Abstract
Aminopeptidase N (APN/CD13) is a 150 kDa membrane-bound ubiquitously expressed protease with a broad functional repertoire. It hydrolyzes small peptide mediators, modulates cell motility and adhesion to extracellular matrix and also acts as a viral receptor. In order to dissect the function of enzymatically active and inactive APN/CD13, substitutions of different enzymatic active amino acid residues were generated by site-directed mutagenesis and stably transfected into human embryonic kidney cells. All APN variants analyzed exhibited a complete loss of enzymatic activity, whereas wild type APN transfectants exerted a strong aminopeptidase-specific activity. Furthermore, wild type APN expression was associated with a significant decrease in proliferation, migration and also reduced anchorage-independent growth when compared to enzymatically inactive APN variants and controls. This appeared to be due to a downregulated mRNA and protein expression of the chemokine receptor CXCR4 and an inhibition of the stromal cell-derived factor (SDF)-1α/CXCL12-mediated migration. Thus, high APN enzyme activity may antagonize the cellular properties regulated by the CXCR4/SDF-1α system in embryonic kidney cells.
| Abbreviations | ||
| Ala-pNA | = | alanine-p-nitroanilide |
| APN | = | aminopeptidase N |
| DPIV | = | dipeptidyl peptidase IV |
| ECL | = | enhanced chemiluminescence |
| EGFP | = | enhanced green fluorescence protein |
| FACS | = | fluorescence activated cell sorter |
| FBS | = | fetal bovine serum |
| G-CSF | = | granulocyte colony stimulating factor |
| HRP | = | horseradish peroxidase |
| JAK/STAT3 | = | Janus kinase/signal tranducer and activator of transcription 3 |
| MAPK | = | mitogen-activated protein kinases |
| HPRT1 | = | hypoxanthine guanine phosphoribosyl transferase 1 |
| mAb | = | monoclonal antibody |
| MFI | = | mean specific fluorescence intensity |
| PAGE | = | polyacrylamide gel electrophoresis |
| PBS | = | phosphate-buffered saline |
| RT-PCR | = | reverse transcriptase-polymerase chain reaction |
| PE | = | phycoerythrin |
| SDF-1 | = | stromal cell-derived factor-1 |
| SDS | = | sodium dodecyl sulfate |
| wt | = | wild type |
Introduction
Aminopeptidase N (APN)/CD13 is a Zn2 + -dependent ectopeptidase of the superfamily of gluzincins with a molecular weight of approximately 150 kDa (Hooper [Citation1994]). The enzyme hydrolyzes oligopeptides and cleaves preferentially neutral amino acids from the N-terminus of various substrates (Turner [Citation1998]). APN is expressed on the surface of a broad variety of cell types including fibroblasts, endothelial cells, committed haematopoietic progenitors, osteoclasts, synaptic membranes of the central nervous system, epithelial cells of the placenta, intestine and kidney as well as on tumors of distinct origin (Look et al. [Citation1989]).
Regarding its structure, APN belongs to the type II membrane proteins with a single-spanning helical transmembrane domain and a short cytoplasmic tail. It is a highly glycosylated protein with ten potential glycosylation sites in the large extracellular domain which contains the catalytic active site with the conserved pentapeptide consensus sequence HELAH representing a zinc binding motif (Vallee & Auld [Citation1990]). The lack of a 39 amino acid segment including the HELAH motif leads to an intracellular retardation and a complete loss of enzymatic activity (Ashmun et al. [Citation1992]). Substitution of the Glu350 residue of the additional consensus sequence GAMEN demonstrated the crucial role of this amino acid for the exopeptidase function of pig APN (Luciani et al. [Citation1998]). The involvement of this glutamate in the catalytic process of aminopeptidase A has also been described (Vazeux et al. [Citation1996]).
APN plays diverse tissue-specific roles and modulates immune responses and inflammation (Riemann et al. [Citation1999]). The enzyme is involved in cell cycle control, cell differentiation, cell motility, degradation of the extracellular matrix, cellular attachment and in the function of immune cells, e.g., by trimming peptides protruding out of the binding groove of MHC class II molecules (Larsen et al. [Citation1996]). In pathophysiological processes, APN serves as a receptor for various viruses, such as the cytomegalovirus (Soderberg et al. [Citation1993]) and different coronaviruses (Delmas et al. [Citation1992], Yeager et al. [Citation1992]). Furthermore, its expression has been associated to tumor angiogenesis and the metastastic potential of tumors (Bauvois [Citation2004], Bhagwat et al. [Citation2001], Fujii et al. [Citation1995]).
The biological activity of APN is not only achieved by the cleavage of peptides, such as neuropeptides, vasoactive peptides, chemokines and growth factor molecules, but also by participation in signal transduction processes (Riemann et al. [Citation2002]). The involvement of membrane peptidases in multimeric protein complexes and their presence in membrane microdomains like rafts and caveolae is a prerequisite for their signaling capacity. Since there exists limited and controversial information on APN function related to its enzymatic activity, the expression pattern, enzyme activity and the biological function of wild type APN (wt-APN) was compared to that of defined APN mutants with different substitutions in the enzymatic active site. Due to the different migration capacity of the APN mutants, the involvement of chemokine receptors and their ligands was also determined.
Materials and methods
Plasmid construction
The full-length cDNA of the human wild type APN (wt-APN) was isolated from total cellular RNA of U937 cells using the APN-specific primers 5′-ATATTTGAGCTCATGGCCAAGGGCTTCTATATTTCCAAGTC-3′ (forward) and 5′-ATATTTGTCGACATTTTGCTGTTTTCTGTGAACCACTGGA-3′ (reverse). The cDNA was cloned into the Sac I and Sal I restriction sites of the mammalian expression vector pEGFP-N1 (Clontech Laboratories Inc., Mountain View, CA, USA), which results in a C-terminal tagging of APN with the enhanced green fluorescent protein (EGFP). APN mutants with modification of the enzymatic active site such as E354G, E354I, E354Q, H387/391C, H387/391I, E388G and E410G, respectively, were generated with the PCR-based Quik Change XL Site-directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) according to the instruction manual. The primers used for cloning and their experimental conditions are listed in the (online version only). In addition, a schematic illustration of the positions of the amino acid substitutions is shown in the (online version only). The integrity of the vector was confirmed by DNA sequencing.
Cell culture, transfection and selection of clones
The human embryonic kidney cell line HEK293 (Deutsche Sammlung fuer Mikroorganismen und Zellkulturen (DSMZ) ACC 305, Braunschweig, Germany) was maintained in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 10 mM HEPES and antibiotics. The different APN plasmids and pEGFP-N1 serving as mock control were stably transfected into HEK293 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. APN-expressing cells were selected in RPMI1640 medium supplemented with 500 µg/ml G-418 (Invitrogen) 48 h post transfection for a period of 2 weeks. Individual single cell clones have been isolated by cell sorting (FACS Vantage, BD Biosciences, San Jose, CA, USA) after staining for APN expression.
Determination of APN enzymatic activity
For protein extracts cell pellets were resuspended in ice-cold 50 mM Tris/HCl (pH 7.4) containing 0.5% Triton X-100. After 1 h incubation on ice cells were disrupted using ultrasonication and debris was removed by a centrifugation step (10,000 g, 4°C). Protein concentrations were determined using the bicinchoninic acid (BCA) method with bovine serum albumine (BSA) as a standard (Smith et al. [Citation1985]). Total cellular APN activity was determined using 8 µg of isolated total protein extracts, whereas surface APN activity was measured employing 5×104 cells. The APN substrate alanine-p-nitroanilide (Ala-pNA; Sigma, St. Louis, MO, USA) was added at various concentrations ranging from 40 µM to 4 mM. The incubation was carried out at 37°C for 15 min in PBS. Ala-pNA cleavage was analyzed by the determination of the hydrolysis of Ala-pNA reflecting the p-nitroaniline concentration in the supernatant at an OD of 405 nm using the ELISA reader MRX II (Dynex Technologies GmbH, Berlin, Germany) (Firla et al. [Citation2002]). Cell-free and enzyme-free supernatants served as controls. The specificity of the Ala-pNA cleavage was monitored by preincubation of cells with 10 µM of the inhibitor actinonin (Sigma, St. Louis, MO, USA) for 30 min at 37°C before adding the substrate (Tieku & Hooper [Citation1992]). All tests were run in duplicates and independently repeated three times. Enzyme rates were used for the determination of the maximal turnover rate by direct fitting to the Michaelis-Menten equation. Statistical analyses were performed using the SigmaPlot software. Enzyme activities were expressed as µmol×min−1×10−6 cells or µmol×min−1×mg−1.
RNA isolation and real time quantitative RT-PCR
Total cellular RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. After DNase I (Invitrogen) treatment, 0.5 µg total RNA was transcribed into cDNA using the RevertAid H Minus First Strand cDNA Synthesis kit (MBI Fermentas, Hanover, MD, USA) and oligo(dT)18 primer at a final volume of 20 µl.
Quantification of gene expression was performed by real time quantitative RT-PCR on the Rotor-Gene 2000 system (Corbett Research, Sydney, Australia) employing the QuantiTect SYBR Green PCR kit (Qiagen) and specific primers listed in the (online version only). Real time quantitative RT-PCR amplifications were performed in a final volume of 20 µl at 95°C for 15 min followed by 40 cycles with denaturation at 95°C for 30 s, annealing at 58°C for 30 s and elongation at 72°C for 30 s. PCR products were finally subjected to a melting curve analysis. The mRNA levels were quantified with the Rotor-Gene analysis software in comparative quantitation mode and normalized to hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1) expression levels. All real time quantitative RT-PCRs were done at least three times using RNA from independent experiments.
Western blot analysis
1×106 cells were lysed in RIPA buffer containing protease and phosphatase inhibitor cocktails (Sigma, St. Louis, MO, USA). 25 µg protein cell extract were separated on 8% SDS-polyacrylamide gels and transferred to nitrocellulose membrane (Schleicher & Schuell Bioscience GmbH, Dassel, Germany) using a semi-dry Fastblot™ apparatus (Biometra, Goettingen, Germany). After blocking with 5% dry milk powder in TBS-T buffer for 1 h, the nitrocellulose membrane was incubated with the primary antibodies anti-CD13 monoclonal antibody (mAb) (clone BF-10), anti-GFP mAb (clone B-2), both purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) or the anti--actin-specific mAb (clone 9A1; Abcam Inc., Cambridge, MA, USA), respectively, at 4°C overnight. After three washing steps with TBS-T buffer, blots were incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (DAKO, Hamburg, Germany) at room temperature for 2 h. The membranes were developed employing the enhanced chemiluminescence (ECL)-based system (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA).
Flow cytometry
For flow cytometric analysis, 5×105 cells were stained either with specific phycoerythrin (PE)-coupled mAb, listed in the (online version only), or with the appropriated isotype controls at room temperature in the dark for 20 min. For CCR10 immunostaining, cells were first incubated with unlabeled primary antibody or isotype control at 4°C for 30 min followed by an incubation with PE-coupled goat F(ab’)2 anti-rabbit IgG at 4°C for additional 30 min. After fixation with 2% paraformaldehyde/phosphate-buffered saline (PBS) for 10 min, PE-fluorescence intensity was determined with the FACS Calibur flow cytometer and CellQuest software (BD Biosciences, Heidelberg, Germany). The results are expressed both as the percentage of expression and as mean specific fluorescence intensity (MFI) of FL2 of three independent experiments.
Cell growth assay
Cell proliferation was determined using the XTT-based colorimetric assay (Cell Proliferation Kit II (XTT), Roche Applied Science, Mannheim, Germany). Briefly, 7.5×103 cells/well were seeded into 96-well micro plates. Cell growth was monitored for the indicated time points by incubation with XTT labeling mixture for 4 h and spectrophotometrically assayed at a wavelength of 492 nm vs. 630 nm. The results of the XTT assay are presented as absorbance at 492 nm. All experiments were performed in triplicates with three independent assays.
Soft agar growth
Anchorage-independent growth was determined by the ability of the transfected cells to form colonies in soft agar as previously described (Courtenay [Citation1976]). Briefly, 2×104 cells/60 mm petri-dish were seeded into 0.3% agar on top of a 0.5% agar base layer, both containing 20% FBS-supplemented RPMI1640. The cultures were maintained in humidity at 37°C with an atmosphere of 5% CO2 for 18 days. The soft agar colonies were visualized by staining each petri-dish with 500 µl iodo-nitrotetrazoliumchloride (5 mg/ml, Sigma, St. Louis, MO, USA) overnight at 37°C and microscopically quantified.
Migration assay
Migration assays were performed in transwell diffusion chambers (Corning Costar, Corning, NY, USA) with a pore size of 8 µm diameter as recently described (Kehlen et al. [Citation2004]). 600 µl RPMI1640 medium containing 10% FBS, 2 mM L-glutamine, 10 mM HEPES and antibiotics as well as Collagen type I (8 µg/ml medium, BD Biosciences, Heidelberg, Germany) were added to the bottom of the 24-well plate. 5×104 cells were inserted into the top chamber. To analyze CXCR4-specific migration, different concentration of recombinant SDF-1α (PeproTech, Rocky Hill, NJ, USA) and of CXCR4-specific inhibitor AMD3100 (Sigma) were added to the system. After 22 h at 37°C, all cells remaining in the transwell chamber were removed by scratching with cotton swabs. The number of migrated cells was determined with the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA) according to the manufacturer's protocol. Luminescence was measured with a Lumat LB 9507 luminometer (Berthold Technologies, Bad Wildbad, Germany). Assays were run in duplicate in at least three independent experiments and results are expressed as percentage of migrated cells compared to the total cell number.
Results
Generation of APN variants with lack of enzymatic activity
Various APN variants with distinct substitutions in the catalytic domain of the enzyme differentially affecting the catalytic substrate cleavage were generated by site-directed mutagenesis: (i) the glutamate residue at the amino acid position 354 in APN known to interact with the free N-terminus of substrates or inhibitors and to stabilize the transition state was mutated to neutral glycine, hydrophobic isoleucine or uncharged, polar glutamine, (ii) the histidines at positions 387 and 391 in the HELAH motif of APN as well as the glutamate at the amino acid position 410 coordinately binding the zinc ion were substituted either by the hydrophobic isoleucine, by cysteine (aa 387/391) or by glycine (aa 410) and (iii) the cleavage-associated glutamate E388 was altered to glycine.
Three independent clones of transfectants and parental HEK293 cells were analyzed for APN expression by real time quantitative RT-PCR, Western blot and/or flow cytometry. As representatively shown for APN-E388G, the APN variants exhibited high APN mRNA and protein levels, which were comparable to that of wt-APN clones ( A, B). With the exception of the variant APN-H387/391I, a CD13 cell surface expression was found in all APN variants as determined by staining with a PE-labeled anti-CD13-specific mAb (). The mock transfectants expressed high levels of intracellular GFP, which interfered with the PE-fluorescence channel. Glycosidase digestion experiments and immunoelectrophoretic blot analysis under reducing conditions demonstrated that most APN variants were present as a 180 kDa polypeptide with a complex glycosylation pattern similar to that of wt-APN. Only the variant APN-H387/391I exhibited a mannose-rich glycosylated protein with a molecular weight of 150 kDa as a result of intracellular retardation in the endoplasmic reticulum (data not shown).
Figure 1. APN expression patterns of representative wt and mutant APN transfectants and respective controls. (A) Determination of the APN mRNA expression. mRNA of cell clones was quantified by real time quantitative RT-PCR (each in triplicate experiments) as described in Materials and methods. The results are expressed as relative APN mRNA expression. HEK293 parental cells are set to 1. (B) APN protein expression in APN transfectants. Equal amounts of proteins were resolved by SDS-PAGE and analyzed by Western blot using APN- and GFP-specific antibodies. Staining of the Western blot with an anti-ß-actin mAb served as loading control. (C) Cell surface enzyme activity of APN transfectants. The enzyme activity of HEK293 cells expressing different APN forms was determined by Ala-pNA cleavage as described in Materials and methods. Results are expressed as µmole×min−1×10−6 cells.
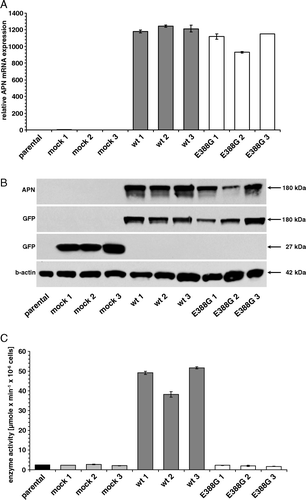
Table I. APN cell surface expression and the enzymatic activity of parental HEK293 cells and transfectants as well as cell extracts.
The enzymatic activities of the APN variant expressing HEK293 cells and their respective controls as well as the corresponding cell extracts were analyzed employing different concentrations of Ala-pNA as substrate. Wt-APN exhibited high levels of substrate cleavage of living cells as well as in total cell extracts with a clonal variation between 97–114 µmole×min−1×10−6 cells and 40–55 µmole×min−1×mg−1, respectively, whereas all APN variants exerted an approximately 20-fold and 50-fold reduced APN-specific activity with a residual cleavage of the Ala-pNA substrate comparable to that of the parental HEK293 cells and mock controls (; C and supplementary Figure I – online version only). The preincubation of cells with the aminopeptidase-specific inhibitor actinonin resulted in a complete inhibition of Ala-pNA cleavage demonstrating the specificity of this assay (data not shown). Since with the exception of APN-H387/391I all other enzymatically inactive APN variants were expressed on the cell surface, APN surface expression is independent of APN enzyme activity.
Functional role of APN enzyme activity
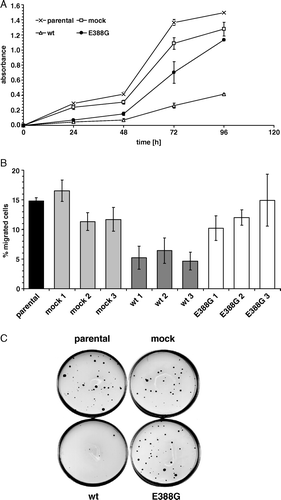
It has been demonstrated that APN overexpression is associated with cell growth and migration capabilities (Kehlen et al. [Citation2003], van Hensbergen et al. [Citation2004]). In order to determine whether enzyme activity is involved in these physiological processes, three individual clones of wt-APN, APN-E388G, vector control as well as parental HEK293 cells were selected for further characterization. Overexpression of wt-APN, but not of APN-E388G caused a significant downregulation of the proliferation rate when compared to controls as determined by XTT assay (A). This reduced cell growth was accompanied by a diminished migration capacity of wt-APN that was approximately 40% of the migration rate of APN-E388G transfectants (B). In addition, the anchorage-independent growth of wt-APN was significantly inhibited when compared to parental HEK293 cells, mock controls and APN-E388G transfectants with a 90% downregulation in the number of soft agar colonies (C).
Figure 2. Cell growth and migration are dependent on APN enzyme activity. (A) Cell proliferation of APN transfectants. Proliferation was measured using a XTT-based colorimetric assay (absorbance at 492 nm vs. 630 nm in time-dependence). One out of four representative experiments is shown. (B) Decreased migration properties of wt-APN transfected cells. Cell migration of APN transfectants and respective controls was assessed in transwell diffusion chambers. After 22 h of chemotaxis to collagen type I, total migrated cells were quantified by a luciferase-based system. Values are given as percentage of migrated cells compared to the number of cells employed. Data are pooled from three independent experiments. (C) Soft agar colony formation. Anchorage-independent growth of APN transfectants was determined in soft agar. Double layer agar cultures in 60-mm petri-dishes were established as described in Materials and methods. 2×104 cells were cultured in humidity at 37°C for 18 days. After staining of viable cells with iodo-nitrotetrazoliumchloride overnight, colonies were microscopically counted. A representative staining pattern of APN transfectants is shown.
Association of chemokine receptor expression with APN expression
Since chemokine receptor expression is involved in migratory and metastatic processes, it was determined whether there exists a link between APN and chemokine receptor expression. First, the mRNA expression pattern of ten different chemokine receptors was analyzed in wt-APN and APN-E388G transfectants as well as mock controls and parental HEK293 cells using real time quantitative RT-PCR ().
Table II. Relative mRNA and cell surface expression of different chemokine receptors in HEK293 cells and transfectants
Supplementary Table I. Primer sequences used for real time quantitative RT-PCR and antibodies for flow cytometry.
Supplementary Table II. Primer sequences used for real time quantitative RT-PCR and antibodies for flow cytometry.
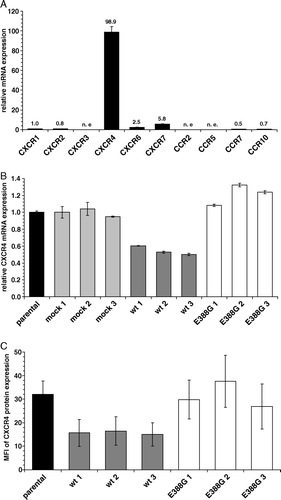
As shown in A, the mRNA expression of the chemokine receptors analyzed was extremely heterogeneous in HEK293 cells ranging from a total lack of CXCR3, CCR2 and CCR5, to low expression levels of CXCR1, 2, 6 and 7 as well as CCR7 and 10 and high expression levels of CXCR4. A distinct chemokine receptor expression was also detected in wt-APN and the APN variant APN-E388G. CXCR1 and CCR10 were upregulated on the cell surface of wt-APN transfectants, but not on APN-E388G cells. In contrast, wt-APN transfectants exhibited a strong downregulation of the CXCR4 mRNA and cell surface expression, whereas APN-E388G transfectants expressed CXCR4 mRNA levels comparable to that of parental HEK293 cells (B, C). It is noteworthy that CXCR2, 6, 7 and CCR7 were not differentially expressed in both transfectants. These data suggest an association between APN enzyme activity and CXCR4 expression. The wt-APN-mediated CXCR4 downregulation was independent of the CXCR4 ligand, the stromal cell-derived factor (SDF)-1α/CXCL12 since neither mRNA nor protein expression was detected by RT-PCR and a SDF-1α-specific ELISA, respectively (data not shown).
Figure 3. The major expressed chemokine receptor CXCR4 is downregulated in wt-APN transfectants on mRNA and protein levels. (A) mRNA expression levels of different chemokine receptors in HEK293 cells using real time quantitative RT-PCR analysis. Values are given as X-fold mRNA expression with CXCR1 expression set to 1. (B) mRNA from parental HEK293 cells as well as transfectants was subjected to real time quantitative RT-PCR analysis using CXCR4-specific primers. Gene expression levels were normalized to HPRT1 expression and then displayed as X-fold mRNA expression with HEK293 parental cells set to 1. (C) CXCR4 cell surface expression of controls and APN transfectants. Cells were detached and directly stained with PE-coupled anti-CXCR4-specific mAb. After fixation with paraformaldehyde PE-fluorescence intensity of cells was determined by flow cytometry. Values are expressed as mean specific fluorescence intensity (MFI) of FL2. Results are shown as average of three independent experiments.
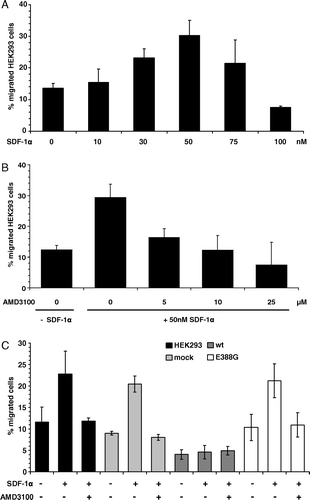
In order to determine whether CXCR4 is responsible for the altered properties, the migration rates of HEK293 parental cells in the presence of different concentrations of recombinant SDF-1α were investigated. A dose-dependent correlation between SDF-1α and migration was observed reaching a maximum with 50 nM SDF-1α (A). The SDF-1α-induced migration was selectively blocked by the CXCR4-specific inhibitor AMD3100 in a dose-dependent manner (B). APN-E388G transfectants as well as mock control cells exhibited similar migration properties as HEK293 cells with a two-fold increase in the presence of 30 nM SDF-1α and an inhibition of migration by treatment with 25 µM AMD3100. In contrast, no effect of SDF-1α on the migration rates of wt-APN transfectants could be demonstrated (C).
Figure 4. HEK293 cells migrate specifically to SDF-1α and APN overexpression inhibits this migration capability. (A) HEK293 cells migrate to recombinant SDF-1α in a dose-dependent manner. Different concentrations of recombinant SDF-1α were used as a chemoattractant in the migration assay as described in Materials and methods. Values are given as percentage of migrated cells compared to the total number of cells employed. (B) SDF-1α-induced migration of HEK293 cells can be blocked by the CXCR4-specific inhibitor AMD3100. Different concentrations of AMD3100 were used in combination with 50 nM SDF-1α in migration assay as described above. (C) Wt-APN expression blocks SDF-1α-induced migration. Wt-APN and APN-388G transfectants as well as vector control cells were analyzed for CXCR4-specific migration using 50 nM SDF-1α in presence or absence of 25 µM AMD3100.
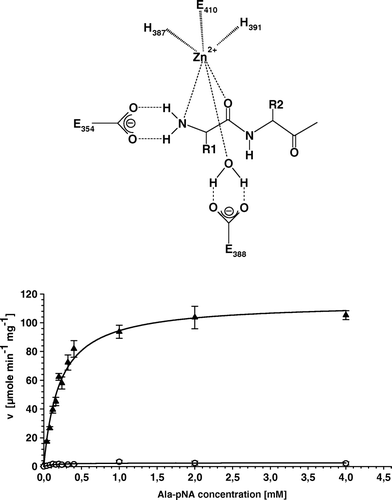
Supplementary Figure 1. All amino acid substitutions in the enzymatic active site of APN cause a complete loss of APN-specific activity. (A) Schematic illustration of the initial step of peptide cleavage by APN. Histidines H387 and H391 as well as glutamate E410 complex the zinc ion. E354 is involved in the polarization of the amino terminus of peptides, whereas E388 polarizes a water molecule, which attacks the carboxyl group of peptide bond. (B) Kinetic plot of cell extracts of APN-WT and APN-E388G expressing transfectants using Ala-pNA as substrate. Replicate determination for APN-WT (▴) and E388G (○) using different substrate concentration and enzyme activities are directly fitted to Michaelis-Menten curves. The curve of the APN-E388G transfectants is characteristic for the other variants exhibiting loss of enzymatic activity.
Discussion
So far, many studies implemented to define APN functions used either microbial aminopeptidase-specific inhibitors, such as bestatin, probestin and actinonin, or the enzyme activity-inhibiting mAb clone WM15 (Marotti et al. [Citation2000], Gabrilovac et al. [Citation2004], Bauvois & Dauzonne [Citation2006]). However, these experimental approaches did not lead to a proper dissection between signals obtained by APN inhibition and signals obtained after ligation of the APN molecule independent of the enzyme activity. For example, incubation with the mAb WM15 provokes a calcium increase, phosphorylation of the mitogen-activated protein kinases (MAPK) Erk, JNK and p38 as well as an upregulation of IL-8 mRNA in monocytic cells (Santos et al. [Citation2000]), whereas the inhibitor bestatin accesses into the cell via a H+-coupled energy-dependent dipeptide transporter (Lee [Citation2000]), which affects the protein kinase C pathway (Kumano & Sugawara [Citation1992]) and the pp60/c-Src tyrosine kinase activity (Murata et al. [Citation1994]). Based on this knowledge, the development of an inhibitor- and antibody-free system is required to characterize the involvement of APN enzyme activity in cellular functions. Therefore, various APN variants with different substitutions in the enzyme active site were generated and stably transfected in HEK293 cells. All single or double substitutions caused a complete loss of APN-specific activity, whereas the wt-APN exhibited high catalytic activity both in protein extracts as well as on the cell surface of intact cells. These data are in accordance with studies of structurally related proteins demonstrating that single amino acid substitutions both in the GAMEN motif and in the zinc-binding motif of human insulin-regulated aminopeptidase resulted in decreased or abolished enzyme activity (Laustsen et al. [Citation2001]). Mutations in the histidines in the HELLGH motif of the rat dipeptidyl peptidase III are associated with an abnormal structure and subsequently loss of enzymatic activity (Fukasawa et al. [Citation1999]). With the exception of H387/391I all APN variants were expressed on the cell surface and showed the same immunoelectrophoretic behavior as wt-APN. Substitutions of both histidine residues to isoleucine at amino acid position 387 and 391 might disrupt zinc binding causing a destabilization of the APN structure, its intracellular retardation and consequently loss of cell surface expression. In contrast, substitutions of these histidines to cysteines might mimic zinc binding thereby leading to cell surface expression of APN. Thus, each amino acid in the enzymatic active site of APN is essential for the catalytic process and their disruption/substitution always leads to a complete loss of APN-specific activity. However, despite the lack of enzyme activity, all variants expressed APN-specific mRNA and protein levels comparable to that of wt-APN.
Overexpression of catalytically active APN in HEK293 cells caused a decreased cell proliferation, migration and soft agar colony formation, whereas cells transfected with the APN variant E388G lacking enzyme activity exhibited growth properties similar to untransfected HEK293 cells. These data indicate the importance of APN enzyme activity, rather than signal transduction via ligation of the membrane enzyme for growth properties. One might speculate that APN either cleaves and inactivates a yet unknown proliferation-promoting substrate, or activates by cleavage an inhibiting mediator. Morphological changes such as epithelial-mesenchymal transition or differences in adhesive features could not be detected in our transfection model (data not shown).
The role of APN expression in tumors is controversially discussed. Diminished or lack of APN expression was described in renal cell cancer (Gohring et al. [Citation1998]), acute myeloid leukaemia (Dybkaer et al. [Citation2001]) as well as in prostate carcinoma (Bogenrieder et al. [Citation1997]) which might be accompanied by an improved anchorage-independent growth or migratory potential. In accordance with our results transfection of an APN-negative ovarian cancer cell line with an APN-expression vector resulted in reduced invasion of cells into matrigel, although the in vitro growth of these cells was not affected (van Hensbergen et al. [Citation2004]). In contrast, a positive correlation of APN with tumor progression was demonstrated in pancreatic (Ikeda et al. [Citation2003]) and hepatocellular carcinoma (Rocken et al. [Citation2004]). Blocking of APN activity by inhibitors or downregulation by small interfering RNA caused a decreased cell growth or metastatic behavior of cell lines of different origin (Kehlen et al. [Citation2003], Kido et al. [Citation2003], Bauvois & Dauzonne [Citation2006]). Contradictory results were obtained for the influence of APN expression in gastric carcinoma: A decreased APN expression associated with a poor prognosis as well as higher metastatic rate in lymph nodes was described by Kawamura and co-authors (Kawamura et al. [Citation2007]), whereas an upregulation of APN expression and a correlation of APN with lymph node metastasis were found by others (Carl-McGrath et al. [Citation2004]).
In order to dissect the molecular mechanisms leading to the altered growth properties, the chemokine receptor status was analyzed in the different APN transfectants and controls. HEK293 cells and the APN-E388G variant, but not wt-APN highly expressed the chemokine receptor CXCR4 and exhibited an increased SDF1α -mediated migration. In contrast, the APN enzyme activity-mediated downregulation of CXCR4 expression completely disrupted the transwell migration to recombinant SDF-1α/CXCL12. These data suggest not only a link between CXCR4 and APN activity, but also the importance of CXCR4 downregulation for the diminished cell proliferation, migration rate as well as the reduced soft agar colony formation. This is in line with a report by Diodovich et al. ([Citation2005]) in which a reduced cell proliferation and clonogenic capability was accompanied by a downregulation of CXCR4 expression in cord blood cells after acrylonitrile exposure. Since chemokine receptors communicate with each other, thereby cross-regulating their function, an indirect regulation of CXCR4 by other chemokines or cytokines inactivated by APN cannot be excluded. APN has been shown to be able to proteolytically process the chemokine CXCL11 which leads to an impaired CXCR3 and CXCR7 binding and signaling and consistently to a reduced cell migration of lymphocytes and endothelial cells (Proost et al. [Citation2007]). However, since SDF-1α has a proline at the penultimate position of the N-terminus, APN should not be able to cleave this chemokine. Bradykinin and substance P possess the Lys-Pro aminoterminus and are natural inhibitors for APN (Xu et al. [Citation1995]). In a cleavage assay of SDF-1α and APN-wt transfectants followed by mass spectrometry we could not detect a processing of recombinant SDF-1α, therefore the degradation of SDF-1α by APN can be excluded (data not shown).
Dipeptidyl peptidase IV (DPIV)/CD26, another member of membrane-bound peptidases known to inactivate SDF-1α (Shioda et al. [Citation1998]), is not expressed on the cell surface of transfected HEK293 cells (data not shown). Therefore, one can exclude a cooperation between APN and DPIV in the cleavage of chemokines. However, it is noteworthy that (i) other proteases in HEK293 cells such as matrix metalloproteinase-9 (MMP-9) or cathepsin G might be involved in the processing of SDF-1α and affect the diminished migration of wt-APN transfectants and that (ii) ligands of neuropeptide receptors as APN substrates might cross-regulate chemokine receptors (Zhang et al. [Citation2003]) resulting in a functionally significant cross-talk between neurohormonal and cytokine signaling.
The molecular mechanisms of the biological functions controlled by CXCR4 are complex. Interaction of SDF-1α/CXCL12 with its G-protein-coupled receptor CXCR4 activates several cellular pathways important for the regulation of adhesion, locomotion or chemotaxis (Kucia et al. [Citation2004]). The most important pathways include the mitogen-activated protein kinase MAPK, the PI-3 kinase/Akt pathway, release of calcium ions, activation of focal adhesion proteins (e.g., p130Cas, paxillin and focal adhesion kinase) as well as signaling via the Janus kinase/signal tranducer and activator of transcription 3 (JAK/STAT3) (Kucia et al. [Citation2004]). Our preliminary results demonstrate that binding of SDF-1α ligand to CXCR4 leads to time-dependent phosphorylation of the MAPK Erk as well as of Akt in all transfectants investigated suggesting that these pathways are not involved in the different migration properties of the wt-APN transfectants in comparison to APN-E388G and control cells (data not shown). Our future investigations have to implement calcium and JAK/STAT3 signaling pathways. Since enzymatically active APN has been associated with membrane protein organization in a recent paper on bradykinin-induced migration of endothelial cells (Petrovic et al. [Citation2007]), we will further study on APN location in rafts/caveolae of transfected HEK293 cells.
In conclusion, to the best of our knowledge this is the first report dissecting the independence of APN expression and enzyme activity. Enzymatic active APN significantly affects cell properties, such as cell proliferation, migration and invasion. This is accompanied by diminished CXCR4 expression and a disruption of SDF-1α-induced migration. However, additional investigations are required to identify APN substrates and to define the molecular mechanism responsible for these effects.
Acknowledgements
This work was supported by the DFG, Bonn, Germany (grant RI 799/2-2) and by the ROUX program of the Martin-Luther-University Halle-Wittenberg, Germany (grant FKZ 4/07). We would like to thank C. Recktenwald for mass spectrometric analysis of cleavage products and C. Stoerr for excellent secretarial help.
References
- Ashmun RA, Shapiro LH, Look AT. Deletion of the zinc-binding motif of CD13/aminopeptidase N molecules results in loss of epitopes that mediate binding of inhibitory antibodies. Blood 1992; 79: 3344–3349
- Bauvois B. Transmembrane proteases in cell growth and invasion: new contributors to angiogenesis?. Oncogene 2004; 23: 317–329
- Bauvois B, Dauzonne D. Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: chemistry, biological evaluations, and therapeutic prospects. Med Res Rev 2006; 26: 88–130
- Bhagwat SV, Lahdenranta J, Giordano R, Arap W, Pasqualini R, Shapiro LH. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 2001; 97: 652–659
- Bogenrieder T, Finstad CL, Freeman RH, Papandreou CN, Scher HI, Albino AP, Reuter VE, Nanus DM. Expression and localization of aminopeptidase A, aminopeptidase N, and dipeptidyl peptidase IV in benign and malignant human prostate tissue. Prostate 1997; 33: 225–232
- Carl-McGrath S, Lendeckel U, Ebert M, Wolter AB, Roessner A, Rocken C. The ectopeptidases CD10, CD13, CD26, and CD143 are upregulated in gastric cancer. Int J Oncol 2004; 25: 1223–1232
- Courtenay VD. A soft agar colony assay for Lewis lung tumour and B16 melanoma taken directly from the mouse. Br J Cancer 1976; 34: 39–45
- Delmas B, Gelfi J, L'Haridon R, Vogel LK, Sjostrom H, Noren O, Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature 1992; 357: 417–420
- Diodovich C, Malerba I, Ferrario D, Bowe G, Bianchi MG, Acquati F, Taramelli R, Parent-Massin D, Gribaldo L. Gene and protein expressions in human cord blood cells after exposure to acrylonitrile. J Biochem Mol Toxicol 2005; 19: 204–212
- Dybkaer K, Olesen G, Pedersen FS, Kristensen JS. Stromal-mediated down-regulation of CD13 in bone marrow cells originating from acute myeloid leukemia patients. Eur J Haematol 2001; 66: 168–177
- Firla B, Arndt M, Frank K, Thiel U, Ansorge S, Tager M, Lendeckel U. Extracellular cysteines define ectopeptidase (APN, CD13) expression and function. Free Radic Biol Med 2002; 32: 584–595
- Fujii H, Nakajima M, Saiki I, Yoneda J, Azuma I, Tsuruo T. Human melanoma invasion and metastasis enhancement by high expression of aminopeptidase N/CD13. Clin Exp Metastasis 1995; 13: 337–344
- Fukasawa K, Fukasawa KM, Iwamoto H, Hirose J, Harada M. The HELLGH motif of rat liver dipeptidyl peptidase III is involved in zinc coordination and the catalytic activity of the enzyme. Biochemistry 1999; 38: 8299–8303
- Gabrilovac J, Cupic B, Breljak D, Zekusic M, Boranic M. Expression of CD13/aminopeptidase N and CD10/neutral endopeptidase on cultured human keratinocytes. Immunol Lett 2004; 91: 39–47
- Gohring B, Holzhausen HJ, Meye A, Heynemann H, Rebmann U, Langner J, Riemann D. Endopeptidase 24.11/CD10 is down-regulated in renal cell cancer. Int J Mol Med 1998; 2: 409–414
- Hooper NM. Families of zinc metalloproteases. FEBS Lett 1994; 354: 1–6
- Ikeda N, Nakajima Y, Tokuhara T, Hattori N, Sho M, Kanehiro H, Miyake M. Clinical significance of aminopeptidase N/CD13 expression in human pancreatic carcinoma. Clin Cancer Res 2003; 9: 1503–1508
- Kawamura J, Shimada Y, Kitaichi H, Komoto I, Hashimoto Y, Kaganoi J, Miyake M, Yamasaki S, Kondo K, Imamura M. Clinicopathological significance of aminopeptidase N/CD13 expression in human gastric carcinoma. Hepatogastroenterology 2007; 54: 36–40
- Kehlen A, Englert N, Seifert A, Klonisch T, Dralle H, Langner J, Hoang-Vu C. Expression, regulation and function of autotaxin in thyroid carcinomas. Int J Cancer 2004; 109: 833–838
- Kehlen A, Lendeckel U, Dralle H, Langner J, Hoang-Vu C. Biological significance of aminopeptidase N/CD13 in thyroid carcinomas. Cancer Res 2003; 63: 8500–8506
- Kido A, Krueger S, Haeckel C, Roessner A. Inhibitory effect of antisense aminopeptidase N (APN/CD13) cDNA transfection on the invasive potential of osteosarcoma cells. Clin Exp Metastasis 2003; 20: 585–592
- Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol 2004; 35: 233–245
- Kumano N, Sugawara S. Ubenimex (Bestatin), an aminopeptidase inhibitor, modulates protein kinase C in K562 cells. J Biol Regul Homeost Agents 1992; 6: 116–120
- Larsen SL, Pedersen LO, Buus S, Stryhn A. T cell responses affected by aminopeptidase N (CD13)-mediated trimming of major histocompatibility complex class II-bound peptides. J Exp Med 1996; 184: 183–189
- Laustsen PG, Vang S, Kristensen T. Mutational analysis of the active site of human insulin-regulated aminopeptidase. Eur J Biochem 2001; 268: 98–104
- Lee VH. Membrane transporters. Eur J Pharm Sci 11 Suppl 2000; 2: S41–50
- Look AT, Ashmun RA, Shapiro LH, Peiper SC. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Invest 1989; 83: 1299–1307
- Luciani N, Marie-Claire C, Ruffet E, Beaumont A, Roques BP, Fournie-Zaluski MC. Characterization of Glu350 as a critical residue involved in the N-terminal amine binding site of aminopeptidase N (EC 3.4.11.2): insights into its mechanism of action. Biochemistry 1998; 37: 686–692
- Marotti T, Balog T, Munic V, Sobocanec S, Abramic M. The link between met-enkephalin-induced down-regulation of APN activity and the release of superoxide anion. Neuropeptides 2000; 34: 121–128
- Murata M, Kubota Y, Tanaka T, Iida-Tanaka K, Takahara J, Irino S. Effect of ubenimex on the proliferation and differentiation of U937 human histiocytic lymphoma cells. Leukemia 1994; 8: 2188–2193
- Petrovic N, Schacke W, Gahagan JR, O'Conor C A, Winnicka B, Conway RE, Mina-Osorio P, Shapiro LH. CD13/APN regulates endothelial invasion and filopodia formation. Blood 2007; 110: 142–150
- Proost P, Mortier A, Loos T, Vandercappellen J, Gouwy M, Ronsse I, Schutyser E, Put W, Parmentier M, Struyf S, Van Damme J. Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signalling and reduces lymphocyte and endothelial cell migration. Blood 2007; 110: 37–44
- Riemann D, Blosz T, Wulfaenger J, Navarrete Santos A. Signal transduction via membrane peptidases. Ectopeptidases, J Langner, S Ansorge. Academic/Plenum Publishers, New York 2002; 141–170
- Riemann D, Kehlen A, Langner J. CD13–not just a marker in leukemia typing. Immunol Today 1999; 20: 83–88
- Rocken C, Carl-McGrath S, Grantzdorffer I, Mantke R, Roessner A, Lendeckel U. Ectopeptidases are differentially expressed in hepatocellular carcinomas. Int J Oncol 2004; 24: 487–495
- Santos AN, Langner J, Herrmann M, Riemann D. Aminopeptidase N/CD13 is directly linked to signal transduction pathways in monocytes. Cell Immunol 2000; 201: 22–32
- Shioda T, Kato H, Ohnishi Y, Tashiro K, Ikegawa M, Nakayama EE, Hu H, Kato A, Sakai Y, Liu H, Honjo T, Nomoto A, Iwamoto A, Morimoto C, Nagai Y. Anti-HIV-1 and chemotactic activities of human stromal cell-derived factor 1alpha (SDF-1alpha) and SDF-1beta are abolished by CD26/dipeptidyl peptidase IV-mediated cleavage. Proc Natl Acad Sci USA 1998; 95: 6331–6336
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 1985; 150: 76–85
- Soderberg C, Giugni TD, Zaia JA, Larsson S, Wahlberg JM, Moller E. CD13 (human aminopeptidase N) mediates human cytomegalovirus infection. J Virol 1993; 67: 6576–6585
- Tieku S, Hooper NM. Inhibition of aminopeptidases N, A and W. A re-evaluation of the actions of bestatin and inhibitors of angiotensin converting enzyme. Biochem Pharmacol 1992; 44: 1725–1730
- Turner AJ. Membrane alanyl aminopeptidase. Handbook of proteolytic enzymes, AJ Barrett, ND Rawlings, JF Woessner. Academic Press, London 1998; 996–1000
- Vallee BL, Auld DS. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 1990; 29: 5647–5659
- van Hensbergen Y, Broxterman HJ, Rana S, van Diest PJ, Duyndam MC, Hoekman K, Pinedo HM, Boven E. Reduced growth, increased vascular area, and reduced response to cisplatin in CD13-overexpressing human ovarian cancer xenografts. Clin Cancer Res 2004; 10: 1180–1191
- Vazeux G, Wang J, Corvol P, Llorens-Cortes C. Identification of glutamate residues essential for catalytic activity and zinc coordination in aminopeptidase A. J Biol Chem 1996; 271: 9069–9074
- Xu Y, Wellner D, Scheinberg DA. Substance P and bradykinin are natural inhibitors of CD13/aminopeptidase N. Biochem Biophys Res Commun 1995; 208: 664–674
- Yeager CL, Ashmun RA, Williams RK, Cardellichio CB, Shapiro LH, Look AT, Holmes KV. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 1992; 357: 420–422
- Zhang N, Hodge D, Rogers TJ, Oppenheim JJ. Ca2 + -independent protein kinase Cs mediate heterologous desensitization of leukocyte chemokine receptors by opioid receptors. J Biol Chem 2003; 278: 12729–12736