Abstract
SLC35A3 encodes a Golgi-resident UDP-N-acetylglucosamine transporter. Here, the porcine SLC35A3 gene was assigned to Sus scrofa chromosome 4 (SSC4) by a combination of radiation hybrid and linkage analysis. Expression profiling using real time RT-PCR showed ubiquitous but variable transcription of SLC35A3 in a selection of tissues. The deduced 325 amino acid sequence revealed a hydrophobic protein with 10 predicted transmembrane helices and the N- and C-terminal tails facing the cytosolic side of the Golgi apparatus. In addition, mutated versions of the UDP-GlcNAc transporter were analyzed in a yeast complementation assay, which allowed us to identify important domains and amino acid residues. Thus, the N-terminal tail was inessential for activity, whereas removal of the first transmembrane domain inhibited yeast complementation. The hydrophilic C-terminus was dispensable while mutant proteins either fully or partially deprived of the last membrane-spanning helix were functionally impaired. The third luminal loop showed modest sequence conservation and appeared structurally flexible as certain deletions were acceptable. In contrast, the fourth luminal loop was more sensitive to changes since the competence of the mutant protein was lowered by mutations. Substitutions of glycines 190, 215 and 254, which are invariant positions in the SLC35A subfamilies affected activity negatively. Interestingly, inhibition of function by a valine to phenylalanine mutation, which has been associated with skeletal malformations, is likely caused by structural incompatibility of the bulky aromatic phenylalanine side chain with the integrity of the transmembrane helix, since substitutions with the smaller aliphatic side chains of leucine and isoleucine were acceptable changes.
| Abbreviations | ||
| NSTs | = | nucleotide-sugar transporters |
| ER | = | endoplasmatic reticulum |
| SLC | = | solute carrier family |
| CDG | = | congenital disorders of glycosylation |
| LAD | = | leukocyte adhesion deficiency |
| GDP-Fuc | = | GDP-fucose |
| CMP-Sia | = | CMP-sialic acid |
| UDP-GlcNAc | = | UDP-N-acetylglucosamine |
| GlcNAc | = | acetylglucosamine |
| CVM | = | complex vertebral malformation |
| EST | = | expressed sequence tags |
| SNP | = | single nucleotide polymorphism |
| SSC4 | = | Sus scrofa chromosome 4 |
| 18S-rRNA | = | 18S-ribosomal RNA |
| TMD | = | transmembrane domain |
| FITC-WGA | = | fluorescein isothiocyanate conjugated wheat germ agglutinin |
| UDP-Gal | = | UDP-galactose |
| UDP-GalNAc | = | UDP-N-acetylgalactosamine |
| GDP-Man | = | GDP-mannose |
| HA | = | hemagglutinin |
Introduction
Availability of nucleotide-sugars for glycoconjugate synthesis is mediated by a group of transmembrane proteins designated nucleotide-sugar transporters (NSTs). Thus, NSTs translocate nucleotide-sugars from the cytosol into the interior lumen of the endoplasmic reticulum (ER) and/or the Golgi apparatus, where the activated sugar molecules are utilized by glycosyltransferases. The translocation occurs as an antiport-based mechanism, which maintains the luminal concentration of nucleotide-sugars by one-to-one exchange with the corresponding nucleoside monophosphate (Capasso & Hirschberg Citation1984, Waldman & Rudnick Citation1990). Several different NSTs are required to translocate the different molecular species of nucleotide-sugars due to substrate specificity of the transporter/antiport cycle (Aoki et al. Citation2001, Aoki et al. Citation2003). Members belonging to the solute carrier family 35 (SLC35) are categorized into subgroups A-F based on amino acid similarities (Ishida & Kawakita Citation2004, Martinez-Duncker et al. Citation2003). Generally, the family members are structurally related membrane-spanning proteins comprised of 325–400 amino acid residues (Eckhardt et al. Citation1999, Hirschberg et al. Citation1998). The conservation of structure across the different substrate specificities of NSTs indicates common features of the basic transport mechanism, however, the structural basis for substrate recognition and transportation is scarcely characterized.
Dysfunctional nucleotide-sugar transport has importance for certain disease states. Thus, congenital disorders of glycosylation (CDG) constitute a group of diseases caused by defects in the synthesis of the glycan moiety of glycoconjugates, and to date two human diseases have been associated with the deficiencies in the entry of nucleotide-sugars into the glycosylation pathway. These are leukocyte adhesion deficiency type II (LAD II or CDG IIc), which is characterized by reduced GDP-fucose (GDP-Fuc) transport (Lubke et al. Citation2001, Luhn et al. Citation2001), and a recently discovered defect in the CMP-sialic acid (CMP-Sia) transporter (CDG IIf) (Martinez-Duncker et al. Citation2005). Furthermore, Thomsen et al. (Citation2006) identified a mutation in the Golgi UDP-N-acetylglucosamine (UDP-GlcNAc) transporter SLC35A3, which is responsible for a lethal disorder in cattle called Complex Vertebral Malformation (CVM) that is characterized by misshapen and fused vertebrae primarily around the cervico-thoracic junction. The severe disease phenotype suggests that inactivation of SLC35A3 impairs several developmental pathways depending on glycosylation such as Notch signal transduction or intracellular signaling involving glycosaminoglycans, for example the Hedgehog and Wnt pathways.
Crystallographic data are not available for NSTs and current understanding of structure-function relationships largely relies on secondary structure predictions and biochemical observations on transporters with natural and site-directed mutations. Here, we have characterized SLC35A3 from the domesticated pig (Sus scrofa) by cDNA cloning and sequencing, tissue expression profiling and chromosomal assignment of the gene. Additionally, conservation of function across evolutionary distant species allowed the porcine UDP-N-acetylglucosamine transporter to complement a Kluyveromyces lactis mutant, which is deficient in the transport of UDP-N-acetylglucosamine into the Golgi apparatus. In this heterologous expression system, we analyzed mutated versions of SLC35A3 harboring truncations, internal deletions and substitutions of specific residues to identify important regions of the transporter. The conserved membrane topology of NSTs suggests that our mutational study of porcine SLC35A3 has importance for understanding the structure-function relationships of other family members as well.
Materials and methods
Single nucleotide polymorphism analysis and chromosomal localization
Single nucleotide polymorphism (SNP) analysis of the coding region of gene SLC35A3 was carried out using genomic DNA from 100 individuals. Exon fragments were amplified by PCR with AmpliTaq Gold DNA polymerase (Applied Biosystems). The amplicons were verified by agarose gel electrophoresis and cleaned up with Exo-SAP (part of the AcycloPrime-FP SNP Detection Kit, Perkin Elmer Life Sciences) prior to sequencing with the BigDye Terminator v.3.1 Cycle Sequencing Kit (PE Applied Biosystems). The DNA sequences were obtained on 3730xl DNA Analyzer (PE Applied Biosystems), and the sequences were analyzed in the “Sequencher” software (version 4.0.5, Gene Codes Corporation).
Candidate SNPs were identified manually and Taqman primers and probes were designed on the basis of a target sequence (Assay-by-design, Applied Biosystems). The SNP was genotyped in pig family-material consisting of 14 boars, approximately 700 sows and 12, 000 offspring using the ABI PRISM 7900HT Sequence Detection System and the SDS version 2.1 software (Applied Biosystems). Gene mapping of SLC35A3 was performed using CRIMAP version 2.4.
The SLC35A3 cDNA sequence has been submitted to GenBank, NCBI, under the accession number DQ883629.
Somatic cell hybrid panel
The somatic cell hybrid panel developed by Yerle et al. (Citation1996) was used for assignment of SLC35A3 to porcine chromosomes. The 27 hybrids were tested in PCR reactions containing 1 ng DNA, 0.5 µM of each primer, 0.2 mM dNTP, 1.5 mM MgCl2, 1×Phusion reaction buffer HF, and 0.2 U Phusion DNA polymerase (Finnzymes) in a total volume of 10 µl. In parallel, the primer specificity was controlled using pig genomic DNA as template. The thermal cycler program included initial denaturation at 98°C in 30 sec; and 30 cycles with denaturation at 98°C in 10 sec, annealing at 60°C in 15 sec, extension at 72°C in 15 sec; and subsequently a final extension at 72°C in 7 min. The PCR products were visualized on 1.5% agarose TBE gel, and data were interpreted in the web-based software on the WWW INRA server (Chevalet et al. Citation1997).
Preparation of RNA
Tissues were stored at −80°C. Samples were prepared from ten different tissues: Thyroid gland, liver, spleen, kidney, lung, vastus intermedius, stomach, fat, heart, and cerebellum. The tissues were dissected in −20°C, dissolved in RNeasy lysis buffer (Qiagen), and homogenized using a Tissue-Tearor rotor-stator homogenizer (Biospec Products Inc). The RNeasy Midi/Maxi Kit (Qiagen) was used for isolation of total RNA, and the yield was quantified at NanoDrop ND-1000 Spectrophotometer (Nanodrop Technologies). The total RNA was diluted to a final concentration of 2 µg/µl, and the purity was verified by agarose gel electrophoresis.
Gene expression analysis
Gene expression of SLC35A3 in different tissues was analyzed by RT-PCR followed by real-time RT-PCR using 18S-rRNA as endogenous standard. Total RNA, 4 µg, was reverse transcribed with 2.5 µM random hexamers (Applied Biosystems) in 20 µl reaction volume by the superscript II RNase H- reverse transcriptase system (Life Technologies, Invitrogen). Exiqon ProbeLibrary probe Human#77 was used for measurements of 18S-rRNA, and SLC35A3 specific TaqMan primers/probe were designed in Primer Express 2.0 software (Applied Biosystems). The primer pairs were included in PCR reactions with the DyNAzyme EXT DNA polymerase kit (Finnzymes) as well as the first-strand cDNA from different tissues as template. The amplicons were visualized on ethidium bromide stained 2% TBE agarose gel.
Real-time PCR was performed on 1 µl cDNA template with the TaqMan Universal PCR Master Mix (Applied Biosystems). Reactions were carried out as triplicates in a volume of 10 µl containing 200 nM primer and 100 nM probe. The cDNA template was diluted 1:1000 before the measurements of 18S-rRNA. The reactions were run in the ABI PRISM 7900HT Sequence Detection System, and the data was treated in SDS version 2.1 software.
Plasmid construction for mutational analysis of SLC35A3
First strand cDNA was synthesized from approximately 4 µg pig liver RNA and 0.5 µg oligo(dT)12–18 primer using the superscript II RNase H− reverse transcriptase system (Life Technologies, Invitrogen). Primers for amplification of cDNA fragments encoding the full length SLC35A3, and N/C-terminal truncated versions of SLC35A3 were designed, and BglII cloning sites were introduced in the ends of the fragments. The SLC35A3 cDNA fragments were prepared by polymerase chain reactions containing: 1.5 mM MgCl2, 0.2 mM dNTP, 0.5 µM primer, and 0.2 U Phusion DNA polymerase (Finnzymes) in a total volume of 10 µl.
The BglII restriction site was used for cloning of the fragments into yeast expression plasmid E4 (pE4), which is described elsewhere (Guillen et al. Citation1998). DNA ligations were performed with T4 DNA ligase (New England Biolabs) and the transformation into E. coli was done by electroporation. The E. coli cells (TOP10, Life Technologies) mixed with plasmid DNA were transferred to a 0.2 cm gap electroporation cuvette (Bio-Rad), and a pulse of 2.1 kV, 25 µF, and 200 ohms was delivered with a Gene Pulser (Bio-Rad). The time constant was 4.6–4.9 msec. The cells were grown on LB/Amp agar overnight at 37°C, and plasmid DNA was isolated from E. coli cells using the QIAprep Spin Miniprep Kit (Qiagen). Sequencing according to the procedure described above validated the constructs.
Targeted deletions and point mutations were introduced into the SLC35A3 cDNA using the QuikChange® XL Site-Directed Mutagenesis Kit (Stratagene). Vector pE4 containing full length SLC35A3 cDNA insert (∼25 ng) was used as template in the mutant strand synthesis reaction, which was run according to the manufacturer's protocol.
Phenotypic correction of yeast mutant measured by flow cytometry
The pE4 constructs were used for expression of porcine SLC35A3 in K. lactis. The K. lactis wild-type strain MG1/2 (MATα, uraA, arg−, lys−K+ pKD1+) and mutant strain KL3 (MATα, uraA, mnn2–2, arg−K+ pKD1+) are described elsewhere (Abeijon et al. Citation1996). The constructs were transformed into yeast by lithium acetate/single stranded carrier DNA/polyethylene glycol method (Gietz & Woods Citation2002), and the cells were selectively grown at SC -URA agar in 30°C incubation. Yeast cells were labeled with FITC-WGA as described in Thomsen et al. Citation2006 using a 0.9% NaCl, 2 mM CaCl2 labeling buffer. The cells were separated on a FACSCanto Flow Cytometer (Becton Dickinson) with FITC excitation at 488 nm and emission at 525 nm. 30,000 events data files were collected for the individual transformants and the files were treated in BD FACSDiva software.
Results
cDNA cloning and chromosomal localization
We performed BLAST searches with the human SLC35A3 cDNA sequence against databases of pig genomic sequences (Wernersson et al. Citation2005) and expressed sequence tags (EST) (Gorodkin et al. Citation2007). This identified sequences for the untranslated regions of both the 5′ end and the 3′ end of the porcine SLC35A3 gene, which permitted us to design primers for amplification and cloning of the full length SLC35A3 cDNA. The 978 bp SLC35A3 cDNA from pig liver (accession number: DQ883629) showed 94%, 93%, 93%, and 89% nucleic acid identities to cattle (XM_599170), dog (NM_001003385), human (NM_012243), and mouse (NM_144902) sequences, respectively. In order to identify single nucleotide polymorphisms (SNP) in exons, we inferred the position of exon-exon junctions in the pig cDNA by sequence comparison with the known gene structures of the human and bovine homologs. Seven primer pairs were designed to amplify exon fragments ranging from 91 to 181 bp, which were analyzed for SNPs in 100 unrelated animals. Two nucleotide variations A378G and C430T were identified, both of which were silent with respect to the codons specifying leucines in position 126 and 144. In addition, an A to G transition was found in the 81 bp intron that separates the second and third coding exon. The C430T polymorphism was utilized as a molecular marker in chromosomal mapping of SLC35A3. To this end, the SNP was genotyped in 14 Landrace/Yorkshire×Duroc one-generation families, comprising approximately 12,000 individuals. The genotyping data showed Mendelian segregation of the SNP with allele frequencies of 0.81 (C) and 0.19 (T). Two-point linkage analysis using the CRIMAP (version 2.4) package with a significant lodscore threshold of more than 5 localized the gene to Sus scrofa chromosome 4 (SSC4). This result was corroborated by assignment of SLC35A3 to SSC4 using a porcine-rodent somatic cell hybrid panel (Yerle et al. Citation1996) in which the regional probabilities indicated a position at either 4q21-q23 (0.45) or 4q24 (0.45) with a correlation of 0.91.
mRNA transcription profile
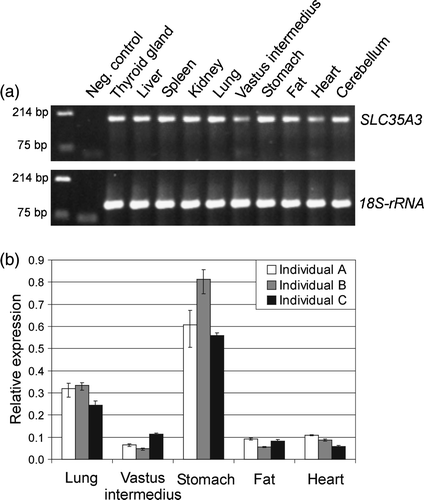
The transcription level of SLC35A3 in ten different tissues was studied by two-step RT-PCR studies. The cDNA synthesis reactions were primed with random hexamers in order to compare with the levels of 18S-ribosomal RNA (18S-rRNA). Detection of genomic DNA contaminations was avoided by using intron-spanning SLC35A3 specific primers. As shown in a, SLC35A3 was transcribed in all examined tissues although variable expression levels were observed. This agrees well with Ishida et al. (Citation1999a), who reported ubiquitous expression of the human SLC35A3 in a series of different tissues. Thorough analysis of the porcine SLC35A3 transcripts in lung, the skeletal muscle vastus intermedius, stomach, fat, and heart was obtained by real-time quantitative RT-PCR data from three individuals (b). The highest expression level was measured in stomach, which was at least ∼5-fold higher than in vastus intermedius, fat, and heart (p<0.001). Also, for each tissue, significant differences between the highest and lowest expression levels were observed among the three animals (p<0.05), indicating some biological variation in gene expression between individuals.
Figure 1. Expression profile of the porcine SLC35A3 mRNA in various tissues. (a) Amplicons from RT-PCR analysis were visualized on ethidium bromide stained 2% TBE agarose gel. SLC35A3 specific primers amplified a product of size 192 bp compared to the 110 bp amplicon of 18S-rRNA. DNA marker was loaded in the first lane followed by a negative control without cDNA template. The sample names are listed above their respective lanes. (b) Real-time RT-PCR data illustrating the relative expression of porcine SLC35A3 mRNA in lung, vastus intermedius, stomach, fat and heart from three individuals. The data were normalized to the amounts of 18S-rRNA.
Sequence analysis
The SLC35A3 cDNA encoded a protein of 325 amino acids with a calculated molecular weight of ∼36 kDa. The amino acid composition of the UDP-GlcNAc transporter revealed a highly hydrophobic protein as expected from its localization in the Golgi membrane. Thus, the hydropathic character of the transporter using the Kyte and Doolittle scale (Kyte & Doolittle Citation1982) suggested ten transmembrane domains, TMD1-10 (). Furthermore, computational prediction of the membrane topology using HMMTOP (Tusnady & Simon Citation1998, Tusnady & Simon Citation2001) indicated the presence of ten membrane-spanning helices with the N- and C-terminal tails facing the cytosolic side of the Golgi apparatus (). Although this model is tentative it does conform to the experimentally determined topology of the murine CMP-Sia transporter, which has 10 transmembrane domains and the N- and C termini oriented towards the cytosol (Eckhardt et al. Citation1999), as well as to the architecture of other NSTs modeled on the basis of theoretical considerations.
Figure 2. Hydrophobicity plot of the porcine UDP-GlcNAc amino acid sequence. The plot was generated using the hydrophobicity values of Kyte and Doolittle with window size 15. The amino acid (aa) position is shown on the x-axis.
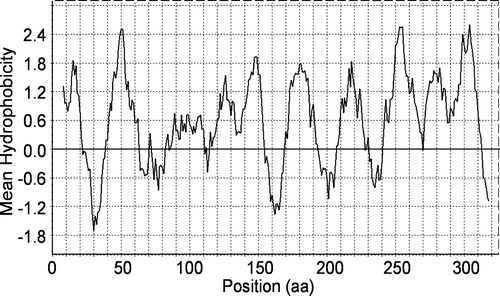
Table I. HMMTOP transmembrane topology prediction of SLC35A3 (Tusnady & Simon Citation1998, Tusnady & Simon Citation2001).
Deletion analysis
Protein alignments showed 95–98% identity with other mammalian orthologues, which suggest strong conservation of structure-function relationships (). To define important domains in the porcine UDP-GlcNAc transporter, we generated a series of SLC35A3 mutants containing terminal truncations, internal deletions and substitutions of putative key residues (). The mutagenized versions of the transporter were evaluated by complementation in a Kluyveromyces lactis mnn2-2 mutant strain, which lacks the ability to transport UDP-GlcNAc into the Golgi apparatus and consequently does not have terminal GlcNAc sugar residues in the cell surface glycans (Abeijon et al. Citation1996). The wild-type and mutant phenotypes can therefore easily be distinguished by flow cytometry combined with cell surface labeling with fluorescein isothiocyanate conjugated wheat germ agglutinin (FITC-WGA), which has affinity for GlcNAc. In agreement with previous studies on the canine and bovine SLC35A3 proteins (Guillen et al. Citation1998; Thomsen et al. Citation2006), we here observed that expression of the porcine wild-type SLC35A3 gene in the K. lactis mutant strain KL3 efficiently restored UDP-GlcNAc transport ().
Figure 3. Comparison of the deduced amino acid sequence of the Golgi UDP-GlcNAc transporter from Sus scrofa to its orthologues in Bos Taurus (accession number: AAO22138), Canis familiaris (AAC39260), Homo sapiens (BAA77841), and Mus musculus (AAH24110). Dots indicate residues identical with the porcine sequence and dashes indicate gaps. Black boxes with white text indicate amino acid substitutions. A plus sign + or a minus sign − indicates the presence or absence of transporter activity; (–) indicates reduced activity. Grey bars denote transmembrane domains predicted on the HMMTOP server (Tusnady & Simon Citation1998, Tusnady & Simon Citation2001). Truncations and internal deletion regions are specified with black arrows.
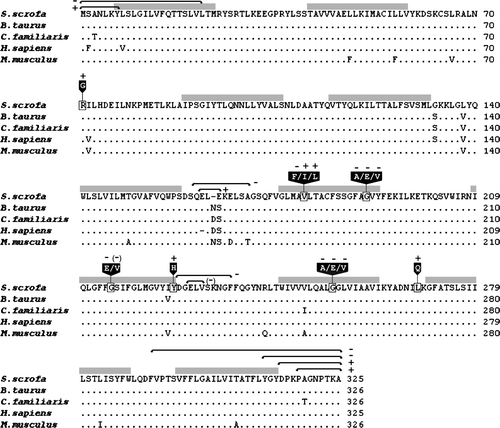
Figure 4. Flow cytometry analysis of labelled Kluyveromyces lactis cells. The mnn2-2 mutant phenotype (KL3) is deficient in UDP-GlcNAc transport, which can be rescued to the level of the yeast wild-type strain MG½ by expression of the porcine wild-type SLC35A3 gene (WT). Yeast transformants were differentially labelled with FITC-conjugated wheat germ agglutinin (WGA), which recognizes terminal GlcNAc residues in cell surface glycans. FITC fluorescence signals are presented on a logarithmic scale on the horizontal axis and the vertical axis shows the counts. Various truncations/deletions/mutations as stated above the individual peak profile were tested in the assay. Yeast cells transformed with the pE4 vector were used as controls.
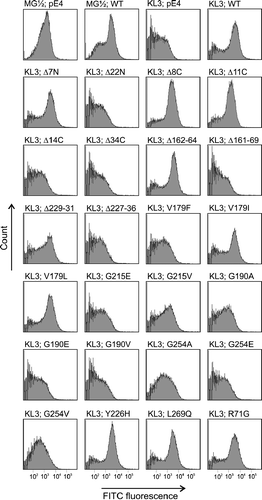
First, we constructed several deletion mutants. Thus, removal of the first seven amino acid residues from the hydrophilic N-terminus (SLC35A3Δ7N construct) did not affect the capability to transport UDP-GlcNAc into the Golgi lumen, however, deletion of further amino acids to also include the first TMD (SLC35A3Δ22N) completely abolished activity. Furthermore, the C-terminal cytosolic residues were dispensable as demonstrated by the ability of SCL35A3Δ8C and SCL35A3Δ11C to complement the yeast mutant. However, constructs with truncations affecting the integrity of TMD10 (SCL35A3Δ14C and SCL35A3Δ34C) were unable to support the expression of glycans containing GlcNAc on the cell surface. Next, we focused on the role of two different loop domains. Thus, the loop between TMD 5–6 is only moderately conserved with variations in both composition and number of amino acids. Consistently, the SLC35A3 transporter tolerated deletion of three amino acids (Δ162–64) in this region but lost its activity when nine residues (Δ161–69) were deleted. In the highly conserved loop connecting the membrane-spanning segments TMD 7–8, two internal deletions (Δ229–31 and Δ227–36) were created. Thus, phenotypic correction was observed after deletion of three amino acids, however, the slightly altered fluorescence peak profile suggested that the transporter activity was affected by the mutation. Deletion of ten amino acids from the loop was detrimental to SLC35A3 function.
Point mutations
The conserved valine residue at position 179 (corresponding to position 180 in cattle) is particularly interesting because its substitution with phenylalanine causes the bovine congenital disease CVM (Thomsen et al. Citation2006). To evaluate the sensitivity of SLC35A3 to amino acid changes at this position, the valine was altered to leucine, isoleucine or phenylalanine. As shown in , the V179F mutation impaired complementation of the mnn2-2 phenotype, thereby confirming previous findings on the bovine SLC35A3. Conversely, we observed that the transmembrane domain was able to accommodate substitution of valine with leucine as well as with isoleucine.
We also studied the effect of changing glycine residues at positions 190, 215 and 254, which are conserved among transporters in the SLC35A subfamily. The fluorescence peak profiles of G190A, G190E, G190V, G215E, G254A, G254E and G254V showed that SLC35A3 function was compromised by these mutations, which points towards essential roles of these glycines. Several substitutions were tolerated. Thus, replacement of glycine 215 with valine resulted in a moderate reduction of fluorescence, suggesting that SLC35A3 may have retained some of its activity. Likewise, mutation of nearby tyrosine 226 to histidine in the end of TMD 7 had no effect on SLC35A3 function.
The murine CMP-Sia transporter and the UDP-GlcNAc transporter encoded by the K. lactisMNN2 gene both contain a leucine zipper motif, which has been hypothesized to be involved in homodimer formation (Abeijon et al. Citation1996). To gain insight into the role of the leucine/isoleucine repeat (amino acids 262–283) near the carboxy terminus of SLC35A3, we generated a L269Q substitution to destroy the heptad motif. However, the data did not reveal any differences in FITC-WGA affinity between cells expressing the L269Q mutation and cells with the wild-type SLC35A3 gene, which decreases the probability of a leucine zipper in this region. Finally, we observed that yeast complementation was unaffected by a R71G substitution in the TMD 2-3 loop region.
Discussion
Gene mapping and ubiquitous expression of SLC35A3
Genes encoding NSTs were first identified a decade ago by complementation studies in yeast or mammalian mutant cell lines lacking the corresponding endogenous nucleotide-sugar transport activity (Abeijon et al. Citation1996, Dean et al. Citation1997, Eckhardt et al. Citation1996, Ishida et al. Citation1996, Ma et al. Citation1997, Miura et al. Citation1996). In the present study, we have cloned and sequenced the full-length cDNA encoding the porcine nucleotide-sugar transporter SLC35A3. It spans 978 bp, which translates into a protein of 325 amino acids with 98% sequence identity to the human and canine SLC35A3 counterparts (). The porcine SLC35A3 was assigned to Sus scrofa chromosome 4 (SSC4) by two-point linkage analysis using CRIMAP and SNP genotypic data, and mapped to chromosome segment 4q21-23 or 4q24 by the use of a porcine-rodent somatic cell hybrid panel. This result agrees well with previous pig-human comparative mapping data, showing extensive conservation of synteny between the distal part of the q arm of SSC4 and the region from human chromosome 1p22 to 1q25 (Fujishima-Kanaya et al. Citation2003, Hiraiwa et al. Citation2003, Moller et al. Citation2004), which includes the human SLC35A3 gene at HSA1p21.2 (Ishida et al. Citation1999a).
Biochemical pathways that add sugar chains to proteins and lipids generate glycosylation patterns that vary in developmental or cell- and tissue-specific manners (Freeze Citation2006). Here, we observed that the SLC35A3 gene is transcribed in all examined tissues, however, at variable levels (). The differences in expression levels may indicate a distinct role of SLC35A3-mediated UDP-GlcNAc uptake in the Golgi in certain cells or tissues. Clearly, embryogenesis without SLC35A3 activity has severe phenotypic consequences (Thomsen et al. Citation2006), demonstrating that other NSTs with the ability to transport UDP-GlcNAc into the Golgi lumen, for example SLC35D2/HFRC1 (Suda et al. Citation2004), cannot compensate for the lack of SLC35A3.
Dispensable cytosolic termini but essential terminal transmembrane domains
The substrate specificity of NSTs is not reflected in their primary sequences (Berninsone & Hirschberg Citation2000). Thus, SLC35A3 and MNN2 are both transporters of UDP-GlcNAc, yet they share only 12% identical residues. Nonetheless, strong conservation of their structure provides the basis for a complementation assay in K. lactis, which we utilized for structural-functional analyses of the porcine UDP-GlcNAc transporter (). The deletion analysis demonstrated that the N-terminal hydrophilic tail was not essential for the activity of SLC35A3. This is consistent with the study of the murine UDP-galactose (UDP-Gal/SLC35A2) transporter, showing that its transporting activity was unaffected by N-terminal truncations (Ishida et al. Citation1999b), as well as the observation that the GDP-mannose (GDP-Man) transporter Vrg4 from Saccharomyces cerevisiae is fully functional without the first 15 amino acids (Gao & Dean Citation2000). Also, studies on chimeras between human transporters of UDP-Gal and CMP-Sia (SLC35A1) showed that the UDP-Gal transporter was insensitive to replacement of its N-terminus with that of the CMP-Sia transporter (Aoki et al. Citation2001). In contrast, expression of the Δ22N truncated version of SLC35A3, which removed the putative first transmembrane domain failed to complement the mnn2-2 yeast phenotype. A probable explanation is that TMD1 is required for localization of the protein to the Golgi apparatus. Thus, it has been demonstrated that the TMD1 of the Schizosaccharomyces pombe UDP-Gal transporter was essential for Golgi targeting (Segawa et al. Citation1999), and additionally that the first and seventh TMD of the human counterpart play a role in protein folding and transport to the Golgi apparatus (Aoki et al. Citation1999). Moreover, a missense mutation in the first membrane helix of the human UDP-Gal transporter caused lower amounts and a diffuse intracellular distribution of the mutant protein (Segawa et al. Citation2002). Likewise, deletion of the N-terminal 44 amino acids from the Vrg4 protein resulted in its mislocation to the ER (Gao & Dean Citation2000).
The transport activity of SLC35A3 was unaffected by a deletion that eliminates the entire cytosolic C-terminus. This agrees with reported observations on the murine UDP-Gal transporter, which also tolerated removal of most of its hydrophilic C-terminus (Ishida et al. Citation1999b). In contrast, mutants of the human GDP-fucose transporter lacking the cytoplasmic C-terminal domain are unable to function in nucleotide-sugar transportation despite correct localization of the protein to the Golgi (Helmus et al. Citation2006, Yakubenia & Wild, Citation2006). Furthermore, mutant SLC35A3 proteins with truncations that reach into (SLC35A3Δ14C) or fully take away (SLC35A3Δ34C) the last membrane-spanning helix showed impaired UDP-GlcNAc transportation. An observation pertinent to this result might be that in certain LAD II patients, the absence of the tenth transmembrane domain in the human GDP-fucose transporter is responsible for subcellular mislocation to the endoplasmic reticulum (Helmus et al. Citation2006, Yakubenia & Wild, Citation2006). Also, it is noteworthy that the SLC35A3Δ14C mutant lacks a conserved tyrosine residue Y312 (), and that a deletion including the corresponding residue in the murine UDP-Gal (Y338) rendered this protein extremely unstable (Ishida et al. Citation1999b). Additionally, it has been shown that the C-terminal cytosolic tail and the predicted last TMD of the yeast GDP-Man transporter play essential roles for protein stability and homodimerization (Abe et al. Citation1999, Gao & Dean Citation2000). Indeed, the functions of several NSTs such as the UDP-N-acetylgalactosamine (UDP-GalNAc) and GDP-Fuc transporters from rat liver (Puglielli et al. Citation1999, Puglielli & Hirschberg Citation1999), as well as the Leishmania GDP-Man transporter (Hong et al. Citation2000) seem to be dependent upon oligomerization. The leucine heptad repeat in SLC35A3 is potentially involved in dimerization of the transporter as has been suggested for other NSTs with zipper motifs (Abeijon et al. Citation1996, Hirschberg et al. Citation1998). However, the L269Q substitution did not obliterate the function of the UDP-GlcNAc transporter, suggesting that this residue is unlikely to be part of a leucine zipper dimerization motif. Similar observations were previously made on the CMP-Sia transporter, which was fully active despite disruption of the leucine zipper motif by insertional mutation (Eckhardt et al. Citation1999).
Figure 5. Multiple alignments of related proteins in the subfamily SLC35A. Besides the UDP-N-acetylglucosamine transporter (SLC35A3; Accession numbers: DQ883629 and AAH24110), the alignment comprises amino acid sequences for the mammalian UDP-sialic acid (SLC35A1; AAI02766 and AAH12252) and UDP-galactose (SLC35A2; AAX46538 and AAH37701) transporters. Sus scrofa is abbreviated Sscr, Bos Taurus: Btau, and Mus musculus: Mmus. Black background indicates conserved residues among the three transporters, whereas grey background indicates similar residues. Arrowheads illustrate SLC35A3 mutations from the present work, and oval symbols illustrate mutations from studies of SLC35A1 and -A2 proteins (Eckhardt et al. Citation1998, Ishida et al. Citation1999b, Oelmann et al. Citation2001). Open symbols indicate maintained transport activity in contrast to filled symbols for inactive transporters.
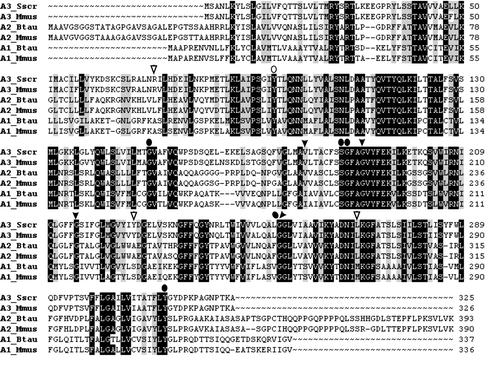
Flexible luminal loops
The hydrophilic region encompassing the third luminal loop of SLC35A3 has only modest sequence conservation with other members of the SLC35A1, -A2, and -A3 subfamilies ( and ). This suggests that this region possesses structural flexibility, which is probably reflected in the observation that deletion of three amino acids from this loop was acceptable, whereas larger deletions eliminated complementation of the yeast mnn2-2 mutant. Importantly, the CMP-Sia transporter tolerated similar structural perturbations, where insertion of additional amino acids in the corresponding luminal loop did not abolish its activity (Eckhardt et al. Citation1999). The fourth luminal loop of SLC35A3 was more sensitive to changes since the competence of the mutant protein was influenced detectably by removal of three amino acids, and was rendered inactive by deletion of ten amino acid residues. Consistently, the equivalent loop in the CMP-Sia transporter could accommodate insertion of a pentapeptide but was inhibited by the larger hemagglutinin (HA) tag.
Importance of invariant glycines and allowable changes at the critical V179 position
Several non-conservative substitutions left the SLC35A3 transporter unaffected. Thus, mutant proteins carrying either Y226H or L269Q effectively supported phenotypic correction of the yeast mutant. Furthermore, substitution of arginine for glycine at position 71 in the loop between TMD2 and TMD3 in SLC35A3 had no negative effect, which agrees well with the observation that the CMP-Sia transporter allowed large insertions in the equivalent region. These data along with the fact that these residues are only weakly conserved between subfamilies () suggest considerable structural freedom at these positions. Conversely, the site-directed missense mutagenesis identified four amino acid residues as functionally important. Thus, substitutions of glycines at positions 190, 215 and 254, which are invariant residues in the SLC35A subfamilies, affected the function of SLC35A3 negatively. It is noteworthy that substitutions at positions that correspond to glycine 254 also inhibit other NSTs. Thus, a G281D mutation in SLC35A2 and a G256D substitution in SLC35A1 have been shown to inactivate transport of UDP-Gal and CMP-Sia, respectively (Oelmann et al. Citation2001). Similarly, introducing glycine to glutamic acid mutations at the homologous positions also affected the activity of the two different C. elegans nucleotide-sugar transporters SFR-3 (UDP-Gal and UDP-GlcNAc) and CO3H5.2 (UDP-GlcNAc and UDP-GalNAc) (Caffaro et al. Citation2006, Hoflich et al. Citation2004). This indicates that these glycines serve functions critical to the activity of all NSTs irrespective of their substrate specificities. It has been determined that motifs with two glycine residues separated by any three residues (GXXXG), or similar motifs in which glycine is replaced by another small residue (e.g. serine), in transmembrane domains are often involved in helix-helix interactions (Senes et al. Citation2004). Both Gly190 and Gly215 in SLC35A3 are part of such motifs within TMD6 and TMD7, respectively, suggesting that they may mediate interactions with neighboring helices. Moreover, the observation that conservative changes of glycines to either alanine or valine at positions 190 and 254 results in loss of function further underscores that these glycine residues are located in regions with strong structural or functional constraints. Importantly, according to the topology predictions G190 colocalizes with V179 in the same transmembrane helix. A significant observation in this context is that replacement of valine 179 with phenylalanine also inhibits the function of porcine SLC35A3 as previously observed in cattle (Thomsen et al. Citation2006) and in C. elegans (Caffaro et al. Citation2006). The data further suggest that inhibition of transporter activity by the pathogenic V179F mutation is caused by structural incompatibility of the bulky aromatic side chain of phenylalanine with the integrity of the transmembrane helix, since substitutions with the smaller aliphatic side chains of leucine and isoleucine were acceptable changes.
SLC35A3 is a monospecific transporter unlike most other NSTs, which have the ability to transport multiple substrates. Despite having overlapping substrate specificities, NSTs are clearly not functionally redundant as evidenced by the diverse and often severe mutant phenotypes observed in different organisms. What molecular features determine substrate recognition and binding is a longstanding question, and analyses of the growing number of identified NSTs have so far failed to establish reliable connections between substrate specificities and primary amino acid sequences. A combination of site-directed mutagenesis and UV cross-linking of a photoaffinity substrate analogue has proven successful for identifying an amino acid motif involved in substrate binding in the yeast Vrg4 GDP-mannose transporter (Gao et al. Citation2001). The panel of SLC35A3 mutants provided in this study might be instrumental in defining roles of specific amino acids in substrate recognition using a similar experimental approach.
References
- Abe M, Hashimoto H, Yoda K. Molecular characterization of Vig4/Vrg4 GDP-mannose transporter of the yeast Saccharomyces cerevisiae. FEBS Lett 1999; 458: 309–312
- Abeijon C, Robbins PW, Hirschberg CB. Molecular cloning of the Golgi apparatus uridine diphosphate-N-acetylglucosamine transporter from Kluyveromyces lactis. Proc Natl Acad Sci USA 1996; 93: 5963–5968
- Aoki K, Ishida N, Kawakita M. Substrate recognition by UDP-galactose and CMP-sialic acid transporters. Different sets of transmembrane helices are utilized for the specific recognition of UDP-galactose and CMP-sialic acid. J Biol Chem 2001; 276: 21555–21561
- Aoki K, Ishida N, Kawakita M. Substrate recognition by nucleotide sugar transporters: further characterization of substrate recognition regions by analyses of UDP-galactose/CMP-sialic acid transporter chimeras and biochemical analysis of the substrate specificity of parental and chimeric transporters. J Biol Chem 2003; 278: 22887–22893
- Aoki K, Sun-Wada GH, Segawa H, Yoshioka S, Ishida N, Kawakita M. Expression and activity of chimeric molecules between human UDP-galactose transporter and CMP-sialic acid transporter. J Biochem (Tokyo) 1999; 126: 940–950
- Berninsone PM, Hirschberg CB. Nucleotide sugar transporters of the Golgi apparatus. Curr Opin Struct Biol 2000; 10: 542–547
- Caffaro CE, Hirschberg CB, Berninsone PM. Independent and simultaneous translocation of two substrates by a nucleotide sugar transporter. Proc Natl Acad Sci USA 2006; 103: 16176–16181
- Capasso JM, Hirschberg CB. Mechanisms of glycosylation and sulfation in the Golgi apparatus: evidence for nucleotide sugar/nucleoside monophosphate and nucleotide sulfate/nucleoside monophosphate antiports in the Golgi apparatus membrane. Proc Natl Acad Sci USA 1984; 81: 7051–7055
- Chevalet C, Gouzy J, SanCristobal-Gaudy M. Regional assignment of genetic markers using a somatic cell hybrid panel: a WWW interactive program available for the pig genome. Comput Appl Biosci 1997; 13: 69–73
- Dean N, Zhang YB, Poster JB. The VRG4 gene is required for GDP-mannose transport into the lumen of the Golgi in the yeast, Saccharomyces cerevisiae. J Biol Chem 1997; 272: 31908–31914
- Eckhardt M, Gotza B, Gerardy-Schahn R. Mutants of the CMP-sialic acid transporter causing the Lec2 phenotype. J Biol Chem 1998; 273: 20189–20195
- Eckhardt M, Gotza B, Gerardy-Schahn R. Membrane topology of the mammalian CMP-sialic acid transporter. J Biol Chem 1999; 274: 8779–8787
- Eckhardt M, Muhlenhoff M, Bethe A, Gerardy-Schahn R. Expression cloning of the Golgi CMP-sialic acid transporter. Proc Natl Acad Sci USA 1996; 93: 7572–7576
- Freeze HH. Genetic defects in the human glycome. Nat Rev Genet 2006; 7: 537–551
- Fujishima-Kanaya N, Toki D, Suzuki K, Sawazaki T, Hiraiwa H, Iida M, Hayashi T, Uenishi H, Wada Y, Ito Y, Awata T. Development of 50 gene-associated microsatellite markers using BAC clones and the construction of a linkage map of swine chromosome 4. Anim Genet 2003; 34: 135–141
- Gao XD, Dean N. Distinct protein domains of the yeast Golgi GDP-mannose transporter mediate oligomer assembly and export from the endoplasmic reticulum. J Biol Chem 2000; 275: 17718–17727
- Gao XD, Nishikawa A, Dean N. Identification of a conserved motif in the yeast golgi GDP-mannose transporter required for binding to nucleotide sugar. J Biol Chem 2001; 276: 4424–4432
- Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 2002; 350: 87–96
- Gorodkin J, Cirera S, Hedegaard J, Gilchrist MJ, Panitz F, Jorgensen CB, Scheibye-Knudsen K, Arvin T, Lumholdt S, Sawera M, Green T, Nielsen BJ, Havgaard JH, Rosenkilde C, Wang J, Li H, Li R, Liu B, Hu S, Dong W, Li W, Yu J, Wang J, Staerfeltd HH, Wernersson R, Madsen LB, Thomsen B, Hornshoj H, Bujie Z, Wang X, Wang X, Bolund L, Brunak S, Yang H, Bendixen C, Fredholm M. Porcine transcriptome analysis based on 97 non-normalized cDNA libraries and assembly of 1,021,891 ESTs. Genome Biol. 2007; 2; 8: R45
- Guillen E, Abeijon C, Hirschberg CB. Mammalian Golgi apparatus UDP-N-acetylglucosamine transporter: molecular cloning by phenotypic correction of a yeast mutant. Proc Natl Acad Sci USA 1998; 95: 7888–7892
- Helmus Y, Denecke J, Yakubenia S, Robinson P, Luhn K, Watson DL, McGrogan PJ, Vestweber D, Marquardt T, Wild MK. Leukocyte adhesion deficiency II patients with a dual defect of the GDP-fucose transporter. Blood 2006; 107: 3959–3966
- Hiraiwa H, Sawazaki T, Suzuki K, Fujishima-Kanaya N, Toki D, Ito Y, Uenishi H, Hayashi T, Awata T, Yasue H. Elucidation of correspondence between swine chromosome 4 and human chromosome 1 by assigning 27 genes to the ImpRH map, and development of microsatellites in the proximity of 14 genes. Cytogenet Genome Res 2003; 101: 84–89
- Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem 1998; 67: 49–69
- Hoflich J, Berninsone P, Gobel C, Gravato-Nobre MJ, Libby BJ, Darby C, Politz SM, Hodgkin J, Hirschberg CB, Baumeister R. Loss of srf-3-encoded nucleotide sugar transporter activity in Caenorhabditis elegans alters surface antigenicity and prevents bacterial adherence. J Biol Chem 2004; 279: 30440–30448
- Hong K, Ma D, Beverley SM, Turco SJ. The Leishmania GDP-mannose transporter is an autonomous, multi-specific, hexameric complex of LPG2 subunits. Biochemistry 2000; 39: 2013–2022
- Ishida N, Kawakita M. Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35). Pflugers Arch 2004; 447: 768–775
- Ishida N, Miura N, Yoshioka S, Kawakita M. Molecular cloning and characterization of a novel isoform of the human UDP-galactose transporter, and of related complementary DNAs belonging to the nucleotide-sugar transporter gene family. J Biochem (Tokyo) 1996; 120: 1074–1078
- Ishida N, Yoshioka S, Chiba Y, Takeuchi M, Kawakita M. Molecular cloning and functional expression of the human Golgi UDP-N-acetylglucosamine transporter. J Biochem (Tokyo) 1999a; 126: 68–77
- Ishida N, Yoshioka S, Iida M, Sudo K, Miura N, Aoki K, Kawakita M. Indispensability of transmembrane domains of Golgi UDP-galactose transporter as revealed by analysis of genetic defects in UDP-galactose transporter-deficient murine had-1 mutant cell lines and construction of deletion mutants. J Biochem (Tokyo) 1999b; 126: 1107–1117
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol 1982; 157: 105–132
- Lubke T, Marquardt T, Etzioni A, Hartmann E, von Figura K, Korner C. Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat Genet 2001; 28: 73–76
- Luhn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet 2001; 28: 69–72
- Ma D, Russell DG, Beverley SM, Turco SJ. Golgi GDP-mannose uptake requires Leishmania LPG2. A member of a eukaryotic family of putative nucleotide-sugar transporters. J Biol Chem 1997; 272: 3799–3805
- Martinez-Duncker I, Dupre T, Piller V, Piller F, Candelier JJ, Trichet C, Tchernia G, Oriol R, Mollicone R. Genetic complementation reveals a novel human congenital disorder of glycosylation of type II, due to inactivation of the Golgi CMP-sialic acid transporter. Blood 2005; 105: 2671–2676
- Martinez-Duncker I, Mollicone R, Codogno P, Oriol R. The nucleotide-sugar transporter family: a phylogenetic approach. Biochimie 2003; 85: 245–260
- Miura N, Ishida N, Hoshino M, Yamauchi M, Hara T, Ayusawa D, Kawakita M. Human UDP-galactose translocator: molecular cloning of a complementary DNA that complements the genetic defect of a mutant cell line deficient in UDP-galactose translocator. J Biochem (Tokyo) 1996; 120: 236–241
- Moller M, Berg F, Riquet J, Pomp D, Archibald A, Anderson S, Feve K, Zhang Y, Rothschild M, Milan D, Andersson L, Tuggle CK. High-resolution comparative mapping of pig Chromosome 4, emphasizing the FAT1 region. Mamm Genome 2004; 15: 717–731
- Oelmann S, Stanley P, Gerardy-Schahn R. Point mutations identified in Lec8 Chinese hamster ovary glycosylation mutants that inactivate both the UDP-galactose and CMP-sialic acid transporters. J Biol Chem 2001; 276: 26291–26300
- Puglielli L, Hirschberg CB. Reconstitution, identification, and purification of the rat liver golgi membrane GDP-fucose transporter. J Biol Chem 1999; 274: 35596–35600
- Puglielli L, Mandon EC, Rancour DM, Menon AK, Hirschberg CB. Identification and purification of the rat liver Golgi membrane UDP-N-acetylgalactosamine transporter. J Biol Chem 1999; 274: 4474–4479
- Segawa H, Ishida N, Takegawa K, Kawakita M. Schizosaccharomyces pombe UDP-galactose transporter: identification of its functional form through cDNA cloning and expression in mammalian cells. FEBS Lett 1999; 451: 295–298
- Segawa H, Kawakita M, Ishida N. Human and Drosophila UDP-galactose transporters transport UDP-N-acetylgalactosamine in addition to UDP-galactose. Eur J Biochem 2002; 269: 128–138
- Senes A, Engel DE, DeGrado WF. Folding of helical membrane proteins: the role of polar, GxxxG-like and proline motifs. Curr Opin Struct Biol 2004; 14: 465–479
- Suda T, Kamiyama S, Suzuki M, Kikuchi N, Nakayama K, Narimatsu H, Jigami Y, Aoki T, Nishihara S. Molecular cloning and characterization of a human multisubstrate specific nucleotide-sugar transporter homologous to Drosophila fringe connection. J Biol Chem 2004; 279: 26469–26474
- Thomsen B, Horn P, Panitz F, Bendixen E, Petersen AH, Holm LE, Nielsen VH, Agerholm JS, Arnbjerg J, Bendixen C. A missense mutation in the bovine SLC35A3 gene, encoding a UDP-N-acetylglucosamine transporter, causes complex vertebral malformation. Genome Res 2006; 16: 97–105
- Tusnady GE, Simon I. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J Mol Biol 1998; 283: 489–506
- Tusnady GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics 2001; 17: 849–850
- Waldman BC, Rudnick G. UDP-GlcNAc transport across the Golgi membrane: electroneutral exchange for dianionic UMP. Biochemistry 1990; 29: 44–52
- Wernersson R, Schierup MH, Jorgensen FG, Gorodkin J, Panitz F, Staerfeldt HH, Christensen OF, Mailund T, Hornshoj H, Klein A, Wang J, Liu B, Hu S, Dong W, Li W, Wong GK, Yu J, Wang J, Bendixen C, Fredholm M, Brunak S, Yang H, Bolund L. Pigs in sequence space: a 0.66X coverage pig genome survey based on shotgun sequencing. BMC Genomics 2005; 6: 70
- Yerle M, Echard G, Robic A, Mairal A, Dubut-Fontana C, Riquet J, Pinton P, Milan D, Lahbib-Mansais Y, Gellin J. A somatic cell hybrid panel for pig regional gene mapping characterized by molecular cytogenetics. Cytogenet Cell Genet 1996; 73: 194–202
- Yakubenia S, Wild MK. Leukocyte adhesion deficiency II. Advances and open questions. FEBS J 2006; 273: 4390–4398