Abstract
The last decade has witnessed an exponential increase in interest in one of the great mysteries of nerve cell biology: Specifically, how do neurons know where to place the ion channels that control their excitability? Many of the most important insights have been gleaned from studies on the voltage-gated potassium channels (Kvs) which underlie the shape, duration and frequency of action potentials. In this review, we gather recent evidence on the expression, trafficking and maintenance mechanisms which control the surface density of Kvs in different subcellular compartments of neurons and how these may be regulated to control cell excitability.
Introduction
The voltage-gated potassium channels (Kvs) are structurally and functionally diverse integral proteins with representatives in all eukaryotic and some prokaryotic organisms Citation[1]. The Kvs play a central role in determining the excitability of tissues including heart, skeletal muscle to brain Citation[1], Citation[2]. By modulating the membrane potential and the magnitude, shape and frequency of action potentials, Kvs ultimately control phenomena such as neurotransmitter release, muscle contractility and signal integration Citation[3], Citation[4]. Malfunction in Kvs underlies a range of inherited or acquired diseases and also mediate the side-effects of many clinically useful drugs Citation[5]. Consequently, Kvs have received much attention both as therapeutic targets and as models of ion channel function Citation[4], Citation[5]. The culmination of such efforts, and one of the great achievements in ion channel biology, has been the description of crystal structures and models for Kv channels which rationalize decades of painstaking electrophysiology Citation[6], Citation[7].
The contribution of Kvs, or indeed any ion channel, to cell physiology, is not merely a function of their intrinsic biophysical characteristics. Rather, the macroscopic current (I) is a product of the channel's discrete current contribution (i) and open probability (p), and also the number of channels expressed at the cell surface (N) – or, more accurately, their surface density Citation[2], Citation[8], Citation[9]. While i and p are biophysical parameters intrinsic to the channel, about which, for Kvs, much is known Citation[1], less is known about the cell biological mechanisms which determine Kv surface densities. In polarized cells, such as neurons, the picture is even less clear, since Kvs are often found in functionally discrete domains including axons and dendrites and their sub-compartments (e.g., synapses) (). How cells control the surface density and distribution of Kvs, has been a mystery whose secrets are now emerging. In this review, we examine the possible mechanisms underlying the control of Kv surface expression and distribution using mammalian neurons as a model.
Table I. Properties of neuronal Kvs. Channels are listed according to IUPHAR and HGNC nomenclatures Citation[1]. Biophysical properties of the corresponding channels are derived from Gutman et al. Citation[1] while the distributions are compiled from references given in the text.
The basics: Kv structure, function and assembly
Through sequence comparisons, mammalian Kvs have been classified into 12 subfamilies (Kv1–12) Citation[1] () of which Kvs 1–4 form functional homo- or hetero-tetramers while Kvs 5–12 are assembled as heteromers Citation[1], Citation[10]. Each Kv monomer – termed the α subunit – has a structure () comprised of an intracellular amino and carboxy- terminus and six transmembrane spanning domains designated as S1–S6. The S1–S4 region, collectively, forms a voltage-sensing domain, while S5–S6 and a re-entrant ‘pore-loop’ region in between them, contain the channel's gating machinery Citation[6], Citation[7] (). Assembly of Kvs occurs within the endoplasmic reticulum (ER), primarily, through tetramerization of T1 domains Citation[11], Citation[12] located in the amino terminus of each subunit Citation[9] (). By stabilizing interactions between appropriate subunits and destabilizing inappropriate subunit interactions, the T1 domain directs assembly of subunits within, but not between, subunit families. For example, Kv1.4 can heterotetramerize with other Kv1 family subunits, but not with those from the Kv2, Kv3 or Kv4 families. Additional intra-subunit interactions are also likely, since removal of the T1 domains can still effect some functional Kv expression, albeit without the subunit family-specificity Citation[12]. A notable exception to the ‘T1-intra-family’ rule, concerns the Kv5,6,8 and 9 ‘electrically silent’ subunits which fail to form functional channels unless co-assembled with Kv2 and/or Kv3 subunits Citation[10], Citation[13–18]. When expressed alone, the electrically silent subunits are retained in the ER Citation[10], Citation[16], most likely by a signal located in the C-terminal end of S6 Citation[19], but are released from the ER upon complexation with Kv2 or Kv3 subunits. Studies on Kv9.1 and Kv9.3 suggest the heteromerization interactions are subunit specific, involving the amino termini for Kv2.1 or the hydrophobic core for Kv3.4 Citation[17].
Figure 1. Structure of the Kv channel. (A) Cartoon of single Kv α-subunit, showing transmembrane helices S1-S6. The N-linked glycosylation site, amino and carboxy termini are designated NLG, N and C, respectively. Side (B) and top (C) (extracellular) view of the structure of the Kv1.2 homotetramer (open state), coloured as in (A). Extra and intracellular loops are shown in blue or black, respectively. Structures were modelled and coloured using co-ordinates given in Pathak et al. (2007) Citation[7].
![Figure 1. Structure of the Kv channel. (A) Cartoon of single Kv α-subunit, showing transmembrane helices S1-S6. The N-linked glycosylation site, amino and carboxy termini are designated NLG, N and C, respectively. Side (B) and top (C) (extracellular) view of the structure of the Kv1.2 homotetramer (open state), coloured as in (A). Extra and intracellular loops are shown in blue or black, respectively. Structures were modelled and coloured using co-ordinates given in Pathak et al. (2007) Citation[7].](/cms/asset/3d28ca14-66c8-41a5-a646-332d90397197/imbc_a_299413_f0001_b.jpg)
Following their assembly, Kv tetramers proceed to the cell surface via the classical secretory pathway, passing through the ER-Golgi Intermediate Compartment (ERGIC), cis, medial and trans-golgi elements and then the Trans Golgi Network Citation[9]. In transit, the Kvs can associate with sub-family specific accessory proteins such as Kvβ subunits Citation[20] and experience post-translational modifications such as glycosylation, which modulate their surface expression and kinetics Citation[9], Citation[21].
From the earliest studies it has been evident that specific Kvs have discrete patterns of subcellular segregation Citation[2], Citation[9], Citation[22], Citation[23] (). In general, Kv1 channels, especially those abundant in brain (Kv1.1, Kv1.2 and Kv1.4), localise to axons and nerve terminals Citation[2], Citation[24]. Although these subunits are often expressed in the same cell, they are thought to form select heterotetramers at specific locales. Thus, Kv1.1 seems to co-localize with Kv1.2 to the exclusion of Kv1.4 in terminals of cerebellar basket cells Citation[25–28] and juxtaparanodal regions of myelinated axons Citation[29], Citation[30], but with Kv1.4 to the exclusion of Kv1.2 in striatal efferents Citation[28]. Some exceptions have been noted, however Citation[28], Citation[31]. The delayed rectifier Kv2 channels are primarily homomers Citation[32] localized to somata and dendrites Citation[33–35] where they are thought to control excitability during periods of high action potential firing Citation[34]. While Kv2.1 channels are often clustered Citation[35], Citation[36], Kv2.2 is distributed more uniformly in dendrites Citation[32]. The distribution of Kv3 channels is complicated by their propensity to form both heteromers and C-terminal splice variants Citation[37]. In general, Kv3 subunits are present in fast-spiking cells, notably interneurons, where they enhance the efficiency of re-polarization Citation[38–40]. Typically, Kv3 channels are found in axon terminals, axons, nodes of Ranvier and somata, but differ in dendritic localization. Thus, the longer splice variant of Kv3.1 – Kv3.1b – is present in proximal dendrites, but its shorter variant – Kv3.1a – is restricted to axons Citation[38], Citation[41]. Interestingly, Kv3.1b seems to be present at the nodes of Ranvier, especially in large myelinated neurons Citation[42]. The Kv3.2 channel is notable by its appearance in subsets of interneurons distinct from those containing Kv3.1 Citation[43]. Both Kv3.3 and Kv3.4 are found in dendrites Citation[44], Citation[45] where they modulate large postsynaptic depolarization events but not the smaller excitatory postsynaptic potentials underlying spatial and temporal summation Citation[44]. The Kv4 channels are invariably expressed in somatodendritic regions Citation[22], Citation[46], Citation[47], especially in distal arbors Citation[47], where they influence dendritic excitability, the back-propagation of action potentials and synaptic plasticity Citation[48], Citation[49]. The Kv7 channels Kv7.2–7.5, underlying the delayed rectifier, M (Muscarinic receptor-modulated)-current have been localized to somatodendrites Citation[50]. However, there is also strong evidence for axonal Kv7 channels especially at the nodes of Ranvier Citation[51] and axon initial segments Citation[52], where they could regulate burst firing. The electrically silent subunits, Kvs 5,6,8 and 9 Citation[1], representing approximately 25% of Kvs, have distributions which generally mirror those of the Kv2 and Kv3 series with whom complexation is obligatory for surface expression Citation[13], Citation[15], Citation[16]. Whether such complexation occurs in neurons awaits confirmation.
Mechanisms for Kv localization
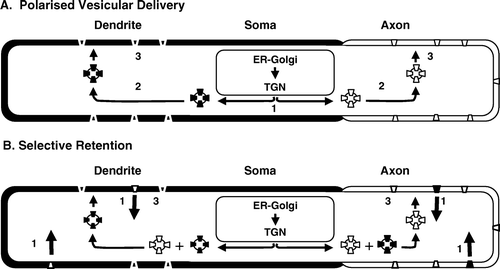
Currently, two major models for neuronal targeting exist () Citation[53], Citation[54]. In the first, targeting occurs via the initial somal sorting of proteins into vesicles which are then trafficked, selectively, to either the axons or dendrites. In the second model, vesicular delivery is not polarized, so proteins are delivered indiscriminately to axons and dendrites and then sorted at the cell surface to retain or remove appropriate or inappropriate components, respectively. Evidence is now accumulating that, the first model controls Kv targeting, especially in axons where, owing to their great lengths, a system of the utmost efficiency is required.
Figure 2. Proposed models for Kv targeting in neurons. In the polarized vesicular trafficking model (A), cargo is sorted at the level of the trans-Golgi network (TGN) into vesicles which are then delivered to axons or dendrites, as appropriate. In the selective retention model (B), vesicular trafficking is not polarised, rather Kvs are trafficked into axons or dendrites, where they are retained in, or removed (large arrows) from, appropriate or inappropriate regions, respectively. Sorting, polarized trafficking and maintenance/retention steps are indicated as 1–3, respectively. Axonal membrane and Kvs are in white, dendritic membrane and Kvs are shown in black.
Selective targeting of Kvs into axons
Seminal work demonstrated that the A-type Kvs, Kv1.4 and Kv4.2, are segregated into axons and dendrites, respectively Citation[22]. Although these Kvs represent good models for targeting, the mechanisms remained elusive until 2002, when sequences within the highly conserved T1 tetramerization domain of Kv1 channels were shown to specify axonal targeting Citation[55]. The T1 domain is also responsible for interaction with the auxiliary Kvβ1 and Kvβ2 subunits Citation[20] and it is these subunits which appear to be both necessary and sufficient for axonal targeting Citation[55], Citation[56]. Significantly, fusion of the T1 domain of Kv1.3 to CD8, a protein normally distributed throughout axons and dendrites causes expression of the T1-CD8 chimera at the surface and within axons, but never in dendrites. Conversely, fusion of the T1 domain to the transferrin receptor (TfR), a protein exclusive to dendrites, localizes the T1-TfR chimera to axons, in accord with the polarized delivery model Citation[57].
Quite how Kvs experience polarized delivery is not fully understood but seems to involve the microtubule (MT)-based cytoskeleton. In neurons MTs have a uni-directional, plus- (distal growing-) end out orientation in nascent axons and dendrites, but, as the neuron acquires its polarized characteristics, the dendrites also, and quite specifically, acquire MTs with a reverse (minus end out) orientation Citation[58]. Thus, it was proposed that proteins might be delivered to axons or dendrites as cargo in vesicles bearing plus or minus end-directed motor proteins. This notion was given considerable support by the recognition that several of the 45 different isoforms of the MT-dependent motor protein, kinesin, were able to direct vesicular trafficking into dendrites Citation[59]. Thus, vectorial delivery of Kvs can be envisaged as reflecting attachment to so-called ‘smart’, destination-specific, MT-dependent motor proteins Citation[53], Citation[59]. The demonstration that the postsynaptic, and hence dendritic, NMDA-subtype of glutamate receptors, associates, via its NR2B subunits, with the vesicular motor protein Kif17, lend powerful support for the smart motor hypothesis and have spurred efforts to identify potential interactions of Kvs with components of the MT-dependent trafficking machinery Citation[60]. In another tour de force of nerve cell biology, Jan's laboratory reported, recently, that axonal trafficking of Kvs requires the docking of Kvβ subunits with the +TIP protein EB1 a member of the TIP family of proteins associated with the growing tips of microtubules Citation[56]. This observation is especially significant as it is now thought that MTs exhibit dynamic growth throughout the axons Citation[56], Citation[61]. Does this preclude a role for vesicular trafficking? It seems not, since these workers also showed that axonal targeting of Kv1 channels also requires an association with KIF3/kinesin II Citation[56]. Thus, it seems likely that Kv1 channels are targeted to axons by selective MT motor-dependent trafficking machines, whose activity also requires or is modulated by complexation with EB1 and, perhaps, other MT binding proteins.
Selective targeting of Kvs into dendrites
Recent experiments show that targeting of the somatodendritic Kv4.2 channel also relies upon MT-based polarized vesicular trafficking. Thus, Kv4.2 is found within and at the surface of dendrites but not axons and, like NMDARs, is trafficked to dendrites via complexation with the MT-motor, Kif17 Citation[62]. Unlike Kv1 channels, however, interaction with Kif17 involves the distal carboxy terminus of Kv4.2 rather than its amino-terminal T1 domains. The underlying mechanism is more complex than this simple interaction suggests, however, since mere Kif17-Kv4.2 association is insufficient to mediate dendritic targeting. Rather, dendritic targeting depends upon an evolutionarily conserved non-endocytic 16 amino acid, dileucine-containing motif within the carboxy terminus of Kv4.2 Citation[63]. Surprisingly, the di-leucine motif is necessary and sufficient for dendritic targeting, but does not interact with Kif17, directly Citation[62]. Consequently, targeting specificity is more likely to reflect regulation of Kif17 by proteins bound to the dileucine motif, than smart motor activity. Indeed, Kif17 mutants, incapable of binding vesicular cargo, are found in both axons and dendrites Citation[64]. Moreover, association with specific cargo-attached proteins can define the motor directionality of kinesins Citation[65].
Maintenance of Kv distributions in axons and dendrites
Irrespective of the precise targeting modality, segregation of Kvs inserted into the surface of axons and dendrites demands mechanisms for their retention in those membrane regions. In epithelial cells, proteins resident in the apical or basolateral surface membrane compartments are prevented from co-mingling, by virtue of the interfacial cuff of cytoskeletal and membrane proteins comprising the tight junctions Citation[66]. Single particle tracking studies suggest a similar cuff exists at the axon hillock forming the boundary of the soma and axon. Thus, the axon hillock is envisaged to act as a picket fence preventing axonal membrane proteins, generally, from diffusing into the somatodendritic regions and vice versa Citation[67]. As discussed below, epigenetic cues may be used to provide additional, more refined, specification of select Kv surface distributions at the nerve surface.
Dynamic regulation of Kv surface expression
A growing body of evidence indicates that Kv surface expression is controlled at the level of both the ER and the plasma membrane. Elegant studies by Deutsch indicate that Kvs attain their overall topology through sequential assembly of biogenic units and association of T1 domains during their co-translational insertion into the ER membrane Citation[12], Citation[68]. Any misfolded Kvs are, in most cases, probably degraded. However, procession of correctly folded Kvs through the secretory pathway does not always occur by default. Thus, the efficiency of ER export of Kv1 channels depends upon the presence of four specific pore-mouth residues, three of which also confer sensitivity to Kv blockade by dendrotoxin (DTX) Citation[69]. Indeed, DTX-sensitive Kvs (Kv1.1, Kv1.2, and Kv1.6) are retained in the ER, while DTX-insensitive ones (Kv1.3, Kv1.4 and Kv1.5) are exported. Expression of DTX in the ER lumen also enhances the cell surface expression of Kv1.1, in homomers or heterotetramers Citation[69] suggesting a DTX-like protein in the ER lumen regulates export of this Kv. Glycosylation also affects the stability and surface expression Citation[9], Citation[21], Citation[70] of some Kvs (Kv1.4) but not others (Kv1.1) and also involves pore determinants Citation[70]. Additional post-translational modifications may also be significant. For example, disruption of thioacylation seems to enhance the degradation of Kv1.5 in its early biogenesis Citation[71].
In addition to ER lumenal mechanisms, export from the ER also seems to require interactions with Kv motifs accessible from the cytoplasm. Thus, mutation of a carboxy terminal VXXSL motif localizes Kv1.4 to the ER Citation[72] by a mechanism which seems to require the presence of residues deep within the Kv pore Citation[73]. In many cases, association of Kvs with modulatory auxiliary subunits also enhances their forward trafficking. Thus, early interactions of Kv1.2 with Kvβ2 and other Kvβ subunits promote N-linked glycosylation and enhance the stability and surface expression of Kv1.2-Kvβ complexes Citation[20], Citation[74].
Forward trafficking is also modulated by the interaction of a subset of Kvs with a second type of auxiliary subunit termed KChap – a member of the Protein Inhibitor of Activated Signal transducer STAT 3 (PIAS3) gene family. In co-expression studies, KChap enhances the functional expression of Kv1.3, Kv2.1, Kv2.2 and Kv4.3 Citation[75], Citation[76] without affecting their kinetics and gating. KChap also increases the total and surface levels of Kv2.1, Kv2.2 and Kv4.3 in a transcription-independent manner Citation[75], Citation[76]. While these data argue that KChap manifests ‘chaperone-like’ properties, the mechanisms are unclear. KChAP, is a cytosolic protein which binds transiently to the cytoplasmic amino termini of its target channels, but, unlike Kvβ subunits is not found at the membrane Citation[75], Citation[76]. Significantly, however, co-expression with Kvβ1.2 subunits reduces the functional effects of KChap, even on those Kvs (Kv2.1 and Kv4.3) which do not bind Kvβ subunits Citation[77]. Conversely, KChap, blocks the actions of Kvβ1.2 on Kv1 subunits, including those (Kv1.4 and Kv1.5) whose functional expression is not enhanced. Since KChap binds Kvβ, it seems likely that Kvβ-KChap complexation blocks either subunit's activity. If this model is correct, the relative levels of Kvβ and KChap, may endow cells with an elegant mechanism for fine-tuning the physiological contribution of its Kvs Citation[77].
A third type of auxiliary subunit – termed KChip – has been identified that, specifically, seems to regulate the forward trafficking of Kv4 channels Citation[78]. The association of KChip proteins, via N-terminal residues and T1 domains in adjacent Kv4.3 α subunits Citation[79], is thought to promote surface trafficking by masking intrinsic ER retention determinants Citation[80]. However, KChip-Kv4 interactions may occur en route to, or within, the golgi apparatus and involve myristoylation Citation[81]. Significantly, the Kv4-KChip interaction site seems to serve as a locus for several proteins which either impair (syntaxin 1A Citation[82]), enhance (frequenin Citation[83]) or modify (long pentraxin PTTX Citation[84]) Kv4 expression or distribution. Enhancement of surface expression is also seen when Kv4 channels interact with certain integral proteins including DPPX (dipeptidyl-aminopeptidase-like protein X, DPP6) a CD26-related homologue of the membrane-bound DPP family of ectopeptidases and also DPPY (DPP10, DRP3) Citation[85]. The DPP-Kv4 interaction appears to involve association of the transmembrane domain of DPP with S1–S2 in Kv4 and can yield ternary complexes with KChip Citation[86]. Another group of single transmembrane auxiliary subunits proteins, termed KCNEs (mink and MiRPs1-3), also promote the surface trafficking of Kv3.1, Kv3.2, Kv4.2, Kv4.3 and Kv11.1 and in at least one case, do so by an early interaction in the secretory pathway Citation[87].
The surface expression of Kvs is also controlled at the end-stages of their trafficking itineraries through endocytic mechanisms. Endocytic removal of Kvs represents a powerful and rapid means to regulate electrical excitability, but, with just a few exceptions Citation[40], Citation[88–91], has not been explored. Most Kvs have motifs conforming to canonical internalization signals (notably di-leucines and NPXY sequences) Citation[92]) recognized by the clathrin-dependent internalization machinery, although other signals and internalization mechanisms probably exist. In HEK293 cells, Kv1.4 internalizes with a half-life of around 90 min Citation[88] and likely involves carboxy-terminal di-leucine motifs Citation[92]. Following internalization, Kv1.5 can experience Rab-GTPase-dependent endocytic recycling, via early endosomes, back to the cell surface, adding an additional level of control to surface expression Citation[89]. Precisely, how Kvs become demarcated for internalization is poorly understood. Phosphorylation appears to be important (see below), but other mechanisms seem likely. Of these, the attachment of ubiquitin to lysine residues – ubiquitylation – is emerging as a key player. Ubiquitylation is known to direct many membrane proteins to the multi-vesicular body/lysosomal degradative pathway Citation[93]. Thus, it is notable that the ubiquitin ligase NEDD-4-2 is reported to down-regulate Kv1.3 Citation[94] and, just recently, Kv7.1-7.5 (KCNQs1-5) Citation[95], Citation[96]. Whether ubiquitylation occurs directly upon binding of NEDD-4-2 Citation[95], Citation[96] and/or involves other ubiquitin ligases or accessory proteins, e.g., KChap awaits clarification Citation[94–96].
Dynamic regulation of Kv surface distributions
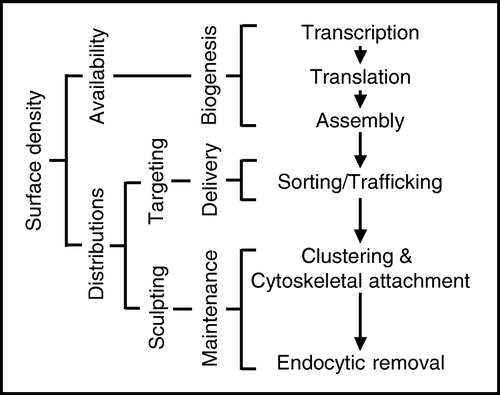
Although vectorial trafficking seems to specify axonal or dendritic targeting, Kvs are clearly not uniformly distributed in axons or dendrites. The simplest explanation is that Kv distributions are determined hierarchically (): Initially, intrinsic programmes specify the vectorial delivery of Kvs into axons or dendrites, and then secondary, epigenetic mechanisms sculpt their distributions according to local need. What might these epigenetic mechanisms be and how would they operate to control local channel topography? The answer seems to lie in an intersection of the mechanisms which stabilize Kvs at the cell surface.
Figure 3. Proposed model for Kv targeting in neurons. Here the overall surface density in specific regions of the nerve membrane is a product of Kv availability and distribution. Biogenic mechanisms determine Kv availability while Kv distributions are determined by sorting, trafficking and maintenance events in two stages. First, Kvs are delivered to axons or dendrites, where their distributions are sculpted according to local need.
In the absence of any restraining factors, membrane proteins are free to diffuse in the plane of the lipid bilayer. In the few cases examined, fluorescence photobleaching studies indicate that Kvs diffuse at rates typical of membrane proteins Citation[97], Citation[98]. However, most Kvs are not freely diffusible, but are immobilized by protein-protein interactions which promote their clustering and attachment to the underlying cytoskeleton Citation[97]. A direct interaction of Kv4.2 with the cytoskeletal protein filamin has been described Citation[99], but less direct interactions through intermediaries such as the actin-binding protein cortactin, and ankyrin have been reported Citation[2], Citation[91]. Many Kvs, including Kv1.4 Citation[88], Citation[100] and Kv4.2 Citation[101], also dock via carboxy terminal motifs to PDZ (Post-Synaptic Density protein 95-Discs Large-ZO1) modules in membrane-associated guanylate kinase (MAGUK) scaffolding proteins Citation[102]. Such interactions control Kv distributions on multiple levels including their incorporation into kinesin-based MT-motor complexes Citation[103], cytoskeletal attachment Citation[102] and clustering Citation[88], Citation[100], Citation[101]. Significantly, the clustering interactions of Kv1.4 with the MAGUK, PSD-95, or cytoskeletal attachment, also prevent Kv internalization Citation[88] and stabilize their axonal expression Citation[104]. Consequently, Kvs probably exist in either free or clustered/immobilized pools, of which only the former are internalization-competent. Thus, the interactions of Kvs with MAGUKs, and/or with specialized regions of the underlying cytoskeleton, permit the mechanisms controlling lateral segregation and surface expression to intersect and effect a site-specific generation of Kv complexes. Precisely where such complexes are formed is unclear, but studies on the concentration of Kvs at the nodes of Ranvier have highlighted a major role for cell contact.
A role for cell contact?
In adult myelinated neurons, Kv1.1, Kv1.2 and Kvβ2 subunits are clustered under the myelin sheath in juxtaparanodal regions (JXPRs) through PDZ-mediated interactions which may involve PSD95 or, more likely, the transmembrane cell adhesion molecule (CAM) Caspr2 Citation[105]. In development, Kv clusters appear concomitantly with myelination Citation[106], Citation[107], while hypomyelination and chronic demyelination induce a more diffuse Kv distribution Citation[106], Citation[107]. In rat sciatic nerve preparations, removal of the myelin sheath by intraneural application of lysolecithin, causes Kv1.1, Kv1.2 and Kvβ2 to re-distribute from the JXPRs and disappear Citation[30]. However, during subsequent re-myelination, the subunits appear transiently at the nodes and then paranodes, after which they re-acquire their adult JXPR distribution. Taken together, these data provide compelling evidence that myelination is required for both the nucleation and maintenance of Kvs at JXPRs. Coordination of Kv distributions in JXPRs is, thus, envisaged to follow a sequence where glial cell adhesion molecules, such as TAG-1, on the surface of oligodendrocytes (CNS) or Schwann cells (periphery), interact with and concentrate, cognate axonal CAMs, notably Caspr2, at sites of axon-glial apposition Citation[108]. The axonal CAMs then serve as sites for the nucleation of complexes of specialized cytoskeletal and scaffolding proteins which stabilize the CAM complexes and allow Kv accumulation. The organization of Kvs by extracellular contact may not be restricted to nodes of Ranvier. For example, Kv2.1, but not Kv2.2, is localized to proximal dendrites at sites close to contact by astrocytic processes Citation[34]. Many Kvs are also expressed at sites of synaptic contact () Citation[46]. Thus, in the visual cortex, Kv4.2 and Kv4.3 are found postsynaptically, opposite GABA terminals in excitatory neurons and interneurons, but at extra-synaptic locations, or peri-synaptically, at sites of excitatory input onto dendrites and spines Citation[47].
Activity-dependent regulation of Kv expression, localization and maintenance
An exciting development has been the discovery that the density and distribution of Kvs which dynamically regulates excitability can in turn be regulated by neuronal activity. Here an intersection of all the machineries controlling Kv surface density is likely. In hippocampal neurons, suppression of synaptic activity, prior to the normal appearance of Kv1.1, Kv1.2, and Kv1.4 in development, appears to inhibit expression of these, but not other, Kv1 channels Citation[109]. Unfortunately, the mechanisms are not well-defined. Indeed, the transcriptional and translational control mechanisms underlying Kv gene expression represent a fertile area, requiring much research. In contrast, much information is beginning to emerge on post-translational control of Kv expression. Thus, the reduction in Kv1.2 currents seen upon phosphorylation of a carboxy-terminal tyrosine residue seems to be due to a weakening of the Kv1.2-cortactin interaction and an enhancement in endocytosis Citation[91]. Neuromodulation by second messenger systems appears to be critical. For example, at basal levels, cyclicAMP (cAMP) controls steady-state Kv1.2 turnover via a PKA-dependent pathway. However, elevations in cAMP increase the surface expression of Kv1.2 via a PKA-independent pathway that blocks endocytosis Citation[110]. Growth factor signalling pathways may also be important as the growth factor receptor TrKB appears to up-regulate Kv1.3 by reducing its rate of internalization Citation[111]. Significantly, the activation of synapses by glutamate Citation[112] or muscarinic Citation[113] receptor stimulation of rat hippocampal neurons, in culture, is reported to cause a rapid dispersal of Kv2.1 from somatodendritic clusters and a shift in its voltage-dependence of activation. The underlying mechanism appears to involve the rapid, calcineurin-mediated dephosphorylation of the Kv2.1 carboxy terminus, triggered by calcium influx from the plasma membrane or IP3 receptor-mediated release from intracellular stores Citation[113]. Here, the Kv2.1 C-terminus seems to be both necessary and sufficient for the phosphorylation-dependent clustering, gating and modulation properties of Kv2.1 and can be transplanted to other Kvs. Activity-dependent regulation of Kv trafficking may also occur through control of ubiquitylation Citation[94]. Recent studies on Kv1.5, however, have revealed another intriguing possibility involving covalent modification with a polypeptide homologous to ubiquitin – the Small Ubiquitin-related Modifier protein, SUMO Citation[114]. Like ubiquitylation, SUMOylation is influenced by, and may be the point of convergence of, diverse signalling pathways on Kvs, many of which contain consensus SUMOylation sequences Citation[114]. In Kv1.5, SUMOylation seems to modulate channel kinetics rather than trafficking. However, the presence of SUMO-specific E3 ligase activity in PIAS proteins, of which KChap is a member Citation[75–77], raises the intriguing possibility that SUMOylation underlies the ability of KChap to enhance trafficking of some Kvs.
Over a decade ago, experimental and computational evidence began to emerge which showed that neurons could modulate channel conductances through feedback mechanisms, requiring sensors and effectors, designed to sustain their firing ‘identities’ Citation[115]. Since the primary means by which voltage changes and neuromodulatory transmission are ‘sensed’ involves Ca2 + entry through voltage-gated Ca2 + channels or release from intracellular stores, respectively, cytosolic Ca2 + may control feedback through trafficking ‘effectors’. Indeed, activation of CamKinase II seems to enhance Kv4.2 surface expression and, thereby, decrease the excitability of hippocampal neurons Citation[116]. Growing evidence suggests the Kv4.2-KChip interaction is a major focal point for activity-dependent regulation of Kv4 trafficking. For example, Ca2 + -dependent conformational changes in KChips, or their phosphorylation by the G-protein receptor kinase, GRK2, may promote forward trafficking of Kv4.2 Citation[81], Citation[117]. As KChip de-phosphorylation seems to involve the Ca2 + -dependent phosphatase, calcineurin, it seems likely that coupled phosphorylation and Ca2 + -dependent dephosphorylation steps control Kv4 trafficking to the cell surface. The forward trafficking of Kv4.2 into dendrites and spines may also be enhanced by a separate mechanism involving the Ca2 + -dependent phosphorylation of the MAGUK, SAP97 Citation[103]. Perhaps the ultimate denouement of how activity may affect Kv trafficking, and its functional significance, has come from very recent evidence showing that dendritic excitability, and long-term potentiation, are enhanced by activity- and Ca2 + -dependent, trafficking of Kv4.2 in hippocampal neurons Citation[45].
Conclusions
In this review we have gathered together evidence, much very recent, describing how the Kvs attain their regionalized subcellular distributions in neurons. We argue that the regionalized expression of Kvs is controlled, hierarchically, through motif-based mechanisms which control the assembly, vectorial delivery and stabilization of Kvs at the cell surface. Regulation of these events occurs through quality control mechanisms within the ER, and an intersection of the mechanisms which relay extracellular cues from synaptic activity and cell contact, with those that define assembly, trafficking and maintenance. Many questions remain unanswered. For example is this model correct? What are all the protein interactions and signalling mechanisms involved and how specific and robust are they? Do neurons acquire their identities by controlling Kv expression and distributions independently or in concert with other channels? Based on precedent, it seems the answers to these questions will be not long coming.
Acknowledgements
This work was supported by funds from the Biotechnology and Biological Sciences Research Council UK (34/C15752). Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, Mckinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stühmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev 2005; 57: 473–508
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nature Neurosci 2006; 7: 548–562
- Manganas LN, Akhtar S, Antonucci DE, Campomanes CR, Dolly JO, Trimmer JS. Episodic ataxia type-1 mutations in the Kv1.1 potassium channel display distinct folding and intracellular trafficking properties. J Biol Chem 2001; 276: 49427–49434
- Delisle BP, Anson BD, Rajamani S, January CT. Biology of cardiac arrhythmias: Ion channel protein trafficking. Circ Res 2004; 94: 1418–1428
- Camerino DC, Tricarico D, Desaphy JF. Ion channel pharmacology. Neurotherapeutics 2007; 4: 184–198
- Long SB, Campbell EB, MacKinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 2005; 309: 897–903
- Pathak MM, Yarov-Yarovoy V, Agarwal G, Roux B, Barth P, Kohout S, Tombola F, Isacoff EY. Closing in on the resting state of the Shaker K+ channel. Neuron 2007; 56: 124–140
- Hille B. Ion channels of excitable membranes. 3rd ed. Sinauer, New York 2001
- Misonou H, Trimmer JS. Determinants of voltage-gated potassium channel surface expression and localization in Mammalian neurons. Crit Rev Biochem Mol Biol 2004; 39: 125–145
- Ottschytsch N, Raes A, Van Hoorick D, Snyders DJ. Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome. Proc Natl Acad Sci USA 2002; 99: 7986–7991
- Pfaffinger PJ, DeRubeis D. Shaker K+ channel T1 domain self-tetramerizes to a stable structure. J Biol Chem 1995; 270: 28595–28600
- Tu L, Wang J, Helm A, Skach WR, Deutsch C. Transmembrane biogenesis of Kv1.3. Biochemistry 1996; 39: 824–836
- Drewe JA, Verma S, Frech G, Joho RH. Distinct spatial and temporal expression patterns of K+ channel mRNAs from different subfamilies. J Neurosci 1992; 12: 538–548
- Post MA, Kirsch GE, Brown AM. Kv2.1 and electrically silent Kv6.1 potassium channel subunits combine and express a novel current. FEBS Lett 1996; 399: 177–182
- Hugnot JP, Salinas M, Lesage F, Guillemare E, de Weille J, Heurteaux C, Mattéi MG, Lazdunski M. Kv8.1, a new neuronal potassium channel subunit with specific inhibitory properties towards Shab and Shaw channels. EMBO J 1996; 15: 3322–3331
- Salinas M, Duprat F, Heurteaux C, Hugnot JP, Lazdunski M. New modulatory alpha subunits for mammalian Shab K+ channels. J Biol Chem 1997; 272: 24371–24379
- Stocker M, Hellwig M, Kerschensteiner D. Subunit assembly and domain analysis of electrically silent K+ channel alpha-subunits of the rat Kv9 subfamily. J Neurochem 1999; 72: 1725–1734
- Zhu XR, Netzer R, Böhlke K, Liu Q, Pongs O. Structural and functional characterization of Kv6.2 a new gamma-subunit of voltage-gated potassium channel. Receptors Channels 1999; 6: 337–350
- Ottschytsch N, Raes AL, Timmermans J-P, Snyders DJ. Domain analysis of Kv6.3, an electrically silent channel. J Physiol 2005; 568: 737–747
- Torres YP, Morera FJ, Carvacho I, Latorre RA. Marriage of convenience: Beta-subunits and voltage-dependent K+ channels. J Biol Chem 2007; 282: 24485–24489
- Watanabe I, Zhu J, Sutachan JJ, Gottschalk A, Recio-Pinto E, Thornhill WB. The glycosylation state of Kv1.2 potassium channels affects trafficking, gating, and simulated action potentials. Brain Res 2007; 1144: 1–18
- Sheng M, Tsaur ML, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron 1992; 9: 271–284
- Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: Contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur J Neurosci 1995; 7: 2189–2205
- Cooper EC, Milroy A, Jan YN, Jan LY, Lowenstein DH. Presynaptic localization of Kv1.4-containing A-type potassium channels near excitatory synapses in the hippocampus. J Neurosci 1998; 18: 965–974
- McNamara NM, Muniz ZM, Wilkin GP, Dolly JO. Prominent location of a K+ channel containing the alpha subunit Kv1.2 in the basket cell nerve terminals of rat cerebellum. Neuroscience 1993; 57: 1039–1045
- Sheng M, Tsaur ML, Jan YN, Jan LY. Contrasting subcellular localization of the Kv1.2 K+ channel subunit in different neurons of rat brain. J Neurosci 1994; 14: 2408–2417
- Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL. Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J Neurosci 1994; 14: 4588–4599
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci 1997; 17: 8246–8258
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal juxtaparanodal regions of neurons. Nature 1993; 365: 75–79
- Rasband MN, Trimmer JS, Schwarz TL, Levinson SR, Ellisman MH, Schachner M, Shrager P. Potassium channel distribution, clustering, and function in remyelinating rat axons. J Neurosci 1998; 18: 36–47
- Juiz JM, Luján R, Domínguez del Toro E, Fuentes V, Ballesta JJ, Criado M. Subcellular compartmentalization of a potassium channel (Kv1.4): preferential distribution in dendrites and dendritic spines of neurons in the dorsal cochlear nucleus. Eur J Neurosci 2000; 12: 4345–4356
- Lim ST, Antonucci DE, Scannevin RH, Trimmer JS. A novel targeting signal for proximal clustering of the Kv2.1 K+ channel in hippocampal neurons. Neuron 2000; 25: 385–397
- Hwang PM, Fotuhi M, Bredt DS, Cunningham AM, Snyder SH. Contrasting immunohistochemical localizations in rat brain of two novel K+ channels of the Shab subfamily. J Neurosci 1993; 13: 1569–1576
- Du J, Tao-Cheng JH, Zerfas P, McBain CJ. The K+ channel, Kv2.1, is apposed to astrocytic processes and is associated with inhibitory postsynaptic membranes in hippocampal and cortical principal neurons and inhibitory interneurons. Neuroscience 1998; 84: 37–48
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience 2001; 108: 69–81
- Scannevin RH, Murakoshi H, Rhodes KJ, Trimmer JS. Identification of a cytoplasmic domain important in the polarized expression and clustering of the Kv2.1 K+ channel. J Cell Biol 1996; 135: 1619–1632
- Luneau CJ, Williams JB, Marshall J, Levitan ES, Oliva C, Smith JS, Antanavage J, Folander K, Stein RB, Swanson R. Alternative splicing contributes to K+ channel diversity in the mammalian central nervous system. Proc Natl Acad Sci USA 1991; 88: 3932–3936
- Weiser M, Bueno E, Sekirnjak C, Martone ME, Baker H, Hillman D, Chen S, Thornhill W, Ellisman M, Rudy B. The potassium channel subunit KV3.1b is localized to somatic and axonal membranes of specific populations of CNS neurons. J Neurosci 1995; 15: 4298–4314
- Sekirnjak C, Martone ME, Weiser M, Deerinck T, Bueno E, Rudy B, Ellisman M. Subcellular localization of the K+ channel subunit Kv3.1b in selected rat CNS neurons. Brain Res 1997; 766: 173–187
- Lien CC, Jonas P. Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J Neurosci 2003; 23: 2058–2068
- Ozaita A, Martone ME, Ellisman MH, Rudy B. Differential subcellular localization of the two alternatively spliced isoforms of the Kv3.1 potassium channel subunit in brain. J Neurophysiol 2002; 88: 394–408
- Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, Scherer SS. Kv3.1b is a novel component of CNS nodes. J Neurosci 2003; 23: 4509–4518
- Chow A, Erisir A, Farb C, Nadal MS, Ozaita A, Lau D, W`elker E, Rudy B. K+ channel expression distinguishes subpopulations of parvalbumin- and somatostatin-containing neocortical interneurons. J Neurosci 1999; 19: 9332–9345
- Martina M, Yao GL, Bean BP. Properties and functional role of voltage-dependent potassium channels in dendrites of rat cerebellar Purkinje neurons. J Neurosci 2003; 23: 5698–5707
- Chang SY, Zagha E, Kwon ES, Ozaita A, Bobik M, Martone ME, Ellisman MH, Heintz N, Rudy B. Distribution of Kv3.3 potassium channel subunits in distinct neuronal populations of mouse brain. J Comp Neurol 2007; 502: 953–972
- Shibasaki K, Nakahira K, Trimmer JS, Shibata R, Akita M, Watanabe S, Ikenaka K. Mossy fibre contact triggers the targeting of Kv4.2 potassium channels to dendrites and synapses in developing cerebellar granule neurons. J Neurochem 2004; 89: 897–907
- Burkhalter A, Gonchar Y, Mellor RL, Nerbonne JM. Differential expression of IA channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses. J Neurosci 2006; 26: 12274–12282
- Chen X, Johnston D. Voltage-gated ion channels in dendrites of hippocampal pyramidal neurons. Pflugers Arch 2006; 453: 397–401
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron 2007; 54: 933–947
- Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci 2001; 21: 9529–9540
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci 2004; 24: 1236–1244
- Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci USA 2006; 103: 8870–8875
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron 2000; 26: 465–472
- Sampo B, Kaech S, Kunz S, Banker G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron 2003; 37: 611–624
- Gu C, Jan YN, Jan LY. A conserved domain in axonal targeting of Kv1 (Shaker) voltage-gated potassium channels. Science 2003; 301: 646–649
- Gu C, Zhou W, Puthenveedu MA, Xu M, Jan YN, Jan LY. The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron 2006; 52: 803–816
- Rivera JF, Chu PJ, Arnold DB. The T1 domain of Kv1.3 mediates intracellular targeting to axons. Eur J Neurosci 2005; 22: 1853–1862
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci USA 1988; 85: 8335–8339
- Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci 2005; 6: 201–214
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 2000; 288: 1796–1802
- Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J Neurosci 2003; 23: 2655–2664
- Chu PJ, Rivera JF, Arnold DB. A role for Kif17 in transport of Kv4.2. J Biol Chem 2006; 281: 365–373
- Rivera JF, Ahmad S, Quick MW, Liman ER, Arnold DB. An evolutionarily conserved dileucine motif in Shal K+ channels mediates dendritic targeting. Nat Neurosci 2003; 6: 243–250
- Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol 2003; 162(6)1045–1055
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein grip1directly steers kinesin to dendrites. Nature 2002; 417: 83–87
- Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature 2003; 422: 766–774
- Nakada C, Ritchie K, Oba Y, Nakamura M, Hotta Y, Iino R, Kasai RS, Yamaguchi K, Fujiwara T, Kusumi A. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol 2003; 5: 626–632
- Robinson JM, Kosolapov A, Deutsch C. Tertiary and quaternary structure formation of voltage-gated potassium channels. Methods Mol Biol 2006; 337: 41–52
- Vacher H, Mohapatra DP, Misonou H, Trimmer JS. Regulation of Kv1 channel trafficking by the mamba snake neurotoxin dendrotoxin K. FASEB J 2007; 21: 906–914
- Watanabe I, Zhu J, Recio-Pinto E, Thornhill WB. Glycosylation affects the protein stability and cell surface expression of Kv1.4 but Not Kv1.1 potassium channels. A pore region determinant dictates the effect of glycosylation on trafficking. J Biol Chem 2004; 279: 8879–8885
- Zhang L, Foster K, Li Q, Martens JR. S-acylation regulates Kv1.5 channel surface expression. Am J Physiol Cell Physiol 2007; 293: C152–161
- Li D, Takimoto K, Levitan ES. Surface expression of Kv1 channels is governed by a C-terminal motif. J Biol Chem 2000; 275: 11597–11602
- Zhu J, Watanabe I, Gomez B, Thornhill WB. Trafficking of Kv1.4 potassium channels: Interdependence of a pore region determinant and a cytoplasmic C-terminal VXXSL determinant in regulating cell-surface trafficking. Biochem J 2003; 375: 761–768
- Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. Beta subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron 1996; 16: 843–852
- Wible BA, Yang Q, Kuryshev YA, Accili EA, Brown AM. Cloning and expression of a novel K+ channel regulatory protein, KChAP. J Biol Chem 1998; 273: 11745–11751
- Kuryshev YA, Gudz TI, Brown AM, Wible BA. KChAP as a chaperone for specific K+ channels. Am J Physiol Cell Physiol 2000; 278: C931–941
- Kuryshev YA, Wible BA, Gudz TI, Ramirez AN, Brown AM. KChAP/Kvbeta1.2 interactions and their effects on cardiac Kv channel expression. Am J Physiol Cell Physiol 2001; 281: C290–299
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature 2000; 403: 553–556
- Wang H, Yan Y, Liu Q, Huang Y, Shen Y, Chen L, Chen Y, Yang Q, Hao Q, Wang K, Chai J. Structural basis for modulation of Kv4 K+ channels by auxiliary KChIP subunits. Nat Neurosci 2007; 10: 32–39
- Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem 2003; 278: 36445–36454
- Hasdemir B, Fitzgerald DJ, Prior IA, Tepikin AV, Burgoyne RD. Traffic of Kv4 K+ channels mediated by KChIP1 is via a novel post-ER vesicular pathway. J Cell Biol 2005; 171: 459–469
- Yamakawa T, Saith S, Li Y, Gao X, Gaisano HY, Tsushima RG. Interaction of syntaxin 1A with the N-terminus of Kv4.2 modulates channel surface expression and gating. Biochemistry 2007; 46: 10942–10949
- Nakamura TY, Pountney DJ, Ozaita A, Nandi S, Ueda S, Rudy B, Coetzee WA. A role for frequenin, a Ca2 + -binding protein, as a regulator of Kv4 K+-currents. Proc Natl Acad Sci USA 2001; 98: 12808–12813
- Duzhyy D, Harvey M, Sokolowski B. A secretory-type protein, containing a pentraxin domain, interacts with an A-type K+ channel. J Biol Chem 2005; 280: 15165–15172
- Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, Goldberg EM, Misumi Y, Ikehara Y, Neubert TA, Rudy B. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron 2003; 37: 449–461
- Ren X, Hayashi Y, Yoshimura N, Takimoto K. Transmembrane interaction mediates complex formation between peptidase homologues and Kv4 channels. Mol Cell Neurosci 2005; 29: 320–332
- Chandrasekhar KD, Bas T, Kobertz WR. KCNE1 subunits require co-assembly with K+ channels for efficient trafficking and cell surface expression. J Biol Chem 2006; 281: 40015–40023
- Jugloff DG, Khanna R, Schlichter LC, Jones OT. Internalization of the Kv1.4 potassium channel is suppressed by clustering interactions with PSD-95. J Biol Chem 2000; 275: 1357–1364
- McEwen DP, Schumacher SM, Li Q, Benson MD, Iñiguez-Lluhí JA, Van Genderen KM, Martens JR. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. J Biol Chem 2007; 282: 29612–29620
- Choi WS, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res 2005; 97: 363–371
- Williams MR, Markey JC, Doczi MA, Morielli AD. An essential role for cortactin in the modulation of the potassium channel Kv1.2. Proc Natl Acad Sci USA 2007; 104: 17412–17417
- McKeown L, Robinson P, Greenwood SM, Hu W, Jones OT. PIN-G – a novel reporter for imaging and defining the effects of trafficking signals in membrane proteins. BMC Biotech 2006; 8: 6–15
- Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev 2006; 86: 669–707
- Henke G, Maier G, Wallisch S, Boehmer C, Lang F. Regulation of the voltage gated K+ channel Kv1.3 by the ubiquitin ligase Nedd4-2 and the serum and glucocorticoid inducible kinase SGK1. J Cell Physiol 2004; 199: 194–199
- Jespersen T, Membrez M, Nicolas CS, Pitard B, Staub O, Olesen SP, Baró I, Abriel H. The KCNQ1 potassium channel is down-regulated by ubiquitylating enzymes of the Nedd4/Nedd4-like family. Cardiovasc Res 2007; 74: 64–74
- Ekberg J, Schuetz F, Boase NA, Conroy SJ, Manning J, Kumar S, Poronnik P, Adams DJ. Regulation of the voltage-gated K+ channels KCNQ2/3 and KCNQ3/5 by ubiquitination. Novel role for Nedd4-2. J Biol Chem 2007; 282: 12135–12142
- Burke NA, Takimoto K, Li D, Han W, Watkins SC, Levitan ES. Distinct structural requirements for clustering and immobilization of K+ channels by PSD-95. J Gen Physiol 1999; 113: 71–80
- O'Connell KM, Rolig AS, Whitesell JD, Tamkun MM. Kv2.1 potassium channels are retained within dynamic cell surface microdomains that are defined by a perimeter fence. J Neurosci 2006; 26: 9609–9618
- Petrecca K, Miller DM, Shrier A. Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin-binding protein filamin. J Neurosci 2000; 20: 8736–8744
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature 1995; 378: 85–88
- Wong W, Newell EW, Jugloff DG, Jones OT, Schlichter LC. Cell surface targeting and clustering interactions between heterologously expressed PSD-95 and the Shal voltage-gated potassium channel, Kv4.2. J Biol Chem 2002; 277: 20423–20430
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci 2004; 5: 771–781
- Gardoni F, Mauceri D, Marcello E, Sala C, Di Luca M, Jeromin A. SAP97 directs the localization of Kv4.2 to spines in hippocampal neurons: regulation by CaMKII. J Biol Chem 2007; 282: 28691–28699
- Arnold DB, Clapham DE. Molecular determinants for subcellular localization of PSD-95 with an interacting K+ channel. Neuron 1999; 23: 149–57
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol 2003; 162: 1149–1160
- Baba H, Akita H, Ishibashi T, Inoue Y, Nakahira K, Ikenaka K. Completion of myelin compaction, but not the attachment of oligodendroglial processes triggers K+ channel clustering. J Neurosci Res 1999; 58: 752–764
- Rasband MN, Trimmer JS, Peles E, Levinson SR, Shrager P. K+ channel distribution and clustering in developing and hypomyelinated axons of the optic nerve. J Neurocytol 1999; 28: 319–331
- Schafer DP, Rasband MN. Glial regulation of the axonal membrane at nodes of Ranvier. Curr Opin Neurobiol 2006; 16: 508–514
- Grosse G, Draguhn A, Höhne L, Tapp R, Veh RW, Ahnert-Hilger G. Expression of Kv1 potassium channels in mouse hippocampal primary cultures: Development and activity-dependent regulation. J Neurosci 2000; 20: 1869–1882
- Connors EC, Ballif BA, Morielli AD. Homeostatic regulation of kv1.2 potassium channel trafficking by cyclic AMP. J Biol Chem 2007; 283: 3445–3453
- Colley BS, Biju KC, Visegrady A, Campbell S, Fadool DA. Neurotrophin B receptor kinase increases Kv subfamily member 1.3 (Kv1.3) ion channel half-life and surface expression. Neuroscience 2007; 144: 531–546
- Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci 2004; 7: 711–718
- Mohapatra DP, Trimmer JS. The Kv2.1 C terminus can autonomously transfer Kv2.1-like phosphorylation-dependent localization, voltage-dependent gating, and muscarinic modulation to diverse Kv channels. J Neurosci 2006; 26: 685–695
- Benson MD, Li QJ, Kieckhafer K, Dudek D, Whorton MR, Sunahara RK, Iñiguez-Lluhí JA, Martens JR. SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5. Proc Natl Acad Sci USA 2007; 104: 1805–1810
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci 2006; 29: 307–323
- Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR, Johnston D, Sweatt JD. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci 2004; 24: 3643–3654
- Ruiz-Gomez A, Mellström B, Tornero D, Morato E, Savignac M, Holguín H, Aurrekoetxea K, González P, González-García C, Ceña V, Mayor F, Jr, Naranjo JR. G protein-coupled receptor kinase 2-mediated phosphorylation of downstream regulatory element antagonist modulator regulates membrane trafficking of Kv4.2 potassium channel. J Biol Chem 2007; 282: 1205–1215