Abstract
Polarized membrane traffic to different domains of the neuron is well documented, and is required for both establishment and maintenance of neuronal polarity. Some soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) proteins, particularly syntaxin 12/13 and TI-VAMP/VAMP7, have known roles in the neuron. We report here that the brain-enriched SNARE syntaxin 16 (Syn 16) is specifically enriched in neuronal dendrites and found at Golgi outposts, thus confirming that Golgi outposts are endowed with a trans-Golgi network (TGN) component. Over-expression of wild type syntaxin 16 moderately stimulates, whereas that of an N-terminal deletion mutant (Syn 16-ΔNt) inhibits, neurite outgrowth in both mouse Neuro-2a cells and primary cortical neurons. Consistent with an inhibited neurite growth, cells over-expressing Syn 16-ΔNt have diminished βIII-tubulin and F-actin labeling. RNA interference-mediated silencing of syntaxin 16 in primary cortical neurons significantly retards neurite outgrowth. Syntaxin 16 may thus play a role in neurite outgrowth and perhaps other specific dendritic anterograde/retrograde traffic.
Introduction
Neuronal dendrites are elaborate, multi-branched structures, and it is conceivable that it would be problematically inefficient if its sole supply of nascent membrane proteins comes only from the cell body. This problem is partly alleviated by having mRNAs targeted to local protein translational machinery of dendrites (Steward & Worley [Citation2001]). The Golgi apparatus is the penultimate membranous organelle within the soma where proteins destined for the secretory pathway, membrane-spanning or otherwise, traverse before reaching the plasma membrane. It is interesting to note that polarization in many eukaryotic cells is often associated with some morphological rearrangement of the Golgi, so as to facilitate the direction of membrane traffic to specialized plasma membrane domains (Horton & Ehlers [Citation2003a]). In neuronal dendrites, this specialization may take the form of small, discrete Golgi-like islands that are not connected with the Golgi apparatus at the soma, termed Golgi outposts.
Membrane structures in post-synaptic differentiations that are immunoreactive for endoplasmic reticulum (ER), ER-Golgi intermediate compartment (ERGIC) and Golgi markers had been shown by both immunocytochemistry and electron microscopy (Gardiol et al. [Citation1999], Pierce et al. [Citation2001]). Aridor and colleagues ([Citation2004]) showed that ER export sites, on which the small GTPase Sar1 and components of coat complex II (COPII) are found, are indeed assembled regularly throughout the dendritic tree. N-methyl D-aspartate (NMDA) receptors were shown to be recruited to these ER export sites upon activation of metabotropic glutamate receptors, indicating that the formation of these sites are physiologically regulated. Horton and Ehlers ([Citation2003b]) demonstrated that the vesicular stomatitis virus G (VSVG) protein, upon release from dendritic ER exit site, moves bidirectionally and fuses with both the somatic Golgi as well as relatively static and long-lived punctuated structures positive for both the cis-Golgi marker GM130 (Nakamura et al. [Citation1995]) and the trans-Golgi marker galactosyltransferase (Nilsson et al. [Citation1991]) in the dendrite. Electron micrographs of high resolution and structural preservation clearly showed that at least some of these consist of Golgi-like cisternae stacks. It was these structures that were termed ‘Golgi outposts’. These Golgi outposts play a role in dendritic growth during morphological differentiation of hippocampal pyramidal neurons (Horton et al. [Citation2005]). Dendritic growth is asymmetric and the Golgi apparatus in the soma is in fact physically oriented towards the longest dendrite. This form of Golgi polarity precedes that of dendritic polarity, and disruption of the Golgi with the over-expression of GRASP65, a protein important for Golgi cisternae stacking (Barr et al. [Citation1997]), resulted in a uniform pattern of dendritic growth.
The existence of Golgi outposts raise many interesting questions, perhaps the most important of which pertains to their origin and function (Horton & Ehlers [Citation2004]). A particularly interesting question is whether these are indeed smaller but nonetheless complete versions of the Golgi at the soma, with a cis- and trans- face. A set of related question is whether Golgi outposts function in receiving both anterograde traffic (ie. from the ER) as well as retrograde traffic (i.e., from the plasma membrane and endosomes), and where such cargo that transits the Golgi outpost is subsequently directed to. To answer these questions it would be helpful to know if Golgi outposts have an associated trans-Golgi network (TGN). It is not particularly clear from the works described above whether the Golgi outposts have a TGN, as morphological tracking of the structures were done using markers that are not specifically TGN-localized, such as the cis-Golgi marker GM130.
Besides localized secretory machinery, polarized membrane traffic in neurons is also necessary for both the establishment and the maintenance of the axonal and somatodendritic plasma membrane domains (Craig & Banker [Citation1994], Tang [Citation2001], Horton & Ehlers 2003a,b). Selective, directed targeting of membrane cargoes in specific domain-bound carriers have been observed for dendritic proteins (Setou et al. [Citation2000], Juttner & Rathjen [Citation2005]). On the other hand, some membrane cargo-ferrying carriers have apparently no specific targeting and can find their way into the associated cytoplasmic region of both axons and dendrites. This is particularly the case for those utilizing motors that travel on plus-end-directed microtubules, which are present in both axons and dendrites (Welte [Citation2004]). For these, selective membrane docking/fusion is presumably required for eventual domain specific incorporation of cargo (Burack et al. [Citation2000]). One of the important players in this selective vesicle trafficking would be the receptors for the N-ethylmaleimide sensitive factor (NSF) attachment protein (SNAP), or SNAREs.
Syntaxin 16 (Syn 16) is a SNARE protein. SNARE proteins have been shown to be involved in early endosome and/or recycling endosome-to-TGN retrograde transport, as dominant-negative α-SNAP inhibited the transport of Shiga toxin B (STxB) subunit, a protein known to undergo retrograde transport to the TGN bypassing the late endosome (Barnard et al. [Citation1997]). Syn 16 was shown to be involved in this retrograde pathway, as antibodies targetted to Syn 16 inhibited STxB transport, as did knockdown of Syn 16 (Mallard et al. [Citation2002]). Given its role in endosomal transport and its localization at the TGN of the somatic Golgi, Syn 16 is a likely candidate to be found in Golgi outposts. Moreover, although ostensibly ubiquitous in the cell body Golgi of all cells, Syn 16 protein is found to be enriched in the brain. We provide evidence below to indicate that Golgi outposts do have an associated TGN component, which contains Syn 16. Since the somatic Golgi and the dendritic Golgi outposts have been shown to be important in the establishment of neuronal polarity as well as dendritic length, we also decided to look at how Syn 16 might be involved in the development of neuronal cells. We show that Syn 16 may have a role in the growth of neurites.
Materials and methods
Antibodies and markers for immunofluorescence and Western blotting
Rabbit antisera against Syn 16 were obtained after repeated immunization with a hexahistidine-tagged bacterial Syn 16 fusion protein. Antibodies for γ-tubulin (Sigma-Aldrich Pte. Ltd., Singapore), syntaxin 6 (Transduction Laboratories, San Diego, CA, USA), the dendritic marker MAP2 (AbCAM, Cambridge, UK), the oligodendrocyte marker CNPase (AbCAM, Cambridge, UK), the astrocyte marker glial fibrillary acidic protein (GFAP) (Sigma-Aldrich Pte. Ltd., Singapore), the Golgi marker GM130 (Sigma-Aldrich Pte. Ltd., Singapore) and the neuronal marker βIII-tubulin (TuJ) (Research Diagnostics Inc., Flanders, NJ, USA) were from the respective commercial sources. γ-tubulin antibodies were from Sigma-Aldrich Pte. Ltd. (Singapore). Antibodies against the vesicular stomatitis virus G protein (VSVG) were described previously (Mallard et al. [Citation2002], Wang et al. [Citation2005]).
Syn 16 silencing
Syn 16 knockdown was performed using a 27-mer double-stranded siRNA (1st-base Pte Ltd., Singapore). The following sequence was used: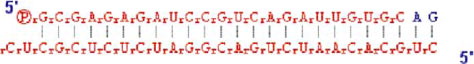
This sequence is shown in colour online in Molecular Membrane Biology online.
Transfection was performed using RNAiMax Lipofectamine (Invitrogen, Singapore) according to manufacturer's protocol.
Syn 16 mutant constructs
DNA manipulations were carried out according to established protocols (Ausubel et al. [Citation1993]). DNA polymerase chain reactions for generating Syn 16 mutant constructs were carried out using high fidelity Pfu polymerase (Promega, Madison, WI). 3 mutant constructs were made: ΔNt – lacking amino acid residues (aa) 1-50, ΔSNARE – lacking aa 247-292, and ΔCt – lacking aa 303-327. These correspond to the N-terminal Vps45-binding region, SNARE domain and transmembrane domain respectively.
Co-immunoprecipitation experiments
HEK293 cells were transfected with the various Syn 16 constructs for 24 hours. Before harvesting, cells were treated with 1mM NEM (Sigma-Aldrich, Pte. Ltd., Singapore) on ice for 15 min, then quenched with 2 mM DTT (Sigma-Aldrich, Pte. Ltd., Singapore) to inactivate NEM. Cells were returned to 37°C for 30 min then harvested. Lysates were incubated for 24 h at 4°C with rabbit Syn 16 polyclonal antibodies. Protein-A sepharose beads were added for 4 h at 4°C. Elution was performed in SDS-PAGE sample buffer and run on 10% SDS-PAGE gel before performing Western blot analysis for Syn 6.
Cell culture and manipulations
(a) Cultured cell lines
HEK293 cells were maintained in RPMI media supplemented with 10% FBS. HeLa and Neuro2a cells (kindly provided by Prof HP Too, National University of Singapore) were maintained in Dulbecco Modified Eagles (DME) culture media supplemented with 10% FBS.
(b) Primary cortical neuron culture
Mouse primary cortical neurons were harvested from E16 embryos and plated onto poly-L-lysine coverslips in Neurobasal medium supplemented with B27 (Invitrogen Singapore Pte. Ltd. (Singapore).
Transfections of constructs into cells were performed using Lipofectamine 2000 (Invitrogen Singapore Pte. Ltd., Singapore), or Effectene (Qiagen GmbH, Hilden, Germany), according to the manufacturers’ protocols.
Western immunoblot analysis
Cells/tissues were lysed in lysis buffer (50 mM Tris (pH 8), 10 mM EDTA and 150 mM NaCl with a protease inhibitor cocktail and 0.1 mM PMSF and 1% Triton X-100) for 1 h. Cell debri was cleared by centrifugation at 14000 rpm for 10 min. Debri from tissue lysates was further cleared by spinning at 50,000 g for 30 min. SDS-PAGE gel electrophoresis was performed on 10% gel for detection of Syn 16 or Syn 6. The membrane was incubated with primary antibody (1:1000 in 3% BSA) for 1 h, followed by secondary antibody for 1 h. Labeled protein bands were visualized using ECL Western blotting substrate (Pierce, Rockford, IL, USA). To normalize the protein loading, mouse monoclonal anti-γ-tubulin antibody (1:3000 in 3% BSA) was used to re-probe stripped membranes.
Neurite outgrowth assessments
(a) In Neuro2a cells
Neuro2a cells at ∼50% confluence were transfected with the Syn 16 constructs and induced to differentiate for 72 h in 1% FBS and 10 µM retinoic acid (Sigma-Aldrich Pte. Ltd., Singapore). Cells were then fixed and prepared for immunofluorescence analysis. Transfected cells were identified by their over-expression of the Syn 16 construct, while untransfected cells expressed the endogenous levels of Syn 16. Cells were co-stained with the neurite marker TuJ. Extent of neurite growth was assessed by the length of TuJ-positive neurites. Cells with at least one neurite greater than 2 cell body lengths were classified as having good neurite growth, while cells with neurites less than 2 cell body lengths were classified as having reduced neurite growth.
(b) In mouse primary cortical neurons
After 3 days of growth, primary cortical neurons were transfected with the Syn 16 constructs using Lipofectamine. At 7DIV cells were fixed and prepared for immunofluorescence analysis. Transfected cells were identified by their over-expression of the Syn 16 construct. Extent of neurite growth was determined by length of βIII-tubulin (TuJ)-positive neurites. Cells with their longest neuritis measuring more than 7 cell body lengths were classified as having good neurite growth, while cells with their longest neurites less than 7 cell body lengths were classified as having reduced neurite growth.
For Syn 16 expression silencing experiments, knockdown was performed on 6DIV cells. At 10DIV cells were fixed. The extent of dendrite growth in wells with Syn 16 siRNA and in control wells was determined by the length of the MAP2 positive dendrites. Cells with their longest dendrite at more than 5 cell body lengths were classified as having good dendrite growth, while cells with longest dendrites less than 5 cell body lengths were classified as having reduced dendrite growth.
Statistical analysis
Experiments were typically conducted in triplicates in three separate experiments. All transfected cells were counted and characterized for neurite growth, while the characteristics of untransfected cells were determined by a sampling of cells in 3 random fields per coverslip. Proportion of neurite growth was calculated by dividing the total number of counted cells with good neurite growth over the total number of counted cells. Comparison between the population of transfected and untransfected cells was made using 2-tailed hypothesis testing, with the null hypothesis that there is no difference between the sampled transfected and untransfected populations.
Transport assays
VSVG transport assay was performed in HeLa cells co-transfected with VSVG and the Syn 16 constructs suing Effectene. Cells were incubated at 40°C for 45 min then transferred to 32°C for 5 h. Localization of VSVG protein was identified using immunofluorescence with anti-VSVG antibody.
Immunohistochemistry and immunofluorescence microscopy
Immunohistochemistry was performed as described previously (Liu et al. [Citation2002]). 20 µm brain tissue sections of adult mice that were OCT (optimal cutting temperature)-embedded (Sakura Finetek USA Inc., Torrance, CA, USA), were made using a cryostat (Leica Mircosystems Singapore, Singapore).
Immunofluorescence microscopy of cultured cells and neurons was performed as described previously (Wang et al. [Citation2005]). Cells were fixed with 4% paraformaldehyde followed by sequential incubation with the primary antibodies and FITC or Texas red-conjugated secondary antibodies. Hoechst 33342 (Molecular Probes, Eugene, OR) was used to mark the nuclei. Fluorescence labeling was visualized using an Axiophot microscope (Carl Zeiss, Oberkochen, Germany) equipped with the Carl Zeiss 510 confocal imaging system. Images were collected in separate z sections and final images presented are typically from collapsed z stacks.
Results
Syntaxin 16 is enriched in neuronal dendrites and can be found in Golgi outposts
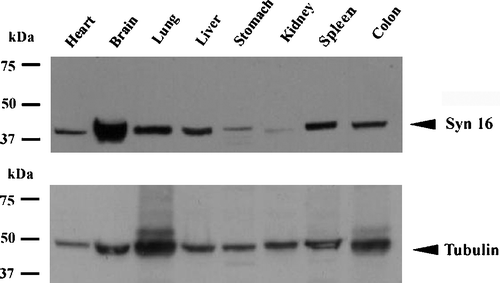
Syntaxin 16 (Syn 16) is a TGN-localized member of the soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) family (Mallard et al. [Citation2002], Wang et al. [Citation2005]). Together with other TGN SNAREs like syntaxin 6, it has been shown to mediate a retrograde transport route from the early/recycling endosome to the TGN (Mallard et al. [Citation2002]), as well as trafficking of the GLUT4 glucose transporter between the endosome and the TGN (Shewan et al. [Citation2003]). Although its function in regulating TGN protein traffic suggests that it is an essential protein and should therefore be ubiquitously present, a tissue survey by Western immunoblot analysis indicate that Syn 16 is surprisingly enriched in the brain ().
Figure 1. Expression of syntaxin 16 (Syn 16) in mouse tissues. 50 µg of detergent lysates of mouse tissues indicated were resolved on a 10% SDS-PAGE, electroblotted and probed with rabbit antibody against Syn 16 (Syn 16) and reprobed with a monoclonal antibody against γ-tubulin (Tubulin).
Immunofluorescence analysis of brain cryosections indicates that Syn 16 is mainly present in neurons, with low to undetectable levels in oligodendrocytes and astrocytes (). Furthermore, besides the expected perinuclear staining at the cell body, Syn 16 also appears to be present in neuronal fibers. Co-staining for the microtubule binding protein MAP2, a dendritic marker, confirmed Syn 16's presence in neuronal dendrites (, top panel). Syn 16's specific enrichment in dendrites is best illustrated by its distinct marking of the elaborate dendritic tree of Purkinje neurons (, bottom panel).
Figure 2. Expression of Syn 16 in different brain cell types. 30 µm cryosections of adult mouse brain were double-labeled with rabbit antibody against Syn 16 (FITC, green) and mouse monoclonal antibodies against neuronal (dendritic) marker MAP-2 (upper panel), the oligodendrocyte marker CNPase (middle panel) and the astrocyte marker GFAP (lower panel) (Texas Red, red). Scale bar = 20 µm.
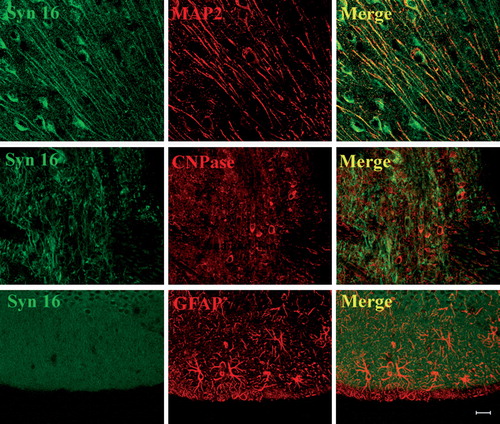
Figure 3. Syn 16 staining is specifically concentrated in dendrites. 30 µm cryosections of adult mouse brain were double-labeled with rabbit antibody against Syn 16 (FITC, green) and mouse monoclonal antibodies against neuronal (dendritic) marker MAP-2 (Texas Red, red). The upper panel illustrates an area densely populated with dendrite. The lower panel shows part of the cerebellum where Purkinje cell bodies and its dendritic tree projection into the molecular layer. Scale bar = 20 µm.
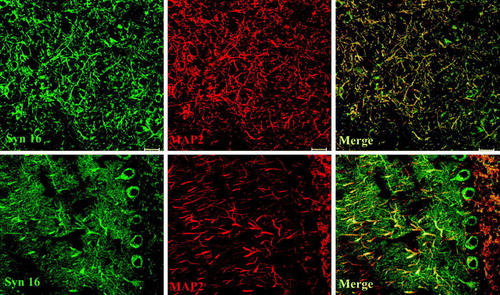
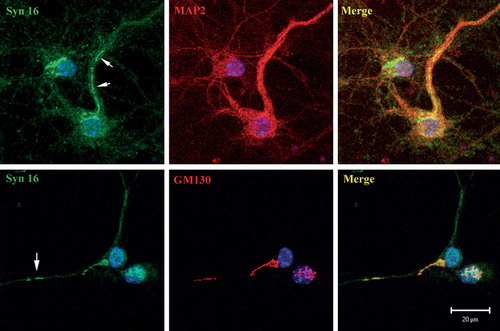
To better determine the subcellular localization of Syn 16, we examined primary cortical neurons in culture. Other than the perinuclear cell body Golgi staining seen in all neurons, Syn 16 could also be found on GM130 positive membrane structures protruding into a few major dendritic trunks that are isolated from the cell body Golgi apparatus (). Syn 16 is therefore present in the dendritic Golgi outposts. It is unclear from the labeling if significant amounts of Syn 16 could also be found on the dendritic plasma membrane. As Syn 16 is a tail-anchored, cytoplasmically oriented polypeptide, we were unable to perform cell surface labeling.
Figure 4. Syn 16 at the dendritic Golgi. Mouse cortical neurons at 8DIV were double- labeled with rabbit antibody against Syn 16 (FITC, green) and mouse monoclonal antibodies against neuronal (dendritic) marker MAP-2 (upper panel) or GM130 (bottom panel) (Texas Red, red). Arrows point to Syn 16 positive dendritic Golgi structures. Scale bar = 10 µm.
Syntaxin 16 and neurite outgrowth
We sought to investigate possible functions of Syn 16 in neurons. Polarized growth of neurites requires vesicular transport, and Syn 16's localization at the somatic TGN and Golgi outposts places it in a position to be involved in such polarized transport processes. Syn 16 has three distinct domains (Dulubova et al. [Citation2002]) – the Vps45-binding N-terminal domain, the SNARE domain involved in the formation of SNARE complexes with the cognate SNARE partners, and the membrane-anchoring transmembrane domain at the C-terminus. Deletion constructs for each of these different domains were generated (a). In order to verify the functionality of these deletion mutants, their ability to co-immunoprecipitate syntaxin 6, a cognate SNARE partner of syntaxin 16, was first examined. While the SNARE domain deletion mutant Syn 16-ΔSNARE is defective in co-immunoprecipitating syntaxin 6, the other two deletion mutants are comparable to wild type Syn 16 in terms of their capacity to interact with syntaxin 6 (b).
Figure 5. Syn 16 mutants inhibit neurite outgrowth of neuroblastoma cells and primary neurons. (a) Schematic diagrams of Syn 16 mutant constructs. Syn 16-ΔNt has the first 50 amino acids of the N-terminal, Vps45-interacting domain deleted. Syn 16-ΔSNARE has the SNARE domain deleted. Syn 16-ΔCt has the transmembrane domain (Tm) deleted. Amino acid numberings at the boundaries of the different domains are shown above. (b) Syn 16 antibodies were used to co-immunoprecipitate Syn 16 and its cognate SNARE partner syntaxin 6 in HEK293 cells over-expressing the Syn 16 constructs. Eluates were resolved on a 10% SDS PAGE gel, Western blotted, and probed with antibodies against syntaxin 6 (left panel). The band intensities were quantified by densitometry, normalized against the band intensity of the input lanes, and presented graphically (right panel) as the relative density of bands compared to that from endogenous Syn 16. (c) Graph showing the effect of Syn 16 construct expressions on neurite outgrowth in Neuro2a cells. Neurite outgrowth is expressed as mean±SD of the proportion of cells with their longest neurite being longer than 2 cell body lengths, over total number of cells counted. Untransfected, n=185; Wild type, n=164; ΔNt, n=143; ΔSNARE, n=165; ΔCt, n=183; ** indicates p<0.001. (d) Graph showing the effect of Syn 16 construct expressions on neurite outgrowth in mouse primary cortical neurons. Neurite outgrowth is expressed as mean±SD of the proportion of cells with their longest neurite being longer than 7 cell body lengths, over total number of cells counted. Untransfected, n=57; WT, n=61 ΔNt, n=52; ΔSNARE, n=41; ΔCt, n=59; **indicates p<0.001.
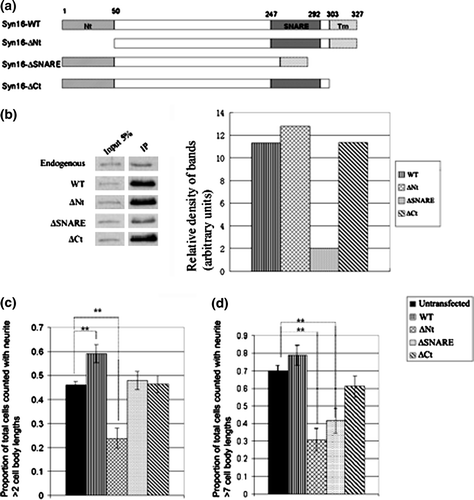
Using these deletion mutant constructs, we first asked if their over-expression may affect neurite outgrowth of Neuro-2a cells that are induced to morphologically differentiate in culture by retinoic acid. Antibodies against βIII-tubulin were used to label the neurites. As shown in c, over-expression of wild type Syn 16 appeared to moderately (and in a statistically significant manner) enhance neurite growth, whereas the Syn 16 mutant with its SNARE domain deleted (ΔSNARE) and one with its membrane spanning domain deleted (ΔCt) had no effect. Interestingly, the Syn 16 mutant with the first 50 amino acids deleted (ΔNt) (thereby rendering it unable to bind mammalian Vps45), significantly inhibited neurite outgrowth when expressed in Neuro2a cells.
To verify if the above observation holds true in a more physiologically relevant system, the Syn 16 wild type and mutant constructs were transfected into mouse primary cortical neurons. Neurite growth is assessed as above by βIII-tubulin labeling. Again there was moderate (albeit statistically insignificant) neurite growth enhancement by wild type Syn 16. Consistent with its effect on Neuro-2a cells, Syn 16-ΔNt also inhibited neurite outgrowth by primary cortical neurons (d). While Syn 16-ΔCt had no effect, Syn 16-ΔSNARE, to a lesser extent when compared to ΔNt, also inhibited neurite outgrowth in primary cortical neurons.
In view of Syn 16-ΔNt's potency in inhibiting neurite growth, we asked if over-expression of the mutant affected morphologies of the exocytic pathway or other cellular components that has to do with polarized membrane traffic. The mutant, when moderately expressed in HeLa, Neuro-2a cells or primary cortical neurons, assumed a normal Golgi-like localization as for wild type Syn 16 (See Supplementary , online version only).
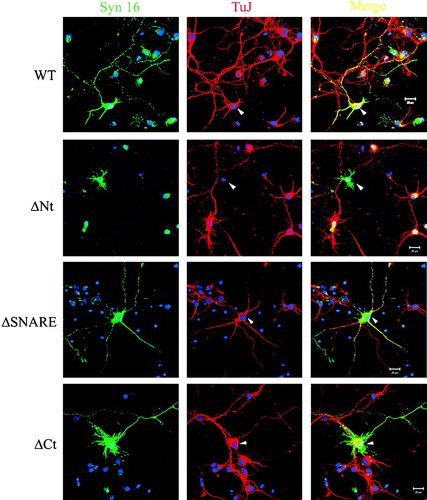
The unimpaired targeting of Syn 16-ΔNt, coupled to the fact that it could interact with the cognate SNARE partner syntaxin 6, suggests that it might act to antagonize the function of endogenous Syn 16 at the TGN. Cortical neurons over-expressing Syn 16-ΔNt had a reduced F-actin labeling by phalloidin (data not shown) and an almost complete loss of βIII-tubulin labeling (). This loss in βIII-tubulin, a neuron-specific isoform, is apparently specific, as a diminished labeling of β-tubulin was not similarly observed (See Supplementary , online version only). These latter observations suggest that Syn 16-ΔNt may inhibit neurite extension by affecting cytoskeletal organization required for polarized transport in neurons.
Figure 6. The effect of Syn 16-ΔNt over-expression on the integrity of neuronal specific βIII-tubulin. Primary cortical neurons transfected at 3DIV and fixed at 7DIV were labeled for Syn 16 (FITC, green) and βIII-tubulin (TuJ) (Texas Red, red). In neurons over-expressing wild type Syn 16, βIII-tubulin staining could be found in both the soma and the neurites (arrowheads, top panel, third panel and bottom panel). In contrast, neurons over-expressing Syn 16-ΔNt appear to have diminished βIII-tubulin staining (arrowhead, second panel). The arrows points to untransfected neighbors of the transfected cell. Scale bar = 20 µm.
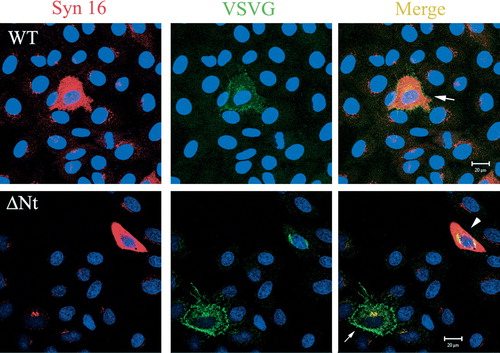
Inhibitory effects observed through the over-expression of dominant-negative mutants could be misleading as these may be due to a gain-of-function effect of the mutant. Indeed, other than the expected inhibition of Shiga toxin B transport to the TGN (data not shown), we have also observed that Syn 16-ΔNt over-expression inhibited TGN-to-surface transport of the vesicular stomatitis virus G protein (VSVG) (see Supplementary , online version only).
We thus sought to further confirm our observations with Syn 16-ΔNt by examining the effect of RNA interference-mediated silencing of Syn 16 expression on neurite outgrowth in primary cortical neurons. Labeling for βIII-tubulin stains all neurites, including both axons and dendrites. Since Syn 16 is enriched in dendrites and is found in dendritic Golgi outposts, we therefore chose to examine specifically the effect on dendrites, labeled by antibodies against MAP2. As shown in , Syn 16-specific siRNA introduced at 6DIV significantly reduced the dendrite length of cortical neurons compared to controls. To see if the effect of the Syn 16 siRNA is specific in inhibiting dendritic growth, and if axons are similarly disrupted, we examined the neurite by βIII-tubulin labeling. As shown in b, there is no shortening of the very long neurites, suggesting that axons were not affected by Syn 16 silencing. These results, taken together, suggest that Syn 16 may indeed have a role in neurite outgrowth, in particular during morphological development of dendrites.
Figure 7. Syn 16 silencing attenuates neurite outgrowth of primary cortical neurons. (a) Syn 16 silencing with siRNA: (i) Neuro-2A cells were either mock transfected, or transfected with either scrambled siRNA or Syn 16 siRNA. Syn 16 proteins levels were analyzed after 48 hours by Western immunoblot with Syn 16 antibody. The blot was reprobed with γ-tubulin to indicate normalized protein loading; and (ii) Mouse primary cortical neurons at 6DIV were transfected with either scrambled siRNA or Syn 16 siRNA. Syn 16 proteins levels were analyzed at 9DIV by Western immunoblot as above. (b) Dendritic outgrowth of primary cortical neurons (6DIV) transfected with scrambled or Syn 16 siRNA were assessed by MAP2 (left panels) and βIII-tubulin (TuJ) labelings. Cells were incubated for 48 h with the siRNA, the transfection media was then removed and the cells were incubated in normal media for another 48 h before fixation and immunofluoresence labeling. Scale bar = 50 µm. (c) Graph comparing the extent of dendrite growth in mouse primary cortical neurons transfected with scrambled (n=170) or Syn 16 siRNA (n=196). Dendrite outgrowth is expressed as mean±SD of the proportion of cells with their longest neurite being longer than 5 cell body lengths, out of total number of cells counted; **indicates p<0.001.
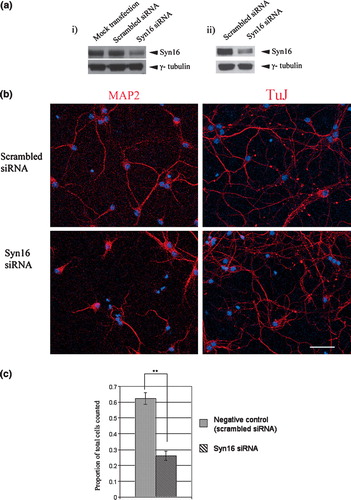
Figure S1. Localization of Syn 16 mutants in Neuro2a. Moderately expressed wild type (WT) Syn 16 (arrowheads, upper panel) and the Syn 16-ΔNt mutant (arrowheads, middle panel) colocalize with the TGN marker GM130 (Texas Red, red). This shows that the N-terminal Vps45-binding region is not necessary for the targeting of Syn 16 to the TGN, and that the Syn 16-ΔNt faithfully targeted to the TGN is in a good position to antagonize the function of endogenous Syn 16. In contrast, Syn 16 ΔCt is not localized to the TGN membrane but, rather, distributed throughout the cytoplasm. Scale bar = 20 µm.
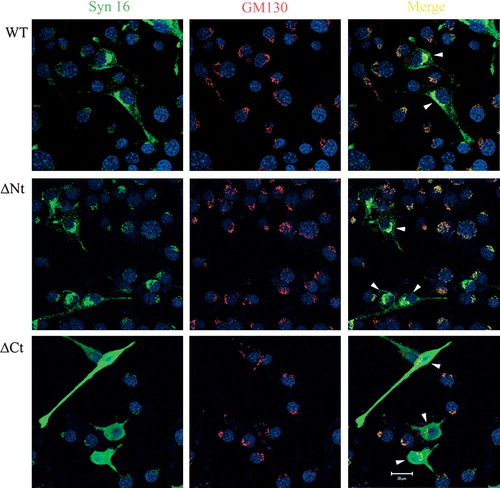
Figure S2. Over-expression of Syn 16 wild type and mutants did not disrupt β-tubulin staining. Primary cortical neurons transfected at 3DIV and fixed at 7DIV were labeled for Syn 16 (FITC, green) and β-tubulin (Texas Red, red). β-tubulin staining is not diminished in cells over-expressing either wild type Syn 16 (WT) or the other mutant constructs (arrowheads). Scale bar = 50 µm.
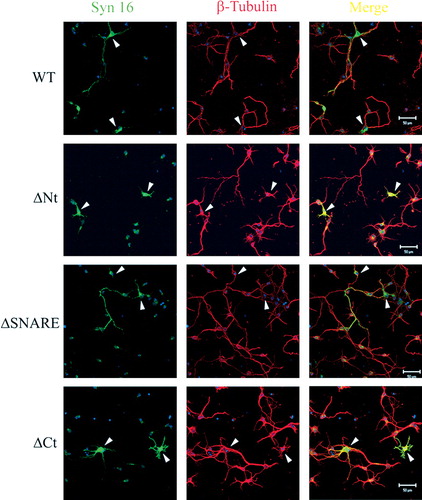
Figure S3. Effect of Syn 16-ΔNt on anterograde VSVG transport. In cells over-expressing the Syn 16 wild type (WT) protein, anterograde transport of VSVG to the cell surface is not significantly affected (arrow, top panel). In cells over-expressing Syn 16 ΔNt, a significant amount of VSVG remains in the perinuclear region (arrowhead, bottom panel). This Golgi/TGN retardation is not as severe in cells expressing only a low amount of Syn 16-ΔNt (arrow, bottom panel).
Discussion
Syn 16 in dendritic Golgi outposts
The demonstration of dendritic concentration of Syn 16 and its presence at dendritic Golgi outposts is conceptually significant in two ways. Firstly, it provides an explanation for the observed brain-enrichment of syntaxin 16. Secondly, it shows that dendritic Golgi outposts are endowed with a TGN component. This point is important because it is conceivable that Golgi outposts are only simplified versions of the Golgi apparatus at the soma, and has evolved for specific targeted anterograde delivery of proteins important for the function of the postsynaptic compartment. The presence of a TGN component at dendritic Golgi outposts therefore makes it more likely for these outposts to be morphologically and functionally equivalent to the Golgi apparatus at the cell body.
There are two known retrograde transport routes involving the TGN. The retrieval of mannose 6-phosphate receptor, for example, occurs via the late endosome. The retrograde transport of nontoxic B-subunit of Shiga toxin and furin and dynamic proteins such as TGN38, occurs directly from the early or recycling endosome to the TGN. This pathway is proposed to be important as it may allow signaling molecules such as CD19, interferon α/β receptors or GPI-anchored receptors such as CD14 to avoid degradation by late endosomes to reach sites where they can interact with their intracellular targets (Dulubova et al. [Citation2002]). Syn 16 has a known role in the early/recycling endosme – TGN retrograde transport. Thus, it may now be appropriate to speculate that dendritic Golgi outposts may also receive cargo from dendritic plasma membranes or dendritic early/recycling endosomes. The presence of the latter, in the form of tubular endosomes and multivesicular bodies (MVBs) has been well-documented (Cooney et al. [Citation2002], Bonifacino & Rojas [Citation2006]). This localized retrograde traffic may serve multiple purposes, for example for the redirection of certain proteins to specific subdomains of the dendrite, such as the spine or to the cell body.
Syn 16 in neurite outgrowth
In probing the function of Syn 16 in neurons, we observed that the Syn 16-ΔNt mutant, which is unable to bind to Vps45, significantly inhibited neurite outgrowth. This effect appears rather specific as wild type Syn 16 itself had a moderate neurite growth-enhancing effect while the presumably soluble Syn 16-ΔCt had no such effect. The inhibitory activity of Syn 16-ΔNt may be dependent on its correct TGN membrane localization (as compared to the largely cytoplasmic Syn 16-ΔCt). Over-expression of the mutant appears to alter βIII-tubulin-based cytoskeletal organization in neuronal cells, resulting in diminished F-actin and βIII-tubulin labeling. It is conceivable that Syn 16-ΔNt may antagonize a function of endogenous Syn 16 that mediates neurite outgrowth, with the latter either participating in a previously undescribed process of TGN-plasma membrane anterograde traffic, or an endosome-TGN retrograde transport process. The molecular basis for the connection of Syn 16-ΔNt to the cytoskeleton, however, remains to be further investigated.
It is possible that high levels of Syn 16-ΔNt at the TGN may actually be acting in a gain-of-function manner to inhibit polarized trafficking from the TGN to the plasma membrane. This is corroborated by our previous observation that Syn 16 silencing, presumably a milder intervention that would reveal only loss-of-function phenotypes, did not significantly affect VSVG cell surface transport in HeLa cells (Wang et al. [Citation2005]). However, our current observation with Syn 16 silencing by siRNA suggests that a loss of Syn 16 function could significantly attenuate neurite outgrowth in primary neurons. It is therefore likely that loss of Syn 16 function may specifically affect certain cargo molecules that are important for polarized neurite growth, particularly that of dendritic extension.
In summary, we have observed an interesting enrichment of Syn 16 in neurons, and our functional studies suggest that one possible role for this SNARE molecule in neurons is the regulation of neurite extension. Syn 16 may have other yet undefined roles, particularly at the dendrite, and pertaining to dendritic endosome-TGN processes. These remain to be explored in future work.
Acknowledgements
We thank Dr Ya Wang for the Syn 16 antibody, and other members of the laboratory for various reagents. The authors declare no commercial conflict of interest.
References
- FM Ausubel, Brent, R, Kingston, RE, Moore, DD, Seidman, JG, Smith, JA, Struhl, K, 1993. Current protocols in Molecular Biology. New York: Green Publishing and Wiley interscience.
- Aridor M, Guzik AK, Bielli A, Fish KN. Endoplasmic reticulum export site formation and function in dendrites. J Neurosci 2004; 24: 3770–3776
- Barnard RJ, Morgan A, Burgoyne RE. Stimulation of NSF ATPase activity by alpha-SNAP is required for SNARE complex disassembly and exocytosis. J Cell Biol 1997; 139: 875–883
- Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 1997; 91: 253–262
- Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol 2006; 7: 568–579
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron 2000; 26: 465–472
- Craig AM, Banker G. Neuronal polarity. Ann Rev Neurosci 1994; 17: 267–310
- Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J Neurosci 2002; 22: 2215–2224
- Dulubova I, Yamaguchi T, Gao Y, Min SW, Huryeva I, Sudhof TC, Rizo J. How Tlg2p/syntaxin 16 ‘snares’ Vps45. EMBO 2002; 21: 3620–3631
- Gardiol A, Racca C, Triller A. Dendritic and postsynaptic protein synthetic machinery. J Neurosci 1999; 19: 168–179
- Horton AP, Ehlers MD. Neuronal polarity and trafficking. Neuron 2003a; 40: 277–295
- Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci 2003b; 23: 6188–6199
- Horton AC, Ehlers MD. Secretory trafficking in neuronal dendrites. Nat Cell Biol 2004; 6: 585–591
- Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 2005; 48: 757–771
- Juttner R, Rathjen FG. Molecular analysis of axonal target specificity and synapse formation. Cell Mol Life Sci 2005; 62: 2811–2827
- Liu H, Ng CE, Tang BL. Nogo-A expression in mouse central nervous system neurons. Neurosci Lett 2002; 328: 257–260
- Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, Antony C, Hong W, Goud B, Johannes L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol 2002; 156: 653–664
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol 1995; 131: 1715–1726
- Nilsson T, Lucocq JM, Mackay D, Warren G. The membrane spanning domain of beta-1,4-galactosyltransferase specifies trans Golgi localization. EMBO J 1991; 10: 3567–3575
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science 2004; 305: 1972–1975
- Pierce JP, Mayer T, McCarthy JB. Evidence for a satellite secretory pathway in neuronal dendritic spines. Curr Biol 2001; 11: 351–355
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 2000; 288: 1796–1802
- Shewan AM, van Dam EM, Martin S, Tang BL, Hong W, Bryant NJ, James DE. GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif. Mol Biol Cell 2003; 14: 973–986
- Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad Sci USA 2001; 98: 7062–7068
- Tang BL. Protein trafficking mechanisms associated with neurite outgrowth and polarized sorting in neurons. J Neurochem 2001; 79: 923–930
- Wang Y, Tai G, Lu L, Johannes L, Hong W, Tang BL. Trans-Golgi network syntaxin 10 functions distinctly from syntaxins 6 and 16. Mol Membr Biol 2005; 22: 313–325
- Welte MA. Bidirectional transport along microtubules. Curr Biol 2004; 14: 525–537