Abstract
Cingulin is a component of the cytoplasmic domain of vertebrate tight junctions (TJ). Mutation or down-regulation of cingulin in cultured cells results in changes in gene expression. Some of these changes are dependent on RhoA, whose activity is regulated by GEF-H1, which is inactivated by binding to cingulin at junctions. To gain further insights on the function of cingulin through dominant-negative effects, we cloned and sequenced canine cingulin, and developed stable MDCK cell lines where either full-length cingulin, or head or rod+tail domains were inducibly overexpressed. Surprisingly, analysis of these clones by immunoblotting, microarray, immunofluorescence, measurement of transepithelial resistance, and cell density showed that the overexpression of either full-length cingulin or its domains does not significantly affect TJ protein levels, gene expression, RhoA activity, cell density, doubling time, and the organization and function of TJ. These results suggest that compensatory mechanisms prevent dominant-negative effects in this model system, and that modulation of cellular functions by cingulin occurs within physiological protein levels.
Introduction
Tight junctions (TJ) are regions of close cell-cell contact located at the apicolateral border of vertebrate epithelial cells. TJ form a semi-permeable seal, which controls the flow of ions, solutes and cells across epithelial monolayers, thus providing tissues with a barrier function. In addition, TJ structurally separate the apical from the basolateral domain of the plasma membrane. The molecular composition of TJ has been clarified in recent years (reviewed in Citation[1]). The four-pass transmembrane proteins claudins form the paracellular pores through which solutes flow, whereas occludin and JAM appear to have regulatory functions (reviewed in Citation[2]). The intracellular domains of TJ transmembrane proteins are anchored to a complex of cytoplasmic adaptor proteins (reviewed in Citation[3]). Evolutionarily conserved proteins, which are involved in the establishment of apico-basal polarity, are also localized at TJ (reviewed in Citation[4]). TJ are connected to the actomyosin cytoskeleton through the interaction of some of the cytoplasmic proteins with actin filaments. Assembly of the junctional actin cytoskeleton is regulated by the small GTPases Rac and Rho, and is of key importance in the establishment and maintenance of TJ integrity and function Citation[5]. TJ are not only involved in barrier function and polarity, but may also contribute to regulating the growth and differentiation of epithelial cells, through the concerted activity of adaptor proteins, signalling proteins, and transcription factors (reviewed in Citation[6]).
Cingulin is a cytoplasmic protein of TJ Citation[7], whose tissue distribution closely mirrors that of TJ Citation[8]. The cingulin molecule is a dimer, each monomer consisting of a globular head domain, a central rod coiled-coil domain, and a small globular tail domain Citation[9]. The head domain is critical for cingulin recruitment into junctions, through its interaction with ZO-1 Citation[10]. The rod domain of cingulin interacts with the RhoA activator GEF-H1 Citation[11] and is responsible for cingulin self-interaction Citation[9], Citation[12]. In order to understand the function of cingulin, we generated knockout and knockdown cultured cell models. Homozygous mutant embryoid bodies (EBs), where the cingulin alleles were mutated by homologous recombination, lacked cingulin at junctions, but the structure and function of TJ appeared normal, suggesting that cingulin does not play a fundamental structural role in TJ Citation[13]. However, mutant cells showed changes in the expression of a large number of genes, including genes involved in endodermal differentiation, and TJ protein genes (claudin-2, claudin-6, claudin-7, occludin) Citation[13]. To study the mechanism through which cingulin affects gene expression, we generated lines of MDCK cells where cingulin was down-regulated by expression of short hairpin RNAs Citation[14]. Analysis of these clones showed that cingulin depletion results in an increase in claudin-2 expression and cell proliferation, which are dependent on the increase in RhoA activity Citation[14]. Other phenotypic effects however, such as the increase in ZO-3 protein levels, are not dependent on the modulation of RhoA activity, suggesting the existence of additional mechanisms through which cingulin can regulate gene expression Citation[14].
To further understand the function of cingulin, we describe here the generation and characterization of stably transfected clones of MDCK cells where either full-length canine cingulin, or its head, or its rod + tail domains fused to GFP are inducibly overexpressed under the control of the Tet operator. The results show that cingulin up-regulation does not disrupt the structure and function of TJ, and indicate that in this model system compensatory mechanisms may prevent the expected decrease in RhoA activity and downstream events.
Materials and methods
Materials
Antibodies against cingulin (36-4401), claudin-2 (32-5600), claudin-3 (34-1700), claudin-7 (34-9100), occludin (71-1500, 33-1500), PAR-3 (36-2301), ZO-1 (61-7300, 33-9100), ZO-2 (71-1400), were from Zymed (Invitrogen). Anti-RhoA (sc-179) and anti-ZO-3 antibody (H-130) were from Santa Cruz Biotechnology. Secondary, Cy3-labelled antibodies for immunofluorescence were from Jackson Laboratories. Other antibodies were as in Citation[13].
Cloning of full-length canine cingulin
The cDNA coding for canine cingulin was obtained by the RACE method. For 3′RACE, 5 µg of RNA (prepared using the RNeasy mini kit, Qiagen) was reverse transcribed with 200U of SuperscriptII Reverse Transcriptase (Invitrogen) in the presence of 5 µM OligodT primer (5′-GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTTV-3′), 10 µM DTT, and 0.5 mM dNTP. RNA was digested by treatment with 2U of Ribonuclease H for 20 min at 37°C. The cDNA thus obtained was used to amplify the 3′ UTR of cingulin by nested PCR using the reverse primer 5′-GGCCACGCGTCGACTAGTAC-3′ and the degenerate forward primers 5′-ccggaattcGAAGCGKCAGGTGGATG-3′ followed by 5′-ccggaattcAGRTCAATGARCAGCTCC-3′, and the Expand High Fidelity PCR Kit (Roche). The remaining part of the cingulin cDNA was amplified in four subsequent steps, using forward degenerate primers based on the known sequences of mammalian cingulin. All PCRs were carried out in triplicate, and cloned independently, to verify sequence consistency. Structure prediction analysis for coiled-coil regions was carried out with the MacStripe and Coils algorithms (www.expasy.ch).
Generation of full-length, head, and rod + tail cingulin constructs
Constructs for Tet-regulated expression of GFP fused to either full-length cingulin, or cingulin head, or rod + tail domains were assembled in four steps. First, the green fluorescent protein (GFP) sequence was amplified from pEGFP-C1 (Clontech), and cloned into the BamHI-NotI sites of pBluescript. Second, the cingulin sequences were amplified, using the Expand High Fidelity PCR kit (Roche), and cloned downstream of the GFP sequence, into the NotI-ClaI sites. Third, a myc tag was inserted into the ClaI-SalI sites. Fourth, the BamHI-SalI inserts were then cloned into the vector pTRE2Hyg (Clontech). The constructs for bacterial expression of myc-tagged proteins were made in pGEX4T1 vector (Amersham). The GFP-head fusion protein was inserted into the BamHI-XhoI sites, and the GFP was removed by cutting with BamHI-BsrGI. The GST-myc protein was generated by the insertion of the myc linker sequence into the EcoRI-XhoI sites. The sequence of all amplified inserts and constructs was checked by DNA sequencing.
Cell culture and transfection
MDCKII (Madin-Darby canine kidney) tet-off cells (Clontech) were cultured in Dulbecco's modified Eagle's medium (DMEM, Sigma) containing 10% fetal bovine serum (FBS), 1×MEM non-essential amino acids (Invitrogen) and 1 µg/ml puromycin (Sigma). Cells were transfected using Lipofectamine 2000 (Invitrogen), following the manufacturer's protocol, and selected with 200 µg/ml hygromycin (Invitrogen). Transgene expression was induced by replacing doxycyclin-containing medium (40 ng/ml) with medium lacking doxycyclin (Dox), for 3 days. Clones were isolated by cloning rings, and screened for low basal GFP expression and high induction of GFP in the absence of Dox, using a fluorescence-activated cell sorter (FACSCalibur, Becton Dickinson). Between three and six clones were isolated for each construct.
Microarray and qRT-PCR analysis
Total RNA was isolated in triplicate from MDCK cells expressing full-length canine cingulin, and cultured either in the absence or in the presence of Dox. 5 µg RNA was converted to double-stranded cDNA using a cDNA synthesis kit (Superscript choice, Invitrogen), with an oligo(dT)24 primer containing a T7 RNA promoter site added 3′ to the poly(T) tract. Biotinylated cRNAs were generated from cDNAs using the Bioarray high yield RNA transcript labeling kit (Enzo Life Science) and purified with the RNeasy kit. 17.5 µg of cRNA probe were fragmented and hybridized to Affymetrix GeneChip Canine Genome Array, containing probe features for 21,700 transcripts. The chips were washed using an Affymetrix GeneChip Fluidics Station 400 according to a standard protocol, scanned, and the fluorescence signals were analyzed with Affimetrix software MAS 5.0. The pairwise analyses of the DNA microarray data obtained with the three RNA extractions from the cells grown in the absence of Dox, and the three RNA extractions from cells grown in the presence of Dox resulted in nine comparison sets. The change in expression was considered as significant when a minimum of 7 out of 9 comparison sets were in the same direction and when the median fold change p-value was <0.05. The expression values were normalized using RMAexpress version 0.3 Citation[15], and relative gene expression was obtained by the LIMM method Citation[16].
Real-time quantitative reverse-transcription PCR (real-time qRT-PCR) was carried out as described in Citation[13], using specific sets of primers for canine cingulin, claudin-1, -2, -3, ZO-1, -2, -3, Lfc, occludin, and ZONAB (see Supplementary Table at end of online version).
RhoA activity assays, measurement of cell density and doubling time
RhoA activity was determined as described in Citation[14], using either 100% or 50% confluent cells. Cell density was determined by seeding an equal number of cells at 50% confluence, culturing for seven days, and counting cell numbers both in a hemacytometer after trypsinization, and on slides after staining the nuclei with DAPI. To measure growth rate, cells were seeded at 5×104 cells per cm2, and counted every 24 h following trypsinization. The doubling time (Dt) was taken as the inverse of the slope of the regression curve calculated during the exponential growth phase, between day 1 and day 3 (Dt = 1/k, where k=(Ln(Nd1/Nd3))/2). Data from two to five independent experiments were averaged.
Calcium switch and measurement of transepithelial resistance
Parallel cultures of cells were incubated either in the absence or in the presence of Dox for three days. Cells were then trypsinized, and plated into duplicate 6.5 mm diameter Transwell filters (Costar) in low calcium medium (S-MEM, Invitrogen) containing 10 mM HEPES, 5% dialyzed FBS, 2 mM L-Glutamine, 2 µM EGTA and 1X MEM non-essential amino acids. 24 h later TER was measured (t = 0 h) using a Millicel ERS apparatus (Millipore), and the low calcium medium was replaced by normal medium (calcium-switch). TER was measured 1, 2, 3, 4, 6, 8, 24, 30, and 48 h after the switch. Data from three independent experiments were averaged, and expressed as ohm.cm2.
Immunoblotting and immunofluorescence
Lysates for immunoblotting were obtained by scraping cells in RIPA buffer (150 mM NaCl, 40 mM Tris-HCl, pH 7.6, 2 mM EDTA, 10% glycerol, 1% Triton X-100, 0.5% sodium deoxycholate, 0.2% SDS, 5 µg/ml antipain, 5 µg/ml leupeptin, 5 µg/ml pepstatin, 1 mM PMSF), followed by sonication (3 sec at 33% amplitude with a Brandon digital sonifier). Protein concentration of samples from induced (−Dox) and non-induced (+Dox) cells was normalized by immunoblotting with anti-tubulin antibodies. After incubation with HRP-labelled secondary antibodies, the reaction was developed using the SuperSignal WestPico chemiluminescence kit (Pierce). For immunofluorescence, cells were permeabilized with PBS containing 0.2% Triton X-100 and 3% paraformaldehyde for 3 min, followed by fixation in PBS containing 3% paraformaldehyde, for 20 min. Incubations with primary and secondary antibodies were for 1 h at 30°C and 45 min at 37°C, respectively. Coverslips were mounted in Vectashield medium (Vector Laboratories) and imaged using either an Axiovert S100 fluorescence microscope, or a Zeiss 510Meta confocal microscope. Confocal images were acquired in multi-tracking mode, to prevent bleedthrough between channels.
Immunoprecipitation
MDCK cell lysates were prepared with CelLytic M reagent (Sigma, 15 min at 4°C), and clarified by centrifugation at 13,000 g 5 min. Lysates were incubated (16 h at 4°C) with 35 µl Dynabeads (anti-mouse, Sigma) that were previously conjugated to 5 µg anti-myc 9E10 mouse monoclonal antibody. The beads were washed with lysis buffer, and eluted proteins were analyzed by SDS-PAGE and immunoblotting with anti-myc and anti-ZO-1 antibodies.
Results
Cloning and sequence analysis of full-length canine cingulin
The amino acid sequence of cingulin is not very highly conserved among vertebrate species Citation[12], raising the possibility that expression of non-canine cingulin in canine (MDCK) cells may result in sequence-dependent artifacts. Thus, in order to examine the effects of cingulin overexpression in MDCK cells, we first obtained a full-length canine cingulin cDNA, and determined its complete nucleotide and derived amino acid sequence (). The sequence we obtained (Genbank n. DQ910799) differs by either 1 (different) or 21 (additional) nucleotides from predicted sequences currently available in GenBank (n. XM_540316.2 and n. XM_856969.1, respectively).
Figure 1. The cingulin sequence is particularly well conserved in the ZIM and rod domains. Alignment of cingulin amino acid sequences from mouse (Accession no. P59242), rat (Accession no. XM_227472), human (Accession no. AF263462) and dog (Accession no. DQ910799) are shown. Boxed sequences correspond to the ZO-1 Interaction Motif (ZIM), and to the rod sequence region (Rod), which shows a predicted coiled-coil structure. Identical residues are highlighted in black.

The sequence of canine cingulin encodes a protein of 1197 amino acids (Mr 135 kDa), which shows an overall 86% identity to human cingulin (). In canine cingulin or the head domain comprises residues 1-338, the coiled-coil rod domain residues 339-1143, and the tail domain residues 1144-1197. As in the case of human versus Xenopus cingulin Citation[12], the sequence identity between human and canine cingulin is greater in the rod + tail domain (89%) than in the head domain (81%). The C-terminal portion of the rod + tail domain (corresponding to amino acids 894-1197 of canine cingulin) is particularly well conserved (95% identity). Within the head domain, the sequence required for in vitro ZO-1 interaction (ZIM = ZO-1 Interaction Motif) Citation[10] is identical between human, rat and mouse sequences, but shows one amino acid change in canine cingulin ().
Inducibly expressed cingulin fused to GFP targets to junctions and interacts with ZO-1
To generate MDCK cell lines where cingulin could be inducibly overexpressed, constructs were prepared in the pTRE2Hyg vector, and transfected into MDCK tet-off cells. The constructs comprised green fluorescent protein (GFP) fused N-terminally either to full-length canine cingulin, or to cingulin head or to cingulin rod + tail domains, in order to visualize directly protein expression by fluorescence microscopy. A construct containing GFP alone was used as a negative control. Doxycycline (Dox) was effective in repressing transgene expression, since little or no GFP fluorescence was detected when cells were cultured in the presence of Dox (A, panels A, C, E, G). Only the nuclei colored with DAPI are visible: Upon removal of Dox, MDCK clones expressing GFP alone showed diffuse cytoplasmic and nuclear fluorescence, indicating that GFP does not associate with junctions (A, panel B). Clones expressing full-length cingulin showed intense fluorescent labeling associated with cell-cell junctions (arrow in A, panel D, see also ). In cells expressing large amounts of full-length cingulin, fluorescent labeling was not only distributed linearly along junctions, but also detected as aggregates in the submembrane cytoplasm (arrowhead in A, panel D, see also ). In clones expressing the cingulin head construct, cells showed strong diffuse cytoplasmic labeling, and weaker junctional labeling (arrow in A, panel F, see also ), consistent with the notion that the head domain contains sequences, which are crucial for cingulin junctional recruitment Citation[10]. No labeling was detected in the nucleus of cells expressing the head domain of cingulin (A, panel F). Finally, clones expressing the rod + tail cingulin construct showed strong cytoplasmic fluorescence, in the form of a few brightly stained aggregates (arrowhead in A, panel H, see also ). In overexposed images, weak labeling was also detected along junctional regions (not shown). In cells expressing lower amounts of GFP-labelled protein, accumulation of labeling in the junctional region was more clearly detectable (arrow in inset of A, panel H), suggesting that the rod + tail domain can be, albeit with low efficiency, recruited into junctions.
Figure 2. Inducible overexpression and localization of GFP-tagged canine cingulin constructs in MDCK cells. Top panel (A) Immunofluorescence micrographs showing the localization of GFP (A, B), GFP-Full-length cingulin (C, D), GFP-head domain (E, F), GFP-rod + tail domains (G, H) in stably transfected clones either in the presence (+: A, C, E, G) or in the absence (−: B, D, F, H) of Dox. Nuclei are stained in blue by DAPI. Note that no GFP labeling is detectable in the presence of Dox, which represses transgene expression. Single arrows (D, F, H) indicate junctional labeling. Arrowheads (D, H) indicate cytoplasmic labeling. Bar = 10 µm. Bottom panel (B) Immunoblot analysis (with anti-ZO-1 and anti-myc antibodies, top and bottom, respectively) of myc immunoprecipitates obtained by incubating myc-tagged GST fusion proteins with anti-mouse Dynabeads, followed by washing and incubation with MDCK cell lysates. Lanes are indicated: input (lysate); beads (no GST fusion proteins); myc (GST-myc); GFP-Head-myc (GST fused to GFP-canine cingulin head-myc); Head-myc (GST fused to canine cingulin head-myc). Asterisks in the Myc immunoblot indicate recombinant, myc-tagged proteins. Other bands are due to non-specific cross-reactivities of the antibodies. Note that the ZO-1 immunoblots show essentially identical signal in the GFP-Head-myc and Head-myc lanes, indicating that the presence of the GFP moiety does not affect the interaction of the cingulin head with ZO-1. This figure is reproduced in colour in Molecular Membrane Biology online.
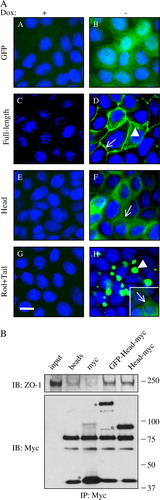
To confirm that the presence of the GFP moiety N-terminal to the cingulin head sequence does not prevent the correct interaction of the ZIM domain of cingulin with ZO-1, we compared the interaction of bacterially expressed recombinant GST fusion proteins, with or without GFP, with ZO-1 in cell lysates, by immunoprecipitation (B). Immunoblot analysis with anti-ZO-1 antibody showed that similar amounts of ZO-1 interacted with the proteins, regardless of wether they contained or not GFP (B). Thus, the presence of the GFP moiety N-terminal to the cingulin sequence does not interfere with correct targeting to junctions and interaction with ZO-1.
Inducible overexpression of either full-length cingulin, or head, or rod + tail domains does not affect TJ protein levels and gene expression
We have previously shown that mutation or down-regulation of cingulin results in changes in the levels of expression of several TJ proteins Citation[13], indicating that a decrease of cellular cingulin below physiological levels has an impact on gene expression, in part through up-regulation of RhoA activity Citation[14]. We therefore hypothesized that an increase in the levels of cingulin, or of its domains, might also affect expression levels of TJ proteins. Total cell lysates from MDCK cell clones cultured in the absence (inducing) or presence (repressing) of Dox were analyzed by immunoblotting with antibodies against GFP (to detect exogenously expressed proteins), and to cingulin, ZO-1, ZO-2, ZO-3, ZONAB, PAR3, claudin-2, claudin-7, occludin, JAM-1 and RhoA (A). In the presence of Dox, anti-GFP antibodies showed barely detectable or undetectable expression of exogeneous protein, indicating good repression of the transgene (A). Upon removal of Dox, anti-GFP revealed intensely stained polypeptides of the expected apparent molecular weight (27 kDa for GFP, 164 kDa for GFP-full length cingulin, 66 kDa for GFP-Head, 127 kDa for GFP-Rod + Tail) (A). By comparing the immunoblot signal from equivalent protein loadings of lysates from non-induced and induced cells, we could not detect significant differences in the protein levels of any of the endogeneous TJ proteins we tested (A).
Figure 3. Overexpression of cingulin and its domains in stable MDCK clones does not result in changes in TJ protein levels and gene expression. (A) Immunoblot analysis of lysates from control MDCK cells, and stable clones expressing either GFP alone, or GFP fused to full-length canine cingulin, head, or rod + tail domains, in the presence (+) or absence (−) of Dox. Proteins are indicated on the left (CGN = cingulin, CLDN = claudin, OCLN = occludin). Numbers on the right correspond to the size (kDa) of prestained molecular weight markers. Note that antibodies against GFP label the exogeneous proteins, which are detected only upon induction of transgene expression, by removal of Dox. Images are representative of three independent experiments for each protein examined. The cingulin immunoblot shows smeared signals in the lanes containing overexpressed exogenous protein. (B) Dot-plot analysis of the normalized expression intensity (expressed in arbitrary units, proportional to the intensity of the fluorescence Affymetrix signal) of the 21,700 canine transcripts in one clone (Clone 2) of MDCK cells stably expressing full-length cingulin, either in the presence or in the absence of Dox. Each transcript is represented by a point. Dotted lines flanking the diagonal represent a 2-fold decrease (top) or increase (bottom) of relative gene expression, in samples without Dox. The four circled points represent transcripts showing >2-fold increase. Note that except for the 4 circled points, all other points are clustered along the diagonal, indicating that overexpression of full-length cingulin does not have major effects on the pattern of gene expression.
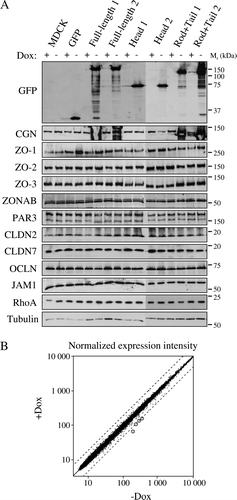
To determine whether cingulin up-regulation may affect gene expression, microarray analysis was carried out to compare transcript levels of non-induced versus induced cells in one clone (Clone 2) of cells overexpressing full-length cingulin. As shown in B, four genes (Genbank accession numbers CO630281, CF407530, CO716576, CF408993) were identified, whose expression in induced cells was over two-fold higher than in non-induced cells (circles in B). To validate this result, real-time quantitative RT-PCR (qRT-PCR) analysis was carried out to evaluate the expression of these genes in induced versus non-induced cells in additional clones, which expressed either full-length cingulin (Clones 1 and 3), or head (Clones 1 and 2), or rod + tail (Clones 1 and 2) constructs. However, this analysis did not reveal any changes in the expression in these genes upon induction (data not shown), indicating that the results of the microarray experiment reflected either clone-dependent or background variations. Similarly, analysis of the expression of several genes coding for TJ proteins (cingulin, claudin-1, -2, -3, ZO-1, -2, -3, Lfc, occludin, ZONAB) by qRT-PCR in the different types of MDCK clones did not reveal any significant differences in their mRNA levels when comparing induced and non-induced cells (data not shown). Taken together, these data indicate that overexpression of either full-length cingulin, or head, or rod + tail constructs does not have significant effects either on overall gene expression, or on regulating the transcript and protein levels of several TJ proteins.
Inducible overexpression of either full-length cingulin, or head, or rod + tail domains does not affect RhoA activity, cell density and doubling time
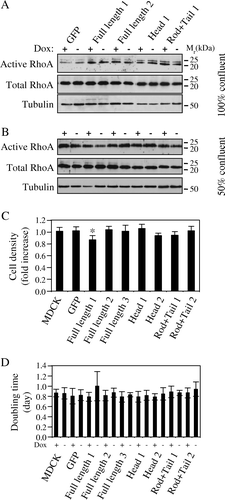
Down-regulation of cingulin results in increased RhoA activity Citation[14]. To test whether inducible cingulin up-regulation in stably transfected clones results in decreased RhoA activity, we carried out rhotekin binding assays on lysates of non-induced and induced confluent MDCK clones, expressing different constructs. As shown in A, the amount of active RhoA was similar in non-induced and induced samples, indicating that increasing the levels of cingulin, or overexpressing its domains, does not affect RhoA activity under these experimental conditions. To test whether the results were different when cells were proliferating more rapidly, e.g. under conditions where RhoA activity is higher, the experiment was repeated with cells growing at 50% confluence. The results show that no major and reproducible changes in the amounts of active RhoA could be detected upon induction of expression of the different constructs (B). This is in agreement with our previous observations on cingulin-depleted cells, where the increase in RhoA activity was detected both in confluent and non-confluent cultures Citation[14]. We next tested whether cingulin up-regulation could affect cell density of confluent monolayers, compared to control cells. When comparing cell density in the absence of Dox (induced cells), versus in the presence of Dox, there was only Clone 1, expressing full-length cingulin, which showed a small, but statistically significant decrease in cell density upon Dox removal (C). In all other clones we tested, Dox removal did not induce statistically significant changes in cell density. When we measured doubling time, which indicates cell proliferation in non-confluent cells, Clone 1 expressing full-length cingulin showed a slight but not statistically significant increase in doubling time upon Dox removal, but no other changes were detected (D). Finally, up-regulation of cingulin and its domains did not have any effect on cell migration, as determined by a wound-healing assay (data not shown).
Figure 4. RhoA activity, cell density and doubling time are not affected by cingulin overexpression. (A) and (B) Immunoblot analysis showing active RhoA (isolated by a rhotekin-binding GST pulldown assay), versus total RhoA and tubulin protein levels, in lysates from MDCK clones expressing either GFP alone, or GFP fused to full-length canine cingulin, head, or rod + tail domains, cultured at 100% confluence (A) or at 50% confluence (B). Numbers on the right correspond to the size (kDa) of prestained molecular weight markers. Note that induction of transgene expression does not result in changes in the levels of active RhoA. (C) Histogram showing the fold-increase in cell density (number of cells in the absence of Dox/number of cells in the presence of Dox) in control MDCK cells, and in stable clones expressing either GFP alone, or GFP fused to full-length canine cingulin, head, or rod + tail domains. (D) Histogram showing the doubling time in the presence (+) or absence (−) of Dox in control MDCK cells, and in the different clones. Values for the two histograms represent the mean of three to five independent experiments (see Materials and methods).
Overexpression of cingulin or its domains has no significant effect on TJ organization and barrier function at steady-state
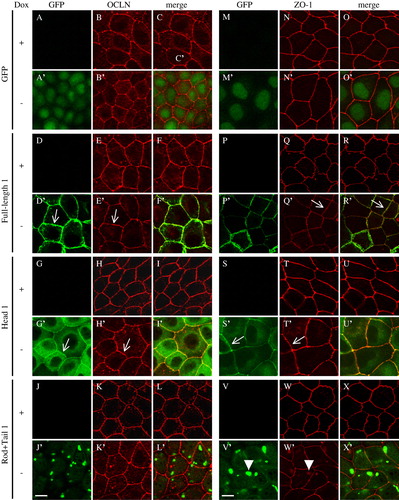
Cingulin is present in a complex with several TJ proteins Citation[9], Citation[17], and it is reasonable to hypothesize that overexpression of cingulin or its domains may alter the molecular organization of TJ by a dominant-negative effect. To test this hypothesis, the localization of occludin and ZO-1, which interact with cingulin Citation[9], Citation[18], was determined in MDCK cells overexpressing different cingulin constructs (). In non-induced cells, ZO-1 showed the continuous linear distribution along cell-cell contact, and occludin showed a mainly junctional localization, with occasional cytoplasmic labeling (B, E, H and 5N, Q, T). In induced cells expressing the GFP alone, the full-length and the head constructs, no changes in the distribution and intensity of immunofluorescent labeling of occludin and ZO-1 were observed (B′, E′, H′ and N′, Q′, T′). However, in cells expressing high levels of the rod + tail construct, ZO-1 labeling was detected mostly at junctions, but occasionally in cytoplasmic aggregates (arrowhead in W′). Most of the aggregates showed co-localization with the rod + tail aggregates (X′), suggesting that the rod + tail construct induces a minor redistribution of ZO-1.
Figure 5. Effect of cingulin overexpression on the localization of occludin and ZO-1 in MDCK cells. Immunofluorescent localization of GFP, GFP fused to full-length canine cingulin, head, or rod + tail domains (green), occludin and ZO-1 (red), either in the presence (+) or absence (−) of Dox. Merge images show co-localization. Single arrows (D′, E′, G′, H′, Q′, R′, S′, T′) indicate junctional labeling. Arrowheads (V′, W′) indicate cytoplasmic labeling. Images are representative of two independent experiments. Bar = 10 µm. This figure is reproduced in colour in Molecular Membrane Biology online.
To determine whether cingulin up-regulation affects the barrier function of TJ, TJ assembly was induced by the calcium switch protocol, and the transepithelial resistance was measured at time intervals after calcium addition in control cells and cells expressing GFP (A), in clones expressing full length cingulin (B), head (C) and rod + tail (D) constructs. The peak in development of TER was between 4 h and 8 h after calcium switch, and the peak values of TER were within a rather broad range, e.g., between 268 and 547 ohm cm2 in the presence of Dox, and between 136 and 435 ohm cm2 in its absence (, ). On the other hand, 30 h after the addition of calcium no difference was observed when comparing the TER of control cells, and clones expressing either GFP or different cingulin constructs (). For each clone and control cells, we compared the peak value of TER during the calcium switch in the absence of Dox versus in its presence, and expressed it as percentage (). Only in control MDCK cells, in one of the clones expressing GFP, and in one of the clones expressing the head construct the peak values of TER were essentially identical with or without Dox. In all other cases, removal of Dox induced either a decrease or, in one case, an increase in the TER value (). Indeed, one of the clones expressing the rod + tail construct showed a 28% increase in the peak value of TER, and the other clone a 29% decrease (). The strongest decrease (61%) in the peak value of TER after Dox removal was observed in Clone 1 expressing full-length cingulin (, ). Since Clone 2 also showed a decrease (26%), we examined a third clone, to check that a decrease in the peak value of TER in the calcium switch was consistently associated with overexpression of full-length cingulin. Clone 3 expressing full-length cingulin showed a small (8%) decrease in the peak value of TER upon Dox removal (). Clone 1, which showed the greatest decrease in the peak value of TER, had a delay in occludin assembly into TJ, as shown by immunofluorescence (E). At 4 h after the calcium switch, all cells expressing GFP showed a continuous occludin labeling around the cell periphery, whereas in the case of Clone 1, several cells showed areas of the cell periphery lacking occludin staining (arrowheads in E). At the end of the calcium switch, occludin labeling (E) and TER values (B) in Clone 1 were the same as control cells, indicating that the delay in occludin assembly was transient. To establish whether the different TER behaviours of the three clones expressing full-length cingulin was due to differences in the expression levels of the exogeneous protein, lysates from cells switched from normal to low extracellular calcium were analyzed by immunoblotting with anti-GFP antibodies (F). The results showed similar levels of expression, suggesting that the differences in TER values observed in the different clones did not correlate with the levels of exogenously expressed cingulin.
Figure 6. Effect of cingulin overexpression on TJ barrier function. Analysis of the transepithelial electrical resistance (TER, expressed in ohm.cm2) of MDCK monolayers during the calcium-switch assay. (A) Wildtype MDCK cells and clones expressing GFP alone, either in the absence (−Dox, empty symbols) or the presence (+Dox, full symbols) of doxycyclin (as indicated in the inset). (B) Clones expressing GFP fused to full-length cingulin. (C) Clones expressing GFP fused to cingulin head domain. (D) Clones expressing GFP fused to cingulin rod + tail domains. Values (see also ) represent the means±S.D. (error bar) of three to five independent experiments. Panel (E) shows the immunofluorescent localization of occludin at time 0, 4 h and 30 h from the beginning of the calcium switch, in Clone 1 expressing full-length cingulin (Full-length 1) and in Clone 1 expressing GFP. Arrowheads indicate discontinuities in occludin labeling observed in cells expressing full-length cingulin. Panel (F) shows immunoblot analysis with anti-GFP antibodies of lysates from three stable clones expressing full-length cingulin, in the presence (+) or absence (−) of Dox-. The anti-tubulin immunoblot was used as a protein loading control. Note that similar amounts of GFP-tagged protein are expressed in the three clones.
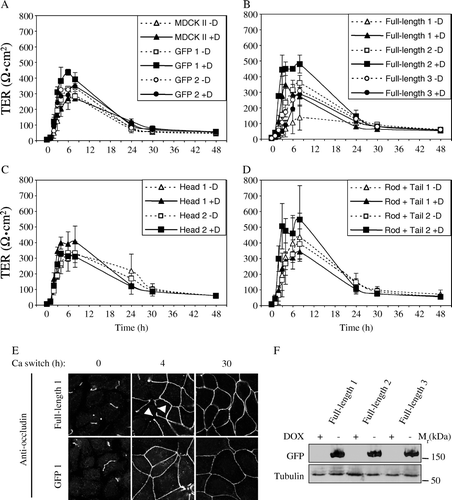
Table I. TER peak* during the Calcium switch.
Discussion
The primary objective of the present study was to develop and characterize stably transfected MDCK cell clones, where the expression of canine cingulin and its domains could be inducibly up-regulated, to generate tools to study cingulin function by a dominant-negative approach. Based on our previous studies, we speculated that cingulin over-expression would affect gene expression and cell density, through an effect on RhoA activity. In addition, since cingulin interacts with several TJ proteins, we sought to investigate whether its overexpression could affect the organization and function of TJ. The results indicate that in this model system cingulin overexpression does not produce major effects on the localization of TJ proteins and the barrier function of TJ at steady state, and that compensatory mechanisms may prevent cingulin overexpression from affecting RhoA activity and downstream events, such as gene expression.
We successfully obtained stable MDCK clones inducibly expressing GFP-tagged full-length, head, and rod-tail domains of cingulin in a tight, Dox-dependent manner. Localization of the recombinant proteins by fluorescence microscopy confirmed and extended previous observations Citation[9], Citation[10]. Full-length cingulin is efficiently targeted to junctions, and excess protein in overexpressing cells accumulates in the sub-junctional area. The cingulin head domain localizes less efficiently to junctions, and is mostly detected in the cytoplasm, indicating that the rod + tail domain improves junctional targeting, due either to its effect on the conformation of the head, or to additional protein interactions, or both. On the other hand, coimmunoprecipitation experiments show that the GFP moiety does not affect interaction of the cingulin head with ZO-1. This observation, and the junctional targeting of GFP-tagged cingulin, shows that the GFP moiety does not modify the functional properties of cingulin, making it possible to use these constructs to investigate the dynamics of cingulin localization in living cells. The protein containing the rod + tail domains was detected as large aggregates in the cytoplasm, but a minor fraction was also detected at junctions. It is possible that the rod + tail domain can associate with junctions via interaction with cingulin itself Citation[12], or GEF-H1 Citation[11]. However, the affinities for these interactions appear too low to allow an efficient junctional recruitment.
Although cingulin interacts with several TJ proteins through the head domain Citation[9], Citation[18] overexpression of full-length cingulin and the head domain did not result in changes in the distribution of occludin and ZO-1. Thus, although ZO-1 interacts with the cingulin head domain with high affinity, its junctional localization is made stable by additional protein interactions. Interestingly, ZO-1 redistribution was observed in transiently transfected Xenopus A6 cells overexpressing full-length Xenopus cingulin Citation[10], suggesting that transiently transfected cells may exhibit different behavior, or that ZO-1 stability at junctions is dependent on cell type. Surprisingly, we observed a minor redistribution of ZO-1 in cells overexpressing the rod + tail construct. It is unclear how this domain, which is not known to interact with ZO-1, could affect ZO-1 distribution. One possible explanation is that the rod + tail domain binds to or affects the activity of another protein, which also binds to ZO-1.
Consistent with the observation that overexpression of cingulin and its domains does not modify ZO-1 and occludin distribution at steady-state, we did not detect significant differences in the TER values of all the clones that we tested, after 30 h from the calcium-switch induced assembly of TJ. Interestingly, all the clones expressing full-length cingulin showed, to different extents, a reduction of the peak value of TER 4 to 8 h after the switch, and Clone 1 showed a delayed assembly of occludin at junctions, suggesting that increased cingulin levels may transiently interfere with the TJ assembly process.
Cingulin mutation or depletion induces changes in gene expression in cultured cells, in part through an increase in RhoA activity Citation[13], Citation[14]. Transient transfection of cultured cells with full-length cingulin or rod + tail constructs leads to inhibition of RhoA activity, through sequestration of the RhoA activator GEF-H1 Citation[11]. Thus, we speculated that inducible overexpression of cingulin would lead to a marked and sustained decrease in RhoA activity, and downstream events controlled by RhoA. Surprisingly, no consistent changes were observed upon Dox removal in protein levels, RhoA activity, gene expression, cell density and doubling time. Only in one out of three clones expressing full-length cingulin, we observed a decreased cell density at confluence, and an increased doubling time. Interestingly, this clone (Clone 1) was the one showing the lowest TER peak during the calcium switch, suggesting that a common mechanism may influence both dynamics of junction formation and proliferative activity. However, since the other clones expressing full-length cingulin had cell density and doubling time similar to control cells, we cannot rule out that the behavior of this particular clone (Clone 1) was not due to clone-dependent variations.
In summary, our results indicate that RhoA activity (and downstream events controlled by RhoA, such as gene expression or cell density) is consistently increased when cingulin levels are decreased, either by siRNA-mediated depletion in MDCK cells, or by mutation of one or both cingulin alleles, in embryoid bodies. On the other hand, inducible up-regulation of cingulin in stably transfected MDCK clones does not inhibit RhoA activity, possibly due to unknown compensatory changes, which offset the effects of cingulin up-regulation on RhoA activity. Interestingly, cingulin expression in certain fibroblastic cells is up-regulated dramatically by differentiating agents such as histone deacetylase inhibitors Citation[19]. So, future studies should address whether cingulin up-regulation in differentiating cells, which undergo mesenchymal-epithelial transition, is linked to the regulation of RhoA activity and cell proliferation.
Supplementary Table. PCR Primers used in qRT-PCR.
Acknowledgements
We thank Laurent Guillemot, Patrick Descombes and Olivier Schaad for advice and discussions. This study was supported by grants from the Swiss National Fund, the Swiss Cancer League (OCS-01390-08-2003), the State of Geneva, and the MIUR (ex-60%).
References
- Schneeberger E.E., Lynch R.D. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 2004; 286: C1213–228
- Tsukita S., Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol 2002; 14: 531–536
- Gonzalez-Mariscal L., Betanzos A., Nava P., Jaramillo B.E. Tight junction proteins. Prog Biophys Mol Biol 2003; 81: 1–44
- Shin K., Fogg V.C., Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 2006; 22: 207–235
- Braga V.M. Cell-cell adhesion and signalling. Curr Opin Cell Biol 2002; 14: 546–556
- Matter K., Aijaz S., Tsapara A., Balda M.S. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol 2005; 17(5)453–458
- Citi S., Sabanay H., Jakes R., Geiger B., Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature 1988; 333: 272–276
- Citi S., Sabanay H., Kendrick-Jones J., Geiger B. Cingulin: characterization and localization. J Cell Sci. 1989; 93: 107–122
- Cordenonsi M., D'Atri F., Hammar E., Party D.A., Kendrick-Jones J., Shore D., Citi S. Cingulin Contains Globular and Coiled-coil Domains and Interacts with ZO-1, ZO-2, ZO-3, and Myosin. J Cell Biol 1999; 147: 1569–1582
- D'Atri F., Nadalutti F., Citi S. Evidence for a functional interaction between cingulin and ZO-1 in cultured cells. J Biol Chem. 2002; 277: 27757–27764
- Aijaz S., D'Atri F., Citi S., Balda M.S., Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and Gl/S phase transition. Dev Cell 2005; 8: 777–786
- Citi S., D'Atri F., Parry D.A.D. Human and Xenopus cingulin share a modular organization of the coiled-coil rod domain: predictions for intra- and intermolecular assembly. J Struct Biol. 2000; 131: 135–145
- Guillemot L., Hammar K., Kaister C., Ritz I., Caille D., Jond L., Bauer C, Meda P., Citi S. Disruption of the cingulin gene does not prevent tight junction formation but alters gene expression. J Cell Science 2004; 117: 5245–5256
- Guillemot L., Citi S. Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase. RhoA Mol Biol Cell 2006; 17: 3569–3577
- Bolstad B.M., Irizarry R.A., Astrand M., Speed T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003; 19: 185–193
- Smyth, G.K. 2004. Linear models and empyrical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Gen Mol Biol, 3: Article 3.
- Bazzoni G., Martinez-Estrada O.M., Orsenigo F., Cordenonsi M., Citi S., Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem 2000; 275: 20520–20526
- Cordenonsi M., Turco F., D'Atri P., Hammar E., Martinucci G., Meggio F., Citi S. Xenopus laevis occludin: identification of in vitro phosphotylation sites by protein kinase CK2 and association with cingulin. Eur J Biochem. 1999; 264: 374–384
- Bordin M., D'Atri F., Guillemot L., Citi S. Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol Cancer Res 2004; 2: 692–701