Abstract
Changes in the cholesterol levels dynamically alter the microenvironment of the plasma membrane and have been shown to modify functions of ion channels. However, the cellular effect of these modifications is largely unknown. In this report, we demonstrate that cholesterol levels modulate neuronal excitability in rat hippocampal neurons. Reduction of cholesterol levels shortened the duration and increased the firing frequency and peak amplitude of action potentials, while enrichment of cholesterol reversed the effect. Furthermore, we showed that reduction of cholesterol levels increased, while enrichment of cholesterol decreased the amplitude of the delayed rectifier IK currents. On the other hand, reduction of cholesterol levels slowed down the inactivation of the fast transient IA currents, but enrichment of cholesterol had no significant effect on the IA currents. Besides, alteration in cholesterol levels had no significant effect on the action potential in the presence of blockers for both IK and IA currents. These observations demonstrate that cholesterol levels bi-directionally regulate the neuronal excitability mainly through modifications of the IK and IA currents, suggesting an optimum level of cholesterol for the optimum excitability of neurons. Alterations in the neuronal cholesterol levels have been associated with aging, cognitive decline, neurodegenerative diseases, etc. Therefore, our findings are important for a deeper understanding of the relationship between the cholesterol level and dysfunctions of the brain at the molecular level.
Introduction
Cholesterol is an essential structural component that influences physicochemical properties of the plasma membrane in animal cells (Pucadyil & Chattopadhyay [Citation2006]). Cholesterol, together with glycosphingolipids, plays a critical role in organizing membrane microdomains, such as rafts and caveolae (Simons & Ikonen [Citation1997]), which provide platforms for protein sorting or signal transduction. In addition to these structural roles in the membrane, changes in the level of cholesterol regulate a variety of signaling molecules including membrane receptors and ion channels (Simons & Ikonen [Citation1997]). Thus, mammals have developed complex mechanisms to maintain the cholesterol levels within a narrow range (Maxfield & Tabas [Citation2005]). Fine tuning of neuronal cholesterol dynamics is essential for the neuronal functions (Barres & Smith [Citation2001], Saher et al. [Citation2005]). In contrast, abnormal regulation of cholesterol can contribute to the dysfunction of the brain and neurodegenerative diseases (Koudinov & Koudinova [Citation2001], Puglielli et al. [Citation2003], Wolozin [Citation2004], Valenza & Cattaneo [Citation2006]). Obviously, such changes in cholesterol levels could affect the microenvironment of the lipid membrane, which in turn could affect the function of membrane proteins.
Ion channels, as integral membrane proteins lodged in the lipid environment, are modulated by the dynamic alterations in the microenvironment of the membrane (Martens et al. [Citation2000], Barrantes [Citation2002], Tillman & Cascio [Citation2003]). Therefore, it is not surprising that cholesterol plays a role in regulating the ion channel functions. On the other hand, ion channels are important determinants of the neuronal excitability through generating and modulating the action potential (AP) shape and frequency (Hille [Citation2001]), so an interesting question is whether cholesterol could regulate the neuronal excitability through modification of ion channels in the neuronal membrane. We therefore explored the hypothesis that modification of ion channels by cholesterol levels may contribute to changes in the neuronal excitability.
Materials and methods
Neuronal cell culture
Hippocampal neurons were acutely dissociated according to a previous method (Brewer et al. [Citation1993]) with slight modification. Briefly, the hippocampi were dissected from neonatal Sprague-Dawley rats (within 24 h of birth; Weitonglihua Animal Center, Beijing). The hippocampal neurons were dissociated by incubation (7 min, 37°C and 5% CO2) in 0.25% Trypsin-EDTA (GIBCO) and triturated in DMEM (GIBCO) supplemented with 10% bovine serum (Hyclone). The resulting hippocampal cells were plated at a density of 2×105 cells/cm2 onto poly-L-lysine (Sigma, St. Louis, MO, USA) coated glass coverslips (Matsunami, Japan). The coverslips were then incubated at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. The medium was replaced 7 h later with NeurobasalTM- A Medium, B-27 (GIBCO) and 0.5 mM glutamine without antibiotic solution. After 48 h the medium was changed to Neurobasal and B-27. Hippocampal pyramidal neurons were identified morphologically by their large pyramidal shaped cell body with a thick stump of apical dendrite.
Whole-cell patch clamp recordings
Whole cell currents or action potentials of hippocampal neurons between 6 and 14 days in vitro were recorded with an EPC-9 amplifier (HEKA, Germany), filtered with a corner frequency of 10 kHz (four pole Bessel filter), sampled at an interval of 10–50 µsec. A program package PULSE + PULSEFIT (HEKA, Germany) was used for data acquisition and analysis. HANKS’ Balanced salts solution (Sigma), containing (in mM) 1.3 CaCl2, 0.8 MgSO4, 5.4 KCl, 0.4 KH2PO4, 136.9 NaCl, 0.3 Na2PO4, 10 D-glucose and 4.2 NaHCO3, was taken as extracellular solution for all electrophysiological recordings. For K+ currents and action potential recordings, the intracellular solution contains (in mM) 140 KCl, 2 MgCl2, 2 CaCl2, 1 EGTA, 2 Na2ATP and 10 HEPES at pH 7.3. For current clamp, neurons were held at potentials between −70 to −60 mV with steady injection of current. Action potentials were evoked by injecting current with duration of 200 ms and amplitude increased from 0 to 200 pA in a step of 50 pA. The components of voltage-dependent K+ currents were isolated using voltage-clamp protocols. The total K+ currents were recorded in response to depolarizing voltage steps from a holding potential of −90 mV to +70 mV in a step of 10 mV, which activated outward currents consisting of two major components: a fast transient IA and a delayed rectifier IK currents. When a 100 ms prepulse to −40 mV preceded the test pulses, almost all of the transient IA current was inactivated, leaving the outward rectifier IK current (Klee et al. [Citation1995]). The transient IA current was obtained by subtracting IK from the total K+ current. The steady-state inactivation voltage protocol consisted of 500-ms preconditioned pulses ranging from −120 to 50 mV followed by 500-ms test pulse depolarizing to −30 mV. Cholesterol content of native hippocampal neurons was modulated by directly adding cholesterol-free or cholesterol-loaded Methyl-beta-cyclodextrin (MβCD) into the recording chamber. Current rundown was apparent at the first 10 min upon formation of the whole cell patches. To overcome this problem, the effect of cholesterol depletion or enrichment on the current was judged by adding different concentrations of MβCD or MβCD-loaded cholesterol in the recording solution only after the current getting stable within a 3 min period, which usually takes about 15–20 min. Cholesterol loading was performed using water-soluble cholesterol (MβCD–cholesterol complexes at ∼8:1 molar ratio; Sigma-Aldrich). Previous work has established that efficient loading of cells occurs when incubated with MβCD–cholesterol complexes prepared at this ratio (Christian et al. [Citation1997]). Currents were corrected offline for a linear leak current measured at −90 mV. Only cells in which both of the electrical and morphological features were confirmed as those of pyramidal neurones were included for analysis. All experiments were performed at room temperature (22–25°C).
Data analysis
Data were analyzed with Origin 7.0 (Origin Lab, Northampton, MA, USA). Relative changes of parameters were measured as a ratio between the value of the parameter at its stable level after and before application of the chemicals. The steady-state inactivation curves were fitted with a Boltzmann equation as follows: I/Imax = 1/{1 + exp[(V − V1/2)/k]}, where I/Imax is the current magnitude normalized to its maximum value, V designates the conditioning voltage, V1/2 is the voltage at which the magnitude is half-maximum, and k is a Boltzmann slope factor, which reflects the voltage sensitivity of steady-state inactivation of the current. The conductance G for activation was estimated according to the following equation: G = I/(V − Vrev), where I is the current magnitude at the test voltage (V), and Vrev is the reversal potential for the current. The activation curve was also fitted with the Boltzmann equation as follows: G/Gmax = 1/{1 + exp[−(V − V1/2)/k]}, where G/Gmax is the conductance magnitude normalized to its maximum value, V designates the test voltage, and other parameters are the same as above. Time course of IA decay was fitted with two exponential functions: I(t)=Afastexp(−t/τfast) + Aslowexp(−t/τslow), where I(t) is the current at time t after the onset of the depolarization, τfast and τslow are the time constants for the fast and slow components of the current time course, and Afast and Aslow are the amplitude for each component. Data are expressed as mean±SEM of n experiments, significance was tested by one-way ANOVA and a difference was considered significant if p<0.05.
Results
Effect of cholesterol levels on neuronal action potentials
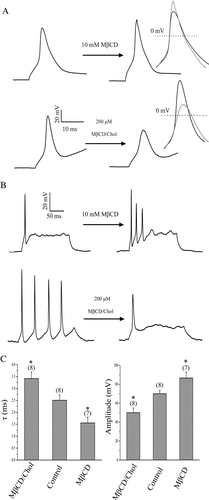
MβCD is a water-soluble cyclic oligosaccharide that enhances the solubility of cholesterol by incorporating it into a hydrophobic cavity (Christian et al. [Citation1997]). Treating cells with cholesterol-free or cholesterol-loaded MβCD (MβCD/Chol) has been widely used to selectively and efficiently reduce or enhance the cholesterol content of the membrane (Christian et al. [Citation1997], Hao et al. [Citation2001]). Using MβCD to manipulate cholesterol level, we studied if cholesterol levels affect neuronal excitability by examining the effects of cholesterol on the AP of the rat hippocampal pyramidal neurons. To begin with, 10 mM MβCD was used to deplete cholesterol level. As shown in A, depletion of cholesterol by MβCD caused a slight but noticeable decrease in the AP duration and increase in the peak AP amplitude in all cells that generated a single action potential. The mean time constant (τ) of AP decay, fitted with single exponential function, was changed from 2.5±0.2 (n=8) of control to 1.6±0.2 ms (n=7) after depletion of cholesterol, while the corresponding peak AP amplitude was increased from 70.1±3.5 to 86.7±6.0 mV (C). Conversely, the above effects of cholesterol depletion could be reversed by increasing cholesterol level of the membrane with cholesterol-loaded MβCD. The mean time constant of AP was slowed down from 2.5±0.2 (n=8) of control to 3.4±0.3 ms (n=8) after enrichment of cholesterol, while the corresponding peak AP amplitude decreased from 70.1±3.5 to 50.0±4.9 mV (A and C). Besides, the firing frequency of the AP was increased by the depletion of membrane cholesterol for some neurons that only generated a single AP in the control condition. In contrast, the firing frequency of the AP was decreased by the cholesterol enrichment for some neurons that generated multiple AP in the control condition (B). These results indicate that cholesterol levels could bi-directionally regulate the neuronal excitability in hippocampal neurons.
Figure 1. Effect of cholesterol levels on action potentials. (A) Effect of cholesterol depletion and enrichment by cholesterol-free (10 mM MβCD) and cholesterol-loaded MβCD (200 µM MβCD/Chol) on action potentials. The inset shows the superimposed AP, which were aligned at the time of maximal upstroke to allow comparison of the time course of the AP at double expanded time scale. (B) Changes in firing patterns of AP induced by cholesterol-free (representative of 6 neurons) and cholesterol-loaded MβCD (MβCD/Chol, representative of 5 neurons). (C) Plots of AP amplitude and time constant for control, depletion (MβCD) and enrichment (MβCD/Chol) of cholesterol (*p<0.05; **p<0.01).
Effect of cholesterol levels on the outward rectifier IK currents
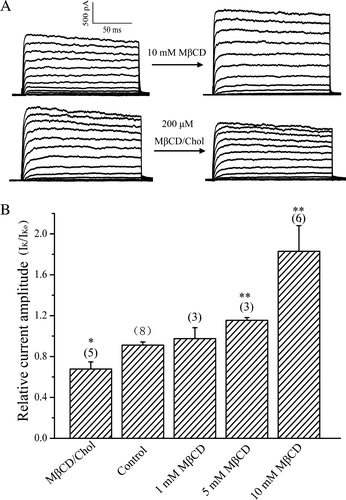
To get insight into the mechanism by which cholesterol modifies the properties of APs, we tested the effects of cholesterol levels on the ion channels that are important for AP in neurons. The above experiments showed that the falling phase of APs was significantly altered by cholesterol levels, suggesting that multiple voltage-dependent K+ channels are involved. Therefore, we tested the action of cholesterol on the outward K+ currents, which could be split into two major components: a fast transient IA and a delayed rectifier IK currents (Klee et al. [Citation1995]). Cholesterol depletion increased, whereas cholesterol enrichment suppressed the IK current at the whole-cell configuration (A). Mean relative IK currents at +10 mV are shown in B for control, cholesterol-depleted and cholesterol-enriched neurons. Exposing neurons into MβCD from concentration of 1 mM to 10 mM resulted in 1.1 to 1.9-fold current increases. In contrast, enrichment with cholesterol with 200 µM cholesterol-loaded MβCD decreased the IK current to 72% of the control (B). These results indicated that the IK currents are sensitive to changes in cholesterol levels altered by MβCD.
Figure 2. Effect of cholesterol levels on the IK currents. (A) Representative current traces for the IK currents for cholesterol-depleted (10 mM MβCD) and enriched (200 µM MβCD/Chol) neurons. (B) Statistics on the ratio (IK/IKo) values of the steady-state outward IK current before (IKo) and after (IK) treatment with MβCD (1–10 mM) or MβCD/Chol. The steady-state outward IK current (+10 mV) was measured as mean value in a range from 85–100% of the current trace.
Effect of cholesterol levels on the transient IA currents
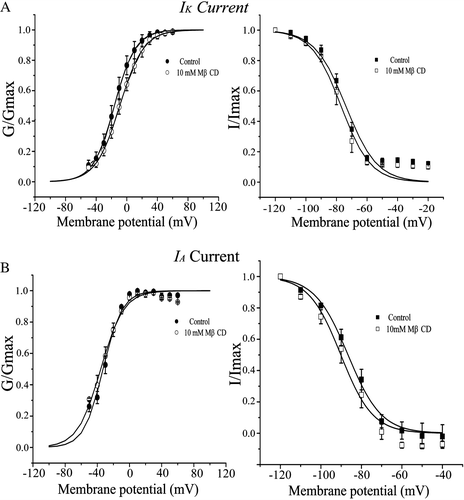
For IA current, the time course of its inactivation relaxed more slowly than that of the control, even though its peak value did not changed significantly by cholesterol depletion. Conversely, cholesterol enrichment almost had no effect on the current at the whole cell level (). At test potential of + 10 mV, only the fast component of time constant (τfast) was significantly affected by cholesterol depletion (10 mM MβCD) according to a double exponential fitting of the time course of IA current decay. The slow component of time constant (τslow) as well as both amplitudes of Afast and Aslow was almost not changed at test potential of +10 mV (B). In contrast, the value of τfast increased 1.8, 2.3 and 2.7 fold with MβCD concentration of 1, 5 and 10 mM, respectively (C). Despite, both activation and inactivation curves of IA and IK were almost not influenced by the cholesterol level compared to that of the control (), suggesting that cholesterol level did not affect the voltage-dependence of the channels. Similar result of cholesterol levels on outward K+ current has been reported in neurons of drosophila mushroom bodies (Gasque et al. [Citation2005]).
Figure 3. Effect of cholesterol levels on the IA currents. (A) Representative current traces for the IA currents for cholesterol depleted and enriched neurons. Inset: Time course of IA decay that was fitted with two exponential functions. Illustrated are representative IA current recorded after cholesterol depletion with 10 mM MβCD. A representative fitted curve is superimposed in grey (shown in red in Molecular Membrane Biology online). (B) Summary of parameters derived from the fitting at a test pulse of +10 mV in the control condition, in the presence of 10 mM MβCD, or in the presence of 200 µM MβCD/chol. (C) Statistics on the ratio (τfast/τfasto) values of the fast time constant component of IA before (τfasto) and after (τfast) treatment with MβCD (1–10 mM) or MβCD/Chol. Times of each experiment are indicated in the parenthesis.
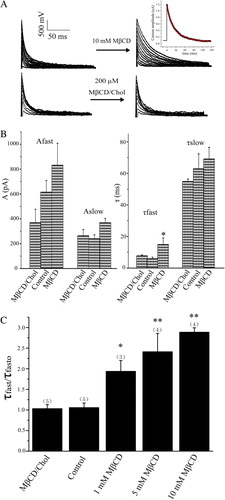
Figure 4. Activation and inactivation curves for IK (A) and IA. (B) in control and MβCD-exposed neurons. For activation curves, the normalized conductance (G/Gmax) is plotted against the membrane potential and fitted with the Boltzmann equation: V1/2 equals to −16.2 and −32.9 mV (control), or −9.2 and −35.5 mV (10 mM MβCD) for IK and IA, respectively. For inactivation curves, the normalized current (I/Imax) is plotted against the conditioning membrane potential and fitted with the Boltzmann equation: V1/2 equals to −73.8 and −86.6 mV (control), or −77.0 and −90.2 mV (10 mM MβCD) for IK and IA, respectively.
Effect of cholesterol on AP in the presence of blockers for IK and IA currents
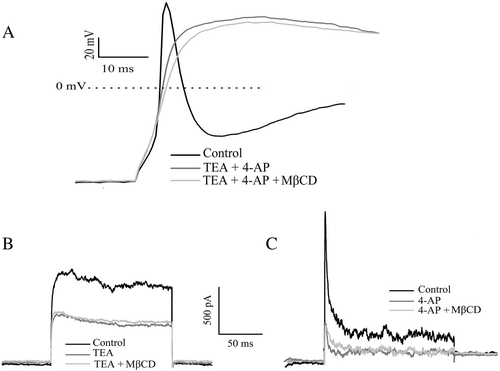
To judge the contribution of IA and IK currents to AP, we examined the effects of changes in cholesterol levels on the AP in the presence of potassium channel blockers. The AP duration was clearly elongated by the externally applied 4-AP and TEA due to blockade of outward K+ currents that are responsible for the falling phase of the AP. Applying MβCD at this condition had no noticed effect on the AP (A). Furthermore, the effect of alteration in cholesterol levels on IK and IA currents was absent when the IK and IA currents were blocked (B and C). These results strongly suggest that the 4-AP sensitive IA and TEA sensitive IK currents are the main contributor for the sensitivity of AP to the cholesterol level.
Figure 5. Effect of cholesterol depletion by MβCD on AP and outward K+ currents in the presence 4-AP and TEA. (A) APs generated from the same neuron in the control condition, in the presence of both TEA (5 mM) and 4-AP (3 mM), and in the presence of 10 mM MβCD after treatment with both TEA and 4-AP (representative of 5 neurons). (B) IK current from the same neuron at +30 mV in the control condition, in the presence of 5 mM TEA, and in the presence of 10 mM MβCD after treatment with TEA (n=4). (C) IA current from the same neuron at +30 mV in the control condition, in the presence of 4 mM 4-AP, and in the presence of 10 mM MβCD after treatment with 4-AP (n=5).
Discussion
In this study, we found that depleting cholesterol resulted in enhancement of IK and slowing of the time course of the IA inactivation, while enhancement with cholesterol reversed the results ( and ). Furthermore, we observed that the effect of alteration in cholesterol level on IK and IA currents was absent when the IK and IA currents were blocked. It has been reported that reduction of the voltage-dependent outward K+ current prolongs AP duration (Vincent et al. [Citation2000]), while increase the current shortens the AP duration (Williams & Sutherland [Citation2004]). In agreement with the reported results, we found that depleting cholesterol resulted in the reduction of AP duration, while enhancement with cholesterol resulted in the prolonging of AP duration (), suggesting that the increases and decreases in IA and IK by cholesterol depletion and enrichment were mainly responsible for the decrease and increase in the AP duration, respectively. It should be noted that both IA and IK may contain several K+ channel types (Storm [Citation1988]). Further work needs to be done to identify which channel types are modulated by the membrane cholesterol levels.
Although we did not directly measure cholesterol levels, numerous studies have shown that cholesterol levels can be modulated by exposing cells to MβCD or cholesterol-loaded MβCD in many cell types. For instance, it has been reported that approximately 30–60% of the total cellular cholesterol can be removed when cells are exposed to 5–10 mM concentration of MβCD for 1 h (Levitan et al. [Citation2000], Sheets et al. [Citation1999], Keller & Simons [Citation1998], Grimmer et al. [Citation2002]). Furthermore, the opposite effects between cholesterol-free and cholesterol-loaded MβCD strongly suggest that the changes in the properties of APs and its related currents were due to changes in the cholesterol level rather than to a direct pharmacological effect of MβCD on the channels. At present, the molecular mechanism of cholesterol on the channels is unclear.
Cholesterol is essential for the formation and stabilization of lipid raft microdomains (Simons & Toomre [Citation2000]). And many pieces of evidence demonstrate that ion channels are located in the microdomains of the cell surface (Martens et al. [Citation2000]). Therefore, it is possible that the effect of cholesterol on the multiple ion channels in neurons is through its action on the raft as reported for other ion channels (Xia et al. [Citation2004], Vial & Evans [Citation2005]). Besides, it has been reported that cholesterol may directly interact with ion channels to influence their functions (Barrantes [Citation2002]) or indirectly affect channel by binding to other related proteins (Huber et al. [Citation2006]), or by altering biophysical properties of the membranes (Pucadyil & Chattopadhyay [Citation2006]).
It has been reported that monkeys who displayed either low or very high firing rates exhibited poor cognitive performance, while those who displayed intermediate firing rates exhibited relatively good cognitive performance (Chang et al. [Citation2005]), implying an optimum excitability for optimum cognitive performance. Our results presented that there is an optimum level of cholesterol for optimum excitability of neurons. As neuronal excitability has coherent relationship with cognitive function of the brain (Chang et al. [Citation2005], Moyer et al. [Citation2000]), we may therefore propose a highly speculative, but intriguing hypothesis that there is an optimum level of cholesterol for the optimum cognitive function of the brain.
In summary, this study highlights a critical role for cholesterol level in bi-directionally modulating neuronal excitability. We also showed that regulation of voltage-dependent IA and IK currents, could account for the effects of cholesterol on neuronal excitability. These data provide insight into the cellular mechanism of how alteration of cholesterol levels influences neuronal excitability. Hopefully, the present study will stimulate further researches designed to elucidate the mechanisms underlying relationships between alteration in cholesterol levels and neuronal excitability-related dysfunction of the brain.
Acknowledgements
This work was partly supported by 973 program (2006CB911003) and NSFC (30470447). We thank Ms M. Y. Huang for technical assistance.
References
- Barrantes FJ. Lipid matters: nicotinic acetylcholine receptor-lipid interactions. Mol Membr Biol 2002; 19: 277–284
- Barres BA, Smith SJ. Neurobiology. Cholesterol–making or breaking the synapse. Science 2001; 294: 1296–1297
- Brewer G.J, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res 1993; 35: 567–576
- Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex 2005; 15: 409–418
- Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res 1997; 38: 2264–2272
- Gasque G., Labarca P, Darszon A. Cholesterol-depleting compounds modulate K + -currents in Drosophila kenyon cells. FEBS Lett 2005; 579: 5129–5134
- Grimmer S, van Deurs B, Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci 2002; 115: 2953–2962
- Hao M, Mukherjee S, Maxfield FR. Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc Natl Acad Sci USA 2001; 98: 13072–13077
- Hille B. 2001. Ionic channels of excitable membranes3rd edn. Sunderland, MA: Sinauer, 814 pp.
- Huber TB, Schermer B, Muller RU, Hohne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K, Shirokova E, Krautwurst D, Harteneck C, Simons M, Pavenstadt H, Kerjaschki D, Thiele C, Walz G, Chalfie M, Benzing T. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci USA 2006; 103: 17079–17086
- Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol 1998; 140: 1357–1367
- Klee R, Ficker E, Heinemann U. Comparison of voltage-dependent potassium currents in rat pyramidal neurons acutely isolated from hippocampal regions CA1 and CA3. J Neurophysiol 1995; 74: 1982–1995
- Koudinov AR, Koudinova NV. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J 2001; 15: 1858–1860
- Levitan I, Christian AE, Tulenko TN, Rothblat GH. Membrane cholesterol content modulates activation of volume-regulated anion current (VRAC) in bovine endothelial cells. J Gen Physiol 2000; 115: 405–416
- Martens JR, Navarro-Polanco R, Coppock EA, Nishiyama A, Parshley L, Grobaski TD, Tamkun MM. Differential targeting of Shaker-like potassium channels to lipid rafts. J Biol Chem 2000; 275: 7443–7746
- Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature 2005; 438: 612–621
- Moyer JRJr, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci 2000; 20: 5476–5482
- Pucadyil TJ, Chattopadhyay A. Effect of cholesterol on lateral diffusion of fluorescent lipid probes in native hippocampal membranes. Chem Phys Lipids 2006; 143: 11–21
- Puglielli LR, Tanzi E, Kovacs DM. Alzheimer's disease: the cholesterol membrane growth. Nat Neurosci 2003; 6: 345–351
- Saher G, Brugger B, Lappe-Siefke C, Mobius W, Tozawa R, Wehr MC, Wieland F, Ishibashi S, Nave KA. High cholesterol level is essential for myelin growth. Nature Neurosci 2005; 8: 468–475
- Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcepsilonRI and their association with detergent-resistant membranes. J Cell Biol 1999; 145: 877–887
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997; 387: 569–572
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000; 1: 31–39
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature 1988; 336: 379–381
- Tillman TS, Cascio M. Effects of membrane lipids on ion channel structure and function. Cell Biochem Biophys 2003; 38: 161–190
- Valenza M, Cattaneo E. Cholesterol dysfunction in neurodegenerative diseases: is Huntington's disease in the list?. Prog Neurobiol 2006; 80: 165–176
- Vial C, Evans RJ. Disruption of lipid rafts inhibits P2X1 receptor mediated currents and arterial vasoconstriction. J Biol Chem 2005; 280: 30705–30711
- Vincent A, Lautermilch NJ, Spitzer NC. Antisense suppression of potassium channel expression demonstrates its role in maturation of the action potential. J Neurosci 2000; 20: 6087–6094
- Williams SH, Sutherland ML. Abbreviated action potential kinetics in a mouse model of potassium channel overexpression during hippocampal development. Cell Mol Neurobiol 2004; 24: 423–441
- Wolozin B. Cholesterol and the biology of Alzheimer's disease. Neuron 2004; 41: 7–10
- Xia F, Gao X, Kwan E, Lam PP, Chan L, Sy K, Sheu L, Wheeler MB, Gaisano HY, Tsushima RG.. Disruption of pancreatic beta-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. J Biol Chem 2004; 279: 24685–24691