Abstract
Members of the CLC ‘chloride channel’ family play vital roles in a wide variety of physiological settings. Research on prokaryotic CLC homologues provided long-anticipated high-resolution structures as well as the unexpected discovery that some CLCs are not chloride channels, but rather are proton-chloride antiporters. Hence, CLCs encompass two functional classes of transport proteins once thought to be fundamentally different from one another. In this review, we discuss the structural features and molecular mechanisms of CLC channels and antiporters. We focus on ClC-0, the most thoroughly studied CLC channel, and ClC-ec1, the prokaryotic antiporter of known structure. We highlight some striking similarities between these CLCs and discuss compelling questions that remain to be addressed. Prokaryotic CLCs will undoubtedly continue to shed light upon this understudied family of proteins.
Introduction
CLC ‘Chloride Channels’ are expressed ubiquitously and perform a striking array of physiological functions Citation[1]. For many years, our understanding of structure-function relationships in this family was limited by the inability to purify these low-abundance eukaryotic membrane proteins in quantity and quality sufficient for high-resolution structure determination. The discovery of prokaryotic homologues Citation[2] and the relative ease of expressing and purifying these proteins greatly mitigated this obstacle. Moreover, research on prokaryotic CLC homologues revealed novel and unexpected functions of CLC proteins.
An early functional analysis demonstrated that the E. coli homologue ClC-ec1 mediates chloride-selective flux, as expected for a CLC protein Citation[3]. A physiological assessment showed that CLC proteins are required for E. coli's resistance to extreme acid conditions Citation[4], a function that parallels roles of mammalian CLCs in acidifying intracellular compartments Citation[5]. This work was followed by a series of groundbreaking studies. First, crystallographic structures of two prokaryotic homologues were determined Citation[6–8]. Second, a more detailed functional analysis revealed that these CLCs are not ion channels as previously supposed but are chloride-proton antiporters Citation[9]. Finally, this finding motivated closer examination of the eukaryotic homologues, and it was discovered that certain mammalian CLCs are also chloride-proton antiporters Citation[10], Citation[11].
Thus, the study of prokaryotic CLC homologues provided the only known high-resolution structure of a CLC family member and effectuated the landmark discovery that the CLC family bridges an evolutionary cusp between two major classes of membrane proteins – channels and antiporters. Studies of CLC mechanisms will improve our understanding of how membrane proteins work, including how they undergo conformational changes that are driven by ion gradients and how similar basic structures are altered to carry out different functions. In this review, we will discuss the structural features and molecular mechanisms of CLC channels and antiporters, highlight some striking similarities between these CLC subclasses, and point out remaining compelling questions about this remarkable protein family.
The molecular design of CLC chloride channels
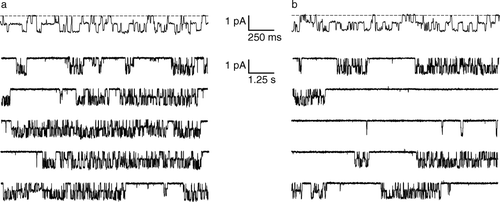
The CLC field has been shaped by mechanistic studies on ClC-0, a homologue from the Torpedo electroplax organ Citation[12–15]. Single-channel analysis revealed a two-pore channel with two gating mechanisms. The pores open and close independently on the millisecond timescale in a process referred to as ‘fast’ gating, and open and close simultaneously on a much slower timescale (seconds to minutes), in a process called ‘slow’ or ‘common’ gating (). Biochemical and mutagenic analysis further revealed that each pore is composed from a single protein subunit.
Figure 1. Single-channel behavior of ClC-0. (a) Top trace: the two pores in ClC-0 are gated independently by the ‘fast gate’. The dashed line corresponds to zero current, and channel openings are downward deflections. Bottom five traces: Fifty seconds of continuous recording, shown on a compressed time scale. The long silent periods correspond to closures of the ‘common gate’. Recordings were at −70 mV; symmetric solutions contained 110 mM NMDG-Cl, pH 7.5 (J. Lisal and M. Maduke, unpublished). (b) Conditions similar to (a) except the extracellular pH has been raised to 8.5. The decrease in proton concentration causes an increased probability of common-gate closure. At this voltage (−70 mV), there is no significant effect of the extracellular pH change on the fast gate.
The homodimeric, two-pore architecture, envisioned long before any direct structural information, depicts a class of channels fundamentally different from that of the well-studied cation-selective ion channels. The latter channels are constructed according to a common plan: they are oligomers with four- or five-fold symmetry (or pseudosymmetry) in which the pore is formed along the axis of symmetry of the oligomer. With this design, the pore is constrained by symmetry to lie perpendicular to the membrane. In contrast, because each CLC channel pore is formed within a single protein subunit, the pore is unconstrained by symmetry; thus, the pore is not required to lie perpendicular to the membrane and will most likely snake its way through each subunit.
Prokaryotic CLC structures and their relevance to eukaryotic CLC channels
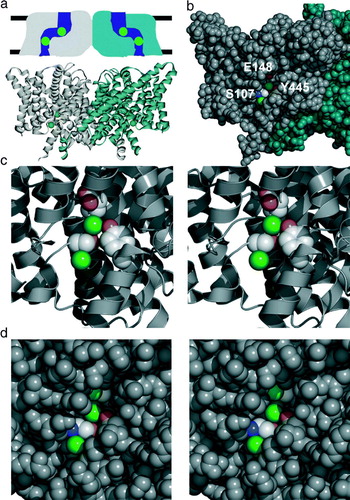
The structure of the E. coli homologue ClC-ec1 Citation[6–8] conforms to the expected homodimeric architecture described above, where a separate pore snakes its way through each subunit (a). In each subunit, four different segments of sequence elegantly converge to form a central chloride binding site. This site is inaccessible to both aqueous spaces (b,c), which originally led to the conclusion that these were chloride channels captured in a closed state. A second chloride-binding site, intracellular to the first, is accessible to the cytoplasm but not to the central (occluded) chloride ion. The two sites can be simultaneously occupied Citation[16], reminiscent of the multi-ion occupancy observed in the ClC-0 channels Citation[17]. Despite the low overall sequence similarity between the prokaryotic and eukaryotic homologues, the residues coordinating these chloride ions are well conserved amongst the CLCs, and these homologues almost certainly share a common fold Citation[18].
Figure 2. CLC structure. (a) Structures are shown from the point of view of the membrane. Above: ‘low resolution’ schematic diagram of CLC structure based on electrophysiological and biochemical studies of ClC-0. The CLC structure was predicted to be a two-pore homodimer, in which the pores are not perpendicular to the membrane. Below: cartoon view of the X-ray crystallographic structure of ClC-ec1 (pdb 1OTS). Each subunit of the homodimer contains two chloride ions (spacefilled in green). (b) Spacefilling view of the ClC-ec1 structure, focused on one of the subunits (shown in grey). Part of the protein has been peeled away to show the two chloride ions. E148, the ‘glutamate gate’ residue, blocks the upper chloride ion from exiting into the extracellular solution. S107 and Y445 directly coordinate the chloride ions, and S107 directly blocks the pathway for chloride to move between the two sites. (c) Close-up stereo view of the selectivity filter. S107, Y445, and E148 side chains are shown. (d) Close-up stereo view of the E148A mutant (pdb 1OTT). A chloride ion is seen in the place previously occupied by the glutamate side chain. The chloride pathway remains obstructed.
Several studies have further elaborated the similarities between prokaryotic and eukaryotic structures. Estevez et al. used the ClC-ec1 structure as a guide to pinpoint inhibitor binding sites in three different eukaryotic CLC homologues Citation[19]. Lin and Chen Citation[20] and Engh and Maduke Citation[21] focused on the pore-lining region of the eukaryotic channel ClC-0, finding that the residues predicted to be near the bound chloride ions are sensitive to thiol-modification and that this modification affects open-channel properties. These studies strongly suggest that the overall structure of the CLC channels and antiporters are similar to one another.
Conformational changes in CLC channels
To understand the similarities and differences between CLC ion channels and CLC antiporters, we begin by examining what is known about conformational changes in CLC ion channels. An early structure-based model proposed that only minimal movement is involved in ClC-0's ‘fast’ gating (the gating process that acts on each pore independently) Citation[8]. This model was based on the combination of three observations. First, in the ClC-ec1 structure, a conserved glutamate (E148) sidechain directly obstructs the chloride exit to the extracellular side (b,c). Second, in ClC-0, mutation of the equivalent glutamate in ClC-0 causes the channel to be open almost all of the time. Third, the structural changes induced by mutation of the ‘glutamate gate’ residue in ClC-ec1 are minimal: the only differences are that the glutamate side chain no longer directly blocks the permeation pathway and that a third chloride ion is seen in the position previously occupied by the carboxylate. Combined, these observations suggested a model in which the glutamate residue acts as a gate in ClC-0. In this model, the glutamate side chain competes with chloride, and chloride binds more readily when the glutamate side chain is protonated. Consistent with such a mechanism, increases in either the concentration of extracellular chloride or protons increase the ClC-0 channel opening rate Citation[14], Citation[15].
Nevertheless, several pieces of evidence support the idea that fast gating in ClC-0 requires more than a simple movement of a glutamate residue. First, the bound chloride ions in the ClC-ec1 E148 mutant structures are still occluded from the intracellular solution (d), and the extracellular access to the new chloride site is also partially restricted. Assuming that the structure of the antiporter is relevant to channel gating, this means that additional changes in the protein must occur. Second, single-channel analysis of ClC-0 fast gating shows that there is a kinetic step in channel opening that occurs subsequent to chloride binding to an external site Citation[22]. If the external site corresponds to the extracellular ‘glutamate gate’ site, then these results suggest that chloride binding induces an additional conformational change in the protein. Analysis of ClC-0 block by state-dependent inhibitors is consistent with such a multi-step process Citation[23]. Finally, mutations in several regions of the protein distant from the glutamate also affect fast gating Citation[24], Citation[25], a finding that fits most readily with a conformational change involving more than a localized movement of a single sidechain.
While the size of the conformational change in ClC-0 fast gating remains to be resolved, the ClC-0 ‘slow’ or ‘common’ gate certainly involves a large conformational change. The ClC-0 common gate is cooperative, acting on both pores simultaneously. Since the two pores are separated by >30 Å, there must be a long-range cooperative interaction. This idea is supported by several experimental observations. First, mutations scattered throughout the protein have effects on common gating Citation[15]. Second, the common gate is extremely sensitive to temperature, having a Q10 of ∼40 Citation[26]. Third, recent FRET measurements suggest a movement of at least 20 Å Citation[27]. Whether such a large, cooperative conformational change also occurs in the prokaryotic homologues remains to be determined and will be discussed below.
Conformational changes in CLC antiporters
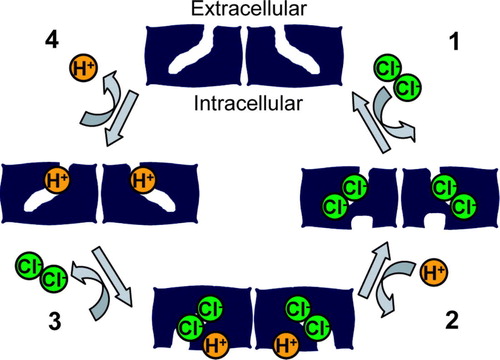
How is it that different CLC family members with similar structures can be either ion channels or antiporters? Unlike ion channels, where a ‘gate’ opens and closes to allow the transport of ions across an aqueous pore, antiporters never have an open pore; if they did, it would not be possible to couple movement of one ion to movement of another ion. Therefore, an antiporter must have two ‘gates’, and these two gates must never be open simultaneously. This means that ion binding must be tightly coordinated with gate opening and closing. It is generally thought that antiporters achieve such coordination by undergoing a series of conformational changes in which opening and closing of these gates is coupled to ion binding and unbinding. A simple model for chloride-proton antiport is shown in . In this model, the antiporter cycles through four conformations, and the conformational changes are induced by the binding and release of chloride and protons.
Figure 3. Model for chloride-proton antiport. In this minimal model, an electrochemical gradient for a transported ion is used to drive the cycle of conformational changes in a specific direction. In Step 1, two chloride ions bind from the extracellular side of the membrane. The binding of these chloride ions triggers a conformational change that allows a proton to bind to an intracellular site (step 2). The binding of this proton induces a conformational change that allows the chloride ions to exit from the intracellular side (step 3). The removal of chloride ions causes a conformational change allowing the transfer of protons from the intracellular side of the protein to the extracellular side of the protein, followed by the exit of protons to the extracellular side of the membrane (step 4). This cycle is repeated, allowing the coupled movement of two chloride ions and one proton in opposite directions. This simple model does not address the question of whether the two-subunit architecture (and the related ‘common’ gating seen in the CLC channels) is essential for CLC antiporter function.
If ClC-ec1 is an antiporter and goes through at least four conformational changes, why is it that every structure that has been crystallized looks nearly identical? In fact, ClC-ec1 and its Salmonella cousin (80% identical in amino acid sequence) have been crystallized at pH values ranging from 4.6 to 9.5 (pH values spanning the activity dependence of ClC-ec1), and the structures are essentially superimposable Citation[7], Citation[8], Citation[16]. One possibility is that not enough different crystallographic conditions have been tested. A second possibility is that only one conformation is stable enough to produce diffracting crystals. If this is the case, inhibitors or modifying reagents may help to stabilize ClC-ec1 in additional conformations and provide ‘snapshots’ of the transport cycle. So far, only one prokaryotic CLC inhibitor has been identified, and its mechanism of action has not yet been determined Citation[28]. A third possibility (which is not mutually exclusive with the first two possibilities) is that the conformational changes are small, perhaps so small that they cannot be resolved at 2.5–3.5 resolution.
Even if the conformational changes in ClC-ec1 are small, they likely involve more than just the movement of the ‘glutamate gate’. As discussed above, antiporters require two gates in order to coordinate coupled movement of the ions. Consistent with this notion, the crystal structure reveals that intracellular residues block access of the central chloride ion to the cytoplasm (b–d); hence, ClC-ec1 must have an intracellular gate. Evidence for movement of intracellular residues comes from observations of accessibility of these residues to water-soluble reagents. The tyrosine residue (Y445) that coordinates the central chloride ion is completely inaccessible to solvent in the crystal structure, yet when mutated to a cysteine, this position is accessible to large thiol-modifying reagents Citation[28]. The simplest explanation of these results is that there is a significant opening of the intracellular vestibule during some point of the transport cycle. However, it is also possible that these large reagents access Y445C during thermal fluctuations that are not correlated with the transport cycle. Consistent with the idea that the transport cycle involves movement of this region, several other residues on the same helix are accessible to modification by large fluorescent reagents, and the fluorescently modified transporters undergo fluorescence changes that are correlated with alteration of either the chloride or proton concentration Citation[29]. These changes are coupled (the magnitude of the chloride-dependent fluorescence change is dependent upon the pH), as would be expected for a coupled transporter. This suggests that the fluorescence changes reflect at least one of the conformational changes necessary for coupled transport.
The relationship between conformational changes in the channels and antiporters
What is the relationship between the conformational changes occurring in the antiporters and those occurring in CLC channel gating? Do the antiporter conformational changes resemble either the fast or common gating observed in the ion channels? Despite the fundamental differences between ion channels and antiporters, the gating properties of CLC ion channels exhibit certain ‘antiporter-like’ qualities.
First, ClC-0 fast gating shares many properties with ClC-ec1 transporter ‘gating’. Both processes are sensitive to chloride and proton concentrations, and both processes lose their sensitivity to protons upon mutation of the glutamate gate residue Citation[8], Citation[9]. This suggests that ClC-0 fast gating and ClC-ec1 antiport may have similar underlying mechanisms for pH-sensitivity. Indeed, many overall features of the proton-, chloride-, and voltage-dependence of ClC-0 fast gating are features that could easily be incorporated into a chloride-proton antiporter mechanism. The corollary is that the ClC-0 fast gating mechanism could be that of a ‘broken transporter’; such a mechanism has been presented with insightful detail in a recent review Citation[30].
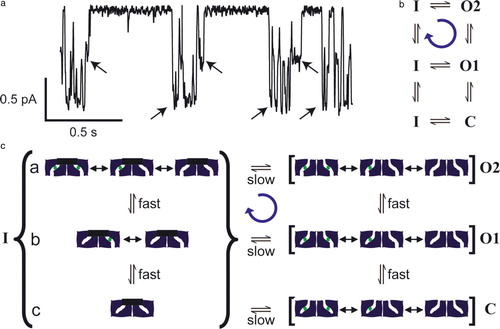
A second ‘antiporter-like’ quality of ClC-0 channel gating is the existence of two gates (the fast gate and the common gate). Since there is no known functional necessity for the existence of two gates in ClC-0, one hypothesis is that this is a remnant of antiporter gating, where two gates are necessary to prevent the formation of an open pore (). Although not necessary, a cooperative ‘common’ gate could serve as one of the gates in the antiporters. Supporting this idea, the common gate shares similarities to ClC-ec1 in being activated by both chloride and protons () Citation[14]. Furthermore, the common gate and fast gate of ClC-0 reveal a cycle of conformational changes that bears striking resemblance to the cycle of conformational changes expected for a coupled transporter (). Specifically, there is a tendency for the common gate to close from a state in which only one of the pores has its fast gate open, and to open from a state in which both pores have their fast gates open (a,b) Citation[31]. This net cycling can be explained by a model in which cycling is driven by the movement of ions down their electrochemical gradient (c). For both channel and antiporter models (c and respectively), the cycles will run in one direction or the other depending on ion gradients. Although it is speculative to conclude that the conformational cycling in CLC channels and antiporters results from structurally similar conformational changes, the very existence of cycling in the CLC channels is nevertheless striking.
Figure 4. Gating asymmetry in ClC-0. (a) A single-channel record of ClC-0. Arrows indicate transitions of the common gate. Channel openings are downward deflections. There is an asymmetry in the gating cycle in that the common gate favors closing when 1 pore is opened and favors opening to a state with both pores open, as shown in this example. Conditions are the same as , except that the extracellular pH is 6.5 (J. Lisal and M. Maduke, unpublished). (b) State diagram indicating a net clockwise cycle from one-pore open (O1) to common-gate closed (inactivated, I) to both pores open (O2). The C to I transition is not measurable. (c) A model that can explain gating asymmetry in ClC-0. This speculative model illustrates that, by making one simple assumption, a channel with two gates can use the energy of a chloride electrochemical gradient to drive net cycling through its conformational states. Such cycling is similar to the cycling required for an antiporter to catalyze ion exchange (). In states O2, O1, and C, the common gate is open. In O2, the fast gates of both subunits are open. In C, the fast gates of both subunits are closed. In O1, the fast gate of only one of the subunits is closed. For simplicity, only one of the two ways of having one fast-gate closed is shown. In all three states, the pores transition very rapidly between different chloride-occupancy states (not all possible states are shown). When the fast gate is closed, chloride from the upper solution equilibrates with the sites in the pore. Channels can enter the inactivated state (I) (common gate closed) from C, O1, or O2. Only the transitions from O1 or O2 are measurable. By assumption, both gates can close only when there is no chloride in the pore. Fast-gate transitions occur during inactivation (substates a, b,c), but are not measurable. When there is a large electrochemical gradient for chloride (assume high chloride in the lower compartment), chloride saturates the binding sites as soon as the fast gate opens. This makes the transition from substate b to substate a essentially unidirectional, and leads to a net cycling in the clockwise direction (blue arrow). This figure is reproduced in colour in Molecular Membrane Biology online.
If the ClC-ec1 transport cycle is related to the ClC-0 asymmetric gating transport cycle described above, then we would expect ClC-ec1 transport properties to share characteristics with the ClC-0 gating processes. For example, if at least one step in the transport cycle of the ClC-ec1 is analogous to the rate-limiting common-gating step in ClC-0, then we might expect the turnover rate of ClC-ec1 transport cycle to be similar to the rate of the ClC-0 common-gate cycle. Contrary to this idea, however, the turnover rate for ClC-ec1 is ∼103 ions/s Citation[32] which is several orders of magnitude faster than the ClC-0 common gate. In fact, this turnover rate is of the same order of magnitude as the rate of the ClC-0 fast-gate. Furthermore, the activation enthalpy for ClC-ec1 transport is ∼50 kJ/mol Citation[33], much closer to that of the ClC-0 fast gate (51 kJ/mol) than to that of the common gate (260 kJ/mol) Citation[26]. These results suggest that the rate-limiting step in the ClC-ec1 transport cycle may be similar to the ClC-0 fast gate mechanism and may not involve the large cooperative conformational change seen with the common gate. However, turnover rate and activation enthalpy do not rigorously define the nature of the conformational change. For example, ClC-1 is known to have a cooperative common gate, but the kinetics of this common gate are very close in magnitude to the kinetics of the fast gate, as are the activation enthalpies (100 and 120 kJ/mol for fast and common gating respectively) Citation[34]. To fully determine whether cooperative conformational changes occur in CLC antiporters and to understand the relationship between CLC channels and antiporters, additional methods to assess the molecular movements in ClC-ec1 must be pursued.
The proton permeation pathway
X-ray crystallography, while providing high-resolution structure of the protein and its chloride binding sites, cannot identify the proton binding sites directly. Currently, mutagenesis studies provide our only guide to discerning the path of the protons. Eleven of the protonatable residues in the transmembrane region of ClC-ec1 have been mutated Citation[9], Citation[35]. Most mutations had little effect on chloride-proton antiport. The two most notable exceptions were E148, the ‘glutamate gate’ located near the extracellular side in the chloride permeation pathway, and E203, near the intracellular side but away from the chloride permeation pathway, towards the subunit interface. Mutation of either of these residues completely abolished proton transport. These results suggest that either: (i) these residues are directly in the proton permeation pathway, or (ii) mutation of these residues induces a conformational change that alters the accessibility or pKa of other residues in the proton pathway, hence indirectly preventing the proton transport. In the second case, one might expect the crystal structures of the E148 or E203 mutant proteins to be different from that of wild-type. In fact, this is not the case – the structures of the E148 and E203 mutant proteins are nearly identical to that of wild-type. Thus, the most straightforward interpretation of these data is that E148 and E203 participate directly in proton permeation. Nevertheless, we should keep in mind the limitations of mutagenesis approaches and the possibility that alternative conformations have simply not yet been crystallized.
E148 and E203 are located ∼15 apart from one other in the structure. Assuming that both of these residues are indeed in the proton permeation pathway, this is too far for protons to cross in a single leap. A conserved tyrosine residue, Y445 (b–d), lies about half-way between these two sites. The hydroxyl group of this tyrosine coordinates the central anion in the ClC-ec1 structure. Surprisingly, mutation of Y445 to phenylalanine, which removes the hydroxyl group, has no effect on the function of the protein Citation[36]. However, mutations of this tyrosine to other residues lead to either loss of proton coupling or to ‘leaky’ proton coupling (i.e., the protein transports less than one proton for every 2 chloride ions). To better understand these effects, structures of multiple mutants were solved. These structures all looked essentially identical to the wild-type structure. In fact, the only noticeable differences were the exact position of the mutant side-chain and the ion occupancy at the central chloride-binding site. Surprisingly, a direct correlation was observed between ion occupancy and coupling efficiency of proton-chloride antiport: the mutants that transported the lowest number of protons per chloride also had the lowest ion occupancy at this central ion-binding site. The mechanistic interpretation of this is not clear, but one possibility is that a proton actually binds to the central chloride ion during transport, and mutations that remove the central anion-binding site prevent the proton from binding at this position. In support of this idea, Nguitragool and Miller discovered that other anions, including thiocyanate and nitrate, disrupt chloride/proton coupling, and that such anions affect occupancy at the central anion-binding site; again, a direct correlation was observed between anion occupancy and proton coupling efficiency Citation[37].
Sequence gazing: a comparison between CLC channels and antiporters
It is interesting to note that E203 (the ‘intracellular glutamate’ discussed above) is conserved in the known CLC antiporters (ClC-ec1, ClC-4, and ClC-5) but is a valine in all of the known CLC channels (ClC-0, ClC-1, ClC-2, ClC-Ka, and ClC-Kb). If this conservation is predictive, the eukaryotic intracellular homologues ClC-3, ClC-6, and ClC-7 would also be chloride-proton antiporters. However, although ClC-6 and ClC-7 have not yet been functionally analyzed, ClC-3 has recently been reported to be a double-barreled channel, like ClC-0 Citation[38]. Given the close relationship between ClC-3, -4, and -5, this suggests that very subtle changes may be sufficient to convert an antiporter to a channel. Regarding the prokaryotic CLCs, over 400 sequences are now known. Less than half of these sequences contain this ‘intracellular glutamate’, while over half contain a hydrophobic residue at this position. Although the results with ClC-3 suggest that the identity of this residue alone cannot be used to deduce whether a CLC is a channel or antiporter, this sequence gazing does suggest the exciting possibility that some prokaryotic CLCs are channels. Further work is necessary to investigate this possibility and to understand the interplay between the residue at 203 and other positions in the protein.
CLC cytoplasmic domains
Although the prokaryotic CLCs of known structure contain all of the ‘core’ chloride-binding residues, they lack the large C-terminal cytoplasmic sequences found in all eukaryotic CLCs. These cytoplasmic sequences always contain a pair of ‘CBS’ domains Citation[39]. Although the precise function of the CBS domains in CLCs is unknown, they are required for proper trafficking Citation[40], Citation[41]. Mutations in these domains cause a number of diseases Citation[1], Citation[42], Citation[43] and affect channel gating Citation[44–46]. Additionally, some CBS domains harbor ATP-binding sites that appear relevant to function Citation[47–50]. Crystal structures of the cytoplasmic domains of ClC-0 and ClC-5 have been determined Citation[50], Citation[51], but it remains to be shown exactly how these cytoplasmic domains interact with the transmembrane portions of the CLC proteins. A few of the prokaryotic CLC homologues do contain cytoplasmic CBS domains, and further study of these homologues may help us to understand the functions of these domains.
Conclusion
The prokaryotic CLC homologues have shed a powerful light on both the structure and function of CLC family members. In addition to providing a high-resolution molecular view of the CLCs, study of the prokaryotic homologues led to the revelation that many CLCs are chloride-proton antiporters, not chloride channels. The challenge now is to carefully distinguish the molecular differences and similarities in the mechanisms of transport in the CLC ion channels and the CLC antiporters; such knowledge will provide fundamental insight into the principles of membrane protein function.
Study of the prokaryotic CLCs will no doubt continue, as these are the only homologues currently accessible to high-resolution methods of both structure and function, and the ability to use both of these techniques on the same protein is very powerful. The availability of milligram quantities of the prokaryotic CLCs in active form makes them readily accessible to biophysical techniques that could be used to probe protein dynamics, thus providing a critical bridge between structural and functional studies. Finally, many additional compelling questions remain: Do the prokaryotic CLC transporters have a ‘common’ gate? Do any of the prokaryotic CLCs that lack the E203 glutamate function as channels rather than antiporters? How are the prokaryotic CLCs regulated in a physiological context? Are there any endogenous ligands for these CLCs? What is the structure and function of the cytoplasmic domains in the prokaryotes? Do the prokaryotic homologues have any accessory subunits, such as the two that are known for mammalian CLCs Citation[52], Citation[53]? How do prokaryotic CLC inhibitors work, and can these help us generate better inhibitors for all of the CLCs? Addressing these questions will likely shed even more light on this enigmatic and ubiquitous family of channels and transporters.
Acknowledgements
We are grateful to M. Walden, A. Accardi, F. Wu, C. Xu, and C. Miller for sharing unpublished data. We thank A. Accardi, G. Martinez, J. Mindell, and R. Reimer for critical review of this manuscript. We also thank G. Martinez for his efforts on .
References
- Jentsch TJ, Neagoe I, Scheel O. CLC chloride channels and transporters. Curr Opin Neurobiol 2005; 15: 319–325
- Jentsch TJ, Friedrich T, Schriever A, Yamada H. The CLC chloride channel family. Pflugers Arch 1999; 437: 783–795
- Maduke M, Pheasant DJ, Miller C. High-level expression, functional reconstitution, and quaternary structure of a prokaryotic ClC-type chloride channel. J Gen Physiol 1999; 114: 713–722
- Iyer R, Iverson TM, Accardi A, Miller C. A biological role for prokaryotic ClC chloride channels. Nature 2002; 419: 715–718
- Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol 2007; 578: 633–640
- Mindell JA, Maduke M, Miller C, Grigorieff N. Projection structure of a ClC-type chloride channel at 6.5 A resolution. Nature 2001; 409: 219–223
- Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 2002; 415: 287–294
- Dutzler R, Campbell EB, MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science 2003; 300: 108–112
- Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature 2004; 427: 803–807
- Picollo A, Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 2005; 436: 420–423
- Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 2005; 436: 424–427
- Jentsch TJ, Steinmeyer K, Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature 1990; 348: 510–514
- Maduke M, Miller C, Mindell JA. A decade of CLC chloride channels: structure, mechanism, and many unsettled questions. Annu Rev Biophys Biomol Struct 2000; 29: 411–438
- Pusch M. Structural insights into chloride and proton-mediated gating of CLC chloride channels. Biochemistry 2004; 43: 1135–1144
- Chen TY. Structure and function of ClC channels. Annu Rev Physiol 2005; 67: 809–839
- Lobet S, Dutzler R. Ion-binding properties of the ClC chloride selectivity filter. Embo J 2006; 25: 24–33
- Pusch M, Ludewig U, Rehfeldt A, Jentsch TJ. Gating of the voltage-dependent chloride channel CIC-0 by the permeant anion. Nature 1995; 373: 527–531
- Fodor AA, Aldrich RW. Statistical limits to the identification of ion channel domains by sequence similarity. J Gen Physiol 2006; 127: 755–766
- Estevez R, Schroeder BC, Accardi A, Jentsch TJ, Pusch M. Conservation of chloride channel structure revealed by an inhibitor binding site in ClC-1. Neuron 2003; 38: 47–59
- Lin CW, Chen TY. Probing the pore of ClC-0 by substituted cysteine accessibility method using methane thiosulfonate reagents. J Gen Physiol 2003; 122: 147–159
- Engh AM, Maduke M. Cysteine accessibility in ClC-0 supports conservation of the ClC intracellular vestibule. J Gen Physiol 2005; 125: 601–617
- Chen TY, Miller C. Nonequilibrium gating and voltage dependence of the ClC-0 Cl− channel. J Gen Physiol 1996; 108: 237–250
- Accardi A, Pusch M. Conformational changes in the pore of CLC-0. J Gen Physiol 2003; 122: 277–294
- Ludewig U, Jentsch TJ, Pusch M. Analysis of a protein region involved in permeation and gating of the voltage-gated Torpedo chloride channel ClC-0. J Physiol 1997; 498(Pt 3)691–702
- Ludewig U, Jentsch TJ, Pusch M. Inward rectification in ClC-0 chloride channels caused by mutations in several protein regions. J Gen Physiol 1997; 110: 165–171
- Pusch M, Ludewig U, Jentsch TJ. Temperature dependence of fast and slow gating relaxations of ClC-0 chloride channels. J Gen Physiol 1997; 109: 105–116
- Bykova EA, Zhang XD, Chen TY, Zheng J. Large movement in the C terminus of CLC-0 chloride channel during slow gating. Nat Struct Mol Biol 2006; 13: 1115–1119
- Matulef K, Maduke M. Side-dependent inhibition of a prokaryotic ClC by DIDS. Biophys J 2005; 89: 1721–1730
- Bell SP, Curran PK, Choi S, Mindell JA. Site-directed fluorescence studies of a prokaryotic ClC antiporter. Biochemistry 2006; 45: 6773–6782
- Miller C. ClC chloride channels viewed through a transporter lens. Nature 2006; 440: 484–489
- Richard EA, Miller C. Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science 1990; 247: 1208–1210
- Walden M, Accardi A, Wu F, Xu C, Williams C, Miller C. Uncoupling and turnover in a Cl−/H+ exchange transporter. J Gen Physiol 2007; 129: 317–329
- Accardi, A. 2007. Temperature dependence of the unitary transport rate of a bacterial CLC transporter. Biophys J abstract. http://www.abstractsonline.com/viewer/?mkey={B65ED1CE-3D1E-400B-BEC0-429A5DA15800}.
- Bennetts B, Roberts ML, Bretag AH, Rychkov GY. Temperature dependence of human muscle ClC-1 chloride channel. J Physiol 2001; 535: 83–93
- Accardi A, Walden M, Nguitragool W, Jayaram H, Williams C, Miller C. Separate ion pathways in a Cl−/H+ exchanger. J Gen Physiol 2005; 126: 563–570
- Accardi A, Lobet S, Williams C, Miller C, Dutzler R. Synergism between halide binding and proton transport in a CLC-type exchanger. J Mol Biol 2006; 362: 691–699
- Nguitragool W, Miller C. Uncoupling of a CLC Cl−/H+ exchange transporter by polyatomic anions. J Mol Biol 2006; 362: 682–690
- Wang XQ, Deriy LV, Foss S, Huang P, Lamb FS, Kaetzel MA, Bindokas V, Marks JD, Nelson DJ. CLC-3 channels modulate excitatory synaptic transmission in hippocampal neurons. Neuron 2006; 52: 321–333
- Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci 1997; 22: 12–13
- Schwappach B, Stobrawa S, Hechenberger M, Steinmeyer K, Jentsch TJ. Golgi localization and functionally important domains in the NH2 and COOH terminus of the yeast CLC putative chloride channel Gef1p. J Biol Chem 1998; 273: 15110–15118
- Carr G, Simmons N, Sayer J. A role for CBS domain 2 in trafficking of chloride channel CLC-5. Biochem Biophys Res Commun 2003; 310: 600–605
- Pusch M. Myotonia caused by mutations in the muscle chloride channel gene CLCN1. Hum Mutat 2002; 19: 423–434
- Dutzler R. The ClC family of chloride channels and transporters. Curr Opin Struct Biol 2006; 16: 439–446
- Estevez R, Pusch M, Ferrer-Costa C, Orozco M, Jentsch TJ. Functional and structural conservation of CBS domains from CLC chloride channels. J Physiol 2004; 557: 363–378
- Hebeisen S, Biela A, Giese B, Muller-Newen G, Hidalgo P, Fahlke C. The role of the carboxyl terminus in ClC chloride channel function. J Biol Chem 2004; 279: 13140–13147
- Yusef YR, Zuniga L, Catalan M, Niemeyer MI, Cid LP, Sepulveda FV. Removal of gating in voltage-dependent ClC-2 chloride channel by point mutations affecting the pore and C-terminus CBS-2 domain. J Physiol 2006; 572: 173–181
- Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 2004; 113: 274–284
- Bennetts B, Rychkov GY, Ng HL, Morton CJ, Stapleton D, Parker MW, Cromer BA. Cytoplasmic ATP-sensing domains regulate gating of skeletal muscle ClC-1 chloride channels. J Biol Chem 2005; 280: 32452–32458
- Wellhauser L, Kuo HH, Stratford FL, Ramjeesingh M, Huan LJ, Luong W, Li C, Deber CM, Bear CE. Nucleotides bind to the C-terminus of ClC-5. Biochem J 2006; 398: 289–294
- Meyer S, Savaresi S, Forster IC, Dutzler R. Nucleotide recognition by the cytoplasmic domain of the human chloride transporter ClC-5. Nat Struct Mol Biol 2007; 14: 60–67
- Meyer S, Dutzler R. Crystal structure of the cytoplasmic domain of the chloride channel ClC-0. Structure 2006; 14: 299–307
- Estevez R, Boettger T, Stein V, Birkenhager R, Otto E, Hildebrandt F, Jentsch TJ. Barttin is a Cl− channel beta-subunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature 2001; 414: 558–561
- Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature 2006; 440: 220–223