Abstract
Our main objective was to analyze the role of lipid rafts in the activation of Vα-14− and Vα-14+ T hybridomas by dendritic cells. We showed that activation of Vα-14+ hybridomas by dendritic cells or other CD1d-expressing cells was altered by disruption of lipid rafts with the cholesterol chelator MβCD. However, CD1d presentation to autoreactive Vα-14− anti-CD1d hybridomas which do not require the endocytic pathway was not altered. Using partitioning of membrane fractions with Brij98 at 37°C, we confirmed that CD1d was enriched in subcellular fractions corresponding to lipid rafts and we describe that α-GalCer enhanced CD1d amount in the low density detergent insoluble fraction. We conclude that the membrane environment of CD1d can influence antigen presentation mainly when the endocytic pathway is required. Flow cytometry analysis can provide additional information on lipid rafts in plasma membranes and allows a dynamics follow-up of lipid rafts partitioning. Using this method, we showed that CD1d plasma membrane expression was sensitive to low concentrations of detergent. This may suggest either that CD1d is associated with lipid rafts mainly in intracellular membranes or that its association with the lipid rafts in the plasma membrane is weak.
Introduction
CD1d molecules are highly glycosylated proteins composed of an α heavy chain (50–55kD) non-covalently associated with β2-microglobulin (β2m). CD1d1 bind hydrophobic peptides, lipids and glycolipids (Zeng et al. Citation1997). All hematopoeitic cells express CD1d including dendritic cells (Mandal et al. Citation1998). CD1d is also expressed by non hematopoeitic cells such as intestinal epithelial cells (van de Wal et al. Citation2003). CD1d-restricted T cells have been first identified as NKT cells in C57BL/6 mouse. They express NK1.1 surface marker and a semi-invariant Vα14-Jα18 T cell receptor (TCR). These cells are activated mainly in the presence of the exogenous antigen α-GalactosylCeramide (α-GalCer) or the endogenous antigen isoglobotrihexosylceramide (iGb3) (Mattner et al. Citation2005, Sandberg & Ljunggren Citation2005). CD1d-αGalCer tetramers allow identification of non-NK1.1 anti-CD1d lymphocytes (Benlagha et al. Citation2000). Anti-CD1d T cells also include autoreactive T cells expressing diverse non Vα14 TCR (Park et al. Citation1998). NKT cells secrete high levels of immunoregulatory cytokines including interferon-γ (IFN-γ) and interleukin-4 (IL-4) and are considered to play intermediary roles bridging innate and acquired immunity. These cells are known to play key roles in a range of different diseases including autoimmune diseases, cancer and infections (Van Kaer & Joyce Citation2005).
CD1d assembly and trafficking are independent of TAP (Brutkiewicz et al. Citation1995) and are partially β2m-dependent whereas a portion of CD1d heavy chains can reach the cell surface in the absence of the light chain. It has been postulated that self lipids such as phosphatidylinositol are loaded during assembly (Joyce et al. Citation1998, Park et al. Citation2004). The majority of CD1d molecules directly reach the membrane. The cytoplasmic tyrosine-based motif of CD1d (De Silva et al. Citation2002) and the adaptator AP-3 drive the CD1d traffic from the surface through the endocytic pathway. Presentation of α-GalCer to Vα14-CD1d restricted T cells requires this traffic through the endocytic pathway whereas this pathway is not required for non Vα14-CD1d autoreactive T cells (Chiu et al. Citation1999; Chiu et al. Citation2002, Jayawardena-Wolf et al. Citation2001).
Lipid rafts contribute to various immune functions, including lymphocyte maturation, lymphocyte signal transduction, immune synapse formation and antigen presentation by MHC class II molecules expressed by APCs (Brown & London Citation2000). Lipid rafts are defined as sphingolipid- and cholesterol-rich clusters in the membrane. They are abundant in the plasma membrane but also in late secretory pathway and endocytic compartments. They can cluster into larger ordered platforms that selectively incorporate or exclude proteins. Typical raft-associated proteins include glycosylphosphatidylinositol(GPI)-anchored proteins and Src family protein tyrosine kinases (Ikonen Citation2001). Numerous proteins are also aggregated in microdomains during the formation of the immunologic synapse (Huby et al. Citation1999, Taner et al. Citation2004) and treatment with appropriate antibodies: FcεRI, co-stimulating molecules CD40, CD28, CD20, CD4/8 (Brown & London Citation2000).
Previous studies showed that CD1d molecules localize to ‘steady-state’ membrane lipid rafts and that they are involved in presentation of α-GalCer (Lang et al. Citation2004, Park et al. Citation2005). This was based on confocal microscopy analysis and western-blot of Triton-X100-insoluble membrane complexes at 4°C from tumoral B cell line, BLC1 and CD1d-transfected cells.
In the present study, we analyzed the lipid rafts in the main APC, dendritic cells and a non-tumoral epithelial cell line expressing high level of CD1d. We showed that disruption of lipid rafts altered the activation of Vα14-positive T cell hybridomas but not Vα14-negative T cell hybridomas. Using partitioning of membrane fractions with Brij98 at 37°C, we confirmed that CD1d was enriched in subcellular fractions corresponding to lipid rafts. Treatment of cells with α-GalCer increased the amount of CD1d recovered in low density detergent insoluble fractions. Moreover, we used flow cytometry techniques which provided additional information on the lipid rafts in plasma membranes and allowed a dynamic follow-up of lipid rafts partitioning even in low expressing CD1d cells such as dendritic cells (Filatov et al. Citation2003, Gombos et al. Citation2004).
Material and methods
Antibodies
Monoclonal antibody (mAb) rat anti-mouse CD1.1 20H2 (IgG1) and 19G11 (IgG2a) were provided by A. Bendelac (University of Chicago, USA). Rabbit anti-CD1d antiserum was obtained by repeated immunizations with peptide containing the intracellular sequence YQDIR of CD1d. PE (phycoerythrine)-labeled anti-mouse CD11c mAb, and PE-labeled rat anti-mouse CD1d 1B1 mAb, PE-labeled anti-CD71 mAb were obtained from Becton Dickinson, Pharmingen, and PE-labeled anti-Rae-1 mAb from R&D systems. Biotin-labeled and FITC-labeled cholera toxin B subunit were purchased from Sigma. Peroxidase-labeled goat anti-rabbit IgG antibody and peroxydase-labeled streptavidin were purchased from Jacskon Laboratories.
Cell cultures
B6 mouse C57SV fibroblast line, CD1.1-transfected C57SV fibroblast line (C57SVCD1.1) Vα14+ hybridomas DN32.D3, P.41 and P.9 and non Vα14 hybridomas TC.B11 and TB.A7 have been previously described (Chiu et al. Citation1999, Park et al. Citation1998) and were cultured in RPMI 1640, 10% Fetal Calf Serum (FCS), 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 µg/ml) (Invitrogen Life Technologies). Monocytic Raw cell line was obtained from ATCC and was cultured in DMEM 10% FCS (Invitrogen Life Technologies).
Dendritic cell cultures were obtained from C57BL/6 mice. Femurs were removed and cut. The bone marrow was flushed with 2 ml of DMEM using a syringe. Cells were washed and cultured in DMEM medium containing 10% FCS, 2 mM glutamine, penicillin (100 U/ml), streptomycin (100 µg/ml) and supplemented with 25% of N1H3T3 supernatant containing GM-CSF (Winzler et al. Citation1997). Fresh medium with GM-CSF was added at Day 3. Immature floating dendritic cells expressing CD11c assessed by flow cytometry (data not shown) were collected at Day 8. Mature dendritic cells were obtained by exposing immature dendritic cells to 1 µg/ml of Escherichia coli lipopolysaccharide (LPS) (Sigma Aldrich) during 24 h.
NOE (nasal olfactory epithelia-derived cell line) was derived from the olfactory mucosa of a C57BL/6 mouse. Olfactory mucosa was dissected and treated during 45 min at 37°C in the presence of 2.4 unit/ml of dispase II (Boehringer). The epithelium was separated from the lamina propria and then incubated with 2.5 mg/ml collagenase H (SERVA) for 10 min, and then mechanically dissociated. The cells were centrifuged and seeded in DMEM F-12 medium. In one well, a clone appeared which was amplified and characterized for different antigens including CD1d.
Isolation of lipid microdomains
Around 30–60×106 cells were treated with 10 mM of MβCD (Methyl-β-cyclodextrin) for 30 min at 37°C or with α-GalCer (200 ng/ml) for 3 h at 37°C. Cells were washed and suspended in 1 ml of hypotonic buffer A [25 mM Hepes (Invitrogen Life Technologies), 150 mM NaCl, 1 mM EDTA (Sigma), 10 mM NaP-P (Sigma), 10 mM NaF (Sigma), 5 mM Na3VO4 (Sigma) and inhibitors of protease (35 µg/ml PMSF, 40 µg/ml O-phenanthroline, 1.2 µg/ml pepstatine, 2 µg/ml aprotinin, 2 µg/ml leupeptin, 37 µg/ml iodoacetamide), Sigma]. We performed sonication (MSE Ultrasonic Desintegrator) 5 w during 5 sec, 5 times and with 30 sec interval, each time on ice. The post-nuclear supernatants were collected after centrifugation at 500 g and warmed for 4 min at 37°C before adding 1% final concentration of Brij 98 (Sigma). The fisrt fraction [2.2 ml of OptiPrep/0.3 M sucrose (43%)] was placed at the bottom of the tube. We added 1 ml layers of Optiprep diluted with buffer A/sucrose 0.3 M to the following concentrations: 36%, 34%, 32%, 30%, 27.5%, 25%, 20% and 0%. The gradients were ultracentrifuged at 75,000 g for 16 h at 4°C in a SW41 rotor (Beckman Instruments Inc.) and each 1 ml fraction were carefully harvested.
Analyses of molecular fractions
All fractions were diluted with sample buffer (1% SDS, 5% β-mercaptoethanol, 62.5 mM Tris pH 6.8, 10% Glycerol) and were denatured for 5 min at 95°C. The samples were loaded onto 10–20% polyacrylamide gradient gel and electrophoresis was run at 100 V. Transfer to the nitrocellulose membranes (Hybond-C extra, Amersham Life Science) were performed with semi-dry transfert Unit (Hoeffer). The membranes were saturated at RT with 5% nonfat milk in washing buffer [15mM Tris, 150mM NaCl, 0.05% Tween20], incubated with rabbit anti-CD1d antiserum (1/750) overnight at 4°C, washed and incubated with peroxidase conjugated goat anti-rabbit IgG (1/5000) 1h at RT. The peroxydase activity was revealed with chemiluminescence SuperSignal Substrate (Pierce). The levels of CD1d in each fraction were assessed by Image Master Software.
N-Glycosidase F (Roche, Germany) digestion was carried out overnight at 37°C by adding 1 µl of N-Glycosidase F into 50 µl of fractions in presence of 0.2% SDS and 1% β-mercaptoethanol.
Flow cytometry analysis
Dendritic cells and adherent cells detached after brief treatment with Trypsin-EDTA were washed with a solution containing PBS, FCS (1%) and sodium azide (0.02%) and were pre-incubated at 4°C for 20 min with rat IgG2 anti mouse-Fcγ receptor (Becton Dickinson (BD), Pharmingen). Cells were incubated with labeled antibodies, unlabeled primary antibodies or isotype controls diluted at 5 µg/ml. After wash, cells were fixed or incubated for 15 min at 4°C with FITC-labeled goat anti rat IgG-Fc or FITC-labeled goat anti mouse IgG-Fc antiserum (Jackson ImmunoResearch) diluted at 10 µg/ml. Cells were washed and fixed in 2% paraformaldehyde. FACS analysis was performed on FACScalibur (Becton Dickinson, Pont-de-Claix, France) using Cellquest Software.
Measurements of detergent solubility were performed in the flow cytometer kinetic mode data acquisition. Cells were labeled during 15 min at 4°C with PE-anti-CD71, PE-anti-CD1d, PE-anti-Rae-1 or FITC-labeled cholera-toxin β subunit and washed in a large volume and analyzed immediately. Labeled-cells were analyzed during 15 sec and then mixed with the detergent. Reaction was analyzed either at 4°C with various concentration of Triton X-100 (0.05, 0.04 or 0.02%) or at 37°C in presence of 1% Brij98. Cells were then immediately recorded in the time-resolved mode of the flow cytometer for 5 min. All data were collected and evaluated by FacsDiva software (Becton-Dickinson, San Jose, CA, USA).
Stimulation of T-cell hybridomas
Adherent cells (8×104 cells per well of flat-bottom 96-well plates) were seeded one day before adding hybridomas. Dendritic cells (8×104 cells per well) were seeded 2 h before. Pretreatment with various concentrations of MβCD was performed in serum free medium at 37°C for 30 min. Cell were then washed in serum containing medium before addition of the T cell hybridomas. The reactivity of hybridomas, either with the presence or absence of 40 ng or 200 ng/ml of α-GalCer, was evaluated 24 h later by IL-2 secretion. Culture supernatants were collected and IL-2 was measured using standard sandwich ELISA according to the manufacturer's protocol (OptEAI, IL-2 kit, BD Pharmingen). Results were expressed as mean±SD of triplicates or expressed as% of inhibition of IL-2 production at various MβCD concentrations.
Measurement of cellular viability
Adherent cells were treated with various concentrations of MβCD for 30 min at 37°C, washed two times and cultured for 24 h. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma] at the final concentration of 0.5 mg/ml was added. After 1 h, the medium was aspirated and the crystals were dissolved in 100 µl of DMSO. Optical densities (OD) were measured at 550 nm. Viability was expressed as mean±SD form triplicates.
Untreated or MβCD treated dendritic cells were cultured during 24 h at the concentration of 8×104 cells per well in 96-well plate. [3H] thymidine (Amersham Pharmacia Biotech) was added at 1 µCi per well during the last 12 h. Cells were harvested on filter mats, dried, and counted using a 1600TR liquid scintillation analyzer (Packard Instruments). 3H incorporation was expressed as mean±SD from triplicates.
Results
Characterization of CD1d expression and hybridomas activation
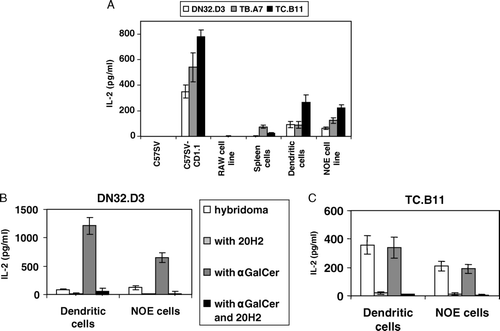
CD1d expression was measured using flow cytometry with 19G11 mAb (). Dendritic cells, the fibroblast cell line C57SV, the Raw cell line and splenocytes expressed similar low levels of CD1d. Expression of CD1d by the NOE cell line was higher and expression by the transfected cell line was even higher. We compared activation of DN32.D3 Vα14-positive hybridoma and TC.B11 and TB.A7 Vα14-negative hybridomas by various cell types (A). NOE and transfected cell lines allowed activation of the three hybridomas. Dendritic cells, despite low expression levels of CD1d, also activated the three hybridomas. Splenocytes activated TB.A7, whereas the other cell types failed to induce any IL-2 production. Pre-treatment with α-GalCer of the NOE cell line and dendritic cells enhanced IL-2 release by DN32.D3 but not by TC.B11 cell line (B and C). Activation of P.41 and P.9 was detected only when α-GalCer was added, as described previously (Chiu et al. Citation1999). Finally, we observed no difference for activation of the hybridomas by either immature or mature dendritic cells (data not shown).
Figure 1. Each cell type was analyzed for CD1.1 expression by incubation with 19G11 or isotype controls diluted at 5 µg/ml. After washing, cells were incubated with FITC-labeled goat anti rat IgG-Fc diluted at 10 µg/ml. Cells were washed, fixed and analyzed on a FACScalibur using Cellquest Software.
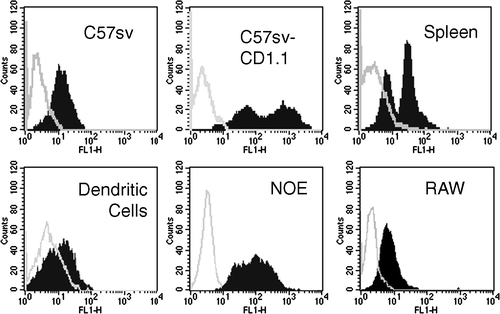
Figure 2. (A) For each cell type, 8×104 cells were incubated with 8×104 T cell hybridomas (DN32.D3, TB.A7 or TC.B11) for 24 h at 37°C in RPMI 1640 medium. Culture supernatants were collected 24 h later and IL-2 was measured. Dendritic cells, NOE cells line (8×104) and DN32.D3 (B) or TC.B11 (C) (8×104) were incubated with either the absence or the presence of α-GalCer (200 ng/ml) and with or without anti-CD1d 20H2 mAb (2 µg/ml). The reactivity of hybridomas was evaluated by IL-2 secretion (±SD from triplicates).
Disruption of lipid rafts inhibits activation of Vα14-positive T cell hybridomas but not Vα14-negative T cell hybridomas
The role of lipid raft microdomains can be investigated with functional studies using several reagents that break their integrity. The membrane cholesterol chelator, MβCD, remains the main reagent used in such studies (Thomas et al. Citation2004). The efficiency of MβCD depends upon its concentration, the time of exposure and the temperature. However, the efficient concentration range is close to the cytotoxic range. We analyzed the ability of MβCD to alter the activation of anti-CD1d T cell hybridoma by different cell types.
With the lowest non toxic concentrations (5 and 10 mM) of MβCD, the activation of DN32.D3 was reduced whereas the activation of the T cell hybridomas TC.B11 and TB.A7 was enhanced (A). The inhibition of activation was then proportional to the amount of MβCD and its effect on cellular viability. We performed another experiment analyzing the effect of MβCD after adding α-GalCer. Dendritic cells were treated with 40 or 200 ng/ml of antigen for 2 h before adding DN32.D3, P.41, P.9 and TC.B11 hybridomas. We observed once again an increase of IL-2 production for TC.B11 hybridoma after treatment with 5 or 10 mM MβCD. The activation of the three V-α14+ hybridomas was reduced with the lowest non toxic MβCD concentrations (B). MβCD toxicity on dendritic cells was assessed by measuring the decrease of 3H incorporation (C).
Figure 3. (A) Dendritic cells were treated with various concentrations of MβCD for 30 min, washed, incubated with DN32.D3, TB.A7 or TC.B11 hybridomas for 24 h and activation was measured by the IL-2 release. (B) Similar experiments were performed with three Vα14-positive hybridomas DN32.D3, P.41 and P.9 and the Vα14-negative hybridoma TC.B11 in the presence of α-GalCer (200 ng/ml). (C) The viability of dendritic cells was indirectly measured by the decrease of 3H incorporation. Results were expressed as% of inhibition of IL-2 production at various concentrations of MβCD as mean±SD from triplicates. (D) C57SV-CD1.1 cell line was treated with various concentrations of MβCD for 30 min, washed, incubated with DN32.D3, TB.A7 or TC.B11 hybridomas for 24 h and activation was measured by the IL-2 release. MβCD cytotoxicity was assessed by MTT test. Results were expressed as% of inhibition of IL-2 production at various MβCD concentrations and% of cellular viability as mean±SD from triplicates. In parallel, CD1d expression was assessed by flow cytometry with incubation of 19G11 mAb and FITC-labeled anti-rat antiserum and illustrated with the concentration of 30 mM of MβCD (insert).
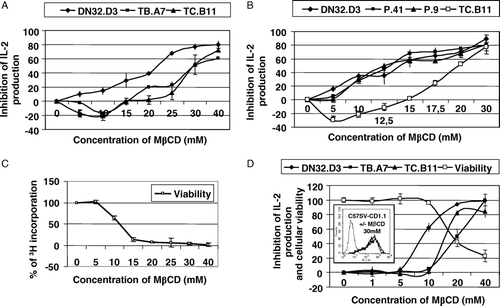
These results allowed us to conclude that the reactivity of Vα14-negative hybridomas was independent of the raft integrity contrary to the Vα14-positive hybridomas. The same observation was done with C57SV-CD1.1 (D) and NOE cells (data not shown). Moreover, whatever the MβCD concentration, CD1d membrane expression measured by flow cytometry was not reduced as illustrated in the insert of D for the concentration of 30 mM.
CD1d is mainly present in low density fractions associated with lipid rafts
Association of CD1d with lipid rafts was analyzed by western-blot after separation of the non-ionic detergent insoluble complexes by gradient ultracentrifugation. We easily detected a 55 kDa band with the polyclonal antiserum in post-nuclear supernatant of C57SV-CD1.1 and NOE cell lines but not in dendritic cells or other low CD1d expressing cell lines (data not shown). Analysis of the different fractions collected after gradient centrifugation revealed that CD1d is present at steady state in the transfected cells or the NOE cell line (A) in fraction 2. This fraction corresponded to the lipid rafts according to the presence of GM1 revealed by the binding of cholera toxin β subunit at the front of the migration. Moreover, pre-treatment of the NOE cell line with 10 mM of MβCD decreased the presence of CD1d in fraction 2 and enhanced its presence in heavy fractions. The α-GalCer induced activation of Vα14-expressing NKT cells which is inhibited by disruption of rafts in presence of MβCD (Lang et al. Citation2004, Park et al. Citation2005). We analyzed the effect of cell treatment with this antigen on CD1d association with lipid rafts. In NOE cells, we observed an enrichment of CD1d in the low density detergent insoluble fraction associated with a reduction in the other fractions except for fraction 9 (A).
Figure 4. (A) C57SV-CD1.1 cell line and the NOE cell line were treated either with MβCD 10 mM for 30 min or α-GalCer (200 ng/ml) for 3 h. Cells were detached, post-nuclear supernatants were prepared in the presence of 1% Brij98 and loaded on Optiprep gradient and ultracentrifuged. The eight fractions were collected and the presence of CD1d and GM1 was analyzed by western-blot using either polyclonal anti-CD1d antiserum and peroxydase-labeled anti-rabbit antiserum or biotinylated cholera toxin and peroxydase-labeled streptavidin. The peroxydase activity was revealed with chemiluminescence. The levels of CD1d in each fraction were assessed by Image Master Software. (B) Fraction 2 from C57SV-CD1.1 cell line and dendritic cells were treated with N-Glycosidase F overnight at 37°C in presence of 0.2% SDS and 1% β-mercaptoethanol. Untreated and treated fractions were then analyzed by western-blot using anti-CD1d polyclonal antiserum, peroxydase-labeled anti-rabbit antiserum and revelation with chemiluminescence.
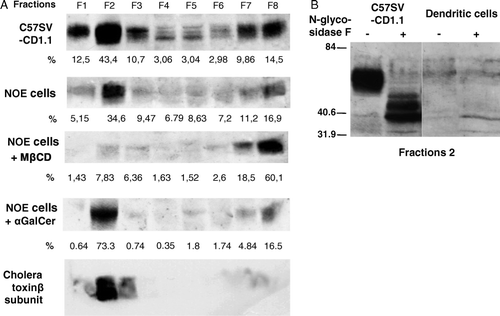
In dendritic cells, only a faint band in fraction 2 was detected (B). The specificity of CD1d signals was further assessed by endoglycosidase F (N-Glycosidase F) treatment of proteins present in fraction 2 of the transfected cell line and dendritic cells. Indeed, we detected a lower molecular weight product corresponding to the unglycosylated form of CD1d (Spada et al. Citation2000).
Flow cytometry kinetic analyses reveal that membrane sensitivity of CD1d to detergent is similar to that of CD71, which is not associated with lipid rafts
Flow cytometry offers an alternative approach for studying the presence of proteins in lipid rafts. CD71 is known to be excluded from lipid rafts (Gombos et al. Citation2004). CD71 displayed a high sensitivity even when very low concentrations of Triton X-100 or 1% Brij 98 were used. This protein was almost completely extracted after less than 1 min with 0.05 or 0.04% of Triton-X100. The rate of extraction was slower when using 1% Brij 98 at 37°C. In contrast, the lipid raft marker ganglioside GM1 and the GPI anchored protein Rae-1 expressed by the NOE cell line were resistant to detergent (A and 5B). Whatever the cell type, CD1d displayed exactly the same sensitivity for detergent extraction as CD71. Using 1% Brij98 at 37°C, we observed the same rate of extraction in the three cell lines for CD71 and CD1d (data not shown).
Figure 5. (A) Measurements of detergent solubility were performed in the flow cytometer kinetic mode data acquisition. NOE cell line were labeled during 15 min at 4°C with PE-anti-CD71, PE-anti-CD1d, PE-anti-Rae-1 or FITC-labeled cholera-toxin β subunit, washed and analyzed immediately. Labeled-cells were analyzed during 15 sec, mixed with the Triton X-100 (0.05, 0.04 or 0.02%) and then recorded for 4 min. Means of fluorescence for each Triton X-100 concentration and molecule were evaluated by FacsDiva software. (B) Results are illustrated with the concentration of 0.04%. (C) NOE cell line was treated in the presence of α-GalCer for 30 min and 3 h at 37°C. The detergent sensitivity of membrane CD1d was measured by flow cytometry kinetic mode data acquisition. NOE cell line was labeled during 15 min at 4°C with PE-anti-CD1d washed and analyzed immediately. Labeled-cells were analyzed during 15 sec, mixed with the Triton X-100 (0.04%) and then recorded for 4 min. Means of fluorescence are reported in the adjacent graphs.
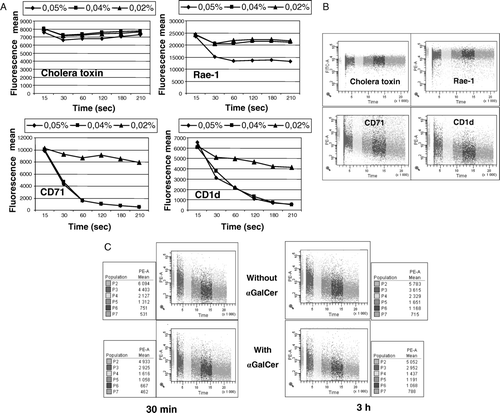
Finally, we compared the detergent sensitivity of membrane CD1d by flow cytometry in either the presence or absence of α-GalCer. Whatever the cell type, the detergent concentration, the temperature, the time of treatment with α-GalCer and the concentration of antigen (25, 50, 100 and 200 ng/ml), we observed no difference in the rate of decrease of fluorescence. We illustrated the results obtained with the NOE cell line incubated with or without 200 ng/ml of α-GalCer in presence of 0.04% of Triton X100 (C). The rate of detergent extraction of CD1d was the same for each condition.
Discussion
Our study describes the constitutive presence of CD1d in lipid rafts/detergent resistant membrane fractions in bone marrow-derived dendritic cells and a new epithelial cell line. CD1d activation of Vα-14 positive hybridomas was altered following lipid raft disruption. However, CD1d presentation to autoreactive Vα-14 negative anti-CD1d hybridomas, which do not require the endocytic pathway, was not altered by disruption of lipid rafts. Moreover, in presence of α-GalCer, the amount of CD1d recovered in low density detergent insoluble fractions was increased. Finally, using flow cytometry analyses, we showed that the detergent sensitivity of CD1d was the same as the non raft CD71 molecule and that addition of α-GalCer did not modify this sensitivity.
In our laboratory, we have developed routine cultures of nasal olfactory mucosa (Feron et al. Citation1999, McCurdy et al. Citation2005). The NOE cell line was established spontaneously and has been maintained in culture several months without losing CD1d expression. This cell line also expresses high levels of MHC-Ia and GPI-anchored MHC-related molecule, Rae-1. The expression of CD1d, quantified by flow cytometry, was higher than in other cell types, including dendritic cells. The reasons are unclear but might be related to the tissue of origin, since we observed, using quantitative PCR, high levels of CD1d mRNA expression in the olfactory bulb (data not shown). Alternatively this cell line might express antigens or molecular partners which facilitate the export and/or the stabilization of CD1d to the membrane. In any case, this cell line is a good model for molecular experiments analyzing CD1d restricted T cell activation. Like the CD1d transfected cell line and dendritic cells, the NOE cell line was able to activate Vα14-positive DN32.D3 hybridoma in absence of exogenous antigen. This response could be explained by either the presence of a specific autoantigen, a particular endocytic pathway (Sandberg & Ljunggren Citation2005) or a specific conformation of CD1d induced by its concentration in specific membrane areas.
Because of their high-lipid content (Brown & London Citation2000, Hooper Citation1999), the detergent-insoluble membrane (DIM) complexes float at low density during gradient centrifugation (Shogomori & Brown Citation2003). The use of detergents leads to the generation of small micelles and large particles that can be separated by centrifugation (Heerklotz Citation2002, Heerklotz et al. Citation2003). However, various methods for lipid raft preparation do not yield identical membrane fractions. For an efficient isolation of raft microdomains, the reaction is performed at low temperature (4°C), where the detergent can be excluded from the mixture of lipids and proteins. It has also been shown that at low temperature the amount of lipids in the plasma membrane rafts is increased (Shogomori & Brown Citation2003). Two previous studies report that CD1d localize in a steady-state in lipid rafts and that microdomains facilitate activation of Vα14-positive NKT cells in presence of α-GalCer. The main procedures used Triton X100, 4°C isolation and ultracentrifugation on sucrose gradient. Some investigators prefer Optiprep to sucrose for the isolation of rafts (Dienz et al. Citation2003, Baron & Coburn Citation2004). This centrifugation procedure yields a fairly consistent product that is enriched in cholesterol and raft marker proteins such as flotillin, glycosphingolipids and GPI-linked proteins. The Brij detergents better preserve the structural integrity of lipid raft–protein complexes, particularly when TCR is the subject of investigation (Drevot et al. Citation2002). Using partitioning at 37°C in the presence of Brij98, we confirmed that CD1d is constitutively present in DIM.
Then, we performed characterization of lipid rafts with flow cytometry method (Filatov et al. Citation2003, Gombos et al. Citation2004). Contrary to the GPI-anchored molecule Rae-1 and the glycosphingolipid GM1, the addition of detergent decreased the fluorescence for CD1d. This result differed from those obtained with flotation gradients. One potential explanation for this discrepancy is that CD1d interacts with plasma membrane partners localized in lipid rafts and that this interaction is not strong enough for resisting detergent. Another explanation is that CD1d is present in a subclass of rafts at the membrane level (Pralle et al. Citation2000). Stabilization of CD1d in lipid rafts would need external stimuli such as cross-linking by antibodies or ligand binding to receptor (Gombos et al. Citation2006). Even if α-GalCer is able to form lipid-CD1d complexes which activate Vα-14 hybridomas, we did not observe modification of detergent sensitivity of CD1d measured by flow cytometry. An alternative explanation is that CD1d could be preferentially associated with lipid rafts during the steps of endocytosis in intracellular organelles. Indeed, if CD1d is localized in lipid rafts in intracellular membranes but not in the plasma membrane, it will not be stained by antibodies in living cells. Further investigations with purified preparations of rafts from plasma and intracellular membranes will help to validate this hypothesis. Another intriguing observation is that α-GalCer favors the localization of CD1d in DIM. The mechanisms explaining the constitutive expression of CD1d in DIM and the action of α-GalCer are unknown. CD1d is not GPI-anchored and no site of palmitoylation is present. Probably, aggregation of CD1d and its interaction with α-GalCer involve other molecules such as members of tetraspanin web do for aggregation of MHC-II molecules (Kropshofer et al. Citation2002, Vogt et al. Citation2002).
MHC-I molecules are not found constitutively in lipid rafts. Adding a GPI anchor to MHC-I molecules leads to a localization in microdomains and a loss of their antigen presenting function (Cariappa et al. Citation1996, Cebecauer et al. Citation1998). Like MHC-I molecules, CD1d molecules reach directly the membrane (Jayawardena-Wolf et al. Citation2001). These CD1d isoforms are recognized by Vα14-negative hybridomas, TCB.11 and TB.A7, and insensitive to cholesterol depletion. Moreover, treatment by MβCD favored the presence of non-aggregated forms of CD1d at the membrane explaining the increase of IL-2 production by these hybridomas when dendritic cells were treated with low concentration of MβCD.
Like MHC-II molecules, CD1d molecules are internalized towards the endosomes and re-expressed at the membrane level. Microdomains are involved in the recycling of these molecules (Simons & Ehehalt Citation2002). The localization of CD1d molecules to membrane rafts helps to concentrate CD1d–lipid complexes in the plasma membrane, and this might enhance antigen presentation to DN32.D3, P41 or P9 hybridomas which are more efficiently activated by CD1d multimers than monomers (Benlagha et al. Citation2000). We described that treatment with MβCD of dendritic cells and CD1d-transfected cell line alters the activation of DN32.D3 hybridoma in the absence or presence of α-GalCer. Park et al. showed that: (i) MβCD treatment reduced the capacity of RBL-CD1d transfected cell line treated with α-GalCer to stimulate DN32.D3 T cell hybridoma (Park et al. Citation2005), and (ii) the effect of MβCD was not observed with high concentrations of α-GalCer (200 ng/ml) which might induce alone CD1d-lipid complexes. The same authors reported that the cell line BCL-1, expressing high level of CD1d, exhibits a raft-localized mCD1d distribution pattern but stimulates DN32.D3 far less efficiently than do transfected RBL-CD1d cells in the presence of αGalCer. They suggested that an unknown additional factor, targeted by MβCD, is required and that the regulation of NKT cells activation is not controlled by CD1d alone. This factor is probably expressed by our epithelial cell line and dendritic cells. This additional factor would be constitutively present in membrane microdomains and associated with CD1d during endocytosis favoring its aggregation and association with exogenous antigen such as α-GalCer. Further studies are required to characterize and elucidate the function of such a factor and to better understand the detailed molecular organization of CD1d in the plasma membrane which influences interaction with Vα14-positive T cell receptor.
Acknowledgements
We thank Drs J. Fantini for review of the manuscript and A. Bendelac and K. Benlagha for providing anti CD1.1 antibodies, CD1.1 transfected fibroblast cell line and hybridomas.
References
- Baron CB, Coburn RF. Smooth muscle raft-like membranes. J Lipid Res 2004; 45: 41–53
- Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med 2000; 191: 1895–1903
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 2000; 275: 17221–17224
- Brutkiewicz RR, Bennink JR, Yewdell JW, Bendelac A. TAP-independent, beta 2-microglobulin-dependent surface expression of functional mouse CD1.1. J Exp Med 1995; 182: 1913–1919
- Cariappa A, Flyer DC, Rollins CT, Roopenian DC, Flavell RA, Brown D, Waneck GL. Glycosylphosphatidylinositol-anchored H-2Db molecules are defective in antigen processing and presentation to cytotoxic T lymphocytes. Eur J Immunol 1996; 26: 2215–2224
- Cebecauer M, Cerny J, Horejsi V. Incorporation of leucocyte GPI-anchored proteins and protein tyrosine kinases into lipid-rich membrane domains of COS-7 cells. Biochem Biophys Res Commun 1998; 243: 706–710
- Chiu YH, Jayawardena J, Weiss A, Lee D, Park SH, Dautry-Varsat A, Bendelac A. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J Exp Med 1999; 189: 103–110
- Chiu YH, Park SH, Benlagha K, Forestier C, Jayawardena-Wolf J, Savage PB, Teyton L, Bendelac A. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail-truncated CD1d. Nat Immunol 2002; 3: 55–60
- De Silva AD, Park JJ, Matsuki N, Stanic AK, Brutkiewicz RR, Medof ME, Joyce S. Lipid protein interactions: the assembly of CD1d1 with cellular phospholipids occurs in the endoplasmic reticulum. J Immunol 2002; 168: 723–733
- Dienz O, Moller A, Strecker A, Stephan N, Krammer PH, Droge W, Schmitz ML. Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa and phospholipase C gamma 1 are required for NF-kappa B activation and lipid raft recruitment of protein kinase C theta induced by T cell costimulation. J Immunol 2003; 170: 365–372
- Drevot P, Langlet C, Guo XJ, Bernard AM, Colard O, Chauvin JP, Lasserre R, He HT. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. Embo J 2002; 21: 1899–1908
- Feron F, Mackay-Sim A, Andrieu JL, Matthaei KI, Holley A, Sicard G. Stress induces neurogenesis in non-neuronal cell cultures of adult olfactory epithelium. Neuroscience 1999; 88: 571–583
- Filatov AV, Shmigol IB, Kuzin II, Sharonov GV, Feofanov AV. Resistance of cellular membrane antigens to solubilization with Triton X-100 as a marker of their association with lipid rafts – analysis by flow cytometry. J Immunol Meth 2003; 278: 211–219
- Gombos I, Bacso Z, Detre C, Nagy H, Goda K, Andrasfalvy M, Szabo G, Matko J. Cholesterol sensitivity of detergent resistance: a rapid flow cytometric test for detecting constitutive or induced raft association of membrane proteins. Cytometry 2004; A61: 117–126
- Gombos I, Kiss E, Detre C, Laszlo G, Matko J. Cholesterol and sphingolipids as lipid organizers of the immune cells’ plasma membrane: their impact on the functions of MHC molecules, effector T-lymphocytes and T-cell death. Immunol Lett 2006; 104: 59–69
- Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J 2002; 83: 2693–2701
- Heerklotz H, Szadkowska H, Anderson T, Seelig J. The sensitivity of lipid domains to small perturbations demonstrated by the effect of Triton. J Mol Biol 2003; 329: 793–799
- Hooper NM. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae (review). Mol Membr Biol 1999; 16: 145–156
- Huby RD, Dearman RJ, Kimber I. Intracellular phosphotyrosine induction by major histocompatibility complex class II requires co-aggregation with membrane rafts. J Biol Chem 1999; 274: 22591–22596
- Ikonen E. Roles of lipid rafts in membrane transport. Curr Opin Cell Biol 2001; 13: 470–477
- Jayawardena-Wolf J, Benlagha K, Chiu YH, Mehr R, Bendelac A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity 2001; 15: 897–908
- Joyce S, Woods AS, Yewdell JW, Bennink JR, De Silva AD, Boesteanu A, Balk SP, Cotter RJ, Brutkiewicz RR. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science 1998; 279: 1541–1544
- Kropshofer H, Spindeldreher S, Rohn TA, Platania N, Grygar C, Daniel N, Wolpl A, Langen H, Horejsi V, Vogt AB. Tetraspan microdomains distinct from lipid rafts enrich select peptide-MHC class II complexes. Nat Immunol 2002; 3: 61–68
- Lang GA, Maltsev SD, Besra GS, Lang ML. Presentation of alpha-galactosylceramide by murine CD1d to natural killer T cells is facilitated by plasma membrane glycolipid rafts. Immunology 2004; 112: 386–396
- Mandal M, Chen XR, Alegre ML, Chiu NM, Chen YH, Castano AR, Wang CR. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol Immunol 1998; 35: 525–536
- Mattner, J, Debord, KL, Ismail, N, Goff, RD, Cantu, C, 3rd, Zhou, D, Saint-Mezard, P, Wang, V, Gao, Y, Yin, N, Hoebe, K, Schneewind, O, Walker, D, Beutler, B, Teyton, L, Savage, PB, Bendelac, A. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature, 434:525–529.
- McCurdy RD, Feron F, McGrath JJ, Mackay-Sim A. Regulation of adult olfactory neurogenesis by insulin-like growth factor-I. Eur J Neurosci 2005; 22: 1581–1588
- Park JJ, Kang SJ, De Silva AD, Stanic AK, Casorati G, Hachey DL, Cresswell P, Joyce S. Lipid-protein interactions: biosynthetic assembly of CD1 with lipids in the endoplasmic reticulum is evolutionarily conserved. Proc Natl Acad Sci USA 2004; 101: 1022–1026
- Park SH, Roark JH, Bendelac A. Tissue-specific recognition of mouse CD1 molecules. J Immunol 1998; 160: 3128–3134
- Park YK, Lee JW, Ko YG, Hong S, Park SH. Lipid rafts are required for efficient signal transduction by CD1d. Biochem Biophys Res Commun 2005; 327: 1143–1154
- Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol 2000; 148: 997–1008
- Sandberg JK, Ljunggren HG. Development and function of CD1d-restricted NKT cells: influence of sphingolipids, SAP and sex. Trends Immunol 2005; 26: 347–349
- Shogomori H, Brown DA. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol Chem 2003; 384: 1259–1263
- Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest 2002; 110: 597–603
- Spada FM, Borriello F, Sugita M, Watts GF, Koezuka Y, Porcelli SA. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur J Immunol 2000; 30: 3468–3477
- Taner SB, Onfelt B, Pirinen NJ, McCann FE, Magee AI, Davis DM. Control of immune responses by trafficking cell surface proteins, vesicles and lipid rafts to and from the immunological synapse. Traffic 2004; 5: 651–661
- Thomas S, Preda-Pais A, Casares S, Brumeanu TD. Analysis of lipid rafts in T cells. Mol Immunol 2004; 41: 399–409
- van de Wal Y, Corazza N, Allez M, Mayer LF, Iijima H, Ryan M, Cornwall S, Kaiserlian D, Hershberg R, Koezuka Y, Colgan SP, Blumberg RS. Delineation of a CD1d-restricted antigen presentation pathway associated with human and mouse intestinal epithelial cells. Gastroenterology 2003; 124: 1420–1431
- Van Kaer L, Joyce S. Innate immunity: NKT cells in the spotlight. Curr Biol 2005; 15: R429–431
- Vogt AB, Spindeldreher S, Kropshofer H. Clustering of MHC-peptide complexes prior to their engagement in the immunological synapse: lipid raft and tetraspan microdomains. Immunol Rev 2002; 189: 136–151
- Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann VS, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med 1997; 185: 317–328
- Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science 1997; 277: 339–345