, and the inhibitor HPP reveals the involvement of the flexible loop in substrate binding. Protein Sci 1999; 8: 1023–1031
- Biegert A, Mayer C, Remmert M, Söding J, Lupas A. The MPI Toolkit for protein sequence analysis. Nucleic Acids Res 2006; 34: W335–W339
- Casal M, Paiva S, Andrade RP, Gancedo C, Leão C. The lactate-proton symport of Saccharomyces cerevisiae is encoded by JEN1. J Bacteriol 1999; 181: 2620–2623
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 1988; 16: 10881–10890
- Fang WG, Zhang YJ, Xiao YH, Ma JC, Yang XY, Pei Y. Isolation and characterization of a carboxylic transport protein JEN1 and its promoter from Metarhizium anisopliae. Yi Chuan Xue Bao 2003; 30: 283–288
- Garcia CK, Goldstein JL, Pathak RK, Anderson RGW, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates – implications for the Cori Cycle. Cell 1994; 76: 865–873
- Gietz RD, Woods RA. Transformation of yeast by the LiAc/SS carrier DNA/PEG method. Methods Enzymol 2002; 350: 87–96
- Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 1999; 15: 1541–1553
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997; 18: 2714–2723
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 1999; 343: 281–299
- Huang YF, Lemieux MJ, Song JM, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science 2003; 301: 616–620
- Koukaki M, Vlanti A, Goudela S, Pantazopoulou A, Gioule H, Tournaviti S, Diallinas G. The nucleobase-ascorbate transporter (NAT) signature motif in UapA defines the function of the purine translocation pathway. J Mol Biol 2005; 350: 499–513
- Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 2001; 305: 567–580
- Lodi T, Fontanesi F, Ferrero I, Donnini C. Carboxylic acids permeases in yeast: two genes in Kluyveromyces lactis. Gene 2004; 339: 111–119
- Ma H, Kunes S, Schatz PJ, Botstein D. Plasmid construction by homologous recombination in yeast. Gene 1987; 58: 201–216
- Marger MD, Saier MHJ. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci 1993; 18: 13–20
- Paiva S, Devaux F, Barbosa S, Jacq C, Casal M. Ady2p is essential for the acetate permease activity in the yeast Saccharomyces cerevisiae. Yeast 2004; 21: 201–210
- Paiva S, Kruckeberg AL, Casal M. Utilization of green fluorescent protein as a marker for studying the expression and turnover of the monocarboxylate permease Jen1p of Saccharomyces cerevisiae. Biochem J 2002; 363: 737–744
- Pantazopoulou A, Diallinas G. The first transmembrane segment (TMS1) of UapA contains determinants necessary for expression in the plasma membrane and purine transport. Mol Membr Biol 2006; 23: 337–348
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 1993; 234: 779–815
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. Cold Spring Harbor. Cold Spring Harbor Laboratory Press, New York 1989
- Soares-Silva I, Paiva S, Kotter P, Entian K D, Casal M. The disruption of JEN1 from Candida albicans impairs the transport of lactate. Mol Membr Biol 2004; 21: 403–411
- Soares-Silva I, Schuller D, Andrade RP, Baltazar F, Cássio F, Casal M. Functional expression of the lactate permease Jen1p of Saccharomyces cerevisiae in Pichia pastoris. Biochem J 2003; 376: 781–787
- Söding J, Biegert, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res 2005; 33: W244–248
- Vimr ER, Troy FA. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J Bacteriol 1985; 164: 845–853
- Wach A, Brachat A, Alberti-Segui C, Rebischung C, Philippsen P. Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast 1997; 13: 1065–1075